Abstract
Background: Leukocyte telomere length (TL) is associated with age-related diseases and early mortality, but there is a lack of data on the determinants of TL in early life. Evidence suggests that dietary intake of marine n–3 (ω-3) polyunsaturated fatty acids (PUFAs) is protective of telomere attrition, yet the effect of methylmercury exposure, also found in fish, on TL is unknown.
Objective: The aim of this study was to investigate the associations between prenatal PUFA status, methylmercury exposure, and TL in mothers and children in the SCDS (Seychelles Child Development Study), for whom fish consumption is high.
Methods: Blood samples collected from 229 mothers (at 28 wk gestation and delivery) and children (at 5 y of age) in the SCDS first nutrition cohort were analyzed for PUFA concentrations. Prenatal mercury was measured in maternal hair collected at delivery. Postnatal mercury was also measured in children’s hair samples with the use of a cumulative metric derived from values obtained at 3–5 y of age. Relative TL was measured in blood obtained from mothers at delivery, in cord blood, and in children at 5 y of age by quantitative polymerase chain reaction. Linear regression models were used to investigate the associations between PUFA status, methylmercury exposure, and TL.
Results: Neither prenatal PUFA status or methylmercury exposure was associated with TL of the mother or child or with TL attrition rate. However, a higher prenatal n–6:n–3 PUFA ratio was significantly associated with longer TLs in the mothers (β = 0.001, P = 0.048). Child PUFA status and methylmercury exposure were not associated with child TL. However, higher family Hollingshead socioeconomic status (SES) scores at 9 mo of age were significantly associated with longer TLs in cord blood (β = 0.005, P = 0.03).
Conclusions: We found no evidence that PUFA status or methylmercury exposure are determinants of TL in either the mother or child. However, our results support the hypothesis that family SES may be associated with child TL.
Keywords: polyunsaturated fatty acid status, methylmercury exposure, telomere length, pregnancy, maternal infant nutrition, fish consumption, Seychelles Child Development Study
Introduction
Telomeres, which are composed of TTAGGG repeats of DNA, act as a protective cap at the end of chromosomes and are essential for chromosome stability and replication (1). Telomeres shorten with each cell division cycle (2) and, as such, shortened telomere length (TL) has been used as an indicator of cell senescence and biological aging (3). Damage to or excessive shortening of telomeres in peripheral blood has been associated with accelerated aging and diseases featuring inflammation and oxidative stress, such as cardiovascular disease (4, 5) and cancer (6, 7). Although TL is largely genetically determined, several environmental influences, such as physical and psychological stress, smoking, body composition, and socioeconomic status (SES), are reported to influence TL (8–10). Furthermore, several studies have reported associations between various dietary components and TL, suggesting that modifying the diet may promote longevity (11–13). There are consistent reports that a Mediterranean dietary pattern, characterized by high fruit and vegetable intake, is associated with greater TLs in various populations (14, 15). Specific nutrients have also been studied in relation to TL. Higher dietary intakes of long-chain n–3 PUFAs, which have anti-inflammatory properties, have been associated with longer TLs in adults (16–18). The balance between the n–3 PUFA and n–6 PUFA families may also be important in relation to the effects on inflammation and TL. A randomized controlled trial with n–3 PUFA supplementation reported that TL increased with decreasing n–6 and n–3 PUFA ratios and concluded that further study of this relation was important to better understand disease prevention through dietary modification (18).
Childhood is the time period of greatest telomere loss in leukocytes, with studies of humans from birth to 90 y of age indicating that the greatest attrition occurs in the first years of life (19–21). Little information exists regarding the natural history of telomere processes in children, and it remains relatively unknown at what life stage dietary or environmental exposures may affect TL (22). However, given the wide interindividual variation in TL at birth and the fact that attrition of TL begins with the first cycle of cell division, it is likely that early life exposures may have an important effect on TL and susceptibility to age-related diseases throughout life, similar to the concept of epigenetics (23, 24).
To our knowledge, no study has yet investigated the effects of exposure to methylmercury from fish consumption on TL. It is understood that methylmercury is a toxin that can induce systemic oxidative stress and inflammation, both of which are associated with an accelerated rate of TL shortening. However, fish is also a rich source of n–3 PUFAs, which may counteract methylmercury-induced inflammation and oxidative stress (25, 26). We have previously reported on the importance of considering prenatal PUFA status when examining the associations between methylmercury exposure and neurodevelopment (27, 28). To clarify the effects of prenatal PUFA status and methylmercury exposure, through fish consumption, on TL and to increase understanding of the determinants of TL at birth and attrition during early life, we set out to investigate the associations between PUFA status, methylmercury exposure, and TL in mothers and their children in the SCDS (Seychelles Child Development Study) first nutrition cohort (NC1). Our primary aim was to investigate the effect of prenatal PUFA status and methylmercury exposure on TLs of the mother and child, and our secondary aim was to examine postnatal PUFA status and methylmercury exposure as potential determinants of child TL at birth and early life.
Methods
Study population.
The SCDS is an observational study conducted in the Republic of Seychelles. It was established to investigate the effects of prenatal exposure to methylmercury, through maternal fish consumption during pregnancy, on child neurodevelopment. The NC1 cohort recruited a total of 300 mothers at their first antenatal appointment on the island of Mahé during 2001, with full details of recruitment and the study setting described previously (27). Maternal height and weight were measured when mothers were enrolled in the study, and in children at 5 y of age, from which BMI was calculated (in kg/m2). Smoking and alcohol use during pregnancy were each measured as a dichotomous variable (some or none). Birth weight (in grams) and gestational age (in weeks) were determined at the child’s birth. Family SES was estimated with the use of the Hollingshead 4-Factor Social Status Index, measured when the child was 9 mo of age and again when the child was 5 y of age. The Hollingshead Index was modified to assess data on the primary caregiver’s education and occupation (mother, father, both, or other) (29), where higher codes indicated higher educational attainment or occupational status (30). We combined occupational and educational codes through a weighted formula into a continuous score (30). The home environment was assessed through the use of the Pediatric Review of Children’s Environmental Support and Stimulation. The study was reviewed and approved by the Seychelles Ethics Board and by the Research Subjects’ Review Board at the University of Rochester.
Blood collection.
Blood samples were collected from mothers at 28 wk gestation and at delivery. Children’s cord blood samples were collected at birth. Blood samples were also collected from the children from the forearm when they were aged ~5 y. All blood samples were venous and nonfasting and collected in EDTA-containing tubes. Whole blood, serum, and plasma aliquots were obtained and stored at −80°C until analysis.
PUFA measurement.
Maternal and child blood samples were maintained and shipped at −80°C to Ulster University for analysis of PUFA status. The description of this protocol has been described in full elsewhere (31). In brief, total lipids were extracted from maternal serum samples through the use of a modified method of Folch et al. (32). FAMEs were prepared by the addition of boron trifluoride in methanol (Sigma-Aldrich) and analyzed with the use of a Thermo Finnigan TRACE mass spectrometer with Xcaliber software (Thermo Finnigan). Precision was ensured by running a reference sample in each batch analysis for which the CV was ≤10%. The limit of detection was 0.01 mg/mL. FAs were detected and quantified with reference to an external linear calibration curve that included 2 standards, C17:0 and C21:0, which were also added to unknown samples as internal standards before extraction, as recommended by Schreiner (33). The correlation coefficient of the calibration curve was r2 = 0.99. Serum total FA concentrations were analyzed in maternal blood to account for the majority of FAs being transported to the fetus as TGs during pregnancy. The geometric mean of the maternal PUFA values measured at 28 wk and delivery was used in these analyzes (27). As previously described, serum concentrations of long-chain n–3 PUFAs measured in NC1 mothers were low, which may be the result of potential oxidation of samples during blood processing (34).
Similarly, blood samples collected from the children at 5 y of age were subject to PUFA analysis by the same method, but we characterized plasma phospholipid PUFA status in this age group and quantified concentrations with an Agilent GC/MS with Chemstation software (Agilent). In both methods, heptadecaenoic acid (17:0) and heneicosaenoic acid (21:0) were used as internal standards and were added before lipid extraction. We quantified in absolute amounts (grams per liter) concentrations of α-linolenic acid (ALA; 18:3n–3), EPA (20:5n–3), DHA (22:6n–3), linoleic acid (LA; 18:2n–6), and arachidonic acid (AA; 20:4n–6). For models using prenatal PUFA status, we summed the total n–3 PUFAs (ALA + EPA + DHA) and total n–6 PUFAs (LA + AA). However, for models using postnatal PUFA status, due to the low concentrations of ALA detected in children’s 5-y blood samples, we replaced the sums of the n–3 PUFAs and n–6 PUFAs with EPA + DHA and AA, respectively, and used the AA:DHA ratio in place of the n–6:n–3 PUFA ratios.
Methylmercury measurement.
Prenatal methylmercury exposure was estimated by measuring total mercury in maternal hair samples collected at delivery with the use of atomic absorption spectroscopy at the University of Rochester, as previously described (28). The limit of detection was 0.5 ng Hg/sample aliquot, and CV was 2.1%. Method accuracy was assessed throughout the analyses by inclusion of standard reference material for hair (IAEA-085 and IAEA-086; International Atomic Energy Agency). The University of Rochester Mercury Analytical Laboratory participated in the recent quality assessment of mercury laboratories with the COPHES/DEMOCOPHES project and served as a reference laboratory for the analysis of mercury in hair (35). Hair was not cleaned before analysis because our previous studies have not shown external contamination to be prevalent, and cleaning hair has been associated with inimitable results (36). Because mercury was measured in the longest hair segment available from maternal hair grown during pregnancy (assuming hair growth of 1.1 cm/mo), this measure represents exposure during the entire pregnancy. Children’s hair samples were obtained at evaluations before age 3 y and at ∼5 y of age. Postnatal mercury exposure was estimated by measuring total mercury in the 1 cm of hair closest to the scalp. For this analysis, we estimated the cumulative (AUC) postnatal mercury exposure between the 3- and 5-y time points, which is reported as parts per million-years.
TL measurement.
Whole blood samples were shipped at −80°C from Ulster University to Lund University for leukocyte TL measurement. We measured TL in blood samples from the mothers at delivery and from their children in cord blood and at 5 y of age. TL was measured in the 229 mothers (and their children) who had both measures of maternal hair mercury and maternal PUFAs. DNA was extracted with the use of the Qiagen mini kit (Qiagen) at the DNA/RNA genotyping laboratory, SWEGEN Resource Center for Profiling Polygenic Disease, Lund University. TL quantification was determined by quantitative PCR as described in detail (37). In short, an aliquot of 5 μL sample DNA (3 ng/μL) was added to each reaction (end volume: 20 μL). A standard curve, reference DNA, and a negative control were included in each run. For each standard curve, 1 calibrator DNA sample was diluted serially by 2-fold per dilution to produce 7 concentrations of 0.25–16 ng/μL. Each sample, standard curve, reference, and negative control were run in duplicates. Master mixes were prepared, containing 0.5 U Taq Platina (Invitrogen), 1×PCR Buffer, 0.8 mmol/L deoxyribonucleotide triphosphate, 1.75 mmol/L MgCl2, 0.3 mmol/L SybrGreen I (Invitrogen), 1×Rox (Invitrogen), and either telomere primers (0.45 μM of each primer) or hemoglobin β-chain primers (0.45 μM for each primer). The PCR was performed on a real-time PCR machine (7900HT; Applied Biosystems). The R2 value for each standard curve was >0.99. SDs [for cycle threshold (Ct) values] were accepted at <0.2.
The TL is an arbitrary value that was obtained through calculating the ratio of the telomere repeat copy number to single-copy gene numbers for each individual with the use of the formula T:S = 2−ΔCt, where ΔCt = Cttelomere − Cthemoglobin β. This ratio was then divided by the ratio of the reference DNA. Reference samples were included in each run and demonstrated a CV of 8.0%, based on 11 runs. The TL attrition rate was calculated as the ratio of the scaled 5-y child TL to the scaled cord blood TL, where scaling divided the TL at that age by the maximum TL at the same age. Because TL shortens with age, this ratio estimates the relative attrition rate, but only when cord blood TL and 5-y-old child TL are measured on the same scale. Scaling each measure was necessary to preserve this interpretation.
Statistical analysis.
Complete data were available for a total of 229 mothers and their children for which at ≥1 TL was measured. Linear regression models were fit to investigate the prespecified associations between TL and covariates as shown in Table 1. Three models investigated prenatal PUFA and mercury exposure as potential determinants of TL in both the mother and child, whereas 2 models considered the child’s postnatal PUFA and mercury exposure. We adjusted for PUFA status in 2 ways: as prenatal n–3 PUFA and n–6 PUFA or postnatal long-chain n–3 (sum of EPA + DHA) and n–6 (AA) PUFAs in primary models and as ratios of prenatal n–6:n–3 PUFA or postnatal AA:DHA ratio in secondary models.
TABLE 1.
Description of linear regression models, their outcomes, and covariates1
| Outcome | Exposure | Covariates |
| TL in mothers | Prenatal n–3 PUFA | Maternal age, maternal BMI, smoking, alcohol consumption, family SES at 9 mo of age |
| Prenatal n–6 PUFA | ||
| Prenatal n–6:n–3 PUFA ratio2 | ||
| Prenatal mercury | ||
| Log(TL in cord blood) | Prenatal n–3 PUFA | Maternal age, maternal BMI, smoking, alcohol consumption, family SES at 9 mo of age, child sex, birth weight, gestational age |
| Prenatal n–6 PUFA | ||
| Prenatal n–6:n–3 PUFA ratio2 | ||
| Prenatal mercury | ||
| TL at 5 y of age | Prenatal n–3 PUFA | Maternal age, maternal BMI, smoking, alcohol consumption, family SES at 5 y of age |
| Prenatal n–6 PUFA | ||
| Prenatal n–6:n–3 PUFA ratio2 | ||
| Prenatal mercury | ||
| TL at 5 y of age | Postnatal EPA+DHA | Child sex, child BMI, home environment, family SES at 5 y of age |
| Postnatal AA | ||
| Postnatal AA:DHA ratio3 | ||
| Postnatal mercury | ||
| Log(TL attrition rate) | Postnatal EPA + DHA | Child sex, child BMI, home environment, family SES at 5 y of age |
| Postnatal AA | ||
| Postnatal AA:DHA ratio3 | ||
| Postnatal mercury |
AA, arachidonic acid; SES, socioeconomic status; TL, telomere length.
Ratio replaced n–3 PUFAs and n–6 PUFAs in the secondary prenatal model.
Ratio replaced EPA + DHA and AA in the secondary postnatal model.
All models were adjusted for possible confounders chosen a priori based on the literature. As shown in Table 1, models that used prenatal PUFA status were adjusted for maternal age, maternal BMI, smoking during pregnancy (yes or no), and alcohol consumption during pregnancy (yes or no), whereas models that used postnatal PUFA status were adjusted for child BMI at 5 y of age and the home environment. Models investigating child TL, cord TL, or their ratio were adjusted for child sex, and the model for cord TL was also adjusted for birth weight and gestational age. Finally, all models were adjusted for SES score either as measured at 9 mo (maternal TL or cord TL) or at 5 y of age (models that use 5-y-old child TL).
Model assumptions were checked through the use of standard methods and included checking whether the residuals had constant variance, were normally distributed, and had approximate linear relations with each continuous covariate. We also checked for outliers and for influential observations as defined by Cook’s distance. If model assumptions were violated, we refit the model using a transformation of the outcome that better satisfied assumptions. All tests were 2-sided, and a P value <0.05 was considered significant.
TL in cord blood and the TL attrition rate required a logarithmic transformation to better meet model assumptions. There were no unduly influential or unduly outlying observations in any models. Due primarily to missing data on ≥1 TL measure and missing data on child PUFA status, models for maternal TL, cord TL, child TL at 5 y of age, and TL attrition rate were fit on data from n = 216, n = 183, n = 202 (adjusted for maternal markers; n = 178 when adjusted for child markers), and n = 141, respectively.
Results
Maternal and child characteristics are presented in Table 2. The mean ± SD TL decreased from 1.18 ± 0.5 T:S in cord blood to 0.71 ± 0.1 T:S at 5 y of age and was lowest in mothers at a mean of 0.64 ± 0.11 T:S. The mean ± SD TL attrition rate was 0.47 ± 0.14 with a range of −0.16 to 0.73. TLs across the 3 time points were only weakly correlated (r = −0.02 for maternal and cord TL, r = 0.06 for maternal and child’s TL at 5 y of age, and r = 0.14 for cord and child’s TL at 5 y of age; P > 0.05 for all correlations).
TABLE 2.
Characteristics of 229 mother-child pairs with ≥1 TL measurement1
| N | Mean | SD | Range | |
| Mothers | ||||
| Age, y | 229 | 27.2 | 5.93 | 15.0–42.0 |
| BMI at enrollment, kg/m2 | 228 | 25.77 | 6.38 | 15.52–50.03 |
| Gestational age, wk | 229 | 38.75 | 1.34 | 34.0–41.0 |
| Family SES score at 9 mo of age | 229 | 33.93 | 11.01 | 13.0–63.0 |
| Family SES score at 5 y of age | 225 | 31.48 | 11.06 | 8.0–63.0 |
| Hair mercury, ppm | 229 | 5.70 | 3.69 | 0.19–18.49 |
| Serum n–3 PUFA, g/L | 229 | 0.03 | 0.01 | 0.01–0.06 |
| Serum n–6 PUFA, g/L | 229 | 1.22 | 0.20 | 0.66–1.72 |
| Serum n–6:n–3 PUFA ratio | 229 | 40.2 | 11.7 | 13.2–90.4 |
| TL, T:S | 218 | 0.64 | 0.11 | 0.39–0.98 |
| Children2 | ||||
| Birth weight, kg | 229 | 3.24 | 0.47 | 1.87–4.45 |
| BMI at 5 y of age, kg/m2 | 220 | 14.96 | 1.98 | 11.61–27.16 |
| Home environment (PROCESS score) | 229 | 152.14 | 14.63 | 113.0–190.0 |
| Postnatal mercury, ppm-y | 220 | 12.83 | 7.32 | 2.52–68.58 |
| Cord TL, T:S | 184 | 1.18 | 0.5 | 0.47–4.66 |
| Plasma AA concentration at 5 y of age, g/L | 201 | 0.05 | 0.01 | 0.02–0.07 |
| Plasma EPA + DHA concentration at 5 y of age, g/L | 201 | 0.04 | 0.01 | 0.01–0.07 |
| Plasma AA:DHA ratio at 5 y of age | 201 | 1.51 | 0.34 | 0.82–2.8 |
| TL at 5 y of age, T:S | 209 | 0.71 | 0.1 | 0.45–0.99 |
| Telomere attrition rate, T:S | 141 | 0.47 | 0.14 | −0.16 to 0.73 |
AA, arachidonic acid; ppm-y, parts per million-years; PROCESS, Pediatric Review of Children’s Environmental Support and Stimulation; SES, socioeconomic status; TL, telomere length; T:S, telomere repeat copy number to single-copy gene number ratio.
Total N = 229 (n = 113 male, n = 16 female).
No significant associations were found between prenatal and postnatal PUFA status, hair mercury, and any of the TL measures, with the exception of the n–6:n–3 PUFA ratio in the mothers, where a greater n–6:n–3 PUFA ratio was significantly associated with longer TL (β = 0.001, P = 0.048; Table 3).
TABLE 3.
Associations between TL at different life stages, PUFA status, and mercury exposure from covariate-adjusted linear regression models1
| Outcome and exposure covariate | β | SE | P |
| TL in mothers (n = 216) | |||
| Prenatal n–3 PUFA | −1.70 | 0.93 | 0.07 |
| Prenatal n–6 PUFA | −0.011 | 0.039 | 0.78 |
| Prenatal mercury | 0.001 | 0.002 | 0.58 |
| Prenatal n–6:n–3 PUFA ratio | 0.001 | 0.001 | 0.0482 |
| Prenatal mercury | 0.001 | 0.002 | 0.71 |
| Log(TL in cord blood) (n = 183) | |||
| Prenatal n–3 PUFA | 4.38 | 3.20 | 0.17 |
| Prenatal n–6 PUFA | −0.031 | 0.14 | 0.82 |
| Prenatal mercury | −0.001 | 0.007 | 0.88 |
| Prenatal n–6:n–3 PUFA ratio | −0.002 | 0.002 | 0.39 |
| Prenatal mercury | 0.001 | 0.007 | 0.93 |
| TL at 5 y of age (n = 202) | |||
| Prenatal n–3 PUFA | 0.081 | 0.92 | 0.93 |
| Prenatal n–6 PUFA | −0.020 | 0.040 | 0.62 |
| Prenatal mercury | −0.002 | 0.002 | 0.23 |
| Prenatal n–6:n–3 PUFA ratio | 0.000 | 0.001 | 0.69 |
| Prenatal mercury | −0.003 | 0.002 | 0.17 |
| TL at 5 y of age (n = 202) | |||
| Postnatal EPA + DHA | −1.19 | 1.06 | 0.26 |
| Postnatal AA | 0.82 | 0.87 | 0.35 |
| Postnatal mercury | 0.001 | 0.001 | 0.26 |
| Postnatal AA:DHA ratio | 0.026 | 0.022 | 0.25 |
| Postnatal mercury | 0.001 | 0.001 | 0.27 |
| Log(TL attrition rate) (n = 141) | |||
| Postnatal EPA + DHA | −2.49 | 4.11 | 0.55 |
| Postnatal AA | −1.33 | 3.63 | 0.72 |
| Postnatal mercury | 0.006 | 0.004 | 0.16 |
| Postnatal AA:DHA ratio | −0.002 | 0.088 | 0.98 |
| Postnatal mercury | 0.005 | 0.004 | 0.20 |
AA, arachidonic acid; TL, telomere length.
Significant P value <0.05.
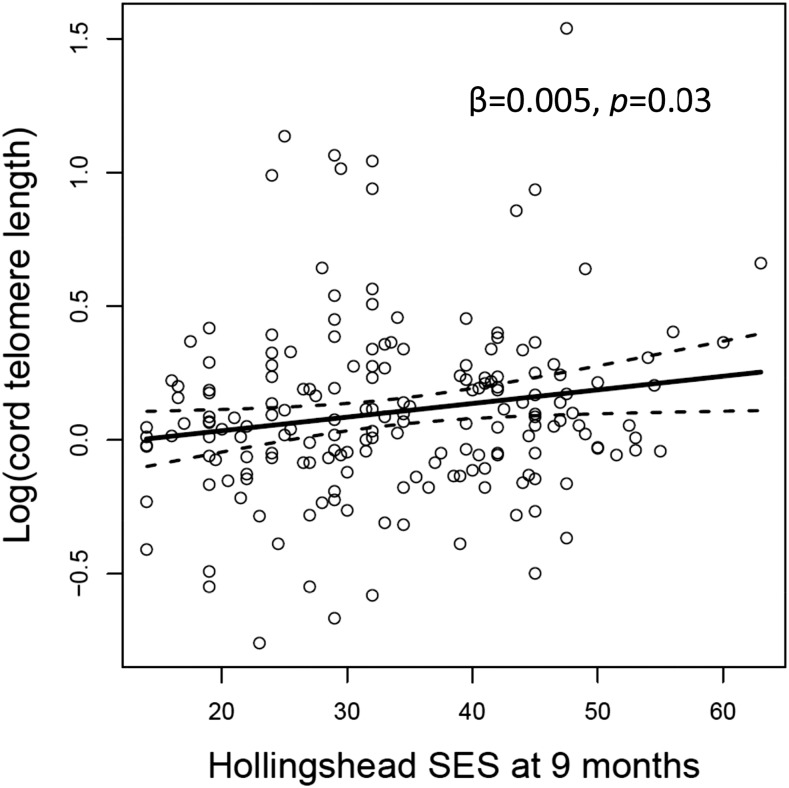
Family SES score at 9 mo was significantly positively associated with TL in cord blood (β = 0.005, P = 0.03; Figure 1). A positive trend was noted between family SES score at 5 y of age and TL at 5 y of age, however this relation was nonsignificant (β = 0.001, P = 0.08). At age 5 y, TL was almost significantly longer in girls than boys (β = 0.026, P = 0.08), and in models adjusted for maternal factors, a positive trend was noted between maternal age and TL of the children at 5 y of age (β = 0.002, P = 0.07); however, both associations were found to be nonsignificant. These associations are from models that adjusted for maternal n–3 and n–6 PUFAs, but similar associations were also found when adjusting for the n–6:n–3 PUFA ratio. No other covariates significantly predicted TL.
FIGURE 1.
The association between the logarithm of the cord telomere length and family Hollingshead SES index measured when the child was 9 mo of age. The superimposed lines show the slopes and 95% CIs from the covariate-adjusted regression. SES, socioeconomic status.
Discussion
This study focused on TL in early life, which, as an indicator of cellular aging, may be related to a range of health outcomes, including risk of developmental disorder in adolescence (38) and age-associated diseases, such as cardiovascular disease, in later life (2, 39). Many populations depend on fish as their primary source of nutrition and are therefore exposed to methylmercury while also consuming n–3 PUFAs. To our knowledge, there are no longitudinal studies that confirm a beneficial effect of fish consumption on TL, either in adults or in children. However, several studies of dietary data have indicated a protective effect of a Mediterranean diet, which is expected to feature high fish intake, on TL in adults (14, 15). We hypothesized that prenatal PUFA status and methylmercury exposure would have conflicting associations with TL, both of the mother and child, through their opposing roles in inflammation and oxidative stress. We found no clear evidence for associations of either prenatal or postnatal PUFA status and methylmercury exposure with TL in Seychellois mothers and their children, despite a uniquely high fish intake in this cohort.
However, we did observe that a higher prenatal n–6:n–3 PUFA ratio was associated with longer TL in mothers. This finding was unexpected given that a higher n–6:n–3 PUFA ratio is generally, but not always, indicative of greater inflammatory insult in the body. Previous studies have reported a protective effect of supplementation with long-chain n–3 PUFAs on telomere shortening in adults (16). However, the relation between PUFAs and TL remains controversial and not fully understood, particularly in pregnancy (12, 40). One intervention study with long-chain n–3 PUFA supplementation found that every 1-U decrease in the n–6:n–3 PUFA ratio was associated with a 20-bp increase in TL (18). Yet, there was no significant difference in the change in TL between placebo and treatment groups in their study. A further intervention study for 6 mo with a relatively small sample size found a significant correlation between erythrocyte DHA status and TL in elderly adults taking DHA supplements, but no significant differences in TL between groups taking supplements of DHA, EPA + DHA, or LA. (17).
The mechanism for a relation between PUFAs and TL is proposed to be via the action of the lipid metabolites derived from PUFAs (e.g., eicosanoids, resolvins, and protectins), which differ in inflammatory properties according to whether their precursor is of the n–3 or n–6 PUFA family. It is possible that our finding of a longer TL with greater maternal n–6:n–3 PUFA ratio is population specific, given that the Seychelles cohort may have a unique genetic background for PUFA metabolism (fatty acid desaturase genotype), as we have previously reported (41). It is evident that the relation between PUFAs and TL is more complex than previously understood, and may be further complicated by altered lipid metabolism during pregnancy. Therefore, it is of interest for future studies to consider the influence of various genotypes that regulate PUFA metabolism when investigating the associations between PUFAs and TL.
This is the first time, to our knowledge, that the relation between methylmercury exposure and TL has been investigated. A major mechanism of methylmercury toxicity in the body is exerted through the promotion of inflammation and oxidative stress (42). Therefore, our finding of a lack of association with TL in either mothers or children is encouraging in that it suggests that methylmercury exposure from fish consumption in the Seychelles is not having a detrimental effect on cell aging.
We observed that a higher family SES score, as measured at 9 mo of age, was associated with longer TL in infants at birth. The association between child TL and SES at 5 y of age was somewhat less strong and was not statistically significant. Other studies have shown that lower SES and social disadvantage during childhood are associated with shorter TL, both in childhood and in adulthood (43–45). Our results confirm the importance of the early home environment for TL in children, a relation that may have lifelong health effects for children in the Seychelles. It is possible that a higher family SES score is an indicator of other environmental factors that could influence TL, such as a higher-quality diet. A focused examination of the postnatal diet of children may elucidate the dietary determinants of TL and potentially explain why we did not find an association between SES at 5 y of age with TL at the same age. Therefore, the clinical implications of a longer TL in early life may relate to lower risk of developmental disorder in adolescence (38, 46) and a variety of conditions in later life (2). To date, the majority of research conducted in this area ascribes these relations to the balance between oxidative stress and antioxidant defenses known to regulate DNA replication and senescence (47, 48).
In all samples, TL was measured and calculated based on the same reference DNA, and therefore, the values were comparable between different groups. We observed that TL was the longest in cord blood, and the shortest in mothers. This pattern supports the general idea that TL could be a biomarker for biological age (39). However, in mothers, there was no evidence of an association between TL and maternal age. The telomere attrition rate between newborn and 5 y-old children was surprisingly large, most likely reflecting their rapid growth, which requires prolific cell division. Robertson et al. (21) found the largest telomere attrition in the first year of life, with a more constant rate of loss thereafter. This high attrition rate could also explain the surprisingly low correlations between TL among mothers and children. We found one child with TL lengthening between birth and 5 y, a phenomenon that has been observed by others (49, 50). It is therefore possible that telomere lengthening processes may be part of overall oscillations in TL, and we speculate that this phenomenon may represent fluctuations in cell types, which it was not possible to account for in our analysis. This represents one of few studies reporting TL in children, and, as such, further investigation is warranted to determine the effect of early life exposures, including diet, on TL and telomere attrition.
This study has several strengths. The mother-child cohort allows investigation of various influential factors on TL, both in the mothers and the offspring at ≤5 y of age. The study population had high fish consumption (51), resulting in a concurrent high intake of n–3 PUFAs and high exposure to methylmercury. Therefore, any possible effects of these factors should have been detected in this study. This study also has limitations. Despite our best efforts at prevention, it is possible that delayed blood processing of maternal samples in this cohort may have resulted in selective oxidation of the more susceptible long-chain PUFAs in a random subset of serum samples. This may account for the relatively low n–3 PUFA concentrations and the higher n–6:n–3 PUFA ratio observed in mothers. As we have previously commented, this may induce nondifferential measurement error, with the result that the observed associations in the models examining prenatal PUFA status within the current study are likely to be closer to the null hypothesis than the true associations (34).
In conclusion, we found no clear evidence that prenatal or postnatal PUFA status or methylmercury exposure are determinants of TL in our high–fish-eating mother-child cohort. However, our results support the hypothesis that early life family SES may influence TL in the child.
Acknowledgments
The authors’ responsibilities were as follows—AJY: had full access to all data in the study (with the exception of mercury data), assisted with data interpretation, prepared the manuscript, and had responsibility for the final content of the manuscript; KB: conceived the overall research concept and designed the analysis plan with SWT and AJY; CFS, GJM, JJS, and PWD: were responsible for the overall study design of the Seychelles Child Development Study first nutrition cohort, and were involved in fieldwork and data collection; SWT: designed and conducted the statistical analysis and assisted with data interpretation; HL: conducted the telomere length analysis and assisted with data interpretation; GEW: took responsibility for the integrity of the mercury data; AJY, MSM, EMM, and JJS: conducted PUFA analysis and assisted with data interpretation; JJS, PWD, EvW, CFS, and GJM: provided the overall study supervision; and all authors: read and approved the final manuscript.
Footnotes
Abbreviations used: AA, arachidonic acid; ALA, α-linolenic acid; Ct, cycle threshold; LA, linoleic acid; NC1, first nutrition cohort; SCDS, Seychelles Child Development Study; SES, socioeconomic status; TL, telomere length.
References
- 1.Blackburn EH. Structure and function of telomeres. Nature 1991;350:569–73. [DOI] [PubMed] [Google Scholar]
- 2.Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 2005;6:611–22. [DOI] [PubMed] [Google Scholar]
- 3.Raynaud CM, Sabatier L, Philipot O, Olaussen KA, Soria JC. Telomere length, telomeric proteins and genomic instability during the multistep carcinogenic process. Crit Rev Oncol Hematol 2008;66:99–117. [DOI] [PubMed] [Google Scholar]
- 4.Balasubramanyam M, Adaikalakoteswari A, Monickaraj SF, Mohan V. Telomere shortening & metabolic/vascular diseases. Indian J Med Res 2007;125:441–50. [PubMed] [Google Scholar]
- 5.Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet 2007;369:107–14. [DOI] [PubMed] [Google Scholar]
- 6.Wu X, Amos CI, Zhu Y, Zhao H, Grossman BH, Shay JW, Luo S, Hong WK, Spitz MR. Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst 2003;95:1211–8. [DOI] [PubMed] [Google Scholar]
- 7.Ma H, Zhou Z, Wei S, Liu Z, Pooley KA, Dunning AM, Svenson U, Roos G, Hosgood HD III, Shen M, et al. Shortened telomere length is associated with increased risk of cancer: a meta-analysis. PLoS One 2011;6:e20466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, Epel ES. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology 2013;38:1835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet 2005;366:662–4. [DOI] [PubMed] [Google Scholar]
- 10.Needham BL, Adler N, Gregorich S, Rehkopf D, Lin J, Blackburn EH, Epel ES. Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999-2002. Soc Sci Med 2013;85:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paul L. Diet, nutrition and telomere length. J Nutr Biochem 2011;22:895–901. [DOI] [PubMed] [Google Scholar]
- 12.Cassidy A, De Vivo I, Liu Y, Han J, Prescott J, Hunter DJ, Rimm EB. Associations between diet, lifestyle factors, and telomere length in women. Am J Clin Nutr 2010;91:1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rafie N, Golpour Hamedani S, Barak F, Safavi SM, Miraghajani M. Dietary patterns, food groups and telomere length: a systematic review of current studies. Eur J Clin Nutr 2017;71:151–8. [DOI] [PubMed] [Google Scholar]
- 14.Crous-Bou M, Fung TT, Prescott J, Julin B, Du M, Sun Q, Rexrode KM, Hu FB, De Vivo I. Mediterranean diet and telomere length in Nurses’ Health Study: population based cohort study. BMJ 2014;349:g6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.García-Calzón S, Martínez-González MA, Razquin C, Arós F, Lapetra J, Martínez JA, Zalba G, Marti A. Mediterranean diet and telomere length in high cardiovascular risk subjects from the PREDIMED-NAVARRA study. Clin Nutr 2016;35:1399–405. [DOI] [PubMed] [Google Scholar]
- 16.Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA 2010;303:250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Callaghan N, Parletta N, Milte CM, Benassi-Evans B, Fenech M, Howe PR. Telomere shortening in elderly individuals with mild cognitive impairment may be attenuated with omega-3 fatty acid supplementation: a randomized controlled pilot study. Nutrition 2014;30:489–91. [DOI] [PubMed] [Google Scholar]
- 18.Kiecolt-Glaser JK, Epel ES, Belury MA, Andridge R, Lin J, Glaser R, Malarkey WB, Hwang BS, Blackburn E. Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: a randomized controlled trial. Brain Behav Immun 2013;28:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frenck RW Jr, Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci USA 1998;95:5607–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rufer N, Brummendorf TH, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, Schulzer M, Lansdorp PM. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med 1999;190:157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robertson JD, Gale RE, Wynn RF, Dougal M, Linch DC, Testa NG, Chopra R. Dynamics of telomere shortening in neutrophils and T lymphocytes during ageing and the relationship to skewed X chromosome inactivation patterns. Br J Haematol 2000;109:272–9. [DOI] [PubMed] [Google Scholar]
- 22.Kark JD, Goldberger N, Kimura M, Sinnreich R, Aviv A. Energy intake and leukocyte telomere length in young adults. Am J Clin Nutr 2012;95:479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnes SK, Ozanne SE. Pathways linking the early environment to long-term health and lifespan. Prog Biophys Mol Biol 2011;106:323–36. [DOI] [PubMed] [Google Scholar]
- 24.Shalev I. Early life stress and telomere length: investigating the connection and possible mechanisms: a critical survey of the evidence base, research methodology and basic biology. BioEssays 2012;34:943–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi AL, Mogensen UB, Bjerve KS, Debes F, Weihe P, Grandjean P, Budtz-Jorgensen E. Negative confounding by essential fatty acids in methylmercury neurotoxicity associations. Neurotoxicol Teratol 2014;42:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahaffey KR, Sunderland EM, Chan HM, Choi AL, Grandjean P, Marien K, Oken E, Sakamoto M, Schoeny R, Weihe P, et al. Balancing the benefits of n–3 polyunsaturated fatty acids and the risks of methylmercury exposure from fish consumption. Nutr Rev 2011;69:493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davidson PW, Strain JJ, Myers GJ, Thurston SW, Bonham MP, Shamlaye CF, Stokes-Riner A, Wallace JM, Robson PJ, Duffy EM, et al. Neurodevelopmental effects of maternal nutritional status and exposure to methylmercury from eating fish during pregnancy. Neurotoxicology 2008;29:767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strain JJ, Yeates AJ, van Wijngaarden E, Thurston SW, Mulhern MS, McSorley EM, Watson GE, Love TM, Smith TH, Yost K, et al. Prenatal exposure to methyl mercury from fish consumption and polyunsaturated fatty acids: associations with child development at 20 mo of age in an observational study in the Republic of Seychelles. Am J Clin Nutr 2015;101:530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davidson PW, Myers GJ, Cox C, Axtell C, Shamlaye C, Sloane-Reeves J, Cernichiari E, Needham L, Choi A, Wang Y, et al. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: outcomes at 66 months of age in the Seychelles Child Development Study. JAMA 1998;280:701–7. [DOI] [PubMed] [Google Scholar]
- 30. Hollingshead AB. Four factor index of social status. New Haven (CT): Yale University; 1975.
- 31.Strain JJ, Davidson PW, Bonham MP, Duffy EM, Stokes-Riner A, Thurston SW, Wallace JM, Robson PJ, Shamlaye CF, Georger LA, et al. Associations of maternal long-chain polyunsaturated fatty acids, methyl mercury, and infant development in the Seychelles Child Development Nutrition Study. Neurotoxicology 2008;29:776–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 33.Schreiner M. Quantification of long chain polyunsaturated fatty acids by gas chromatography. Evaluation of factors affecting accuracy. J Chromatogr A 2005;1095:126–30. [DOI] [PubMed] [Google Scholar]
- 34.Strain JJ, Davidson PW, Thurston SW, Harrington D, Mulhern MS, McAfee AJ, van Wijngaarden E, Shamlaye CF, Henderson J, Watson GE, et al. Maternal PUFA status but not prenatal methylmercury exposure is associated with children’s language functions at age five years in the Seychelles. J Nutr 2012;142:1943–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esteban M, Schindler BK, Jimenez JA, Koch HM, Angerer J, Rosado M, Gomez S, Casteleyn L, Kolossa-Gehring M, Becker K, et al. Mercury analysis in hair: comparability and quality assessment within the transnational COPHES/DEMOCOPHES project. Environ Res 2015;141:24–30. [DOI] [PubMed] [Google Scholar]
- 36.Nuttall KL. Interpreting hair mercury levels in individual patients. Ann Clin Lab Sci 2006;36:248–61. [PubMed] [Google Scholar]
- 37.Li H, Jonsson BA, Lindh CH, Albin M, Broberg K. N-nitrosamines are associated with shorter telomere length. Scand J Work Environ Health 2011;37:316–24. [DOI] [PubMed] [Google Scholar]
- 38.Costa Dde S, Rosa DV, Barros AG, Romano-Silva MA, Malloy-Diniz LF, Mattos P, de Miranda DM. Telomere length is highly inherited and associated with hyperactivity-impulsivity in children with attention deficit/hyperactivity disorder. Front Mol Neurosci 2015;8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanders JL, Newman AB. Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol Rev 2013;35:112–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das UN. Telomere length and polyunsaturated fatty acids. Nutrition 2014;30:1218–21. [DOI] [PubMed] [Google Scholar]
- 41.Yeates AJ, Love TM, Engstrom K, Mulhern MS, McSorley EM, Grzesik K, Alhamdow A, Wahlberg K, Thurston SW, Davidson PW, et al. Genetic variation in FADS genes is associated with maternal long-chain PUFA status but not with cognitive development of infants in a high fish-eating observational study. Prostaglandins Leukot Essent Fatty Acids 2015;102–103:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farina M, Aschner M, Rocha JB. Oxidative stress in MeHg-induced neurotoxicity. Toxicol Appl Pharmacol 2011;256:405–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen S, Janicki-Deverts D, Turner RB, Marsland AL, Casselbrant ML, Li-Korotky HS, Epel ES, Doyle WJ. Childhood socioeconomic status, telomere length, and susceptibility to upper respiratory infection. Brain Behav Immun 2013;34:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchell C, Hobcraft J, McLanahan SS, Siegel SR, Berg A, Brooks-Gunn J, Garfinkel I, Notterman D. Social disadvantage, genetic sensitivity, and children’s telomere length. Proc Natl Acad Sci USA 2014;111:5944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Needham BL, Fernandez JR, Lin J, Epel ES, Blackburn EH. Socioeconomic status and cell aging in children. Soc Sci Med 2012;74:1948–51. [DOI] [PubMed] [Google Scholar]
- 46.Henje Blom E, Han LK, Connolly CG, Ho TC, Lin J, LeWinn KZ, Simmons AN, Sacchet MD, Mobayed N, Luna ME, et al. Peripheral telomere length and hippocampal volume in adolescents with major depressive disorder. Transl Psychiatry 2015;5:e676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glade MJ, Meguid MM. A glance at ... telomeres, oxidative stress, antioxidants, and biological aging. Nutrition 2015;31:1447–51. [DOI] [PubMed] [Google Scholar]
- 48.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci 2002;27:339–44. [DOI] [PubMed] [Google Scholar]
- 49.Wojcicki JM, Shiboski S, Heyman MB, Elwan D, Lin J, Blackburn E, Epel E. Telomere length change plateaus at 4 years of age in Latino children: associations with baseline length and maternal change. Mol Genet Genomics 2016;291:1379–89. [DOI] [PubMed] [Google Scholar]
- 50.Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, Mill J, Arseneault L, Caspi A. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol Psychiatry 2013;18:576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonham MP, Duffy EM, Wallace JM, Robson PJ, Myers GJ, Davidson PW, Clarkson TW, Shamlaye CF, Strain JJ. Habitual fish consumption does not prevent a decrease in LCPUFA status in pregnant women (the Seychelles Child Development Nutrition Study). Prostaglandins Leukot Essent Fatty Acids 2008;78:343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]