Abstract
Please cite this paper as: Chadha et al. (2011) Multi site Virological Influenza Surveillance in India: 2004–2008. Influenza and Other Respiratory Viruses 6(3), 196–203.
Background Influenza surveillance is important to identify circulating, emerging/reemerging strains and unusual epidemiological trends. With these objectives, a multisite human influenza surveillance network was initiated in India in 2004.
Methods Epidemiologic data and throat swabs for laboratory testing were collected from patients with influenza‐like illness (ILI) and severe acute respiratory infections (SARI). Virus isolation was carried out in Madin–Darby canine kidney cells and strains identified by hemagglutination inhibition assay. Meteorological data were collected.
Results From September 2004 to December 2008, 617 (4·43%) of 13928 cases yielded isolates: 27·8% were influenza A(H1N1), 29·8% were type A(H3N2), and 42·3% were type B. The yearly type and subtype distribution varied significantly from site to site. Peak influenza activity was observed from June to August in Delhi, Pune, and Kolkata and October to December in Chennai. Maximum influenza activity was seen during the rains in Delhi, Pune, Chennai, and Kolkata in correlation with virus isolations. Multivariate analysis of ILI cases showed chill/rigors, cough, fatigue, and ILI in family, correlated positively with isolation. Genetic analysis of Indian isolates revealed that viruses matched with vaccine strains by and large. Overlapping between circulating and vaccine component strains of consecutive years was also observed.
Conclusions Seasonal influenza A(H1N1), H3N2, and type B co‐circulated in all regions without any particular pattern of movement of any subtype. Year‐round limited influenza activity with peaks during rains was observed. Genetic drifts and varying seasonality in different parts of the country suggest that a staggered timing of vaccination may be appropriate for India.
Keywords: India, influenza strains, multisite surveillance, vaccine strains
Introduction
Influenza is a widespread viral infection that can also cause severe or fatal disease in the elderly, the very young, pregnant, and those with underlying illness. 1 , 2 Globally, the World Health Organization (WHO) estimates the burden of influenza at approximately 3–5 million cases of severe illness and >300 000 deaths annually. 3 In temperate regions, seasonal influenza epidemics occur during the winters, often resulting in increased hospitalizations, deaths, and significant economic losses owing to workplace absenteeism and cost of medical care. 4 Because of annual outbreaks and occasional pandemics, control of influenza has become a major public health challenge. 4 Of even greater concern is the ability of influenza A viruses to cause pandemics, as has been observed with recent H1N1/2009 pandemic. 5 , 6 , 7
Influenza surveillance in the community is an important tool for monitoring circulating strains, detection of emerging/reemerging viruses, and epidemiological trends and to define seasonality in different geographical areas. It is also relevant for the evaluation of antigenic and genetic characteristics of circulating strains in comparison with recommended vaccine strains.
The influenza virus surveillance system has been well established in many countries and has greatly contributed to the control of influenza virus infections. In temperate regions, outbreaks consistently occur during the late autumn and winter months; in November–March in the Northern Hemisphere; and in May–September in the Southern Hemisphere. 4 This consistent pattern helps in carrying out vaccination programs at the same time year after year, making it an efficient control measure. In some tropical and subtropical countries, influenza activity is noted year round with peaks in the rainy season. 8 However, in places like Hong Kong, besides the peak during the rainy season, additional activity was observed in the summer months. 9
Limited surveillance had been conducted in India. Several outbreaks in Pune, Himachal Pradesh, 10 Delhi, 11 and Kolkata 12 have been investigated. Seasonality data have shown that major peaks of influenza activity were during rainy season in Pune and winters in Delhi. 11 Not much is known about the prevalence and burden of influenza in India because of inadequate and limited data. A systematic laboratory‐based surveillance network of influenza viruses was established by the Indian Council of Medical Research, India, in 2004. This network included seven clinical virology laboratories geographically distributed in Northern, Western, Eastern, Southern, and Central India. In this report, we summarize surveillance data from these seven sites over a period of 4 years.
Materials and methods
Study sites
The participating centers were National Institute of Virology(NIV), Pune (Western India, 18°31′25″N, 73°50′52″E), All India Institute of Medical Sciences(AIIMS), New Delhi (North India, 28°36′36″N, 77°13′48″E, King Institute of Preventive Medicine(KIPM), Chennai (South India, 13°5′2″N, 80°16′12″E), National Institute for Cholera and Enteric Diseases (NICED) (Eastern India, 22°34′11″N, 88°22′11″E), and Regional Medical Research Center(RMRC), Dibrugarh (27°29′N, 94°54′E). Surveillance was carried out among patients attending outpatient departments (OPD) with influenza‐like Illness (ILI).
In 2006, 2 more sites were added to the network: Indira Gandhi Medical College (IGMC), Nagpur (Central India) (21·07°N, 79·27°E), for ILI surveillance and Christian Medical College and Hospitals (CMCH), Vellore (South, 12·868719°N, 79·119000°E), for both ILI and severe acute respiratory illness (SARI) surveillance in hospitalized patients. NIV, Pune, was the referral center for the entire study.
Study population
Patients with ILI presenting in outpatient departments of dispensaries/hospitals were included in the study. In the centers specified for the surveillance of SARI, hospitalized patients were studied. Each center was required to randomly collect 5–10 specimens per week throughout the study period.
Case definition of ILI
A person presenting with sudden onset of fever >38°C or history of sudden onset of fever in the recent past (<3 days), cough or sore throat, and/or rhinorrhea. 13
Severe acute respiratory infections was defined as an ILI case with breathlessness or difficulty in breathing/tachypnea or clinically suspected pneumonia (in children) with increased respiratory rates as per Integrated Management of Childhood Illness (IMCI). 14 A confirmed influenza case was an ILI/SARI patient from whose clinical specimen influenza virus could be isolated.
Patients were identified, and their demographic and clinical details were recorded on a preset proforma, which was uniform for all the study sites. Throat and/or nasal swabs were collected in virus transport medium, supplemented with Hanks’ balanced salt solution consisting of antibiotics and bovine serum albumin, transported to the laboratory on ice within 4 h and maintained at +4° till processed for virus isolation within 24 h.
Virus isolation and identification
Madin–Darby canine kidney (MDCK) cells were maintained in minimum essential medium (supplemented with 10% fetal bovine serum, penicillin [100 U/ml], streptomycin [100 μg/ml], and amphotericin B [0·25 μg/ml]) and incubated at 37°C with 5% CO2. Three microliters of respiratory samples was inoculated into MDCK cells in T25 culture flask in the presence of viral growth medium (minimal essential medium with 2 μg/ml TPCK‐treated trypsin) and incubated for 1 h to allow adsorption followed by the addition of viral growth medium. Cells were observed for 7 days and harvested at the end of 7 days or earlier if cytopathic effect was evident. Supernatants from all flasks were subjected to hemagglutination (HA) test using standard method. Subtype identification of HA‐positive isolates was performed by hemagglutination inhibition (HI) test. 15
Virus isolates were re‐grown in MDCK cells and reconfirmed at the referral center. Representative isolates were subsequently shipped to the WHO Collaborating Center at Center for Disease Control and Prevention, Atlanta, USA, for further antigenic and genetic characterization.
Meteorological data were collected from the governmental meteorological departments by all centers. Weekly data were collected for relative humidity, average rainfall, and temperature for all the sites for entire study period including rainy and other seasons. Univariate regression analysis was carried out with average number of confirmed cases per month as a dependent variable and average rainfall, average temperature, and average relative humidity as independent variables. The analysis was carried out using monthly mean value of each variable. Multivariate regression analysis using stepwise method was also carried out with only independent variables showing statistically significant correlation with average number of cases per month. Similarly, correlation analysis between monthly pooled percentage of isolates and averaged meteorological variables was carried out.
Genetic analysis
HA1 gene sequencing and phylogenetic analysis
Representatives of H1N1 (n = 145), H3N2 (n = 133), and type B (n = 169) isolates from all sites to represent all the geographical regions of India temporally were sequenced for HA1 gene and compared with corresponding Northern Hemisphere vaccine components phylogenetically.
RNA extraction and RT‐PCR and nucleotide sequencing
Viral RNA was extracted using commercially available QIAamp Viral RNA Mini Kit (Qiagen GmbH, Hilden, Germany) as per manufacturer’s instructions. For the extraction of viral RNA, 140 ul of MDCK cultured fluid was used. To amplify HA1 gene, 5 μl of RNA was added to 50 ul of master mix containing 25 μl of 2 × enzyme buffer, 2 μl Invitrogen Superscript III enzyme, 1 μl of each reverse and forward primers (previously published), 1 μl RNasin (Promega, Madison, WI, USA), and 15 ul molecular‐grade water. The reaction conditions were reverse transcription at 55°C for 40 min, initial denaturation at 94°C for 3 min followed by 35 cycles of 94°C for 30 s, 50°C for 1 min, and 68°C for 1 min, with a final extension at 68°C for 5 min. Nucleotide (nt) sequencing was carried out in an ABI Prism 3730 DNA Analyzer (PE Applied Biosystems, Foster City, CA, USA) using published gene‐specific forward and reverse primers. Sequences were curetted using sequencing analysis version 5.3. Sequence analysis, including pairwise sequence alignment and protein translation, was performed with the MEGA (version 4, Arizona State University, AZ, USA) program.
Nucleotide sequencing
The resulting amplicons for HA1 segments were analyzed by 1% agarose gel electrophoresis. The expected size products that appeared as single bands were purified directly using PCR Purification kits (Qiagen). DNA sequencing was carried out using Big Dye terminator V 3.1 cycle sequencing ready reaction kit (ABI, Foster City, CA, USA) together with corresponding internal primers, which were designed de novo to ensure specificity for each sequence. Subsequently, any unincorporated labeled dNTPs were removed using Dye‐x removal column purification kit (Qiagen). The sequencing was carried out on ABI 3730 DNA analyzer.
Phylogenetic analysis
MEGA version 4 was used for constructing neighbor‐joining (NJ) trees using the Kimura’s two‐parameter distance model, with 1000 bootstrap replicates.
Results
Influenza surveillance in India
From September 2004 to December 31, 2008, 13928 specimens were collected from ILI and/or SARI cases from seven surveillance sites. Influenza viruses were isolated from 617 (4·43%) of the samples (Table 1). The average isolation rates ranged from 1·06% to 5·99% in different sites over the entire study period. Of these, 356 (57·7%) were influenza A, and 261 (42·3%) were influenza B viruses. Of 356 influenza A viruses, 172 (48%) were of seasonal A(H1N1) and 184 of A(H3N2) subtypes (Table 1).
Table 1.
Patients analyzed from various sites
| Center | Samples collected | Isolates (%) | H3 | H1 | Type B | |
|---|---|---|---|---|---|---|
| Victoria | Yamagata | |||||
| Delhi | 3075 | 149 (4·84) | 43 | 33 | 51 | 22 |
| Chennai | 3712 | 198 (5·33) | 72 | 52 | 26 | 48 |
| Dibrugarh | 1694 | 18 (1·06) | 6 | 5 | 6 | 1 |
| Kolkata | 2144 | 106 (4·9) | 27 | 34 | 17 | 28 |
| Nagpur | 539 | 8 (1·48) | 1 | 5 | 1 | 1 |
| Pune | 2230 | 106 (4·75) | 24 | 42 | 34 | 6 |
| Vellore | 534 | 32 (5·99) | 11 | 1 | 14 | 6 |
| Total | 13928 | 617 (4·43) | 184 | 172 | 149 | 112 |
Isolation rates in ILI cases were significantly higher than rates in SARI cases at the Delhi (5·2% and 1·6%, respectively, P < 0·002) and Vellore (9·5% and 2·5%, respectively, P < 0·001) sites.
Clinico‐epidemiological characteristics
The median age of ILI/SARI patients enrolled in the study was 14·66 years (range: 1 month–94 years). The highest number of ILI/SARI samples was in the age group of <5 year old (n = 6462), of which 4·3% (276) were influenza positive. There was no statistically significant difference in the influenza virus isolation rates among different age groups (<5, >5–15, >15–45 or >45 years old) (Figure 1).However, frequency of type B influenza was significantly higher among children (≤15 years) than among adults [2·1% among children and 1·4% among adults P = 0·0046, OR 1·5 (95% CL and CI 1·13–2·02)]. Such a difference was not observed for influenza A.
Figure 1.
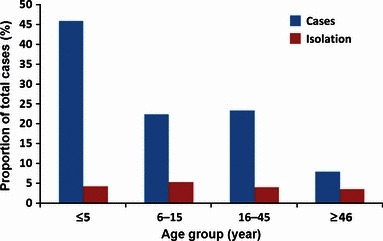
Age‐related distribution of influenza‐like illness (ILI) cases.
Overall, the male‐to‐female ratio of influenza positives individuals was 1·5–1; there was no significant difference in different age groups.
Clinical features were analyzed for 13737 cases, which included 11669 of ILI and 2068 of SARI patients. Among 11 669 ILI cases, 550 (4·7%) were influenza positive, and of 2068 SARI cases, 66 (3·2%) had influenza.
In ILI cases, fever, cough, nasal discharge, headache, body ache, sore throat, chills/rigors, expectoration, and fatigue were noted in 96·3, 91·8, 78·4, 32·8, 29·8, 18·7, 13·6, 12, and 11·4 percent cases, respectively. Frequency of cough, fever, nasal discharge, headache, body ache, sore throat, fatigue, expectoration, and chills/rigors was noted in 96·5, 94·9, 76·4, 38·7, 36, 34·9, 26·7, 24·9 and 24 percent cases, respectively, in SARI cases.
Further, univariate analysis for ILI cases with and without influenza positivity showed that body ache, fatigue, chill/rigors, cough, sore throat, ILI in family, expectoration, and headache had a positive correlation with influenza positivity. Multivariate analysis showed that chill/rigors, cough, fatigue, and ILI in family had a significant correlation with influenza positivity among ILI cases (Table 2). Univariate analysis for SARI cases showed that only body ache to have a positive correlation with isolation; therefore, a multivariate analysis was not carried out.
Table 2.
Multivariate analysis for isolation positives [influenza‐like illness (ILI) Cases]
| Odds ratio | 95% C.I. | P‐Value | |
|---|---|---|---|
| Body ache | 1·2135 | 0·9466, 1·5556 | 0·1268 |
| Chills/rigors | 1·5815 | 1·2707, 1·9684 | <0·001 |
| Cough | 1·8449 | 1·2247, 2·7792 | 0·0034 |
| Expectoration | 0·9424 | 0·7253, 1·2246 | 0·6572 |
| Fatigue | 1·8002 | 1·4045, 2·3074 | <0·001 |
| Headache | 1·1055 | 0·8739, 1·3984 | 0·4032 |
| ILI family | 2·5991 | 1·6502, 4·0937 | <0·001 |
| Sore throat | 0·9835 | 0·7869, 1·2291 | 0·8834 |
Seasonality
Month‐wise distribution in four major sites, i.e., north (Delhi), west (Pune), east (Kolkata), and south (Chennai), of India revealed differences in the influenza seasonality and the presence of influenza types and subtypes over time (Figure 2a–d).
Figure 2.
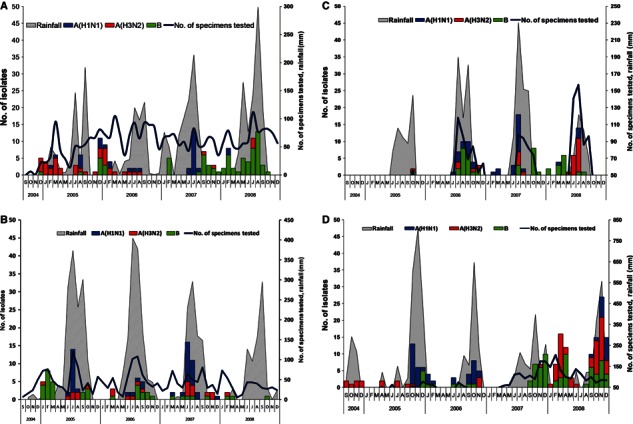
Month‐wise distribution of influenza subtypes in four major sites (a) New Delhi, (b) Pune, (c) Kolkata and (d) Chennai, India, in correlation with rainfall.
In Delhi (North India), two peaks were consistently observed, one from July to Sept (rainy season) and another from January to March (Figure 2a). In Delhi, from late 2004 to the middle of 2006, there was predominance of H3N2 viruses with co‐circulation of H1N1 and influenza B. From August 2006 up to September 2007, there was no H3N2 positivity for 1 year. In June–July 2007, we observed a major H1N1 activity, which was followed by predominance of influenza B consistently from September 2007 to 2008.
In Pune, the predominant influenza activity was observed during July and August (rainy season) with minor activity in the months of January to March in years 2005, 2006, and 2007. However, there was very limited influenza activity (isolation rate was 1%) in year 2008. While all three viruses, H1N1, H3N2, and influenza B circulated over the years in Pune, there was predominance of H1N1 in 2005 and 2007 (Figure 2b).
In Kolkata, major peak was observed from June to August, which coincides with rainy season (Figure 2c). All three types/subtypes circulated. Influenza B was predominant in 2006 and later part of 2007 and early 2008. Further, there was a surge of H1N1 in July 2007 and predominance of H3N2 in July 2008.
In Chennai, predominant activity of influenza viruses occurred from September to December (rainy season) (Figure 2d), with a predominance of H1 in 2005 and 2006 and influenza B in 2007 and 2008, with co‐circulation of H3N2 in 2008. In 2008, an additional peak was observed from February to April with co‐circulation of influenza B and H3N2 viruses.
Influenza activity was consistent with the rainy season at all sites. New Delhi had an additional peak of influenza activity in the winter season. It is located in the north of India, which has a marked winter season as compared to other parts of the country.
In New Delhi, isolation percentage had positive correlation with relative humidity (r 2 = 0·42, P = 0·025). A positive correlation with rainfall was observed (r 2 = 0·31, P = 0·064). In Pune, isolation had positive correlation with rainfall (r 2 = 0·33, P = 0·056). In Kolkata, percentage of isolation had positive correlation with rainfall (r 2 = 0·32, P = 0·057) also with temperature (r 2 = 0·33, P = 0·056). In Chennai, percentage of isolation had positive correlation with rainfall (r 2 = 0·67, P = 0·001) as well as with relative humidity (r 2 = 0·56, P = 0·006). In multivariate analysis, only relative humidity had significant positive correlation (adjusted r 2 = 0·80, P = 0·043) (Figure S1a–d).
Antigenic and genetic comparison with vaccine strains
We compared the circulating strains from all sites in India with recommended Northern Hemisphere vaccine component during corresponding periods.
On genetic comparison of H1N1 isolates with recommended vaccine strains for the study period, two distinct clusters representing A/New Caledonia/20/1999, which was 2004–2007 vaccine component, and A/Brisbane/59/2007, which was 2008–2009 vaccine component, were observed. Majority (n = 32) of 2005 isolates of Delhi, Pune, and Chennai and 23 2006 isolates up to November from Delhi and Chennai grouped and matched with A/New Caledonia/20/1999 vaccine component. None of the 2007–2008 isolates clustered with vaccine component A/Solomon Islands/3/2006 of the corresponding period. However, they clustered with A/Brisbane/59/2007, which was 2008–2009 vaccine component (Figure 3a).
Figure 3.
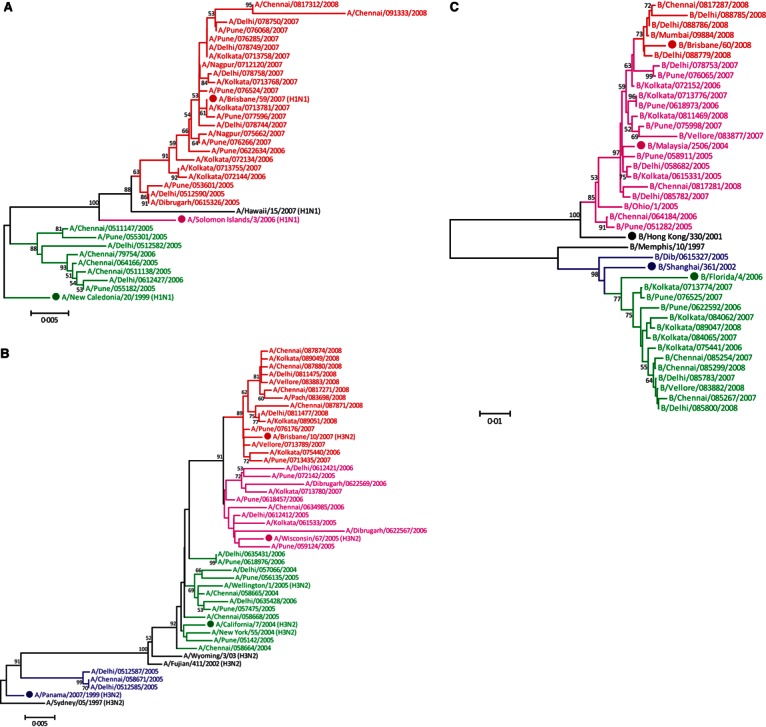
(a) Phylogenetic tree comparing HA1 gene of H1N1 Indian isolates and vaccine components for the study period using the neighbor‐joining method. Scale bar indicates number of nucleotide substitutions per site. Solid dot indicates Northern Hemisphere vaccine components (Accession Nos. CY090286CY090430). (b) Phylogenetic tree comparing HA1 gene of H3N2 Indian isolates and vaccine components for the study period using the neighbor‐joining method. Scale bar indicates number of nucleotide substitutions per site. Solid dot indicates Northern Hemisphere vaccine components (Accession Nos. CY090431CY090572). (c) Phylogenetic tree comparing HA1 gene of influenza type B Indian isolates and vaccine components for the study period using the neighbor‐joining method. Scale bar indicates number of nucleotide substitutions per site. Solid dot indicates Northern Hemisphere vaccine components (Accession Nos. CY090573CY090737).
As seen in Figure 3b, H3N2 isolates when compared with vaccine strains of the corresponding period showed four distinct clusters. Fifteen 2004–2005 isolates from Delhi and Chennai clustered with A/Panama/2007/1999, which was 2000–2004 Northern Hemisphere vaccine component. Further, 2005–2008 isolates clustered with the corresponding vaccine strains; A/New Caledonia/20/1999, A/Wisconsin/67/2005, A/Brisbane/10/2007, respectively.
Phylogenetic analysis of influenza type B isolates (Figure 3c) showed that during all 4 years, both the lineages were circulating in India. Majority strains however clustered with corresponding vaccine component.
Discussion
Although limited influenza activity was seen the year round, a positive correlation with the rainy season was noted at sites like Pune, Kolkata, and Chennai with a secondary peak in cooler winter months. However, in Delhi, a peak of influenza activity in winter with enhanced influenza activity during the rains was observed throughout the study period. Delhi, being located in the north of the country and significantly cooler in the winter months, shows an influenza activity similar to temperate regions. Our findings of seasonal peaks in influenza virus activity in India are consistent with data reported from other Southeast Asian countries. 16 , 17 , 18 The peak influenza activities coincide with rainy seasons in Thailand, 19 Singapore, 20 Indonesia, 21 Myanmar, 22 and China. 23 , 24 Regional differences in peak circulation of influenza viruses in India might be due to differences in climate as Northern India has distinct seasons and lower temperatures in winters, while the south has a more tropical climate with rainy and dry seasons. These data illustrate the difficulty in having effective uniform vaccination timing for a vast country like India and have implications when formulating vaccination policies.
Isolation rates of 4·43% observed during our study concurred with earlier surveillance activity conducted in Pune since 1976. 25 , 26 Isolation rates varied from site to site from 1·06% to 5·99%. Health‐seeking behavior or access to medical care is not uniform in geographically diverse areas of India. Certain sites like Dibrugarh, in the northeast, have a particularly difficult terrain, and patients may have sought medical attention after a lag of few days after onset of illness leading to a lower isolation rates. It is known that successful isolation and identification of influenza virus in clinical specimens depend on specimen quality, time lag from illness to specimen collection, proper transport, and storage of specimens till processing for isolation.
Higher isolation rates in ILI cases than among SARI cases at Delhi and Vellore could be attributed to the fact that both these centers are tertiary care centers and hospitalization may be delayed or sought owing to secondary reasons such as superadded bacterial infections. Higher frequency of influenza B in children could be attributed to seeking of medical attention in case of children, even with mild symptoms known in influenza B, in comparison to adults.
Fever is one of the symptoms in the case definition; however, fever was recorded in 93·3% and 94·9% of ILI and SARI cases. This could be due to the use of antipyretics, which is common in India before seeking medical attention.
Influenza A(H1N1), A(H3N2), and type B virus co‐circulated in different regions during the study period. In spite of the land expanse, no particular pattern or direction of movement of increased influenza activity or prevalence of any subtype was observed, as has been observed in studies in Europe where there was clear indication of west–east or north–south spread across Europe. 27
Diversity in circulating influenza types and subtypes poses a real challenge to vaccine strategies. 6 , 28 Comparative analysis of the genetic characteristics of viral isolates over time with the antigenically selected influenza vaccine for each year revealed homology with vaccine strain component by and large. Seasonal influenza A(H1N1) isolates matched well with the vaccine component up to 2007. None of our isolates clustered with A/Solomon Islands/3/2006. This could be due to very low activity of influenza A(H1N1) during the corresponding year throughout India. From late 2005, we observed A/Brisbane/59/2007‐like viruses in circulation along with A/New Caledonia/20/1999, indicating early seeding of these viruses in India.
For influenza A(H3N2), A/Panama/2007/99 was the vaccine component from 2000 to 2004; however, it circulated in India up to September 2005 in Delhi. This persistence of virus could be due to continuous circulation of H3N2 influenza in India as reported from tropical countries, 27 with an upsurge of influenza activity during the rainy season. A/Wyoming/3/2003, vaccine component in 2004–2005, was not observed in India; however, 2004–2005 H3N2 isolates from Chennai and Pune clustered with A/California/7/2004.A/Wellington/1/2004, the vaccine component for the Southern Hemisphere in 2005 circulated in India in Pune (March 2006) and Delhi (August 2006). This virus could have been seeded into the country owing to high frequency of international travel from the all over the world; however, this strain did not persist for a long time nor circulated extensively in India.
A/H3N2 viruses are more heterogeneous owing to continuous antigenic drifts. 28 Our genetic data suggest co‐circulation of and overlapping of vaccine strains of consecutive years.
Antigenic drifts of circulating influenza viruses in India, together with the temporal peaks in seasonality of influenza in different parts of the country, illustrate the difficulty in having effective uniform vaccines and vaccination timing for a vast country like India.
Although the surveillance data demonstrate that the influenza virus is an important cause of ILI among outpatients, our findings might be an underestimate. The specimens collected from ILI patients and limited number of SARI cases were sample of convenience (5–10 samples per week per center), whereas a systemic broad sentinel site–based surveillance is needed to get better estimate of influenza burden estimates in India. Our sampled population was not representative of the general population as the median age of ILI patients tested was 14 years, whereas the median age of the India population is 25·1 years. 29
Continued surveillance will help to better define any seasonal patterns in the circulation of influenza A and B viruses and any regional differences in influenza seasonality in India, as well as to determine optimal periods to implement influenza vaccination programs among priority populations.
In conclusion, our study clearly shows that influenza is an important cause of ILI and SARI. There is a need to formulate a national vaccination policy especially for high‐risk populations. The evidence of antigenic drifts of circulating influenza viruses in India, together with the temporal peaks in seasonality of influenza in different parts of the country, illustrate the need for a staggered approach in vaccination timing for a vast country like India.
Addendum
S. Broor, C. P. Gunassekaran, A. Krishnan, M. Chawla‐Sarkar, D. Biswas, A. M. Abraham, and S. V. Jalgaonkar carried out the study including identification of patients, isolation, and monitoring trends. V. A. Potdar carried out genetic analysis. H. Kaur coordinated the study at the Indian Council of Medical Research. A. Klimov confirmed the subtyping and quality control at the Collaborating Centre, Centers for Disease Control and Prevention, Atlanta, USA. R. B. Lal and A. Moen coordinated and regulated financial aspects on behalf of Centers for Disease Control and Prevention, Atlanta, USA. L. Kant is the overall coordinator of study. A. C. Mishra is the principal investigator of the study and provided inputs for study design and study implementation and intellectual inputs for manuscript preparation.
Acknowledgements
Authors acknowledge contributions of the staff of Regional and Referral centers. This study was funded by DHHS‐CDC Co – operative Agreement Grant Number: 5U50C1024407 and Indian Council of Medical Research, New Delhi.
Supporting information
Figure S1. Seasonality of influenza in India: 2004?2008.
Please note: Wiley‐Blackwell are not responsible for thecontent or functionality of any supporting materials suppliedby the authors. Any queries (other than missingmaterial) should be directed to the corresponding authorfor the article.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
References
- 1. Cox NJ, Subbarao K. Global epidemiology of influenza: past and present. Annu Rev Med 2000; 51:407–421. [DOI] [PubMed] [Google Scholar]
- 2. Rambaut A, Pybus OG, Nelson MI, Viboud C, Taubenberger JK, Holmes EC. The genomic and epidemiological dynamics of human influenza A virus. Nature 2008; 29:615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . 2007. Acute Respiratory Infections: Influenza. Available from websource: http://www.who.int/vaccine_research/diseases/ari/en/index.html.
- 4. Simonsen L. The global impact of influenza on morbidity and mortality. Vaccine 1999; 7(Suppl. 1):S3–S10. [DOI] [PubMed] [Google Scholar]
- 5. Novel Swine‐Origin Influenza A (H1N1) Virus Investigation Team , Dawood FS, Jain S, Finelli L, et al. Emergence of a novel swine origin influenza A (H1N1) virus in humans. N Engl J Med 2009; 360:2605–2615. [DOI] [PubMed] [Google Scholar]
- 6. Garten RJ, Davis T, Colin A et al. Antigenic and genetic characteristics of swine‐origin 2009 A (H1N1) influenza viruses circulating in humans. Science 2009; 325:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mishra AC, Chadha MS, Choudhary ML, Potdar VA. Pandemic influenza (H1N1) 2009 is associated with severe disease in India. PLoS One 2010; 5:e10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mardy S, Ly S, Heng S et al. Cambodia Influenza activity in Cambodia during 2006–2008. BMC Infect Dis 2009; 9:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paul KSC, Mok HY, Lee TC, Chu IMT, Lam W‐Y, Sung JJY. Seasonal influenza activity in Hong Kong and its association with meteorological variations. J Med Virol 2009; 81:1797–1808. [DOI] [PubMed] [Google Scholar]
- 10. Rao BL. Epidemiology and control of influenza. Natl Med J India, 2003; 16:143–149. [PubMed] [Google Scholar]
- 11. Hampson AW. Epidemiological data on influenza in Asian countries. Vaccine 1999; 17:S19–S23. [DOI] [PubMed] [Google Scholar]
- 12. Chatterjee S, Mukherjee KK, Mondal MC, Chakraborty MS. A study of influenza A virus in the city of Calcutta, India, highlighting the strain prevalence. Acta Microbiol Pol 1996; 45:279–283. [PubMed] [Google Scholar]
- 13. World health organization . A Practical guide to harmonizing Virological and epidemiological influenza surveillance. World Health Organization, Regional office for the Western Pacific, manila, Philippines. 2008. Available at http://www.wpro.who.int/internet/resources.ashx/CSR/Publications/GuideToHarmonizingInfluenzaSurveillance‐revised2302.pdf (Accessed 20 September 2010). [Google Scholar]
- 14. World Health Organization . Handbook: IMCI Integrated Management of Childhood Illness. 2005. Available from http://whqlibdoc.who.int/publications/2005/9241546441.pdf (accessed on 20 September 2010).
- 15. Kendal AP, Pereira MS, Skehel JJ. In Concepts and Procedures for Laboratory‐based Influenza Surveillance. Atlanta, GA, USA, Centre for Disease Control and Prevention, 1982. [Google Scholar]
- 16. Park AW, Glass K. Dynamic patterns of avian and human influenza in east and Southeast Asia. Lancet Infect Dis 2007; 7:543–548. [DOI] [PubMed] [Google Scholar]
- 17. Viboud C, Alonso WJ, Simonsen L. Influenza in tropical regions. PLoS Med 2006; 3:e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Finkelman BS, Viboud C, Koelle K, Ferrari MJ, Bharti N, Grenfell BT. Global patterns in seasonal activity of influenza A/H3N2, A/H1N1, and B from 1997 to 2005: viral coexistence and latitudinal gradients. PLoS One 2007; 2:e1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waicharoen S, Thawatsupha P, Chittaganpitch M, Maneewong P, Thanadachakul T, Sawanpanyalert P. Influenza viruses circulating in Thailand in 2004 and 2005. Jpn J Infect Dis 2008; 61:321–323. [PubMed] [Google Scholar]
- 20. Doraisingham S, Goh KT, Ling AE, Yu M. Influenza surveillance in Singapore: 1972–86. Bull World Health Organ 1988; 66:57–63. [PMC free article] [PubMed] [Google Scholar]
- 21. Beckett CG, Kosasih H, Ma’roef C et al. Influenza surveillance in Indonesia: 1999–2003. Clin Infect Dis 2004; 39:443–449. [DOI] [PubMed] [Google Scholar]
- 22. Hasegawa G, Kyaw Y, New HM et al. Epidemiological study of influenza virus infections in Yangon, Myanmar. Trop Med Health 2006; 34:3–6. [Google Scholar]
- 23. Yang L, Wong CM, Lau EH, Chan KP, Ou CQ, Peiris JS. Synchrony of clinical and laboratory surveillance for influenza in Hong Kong. PLoS ONE 2008; 3:e1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang P, Ni H, Shen G, Zhou H, Peng G, Liu S. Analysis of the 1991–2000 influenza epidemic in Guangdong Province, China. Southeast Asian J Trop Med Public Health 2001; 32:787–790. [PubMed] [Google Scholar]
- 25. Rao BL, Kadam SS, Pawar MS. Isolation of recent variant influenza types A(H3N2), A(H1N1) and B strains in Pune, India 2000. Indian J Med Res 2001; 114:157–159. [PubMed] [Google Scholar]
- 26. Yeolekar LR, Kulkarni PB, Pawar SD, Rao BL. Influenza surveillance in Pune, India: reappearance of B/Victoria/2/87‐ like influenza virus strain in 2002. Curr Sci 2004; 86:966–968. [Google Scholar]
- 27. Paget J, Marquet R, Meijer A, Van der Velden K. Influenza activity in Europe during eight seasons (1999–2207): an evaluation of the indicators used to measure activity and an assessment of the timing, length and course of peak activity (spread) across Europe. BMC Infect Dis 2007; 7:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Russell CA, Jones TC, Barr IG et al. The global circulation of seasonal influenza A (H3N2) viruses. Science 2008; 320:340–346. [DOI] [PubMed] [Google Scholar]
- 29. Census of India: Census Data 2001: India at a glance.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Seasonality of influenza in India: 2004?2008.
Please note: Wiley‐Blackwell are not responsible for thecontent or functionality of any supporting materials suppliedby the authors. Any queries (other than missingmaterial) should be directed to the corresponding authorfor the article.
Supporting info item
Supporting info item
Supporting info item
Supporting info item


