Abstract
Background
Ex-vivo normothermic perfusion strategies are a promising new instrument in organ transplantation. The perfusion conditions are designed to be protective however the artificial environment can induce a local inflammatory response. The aim of this study was to determine the effect of incorporating a Cytosorb adsorber into an isolated kidney perfusion system.
Methods
Porcine kidneys were subjected to 22 h of cold ischaemia then reperfused for 6 h on an ex vivo reperfusion circuit. Pairs of kidneys were randomised to either control (n = 5) or reperfusion with a Cytosorb adsorber (n = 5) integrated into the circuit. Tissue, blood and urine samples were taken for the measurement of inflammation and renal function.
Results
Baseline levels of cytokines (IL-6, TNFα, IL-8, IL-10, IL-1β, IL-1α) were similar between groups. Levels of IL-6 and IL-8 in the perfusate significantly increased during reperfusion in the control group but not in the Cytosorb group (P = 0.023, 0.049). Levels of the other cytokines were numerically lower in the Cytosorb group; however, this did not reach statistical significance. The mean renal blood flow (RBF) was significantly higher in the Cytosorb group (162 ± 53 vs. 120 ± 35 mL/min/100 g; P = 0.022). Perfusate levels of prostaglandin E2 were significantly lower in the Cytosorb group (642 ± 762 vs. 3258 ± 980 pg/mL; P = 0.0001). Levels of prostacyclin were significantly lower in the Cytosorb group at 1, 3 and 6 h of reperfusion (P = 0.008, 0.003, 0.0002). Levels of thromboxane were also significantly lower in the Cytosorb group throughout reperfusion (P = 0.005). Haemoadsorption had no effect on creatinine clearance (P = 0.109).
Conclusion
Haemoadsorption can reduce the inflammatory response and improve renal blood flow during perfusion. Nonetheless, in this model haemoadsorption had no influence on renal function and this may relate to the broad-spectrum action of the Cytosorb adsorber that also removes potentially important anti-inflammatory mediators.
Keywords: Kidney, Ex vivo perfusion, Haemoadsorption, Inflammation
Background
The adoption of ex vivo normothermic perfusion technologies in clinical transplantation offers significant advantages compared to hypothermic techniques [1–3]. Restoring function ex vivo upregulates protective mechanisms [4], replenishes cellular energy [5] and allows a functional assessment of an organ prior to transplantation [6]. Although organs are perfused in a protective environment without leukocytes or complement, inflammatory processes are upregulated [4]. This is possibly due to the mechanical process of perfusion and direct contact of the perfusate with artificial surfaces. The consequences of this heightened inflammatory response in this setting are unknown. Although they appear not to have a detrimental effect on graft outcome, removing them during perfusion may be beneficial. Inflammatory mediators play an important role in exacerbating the severity of renal ischaemia reperfusion injury (IRI) [7]. IRI is a complicated multifactorial process causing damage to the vascular endothelial cells and tubular epithelium due to the production of reactive oxygen species (ROS), upregulation of pro-inflammatory cytokines, recruitment of neutrophils, and complement and platelet activation [8–10]. In transplantation, periods of warm and cold ischaemia exacerbate IRI causing graft dysfunction and reducing graft survival [11, 12].
The removal of cytokines using haemoadsorption has been advocated in the management of severe inflammatory-driven disease states such as sepsis [13] and severe systemic inflammatory response syndrome (SIRS) [14–16]. Although haemoadsorption effectively reduces the concentration of circulating cytokines and improves survival, its effectiveness in other circumstances, such as during cardiopulmonary bypass, remains inconclusive [17].
Locally within the kidney, circulating cells and tubular epithelial cells produce numerous cytokines. Pro-inflammatory cytokines such as IL-1β, IL-6, TNFα and IL-8 are expressed in the early revascularisation phase after transplantation [18–20]. They orchestrate a network of interlinked responses causing tissue damage. The Cytosorb adsorber is most effective for removing molecules in the 10–50 kDa range, which includes many anti- but also pro-inflammatory cytokines [21]. Therefore, the overall effect of the Cytosorb adsorber will depend on the balance between removal of beneficial and deleterious mediators. The aim of this study was to examine the effect of cytokine haemoadsorption in an isolated kidney perfusion system.
Methods
Kidney retrieval
Under Home Office ‘The Animals’ (Scientific Procedures) Act 1986 in the UK, five landrace cross pigs weighing 61.6 ± 7.9 kg underwent a general anaesthesia and midline laparotomy. Both renal pedicles were exposed and the kidneys dissected. A bolus injection of 25000 IU heparin was given 5 min before the renal artery and renal veins were ligated and the kidneys removed. One litre of blood was collected from the aorta into two citrate–phosphate-dextrose-adenine blood bags before the animal was culled with an overdose of barbiturate.
Preservation
Immediately after retrieval the kidneys were flushed with 500 mL of ice-cold University of Wisconsin (UW) preservation solution at a hydrostatic pressure of 100 mmHg. Kidneys were then placed in bags containing ice-cold UW solution, packed in ice and stored for 22 h.
Ex-vivo perfusion system
After removal from cold storage the kidneys were weighed and prepared for reperfusion on the ex vivo perfusion circuit. The renal artery, vein and ureter were cannulated and the kidney was flushed with 200 mL of cold Ringer’s solution to remove the preservation solution.
Perfusion was carried out using an adapted paediatric cardiac bypass system (Medtronic, Bioconsole 560) as described previously [4, 5]. The ex vivo perfusion system was primed with 300 mL Ringer’s solution (Baxter Healthcare, Thetford UK), 2.5 g Mannitol (Baxter Healthcare), 12 mL sodium bicarbonate 8.4% (Fresenius Kabi, Runcorn, UK) and 3000 IU heparin (LEO Pharma A/S, Ballerup, Denmark). Whole blood (300 mL) was then added and recirculated to a temperature of 37.4 °C. The blood-based solution was oxygenated with a balance of 95% oxygen/5% CO2 at a flow rate of 0.1 L/min.
The blood-based solution was circulated continually through the kidney via the renal artery at a mean arterial pressure of 85 mmHg and pump speed of 1500 RPM. A nutrient solution (Synthamin 17 10%, Baxter Healthcare) with 15 mL of sodium bicarbonate 8.4% and 100 IU of insulin added (Actrapid, Novo Nordisk, London, UK) was infused at a rate of 20 mL/h. Glucose 5% (Baxter Healthcare) was infused at a rate of 5 mL/h and Ringer’s solution was used to replace urine output (mL for mL).
Study design
A computerised sequence was generated and pairs of kidneys randomised to either control or Cytosorb adsorber (Cytosorb, LINC Medical Systems Ltd, Leicester, UK) (n = 5 kidneys per group).
The Cytosorb adsorber was flushed and primed with Ringer’s solution then added to the circuit by connecting it to the line from the oxygenator allowing the blood to flow through the adsorber back into the venous reservoir in parallel with the main flow to the renal artery (Fig. 1).
Fig. 1.
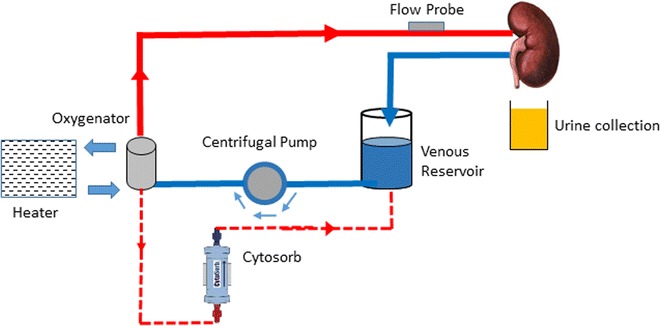
A schematic diagram of the perfusion circuit. The blood based perfusate circulates from the venous reservoir into the centrifugal pump. It is pumped into the membrane oxygenator at a set pump speed and mean arterial pressure were it is oxygenated and warmed to 37.4 °C before entering into the arterial arm of the circuit and into the kidney via the renal artery. The Cytosorb was attached to a line from the oxygenator then fed back into the venous reservoir allowing a collateral circulation of the perfusate without altering the renal blood flow
Outcome measures
The renal blood flow (RBF) was recorded every 5 min for the 30 min and thereafter every 30 min. Samples of perfusate (arterial and venous) were collected pre-perfusion then hourly. Urine samples were collected hourly.
Samples of perfusate and urine were sent to biochemistry for the measurement of urea and electrolytes. Samples of perfusate were also sent for haematology analysis.
Samples of venous perfusate and urine were centrifuged at 1600 rpm for 10 min at 4 °C. The supernatant was collected and frozen in liquid nitrogen then stored at − 70 °C until analysed.
Samples of arterial and venous perfusate were collected at 1, 3 and 6 h of reperfusion for blood gas analysis (OPTI-CCS). Oxygen consumption was calculated (arterial PaO2 – venous PaO2 × RBF/weight of kidney).
RNA extraction and relative gene expression
Core biopsies were taken from each kidney (n = 10) in situ (baseline) and after 6 h of perfusion. Biopsies were stored in RNA later solution (Invitrogen RNA later™ Soln.) for fixing. The tissue was lysed (Precellys Lysing Kit MK28-R) and RNA was extracted (Invitrogen PureLink™ RNA Mini Kit & Invitrogen TURBO DNA-free™ Kit). The RNA was used to generate the cDNAs for each sample by reverse transcription (Applied Biosystems High Capacity cDNA Reverse Transcription Kit). Primers for ICAM-1, IL-1β, IL-6, IL-8, HMGB-1, TLR4 and RPL4 were designed using NCBI Primer Blast (Appendix: Table 2). Relative qPCR (Stratagene Mx3005P) was then performed using SYBR Green (Applied Biosystems Power SYBR® Green PCR Master Mix) on baseline and 6 h perfusion samples for all kidneys and all genes. Cycling conditions are shown in Appendix: Table 3. Samples were assayed in triplicate and Ct values were averaged for each gene. The housekeeping gene (RPL4) Ct value was subtracted from the average Ct value of other genes within that sample (ΔCt). Since the kidneys were paired, the control ΔCt value was subtracted from the Cytosorb adsorber ΔCt value (ΔΔCt). The relative fold change for each gene between treatment and control was then calculated using 2−ΔΔCt. The same analysis was performed comparing the relative expression difference within each kidney between baseline and 6 h.
Table 2.
qPCR primers
| Gene | NCBI accession number | Primer sequences |
|---|---|---|
| ICAM-1 | NM_213816.1 | TCAATGTGGCCCCTAAACACC |
| GTCTCTAGGCCAAAGCTGGT | ||
| IL-1β | NM_214055.1 | CCTTGAAACGTGCAATGATGACT |
| GCCAGCCAGCACTAGAGATT | ||
| IL-6 | NM_001252429.1 | GGGTTCAATCAGGAGACCTGC |
| CGGCCTCGACATTTCCCTTA | ||
| IL-8 | NM_213867.1 | AGAGTGGACCCCACTGTGAA |
| TGTTGTTGCTTCTCAGTTCTCTT | ||
| HMGB-1 | NM_0010040134.1 | GCTCAGAAAGGTGGAAGACCAT |
| TCATAACGGGCCTTGTCCG | ||
| RPL4 | XM_005659862.2 | TGAGCTCTATGGCACTTGGC |
| GAATCTTCTTGCGTGGTGCG | ||
| TLR4 | NM_001113039.2 | TCATGCTTTCTCCGGGTCAC |
| TAGGAACCACCTGCACGCAA |
Table 3.
PCR thermocycler conditions
| Segment | Temperature (°C) | Time (s) |
|---|---|---|
| Segment 1 1 cycle |
95 | 600 |
| Segment 2 40 cycles |
95 | 30 |
| 59 | 60 | |
| Segment 3 1 cycle |
95 | 60 |
| 59 | 30 | |
| 95 | 30 |
Protein analysis
Perfusate levels of the following cytokines and inflammatory/injury markers were measured by ELISA as per the manufacturer’s instructions; IL-1α, IL-1β, IL-6, IL-8, IL-10, TNFα (R&D Systems), CRP (R&D Systems Porcine C-Reactive Protein/CRP) and neutrophil gelatinase-associated lipocalin (NGAL) (Elabscience Porcine NGAL ELISA). Perfusate levels of IL-1RA (Elabscience Porcine IL-1RA ELISA Kit) Prostaglandin E2 Assay, prostacyclin (PG12) and thromboxane B1 (R&D Systems), HMGB1 (Cusabio Pig High Mobility Group Protein B1), heme (BioVision Heme Colorimetric Assay Kit) and urinary levels of NGAL were also measured.
Statistics
Values are presented as mean ± standard deviation. Continuous variables such as RBF were plotted against time. Values were compared using a paired t test. P ≤ 0.050 was considered statistically significant. Statistical analysis was performed using Microsoft Excel and Graphpad Prism 7 (GraphPad Software Inc., La Jolla, CA, USA).
Results
The mean cold ischaemic times in the Cytosorb and control groups were 21.7 ± 0.3 h and 22.9 ± 0.3 h respectively (P = 0.196).
Haemodynamics
The haematocrit (HCT) and platelet count were significantly lower in the Cytosorb group at the start of perfusion compared to the control (P = 0.046, 0.008, 0.003, respectively; Table 1). At 6 h there was no significant difference in the HCT or Hb counts (P = 0.650, 0.626, 0.444, respectively; Table 1). However, the platelet count was significantly lower in the Cytosorb group (P = 0.0003), falling by 50% compared with 40% in the control during perfusion.
Table 1.
Haemoglobin (Hb), haematocrit (HCT), platelet count, white cell count (WCC) and heme before and after 6 h of reperfusion
| Parameters | Pre-perfusion | After 6 h of perfusion | ||||
|---|---|---|---|---|---|---|
| Control | Cytosorb | P value | Control | Cytosorb | P value | |
| Hb (g/L) | 45.2 ± 6.5 | 38.0 ± 5.2 | 0.115 | 37.0 ± 9.5 | 34.6 ± 7.6 | 0.626 |
| HCT (L/L) | 0.16 ± 0.02 | 0.13 ± 0.01 | 0.046* | 0.14 ± 0.03 | 0.13 ± 0.02 | 0.845 |
| Platelets (10 × 9/L) | 172 ± 27 | 106 ± 22 | 0.008* | 106 ± 28 | 52 ± 26 | 0.003* |
| WCC (10 × 9/L) | 2.6 ± 0.9 | 2.4 ± 0.1 | 0.611 | 1.7 ± 1.4 | 1.2 ± 0.7 | 0.262 |
| Heme (pmol/L) | 33.2 ± 1.6 | 33.1 ± 1.3 | 0.785 | 38.8 ± 9.3 | 67.6 ± 56.1 | 0.342 |
Kidneys were reperfused with autologous blood for 6 h on an ex vivo circuit * P <0.050
Baseline levels of heme in the perfusate were similar between groups (P = 0.785; Table 1). There was a numerical increase in the control group during perfusion (P = 0.356) and levels were numerically higher in the control group compared to the Cytosorb group at 6 h (P = 0.342; Table 1).
At the start of perfusion the mean RBF was similar between the groups (Fig. 2). In the control group during the first 20 min of perfusion, the RBF fell and then remained stable before starting to increase after 3 h. In the Cytosorb group there was an increase in the RBF over the first 30 min of perfusion. The RBF then fell abruptly remaining stable before gradually recovering at 2 h (Fig. 2). The mean RBF was significantly lower in the control group (control 120 ± 35 vs. Cytosorb 162 ± 52 mL/min/100 g; P = 0.022).
Fig. 2.
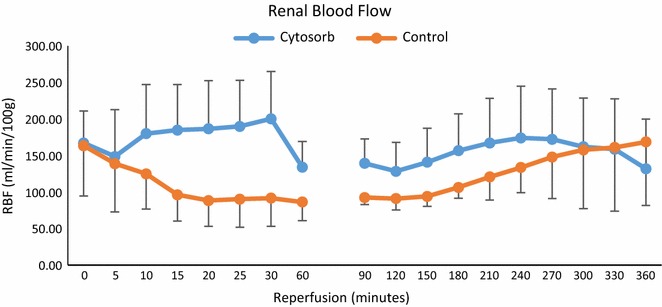
Mean renal blood flow during 6 h of reperfusion in the control and Cytosorb groups
Levels of oxygen consumption were significantly higher in the Cytosorb group at 1 h (96.4 ± 28.1 vs. 59.1 ± 17.2 mL/min/g; P = 0.027) and numerically higher at 3 h (104.4 ± 31.1 vs. 72.0 ± 8.7 mL/min/g; P = 0.057) of perfusion. There was no significant difference in levels at 6 h (P = 0.132).
Renal function
There was no significant difference in the total amount of urine produced between the groups (control 1321 ± 497 mL vs. Cytosorb 1462 ± 515 mL; P = 0.644). Levels of creatinine clearance were similar between groups [area under the curve (AUC) control 31.7 ± 12.3 vs. Cytosorb 40.5 ± 17.9 mL/min/100 g; P = 0.109]. Tubular function was also similar between groups (AUC fractional excretion of sodium, control 219 ± 60.3 vs. Cytosorb 202 ± 93%; P = 0.561).
NGAL
Levels of NGAL in the perfusate were numerically lower in the Cytosorb group at 6 h but did not reach statistical significance (Cytosorb 18 ± 7 vs. control 107 ± 77 ng/mL; P = 0.055). However, urinary NGAL levels were significantly lower in the Cytosorb group throughout reperfusion (6 h 1.1 ± 1.7 vs. 173.5 ± 115.5 ng/mL; P = 0.030).
Cytokines
Baseline levels of cytokines (IL-1β, IL-1α, IL-receptor antagonist (IL-RA), TNFα, IL-6, IL-8, IL-10 and C-reactive protein (CRP) were similar among groups (P > 0.05). There was a numerical increase in the levels of IL-1β, IL-1α, IL-RA, TNFα and IL-10 in the control group throughout perfusion, whereas levels remained low in the Cytosorb group. Levels of IL-6 were significantly lower in the Cytosorb group at 3 and 6 h of perfusion (P = 0.040, 0.023, respectively, Fig. 3). Levels of IL-8 were numerically lower at 6 h of perfusion in the Cytosorb group (P = 0.052). CRP was significantly lower in the Cytosorb group at 1, 3 and 6 h perfusion. There was no significant difference in levels of IL-1β, IL-1α, IL-RA and IL-10 between the groups at 1, 3 and 6 h of perfusion (P > 0.05). The Cytosorb adsorber reduced IL-1β by 39 ± 54%, IL-1α by 48 ± 25%, IL-RA by 52 ± 67%, TNFα by 39 ± 54%, IL-6 by 87 ± 12%, IL-8 by 59 ± 54%, IL-10 by 40% and CRP by 75 ± 31% after 6 h of perfusion compared to the control group.
Fig. 3.
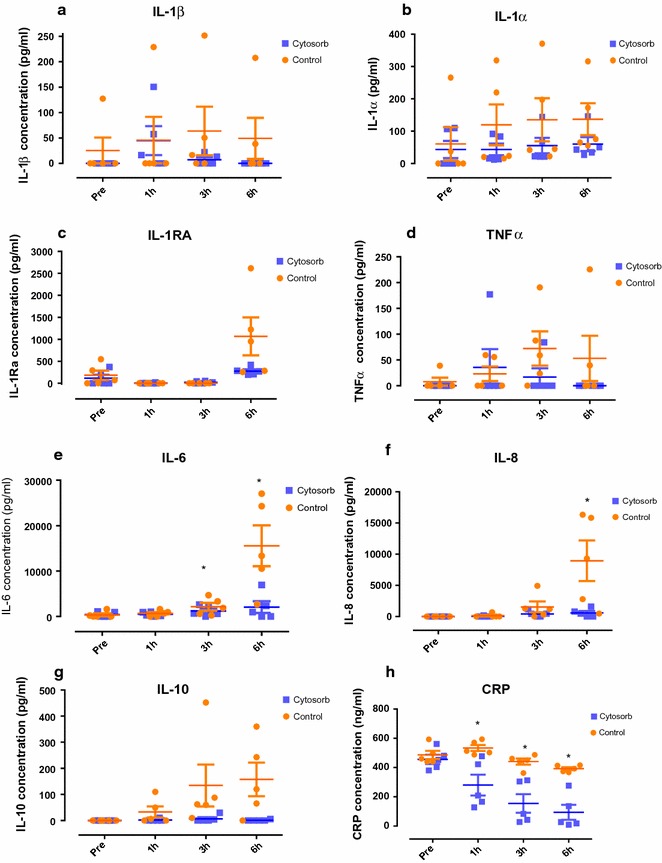
Perfusate levels of a IL-1β, b IL-1α, c IL-RA, d TNFα, e IL-6, f IL-8, g IL-10, and h CRP pre, 1, 3 and 6 h of reperfusion in the control and Cytosorb groups measured by ELISA. *P < 0.05
Prostaglandins
Baseline levels of prostaglandin E2 and prostacyclin were similar in both groups (P = 0.166, 0.236, respectively; Fig. 4), whereas baseline levels of thromboxane were significantly lower in the Cytosorb group (P = 0.024; Fig. 4). There was a numerical decrease in levels of prostaglandin E2 in both groups during perfusion (control P = 0.11, Cytosorb P = 0.066; Fig. 4a) and levels were significantly lower in the Cytosorb group compared to controls at 3 and 6 h (P = 0.023, 0.001; Fig. 4a). Levels of prostacyclin fell significantly in the Cytosorb group (P = 0.033; Fig. 4b) but there was only a numerical fall in the control group (P = 0.095; Fig. 4b). Prostacyclin levels were significantly lower in the Cytosorb group at 1, 3 and 6 h of perfusion compared to controls (P = 0.008, 0.003, 0.0002; Fig. 4b). Levels of thromboxane B2 were significantly lower in the Cytosorb group throughout perfusion (1 h P = 0.035, 3 h P = 0.025 and 6 h P = 0.005; Fig. 4c).
Fig. 4.
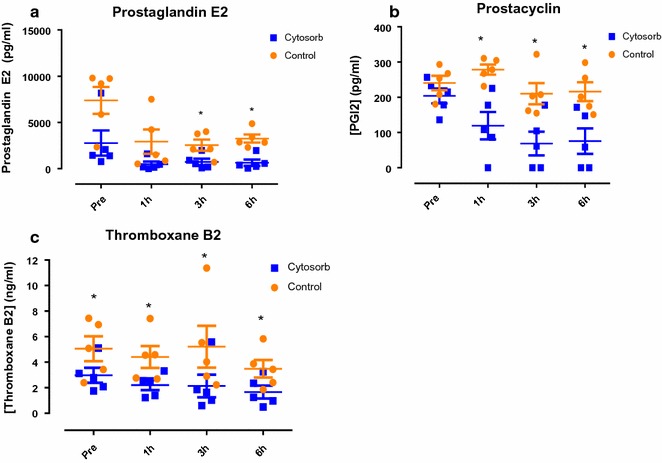
Perfusate levels of a Prostaglandin E2 (PGE2), b Prostacyclin I2 (PGI2), and c Thromboxane B2 (TXB2) measured by ELISA. *P < 0.05
Gene expression
Paired kidney analysis showed a significant downregulation of renal tissue levels of IL-6 (P = 0.017; Fig. 5) in the Cytosorb group relative to the control group after 6 h of perfusion. There was also a numerical decrease in renal IL-8 expression at 6 h in the Cytosorb group relative to the control.
Fig. 5.
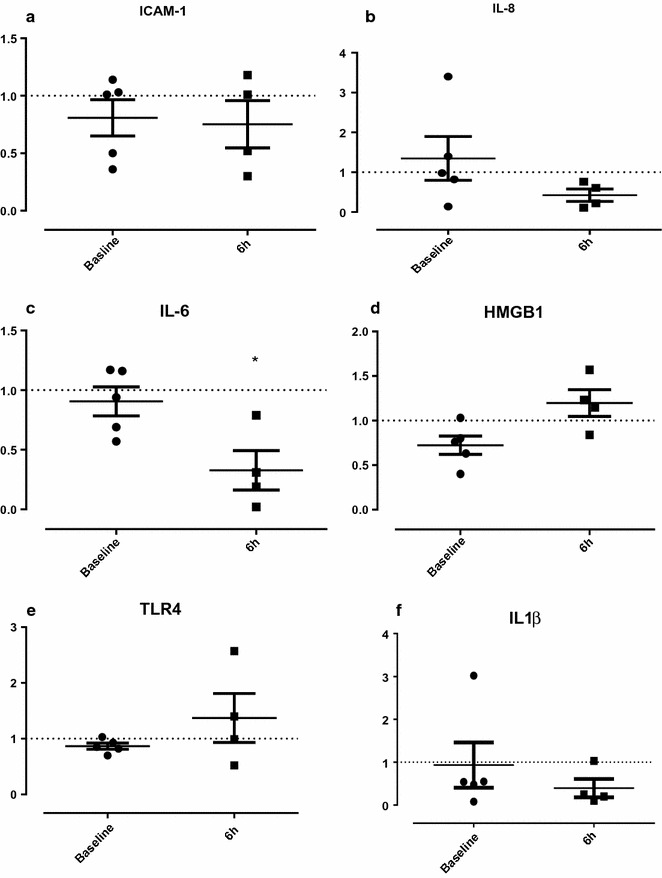
Gene expression in the Cytosorb group relative to the control group after 6 h reperfusion, normalised to RPL4. The relative fold change for each gene between treatment and control was calculated using 2−ΔΔCt. The same analysis was performed comparing the relative expression difference within each kidney between in situ (baseline) and 6 h. *P < 0.05
Individual kidney analysis of gene expression at 6 h relative to baseline showed a statistically significant increase in IL-6 (P = 0.017), but there were no differences in IL-8 (P = 0.176) and IL-1β (P = 0.448) in the control group relative to the adsorber.
Discussion
The Cytosorb adsorber has been used widely in the clinic with the main indication for the treatment of sepsis [21]. Numerous studies have shown that it is a safe and effective way of reducing the systemic inflammatory response. Suppression of inflammation by inhibiting specific inflammatory mediators can reduce the effects of IRI [22]. Haemoadsorption has the advantage that a range of the pro-inflammatory cytokines are effectively reduced rather than targeting individual cytokines. This had a number of beneficial effects in this isolated kidney model. During perfusion there was an increase in the level of circulating pro-inflammatory cytokines, particularly IL-6 and IL-8 after 3 h, but haemoadsorption significantly abrogated this effect. IL-6 and IL-8 are produced as a result of NFκB signalling, typically initiated via IL-1β and TNFα dependent pathways [23]. There was also a significant reduction in IL-6 gene expression and a numerical decrease in IL-8, suggesting a sustained effect in altering the pro-inflammatory profile. However, longer follow up would be needed to determine the full effect of this. Levels of IL-1β and TNFα also increased during reperfusion, although to a lesser extent. This may reflect the study of an isolated perfused organ as opposed to a whole organism, where a larger systemic effect might be anticipated. Nonetheless, haemoadsorption resulted in a numerical reduction in circulating levels.
The Cytosorb adsorber is not specific for pro-inflammatory cytokines and as a consequence the anti-inflammatory cytokine IL-10 along with the receptor antagonist IL-1RA were also reduced by haemoadsorption. IL-10 is essential for maintaining the integrity and homeostasis of tissue epithelial layers and suppressing the pro-inflammatory response [24, 25]. Receptor antagonists inhibit the binding of their corresponding cytokines to prevent their action [26]. IL-1RA can reduce the expression of intracellular adhesion molecule-1 (ICAM-1), and reduce apoptosis [24, 25]. This may explain why we found no difference in the expression of ICAM-1, other pro-inflammatory cytokines or TLR-4 after 6 h of perfusion. Further investigation into the balance of pro- and anti-inflammatory mediators is necessary.
The results of haemoadsorption in the treatment of other inflammatory states such as that induced by cardiopulmonary bypass has been variable. A randomised controlled trial demonstrated no significant differences in pro-inflammatory cytokine levels and no effect on patient outcome [16]. One explanation for the lack of effect may be the duration of the bypass procedure. Patients underwent bypass for a median of 191 min with cytokine levels peaking at 2 h after treatment. Our data show that the majority of cytokines are upregulated after 3 h of perfusion. Therefore, the addition of the Cytosorb adsorber during cardiopulmonary bypass may be beneficial for more prolonged cases.
In spite of the reduction of pro-inflammatory cytokines in this study, haemoadsorption had no effect on renal function. All kidneys demonstrated a similar level of glomerular and tubular function. NGAL, a reliable marker of proximal tubular damage [26, 27], was high in the control kidneys corresponding to the level of cold ischaemic injury. NGAL was reduced in the Cytosorb group, but rather than indicating a reduction in injury during reperfusion this was probably due to direct haemoadsorption of the NGAL molecule, which has a molecular mass of 23 kDa.
The vascular endothelium plays an important role in regulating RBF. In response to ischaemic injury there is a reduction in vasodilatory mediators such as prostacyclin and prostaglandin E2 and enhanced expression of thromboxane, which promotes vasoconstriction, thus reducing blood flow [28]. A reduction in the RBF and oxygen consumption was evident in the control group throughout perfusion and this corresponded with higher thromboxane levels. Haemoadsorption improved overall blood flow throughout 6 h of reperfusion, but at 30 min there was a noticeable rapid decrease in blood flow. This may be explained by the finding of lower levels of thromboxane and a fall in prostacyclin and prostaglandin E2 in the first hour of haemoadsorption. This suggests that the beneficial effect of filtering thromboxane was counteracted by the filtration of prostacyclin and prostaglandin E2.
Platelet activation can enhance IRI and reduce blood flow through endothelial adhesion and release of vasoconstriction mediators [29]. The platelet count was significantly lower at baseline in the Cytosorb group due to the extra volume required to fill the adsorber. This may have enhanced the blood flow at the beginning of perfusion. Nonetheless, the platelet count fell in both the Cytosorb and control groups but the decrement was 10% greater after haemoadsorption and this may be another factor in improving RBF. Heme is associated with the activation of pro-inflammatory, apoptotic and oxidant pathways and can result in further organ damage [30] but in this study was not significantly reduced by haemoadsorption.
Cytokine filtration has recently been applied to ex vivo lung perfusion [31]. Iskender et al. demonstrated that porcine lungs perfused ex vivo for 12 h with the Cytosorb adsorber incorporated into the circuit developed less oedema, had improved electrolyte balance and lower neutrophil infiltration [31]. This present study suggests that haemoadsorption could be beneficial to the kidney during ex vivo perfusion. Ex-vivo normothermic perfusion has recently been introduced into clinical practice in kidney transplantation [1]. Adding a Cytosorb adsorber to the system would enable a proportion of the inflammatory mediators to be removed. However, supplementation particularly of vasodilatory mediators would be necessary to maintain a stable environment. We chose to examine the effect of adding the adsorber into the circuit in the most practical and convenient way. Whole blood rather than leucocyte depleted or packed red cells was used to determine the full effect of the adsorber. This provided important information on the preservation conditions, particularly in regard to the removal of prostaglandins. A single centrifugal pump circulated the blood based solution at a set pump speed and pressure. The Cytosorb was placed in the arterial side of the circuit from the membrane oxygenator back into the reservoir forming a collateral circulation. The adsorber may be more effective if it was placed on the venous arm of the system. However, this would require the addition of another pump which may alter the perfusion of the kidney. The degree of reduction in the levels of cytokines was within range of the described effects of the Cytosorb and with the low volume in the system it is likely that the blood based solution did effectively pass through the adsorber.
Conclusion
In an isolated renal perfusion model, haemoadsorption reduced the inflammatory response and improved RBF. Nonetheless, it had no influence on renal function and this may relate to the broad-spectrum action of the Cytosorb adsorber that also removes potentially important anti-inflammatory mediators.
Authors’ contributions
SAH designed the study carried out the experiments, analysed the results and wrote the manuscript. TM carried out the experiments analysed the samples and co-wrote the manuscript. TK analysed the samples and results. TA carried out the experiments and analysed the samples. MLN designed the study carried out the experiments, and co-wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank Rachel Brown with her assistance in editing the manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was conducted Under Home Office ‘The Animals’ (Scientific Procedures) Act 1986 in the UK.
Funding
The study was funded by the University of Cambridge.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Contributor Information
Sarah A. Hosgood, Phone: +44(0) 1223 763105, Email: sarahhosgood@hotmail.com
Tom Moore, Email: tm401@cam.ac.uk.
Theresa Kleverlaan, Email: theresa_kleverlaan@hotmail.co.uk.
Tom Adams, Email: thom.d.adams@gmail.com.
Michael L. Nicholson, Email: mln31@cam.ac.uk
References
- 1.Nicholson ML, Hosgood SA. Renal transplantation after ex vivo normothermic perfusion: the first clinical study. Am J Transplant. 2013;13(5):1246–1252. doi: 10.1111/ajt.12179. [DOI] [PubMed] [Google Scholar]
- 2.Barbas AS, Goldaracena N, Dib MJ, Selzner M. Ex-vivo liver perfusion for organ preservation: recent advances in the field. Transplant Rev. 2016;30(3):154–160. doi: 10.1016/j.trre.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Cypel M, Keshavjee S. Extracorporeal lung perfusion (ex vivo lung perfusion) Curr Opin Organ Transplant. 2016;21(3):329–335. doi: 10.1097/MOT.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 4.Hosgood SA, Patel M, Nicholson ML. The conditioning effect of ex vivo normothermic perfusion in an experimental kidney model. J Surg Res. 2013;182:153–160. doi: 10.1016/j.jss.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Bagul A, Hosgood SA, Kaushik M, Kay MD, Waller HL, Nicholson ML. Experimental renal preservation by normothermic resuscitation perfusion with autologous blood. Br J Surg. 2008;95(1):111–118. doi: 10.1002/bjs.5909. [DOI] [PubMed] [Google Scholar]
- 6.Hosgood SA, Barlow AD, Hunter JP, Nicholson ML. Ex vivo normothermic perfusion for quality assessment of marginal donor kidney transplants. Br J Surg. 2015;102(11):1433–1440. doi: 10.1002/bjs.9894. [DOI] [PubMed] [Google Scholar]
- 7.Malek M, Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Renal Inj Prev. 2015;4:20–27. doi: 10.12861/jrip.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yano T, et al. The pathological role of IL-18Rα in renal ischemia/reperfusion injury. Lab Invest. 2015;95:78–91. doi: 10.1038/labinvest.2014.120. [DOI] [PubMed] [Google Scholar]
- 9.Jang HR, Ko GJ, Wasowska BA, Rabb H. The interaction between ischemia-reperfusion and immune responses in the kidney. J Mol Med. 2009;87:859–864. doi: 10.1007/s00109-009-0491-y. [DOI] [PubMed] [Google Scholar]
- 10.Kamińska D, et al. The influence of warm ischemia elimination on kidney injury during transplantation—clinical and molecular study. Sci Rep. 2016;6:36118. doi: 10.1038/srep36118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datta N, Devaney SG, Busuttil RW, Azari K, Kupiec-Weglinski JW. Prolonged cold ischemia time results in local and remote organ dysfunction in a murine model of vascularized composite transplantation. Am J Transplant. 2017 doi: 10.1111/ajt.14290. [DOI] [PubMed] [Google Scholar]
- 12.Kogelmann K, Jarczak D, Scheller M, Drüner M. Hemoadsorption by CytoSorb in septic patients: a case series. Crit Care. 2017;21:74. doi: 10.1186/s13054-017-1662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Träger K, et al. Treatment of post-cardiopulmonary bypass SIRS by hemoadsorption: a case series. Int J Artif Organs. 2016;39:141–146. doi: 10.5301/ijao.5000492. [DOI] [PubMed] [Google Scholar]
- 14.Houschyar KS, Nietzschmann I, Siemers F. The use of a cytokine adsorber (CytoSorb) in a patient with septic shock and multi-organ dysfunction (MODS) after a severe burn injury. Handchir Mikrochir Plast Chir. 2017;49:123–126. doi: 10.1055/s-0042-111965. [DOI] [PubMed] [Google Scholar]
- 15.David S, Thamm K, Schmidt BMW, Falk CS, Kielstein JT. Effect of extracorporeal cytokine removal on vascular barrier function in a septic shock patient. J Intensive Care. 2017;5:12. doi: 10.1186/s40560-017-0208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernardi MH, et al. Effect of hemoadsorption during cardiopulmonary bypass surgery—a blinded, randomized, controlled pilot study using a novel adsorbent. Crit Care. 2016;20:96. doi: 10.1186/s13054-016-1270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menke J, Sollinger D, Schamberger B, Heemann U, Lutz J. The effect of ischemia/reperfusion on the kidney graft. Curr Opin Organ Transplant. 2014;19:395–400. doi: 10.1097/MOT.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 18.Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072. doi: 10.1155/2009/137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thurman JM. Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol. 2007;123:7–13. doi: 10.1016/j.clim.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houschyar KS, et al. Continuous hemoadsorption with a cytokine adsorber during sepsis—a review of the literature. Int J Artif Organs. 2017;40:205–211. doi: 10.5301/ijao.5000591. [DOI] [PubMed] [Google Scholar]
- 21.Lutz J, Thürmel K, Heemann U. Anti-inflammatory treatment strategies for ischemia/reperfusion injury in transplantation. J Inflamm. 2010;7:27. doi: 10.1186/1476-9255-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabryšová L, Howes A, Saraiva M, O’Garra A. The regulation of IL-1 expression. Curr Top Microbiol Immunol. 2014;380:157–190. doi: 10.1007/978-3-662-43492-5_8. [DOI] [PubMed] [Google Scholar]
- 24.Frangogiannis NG, et al. Cytokines and the microcirculation in ischemia and reperfusion. J Mol Cell Cardiol. 1998;30:2567–2576. doi: 10.1006/jmcc.1998.0829. [DOI] [PubMed] [Google Scholar]
- 25.Rusai K, et al. Administration of interleukin-1 receptor antagonist ameliorates renal ischemia-reperfusion injury. Transpl Int. 2008;21:572–580. doi: 10.1111/j.1432-2277.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 26.Hosgood SA, Nicholson ML. An assessment of urinary biomarkers in a series of declined human kidneys measured during ex vivo normothermic kidney perfusion. Transplantation. 2016 doi: 10.1097/TP.0000000000001504. [DOI] [PubMed] [Google Scholar]
- 27.Au V, Feit J, Barasch J, Sladen RN, Wagener G. Urinary neutrophil gelatinase-associated lipocalin (NGAL) distinguishes sustained from transient acute kidney injury after general surgery. Kidney Int Rep. 2016;1:3–9. doi: 10.1016/j.ekir.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yun SH, Sim EH, Goh RY, Park JI, Han JY. Platelet activation: the mechanisms and potential biomarkers. Biomed Res Int. 2016;9060143:2016. doi: 10.1155/2016/9060143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Immenschuh S, Vijayan V, Janciauskiene S, Gueler F. Heme as a target for therapeutic interventions. Front Pharmacol. 2017;8:146. doi: 10.3389/fphar.2017.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iskender I, et al. Cytokine filtration modulates pulmonary metabolism and edema formation during ex vivo lung perfusion. J Heart Lung Transplant. 2017;17(S1053–2498):31802–31808. doi: 10.1016/j.healun.2017.05.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


