Abstract
PURPOSE:
The purpose of this study is to assess clinical characteristics, risk factors, and management outcomes of retinal changes similar to retinopathy of prematurity (ROP), seen in full-term infants.
PATIENTS AND METHODS:
This is a retrospective review of 46 eyes of 23 patients, born at full term or near full term and diagnosed to have active ROP-like retinopathy or sequelae of ROP-like retinopathy.
RESULTS:
Mean birth weight (BW) and gestational age (GA) were 2342 ± 923 g (range, 1200–4160 g) and 38.5 ± 1.85 weeks (range, 37–40 weeks). Mean age at the time of diagnosis was 3.5 ± 8.75 years (range, 1 month–16 years). Stage 1 and 2 of retinopathy was seen in 10 eyes (21.7%), threshold disease with plus disease in 12 eyes (26%) and Stage 4 or 5 in 14 eyes (30.4%). Involutional sequelae were noted in 10 eyes (21.7%). Twenty-one eyes (45.6%) underwent appropriate treatment in the form of laser, cryotherapy, or retinal detachment surgery. Eight eyes (17.4%) with advanced sequelae such as total closed funnel retinal detachment and macular fold were not treated. Mean follow-up was 3 years (range, 1 month to 12 years). At the last follow-up, 29 eyes (63%) had a favorable structural outcome (P < 0.001). Among the patients in whom visual acuity could be assessed (16 eyes), favorable visual outcome was noted in 9 eyes (56.2%). Low BW (P = 0.038), multiple births (P = 0.013), respiratory distress syndrome (RDS) (P = 0.001), phototherapy (P = 0.001), and oxygen administration (P < 0.001) were significantly associated with the development of ROP-like retinopathy in these full-term infants.
CONCLUSIONS:
ROP-like retinopathy can occur in full-term and near full-term infants and can potentially lead to permanent visual impairment. Screening of infants with risk factors such as oxygen administration, RDS, multiple births, and low BW, regardless of GA, may reduce visual impairment.
Keywords: Birth weight, prematurity, retinopathy of prematurity, term birth
Introduction
Retinopathy of prematurity (ROP) is a disease characterized by abnormal neovascularization of the retina which shows distinct stages of development.[1] In the early stages, it can regress spontaneously without leaving any sequelae. However, in the late stages, spontaneous regression is uncommon, and oftentimes cicatricial sequelae such as dragged macula, macular fold, or retinal detachment can be seen.[2] Smith and Tasman reported some residual posterior segment pathology in nearly 88.4% of eyes with regressed ROP.[3] Since it was first reported by Terry in 1942, our understanding of its pathogenesis has increased immensely. High oxygen saturation in the postnatal period was recognized as a causative factor for ROP.[4] The oxygen–induced retinopathy model developed in mouse pups has helped researchers immensely in understanding the complex molecular and cellular mechanisms involved.[5] This led to the development of protocols restricting the oxygen saturation in the Neonatal Intensive Care Units (NICU). However, these approaches have not eliminated ROP, and it still remains a significant cause of childhood blindness in middle-income countries worldwide, especially in Asia.[6]
Other than oxygen saturation, prematurity and low birth weight (BW) are the most common factors associated with ROP.[7] However, ROP is not exclusive to preterm or low BW infants. Several case reports, mainly from developing countries have shown the occurrence of ROP in heavier BW babies with or without the presence of comorbidities.[8] Furthermore, case reports in the past have described changes similar to ROP in healthy full-term and near full-term infants.[9,10,11] Two recent reports by Li et al.[12] and Vinekar et al.,[13] describing their experience in the universal screening of all neonates, have also shown the occurrence of ROP-like retinopathy in a small percentage of healthy full-term infants.
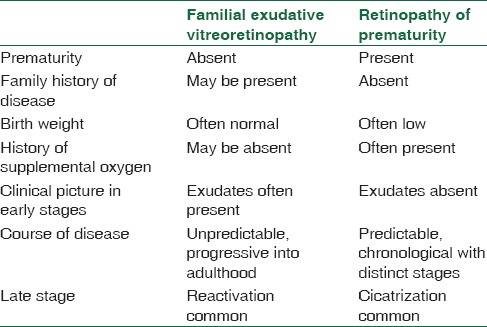
However, till now there is no consensus regarding the existence of ROP-like retinopathy in full term and near full term infants. The diagnosis of ROP-like retinopathy in full term is made after exclusion of ROP-mimicking retinopathy which includes persistent hyperplastic primary vitreous (PHPV), incontinentia pigmenti, familial exudative vitreoretinopathy (FEVR), and Norrie disease.[9] PHPV, incontinentia pigmenti and Norrie disease are relatively uncommon and are easy to distinguish from ROP due to their specific features. PHPV is associated with microphthalmos and shows a vascularized stalk extending from the optic disc till the back of the lens. There are pigmented cutaneous lesions associated with totally dysmorphic retina in incontinentia pigmenti. Norrie disease also shows dysmorphic detached retina, leading to a fibrous ball like structure behind the lens. It might be difficult to distinguish between ROP and FEVR; however, a careful history will help. Table 1 lists the salient features to differentiate these two diseases. When in doubt, a fluorescein angiography can delineate the vascular abnormalities of FEVR, leakage, and peripheral avascular retina well. Unlike in ROP, there is no growth of normal vessels in the peripheral avascular retina after laser photocoagulation. And further follow-up can reveal continued exudation and reactivation in FEVR.
Table 1.
Salient features of differentiation between familial exudative vitreoretinopathy and retinopathy of prematurity
Trying to look for answers to this puzzling existence of ROP-like retinopathy in full-term and near full-term infants, we retrospectively analyzed infants born at full term who presented to us with ROP-like retinopathy or its sequelae with the purpose of assessing the clinical characteristics, potential risk factors, and management outcomes.
Patients and Methods
The study was a retrospective, nonrandomized, observational clinical case series, approved by the institutional review board. The records of 46 eyes of 23 patients who were born at full term and diagnosed to have active ROP-like retinopathy or ROP-like involutional sequelae were analyzed. These patients were taken from the database of patients referred to the Department of Vitreoretinal diseases of our tertiary care eye institute for screening or treatment, over a 13-year period from 2000 to 2013. Some of these patients attended the pediatric ophthalmology outpatient department and were found to have the retinal changes. These patients underwent eye examination and appropriate management in the department of vitreoretinal diseases of our institute situated in Southern India. The follow-up ranged from 1 month to 12 years (mean - 3 years).
The inclusion criteria were a history of full term birth with gestational age (GA) of 37 weeks or more with clinical findings similar to active or regressed ROP. Patients with a family history of similar retinal changes or any known causes of retinopathy were excluded. A detailed history was taken, and cases with any ambiguity regarding the GA or postmenstrual age, or unavailability of clear family history were excluded. We noted various parameters including BW, period of gestation, oxygen exposure, inborn and outborn status. A careful review of charts was done for the presence of neonatal illnesses which included respiratory distress syndrome (RDS), sepsis, neonatal jaundice, multiple births, apneic episodes, anemia, pneumonia, polycythemia, metabolic acidosis, hypoglycemia, hydrocephalus, congenital heart disease, and others. Treatment received in NICU was recorded and analyzed.
Ophthalmologic examination was done in the outpatient department. Pupillary dilation was done with commercially available eye drops for ROP containing tropicamide 0.5% and phenylephrine 2.5%. Binocular indirect ophthalmoscopy was done with the use of lid speculum and scleral depressor under topical anesthesia. Some of the children who presented at an older age were examined under general anesthesia in the operation theater. Care was taken to exclude FEVR. More exudation was in favor of FEVR. Fluorescein angiography was done occasionally. Special note was made about the progression of normal vascularization reaching up to the ora following laser photocoagulation which is not seen in FEVR. In case of absence of fundal view, a B-scan ultrasonography was done. Retinopathy was graded according to the international classification of ROP revisited classification.[1] Plus disease represented significant dilation and tortuosity of posterior polar blood vessels, iris engorgement, and pupillary rigidity. Detailed note of the fundus findings was done with the help of specially designed ROP recording charts or fundus drawings of children who underwent an examination under anesthesia and fundus images whenever available. Treatment details analyzed included surgical and nonsurgical treatment. Laser photocoagulation was performed under topical anesthesia while the infant was continuously monitored by an anesthetist, in the designated area of the operation theater complex. Cryotherapy was done under general anesthesia. The absence of treatment in cases with spontaneous regression or advanced untreatable sequelae was also noted. In the active stage, the follow-up was done weekly till complete regression was seen. Regressed cases were then followed up annually.
Statistical analysis
Qualitative variables such as presence or absence of neonatal risk factors were tested for statistical significance using Chi-square test and Fisher tests. Quantitative data such as GA and BW was analyzed using the ANOVA test. In all cases, P < 0.05 was taken to indicate statistical significance. All statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS, Chicago, SPSS, Ine) 14.1 version software.
Results
Forty-six eyes of 23 children born at full term which showed ROP-like changes, either active or involutional sequelae, without any family history or presence of any known causes of retinopathy were included in the study. The mean BW and GA were 2342 ± 923 g (range, 1200–4160 g) and 38.5 ± 1.85 weeks (range, 37–40 weeks), respectively. Mean chronological age at the time of diagnosis was 3.5 years. Mean follow-up was 3 years (range, 1 month to 12 years).
ROP-like retinopathy Stage 1 and 2 was seen in 10 eyes (21.7%) whereas 12 eyes (26.1%) had Stage 3 plus or threshold disease. The changes were seen in anterior zone 2 or 3 except for 1 child who had aggressive posterior ROP in both the eyes [Figure 1] which is a particularly severe form of ROP seen in zone 1 with plus disease which progresses rapidly. Stage 4 ROP-like retinopathy was seen in 3 eyes (6.5%) and 11 eyes (23.9%) had Stage 5 ROP-like retinopathy. ROP involutional sequelae were noted in 10 eyes (21.7%) [Figure 2]. Involutional sequelae included peripheral folds in 1 eye (2.1%), vitreous membranes in 3 eyes (6.5%), lattice-like degeneration in 1 eye (2.1%), retinal breaks in 2 eyes (4.3%), straightening of vessels of temporal arcade in 4 eyes (8.6%), distortion and ectopia of macula in 2 eyes (4.3%), failure of peripheral retinal vascularization in 2 eyes (4.3%), stretching and folding of retina in macular region in 3 eyes (6.5%), dragging of retina over optic disc in 3 eyes (6.5%), and localized, peripheral tractional retinal detachment in 2 eyes (2.1%). More than one finding was seen in these eyes. All patients showed bilateral disease with various degree of asymmetry.
Figure 1.
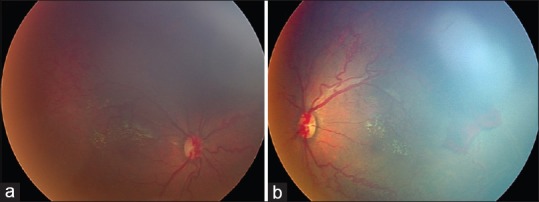
(a and b) Right and left eye of a child born at 40 weeks gestation, with birth weight of 4160 g showing aggressive form of posterior retinopathy of prematurity. The child had aspiration at birth followed by hypoxia and was also diagnosed to have valvular pulmonic stenosis. The retinopathy regressed with 2 sittings of laser photocoagulation. After 29 months follow up, child had favorable visual outcome with 3 diopters myopia
Figure 2.
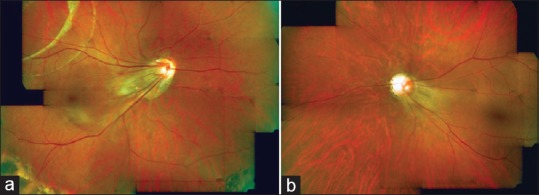
(a) Right eye of a patient underwent scleral buckle, shows retinal scarring, straightening of vessels and dragging of macula. (b) The left eye shows macular ectopia and straightening of vessels
Table 2 presents the relation between BW and GA in different groups with ROP-like retinopathy. Mean BW among Stage 1 or 2 was 1546 g, Stage 3 or threshold was 2732 g, Stage 4 was 2333 g, Stage 5 was 2331 g, while for involutional sequelae it was 3483 grams (P = 0.002). Mean GA among Stage 1 or 2 was 37.2 weeks, Stage 3 or threshold disease was 38.2 weeks, Stage 4 was 38.6 weeks, Stage 5 was 39.6 weeks and in involutional sequelae group was 39.2 weeks (P = 0.004).
Table 2.
Birth weight and gestational age in retinopathy of prematurity like retinopathy stages
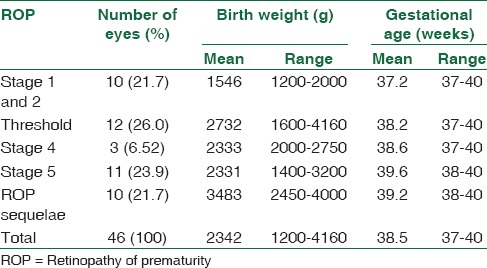
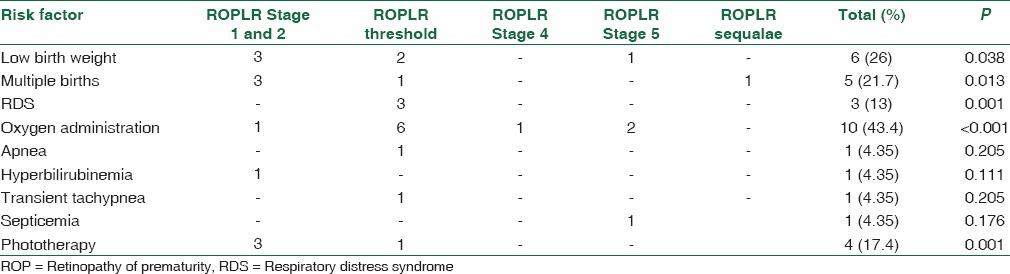
Table 3 presents main risk factors seen in these patients. Risk factors included low BW in 6 patients (P = 0.038), multiple births in 5 patients (P = 0.013), RDS in 3 patients (P = 0.001), oxygen administration in 10 patients (P < 0.001), apnea in 1 patient (P = 0.205), hyperbilirubinemia in 1 patient (P = 0.111), transient tachypnea in 1 patient (P = 0.205), septicemia in 1 patient (P = 0.176), phototherapy in 4 patients (P = 0.001). One child with respiratory distress had congenital valvular pulmonic stenosis. Low BW, multiple births, RDS, and use of phototherapy were seen to be significantly and oxygen administration to be highly significantly associated with the development of ROP-like retinopathy in full term and near full term infants.
Table 3.
Risk factors for retinopathy of prematurity like retinopathy
Twenty-one eyes (45.6%) underwent appropriate treatment. Laser photocoagulation was done to avascular retina in 14 eyes. Laser photocoagulation along with transscleral cryotherapy was done in 1 eye with multiple retinal breaks. Lensectomy with vitrectomy was done in 4 eyes with progressive Stage 4 and Stage 5 ROP-like retinopathy. Scleral buckle was done in 2 eyes with subtotal retinal detachment. In 17 eyes (36.9%), no treatment was required and 8 eyes (17.4%) with advanced sequelae of total closed funnel retinal detachment and macular fold were advised to be best left alone without any treatment. At the last follow-up visit, 29 eyes (63.0%) had a favorable structural outcome (P < 0.001). Among the patients in whom visual acuity could be assessed (16 eyes), a favorable visual outcome was noted in 9 eyes (56.3%). However, in 7 eyes (43.7%) visual outcome was poor.
Discussion
ROP according to disease terminology occurs principally, but not exclusively, in premature infants. It has been reported in full-term infants also.[9,10,11] In this study, we found that factors which could predispose a full term infant to develop ROP-like changes were similar to those seen in prematurely born infants, namely, low BW, multiple births, RDS, oxygen administration, and phototherapy. These were significantly associated with the development of ROP-like retinopathy even in full-term infants. In their study of 816 full-term infants, Chen et al.[14] found the risk factors for developing retinopathy were pregnancy related hypertension, asphyxia, hypoxic ischemic encephalopathy, and low BW. However, the retinal changes observed in their series were mainly retinal hemorrhages, pale optic disc, vascular tortuosity, and disc edema. None had ROP-like changes.
Clearly, low BW, prematurity, or oxygen administration are not the only factors responsible for ROP and its pathogenesis appears to be multifactorial. Schulman et al.[11] have hypothesized that spontaneous closure of both ductus arteriosus and foramen ovale at birth causes a rise in the arterial oxygen tension which may cause ROP-like retinopathy in full-term infants. They suggested that this increase in oxygen tension may be sufficient to cause retinal vasoconstriction, vaso-obliteration, and finally ROP-like proliferation. Kim and Yu[9] believe that unknown factors during the in utero developmental stage lead to the arrest of retinal vascular maturation even in infants born at full term. They found a positive correlation between systemic abnormalities, in particular brain anomalies and defects in the central nervous system and ROP.
Smith et al.[15] believe that the increased metabolic demand of the retina after birth, if not met properly by the nutritional supply, may lead to imbalance. Decreased insulin like growth factor-1 leading to poor weight gain after birth, has already been shown to be highly associated with risk of ROP. Reduced levels of omega 3 fatty acids in particular docosahexaenoic acid (DHA) may play a role. Just before birth, there is a massive transfer of nutritional factors including DHA from the maternal side to the infant through the placenta. After birth, DHA is exogenously derived from the diet. Poor maternal nutrition may result in a lack of DHA which may be responsible for ROP-like changes in the full-term infant. They further suggest that supplementation with DHA may prevent ROP.
Low BW has been considered as a major risk factor for the development of ROP. The average BW in our series was 2342 g, and some even exceeded 4000 g. Babies with higher BW in our study were seen to have more severe retinal changes. Sanghi et al.[16] suggested that in India, severe ROP affects much larger and heavier infants than their western counterparts. The mean GA and BW in their series are higher than the earlier published western guidelines of aggressive posterior ROP. In their series, 15.91% of infants had a BW >1500 g and 9.1% were born after 32 weeks of gestation. Vinekar et al.[8] noted that babies born from centers with poor NICU care are at risk of developing Stage 5 ROP even with a BW of more than 1500 g. They also noted that screening of “heavy” babies with ROP may be missed when adhered to the western guidelines. They showed that 17.7% of babies with severe ROP would have been missed using American guidelines (≤1500 g BW or ≤32 weeks GA) and 22.6% of babies with severe ROP would have been missed using the British screening guidelines (≤1500 g or ≤31 weeks). In their study, on multivariate analysis respiratory distress and exchange transfusion emerged as independent risk factors for severe ROP.
Our observations suggest that ROP-like retinopathy can occur in full-term infants and can lead to permanent visual impairment if not screened and treated at the appropriate time. Factors apart from prematurity and low BW may be responsible for ROP-like retinopathy. Our study is limited on account of its retrospective nature and small sample size. A larger study would help solve the puzzle of ROP-like retinopathy occurring in full term infants. Recently, two reports have looked at the idea of universal screening of all neonates whether born at full term or prematurely. In a large study of 3573 healthy, term newborns, Li et al.[12] found an abnormality in 871 cases (24.4%). Most common abnormality noted was retinal hemorrhages (21.1%), and some of these hemorrhages situated at the fovea could be sight threatening. Nearly 15 (0.42%) of these healthy, full-term infants had ROP-like retinopathy. Vinekar et al.[13] recently published their results of universal screening of neonates. They consecutively enrolled 1021 healthy, term infants and screened them using a wide-angle fundus viewing system-the RetCam shuttle. They noted ROP-like ridge in 9 babies, 0.9% of all screened and 18.8% of all abnormalities.
Our study highlights the need for screening of term babies for possible sight-threatening ROP. It also points to the lacunae in our understanding of the pathogenesis of ROP. Our study implies the need for further research on the factors responsible for ROP and the ways to prevent them.
Conclusion
In conclusion, ROP like retinopathy can occasionally occur in full term infants. The risk factors for development of such retinopathy changes in term infants appear to be similar to those seen in premature infants namely, oxygen administration, low birth weight, multiple births, hypoxia, sepsis, etc. However, there appears to be a complex interplay of factors which can potentially cause ROP like changes in term infants. These interactions need to be further explored. There exists a potential risk of severe visual impairment in such children. The idea of universal screening of neonates therefore is appealing and is gaining rapid attention.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123:991–9. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 2.Kaiser RS, Trese MT, Williams GA, Cox MS., Jr Adult retinopathy of prematurity: Outcomes of rhegmatogenous retinal detachments and retinal tears. Ophthalmology. 2001;108:1647–53. doi: 10.1016/s0161-6420(01)00660-1. [DOI] [PubMed] [Google Scholar]
- 3.Smith BT, Tasman WS. Retinopathy of prematurity: Late complications in the baby boomer generation (1946-1964) Trans Am Ophthalmol Soc. 2005;103:225–34. [PMC free article] [PubMed] [Google Scholar]
- 4.Owen LA, Hartnett ME. Current concepts of oxygen management in retinopathy of prematurity. J Ophthalmic Vis Res. 2014;9:94–100. [PMC free article] [PubMed] [Google Scholar]
- 5.Scott A, Fruttiger M. Oxygen-induced retinopathy: A model for vascular pathology in the retina. Eye (Lond) 2010;24:416–21. doi: 10.1038/eye.2009.306. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert C, Wormald R, Fielder A, Deorari A, Zepeda-Romero LC, Quinn G, et al. Potential for a paradigm change in the detection of retinopathy of prematurity requiring treatment. Arch Dis Child Fetal Neonatal Ed. 2016;101:F6–9. doi: 10.1136/archdischild-2015-308704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah PK, Prabhu V, Ranjan R, Narendran V, Kalpana N. Retinopathy of prematurity: Clinical features, classification, natural history, management and outcome. Indian Pediatr. 2016;53(Suppl 2):S118–22. [PubMed] [Google Scholar]
- 8.Vinekar A, Dogra MR, Sangtam T, Narang A, Gupta A. Retinopathy of prematurity in Asian Indian babies weighing greater than 1250 grams at birth: Ten year data from a tertiary care center in a developing country. Indian J Ophthalmol. 2007;55:331–6. doi: 10.4103/0301-4738.33817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HY, Yu YS. Retinopathy of prematurity-mimicking retinopathy in full-term babies. Korean J Ophthalmol. 1998;12:98–102. doi: 10.3341/kjo.1998.12.2.98. [DOI] [PubMed] [Google Scholar]
- 10.Dhaliwal CA, Fleck BW, Wright E, Graham C, McIntosh N. Retinopathy of prematurity in small-for-gestational age infants compared with those of appropriate size for gestational age. Arch Dis Child Fetal Neonatal Ed. 2009;94:F193–5. doi: 10.1136/adc.2008.143552. [DOI] [PubMed] [Google Scholar]
- 11.Schulman J, Jampol LM, Schwartz H. Peripheral proliferative retinopathy without oxygen therapy in a full-term infant. Am J Ophthalmol. 1980;90:509–14. doi: 10.1016/s0002-9394(14)75020-2. [DOI] [PubMed] [Google Scholar]
- 12.Li LH, Li N, Zhao JY, Fei P, Zhang GM, Mao JB, et al. Findings of perinatal ocular examination performed on 3573, healthy full-term newborns. Br J Ophthalmol. 2013;97:588–91. doi: 10.1136/bjophthalmol-2012-302539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vinekar A, Govindaraj I, Jayadev C, Kumar AK, Sharma P, Mangalesh S, et al. Universal ocular screening of 1021 term infants using wide-field digital imaging in a single public hospital in India – A pilot study. Acta Ophthalmol. 2015;93:e372–6. doi: 10.1111/aos.12685. [DOI] [PubMed] [Google Scholar]
- 14.Chen LN, He XP, Huang LP. A survey of high risk factors affecting retinopathy in full-term infants in China. Int J Ophthalmol. 2012;5:177–80. doi: 10.3980/j.issn.2222-3959.2012.02.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith LE, Hard AL, Hellström A. The biology of retinopathy of prematurity: How knowledge of pathogenesis guides treatment. Clin Perinatol. 2013;40:201–14. doi: 10.1016/j.clp.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanghi G, Dogra MR, Das P, Vinekar A, Gupta A, Dutta S. Aggressive posterior retinopathy of prematurity in Asian Indian babies: Spectrum of disease and outcome after laser treatment. Retina. 2009;29:1335–9. doi: 10.1097/IAE.0b013e3181a68f3a. [DOI] [PubMed] [Google Scholar]


