Abstract
Tick-borne pathogens transmitted by Ixodes scapularis Say (Acari: Ixodidae), also known as the deer tick or blacklegged tick, are increasing in incidence and geographic distribution in the United States. We examined the risk of tick-borne disease exposure in 9 national parks across six Northeastern and Mid-Atlantic States and the District of Columbia in 2014 and 2015. To assess the recreational risk to park visitors, we sampled for ticks along frequently used trails and calculated the density of I. scapularis nymphs (DON) and the density of infected nymphs (DIN). We determined the nymphal infection prevalence of I. scapularis with a suite of tick-borne pathogens including Borrelia burgdorferi, Borrelia miyamotoi, Anaplasma phagocytophilum, and Babesia microti. Ixodes scapularis nymphs were found in all national park units; DON ranged from 0.40 to 13.73 nymphs per 100 m2. Borrelia burgdorferi, the causative agent of Lyme disease, was found at all sites where I. scapularis was documented; DIN with B. burgdorferi ranged from 0.06 to 5.71 nymphs per 100 m2. Borrelia miyamotoi and A. phagocytophilum were documented at 60% and 70% of the parks, respectively, while Ba. microti occurred at just 20% of the parks. Ixodes scapularis is well established across much of the Northeastern and Mid-Atlantic States, and our results are generally consistent with previous studies conducted near the areas we sampled. Newly established I. scapularis populations were documented in two locations: Washington, D.C. (Rock Creek Park) and Greene County, Virginia (Shenandoah National Park). This research demonstrates the potential risk of tick-borne pathogen exposure in national parks and can be used to educate park visitors about the importance of preventative actions to minimize tick exposure.
Keywords: Ixodes scapularis, Borrelia burgdorferi, tick-borne disease, recreational exposure
In the eastern United States, the blacklegged tick, Ixodes scapularis Say, is the primary vector of Borrelia burgdorferi, the causative agent of Lyme disease, which is the most commonly reported vector-borne disease in the United States (Mead 2015). Ixodes scapularis also vectors other pathogens that can cause potentially serious disease, including Borrelia miyamotoi, Anaplasma phagocytophilum, and Babesia microti (Barbour and Fish 1993, Homer et al. 2000, Jin et al. 2012, Krause et al. 2015). Established blacklegged tick populations are nearly continuous across counties in the Northeastern and North-Central United States where the majority of I. scapularis-borne disease cases are reported (Mead 2015, Eisen et al. 2016). The risk of acquiring Lyme disease is influenced by spatiotemporal variation in the density of host-seeking infected nymphs (Diuk-Wasser et al. 2012). This metric often correlates with Lyme disease incidence, though to varying degrees (Mather et al. 1996, Stafford et al. 1998, Falco et al. 1999, Pepin et al. 2012). Human behavior, including time spent in tick-infested areas or engaged in behaviors that enhance or reduce the likelihood of encounters with ticks (Orloski et al. 2000, Connally et al. 2009), also influences the likelihood of acquiring Lyme disease and may explain some of the lack of concordance between measures of density of infected host-seeking nymphs and Lyme disease incidence (Pepin et al. 2012).
Understanding where people may come into contact with infected vector-competent ticks is central to mitigating tick-borne disease risk. For example, in the Mid-Atlantic and Northeastern United States, peridomestic exposure to I. scapularis likely occurs frequently (Falco and Fish 1988, Maupin et al. 1991, Klein et al. 1996, Connally et al. 2006, Feldman et al. 2015), whereas in the North-Central United States, recreational exposures are believed to be more common than peridomestic exposures (Kitron and Kazmierczak 1997, Paskewitz et al. 2001). Regardless of geographic region, previous studies have demonstrated a risk of human exposure to infected host-seeking I. scapularis nymphs in recreational settings (Falco and Fish 1989, Schulze et al. 1992, Oliver and Howard 1998, Paskewitz et al. 2001, Han et al. 2014, Prusinski et al. 2014, Ford et al. 2015). National parks are popular recreation destinations and may represent areas of elevated acarological risk, yet one cannot adequately infer the risk of tick-borne disease for park visitors or employees from the epidemiological surveillance conducted at the county spatial scale (Eisen et al. 2013). National parks often vary ecologically from surrounding areas, and thus the density of infected ticks may differ between settings; further, human behavior within the parks may differ from behavior in surrounding communities.
In this study, we sought to characterize the acarological risk, that is, the risk of human exposure to tick-borne pathogens, in national parks in the Eastern United States. We surveyed frequently used trails in national park units across six Northeastern and Mid-Atlantic States and the District of Columbia, ranging from Maine in the north to Virginia in the south. Our collection efforts focused on the nymphal stage of I. scapularis. This stage likely poses the greatest threat of transmission of B. burgdorferi and other pathogens to humans, as peak activity of questing nymphs occurs in late spring and early summer which coincides with peak onset of human disease (Piesman 1989, Fish 1993, Falco et al. 1999, Mead 2015). Here, we describe the diversity of ticks collected by drag sampling during summer months, density of host-seeking I. scapularis nymphs, and diversity and prevalence of B. burgdorferi, B. miyamotoi, A. phagocytophilum, and Ba. microti infection in I. scapularis nymphs.
Materials and Methods
Tick Collection
Ticks were collected between 29 May and 1 July, 2014, by drag sampling in seven National Park Service (NPS) units in the Northeastern United States. In 2015, Acadia and Shenandoah National Parks were added. Shenandoah National Park in Virginia represents an area of expansion of human Lyme disease cases as well as geographic expansion of I. scapularis. Acadia National Park has a history of I. scapularis expansion on the island and also represents a site where tick phenology differs, likely due to climate. In 2015, all sites except Acadia National Park were sampled between 27 May and 2 July (Table 1, Fig. 1). At Acadia National Park, the northern-most study site, the peak activity of questing I. scapularis nymphs occurs later in the year (Rand et al. 2007). Therefore, we visited each transect at this site three times; sampling was initiated in June to synchronize with other parks being sampled and sampling occurred again in mid-July and early August when peak questing activity was expected (Rand et al. 2007). In each park unit, we consulted NPS staff to choose high visitor use areas with suitable habitat and a history of reports from visitors or staff about tick abundance as our sampling sites. We aimed to sample three 750-m transects in each NPS unit twice per year. However, at Monocacy National Battlefield, only a single transect was sampled in 2014 and only two transects were sampled in 2015. Further, due to poor sampling conditions, transects at some parks were only sampled on a single occasion in a given year (Table 1). To best represent the risk to park visitors of encountering host-seeking I. scapularis nymphs, transects were located along established trails in closed canopy deciduous forest with ample leaf litter on or near the trail. Because we sought to maximize the number of host-seeking I. scapularis nymphs captured, we targeted timing and site selection to this species. Although we did not conduct phenology studies at our study sites, sampling corresponded with the period of peak I. scapularis nymphal host-seeking activity (Wilson and Spielman 1985, Piesman et al. 1987, Sonenshine and Mather 1994, Ostfeld et al. 1996, Diuk-Wasser et al. 2006, Gatewood et al. 2009, Orr et al. 2013) and the time of year when most human cases of Lyme disease are reported (Mead 2015). We report the highest number of nymphs encountered during a single visit to represent the “peak” density of nymphs (DON). All other ticks captured were considered incidental captures.
Table 1.
Dates, number, and area of transects sampled and number of ticks collected from eastern United States national parks—2014–2015
| Sitea/year | Sample date range | No. transects sampledb | Total area (m2) | Ixodes scapularis | Dermacentor variabilis | Amblyomma americanum | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| Larva | Nymph | Adult | Nymph | Adult | Larva | Nymph | Adult | ||||
| ACAD | |||||||||||
| 2015 | 6/30–8/14 | 6 | 6,750 | 116 | 154 | 1 | 0 | 0 | 0 | 0 | 0 |
| CATOc | |||||||||||
| 2014 | 6/17–7/1 | 6 | 4,500 | 0 | 104 | 2 | 0 | 2 | 0 | 0 | 0 |
| 2015 | 6/11–6/25 | 6 | 4,500 | 15 | 46 | 2 | 0 | 0 | 0 | 0 | 0 |
| FIISc,d | |||||||||||
| 2014 | 5/29–6/12 | 6 | 4,500 | 6 | 339 | 9 | 0 | 6 | 24 | 2,628 | 887 |
| 2105 | 5/29–6/19 | 6 | 4,500 | 1 | 539 | 10 | 0 | 6 | 1 | 1,630 | 1,027 |
| GETTc | |||||||||||
| 2014 | 6/4–6/19 | 4 | 3,000 | 0 | 63 | 0 | 1 | 30 | 0 | 0 | 0 |
| 2015 | 6/1–7/2 | 6 | 4,500 | 19 | 51 | 2 | 0 | 37 | 0 | 0 | 0 |
| MANAe,f | |||||||||||
| 2014 | 6/2–6/18 | 6 | 4,500 | 0 | 196 | 2 | 0 | 77 | 0 | 610 | 224 |
| 2015 | 5/27–6/16 | 6 | 4,500 | 0 | 106 | 1 | 1 | 23 | 0 | 217 | 139 |
| MONO | |||||||||||
| 2014 | 6/3–6/13 | 2 | 1,500 | 0 | 10 | 0 | 0 | 1 | 0 | 0 | 0 |
| 2015 | 6/25 | 2 | 1,500 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| PRWIc | |||||||||||
| 2014 | 6/1–6/10 | 6 | 4,500 | 0 | 79 | 2 | 0 | 15 | 0 | 139 | 50 |
| 2015 | 5/28–6/11 | 6 | 4,500 | 1 | 62 | 6 | 0 | 0 | 0 | 29 | 16 |
| ROCR | |||||||||||
| 2014 | 6/7–6/19 | 6 | 4,500 | 0 | 514 | 0 | 0 | 0 | 0 | 2 | 0 |
| 2015 | 5/26–6/5 | 6 | 4,500 | 0 | 124 | 3 | 0 | 0 | 0 | 0 | 3 |
| SHEN | |||||||||||
| 2015 | 5/28–6/24 | 6 | 4,500 | 30 | 190 | 4 | 2 | 0 | 0 | 22 | 9 |
ACAD, Acadia National Park; CATO, Catoctin Mountain Park; FIIS, Fire Island National Seashore; GETT, Gettysburg National Military Park; MANA, Manassas National Battlefield Park; MONO, Monocacy National Battlefield; PRWI, Prince William Forest Park; ROCR, Rock Creek Park; SHEN, Shenandoah National Park.
This represents the total number of 750-m transects sampled at each park. Most sites had three transects sampled two times (N =6).
Transects from which extra sampling in surrounding suitable habitat was conducted in effort to obtain ≥50 nymphal I. scapularis for B. burgdorferi infection prevalence estimates; extra ticks collected off transect were not included in density estimates.
One Rhipicephalus sanguineus male and one Haemaphysalis leporispalustris nymph caught in 2015.
Transects 1 and 2 at Monocacy National Battlefield were only sampled a single time in 2015.
One A. maculatum male caught in 2014.
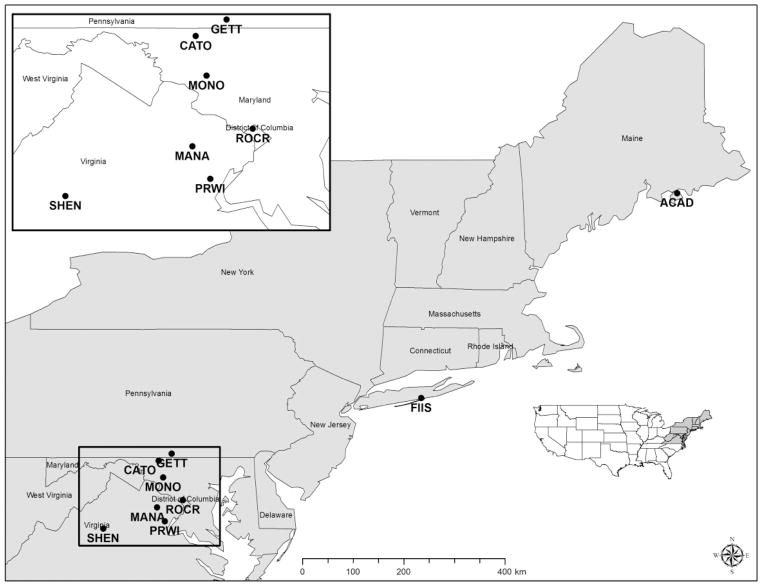
Fig. 1.
Nine national park units with established populations of I. scapularis in 2014 and 2015. ACAD, Acadia National Park; CATO, Catoctin Mountain Park; FIIS, Fire Island National Seashore; GETT, Gettysburg National Military Park; MANA, Manassas National Battlefield Park; MONO, Monocacy National Battlefield; PRWI, Prince William Forest Park; ROCR, Rock Creek Park; SHEN, Shenandoah National Park.
Ticks were collected from the leaf litter directly adjacent to the trail edge by dragging a 1-m2 rubber-bonded cotton sheet (JoAnn Fabric #1491315) with a rope attached to a 48″ dowel inside the top edge and washers were sewn into the bottom edge to enhance ground contact. Trail transects were 750 m in length except at Manassas National Battlefield Park, where one transect was split into two 375-m segments. To minimize the number of ticks missed due to falling off the drag, we checked the drags and removed ticks every 15 m. All species of ticks encountered were collected and preserved immediately in RNAlater (Ambion, Austin, TX) or 70% ethanol, with the exception of five I. scapularis nymphs from Catoctin Mountain Park that were initially collected on tape and later transferred to RNAlater. For pathogen detection, we aimed to collect at least 50 I. scapularis nymphs from each transect. If a total of 50 I. scapularis nymphs had not been collected after both sampling sessions in a given year, additional sampling was done; nearby suitable habitat was selected for sampling and often the other side of the trail was sampled or suitable habitat parallel with and <5 m off the trail. Nymphs collected during extra sampling were not included in density calculations.
DNA Extraction and Pathogen Detection, Ixodes scapularis
We prepared 375-μl triturates from up to 155 individual I. scapularis nymphs per park and extracted DNA from a 150-μl aliquot of each triturate as described in Graham et al. (2016). Leftover triturate was stored at 4 °C or −80 °C. For every 18 field-collected samples, we included one tick-free extraction as a negative control. Extracts were stored at 4 °C or −80 °C or tested immediately for B. burgdorferi, A. phagocytophilum, and Ba. microti using a pair of previously described multiplex real-time polymerase chain reaction (PCR) assays (Hojgaard et al. 2014). Each of the two assays, hereafter M1 and M2, included one target for each pathogen. M1 also targeted the I. scapularis actin gene to allow us to verify the presence of amplifiable DNA in each tick extract (Hojgaard et al. 2014). Each 10-μl reaction contained 4.8 μl eluate (~2% of the original tick), 1X iQ Multiplex Powermix (Bio-Rad, Hercules, CA), 300 nM each forward and reverse primer, and 200 nM each probe. Cycling conditions comprised a 3-min denaturation at 95 °C followed by 40 cycles of 95 °C for 10 s and 60 °C for 45 s. We tested each extract for B. miyamotoi using a second pair of real-time PCR assays targeting the adenylosuccinate lyase (purB) and glycerophosphodiester phosphodiesterase (glpQ) genes (Graham et al. 2016). One of the paired B. miyamotoi real-time PCR assays also detected the I. scapularis actin gene, which allowed us to verify the integrity of DNA that had been held at −80 °C for up to a year or at 4 °C for up to 2 mo after extraction and testing with M1 and M2.
Each set of real-time PCRs included negative extraction controls and a no-template control. Positive controls were composed of DNA from plasmid constructs containing the pathogen target sequences (Hojgaard et al. 2014, Graham et al. 2016). We carried out sample DNA extractions, PCR set-up, and amplification in three separate rooms. All real-time reactions were run on a C1000 Touch thermal cycler with a CFX96 real-time system (Bio-Rad).
We analyzed samples using CFX Manager 3.1 software (Bio-Rad) with the quantitation cycle (Cq) determination mode set to regression. To verify the integrity of each sample, we analyzed the distribution of I. scapularis actin Cq values for all samples collected in a single year and identified outliers by constructing an outlier box plot using JMP 11 statistical software (v. 11.1.1 SAS Institute Inc. 2013). Samples with an I. scapularis actin Cq greater than the upper whisker value (3rd quartile +1.5 [interquartile range]) were repeated. We also repeated any sample if the actin amplification curve had an end relative fluorescent unit (RFU) value <400. If the quality of the DNA was still suspect upon repeat, we extracted fresh DNA from 150 μl of the remaining tick triturate. If the second isolate repeatedly failed to yield acceptable Cq and RFU values for the I. scapularis actin target, that sample was not included in infection prevalence analyses. A sample that showed acceptable I. scapularis actin amplification and tested positive (Cq ≤40) for both pathogen targets was considered positive for that pathogen. We repeated or reextracted any sample that initially yielded inconsistent results for one or more pathogens.
In our hands, DNA extracted from B. burgdorferi (ss) strains typically yields similar Cq values for both B. burgdorferi targets employed in the Hojgaard et al. (2014) real-time PCR panels: a segment of the flagellar filament cap gene (fliD) and a segment of the B. burgdorferi chromosome containing partial tRNA coding sequences (gB31). DNA from other B. burgdorferi sensu lato species, however, may test positive for neither, one, or both targets, and some strains yield dramatically different Cq values for fliD and gB31 (data not shown). We therefore amplified and sequenced segments of the clpA and pepX genes to verify the species identification of at least one fliD- and gB31-positive sample from each park, including any sample that yielded fliD and gB31 Cq values that differed by ≥2. We employed a (semi-)nested approach based on the MLST typing protocol originally developed by Margos et al. (2008), and detailed by Wang et al. (2014), with minor modifications, to amplify both targets. Each 25-μl outer reaction contained 12.5 μl 2X HotStar Taq Master Mix (Qiagen, Valencia, CA), 500 nM each outer primer, 2.0 mM MgCl2, and 5–10 μl template. Cycling conditions included a 15-min activation and denaturation at 95 °C followed by 8 cycles (clpA) or 9 cycles (pepX) of 30 s at 94 °C, 30 s at 55 °C to 48 °C (clpA) or 60 °C to 52 °C (pepX), decreasing 1 °C each cycle, and 1 min at 72 °C. This was followed by an additional 20–30 cycles of 30 s at 94 °C, 30 s at 48 °C (clpA) or 52 °C (pepX), and 1 min at 72 °C, and a final 5 min extension at 72 °C. Each 50-μl inner reaction contained 25 μl 2X HotStar Taq Master Mix (Qiagen), 500 nM each outer primer, 2.0 mM MgCl2, and 5–10 μl product from the outer reaction. Cycling conditions were as indicated in the Wang et al. (2014) simplified (semi-)nested PCR protocol, with the denaturation step extended to 30 s and 50 °C and 52 °C annealing temperatures for clpA and pepX, respectively. We verified the presence of an ~850-nt clpA amplicon or an ~668-nt pepX amplicon by visualizing 10 μl of the inner product on a 1% agarose gel. The remaining product was purified using the QIAquick PCR Purification Kit (Qiagen). We sequenced ~4 ng of each amplicon in both directions using the inner amplification primers and BigDye Terminator 3.1 Ready Reaction Mix (ThermoFisher). The BigDye Xterminator Kit (ThermoFisher) was used to remove unincorporated dyes before analyzing the samples on an ABI 3130XL genetic analyzer. We used Lasergene 12 software (DNASTAR, Madison) to construct a consensus sequence for each amplicon based on at least double coverage of every nt and queried the GenBank and pubMLST (http://pubmlst.org/borrelia/; Margos et al. 2015) (accessed July 2016) databases for similar sequences. We verified the species identification of all samples that tested positive for B. miyamotoi by using a similar approach, described in Graham et al. (2016), to amplify, sequence, and analyze a segment of the clpA gene.
Molecular Verification of Tick Species Identification
It is difficult to distinguish I. scapularis nymphs from Ixodes affinis Neumann nymphs using morphologic keys, and some of our collection sites were in areas in which I. affinis may be expanding its range (Nadolny et al. 2011). We therefore tested a subset (378/679) of samples from our 2014 collection using a single-tube molecular assay to distinguish I. scapularis from I. affinis (Wright et al. 2014). Each 15-μl reaction contained 1X SsoAdvanced SYBR Green Supermix (Bio-Rad), 500 nM forward and reverse I. affinis primers, 500 nM forward and reverse I. scapularis primers, and 2 μl template. Reactions were performed on a C1000 Touch thermal cycler with a CFX96 real-time system (Bio-Rad), as previously described (Wright et al. 2014). We analyzed samples using CFX Manager 3.1 (Bio-Rad) with the Cq determination mode set to single threshold. Wright et al. (2014) reported that a melting peak range of 84.0–85.5°C was characteristic of I. scapularis, whereas a range of 81.5–82.5°C was characteristic of I. affinis. In our hands, DNA from laboratory-reared I. scapularis (Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention, Fort Collins, CO) processed alongside field-collected samples occasionally yielded melting peaks as high as 86.0°C. We therefore considered a Cq ≤30 and a melting temperature between 84.0 and 86.0°C confirmation that a specimen had been correctly identified as I. scapularis.
Statistical Analyses
We calculated the density of I. scapularis nymphs for each collection period by dividing the number of nymphs collected on each transect by the total drag area. To represent the highest potential risk in a given area, we then identified the single collection period during each year that yielded the highest nymphal density. We report this observed peak nymphal density as the density of nymphal I. scapularis (DON). We report the percent of all I. scapularis nymphs collected at a site over all collection periods within a year infected with B. burgdorferi, B. miyamotoi, A. phagocytophilum, or Ba. microti as the nymphal infection prevalence (NIP). We calculated the peak density of infected nymphs (DIN) by multiplying NIP by the peak DON to estimate the number of infected I. scapularis nymphs per 100 m2. We used the Wilcoxon signed rank tests to detect differences in DON and DIN between years. We used a likelihood ratio test with a chi-square approximation to test for differences in the proportion of infected ticks between years. Differences in density of infected nymphs among pathogens were analyzed using the non-parametric Kruskal–Wallis rank sums test with the Steel–Dwass correction for multiple comparisons. We assessed pathogen diversity using two diversity indices, Shannon–Wiener Index (H) and Simpson’s Index (D). These two indices measure species diversity in distinct ways, each accounting for richness and evenness differently. Simpson’s Diversity Index is a dominance index that accounts for number and abundance of each species and is sensitive to dominant or abundant species. The Shannon–Wiener Diversity Index is an evenness index that is equally sensitive to rare and abundant species. We used Pearson correlation (r) to detect relationships between the geographic location (latitude and longitude) of each park and pathogen diversity and the abundance of coinfected ticks. We also used Pearson correlation to examine the relationship between the numbers of coinfected ticks and peak DON. All statistical tests were carried out at a significance level of α =0.05 and performed using JMP statistical software (v. 11.1.1 SAS Institute, Inc. 2013).
Results
Seven national parks were sampled between late May and early August in 2014 and 2015; two additional parks were sampled only in 2015, for a total of nine parks sampled during the duration of the 2-yr study. Ticks characterized as I. scapularis based on morphology were found at all sites and in both years (Table 1). Given the morphological similarities, particularly among immature life stages, and coincidental timing of host-seeking activity between I. scapularis and I. affinis, we tested a subset of nymphs using molecular methods to confirm morphological identification. All nymphs tested were identified as I. scapularis.
We collected a total of 1,305 I. scapularis nymphs by drag sampling at seven national parks in 2014, and we collected 1,282 I. scapularis nymphs from nine national parks in 2015 (Table 1). Among the national parks sampled in 2014, we observed the lowest DON (0.67 nymphs per 100 m2) on the single transect sampled at Monocacy National Battlefield and the highest peak DON (13.73 nymphs per 100 m2) at Rock Creek Park. In 2015, Gettysburg National Military Park yielded the lowest DON (0.27 nymphs per 100 m2), whereas the highest DON (20.40 nymphs per 100 m2) occurred at Fire Island National Seashore, William Floyd Estate (Table 2). There was considerable variation among transects within national parks. The largest amount of variation in peak DON within a single national park was at Manassas in 2014, where there was an eightfold difference in DON among transects, and at Rock Creek Park in 2015, where there was a sevenfold difference in DON between the highest and lowest density transects. National parks showing the least amount of variation in DON across transects in both years were Monocacy National Battlefield and Prince William Forest Park, which had variation among transects of <1 nymphs per 100 m2 (Table 2). The median DON over all sites and years was 2.4 I. scapularis nymphs per 100 m2. Although a trend of fewer I. scapularis nymphs was noted in 2015 when the median peak DON was 1.6 nymphs per 100 m2, as compared with 3.0 nymphs per 100 m2 in 2014, there was no significant difference in the number of I. scapularis nymphs or DON detected between years (Wilcoxon signed ranks; W =10.00, P =0.11).
Table 2.
Density and infection prevalence of nymphal I. scapularis collected at national parks in the eastern United States—2014–2015
| Parka/Year | Peak DONb/100 m2 median (range) | No. nymphs PCR | Borrelia burgdorferi | Borrelia miyamotoi | Babesia microti | Anaplasma phagocytophilum | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||
| NIPc | Peak DINd/100 m2 (range) | NIP | Peak DIN/100 m2 (range) | NIP | Peak DIN/100 m2 (range) | NIP | Peak DIN/100 m2 (range) | ||||
| ACAD | 2015 | 3.20 (1.60–4.93) | 154 | 0.18 | 0.56 (0.28–1.48) | 0.01 | 0.02 (0.00–0.07) | 0.04 | 0.13 (0.00–0.19) | 0.03 | 0.10 (0.00–0.06) |
| CATO | 2014 | 2.93 (1.33–3.47) | 103 | 0.19 | 0.57 (0.39–0.80) | 0.02 | 0.06 (0.00–0.14) | 0.00 | NA | 0.03 | 0.09 (0.00–0.20) |
| 2015 | 1.33 (0.27–2.53) | 45 | 0.36 | 0.47 (0.09–0.88) | 0.04 | 0.06 (0.00–0.22) | 0.00 | NA | 0.02 | 0.03 (0.00–0.07) | |
| FIIS | 2014 | 10.67 (2.40–13.07) | 149 | 0.15 | 1.57 (0.20–3.14) | 0.04 | 0.43 (0.05–1.05) | 0.09 | 0.93 (0.20–1.60) | 0.07 | 0.79 (0.15–1.49) |
| 2015 | 12.00 (8.13–20.40) | 150 | 0.19 | 2.24 (1.20–5.71) | 0.02 | 0.24 (0.000–0.48) | 0.15 | 1.81 (0.72–5.71) | 0.11 | 1.28 (0.48–1.46) | |
| GETT | 2014 | 2.27 (1.33–4.00) | 63 | 0.29 | 0.65 (0.000–0.93) | 0.00 | NA | 0.00 | NA | 0.02 | 0.04 (0.00–0.04) |
| 2015 | 0.67 (0.27–2.67) | 51 | 0.16 | 0.11 (0.05–0.89) | 0.00 | NA | 0.00 | NA | 0.04 | 0.03 (0.00–0.05) | |
| MANA | 2014 | 4.87 (1.47 – 12.53) | 120 | 0.17 | 0.81 (0.29–1.33) | 0.03 | 0.12 (0.00–0.12) | 0.00 | NA | 0.00 | NA |
| 2015 | 3.87 (1.33–8.53) | 87 | 0.24 | 0.93 (0.42–2.05) | 0.02 | 0.09 (0.00–0.34) | 0.00 | NA | 0.00 | NA | |
| MONOe | 2014 | 0.67 (NA) | 10 | 0.10 | 0.07 | 0.00 | NA | 0.00 | NA | 0.00 | NA |
| 2015 | 0.67 (0.40–0.93) | 10 | 0.20 | 0.13 (0.00–0.27) | 0.00 | NA | 0.00 | NA | 0.00 | NA | |
| PRWI | 2014 | 1.47 (1.47–2.00) | 79 | 0.05 | 0.07 (0.06–0.12) | 0.03 | 0.04 (0.00–0.06) | 0.00 | NA | 0.03 | 0.04 (0.00–0.06) |
| 2015 | 1.33 (1.33–2.00) | 62 | 0.03 | 0.04 (0.00–0.10) | 0.02 | 0.02 (0.00–0.10) | 0.00 | NA | 0.00 | NA | |
| ROCR | 2014 | 12.13 (7.73 – 13.73) | 155 | 0.27 | 3.29 (1.69–3.88) | 0.01 | 0.16 (0.00–0.28) | 0.00 | NA | 0.00 | NA |
| 2015 | 3.60 (1.33–9.47) | 98 | 0.20 | 0.73 (0.61–0.97) | 0.00 | NA | 0.00 | NA | 0.01 | 0.04 (0.000–0.19) | |
| SHEN | 2015 | 1.33 (1.33–2.93) | 124 | 0.12 | 0.16 (0.08–0.39) | 0.00 | NA | 0.00 | NA | 0.02 | 0.03 (0.00–0.11) |
ACAD, Acadia National Park; CATO, Catoctin Mountain Park; FIIS, Fire Island National Seashore; GETT, Gettysburg National Military Park; MANA, Manassas National Battlefield Park; MONO, Monocacy National Battlefield; PRWI, Prince William Forest Park; ROCR, Rock Creek Park; SHEN, Shenandoah National Park.
DON, density of nymphal I. scapularis (range across transects).
NIP, nymphal infection prevalence.
DIN, density of infected nymphs (NIP × DON; range across transects).
Only one transect sampled at MONO in 2014.
We tested 679 and 781 I. scapularis nymphs collected in 2014 and 2015, respectively, for a suite of zoonotic I. scapularis-borne pathogens (Table 2). Borrelia burgdorferi was the most widespread pathogen, and was detected in nymphs collected at all sites in both years of the study. We were able to amplify and sequence two Borrelia targets (clpA and pepX) from 31 of the nymphs that had tested positive for B. burgdorferi by real-time PCR, and at least one target from two additional samples. This included at least one sample from each park for each collection year and all samples that had yielded suspect real-time PCR results. BLAST analysis indicated that all target sequences were ≥99% identical to homologous sequences from B. burgdorferi sensu stricto (ss) isolates, and ≤96% identical to the corresponding sequences from all other species, including other B. burgdorferi sensu lato species, in the GenBank database. In three cases, the sequence data indicated that the sample contained a mix of more than one species or strain, so we could not conclusively identify the infecting species. The sequence data were consistent, however, with coinfection with multiple B. burgdorferi (ss) strains. All pepX amplicons contained 570-nt segments identical to pepX alleles associated exclusively with B. burgdorferi (ss) isolates in the pubMLST database (alleles 1, 5, 6, 7, 8, and 18). All but two of the clpA amplicons contained a 579-nt segment identical to clpA alleles also associated exclusively with B. burgdorferi (ss) isolates (alleles 1, 4, 5, 6, 7, 9, 10, 18, 24, and 158). Two of the nymphs yielded identical clpA alleles that differed by a single nucleotide from clpA allele 21 (426A >G). Allele 21 is also associated only with B. burgdorferi (ss). Given that we positively identified 30 (11%) of the B. burgdorferi-positive samples as B. burgdorferi (ss), and given that we did not identify any other Borrelia spp. in any of the samples, we considered all B. burgdorferi-positive nymphs positive for B. burgdorferi (ss).
Borrelia burgdorferi (ss) NIP per park in I. scapularis ranged from 5.1% at Prince William Forest Park to 28.6% at Gettysburg National Military Park in 2014, and from 3.2% at Prince William Forest Park to 35.6% at Catoctin Mountain Park in 2015 (Table 2). There was no difference in the NIP with B. burgdorferi between years (likelihood ratio: χ2 =0.20, df =1, P =0.65). Among parks that were sampled in consecutive years, there was no significant difference detected in the density of I. scapularis nymphs infected with B. burgdorferi between years (Wilcoxon signed ranks; W =2.00, P =0.81); DIN was lowest at Prince William Forest Park in both years, with 0.07 and 0.04 infected nymphs per 100 m2 in 2014 and 2015, respectively. The highest DIN was detected at Rock Creek Park in 2014 (3.3 infected nymphs per 100 m2) and from Fire Island National Seashore, William Floyd Estate in 2015 (2.2 infected nymphs per 100 m2).
We also tested Ixodes scapularis nymphs for B. miyamotoi, A. phagocytophilum, and Ba. microti infection. Amplification and sequencing of the clpA target confirmed all B. miyamotoi-positives. Amplicon sequences from all B. miyamotoi-positive nymphs were identical to homologous sequences from B. miyamotoi CT14D4 (CP010308.1) and LB-2001 (CP006647.2), and all amplicons contained a 570-nt segment identical to clpA allele 200 in pubMSLT. As of July 26, 2016, this allele was associated with only one isolate in the pubMLST database, B. miyamotoi M1029. Borrelia miyamotoi-infected nymphs had a more sporadic distribution than nymphs infected with B. burgdorferi and were found at 66.7% of the sites sampled (Table 2). When present, NIP across all sites ranged from <1% at Acadia National Park to as high as 4.4% at Catoctin Mountain Park. Anaplasma phagocytophilum-infected nymphs were found at 77.8% of sites; NIP among sites ranged from 1% at Rock Creek Park to 10.7% of I. scapularis nymphs tested from Fire Island National Seashore, William Floyd Estate. Babesia microti was the least commonly detected pathogen and was only detected in the two most northerly national parks. At Acadia National Park, NIP for Ba. microti in 2015 was 4% and as many as 9% of I. scapularis nymphs from a given transect tested positive. NIP rates with Ba. microti at Fire Island National Seashore in 2014 and 2015 were 8.7% and 15.3%, respectively, with as many as 28% of nymphs infected from a single transect in 2015 (Table 2).
We detected multiple pathogens in I. scapularis from all sites except Monocacy National Battlefield, where we only detected B. burgdorferi. We calculated the Shannon–Wiener (H) and Simpson’s (D) Diversity Indices for all national parks and both years. Based on these indices, pathogen diversity in I. scapularis was lowest at Monocacy National Battlefield, whereas Fire Island National Seashore harbored the greatest pathogen diversity. There were no significant differences in Shannon–Wiener Diversity or Simpson’s Diversity among sites (Kruskal–Wallis; |z|≤1.29, P ≥0.96), and neither index of diversity was related to the density of I. scapularis nymphs (Pearson’s correlation: r ≤0.48, P ≥0.07). Further, there was no association between diversity and latitude (Pearson’s correlation: r ≤0.37, P ≥0.17); however, a significant association was found between longitude and both measures of species diversity (Pearson’s correlation: r ≤0.53, P ≥0.03). Higher diversity sites were located closer to the coast, although geographic position only explained ~50% of the observed variation in diversity.
A small percentage (2.7%) of I. scapularis nymphs tested were coinfected with multiple pathogens (Table 3). The majority of coinfected nymphs, 97.5%, were simultaneously infected with two different pathogens; coinfections with B. burgdorferi and Ba. microti were the most common, occurring in 16 of 39 (41.0%) coinfected nymphs, followed by coinfections with B. burgdorferi and A. phagocytophilum, which occurred in 14 of 39 (35.9%). We only observed coinfections with B. burgdorferi and B. miyamotoi in a single nymph collected from Manassas National Battlefield Park and a single nymph from Rock Creek Park. Other pathogen combinations in coinfected nymphs are shown in Table 3. Two parks accounted for the majority of coinfected nymphs; 62% were collected at Fire Island National Seashore and 26% at Acadia National Park. Fire Island National Seashore had the highest pathogen diversity of all sites and also had the highest rate of coinfected ticks; one tick found there was infected with three pathogens: B. burgdorferi, Ba. microti, and A. phagocytophilum. We were significantly more likely to observe coinfected ticks as DON increased (Pearson’s correlation: r =0.65, P =0.01), and coinfected ticks were most likely to occur at northern (Pearson’s correlation: r =0.55, P =0.03) and coastal sites (Pearson’s correlation: r =0.72, 0.48, P =0.002).
Table 3.
Coinfection of I. scapularis nymphs and density per 100 m2 from eastern United States national parks—2014–2015
| Sitea | Borrelia burgdorferi | Babesia microti | Anaplasma phagocytophilum | Borrelia miyamotoi | No. of ticks/total tested | Coinfection prevalence | Density of coinfected nymphs per 100 m2 |
|---|---|---|---|---|---|---|---|
| ACAD | |||||||
| 2015 | + | + | 5/154 | 0.032 | 0.10 | ||
| + | + | 5/154 | 0.032 | 0.10 | |||
| FIIS | |||||||
| 2014 | + | + | 3/149 | 0.020 | 0.17 | ||
| + | + | 5/149 | 0.034 | 0.30 | |||
| + | + | 1/149 | 0.007 | 0.06 | |||
| 2015 | + | + | 8/150 | 0.053 | 0.72 | ||
| + | + | 3/150 | 0.020 | 0.27 | |||
| + | + | 2/150 | 0.013 | 0.18 | |||
| + | + | 1/150 | 0.007 | 0.09 | |||
| + | + | + | 1/150 | 0.007 | 0.09 | ||
| GETT | |||||||
| 2014 | + | + | 1/63 | 0.016 | 0.04 | ||
| MANA | |||||||
| 2015 | + | + | 1/87 | 0.011 | 0.05 | ||
| PRWI | |||||||
| 2014 | + | + | 1/79 | 0.013 | 0.02 | ||
| ROCR | |||||||
| 2014 | + | + | 1/155 | 0.006 | 0.07 | ||
| SHEN | |||||||
| 2015 | + | + | 1/124 | 0.008 | 0.01 | ||
ACAD, Acadia National Park; FIIS, Fire Island National Seashore; GETT, Gettysburg National Military Park; MANA, Manassas National Battlefield Park; MONO, Monocacy National Battlefield; PRWI, Prince William Forest Park; ROCR, Rock Creek Park; SHEN, Shenandoah National Park.
Although we sampled habitat conducive to I. scapularis survival, we encountered and collected other species of ticks at most national parks sampled. Ixodes scapularis was the only species collected at Acadia National Park, whereas at least one other species was encountered at each of the other parks (Table 1). Dermacentor variabilis (Say) adults were collected from six national parks in 2014 and from three parks in 2015; a single D. variabilis nymph was collected at Gettysburg National Military Park and two D. variabilis nymphs were collected from Shenandoah National Park in 2015 (Table 1). Three sites accounted for the majority of the Amblyomma americanum (L.) found, and when present, A. americanum often occurred in high numbers. We collected >3,000 A. americanum adults and nymphs and >2,500 adults and nymphs at Fire Island National Seashore in 2014 and 2015, respectively (Table 1). A single Rhipicephalus sanguineus (Latreille) male and one Haemaphysalis leporispalustris (Packard) nymph was caught in 2015 at Fire Island National Seashore and one Amblyomma maculatum Koch was found in 2014 at Manassas National Battlefield Park (Table 1).
Discussion
Avoiding tick bites is essential to reducing the risk of tick-borne pathogen exposure, and this is best accomplished, particularly in recreational settings, by avoiding high-risk habitats during peak nymphal tick activity (Piesman and Eisen 2008). Assessing when and where people are at highest risk for exposure to vectors and what pathogens are present in those vectors are primary steps in risk assessment and prevention (Piesman and Eisen 2008, Eisen et al. 2013). In this study, we documented acarological risk for exposure to I. scapularis-borne pathogens on frequently used hiking trails in nine eastern national parks, but we observed great variability in acarological risk within and among parks. Compared with B. burgdorferi, ticks infected with B. miyamotoi, A. phagocytophilum, and Ba. microti were less widespread and less prevalent.
The results presented here for host-seeking I. scapularis distribution are generally consistent with previous studies conducted near the national parks we sampled. The establishment of I. scapularis is well documented across much of the study area (Eisen et al. 2016). However, to our knowledge, previous studies had not documented established I. scapularis populations in Washington, D.C., where we collected I. scapularis at Rock Creek Park, or in Greene County, Virginia, where we collected I. scapularis at Shenandoah National Park. Additionally, we confirmed recent reports of established I. scapularis populations in Albemarle, Warren, and Prince William Counties in Virginia, and in Adams County, Pennsylvania (Han et al. 2014, Eisen et al. 2016). National park units in Pennsylvania and Maryland are located within counties that have been considered high Lyme disease incidence counties since the mid- to late 1990s, while the District of Columbia and counties sampled in Virginia have only more recently achieved high-incidence Lyme disease status (Kugeler et al. 2015, Nelson et al. 2015).
Pathogen prevalence was also consistent with findings from previous research conducted at locations near our study sites. Han et al. (2014) reported an NIP of B. burgdorferi of 18% at Gettysburg National Military Park, Adams County, Pennsylvania. Similarly, investigations in Cumberland and York Counties, which border Adams County to the north and east, respectively, found a 39% to 52% infection prevalence of I. scapularis with B. burgdorferi (Diuk-Wasser et al. 2012, Hutchinson et al. 2015). In this study, I. scapularis nymphal infection prevalence with B. burgdorferi was 20–30% at Gettysburg National Military Park. At the same site, as many as 3% of nymphs collected in 2014 and 8% of nymphs collected in 2015 were found to be infected with A. phagocytophilum. A single I. scapularis nymph collected at this park was coinfected with B. burgdorferi and A. phagocytophilum, a coinfection prevalence of 1.6%. This is the first report of A. phagocytophilum from this park, but in Cumberland County, Pennsylvania, bordering Adams County to the north, B. burgdorferi, Ba. microti, and A. phagocytophilum are known to infect I. scapularis, and investigators have observed B. burgdorferi and A. phagocytophilum coinfections in about 1.5% of adult ticks (Hutchinson et al. 2015). We documented B. burgdorferi in 12–26% of I. scapularis nymphs from Acadia National Park, results which are consistent with other studies that reported infection prevalence of 11.8% (Ginsberg 1992) to 23% (Diuk-Wasser et al. 2012) in host-seeking nymphs, and as high as 45% in nymphs collected from field-caught rodents (Rand et al. 1993). Previous studies conducted at Fire Island National Seashore and Long Island reported higher infection prevalence (20–30%) of B. burgdorferi infecting I. scapularis nymphs (Ginsberg 1992, Diuk-Wasser et al. 2012); our results show NIP on individual transects at Fire Island National Seashore ranged from 8% to 28%. Across all transects, 14% and 19% of nymphs caught in 2014 and 2015 Fire Island National Seashore were infected with B. burgdorferi. Across study sites, coinfection in ticks was observed in 2.6% of nymphs tested. The most common coinfections were ticks infected with B. burgdorferi and Ba. microti (1.1% of all nymphs tested) or B. burgdorferi and A. phagocytophilum (0.7% of all nymphs tested). Coinfections of B. burgdorferi and Ba. microti were only documented in ticks collected from Acadia National Park and Fire Island National Seashore, whereas coinfections of B. burgdorferi and A. phagocytophilum occurred at Acadia National Park, Fire Island National Seashore, Gettysburg National Military Park, and Shenandoah National Park (Table 3). The rates of coinfection documented here are in general agreement with published rates of coinfections in I. scapularis nymphs with B. burgdorferi and Ba. microti or B. burgdorferi and A. phagocytophilum (Diuk-Wasser et al. 2016, Feldman et al. 2015). The highest rate of coinfection with B. burgdorferi and Ba. microti at our study sites was 5.3% at Fire Island National Seashore, whereas coinfection rates as high as 7.7% have been documented at residential sites elsewhere in New York (Feldman et al. 2015).
Over the past two decades, the distribution of Virginia counties reporting Lyme disease cases has expanded to the southwest, as has the distribution of counties classified as having established I. scapularis populations (Brinkerhoff et al. 2014, Eisen et al. 2016). Diuk-Wasser et al. (2012) reported the highest DON and DIN in the northeastern portion of Virginia, and they reported few I. scapularis nymphs and did not detect B. burgdorferi at sites located throughout the rest of the state. They sampled from 2004 to 2006, however, when Lyme disease cases occurred less frequently in central and western Virginia than in recent years (Sonenshine et al. 1995, Casteel and Sonenshine 1996, Brinkerhoff et al. 2014). In 2013, Ford et al. (2015) did not find I. scapularis at any of six sampling areas along the Appalachian Trail in west, central, and north-central Virginia. We sampled Shenandoah National Park in the north-central part of the state and Manassas National Battlefield and Prince William Forest park in the northeast. We documented I. scapularis and B. burgdorferi on each transect at all three national parks sampled in Virginia, with the exception of a single transect at Prince William Forest Park from which 0/26 nymphs tested positive for any pathogen in 2015. Across the state, prevalence of B. burgdorferi ranged from 6 to 29%, and prevalence of A. phagocytophilum was as high as 8% at three transects at Shenandoah National Park. Findings were similar <100 km east at Manassas National Battlefield Park, where the number of I. scapularis nymphs was comparable, prevalence of B. burgdorferi ranged from 8 to 39%, and prevalence of B. miyamotoi was as high as 8%. Interestingly, Manassas National Battlefield Park and Prince William Forest Park are located <40 km apart in Prince William County in northeast Virginia, yet the DON was roughly three times greater at Manassas National Battlefield Park than at Prince William Forest Park, and NIP with B. burgdorferi was 10 times higher at Manassas National Battlefield Park than at Prince William Forest Park in 2014 and 20 times higher at Manassas National Battlefield Park than at Prince William Forest Park in 2015. It is not surprising to observe considerable differences in NIP among park units located in close proximity, as national park units are often quite different from surrounding areas and may be managed differently depending on the type or park unit, e.g., battlefield park versus forest park, and thus the density of infected ticks may differ between settings. Thus, it is difficult to infer risk of surrounding areas based on a single measurement and inference to the risk of tick-borne disease for park visitors or employees should be based on park-specific surveillance efforts. Generally, our findings thus suggest that the distribution of B. burgdorferi-infected I. scapularis, like the distribution of counties reporting Lyme disease, is expanding westward from known human disease foci in the eastern United States (Nelson et al. 2015).
For this study, we aimed to sample for ticks during the period of peak I. scapularis nymphal host-seeking activity which occurs between May and August in the Mid-Atlantic and Northeastern United States (Wilson and Spielman 1985, Piesman et al. 1987, Sonenshine and Mather 1994, Ostfeld et al. 1996, Diuk-Wasser et al. 2006, Gatewood et al. 2009, Orr et al. 2013). Although this was our aim and we report peak observed nymphal density, we did not conduct phenology studies simultaneously to document the nymphal peak and therefore cannot infer that the numbers of ticks reported here represent the absolute peak in nymphal host-seeking density each year. Peak density of I. scapularis nymphs was highly variable across national park units sampled and ranged from a low of less than one nymph per 100 m2 at Monocacy National Battlefield, to 12 nymphs per 100 m2 at Fire Island National Seashore, William Floyd Estate, in both years of the study. Most other sites had between two and five I. scapularis nymphs per 100 m2. Moreover, although we targeted I. scapularis habitat, primarily deciduous forests with canopy and adequate leaf litter (Ginsberg and Ewing 1989, Siegel et al. 1991), both A. americanum and D. variabilis overlap with I. scapularis in both questing activity timing and habitat (Bishopp and Trembley 1945, Sonenshine 1991), and thus were incidental captures.
This research demonstrates the potential risk of tick-borne disease in eastern national parks and can be used to promote awareness among park visitors of the potential for recreational tick and tick-borne pathogen exposure. This work represents a step toward understanding public health risks in these national parks, and the data will aid in improving prevention and education programs for park visitors. To reduce vector-borne disease exposures, national parks focus on encouraging visitors to use appropriate personal protective measures. Strategies include informing visitors when and where they are at highest risk, which pathogens are present and what symptoms result from infection (National Park Service 2006, Piesman and Eisen 2008, Eisen et al. 2013), and providing guidance on personal protective measures to prevent tick bite (http://www.cdc.gov/ticks/avoid/on_people.html) (accessed July 2016), the importance of prompt and safe removal of attached ticks (http://www.cdc.gov/ticks/removing_a_tick.html) (accessed July 2016) and seeking medical attention if signs and symptoms occur after being bitten by a tick (http://www.cdc.gov/ticks/symptoms.html) (accessed July 2016). Promoting prevention efforts in these areas may be particularly important for those visitors traveling from nonendemic areas that may not be as mindful to the prevention of tick bites or symptoms of tick-borne diseases. Although this work identified the diversity, density, and distribution of ticks and medically important tick-borne pathogens, further studies are needed to fully assess acarological risk, including studies that incorporate communication with visitors to evaluate how frequently people encounter ticks in national parks and to identify specific behaviors that may result in increased risk of tick encounter.
Acknowledgments
We thank Courtney Asher, Andrew Banasik, Jessica Carson, Megan Casey, Bruce Connery, Lindsey Donaldson, Ken Ferebee, David Gaines, Dorothy Geyer, Bridgette Gleason, Bryan Gorsira, Christopher Heilakka, Katelyn Kerr, Eric Kelley, Cara Bicking Kinsey, Randy Krichten, Adam Kramer, Rebecca Lancosky, Allison Longenberger, Kevin Miller, Charlie Newton, Tony Nguyen, Amy Thomas, Liz Thomas, Rick Toomey, Erickson Smith, Andre Weltman, Bik Wheeler, Alan Williams, Ahna Wilson, Bill Yeaman, and Andy Yu for assistance with tick collections.
References Cited
- Barbour AG, Fish D. The biological and social phenomenon of Lyme disease. Science. 1993;260:1610–1616. doi: 10.1126/science.8503006. [DOI] [PubMed] [Google Scholar]
- Bishopp F, Trembley HL. Distribution and hosts of certain North American ticks. J Parasitol. 1945;31:1–54. [Google Scholar]
- Brinkerhoff RJ, Gilliam WF, Gaines D. Lyme disease, Virginia, USA, 2000–2011. Emerg Infect Dis. 2014;20:1661–1668. doi: 10.3201/eid2010.130782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteel M, Sonenshine DE. Abundance of adult Ixodes scapularis and infection with Borrelia burgdorferi in eastern Virginia. VA J Sci. 1996;47:293–301. [Google Scholar]
- Connally NP, Ginsberg HS, Mather TN. Assessing peridomestic entomological factors as predictors for Lyme disease. J Vector Ecol. 2006;31:364–370. doi: 10.3376/1081-1710(2006)31[364:apefap]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Connally NP, Durante AJ, Yousey-Hindes KM, Meek JI, Nelson RS, Heimer R. Peridomestic Lyme disease prevention: Results of a population-based case–control study. Am J Prev Med. 2009;37:201–206. doi: 10.1016/j.amepre.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Diuk-Wasser MA, Gatewood AG, Cortinas MR, Yaremych-Hamer S, Tsao J, Kitron U, Hickling G, Brownstein JS, Walker E, Piesman J, et al. Spatiotemporal patterns of host-seeking Ixodes scapularis nymphs (Acari: Ixodidae) in the United States. J Med Entomol. 2006;43:166–176. doi: 10.1603/0022-2585(2006)043[0166:spohis]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Diuk-Wasser MA, Hoen AG, Cislo P, Brinkerhoff R, Hamer SA, Rowland M, Cortinas R, Vourc’h G, Melton F, Hickling GJ, et al. Human risk of infection with Borrelia burgdorferi, the Lyme disease agent, in eastern United States. Am J Trop Med Hyg. 2012;86:320–327. doi: 10.4269/ajtmh.2012.11-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diuk-Wasser MA, Vannier E, Krause PJ. Coinfection by Ixodes tick-borne pathogens: Ecological, epidemiological, and clinical consequences. TREE. 2016;32:30–42. doi: 10.1016/j.pt.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, Wong D, Shelus V, Eisen RJ. What is the risk for exposure to vector-borne pathogens in United States national parks? J Med Entomol. 2013;50:221–230. doi: 10.1603/me12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Beard CB. County-Scale Distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Continental United States. J Med Entomol. 2016;53:349–386. doi: 10.1093/jme/tjv237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco RC, Fish D. Prevalence of Ixodes dammini near the homes of Lyme disease patients in Westchester County, New York. Am J Epidemiol. 1988;127:826–830. doi: 10.1093/oxfordjournals.aje.a114865. [DOI] [PubMed] [Google Scholar]
- Falco RC, Fish D. Potential for exposure to tick bites in recreational parks in a Lyme disease endemic area. Am J Pub Health. 1989;79:12–15. doi: 10.2105/ajph.79.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco RC, McKenna DF, Daniels TJ, Nadelman RB, Nowakowski J, Fish D, Wormser GP. Temporal relation between Ixodes scapularis abundance and risk for Lyme disease associated with erythema migrans. Am J Epidemiol. 1999;149:771–776. doi: 10.1093/oxfordjournals.aje.a009886. [DOI] [PubMed] [Google Scholar]
- Feldman KA, Connally NP, Hojgaard A, Jones EH, White JL, Hinckley AF. Abundance and infection rates of Ixodes scapularis nymphs collected from residential properties in Lyme disease-endemic areas of Connecticut, Maryland, and New York. J Vector Ecol. 2015;40:198–201. doi: 10.1111/jvec.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish D. Population ecology of Ixodes dammini. In: Ginsberg H, editor. Ecology and Environmental Management of Lyme Disease. Rutgers University Press; New Brunswick, NJ: 1993. pp. 25–42. [Google Scholar]
- Ford K, Nadolny R, Stromdahl E, Hickling G. Tick surveillance and disease prevention on the Appalachian trail. Park Sci. 2015;32:36. [Google Scholar]
- Gatewood AG, Liebman KA, Vourc’h G, Bunikis J, Hamer SA, Cortinas R, Melton F, Cislo P, Kitron U, Tsao J, et al. Climate and tick seasonality are predictors of Borrelia burgdorferi genotype distribution. Appl Environ Microb. 2009;75:2476–2483. doi: 10.1128/AEM.02633-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg HS. Ecology and management of ticks and Lyme disease at Fire Island National Seashore and selected eastern national parks. US National Park Service; 1992. [Google Scholar]
- Ginsberg HS, Ewing CP. Habitat distribution of Ixodes dammini (Acari: Ixodidae) an Lyme disease spirochetes on Fire Island, New York. J Med Entomol. 1989;26:183–189. doi: 10.1093/jmedent/26.3.183. [DOI] [PubMed] [Google Scholar]
- Graham CB, Pilgard MA, Maes SE, Hojgaard A, Eisen RJ. Paired real-time PCR assays for detection of Borrelia miyamotoi in North American Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) Ticks Tick Borne Dis. 2016;7:1230–1235. doi: 10.1016/j.ttbdis.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han GS, Stromdahl EY, Wong D, Weltman AC. Exposure to Borrelia burgdorferi and other tick-borne pathogens in Gettysburg National Military Park, south-central Pennsylvania, 2009. Vector Borne Zoonotic Dis. 2014;14:227–233. doi: 10.1089/vbz.2013.1363. [DOI] [PubMed] [Google Scholar]
- Hojgaard A, Lukacik G, Piesman J. Detection of Borrelia burgdorferi, Anaplasma phagocytophilum and Babesia microti, with two different multiplex PCR assays. Ticks Tick Borne Dis. 2014;5:349–351. doi: 10.1016/j.ttbdis.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Homer MJ, Aguilar-Delfin I, Telford SR, 3rd, Krause PJ, Persing DH. Babesiosis. Clin Microbiol Rev. 2000;13:451–469. doi: 10.1128/cmr.13.3.451-469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson ML, Strohecker MD, Simmons TW, Kyle AD, Helwig MW. Prevalence rates of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in host-seeking Ixodes scapularis (Acari: Ixodidae) from Pennsylvania. J Med Entomol. 2015;52:693–698. doi: 10.1093/jme/tjv037. [DOI] [PubMed] [Google Scholar]
- Jin H, Wei F, Liu Q, Qian J. Epidemiology and control of human granulocytic anaplasmosis: A systematic review. Vector Borne Zoonotic Dis. 2012;12:269–274. doi: 10.1089/vbz.2011.0753. [DOI] [PubMed] [Google Scholar]
- Kitron U, Kazmierczak JJ. Spatial analysis of the distribution of Lyme disease in Wisconsin. Am J Epidemiol. 1997;145:558–566. doi: 10.1093/oxfordjournals.aje.a009145. [DOI] [PubMed] [Google Scholar]
- Klein JD, Eppes SC, Hunt P. Environmental and life-style risk factors for Lyme disease in children. Clin Pediatr. 1996;35:359–363. doi: 10.1177/000992289603500705. [DOI] [PubMed] [Google Scholar]
- Krause PJ, Fish D, Narasimhan S, Barbour AG. Borrelia miyamotoi infection in nature and in humans. Clin Microbiol Infect. 2015;21:631–639. doi: 10.1016/j.cmi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugeler KJ, Farley GM, Forrester JD, Mead PS. Geographic distribution and expansion of human Lyme disease, United States. Emerg Infect Dis. 2015;21:1455–1457. doi: 10.3201/eid2108.141878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margos G, Gatewood AG, Aanensen DM, Hanincová K, Terekhova D, Vollmer SA, Hurn MA. MLST of housekeeping genes captures geographic population structure and suggests a European origin of Borrelia burgdorferi. PNAS. 2008;105:8730–8735. doi: 10.1073/pnas.0800323105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margos G, Binder K, Dzaferovic E, Hizo-Teufel C, Sing A, Wildner M, Fingerle V, Jolley KA. PubMLST. org-The new home for the Borrelia MLSA database. Ticks Tick Borne Dis. 2015;6:869. doi: 10.1016/j.ttbdis.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Mather TN, Nicholson MC, Donnelly EF, Matyas BT. Entomologic index for human risk of Lyme disease. Am J Epidemiol. 1996;144:1066–1069. doi: 10.1093/oxfordjournals.aje.a008879. [DOI] [PubMed] [Google Scholar]
- Maupin GO, Fish D, Zultowsky J, Campos EG, Piesman J. Landscape ecology of Lyme disease in a residential area of Westchester County, New York. Am J Epidemiol. 1991;133:1105–1113. doi: 10.1093/oxfordjournals.aje.a115823. [DOI] [PubMed] [Google Scholar]
- Mead PS. Epidemiology of Lyme disease. Infect Dis Clin North Am. 2015;29:187–210. doi: 10.1016/j.idc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Nadolny RM, Wright CL, Hynes WL, Sonenshine DE, Gaff HD. Ixodes affinis (Acari: Ixodidae) in southeastern Virginia and implications for the spread of Borrelia burgdorferi, the agent of Lyme disease. J Vector Ecol. 2011;36:464–467. doi: 10.1111/j.1948-7134.2011.00191.x. [DOI] [PubMed] [Google Scholar]
- National Park Service. Management policies 2006. United States Government Printing Office; Washington, DC: 2006. [Google Scholar]
- Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley AF, Mead PS. Incidence of clinician-diagnosed Lyme disease, United States, 2005–2010. Emerg Infect Dis. 2015;21:1625–1631. doi: 10.3201/eid2109.150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J, Howard JJ. Occurrence of Ixodes scapularis (Acari: Ixodidae) on a selected segment of the Appalachian Trail. J Med Entomol. 1998;35:54–58. doi: 10.1093/jmedent/35.1.54. [DOI] [PubMed] [Google Scholar]
- Orloski KA, Hayes EB, Campbell GL, Dennis DT. Surveillance for Lyme disease–United States, 1992–1998. MMWR Surveill Summ. 2000;49:1–11. [PubMed] [Google Scholar]
- Orr JM, Smith JD, Zawada SG, Arias JR. Diel and seasonal activity and trapping of ticks (Acari: Ixodidae) in Northern Virginia, USA. Syst Appl Acarol. 2013;18:105–111. [Google Scholar]
- Ostfeld RS, Hazler KR, Cepeda OM. Temporal and spatial dynamics of Ixodes scapularis (Acari: Ixodidae) in a rural landscape. J Med Entomol. 1996;33:90–95. doi: 10.1093/jmedent/33.1.90. [DOI] [PubMed] [Google Scholar]
- Paskewitz SM, Vandermause M, Belongia EA, Kazmierczak JJ. Ixodes scapularis (Acari: Ixodidae): Abundance and rate of infection with Borrelia burgdorferi in four state parks in Wisconsin. J Med Entomol. 2001;38:33–38. doi: 10.1603/0022-2585-38.1.33. [DOI] [PubMed] [Google Scholar]
- Pepin KM, Eisen RJ, Mead PS, Piesman J, Fish D, Hoen AG, Barbour AG, Hamer S, Diuk-Wasser MA. Geographic variation in the relationship between human Lyme disease incidence and density of infected host-seeking Ixodes scapularis nymphs in the Eastern United States. Am J Trop Med Hyg. 2012;86:1062–1071. doi: 10.4269/ajtmh.2012.11-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J. Transmission of Lyme disease spirochetes (Borrelia burgdorferi) Exp Appl Aacarol. 1989;7:71–80. doi: 10.1007/BF01200454. [DOI] [PubMed] [Google Scholar]
- Piesman J, Eisen L. Prevention of tick-borne diseases. Ann Rev Entomol. 2008;53:323–343. doi: 10.1146/annurev.ento.53.103106.093429. [DOI] [PubMed] [Google Scholar]
- Piesman J, Mather TN, Dammin GJ, Telford SR, Lastavica CC, Spielman A. Seasonal variation of transmission risk of Lyme disease and human babesiosis. Am J Epidemiol. 1987;126:1187–1189. doi: 10.1093/oxfordjournals.aje.a114757. [DOI] [PubMed] [Google Scholar]
- Prusinski M, Kokas J, Hukey K, Kogut S, Lee J, Backenson P. Prevalence of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in Ixodes scapularis (Acari: Ixodidae) collected from recreational lands in the Hudson Valley region, New York state. J Med Entomol. 2014;51:226–236. doi: 10.1603/me13101. [DOI] [PubMed] [Google Scholar]
- Rand PW, Lacombe EH, Smith RP, Jr, Rich SM, Kilpatrick CW, Dragoni CA, Caporale D. Competence of Peromyscus maniculatus (Rodentia: Cricetidae) as a reservoir host for Borrelia burgdorferi (Spirochaetares: Spirochaetaceae) in the wild. J Med Entomol. 1993;30:614–618. doi: 10.1093/jmedent/30.3.614. [DOI] [PubMed] [Google Scholar]
- Rand PW, Lacombe EH, Dearborn R, Cahill B, Elias S, Lubelczyk CB, Beckett GA, Smith RP. Passive surveillance in Maine, an area emergent for tick-borne diseases. J Med Entomol. 2007;44:1118–1129. doi: 10.1603/0022-2585(2007)44[1118:psimaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Taylor GC, Vasvary LM, Simmons W, Jordan RA. Effectiveness of an aerial application of carbaryl in controlling Ixodes dammini (Acari: Ixodidae) adults in a high-use recreational area in New Jersey. J Med Entomol. 1992;29:544–547. doi: 10.1093/jmedent/29.3.544. [DOI] [PubMed] [Google Scholar]
- Siegel JP, Kitron U, Bouseman JK. Spatial and temporal distribution of Ixodes dammini (Acari: Ixodidae) in a northwestern Illinois state park. J Med Entomol. 1991;28:101–104. doi: 10.1093/jmedent/28.1.101. [DOI] [PubMed] [Google Scholar]
- Sonenshine D. Biology of ticks. Vol. 1. Oxford University Press; New York, NY: 1991. Tick life cycles; pp. 51–66. [Google Scholar]
- Sonenshine DE, Mather TN. Ecological dynamics of tick-borne zoonoses. Oxford University Press on Demand; New York, NY: 1994. [Google Scholar]
- Sonenshine DE, Ratzlaff RE, Troyer J, Demmerle S, Demmerle ER, Austin WE, Tan S, Annis BA, Jenkins S. Borrelia burgdorferi in eastern Virginia: Comparison between a coastal and inland locality. Am J Trop Med Hyg. 1995;53:123–133. doi: 10.4269/ajtmh.1995.53.123. [DOI] [PubMed] [Google Scholar]
- Stafford KC, Cartter ML, Magnarelli LA, Ertel SH, Mshar PA. Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. J Clin Microbiol. 1998;36:1240–1244. doi: 10.1128/jcm.36.5.1240-1244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Liveris D, Mukherjee P, Jungnick S, Margos G, Schwartz I. Molecular typing of Borrelia burgdorferi. Curr Prot Microbiol. 2014;34:12C. 15.11–12C. 15.31. doi: 10.1002/9780471729259.mc12c05s34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ML, Spielman A. Seasonal activity of immature Ixodes dammini (Acari: Ixodidae) J Med Entomol. 1985;22:408–414. doi: 10.1093/jmedent/22.4.408. [DOI] [PubMed] [Google Scholar]
- Wright CL, Hynes WL, White BT, Marshall MN, Gaff HD, Gauthier DT. Single-tube real-time PCR assay for differentiation of Ixodes affinis and Ixodes scapularis. Ticks Tick Borne Dis. 2014;5:48–52. doi: 10.1016/j.ttbdis.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]