Abstract
Retinal expression of transgenes was examined in four mouse lines. Two constructs were driven by the choline acetyltransferase (ChAT) promoter: green fluorescent protein conjugated to tau protein (tau-GFP) or cytosolic yellow fluorescent protein (YFP) generated through CRE recombinase-induced expression of Rosa26 (ChAT-CRE/ Rosa26YFP). Two other constructs targeted inhibitory interneurons: GABAergic horizontal and amacrine cells identified by glutamic acid decarboxylase (GAD65-GFP) or parvalbumin (PV) cells (PV-CRE/Rosa26YFP). Animals were transcardially perfused and retinal sections prepared. Antibodies against PV, calretinin (CALR), calbindin (CALB), and tyrosine hydroxylase (TH) were used to counterstain transgene-expressing cells. In PVxRosa and ChAT-tauGFP constructs, staining appeared in vertically oriented row of processes resembling Müller cells. In the ChATxRosa construct, populations of amacrine cells and neurons in the ganglion cell layer were labeled. Some cones also exhibited GFP fluorescence. CALR, PV and TH were found in none of these cells. Occasionally, we found GFP/ CALR and GFP/PV double-stained cells in the ganglion cell layer (GCL). In the GAD65-GFP construct, all layers of the neuroretina were labeled, except photoreceptors. Not all horizontal cells expressed GFP. We did not find GFP/TH double-labeled cells and GFP was rarely present in CALR-and CALB-containing cells. Many PV-positive neurons were also labeled for GFP, including small diameter amacrines. In the GCL, single labeling for GFP and PV was ascertained, as well as several CALR/PV double-stained neurons. In the GCL, cells triple labeled with GFP/CALR/ CALB were sparse. In conclusion, only one of the four transgenic constructs exhibited an expression pattern consistent with endogenous retinal protein expression, while the others strongly suggested ectopic gene expression.
Keywords: Expression mismatch, ChAT-tauGFP, ChAT-CRE/Rosa26YFP, GAD65-GFP, PV-CRE/Rosa26YFP
Introduction
The availability of transgenic animal strains, especially mice, has changed the research strategies of many laboratories. Exploiting the fact that expression of particular proteins can be highly specific, targeting of formally characterized cell types to obtain more detailed information on anatomy, physiology and cell biology has become a powerful resource in biomedical research.
In recent years, a number of transgenic mouse lines that selectively express fluorescent proteins (FPs) in retinal cell types have been introduced. For example, in a transgenic mouse line containing sequences upstream of the human red and green visual pigments, cone photoreceptors can be engineered to express green FP (GFP; Fei and Hughes 2001). Under the control of the metabotropic glutamate receptor (mGluR) 6 promoter, both rod and cone ON-bipolar cells can be labeled with GFP (Dhingra et al. 2008). GABAergic amacrine cells express GFP under the control of the glutamic acid decarboxylase (GAD) promoter (May et al. 2008) while glycinergic amacrine cells can be visualized under the control of the glycine transporter 1 promoter (Zeilhofer et al. 2005). Moreover, cholinergic amacrine cells can be labeled by expressing GFP fused to the human interleukin 2-subunit under the control of the mGluR2 promoter (Yoshida et al. 2001; Wang et al. 2007), while dopaminergic amacrine cells are visualized using human placental alkaline phosphatase under the control of the tyrosine hydroxylase (TH) promoter (Gustincich et al. 1997). In the mouse retina, the majority of amacrine cells, including the cholinergic cells (Haverkamp and Wässle 2000), contain calretinin (CALR). These neurons expressed GFP in CD44 (cluster of differentiation 44) mice (Sarthy et al. 2007). Badea and Nathans (2004) developed a genetic method for revealing cell morphologies in the mouse retina by using alkaline phosphatase as histochemical reporter and performed a quantitative analysis of bipolar, amacrine, and ganglion cells. Huberman and colleagues (2008) have screened the library of bacterial artificial chromosome transgenic mice with GFP expressed under the control of different promoters (Gong et al. 2007). Others observed transient OFF alpha ganglion cells in CALR-EGFP mice (Pang et al. 2003).
However, not all the experiments brought the expected results. In a paper of Haverkamp and colleagues (2009), four transgenic mouse lines were examined in detail. Expression of enhanced GFP (EGFP) driven by the CALR and parvalbumin (PV) promoter was found in amacrine, displaced amacrine and ganglion cells. Comparison of EGFP expression and CALR/PV immunolabeling showed that not all cells were double labeled. Expression of EGFP under the control of the choline acetyltransferase (ChAT) promoter was found in amacrine cells; however, the fluorescent cells did not correspond to the well-characterized cholinergic (starburst) cells of the mouse retina. Similarly, the expression of EGFP driven by the promoter for the serotonin (5-HT) 3A receptor (5-HT3AR) was restricted to type 5 bipolar cells. In contrast, immunostaining for 5-HT3AR was found in synaptic hot spots in sublamina 1 of the inner plexiform layer and was not related to type 5 bipolar cells. Despite these results, the authors concluded that these mouse lines are extremely useful for microelectrode targeting of well-defined retinal neuron types; they have investigated one such transgenic cell type in detail, identifying it as a wide-field ON–OFF gamma-aminobutyric acid (GABA)-containing cell type (Knop et al. 2014).
In this study, retinal expression of fluorescent transgenes is examined in four transgenic mouse lines. Two constructs are related to the cholinergic system: one is expressing GFP conjugated to the axonal tau protein (ChAT-tauGFP mouse line; Grybko et al. 2011), while the other expresses yellow FP (YFP) in ChAT-CRE neurons (Chat-RosaYFP; Ivanova et al. 2010; Gong et al. 2007; Hao et al. 2013). The other two constructs are related to the most frequent inhibitory interneuron populations: the GABAergic horizontal and amacrine cells which can be identified by their glutamic acid decarboxylase 65 (GAD65) content (GAD65-GFP; Lopez-Bendito et al. 2004) while the other is the PV-containing amacrine cell population (PVRosa-YFP; Hippenmeyer et al. 2005) and some ganglion cell types (Haverkamp and Wässle 2000). These constructs can possibly be useful for microanatomical, physiological and pharmacological research. Exact identification of the transgene-expressing cells may facilitate the use of these animals in studying the development of certain retinal cell types, from early embryonic age through adulthood, since neuron generation in the mouse retina lasts for more than 20 days. Several characteristic endogenous markers are expressed only late, often only after birth (Young et al. 1985; Bagnoli et al. 2003), and many genes switch on temporarily (Zhang et al. 2006). It may also be possible to follow certain cell types involved in retinal degenerative disorders to examine the effects of therapeutic intervention.
Materials and methods
Transgenic mice
All procedures were performed in accordance with the University of Montana Institutional Animal Care and Use Committee (AUP 026-11). After wean, mice were socially housed in gender-specific groups of 4–5 littermates per cage. Mice were housed in a room equipped with light timers set on a 12:12 light/dark cycle. The animals used in this study were between 10 and 20 weeks of age. Four mouse lines were used in this study:
Chat-tauGFP mice (n = 4) in which GFP is conjugated to the axonal protein tau (Grybko et al. 2011).
Chat-Rosa mice (n = 6; Ivanova et al. 2010; Yi et al. 2015) which were obtained by crossing homozygous ChAT-CRE (GM24 founder line, MMRRC 017269-UCD; Gong et al. 2007) with homozygous Rosa26YFP mice (Jackson Labs stock number 007920; Soriano 1999; Madisen et al. 2009).
PV-Rosa mice (n = 4; Yi et al. 2014), which were obtained by crossing homozygous PV-CRE mice (Hippenmeyer et al. 2005; stock #008069; Jackson Labs, Bar Harbor, ME) with homozygous Rosa26YFP mice.
GAD65-GFP mice (n = 6), in which GFP is driven by the GABA synthesizing enzyme GAD65 (Lopez-Bendito et al. 2004).
Each of these transgenic mouse lines yields GFP or YFP fluorescence in surviving slices in subpopulations of neurons throughout the central nervous system.
Tissue preparation
Animals were anesthetised with isoflurane and transcardially perfused first with physiological saline and then with ice-cold 4 % paraformaldehyde dissolved in 0.1 M phosphate buffer. The eyes were dissected from the orbits, cut open along the edge of the cornea and the lens was removed. Postfixation was carried out overnight. After fixation, the samples were thoroughly washed in phosphate buffered saline (PBS) for 4–6 h. Samples were then immersed in 30 % sucrose in phosphate buffer, sectioned in a cryostat at 15 µm, collected on positively charged glass slides, and stored at −20 °C until use.
Immunohistochemistry
After bringing tissues to room temperature, sections were preincubated for 1 h in an antibody diluent solution described previously (Gábriel et al. 1992). Double- and triple-labeling experiments were designed for each construct based on the transgenes inserted (Table 1). The primary antibodies were from commercial sources and listed in Table 2. In our experience, the antibody used to label GFP fully cross-reacts with YFP. As a procedure common to all experiments, the GFP/YFP marker linked to the transgene was developed with fluorescent secondary antibodies emitting in the green wavelength. Based on a previous thorough description of the neurochemical markers in mice (Haverkamp and Wässle 2000), an anti-calretinin antibody was utilized to label, among others, the cholinergic cell populations; an anti-calbindin 28 kDa antibody labeled the horizontal and AII amacrine cells. Dopaminergic cells were marked with an anti-TH antibody, while an anti-parvalbumin antibody labeled a number of amacrine and ganglion cell types (Table 2). Final dilutions of the primaries were made with the antibody diluent. After overnight incubation, samples were washed in PBS for 6 × 10 min and the secondary antibodies were applied as follows: donkey anti-chicken Alexa 488 (1:400; Life Technologies); donkey anti-rabbit Alexa 647 (1:200; Life Technologies) and donkey anti-goat Alexa 350 (1:200; Life Technologies). Controls have been made by omitting the primary antibodies, in which case relatively weak or no intrinsic fluorescence of the genetically modified cells was seen in the green wavelength, with the exception of the GAD65-GFP construct, where the intrinsic GFP fluorescence remained strong even after fixation of the tissue. Crossreactivity of the non-corresponding secondary antibodies have also been checked in pairs (the chicken primary against the anti-goat and anti-rabbit secondary, the goat primary against the anti-rabbit, and anti-chicken secondary and rabbit primary against the anti-chicken and anti-goat secondary). No crossreactivity was observed in these experiments. In some experiments, we utilized peanut agglutinin (PNA) conjugated to rhodamine (Vector Laboratories) to selectively label cone photoreceptors (Mieziewska et al. 1991). PNA-rhodamine was mixed with the anti-GFP primary antibody at a concentration of 1:200–1:500.
Table 1.
Experimental designs for the different transgenic strains
| Strain/experiments | Single label GFP | Double labeling | Triple labeling | Without antibody staining |
|---|---|---|---|---|
| ChAT-tauGFP | Done | Not applied | Not applied | No intrinsic label |
| ChAT-Rosa | Done | GFP/CR, GFP/TH, GFP/Calb, GFP/PV | GFP/CALR/CALB | Weak intrinsic label |
| GFP/CALR/PV | ||||
| GAD65-GFP | Done | GFP/CR, GFP/TH, GFP/Calb, GFP/PV | GFP/CALR/CALB | Strong intrinsic label |
| GFP/CALR/PV | ||||
| PV-Rosa | Done | Not applied | GFP/CALR/PV | No intrinsic label |
Table 2.
Primary antibodies used in double- and triple labeling experiments
| Antigen | Host species | Dilution | Supplier | Remark |
|---|---|---|---|---|
| Green fluorescent protein | Chicken | 1:4000 | Aves labs | Against recombinant GFP, affinity-purified |
| Tyrosine hydroxylase | Rabbit | 1:500 | Abcam | Synthesized non-phosphopeptide tyrosine hydroxylase near phosphorylation site of serine 19 (human) |
| Calretinin | Goat | 1:1000 | Swant | Against human recombinant protein |
| 28 kDa calbindin | Rabbit | 1:1000 | Swant | Rat recombinant calbindin 28 KDa |
| Parvalbumin | Mouse | 1:1000 | Swant | Against skeletal muscle parvalbumin |
Imaging
Images were acquired with the Fluoview confocal imaging system (FV-1000; Olympus America, Center Valley, PA, USA) with the lasers and the filter settings optimized for the above described Alexa dyes. Individual and serial optical slices (0.5–2 µm thick) were scanned using the Fluoview 3.1 program. Images were adjusted for contrast only. Further processing (assembling tables and labeling individual figures) was made with the functions of Adobe Photoshop 7.0 (Adobe Systems Inc., San Jose, CA, USA).
Results
Retinal expression in the ChAT-tauGFP mouse strain
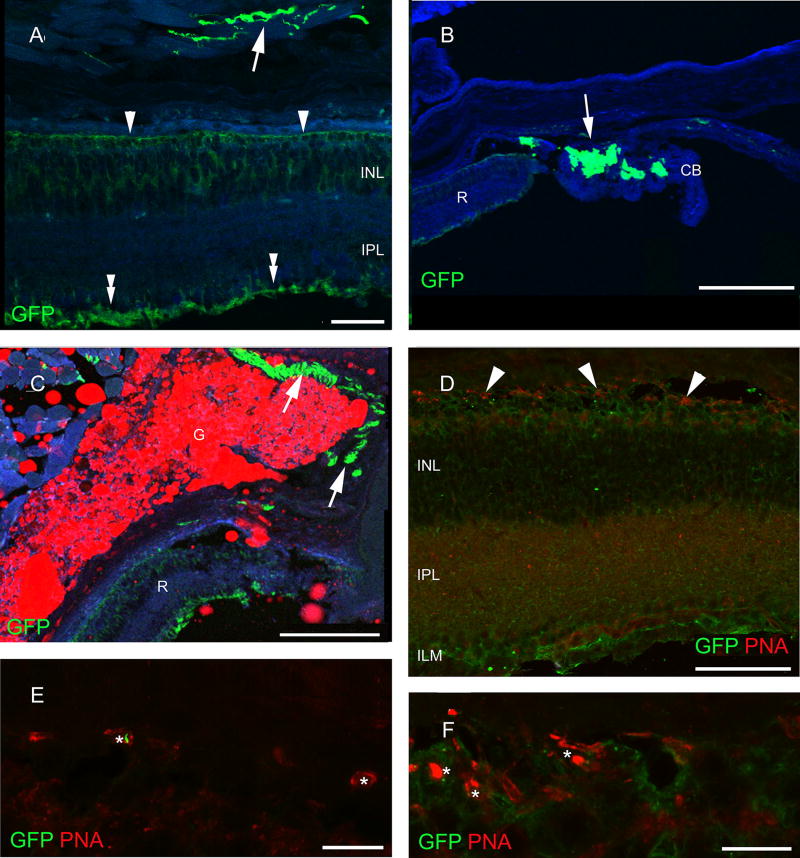
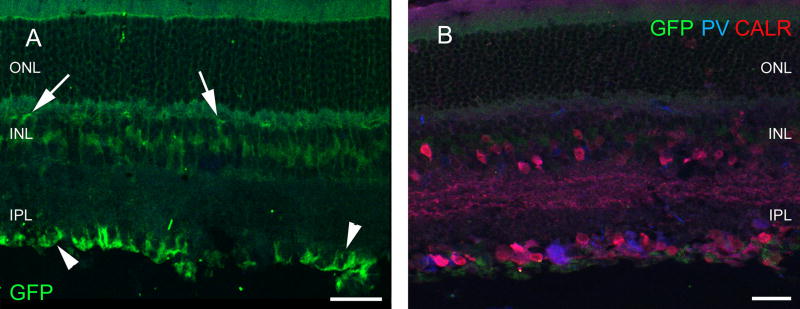
In the retina of ChAT-tauGFP mice, we saw no labeling without antibody application and observed only weak staining when we examined the sections after anti-GFP immunocytochemistry. The tauGFP staining spanned the entire retina width, reminiscent of the Müller glial cells (Fig. 1a). The inner and outer limiting membranes are clearly labeled (Fig. 1a). No neuronal labeling of any kind was ascertained in the retina. However, tauGFP labeling appeared in the nerve fibers innervating the extraocular muscles (Fig. 1a), the ciliary body (Fig. 1b) and the palpebral gland (Fig. 1c), indicating that the somatomotoric and secretomotoric cholinergic fibers indeed expressed the tauGFP gene. However, retinal layering did not follow the general rule: the outer retina seemed to be intermingled with the inner nuclear layer (Fig. 1d). To investigate the arrangement and labeling pattern of cones, we performed PNA labeling in these sections. Cones were located among other cells in the outer row of the inner nuclear layer (Fig. 1e). Only patchy labeling with PNA was observed (compare to Fig. 2c), indicating that both the number and the integrity of the cones were severely compromised in ChAT-tauGFP. Outer segment fragments were only occasionally seen, and staining of the cone terminals was also irregular (Fig. 1f). Therefore, the retina of ChAT-tauGFP mice do not exhibit normal retinal layering, suggesting that vision is greatly impaired due to degeneration and/or developmental abnormalities of the outer retina.
Fig. 1.
Retinal expression of the GFP transgene in the ChAT-tauGFP mouse line. Labeling was seen in the outer (arrowhead) and inner limiting membranes (double arrowhead) as well as in the presumed cholinergic nerves of the external eye muscles (a). b GFP-positive nerves (arrows) are also present in the ciliary body (CB) close to the retinal edge (R). c The palpebral glands (G) are also innervated by labeled fibers. The red color is autofluorescence and is emitted by the product of the gland. d The remainder of the photoreceptors (arrowheads) sit right on the top of INL. e Small, possibly degenerating PNA-positive cone outer segments (asterisks) were rare. f These occasionally formed nests. Scale bars 90 µm in a, 40 µm in b, 180 µm in c and d, 30 µm in e and 10 µm in f. PRL photoreceptor layer, ONL outer nuclear layer, OPL outer plexiform layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer, GFP green fluorescent protein, PNA peanut agglutinin, TH tyrosine hydroxylase, CALR calretinin, CALB calbindin 28 kDa, PV parvalbumin
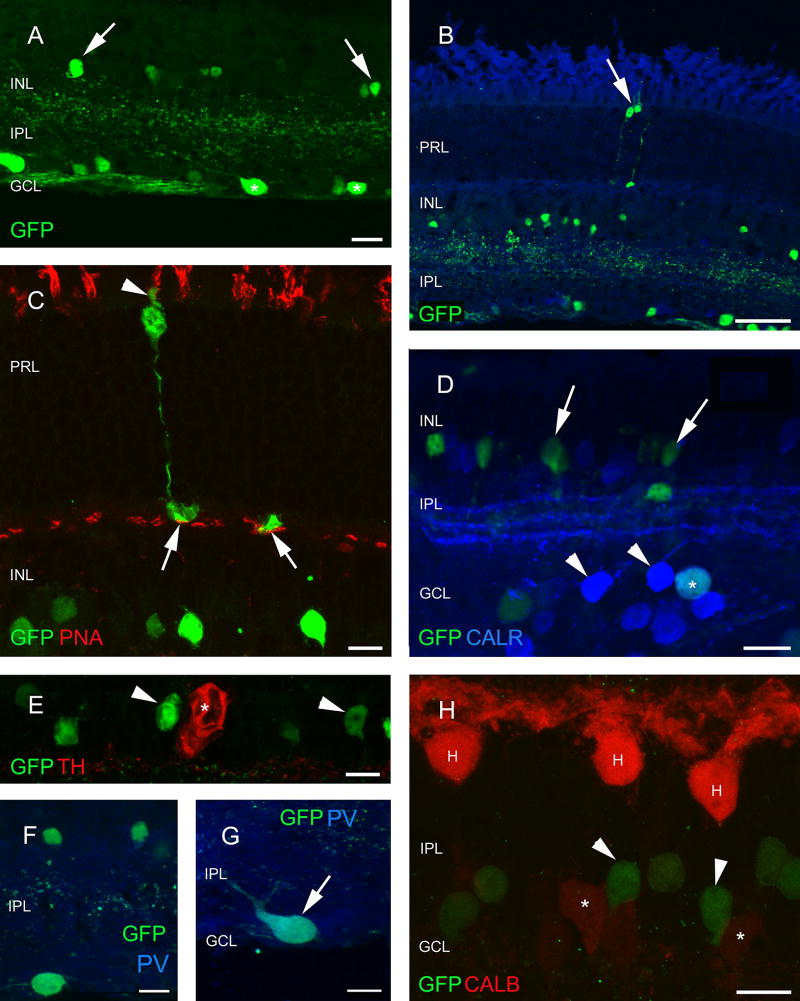
Fig. 2.
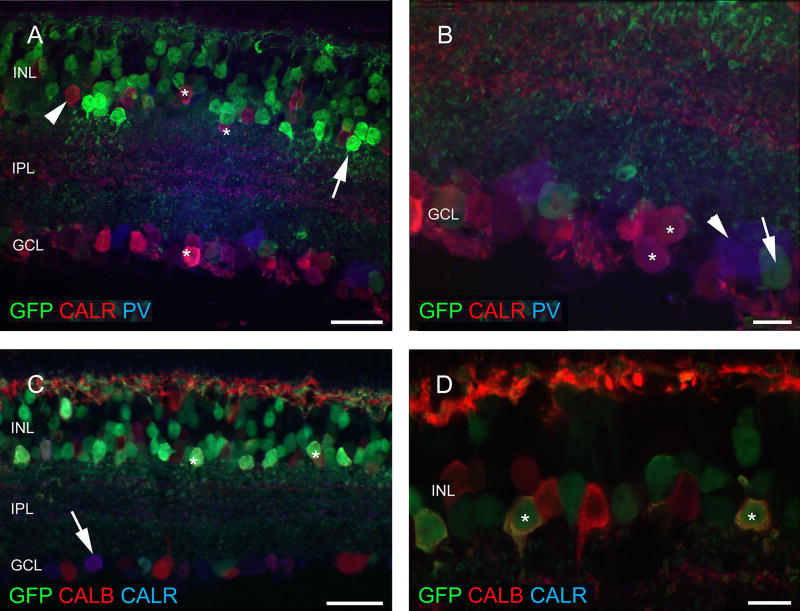
Analysis of neuron types in the retina of ChAT-RosaYFP mice. YFP has been labeled with antibodies against GFP, therefore, YFP-expressing cells will be referred as GFP-positive/labeled cells below. The YFP transgene is expressed in the inner retinal layers, mostly in amacrine (arrows) and ganglion (asterisks) cells (a). Besides the regularly observed elements, rarely photoreceptors are also seen to contain the GFP label (arrow in b) which could be identified as cones based on the PNA label in their outer segments (arrowhead) and endfeet (arrows) (c). GFP-labeled amacrine cells (arrows) do not contain CALR (d) or TH (asterisk in e), whereas only a fraction of the CALR-positive cells in the GCL (arrowheads) label for GFP (asterisk in d) The same is true for PV (f, g). The small GFP cells in the GCL are single-labeled and the large PV-positive cells located in the GCL, which are likely to be ganglion cells contain GFP (f, g). h Horizontal cells (H) and CALB-positive amacrine cells (asterisks) never contain GFP-label (GFP-positive cells are marked with arrowheads). Scale bars 20 µm in a and d, 50 µm in b, 10 µm in c and e–h. PRL photoreceptor layer, ONL outer nuclear layer, OPL outer plexiform layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer, GFP green fluorescent protein, PNA peanut agglutinin, TH tyrosine hydroxylase, CALR calretinin, CALB calbindin 28 kDa, PV parvalbumin
Retinal expression in the ChAT-Rosa strain
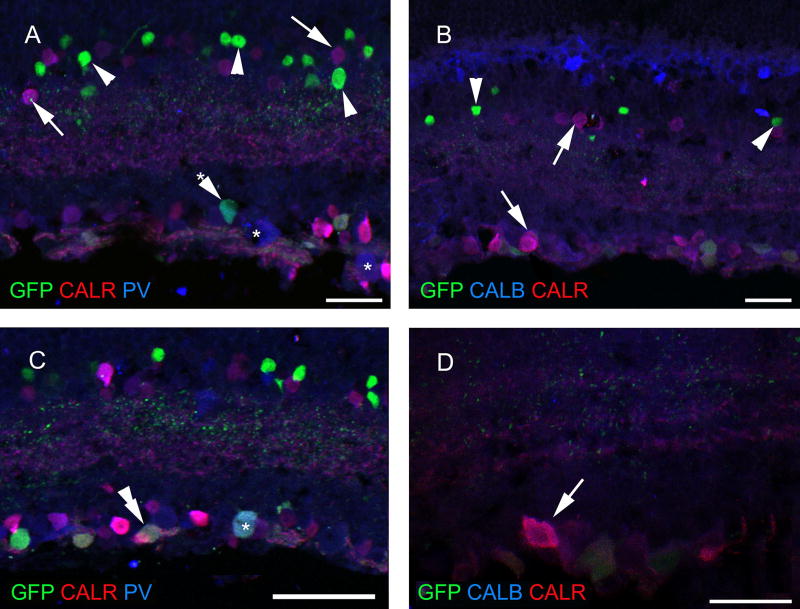
When the retina sections of the ChAT-Rosa mouse were examined, it was apparent that populations of amacrine cells and cells in the GCL were sparsely labeled (Fig. 2a). Occasionally, some photoreceptors also showed GFP fluorescence (Fig. 2b). Based on morphology, these cells were tentatively identified as cones. We attempted to ascertain the identity of these cells with a double-labeling experiment executed with a PNA-rhodamine conjugate. Indeed, the synaptic surface of cone terminals visualized by GFP immunofluorescence was matching with the localization of the PNA-rhodamine staining (Fig. 2c). To identify the labeled amacrine cell type(s), first we performed a double-labeling experiment with CALR and GFP, since CALR is known to be a secondary marker of the cholinergic starburst amacrine cells in mice (Haverkamp and Wassle Haverkamp and Wässle 2000). There was no colocalization of CALR and GFP fluorescence in the inner nuclear layer (INL); however, occasionally we observed double-stained cells in the GCL (Fig. 2d, g). Based on soma size, we did not anticipate that the GFP-positive cells colocalize TH, which was confirmed in our observations (Fig. 2e). No overlap was found between CALB and GFP either (Fig. 2h). Within the amacrine cell population, none of the GFP-immunoreactive cells contained PV (Fig. 2f), whereas in the GCL, several, but not all large GFP-immunoreactive cells were PV-positive (Fig. 2g). In GFP/ CALR/PV triple-labeling experiments, we corroborated our initial observation in double staining experiments that GFP-postive amacrine cells appeared to label a distinct class of amacrine cells lacking calcium-binding proteins (Fig. 3a, b). In the case of cells in the GCL, a much more diverse picture emerged. We observed single-labeled GFP and PV-positive cells, double-labeled CALR/PV and GFP/ PV cells, as well as triple-stained cells (Fig. 3c). In the GFP/CALR/CALB triple-labeling, we found no evidence that GFP-positive cells co-localized for both CALR and CALB. However, a subset of GFP-negative cells was double-stained for CALR/CALB (Fig. 3d).
Fig. 3.
Colocalization pattern of calcium-binding proteins in amacrine and ganglion cells of ChAT-RosaYFP mice. YFP has been labeled with antibodies against GFP, therefore, YFP-expressing cells will be referred as GFP-positive/labeled cells below. None of the GFP-positive amacrine cells (arrowheads) colocalized CALR, CALB or PV, while co-localization of calcium-binding proteins (arrows) were common in amacrine cells. a, b Asterisks single-labeled large CALR-positive cells in the GCL. Asterisk with arrowhead GFP + PV positive cell in the GCL. Besides single-labeled GFP- and CALR-positive cells in the GCL we also found colocalization of GFP with PV (double arrowhead in c) and also a triple-labeled cell (asterisk), but not with CALB (d, arrow shows a CALR/CALB double-labeled cell). Scale bars 30 µm in a and b, 50 µm in c and 20 µm in d. PRL photoreceptor layer, ONL outer nuclear layer, OPL outer plexiform layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer, GFP green fluorescent protein, PNA peanut agglutinin, TH tyrosine hydroxylase, CALR calretinin, CALB calbindin 28 kDa, PV parvalbumin
Retinal expression in the GAD65-GFP strain
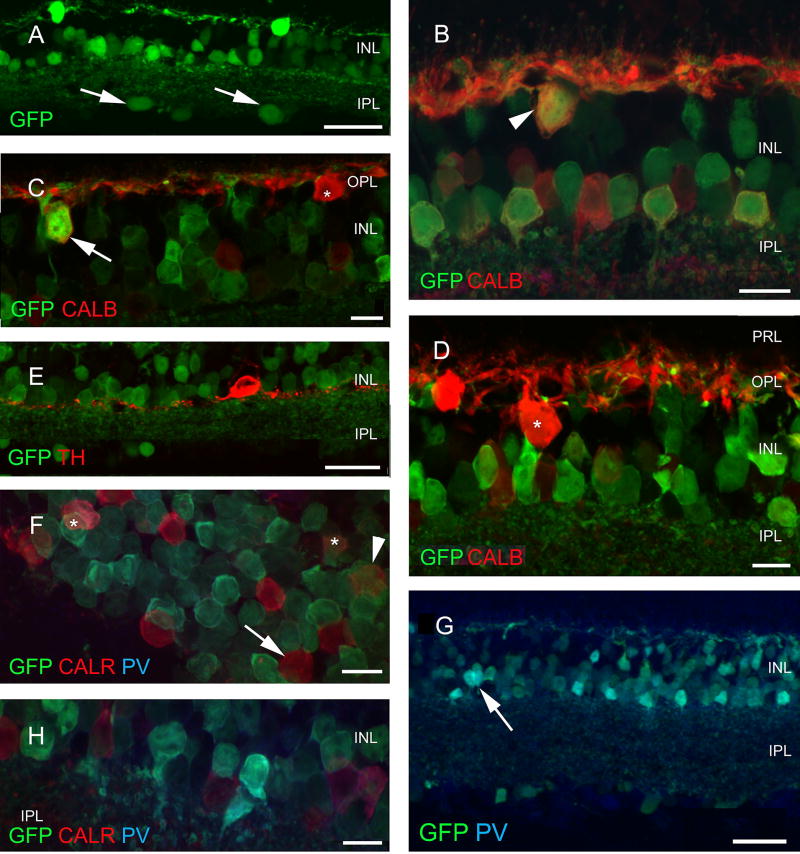
There was strong intrinsic GFP labeling of several cell populations in the retina of the GAD65-GFP mouse line, in almost all layers of the neuroretina, with the exception of the photoreceptor layer (Fig. 4a). In the INL, horizontal, bipolar and amacrine cells were labeled (Fig. 4b). Interestingly, not all the horizontal cells expressed GFP, which could be demonstrated with double-labeling for CALB (Fig. 4c). There was considerable variability both GFP and CALB labeling intensity, including evidence that some horizontal cells expressed CALB only (Fig. 4d). No GFP/ TH double-labeled cells were found (Fig. 4e). Among calcium-binding protein-containing amacrine cells, GFP was observed to be consistently absent from CALB-positive (Fig. 4b) and CALR-positive (Fig. 4f) neurons. However, many GFP-positive cells contained PV (Fig. 4g, h). In most of the cases, small diameter amacrine cells were double labeled (Fig. 4h).
Fig. 4.
Retinal expression pattern of GAD65-GFP construct. Several cell types show GFP expression in the GAD65-GFP construct, including large cells in the ganglion cell layer (arrows in a). Among interneurons, some (but not all) horizontal [arrowhead weak label in b, arrow strong positivity in c and none (asterisk) in d] cells express GFP. Among the amacrines, TH- (e) and CALB-containing cells (b) do not, while CALR-containing cells (arrowhead and asterisks in f) rarely contain GFP; no colocalization is seen in g (arrow) while small, narrow-field PV-positive cells (arrow) frequently express GFP (g, h). Scale bars 30 µm in a, e and h and 10 µm in all other figures. PRL photoreceptor layer, ONL outer nuclear layer, OPL outer plexiform layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer, GFP green fluorescent protein, PNA peanut agglutinin, TH tyrosine hydroxylase, CALR calretinin, CALB calbindin 28 kDa, PV parvalbumin
In GFP/CALR/PV triple-labeling experiments, GFP overlapped with PV but not CALR (Fig. 5a), confirming double-labeling results. In the GCL, single labeling for GFP and PV was observed, as well as several GFP-negative CALR/PV double-stained neurons. No triple-labeled cells were observed in this preparation (Fig. 5b). When GFP/CALR/CALB triple labeling was performed, we corroborated several findings of the double-labeling experiments. Namely, we observed GFP cells extensively co-labeled with CALB or CALR in the INL and GCL (Fig. 5c). Surprisingly, at high magnification and near saturating laser intensities, we saw, only a few, triple labeled cells (Fig. 5d).
Fig. 5.
Calcium-binding proteins are often co-localized with each other in amacrine and ganglion cells, but not with GFP, in the GAD65-GFP construct. Among amacrine cells, GFP (arrow) and CALR (arrowhead) is localized to different cell populations (a). However, in ganglion cells CALR/PV colocalization is frequent (asterisks in a, b). At the same time, single GFP- and PV-labeled cells are also seen (arrows in a, b, respectively). Besides the numerous single-labeled GFP-positive cells in the INL (asterisks), CALB/CALR colocalization (arrow) was also often observed in the GCL (c). A few triple-labeled cells (asterisks) were also seen among amacrine cells (d). Scale bars 30 µm in a and c and 10 µm in b and d. PRL photoreceptor layer, ONL outer nuclear layer, OPL outer plexiform layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer, GFP green fluorescent protein, PNA peanut agglutinin, TH tyrosine hydroxylase, CALR calretinin, CALB calbindin 28 kDa, PV parvalbumin
Retinal expression in the PV-Rosa strain
Similar to the retinas of ChAT-tauGFP mice, no specific labeling was observed in these retinas (Fig. 6a). At high laser power, some background staining was observed in Müller cells. As an internal control, we performed triple labeling with anti-GFP, anti-CALR and anti-PV antibodies. Except for the usual pattern of staining for these calcium-binding proteins, no other specific staining was observed with the anti-GFP antibody (Fig. 6b).
Fig. 6.
Retina of the PVRosa-YFP strain. GFP is present in the glial cells only (arrows), labeling is strong in the endfeet of glial cells (arrowheads) in the inner limiting membrane (a). At the same time, cells can be labeled for PV and CALR in the retina of this strain as usual (b). Scale bars 40 µm in a and 30 µm in b. PRL photoreceptor layer, ONL outer nuclear layer, OPL outer plexiform layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer, GFP green fluorescent protein, PNA peanut agglutinin, TH tyrosine hydroxylase, CALR calretinin, CALB calbindin 28 kDa, PV parvalbumin
Discussion
Retinal expression of transgenes has been examined in several previous studies; almost all of these studies have described some mismatches compared to expectations based on results of experiments performed with more traditional immunocytochemistry (Haverkamp et al. 2009; Huberman et al. 2008; Pang et al. 2003). In the present paper, we report that only one of the four examined mouse lines fulfilled the expectation closely, while three others exhibited transgene expression that was either conspicuously ectopic or absent.
Similarity to formerly neurochemically identified cells
Only the GAD65-GFP transgenic mouse met expectations for the retinal expression of GFP. GABA has been shown to be consistently present in all horizontal and about half of the amacrine cells in the mammalian retina (Mosinger et al. 1986). Overall our results in the GAD65-GFP mouse are consistent with an earlier study that found GFP labeling in the INL robustly labeling horizontal, amacrine, and bipolar cells (Deniz et al. 2011). In addition, we note that GAD65-GFP mice label a subset of these cell types, as indicated by evidence of calbindin-positive, GFP-negative cell types present. The expression pattern of GFP suggests that the ratio of GAD65/GAD67 isoform varies widely in horizontal cells, spanning mutually exclusive to complete overlap of GAD65 and GAD67 isoforms (Deniz et al. 2011). There is one inconsistency; however, in the general pattern of GAD65-GFP in the retinal GABAergic system. PV is usually not present in any of the GABAergic elements in the retina. This observation should be further addressed in future studies.
Glial labeling in the ChAT-tauGFP animals and PV-Rosa-YFP animals was entirely surprising. The most surprising, however, was the evidence of photoreceptor degeneration in ChAT-tauGFP mice. Despite the fact that the transgene expression is widespread in the brain of both ChAT-tauGFP and PV-Rosa animals (Grybko et al. 2011; Yi et al. 2015), these two lines of mice, therefore, would not be useful in further retinal studies. The photoreceptor degeneration observed in the ChAT-tauGFP mice is especially relevant to the study of central cholinergic mechanisms, as these mice may be visually impaired during active learning tasks. Haverkamp and colleagues (2009) found no evidence of retinal degeneration in ChAT-GFP mice. Therefore, there is a possibility that overexpression of tau-GFP may contribute to the retinal neurodegeneration observed. However, the failure of ChAT-Rosa mice to label cholinergic amacrine cells was similar to that observed in ChAT-GFP mice (Haverkamp et al. 2009; Knop et al. 2014). The ectopic expression of fluorescent proteins in cells driven by the ChAT promoter also occurs in the cortex (von Engelhardt et al. 2007) and hippocampus (Yi et al. 2015). Therefore, even if ChAT is only transiently expressed during development, some regulatory elements may be missing in the transgene CRE expression that drives YFP expression in ChAT-Rosa mice. This may lead to expression of YFP in the adult animals even if ChAT expression is strongly downregulated at older ages. Such a mismatch between the endogenous and transgenic proteins can therefore gain insight into the multipotency of ChAT-expressing cell types earlier in development.
Similarity to other transgenic mice with the same transgene inserted to identical promoters
Among the four mouse lines examined in this study, two of them were similar in some aspects to formerly studied transgenic mice lines. In fact the results of the present study are strikingly similar to those described by Haverkamp et al. (2009) for the ChAT-GFP transgenic mice generated by using the artificial bacterial chromosome system (von Engelhardt et al. 2007). Interestingly, in another report of the same line, the researchers found weakly GFP-labeled cell bodies also immunoreactive for ChAT (Knop et al. 2014). However, we could confirm another finding from this study, namely that we also identified GFP-positive wide-field amacrine cells. Our other finding that some ganglion cells may also be GFP-positive and represent a subtype of CALR-positive ganglion cells in this construct needs further corroboration.
In the case of PV-EGFP mice (Meyer et al. 2002), Haverkamp and co-workers (2009) could identify PV-containing EGFP-expressing amacrine and ganglion cells. They found also a mismatch among the amacrine cells (cells expressing EGFP but not PV immunoreactivity). In our PV-Rosa mice, it was surprising that we could not confirm neuronal GFP expression in the retina. Again, YFP fluorescence was present in living brain tissue which could be enhanced by antibody labeling in fixed material (Yi et al. 2014). At the same time, PV was present in the retinal tissue. Therefore, it seems plausible that the PV promoter inserted with the transgene was not able to switch on in the retina, while endogenous PV expression appeared to function normally.
Retinal development and transgene expression
Although the retina is the part of the central nervous system, its development is quite independent from the rest of the brain. Regulated by a set of the homeobox genes (Zagozewski et al. 2014), the eyecup is formed very early (E6–8) and the neurochemical specification of the neurons start as early as E14–15. However, some cells are born quite late in development, even well after birth (P5–6). Thus, about two dozen genes will determine the cell number, distribution, connectivity and neurochemistry of the mammalian retinal cells (Rapaport et al. 2004). The intimate network of signaling molecules may be able to up-or downregulate the transgene expression independently from those genes that occur naturally in the retinal tissue. One reason might be the long period (over 20 days) of cell generation in the mouse retina (Young et al. 1985; Bagnoli et al. 2003) during which many genes switch on temporarily and possibly more than one time (Zhang et al. 2006). These genes include transcription factors like Nanog and Pax6 which promote the multipotent state and inhibit cell differentiation (Marquardt et al. 2001; Hamazaki et al. 2006; Pereira et al. 2006). On the other hand, the members of the Wnt pathway promote differentiation and synapse formation (Kiecker and Niehrs 2001), just like NeuroD1 (Cepko 1999; Morrow et al. 1999; Inoue et al. 2002). These above factors may all influence cell fate and, if present, their effect will depend on the actual molecular ratio. Being at a certain position at a certain time, cell populations may be exposed to multiple influences that could change phenotype. Thus, transgene expression during development may alter the final expression of a number of phenotypes, which could account for why the transgenes considered in this study failed to highlight the expected cell populations. The difference between expression of the transgene and expression of endogenous proteins seen here should not be carelessly dismissed as ectopic expression due to the artificial nature of the transgene, but may provide insights into the complexity of development of specific cell types. These processes may also be in the background of the recent finding that different constructs for expression of dopaminergic markers led to heterogenous expression in the retina (Vuong et al. 2015). Nevertheless, a specific but consistent pattern of expression may help researchers execute a detailed study on the development of certain retinal cell populations, making it also possible to follow different retinal degenerative events due to aging or diseases.
Acknowledgments
This study was supported by NAP KTIA_13_ NAP-A-I/12 (R.G.) and NIH R01 NS069689 (J.J.L.) Grants. M.W. was in receipt of a short-term fellowship from the College of Health Professions and Biomedical Sciences, University of Montana. R.G. was a Fulbright Fellow in this institution. This project was also supported through Grants from the Center for Environmental Health Sciences COBRE P20RR017670, Center for Biomolecular Structure and Dynamics P20GM103546, COBRE Center for Structural and Functional Neuroscience P20RR015583, and an internal Grant from the University of Montana, Department of Biomedical Sciences. We thank Feng Yi and Elizabeth Catudio-Garrett for help with the transgenic animals, Sukumar Vijayaraghavan (UC-Denver) for ChAT-tauGFP mice, and Edit Kiss with the preparation of the figures.
Footnotes
Compliance with ethical standards
Ethical approval All procedures performed in studies involving animals were in accordance with the ethical standards of the institution.
Conflict of interest The authors declare that they have no conflict of interest.
References
- Badea TC, Nathans J. Quantitative analysis of neuronal morphologies in the mouse retina visualized by using a genetically directed reporter. J Comp Neurol. 2004;480:331–351. doi: 10.1002/cne.20304. [DOI] [PubMed] [Google Scholar]
- Bagnoli P, Dal Monte M, Casini G. Expression of neuropeptides and their receptors in the developing retina of mammals. Histol Histopathol. 2003;18:1219–1242. doi: 10.14670/HH-18.1219. [DOI] [PubMed] [Google Scholar]
- Cepko CL. The roles of intrinsic and extrinsic cues and bHLH genes in the determination of retinal cell fates. Curr Opin Neurobiol. 1999;9:37–46. doi: 10.1016/s0959-4388(99)80005-1. [DOI] [PubMed] [Google Scholar]
- Deniz S, Wersinger E, Schwab Y, Mura C, Erdelyi F, Szabo G, Rendon A, Sahel JA, Picaud S, Roux MJ. Mammalian retinal horizontal cells are unconventional GABAergic neurons. J Neurochem. 2011;116:350–362. doi: 10.1111/j.1471-4159.2010.07114.x. [DOI] [PubMed] [Google Scholar]
- Dhingra A, Sulaiman P, Xu Y, Fina ME, Veh RW, Virdi N. Probing neurochemical structure and function of retinal ON bipolar cells with a transgenic mouse. J Comp Neurol. 2008;510:484–496. doi: 10.1002/cne.21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei Y, Hughes TE. Transgenic expression of the jellyfish green fluorescent protein in the cone photoreceptors of the mouse. Vis Neurosci. 2001;18:615–623. doi: 10.1017/s0952523801184117. [DOI] [PubMed] [Google Scholar]
- Gábriel R, Wilhelm M, Straznicky C. Microtubule-associated protein 2 (MAP2)-immunoreactive neurons in the retina of Bufo marinus: colocalisation with tyrosine hydroxylase and serotonin in amacrine cells. Cell Tissue Res. 1992;269:175–182. doi: 10.1007/BF00384738. [DOI] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;12:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grybko MJ, Hamh E, Perrine W, Parnes JA, Chick WS, Sharma G, Finger TE, Vijayaraghavan S. A transgenic mouse model reveals fast nicotinic transmission in hippocampal pyramidal neurons. Eur J Neurosci. 2011;33:1786–1798. doi: 10.1111/j.1460-9568.2011.07671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustincich S, Feigenspan A, Wu D-DK, Koopman LJ, Raviola E. Control of dopamine release in the retina: a transgenic approach to neural networks. Neuron. 1997;18:723–736. doi: 10.1016/s0896-6273(00)80313-x. [DOI] [PubMed] [Google Scholar]
- Hamazaki T, Kehoe SM, Nakano T, Terada N. The Grb2/Mek pathway represses Nanog in murine embryonic stem cells. Mol Cell Biol. 2006;26:7539–7549. doi: 10.1128/MCB.00508-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao MM, Bornstein JC, Young HM. Development of myenteric cholinergic neurons in ChAT-Cre;R26R-YFP Mice. J Comp Neurol. 2013;531:3358–3370. doi: 10.1002/cne.23354. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H. Immunocytochemical analysis of the mouse retina. J Comp Neurol. 2000;424:1–23. [PubMed] [Google Scholar]
- Haverkamp S, Inta D, Monyer H, Wässle H. Expression analysis of green fluorescent protein in retinal neurons of four transgenic mouse lines. Neuroscience. 2009;160:126–139. doi: 10.1016/j.neuroscience.2009.01.081. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Manu M, Koch SM, Susman MW, Lutz AB, Ullian EM, Baccus SA, Barres BA. Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron. 2008;59:425–438. doi: 10.1016/j.neuron.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Hojo M, Bessho Y, Tano Y, Lee JE, Kageyama R. Math3 and NeuroD regulate amacrine cell fate specification in the retina. Development. 2002;129:831–842. doi: 10.1242/dev.129.4.831. [DOI] [PubMed] [Google Scholar]
- Ivanova E, Hwang G-S, Pan Z-H. Characterization of transgenic mouse lines expressing Cre recombinase. Neuroscience. 2010;135:233–243. doi: 10.1016/j.neuroscience.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128:4189–4201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- Knop GC, Pottek M, Monyer H, Weiler R, Dedek K. Morphological and physiological properties of enhanced green fluorescent protein (EGFP)-expressing wide-field amacrine cells in the ChAT-EGFP mouse line. Eur J Neurosci. 2014;39:800–810. doi: 10.1111/ejn.12443. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Sturgess K, Erdélyi F, Szabó G, Molnár Z, Paulsen O. Preferential origin and layer destination of GAD65-GFP cortical interneurons. Cereb Cortex. 2004;14:1122–1133. doi: 10.1093/cercor/bhh072. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AS, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature Neurosci. 2009;13:133–142. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- May CA, Nakamura K, Fujiyama F, Yanagawa Y. Quantification and characterization of GABA-ergic amacrine cells in the retina of GAD67-GFP knock-in mice. Acta Ophthalmol. 2008;86:395–400. doi: 10.1111/j.1600-0420.2007.01054.x. [DOI] [PubMed] [Google Scholar]
- Meyer AH, Katona I, Blatow M, Rozov A, Monyer H. In vivo labeling of parvalbumin-positive interneurons and analysis of electrical coupling in identified neurons. J Neurosci. 2002;22:7055–7064. doi: 10.1523/JNEUROSCI.22-16-07055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieziewska KE, van Veen T, Murray JM, Aguirre GD. Rod and cone specific domains in the interphotoreceptor matrix. J Comp Neurol. 1991;308:371–380. doi: 10.1002/cne.903080305. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Furukawa T, Lee JE, Cepko CL. NeuroD regulates multiple functions in the developing neural retina in rodent. Development. 1999;126:23–36. doi: 10.1242/dev.126.1.23. [DOI] [PubMed] [Google Scholar]
- Mosinger JL, Yazulla S, Studhulme KM. GABA-like immunoreactivity in the vertebrate retina: a species comparison. Exp Eye Res. 1986;42:631–644. doi: 10.1016/0014-4835(86)90052-7. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Light-evoked excitatory and inhibitory synaptic inputs to ON and OFF ganglion cells in the mouse retina. J Neurosci. 2003;23:6063–6073. doi: 10.1523/JNEUROSCI.23-14-06063.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L, Yi F, Merrill BJ. Repression of Nanog gene transcription by Tcf3 limits embryonic stem cell self-renewal. Mol Cell Biol. 2006;26:7479–7491. doi: 10.1128/MCB.00368-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport DH, Wong LL, Wood ED, Yasumura D, LaVail MM. Timing and topography of cell genesis in the rat retina. J Comp Neurol. 2004;474:304–324. doi: 10.1002/cne.20134. [DOI] [PubMed] [Google Scholar]
- Sarthy V, Hoshi H, Mills S, Dudley VJ. Characterization of green fluorescent protein expressing retinal cells in CD44transgenic mice. Neuroscience. 2007;144:1087–1093. doi: 10.1016/j.neuroscience.2006.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- von Engelhardt J, Eliava M, Meyer AH, Rozov A, Monyer H. Functional characterization of intrinsic cholinergic interneurons in the cortex. J Neurosci. 2007;27:5633–5642. doi: 10.1523/JNEUROSCI.4647-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong HE, Sevilla Müller LP, Hardi CN, McMahon DG, Brecha NC. Heterogeneous transgene expression in the retinas of the TH-RFP, TH-Cre, TH-BAC-Cre and DAT-Cre mouse lines. Neuroscience. 2015;307:319–337. doi: 10.1016/j.neuroscience.2015.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-T, Blankenship AG, Anishchenko A, Elstrott J, Fikhman M, Nakanishi S, Feller MB. GABAA receptor-mediated signaling alters the structure of spontaneous activity in the developing retina. J Neurosci. 2007;27:9130–9140. doi: 10.1523/JNEUROSCI.1293-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Ball J, Stoll KE, Satpute VC, Mitchell SM, Pauli JL, Holloway BB, Johnston AD, Nathanson NN, Deisseroth K, Gerber DJ, Tonegawa S, Lawrence JJ. Direct excitation of parvalbumin-positive interneurons by M1 muscarinic acetylcholine receptors: roles in cellular excitability, inhibitory transmission and cognition. J Physiol. 2014;592:3463–3494. doi: 10.1113/jphysiol.2014.275453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Catudio-Garrett E, Gabriel R, Wilhelm M, Erdelyi F, Szabo G, Deisseroth K, Lawrence JJ. Hippocampal “cholinergic interneurons” visualized with the choline acetyltransferase promoter: anatomical distribution, intrinsic membrane properties, neurochemical characteristics, and capacity for cholinergic modulation. Front Synaptic Neurosci. 2015;7:4. doi: 10.3389/fnsyn.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Watanabe D, Ishikane H, Tachibana M, Pastan I, Nakanishi S. Neuron A key role of starburst amacrine cells in originating retinal directional selectivity and optokinetic eye movement. Neuron. 2001;30:771–780. doi: 10.1016/s0896-6273(01)00316-6. [DOI] [PubMed] [Google Scholar]
- Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- Zagozewski JL, Zhang Q, Pinto VI, Wigle JT, Eisenstat DD. The role of homeobox genes in retinal development and disease. Dev Biol. 2014;393(2):195–208. doi: 10.1016/j.ydbio.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Zeilhofer HU, Studler B, Arabadzisz D, Schweizer C, Ahmadi S, Layh B, Bosl MR, Fritschy JM. Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. J Comp Neurol. 2005;482:123–141. doi: 10.1002/cne.20349. [DOI] [PubMed] [Google Scholar]
- Zhang SSM, Xu X, Liu MG, Zhao H, Soares MB, Barnstable CJ, Fu XY. A biphasic pattern of gene expression during mouse retina development. BMC Dev Biol. 2006;6:48. doi: 10.1186/1471-213X-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]