Abstract
Methamphetamine (meth) addiction is a prevalent health concern worldwide, yet remains without approved pharmacological treatments. Preclinical evidence suggests that oxytocin may decrease relapse, but the neuronal underpinnings driving this effect remain unknown. Here we investigate whether oxytocin’s effect is dependent on presynaptic glutamatergic regulation in the nucleus accumbens core (NAcore) by blocking metabotropic glutamate receptors 2/3 (mGluR2/3). Male and female Sprague-Dawley rats self-administered meth or sucrose on an escalating fixed ratio, followed by extinction and cue-induced reinstatement sessions. Reinstatement tests consisted of systemic (Experiment 1) or site-specific application of the drugs into the NAcore (Experiments 2 and 3). Before reinstatement sessions, rats received LY341495, an mGluR2/3 antagonist, or its vehicle followed by a second infusion/injection of oxytocin or saline. As expected, both males and females reinstated lever pressing to meth associated cues, and LY341495 alone did not impact this behavior. Oxytocin injected systemically or infused into the NAcore decreased cued meth seeking. Importantly, combined LY341495 and oxytocin administration restored meth cued reinstatement. Interestingly, neither oxytocin nor LY341495 impacted sucrose-cued reinstatement, suggesting distinct mechanisms between meth and sucrose. These findings were consistent between males and females. Overall, we report that oxytocin reduced responding to meth-associated cues and blocking presynaptic mGluR2/3 reversed this effect. Further, oxytocin’s effects were specific to meth cues as NAcore oxytocin was without an effect on sucrose cued reinstatement. Results are discussed in terms of oxytocin receptor localization in the NAcore and modulation of presynaptic regulation of glutamate in response to drug associated cues.
1. Introduction
Oxytocin is synthetized in the paraventricular and supraoptic nuclei of the hypothalamus, released in the circulatory system by the posterior pituitary and binds to widespread oxytocin receptors throughout the brain (Stoop, 2012; Vargas-Martinez et al., 2014). Traditionally, oxytocin is known to promote numerous prosocial behaviors in humans (Heinrichs and Domes, 2008; Kosfeld et al., 2005) and rodents (Bielsky and Young, 2004; Dölen et al., 2013; Ross and Young, 2009). But, recently, particular attention has been drawn to its therapeutic potential in anxiety-related disorders and autism (Andari et al., 2010; Guastella et al., 2010) as well as substance abuse and drug addiction (Lee and Weerts, 2016; Sarnyai and Kovacs, 2014).
In studies with human addicts, intranasal oxytocin alleviated cue induced craving for alcohol in patients with alcohol use disorder comorbid with high anxiety (Mitchell et al., 2016) and decreased alcohol withdrawal symptoms (Pedersen et al., 2013). In cannabis-dependent individuals, oxytocin reduced marijuana craving and stress scores (McRae-Clark et al., 2013). Finally, relative to placebo, oxytocin increased desire to use but had no effect on cue induced craving in a cocaine-dependent population (Lee et al., 2014). Recently, studies examining the effects of oxytocin on drug related behaviors have been comprehensively reviewed (Baracz and Cornish, 2016). In summary, oxytocin has therapeutic potential for multiple abused substances including methamphetamine (meth). Meth is a commonly used and addictive drug that is currently without approved pharmacotherapy to reduce use and relapse. In preclinical studies using rats trained to self-administer meth, oxytocin decreased motivation for meth, decreased drug intake, and reduced drug primed reinstatement (Carson et al., 2010a; Cox et al., 2017). Consistently, our laboratory has reported that intraperitoneal (ip) oxytocin decreased lever responding for meth-associated cues using a model of self-administration, extinction, and reinstatement (Cox et al., 2017; Cox et al., 2013). The mechanisms by which oxytocin reduces responding to drug conditioned cues have not been identified within brain areas involved in the addiction circuitry.
Oxytocin receptors in the nucleus accumbens core (NAcore) are localized on serotonin neurons, parvalbumin interneurons, and astrocytes (Dolen et al., 2013) and greater levels of oxytocin receptor mRNA are found in males relative to females (Dumais and Veenema, 2016). Oxytocin infusion into the NAcore decreased heroin self-administration in heroin tolerant rats (Ibragimov et al., 1987) and reduced cocaine-induced sniffing behavior (Sarnyai et al., 1991). More recently, our laboratory (Cox et al., 2017) and others (Baracz et al., 2012, 2014, 2015) have shown that oxytocin acts in the NAcore to decrease meth taking and seeking. Specifically, oxytocin infused directly into the NAcore decreased cued meth seeking, and co-treatment with an oxytocin receptor antagonist blocked this effect (Cox et al., 2017).
Psychostimulant exposure causes maladaptive changes in glutamatergic regulation of the NAcore, which may contribute to the cyclical aspect of addiction (reviewed in Scofield et al., 2016). Escalating meth self-administration increased basal levels of extra synaptic glutamate in the NAcore following 1–3 weeks of forced abstinence (Lominac et al., 2012). In contrast, basal levels of extracellular glutamate in the NAcore were reduced in meth self-administration animals that have also undergone extinction of the lever response (Parsegian and See, 2014), and presentation of meth-paired cues during reinstatement increased NAcore glutamate (Parsegian and See, 2014). Given the critical role for glutamatergic signaling in reinstatement behavior, we hypothesize that oxytocin signaling in the NAcore can modulate glutamatergic transmission to reduce cued reinstatement. To begin to test this notion pharmacologically, we targeted metabotropic glutamate receptors (mGluR) subtype 2/3 which are located in the presynaptic part of glutamatergic terminals. These are inhibitory auto receptors on glutamate terminals that regulate neurotransmitter release and activation decreases synaptic neurotransmitter release (reviewed in Scofield et al., 2016). For example, activation of presynaptic mGluR2/3 with an agonist decreased cued meth seeking (Caprioli et al., 2015; Kufahl et al., 2013) presumably through inhibiting presynaptic glutamate release. In these studies, we examined the hypothesis that blockade of mGluR2/3 would prevent oxytocin from reducing cued reinstatement. To this end, we administered the mGluR2/3 antagonist LY341495 or vehicle before treatment with oxytocin or saline and then evaluated responding for meth conditioned cues. In separate experiments we administered the compounds via systemic injection and directly into the NAcore. An additional experiment with sucrose as the primary reinforcer was conducted to determine if oxytocin’s effects were meth specific in the NAcore.
2. Material and Methods
2.1. Subjects
Male and female Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) weighing 275–300g (males) and 205–250g (females) on arrival were used in all experiments. Rats were individually housed on a reversed 12:12 light-dark cycle in a temperature and humidity controlled vivarium. All experiments were conducted during the dark cycle. Water was available ad libitum throughout the study and rat chow (Harlan, Indianapolis, IN, USA) was delivered daily (10–30g). Food was restricted during the experiments based on sex and daily body weights while ensuring for continual weight gain throughout the experiments and maintenance of animal health. Females on average were fed 10–25g and males 15–30g: the minimal amount was used only during the first 3 days of drug self-administration, while the maximal amount was used to prevent excessive weight gain and thus to maintain catheter patency. All experimental protocols were approved by the Institutional Animal Care and Use Committee, and were in accordance with the “Guide for the Care and Use of Laboratory Animals” (National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals. et al., 2011).
2.2. Surgery
Rats were anesthetized with ketamine (57 mg/kg, ip, Vedco Inc., USA), xylazine (8.7 mg/kg, ip, Bimeda, USA) and equithesin (0.7ml/kg ip, sodium pentobarbital 4 mg/kg, chloral hydrate 17 mg/kg, and 21.3 mg/kg magnesium sulfate heptahydrate dissolved in 44% propylene glycol, 10 % ethanol solution). Ketorolac (2.0 mg/kg, ip, Sigma, USA) was administered preoperatively for analgesia, and cefazolin (200 mg/kg, sc, Schein Pharmaceuticals, USA) was used to protect against post-surgical infection. One end of a silastic catheter was inserted 33 mm into the external right jugular and secured with 4.0 silk sutures. The other end ran subcutaneously, exited from a small incision on the back, and attached to an infusion cannula (PlasticsOne Inc. Roanoke, VA). The rats were flushed with 0.05 ml of TCS catheter lock solution (Access Technologies, USA) post-surgery to prevent clots and microbial growth in the catheter. Meth self-administration began at least 5 days after recovery from surgery.
Following intravenous surgery, some rats also received bilateral intracranial surgery. Ketamine boosts (1/6th of initial dose) and local lidocaine (0.02 µl of 2% Lidocaine) administration were used to ensure a continuous state of sedation and analgesia. Bilateral guide cannulae (26 gauge, 10 mm Plastics One, Roanoke, VA) were stereotaxically implanted into the NAcore. Coordinates were measured from bregma at the skull surface based on a rat brain atlas (Paxinos and Watson, 1998) (+1.2 posterior, ±2.4 medial–lateral, −5.2 ventral), and cannulae were inserted with a 6° angle; dorsoventral coordinates took into account that microinjectors extended 2mm beyond the cannulae. Cannulae were secured to the skull with four jeweler’s screws and dental acrylic. Dummy cannulae were placed into the guide cannulae to prevent occlusion and minimize the risk of infection. Rats were allowed to recover for at least 5 days before initiating behavioral procedures.
2.3. Drugs and microinfusions
Methamphetamine HCl (Sigma, St Louis, MO) was dissolved in 0.9% sterile saline. For both systemic and intracranial administrations, oxytocin (Cell Sciences, Canton, MA) was dissolved in 0.9% sterile saline, and the mGluR2/3 antagonist LY341495 (Tocris bioscience, Bristol, UK) was first dissolved in HCl and then saline. All drug doses were derived from previous work from our laboratory and others. For systemic injections oxytocin was administered at 1 mg/kg (Cox et al., 2013, Zhou et al., 2015; Leong et al., 2016) and LY341495 was injected at 1 mg/kg (Scofield et al., 2015; Li et al., 2010). For intracranial administration, oxytocin was infused at a concentration of 0.6nmol/0.25µl/side (Baracz et al., 2012; Cox et al., 2017). LY341495 was infused at a concentration of 1.3nmol/0.25µl/side based on a range of two efficient doses (Kim et al, 2015; Richard and Berridge, 2011). Micro infusions were made by inserting a 33-gauge stainless steel injector (Plastics One, Roanoke, VA) into the guide cannulae. The injector was connected to a polyethylene tube (PE-20) and fitted to a 1 µl Hamilton syringe (Hamilton, NV, USA). Each rat received two consecutive microinjections of either LY341495 or vehicle followed by oxytocin or saline over a 1 min period. Injectors were left in place an additional minute to allow for diffusion.
2.4. Apparatus
Self-administration, extinction, and reinstatement were performed in standard operant chambers (30 × 20 × 20 cm) enclosed in sound attenuating cubicles with a ventilation fan (Med Associates, St. Albans, Vermont) and linked to a computerized data collection program (MED PC, Med Associates). Each chamber was equipped with two retractable levers, a white stimulus light above each lever, house light, and tone generator (78 dB, 4.5 kHz). A house light remained on throughout each experimental session. For meth self-administration, infusion tubing was enclosed in steel spring leashes (Plastics One Inc., Roanoke, VA) and connected to the infusion harness. A weighted swivel apparatus (Instech, Plymouth Meeting, PA) was suspended above the box to allow for free movement within the chamber. For sucrose self-administration, a food magazine was inserted between levers, allowing the delivery of a sucrose pellet (45mg, Bioserve, MD).
2.5. Meth or Sucrose Self-administration
Self-administration procedures were based on our recent study in male and female rats (Cox et al., 2013). Sessions were conducted in daily 2-hour sessions beginning with an FR1 for 5 sessions, then advanced to FR3 for 3 sessions, and finally maintained on an FR5 for 5 sessions. The response requirement to increase the FR value was set at a minimum of 10 daily infusions. An FR5 was selected in order to clearly define lever discrimination in both sexes (Reichel et al., 2012) and promote responding for cue-induced reinstatement. During drug self-administration sessions, a response on the active lever resulted in activation of the infusion pump and delivery of a 2-sec meth infusion (17.5 µg/50 µl bolus for females and 20 µg/50 µl bolus for males), together with a 5-sec presentation of a stimulus complex (illumination of the light over the active lever and activation of the tone generator), and followed by a 20-sec time-out. During the time-out period, responses on the active and inactive levers were recorded, but had no scheduled consequences. Pressing on the inactive lever produced no consequence. For sucrose self-administration, pressing the active lever resulted in the delivery of a sucrose pellet in the food magazine, together with the same light+tone complex and time-out period that occurred during drug self-administration.
Following self-administration, the lever press response was extinguished in daily 2 hr sessions. During extinction, responding on the active lever had no scheduled consequences (i.e. no light + tone administration or drug). A minimum of 8 daily sessions and criteria of less than 25 presses on two consecutive days was required before reinstatement testing, consistent with our previous publications (Reichel et al., 2012; Cox et al., 2013; Leong et al., 2016). Active lever responding during the cued-induced reinstatement tests resulted in the delivery of the same light+tone stimulus complex originally paired with the primary reinforcer, but no drug or sucrose were delivered. Pressing the inactive lever was still without consequence. Before tests, rats were treated with the mGluR2/3 antagonist or vehicle (details in the next section for each experiment) before oxytocin or saline to result in four conditions: Veh/Sal, LY/Sal, Veh/Oxy, and LY/Oxy. This nomenclature will be used to define the test conditions throughout (first treatment/second treatment). Each rat was tested twice with at least 2 additional extinction sessions to criteria in between tests. The extinction means (± SEM) for the last two days before testing are presented in Table 1. Test condition and test order were randomly assigned without replacement until the groups were full. The number of reinstatement tests was limited to 2 in order to avoid extinction of the drug-conditioned cues during the test sessions. Previous studies have confirmed the absence of a test order effect (Reichel et al., 2012; Cox et al., 2013; Zhou et al., 2015; Leong et al., 2016).
Table 1.
Average lever responses during the last two days of extinction before each reinstatement test.
| All subjects (Mean ± SEM) |
Males (Mean ± SEM) |
Females (Mean ± SEM) |
||
|---|---|---|---|---|
| Experiment 1 | ||||
| Test 1: Active | 11.57 ± 1.20 | 13.81 ± 2.12 | 9.63 ± 1.04 | |
| Inactive | 6.32 ± 1.28 | 7.00 ± 1.93 | 5.73 ± 1.76 | |
| Test 2: Active | 15.17 ± 1.09 | 15.14 ± 1.42 | 15.2 ± 1.70 | |
| Inactive | 5.44 ± 0.77 | 6.32 ± 0.93 | 4.2 ± 1.28 | |
|
| ||||
| Experiment 2 | ||||
| Test 1: Active | 15.95 ± 1.02 | 18.48 ± 1.65 | 13.52 ± 1.02 | |
| Inactive | 6.79 ± 0.64 | 8.52 ± 0.94 | 5.20 ± 0.78 | |
| Test 2: Active | 15.25 ± 0.80 | 15.10 ± 0.91 | 15.50 ± 1.53 | |
| Inactive | 6.66 ± 0.91 | 7.69 ± 1.32 | 5.00 ± 0.91 | |
|
| ||||
| Experiment 3 | ||||
| Test 1: Active | 14.23 ± 1.16 | 14.23 ± 1.00 | 14.23 ± 0.99 | |
| Inactive | 6.95 ± 1.02 | 9.05 ± 1.42 | 5.05 ± 1.25 | |
| Test 2: Active | 16.95 ± 1.13 | 17.17 ± 1.97 | 16.77 ± 1.37 | |
| Inactive | 6.85 ± 1.40 | 6.89 ± 1.03 | 6.82 ± 2.47 | |
After the second test, rats were decapitated, and brains were collected for histological assessment of cannula placement. In brief, rats were deeply anesthetized with Equithesin and then transcardially perfused with 150–200 ml cold 0.9% saline followed by 400–500 ml of 4% paraformaldehyde. Brains were removed and post-fixed in 4% paraformaldehyde for 24 hr, submerged in 20% sucrose for 48 hr, and then sectioned on a vibratome. Sections were mounted on microscope slides and cannula placement was verified within the NAcore based on the atlas by Paxinos and Watson, 1998.
2.6. Experimental Procedures
2.6.1. Systemic mGluR2/3 antagonism before oxytocin on meth cue-induced reinstatement
Male (n=13) and female (n=15) rats underwent catheter surgery, meth self-administration and extinction as described above. Following extinction, rats were tested twice for cue-induced reinstatement. To test the effect of blocking mGluR2/3, rats received LY341395 or vehicle (ip) and 5 min later oxytocin or saline (ip). Thirty minutes after the second injection rats tested for cued-reinstatement. This injection to placement interval allows the locomotor suppressing effects of systemic oxytocin to diminish before chamber placement. Previously, we have shown that IP injections of oxytocin (0.1–3 mg/kg) decreased locomotor activity for the first 30 min of exposure in males and females (Leong et al., 2016).
2.6.2. Antagonism of mGluR2/3 in the NAcore before oxytocin and meth cue-induced reinstatement
Male (n=25) and female (n=24) rats underwent catheter and intracranial surgery, meth self-administration and extinction as described above. Rats were then reinstated with contingent presentations of the drug-associated cues. Before reinstatement, rats received NAcore microinjections of either vehicle or LY341495 followed by oxytocin or saline. Five minutes after the infusions, rats were placed into the chamber for the cued reinstatement test. The injection to placement interval for intracranial oxytocin was five minutes because we do not observe locomotor suppression following microinjections (unpublished data).
2.6.3. Antagonism of mGluR2/3 in the NAcore before oxytocin and sucrose cue-induced reinstatement
Male (n=10) and female (n=11) rats underwent intracranial surgery, sucrose self-administration, and extinction. Following extinction rats were reinstated with contingent presentations of the sucrose-associated cue. Before reinstatement, rats received NAcore microinjections of either vehicle or LY341495 followed by oxytocin or saline. Five minutes after the infusion, rats were placed into the chamber for the cued reinstatement test.
2.7. Statistical Analyses and Behavioral Observations
Lever presses and drug intake were the primary dependent measures. For drug self-administration and extinction, data were analyzed by two-way mixed analysis of variance (ANOVA) with sex as the between subjects variable and day as the repeated measures variable. Holm-Sidak’s post hoc comparisons were used to adjust for family wise error when appropriate. Test data were first analyzed with two-way between subjects ANOVA with sex (male and female) and test condition (Veh/Sal, LY/Sal, Veh/Oxy, and LY/Oxy) to determine whether sex differences existed in lever responding to meth-conditioned cues. If males and females did not differ, a two-way between subjects ANOVA was conducted with pre-treatment (Veh vs LY) and treatment (Sal vs Oxy) as the independent variables. To directly test the hypothesis that LY341495 blocks oxytocin’s effect an a-priori planned comparison was conducted between group Veh/Oxy and Ly/Oxy. Significant interactions and main effects were Holm-Sidak’s post hoc comparisons.
3. Results
3.1. Systemic mGluR2/3 antagonism prevented oxytocin from reducing meth cue-induced reinstatement
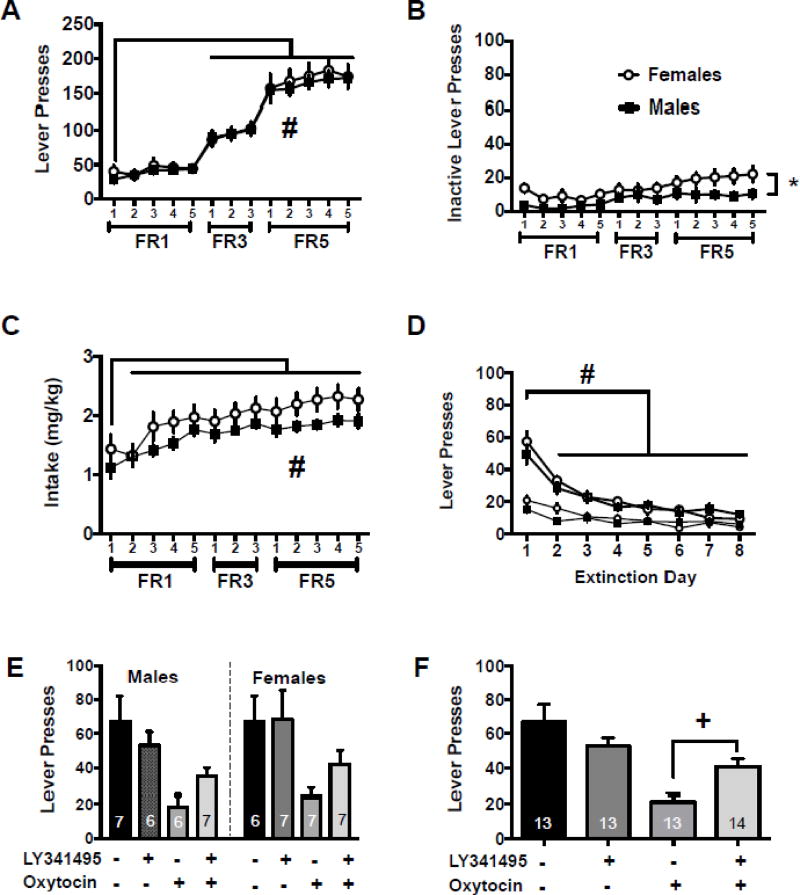
Male and female rats underwent catheter surgery, meth self-administration, and extinction as described above. Figure 1A depicts the active lever presses throughout the self-administration period. There was a main effect of day [F(12,312)=139.7, p<0.001] with days 6–13 being significantly higher than day 1 (Holm-Sidak’s, p<0.05). There were no sex differences on active lever presses during the meth self-administration period, nor was there a sex by day interaction. Figure 1B shows inactive lever presses throughout self-administration. On the inactive lever there was a main effect of day [F(12,312)=6.64, p<0.001] and a main effect of sex [F(1,26)=5.61, p<0.027] with females pressing the inactive lever more than males. Males and females had similar meth intake during self-administration as both groups increased intake after the first day [main effect of day, Figure 1C, F(12,300)=18.27, p<0.001]; specifically meth intake increased following day 1 on all subsequent days (Holm-Sidak’s, p<0,05). The main effect of sex and the sex by day interaction were not significant.
Figure 1.
Systemic mGluR2/3 antagonism prevented oxytocin from reducing meth cue-induced reinstatement. A) Active lever presses throughout the self-administration period for males and females. B) Inactive lever presses throughout the self-administration period for males and females. C) Drug intake in mg/kg in males and females. D) Lever presses during extinction for males and females. E) Lever presses during cued reinstatement for males and females presented separately for comparison purposes. F) Lever presses during cued reinstatement for males and females combined.
−− indicates Veh/Sal, +− indicates LY/Sal, −+ indicates Veh/Oxy, ++ indicates LY/Oxy
# significantly different from day 1, p<0.05
* significant difference between males and females, p<0.05
+ significantly different from Veh/Oxy group, p<0.05
During extinction, there was a main effect of extinction day [Fig 1D, F(7,182)=46.75, p<0.0001]. Specifically, active lever responses on days 2–8 were lower than extinction day 1 (Holm-Sidak’s, p<0.05). The main effect of sex and the sex by extinction day interaction were not significant. Inactive lever responding during extinction resulted in an interaction between sex and extinction day [Fig 1D, F(1,182)=2.92, p<0.007]; however post hoc comparisons did not reach statistical significance. Both males and females decreased responding on the inactive lever over the sessions [main effect of day, F(1,182)=13.07, p<0.001]. Extinction values between test sessions are presented in Table 1.
On cued reinstatement responding, the two-way ANOVA of sex × test condition resulted in a main effect of test condition [F(3,45)=6.76, p<0.001], but no significant main effect of sex or significant sex × test condition interaction (Figure 1E). The two-way ANOVA collapsed across sex revealed a main effect of Treatment [F(1,49)=19.6, p<0.0001], but the interaction and main effect of pretreatment were not significant (Figure 1F). The planned comparison between Veh/Oxy and LY/Oxy was significant [t(24)=3.32, p<0.003]. Table 2 presents the inactive lever data from the cued reinstatement tests conducted in Experiment 1. The interaction was not significant on the inactive lever, nor was the main effect of treatment. There was, however, a main effect of pretreatment [F(1,49)=7.45, p<0.008].
Table 2.
Average inactive lever responses during reinstatement tests.
| All subjects (Mean ± SEM) |
Males (Mean ± SEM) |
Females (Mean ± SEM) |
|
|---|---|---|---|
| Experiment 1 | |||
| Veh/Sal | 8.69 ± 2.10 | 7.86 ± 3.23 | 9.67 ± 2.81 |
| LY/Sal | 6.57 ± 1.58 | 7.29 ± 2.41 | 5.86 ± 2.20 |
| Veh/Oxy | 2.23 ± 0.44 * | 2.50 ± 0.89 | 2.00 ± 0.38 |
| LY/Oxy | 4.36 ± 1.26 | 5.71 ± 2.38 | 3.00 ± 0.79 |
|
| |||
| Experiment 2# | |||
| Veh/Sal | 11.20 ± 2.27 | 12.00 ± 3.99 | 10.55 ± 2.69 |
| LY/Sal | 12.71 ± 1.85 | 15.11 ± 2.41 | 10.00 ± 2.69 |
| Veh/Oxy | 6.78 ± 1.69 + | 8.60 ± 2.82 | 4.50 ± 1.2 |
| LY/Oxy | 15.00 ± 2.09 | 17.00 ± 2.85 | 12.75 ± 3.07 |
|
| |||
| Experiment 3 | |||
| Veh/Sal | 4.67 ± 1.78 | 6.00 ± 1.29 | 3.60 ± 3.12 |
| LY/Sal | 10.22 ± 1.81 | 13.60 ± 1.75 | 6.00 ± 1.96 |
| Veh/Oxy | 9.00 ± 2.22 | 10.75 ± 3.68 | 7.83 ± 2.94 |
| LY/Oxy | 17.60 ± 6.31 | 21.80 ± 10.85 | 13.40 ± 7.26 |
significantly different from Veh/Sal
significantly different from LY/Oxy
significant main effect of sex
3.2. mGluR2/3 antagonism in the NAcore prevented oxytocin from reducing meth cue-induced reinstatement
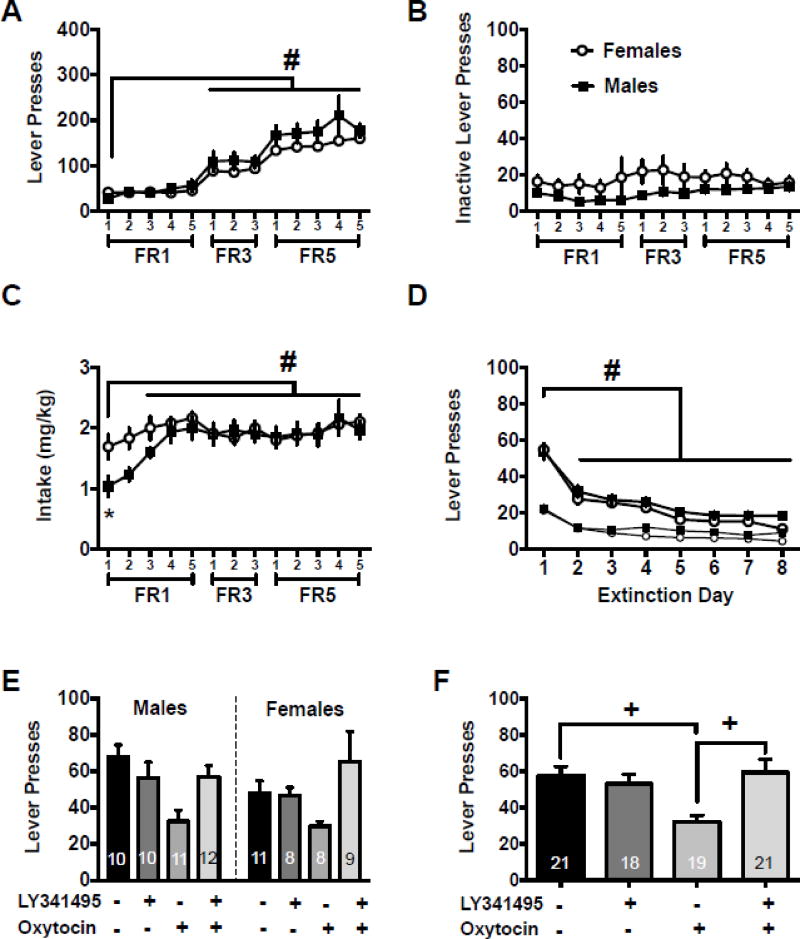
Male and female rats underwent catheter and intracranial surgery, meth self-administration, and extinction as described above. Figure 2A represents the active lever presses during self-administration. There was a main effect of day [F(12,611)=32.07, p<0.05] with days 6–13 reaching significantly higher values than day 1 (Holm-Sidak’s, p<0.05). There was also a main effect of sex [F(1,611)=10.85, p<0.001] with males having higher lever responding than females. Figure 2B shows that there were no differences detected in inactive lever responding during self-administration. Males and females had similar meth intake during self-administration as both groups increased intake after the first day [main effect of day, Figure 1C, F(12,300)=18.27, p<0.001]; specifically meth intake increased on days 3–13 relative to day one (Holm-Sidak’s, p<0.05). Females also took more meth on day one than males [main effect of sex, F(1,546)=6.02, p<0.015].
Figure 2.
NAcore mGluR2/3 antagonism prevented oxytocin from reducing meth cue-induced reinstatement. A) Active lever presses throughout the self-administration period for males and females. B) Inactive lever presses throughout the self-administration period for males and females. C) Drug intake in mg/kg in males and females. D) Lever presses during extinction for males and females. E) Lever presses during cued reinstatement for males and females presented separately for comparison purposes. F) Lever presses during cued reinstatement for males and females combined.
−− indicates Veh/Sal, +− indicates LY/Sal, −+ indicates Veh/Oxy, ++ indicates LY/Oxy
# significantly different from day 1, p<0.05
* significant difference between males and females, p<0.05
+ significantly different from Veh/Oxy group, p<0.05
Figure 2D depicts active lever presses during extinction. There was a main effect of extinction day [F(7,322)=86.85, p<0.0001]. Specifically, active lever responses on days 2–8 were lower than extinction day 1 (Holm-Sidak’s, p<0.05). There was also a main effect of extinction day on the inactive lever [F(7,322)=39.33, p<0.0001], with responding significantly lower on days 2–8 relative to extinction day 1. The main effects of sex and sex by extinction day interactions were not significant on either lever. Extinction values between test sessions are presented in Table 1.
On the cued reinstatement test, the two-way ANOVA resulted in a main effect of test condition [Fig 2E, F(3,67)=5.18, p<0.003], but no significant main effect of sex or significant sex by test condition interaction. The two-way ANOVA collapsed across sex resulted in a pretreatment×treatment interaction [Figure 2F, F(1,71)=10.5, p<0.0018]. Post hoc comparisons show that Veh/Oxy had significantly lower lever responding than Veh/Sal and LY/Oxy conditions (Holm-Sidak’s, p<0.05). Table 2 presents the inactive lever data from the cued reinstatement tests conducted in Experiment 2. The interaction between pre-treatment and treatment was not significant, nor was the mail effect of treatment. There was however a main effect of pretreatment [F(1,68)=5.82, p<0.02].
3.3. NAcore oxytocin did not impact sucrose cue-induced reinstatement
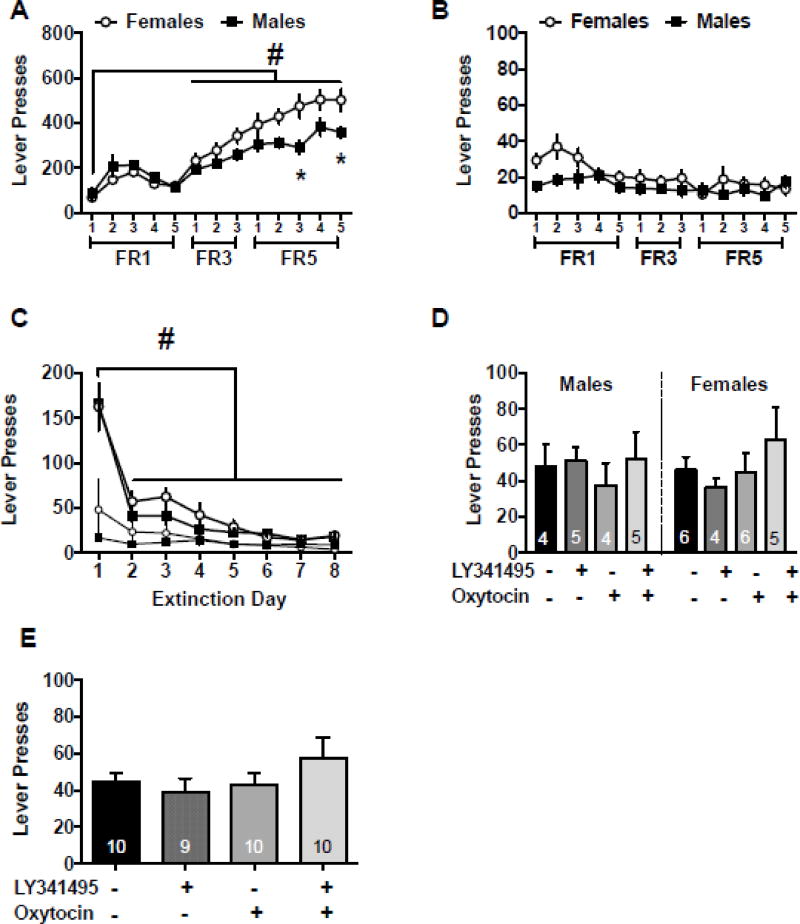
Male and female rats underwent intracranial surgery, sucrose self-administration, and extinction as described above. Figure 3A depicts the active lever presses during the self-administration period. There was a significant sex by day interaction [F(12,228)=6.11, p<0.0001]. Post hoc comparisons show that females pressed the lever more than males on days 11 and 13 (Holm-Sidak’s, p<0.05). There was also a main effect of day [F(12,228)=62.68, p<0.0001] with days 6–13 reaching significantly higher values than day 1 (Holm-Sidak’s, p<0.05). Figure 3B shows inactive lever responding during sucrose administration. There was a sex by day interaction with females pressing the lever more on day two than males [F(12,228)=2.06, p<0.021 and Holm-Sidak’s p<0.05]. There was also a main effect of day [F(12,228)=4.85, p<0.001]. The main effect of sex was not significant on either lever.
Figure 3.
NAcore oxytocin and mGluR2/3 antagonism had no effect on sucrose cue-induced reinstatement. A) Active lever presses throughout the self-administration period for males and females. B) Inactive lever presses throughout the self-administration period for males and females. C) Lever presses during cued reinstatement for males and females presented separately for comparison purposes. E) Lever presses during extinction for males and females. D) Lever presses during cued reinstatement for males and females combined. −− indicates Veh/Sal, +− indicates LY/Sal, −+ indicates Veh/Oxy, ++ indicates LY/Oxy # significantly different from day 1, p<0.05
Both males and females reduced responding on the active levers over the 8 days of extinction, as demonstrated by a main effect of extinction day [Fig 3C, F(7,133)=70.30, p<0.0001]. Specifically, lever responses on days 2–8 were lower than extinction day 1 (Holm-Sidak’s, p<0.05). The main effect of sex and sex by extinction day interaction was not significant on the active lever and there were no differences found on the inactive lever. Extinction values between test sessions are presented in Table 1. On the sucrose cued test day there were no significant effects on the active or inactive levers (Fig 3D and 3E, Table 2).
4. Discussion
Numerous reports have shown that oxytocin decreased motivation to take meth and reduced drug seeking (Carson et al., 2010a; Carson et al., 2010b; Cox et al., 2017; Cox et al., 2013; Leong et al., 2016; Zhou et al., 2015). Here we report that oxytocin decreased meth seeking during cued reinstatement and that co-treatment with an mGluR2/3 antagonist blocked this effect. Similar reversal of oxytocin’s effect was observed with peripheral and central administration of the drugs as well as in males and females. Importantly, the intracranial findings were specific to meth because sucrose-cued reinstatement was unchanged regardless of treatment condition. Our results support the idea that oxytocin may be a viable therapeutic to treat meth addiction.
Previously, our laboratory demonstrated that peripheral oxytocin decreased meth seeking via a central mechanism (Cox et al., 2017). An intracerebroventricular (ICV) infusion of an oxytocin receptor antagonist completely blocked the effect of systemic oxytocin on meth seeking. Similarly, ICV oxytocin antagonism reversed the effects of systemic oxytocin on cocaine induced sniffing behavior (Sarnyai et al., 1991). Taken together, this evidence indicates that systemic oxytocin is producing its effects via a central mechanism; however, the mechanisms that produce these effects are unclear. Systemic oxytocin activates oxytocin cell bodies in the paraventricular nucleus (PVN) (Carson et al., 2010b; Leong et al., in press). This activation results in local dendritic release of oxytocin and an ensuing positive-feedback effect promoting prolonged activation of the oxytocin system (Ludwig et al., 2002; Rossoni et al., 2008), which is presumed to cause brain wide oxytocin release, including areas associated with addiction. However, it remains unclear exactly where systemic oxytocin acts to activate the central oxytocin neurons because negligible amounts of peripherally administered oxytocin cross the blood brain barrier (Ermisch et al., 1985; Landgraf et al., 1979; Mens et al., 1983) and the half-life of the oxytocin peptide in the periphery is only minutes (Mens et al., 1983). It is believed that the small amounts of oxytocin initiate a feed-forward mechanism to propagate endogenous oxytocin production (Rossoni et al., 2008). Recently, Neumann and colleagues (2013) used microdialysis in rats to demonstrate that IP administration of oxytocin caused rapid peak levels in brain dialysates 30 min post injection, providing direct evidence that systemic oxytocin increases central oxytocin levels. Consistently, intravenous oxytocin (5 IU/kg) increased oxytocin levels in cerebral spinal fluid, peaking at 15 min post-dose and gradually returning to baseline by 120 min in rhesus macaques (Freeman et al., 2016). However, it does remain unclear how peripheral administration of oxytocin propagates central oxytocin function because Lee et al. (2017) demonstrated that oxytocin administered through intranasal and intravenous administration increased oxytocin in cerebral spinal fluid, but did not activate a feed forward mechanism to elevate endogenous oxytocin. Further studies are needed to examine the mechanism by which systemically administered oxytocin is acting to decrease meth seeking.
In both meth experiments male and female rats pressed the active lever to the same degree with sex differences emerging in drug intake (females > males) when the number of infusions was adjusted per body weight. Consistent responding on the active lever during self-administration ensures that the groups were similar in their behavioral output prior to reinstatement testing, in which the primary dependent variable is the active lever press. Interestingly, meth females also responded more on the inactive lever during self-administration. We have previously reported increased inactive lever responding in females relative to males using a limited access protocol (Reichel et al., 2012). Male and female rats exhibited discrimination between active and inactive response patterns so we attribute this finding to greater general activity of female rats relative males (Zhou et al., 2015; Craft et al., 2006; Caldarone et al., 2008; Wooters et al., 2006) and greater locomotor responses to stimulant drugs (Caldarone et al., 2008; Wooters et al., 2006).
The analysis presented herein revealed that male and female rats reinstated equally in response to meth cues. This may contrast the popular notion that females are more responsive to meth-associated stimuli. In general, freely cycling female rats reinstate to a drug prime to a greater degree than males (Reichel et al., 2012; Anker et al., 2008; Kippin et al., 2005; Lynch and Carroll, 2000); however, in response to psychostimulant-associated cues, sex and circulating hormones have less effect on reinstated behavior (Feltenstein et al., 2011; Fuchs et al., 2005; Leong et al., 2016). There are also methodological differences in specific training procedures that could account for discrepancies in regards to cued reinstatement. Previous studies reported that enhanced cue-induced reinstatement in males is dependent on training dose (Fuchs et al., 2005); whereas, enhanced responding in females relies on the response requirement during the conditioned cue test (Cox et al., 2017; Cox et al., 2013). Additionally, our studies show that in both males and females NAcore oxytocin did not impact sucrose-cued reinstatement, thereby suggesting a drug-specific action for oxytocin in the NAcore. Further, mGluR2/3 antagonism was without an effect on sucrose-cued reinstatement (Myal et al., 2015) with or without oxytocin (current report). In contrast to NAcore oxytocin administration, our laboratory has already reported that systemic oxytocin decreased sucrose-cued reinstatement (Cox et al., 2013; Zhou et al., 2015). This current finding suggests that cued reinstatement following systemic oxytocin is not mediated within the NAcore.
In previous studies, oxytocin infused into NAcore blocked conditioned place preference for meth (Baracz et al., 2012), decreased meth-primed (Baracz et al., 2014), and meth and cocaine cued reinstatement (Cox et al., 2017; Leong et al., 2016). Here, we demonstrated that intra-accumbens core microinfusions of oxytocin decreased cue-induced reinstatement of meth seeking in males and females. Importantly, intra-NAcore oxytocin attenuated meth seeking in a manner similar to systemic treatment, suggesting a common mechanism. A previous study from our lab (Cox et al., 2017) demonstrated that an oxytocin receptor antagonist infused into the NAcore blocked the effects of systemic oxytocin. Further, we have shown that systemic administration of oxytocin normalizes conditioned cued-induced fos expression within the addiction cirtictry (Leong et al., in press). While the precise mechanism of oxytocin in the NAcore remains to be determined, our finding that mGluR2/3 antagonism blocks oxytocin’s impact on cued meth seeking suggests oxytocin may modulate presynaptic glutamate function.
In the NAcore, Dölen and colleagues (2013) used oxytocin receptor Venus reporter mice to provide a detailed distribution of the oxytocin receptor protein. This information was previously lacking due to a non-specificity of oxytocin receptor antibodies until the development of the Venus knock-in mouse for the oxytocin receptor gene (Yoshida et al., 2009). Using the Venus fluorescence signal, under control of the endogenous oxytocin receptor promoter, the detailed tissue distribution of oxytocin receptors in the NAcore revealed that oxytocin receptors are localized on astrocytes and parvalbumin (PV)+ interneurons, but not on median spiny neurons, cholinergic interneurons, or nitric oxide synthase 1 expressing interneurons (Dölen et al., 2013). Recently, Scofield and colleagues (2015) demonstrated that specific activation of astrocytes (via Gq coupled DREADDs) in NAcore decreased cued reinstatement of cocaine seeking through an mGluR2/3-dependent mechanism. Here, we speculate that the localization of astroglial oxytocin receptors reduces relapse via Gq signaling that can activate transduction pathways including IP3 receptors and the release of intracellular calcium stores resulting in glial transmitter release, including glutamate (Di Scala-Guenot et al., 1994; Di Scala-Guenot and Strosser, 1992; Hamilton and Attwell, 2010). Extrasynaptic glutamate can thereby activate mGluR2/3 receptors located presynaptically on cortical glutamatergic axons, inhibiting synaptic release of glutamate, thus restoring glutamatergic tone (Moussawi and Kalivas, 2010).
Alternatively, Gq coupled oxytocin receptors localized on PV+ interneurons may also be a mechanism by which the peptide reduces cued reinstatement. Several studies have shown that oxytocin mediates GABA release through interneurons both in the rat brain (Levesque and Parent, 2005; Zaninetti and Raggenbass, 2000) and spinal cord (Condes-Lara et al., 2009; Rojas-Piloni et al., 2007). In the NAcore, greater than 90% of cells are median spiny neurons with only a small but important percent of cells comprised of interneurons. As oxytocin receptors are Gq coupled, activation of PV+ interneurons inhibit median spiny neurons containing dopamine D1 and D2 receptors in the NAcore and inhibition of neurons in the NAcore decreases cocaine-seeking (Stefanik et al., 2016). However, this hypothesis can not account for the mGluR2/3-dependence of oxytocin’s effect on cued reinstatement. Given the peptides’s receptor distribution, it is possible that oxytocin regulates cued reinstatement through multiple independent or interdependent mechanisms, including mGluR2/3 regulation of glutamate tone. Future studies focused on cell-type specific manipulations of oxytocin function will be required to test these hypotheses.
Taken together, these studies show that both systemic and NAcore site-specific oxytocin decreased cued reinstatement in males and females. The ability of NAcore oxytocin to decrease cued responding was specific to meth cues because sucrose animals continued to respond regardless of oxytocin treatment. Further, mGlu2/3 antagonism prevented oxytocin from reducing meth seeking. Although a synaptic mechanism by which oxytocin reduces drug seeking remains to be determined, it is evident that oxytocin acts within the NAcore. Future studies are needed, as technology advances, to determine cell-site specific manipulation of oxytocin receptors in rat models of self-administration and relapse.
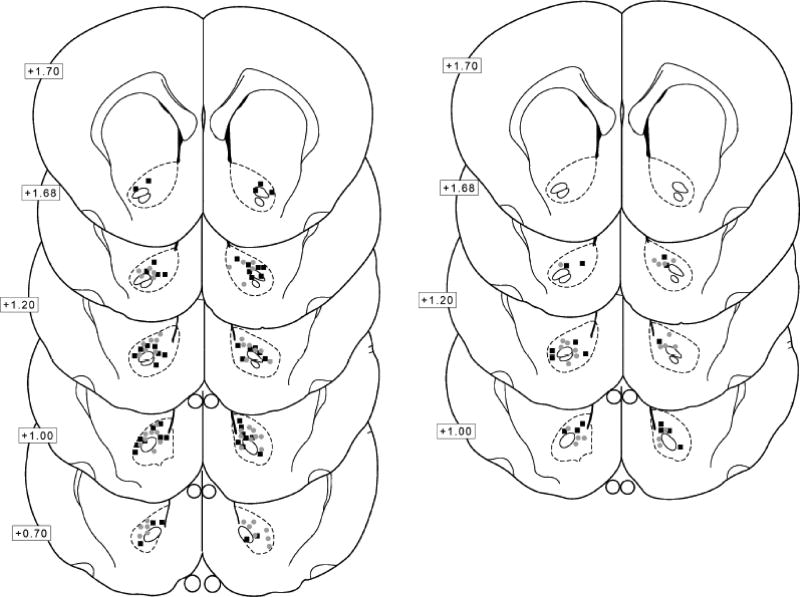
Figure 4.
A depiction of the terminal point of the injectors used to infuse compounds before reinstatement tests, with numbers next to brain sections detailing the mm anterior to Bregma. Gray circles represent females and black squares represent the males. The placements for Experiment 2 and Experiment 3 are on the left and right, respectively.
Highlights.
Oxytocin decreased cued meth seeking via systemic and site-specific application into the nucleus accumbens core in male and female rats.
Systemic and site-specific blockade of metabotropic glutamate 2/3 receptors reversed oxytocin’s effect on cued reinstatement.
Site-specific application of oxytocin into the nucleus accumbens core did not impact sucrose seeking.
Overall, these findings suggests that oxytocin may impact presynaptic regulation of glutamate in response to drug associated cues.
Acknowledgments
We thank Shannon Ghee and Rachel Anderson for technical assistance. We thank Dr. Rachel Penrod for her contributions on an earlier edition of the manuscript.
Funding
This study has been supported by NIH/NIDA grant R01DA033049 (CMR), P50DA016511 (CMR), and T32DA728823 (KCL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors declare no financial disclosures.
References
- Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci U S A. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Holtz NA, Zlebnik N, Carroll ME. Effects of allopregnanolone on the reinstatement of cocaine-seeking behavior in male and female rats. Psychopharmacology (Berl) 2008:63–72. doi: 10.1007/s00213-008-1371-9. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Cornish JL. The neurocircuitry involved in oxytocin modulation of methamphetamine addiction. Front Neuroendocrinol. 2016 doi: 10.1016/j.yfrne.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Everett NA, McGregor IS, Cornish JL. Oxytocin in the nucleus accumbens core reduces reinstatement of methamphetamine-seeking behaviour in rats. Addict Biol. 2014 doi: 10.1111/adb.12198. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Rourke PI, Pardey MC, Hunt GE, McGregor IS, Cornish JL. Behav Brain Res. Elsevier B. V.; 2012. Oxytocin directly administered into the nucleus accumbens core or subthalamic nucleus attenuates methamphetamine-induced conditioned place preference; pp. 185–193. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, King SL, Picciotto MR. Sex differences in anxiety-like behavior and locomotor activity following chronic nicotine exposure in mice. Neurosci Letters. 2008;439:187–91. doi: 10.1016/j.neulet.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Venniro M, Zeric T, Li X, Adhikary S, Madangopal R, Marchant NJ, Lucantonio F, Schoenbaum G, Bossert JM, Shaham Y. Effect of the Novel Positive Allosteric Modulator of Metabotropic Glutamate Receptor 2 AZD8529 on Incubation of Methamphetamine Craving After Prolonged Voluntary Abstinence in a Rat Model. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson DS, Cornish JL, Guastella AJ, Hunt GE, McGregor IS. Neuropharmacology. Elsevier Ltd; 2010a. Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats; pp. 38–43. [DOI] [PubMed] [Google Scholar]
- Carson DS, Hunt GE, Guastella AJ, Barber L, Cornish JL, Arnold JC, Boucher AA, McGregor IS. Systemically administered oxytocin decreases methamphetamine activation of the subthalamic nucleus and accumbens core and stimulates oxytocinergic neurons in the hypothalamus. Addict Biol. 2010b:448–463. doi: 10.1111/j.1369-1600.2010.00247.x. [DOI] [PubMed] [Google Scholar]
- Condes-Lara M, Rojas-Piloni G, Martinez-Lorenzana G, Lopez-Hidalgo M, Rodriguez- Jimenez J. Hypothalamospinal oxytocinergic antinociception is mediated by GABAergic and opiate neurons that reduce A-delta and C fiber primary afferent excitation of spinal cord cells. Brain Res. 2009;1247:38–49. doi: 10.1016/j.brainres.2008.10.030. [DOI] [PubMed] [Google Scholar]
- Cox BM, Bentzley BS, Regen-Tuero H, See RE, Reichel CM, Aston-Jones G. Oxytocin Acts in Nucleus Accumbens to Attenuate Methamphetamine Seeking and Demand. Biol Psychiatry. 2017 doi: 10.1016/j.biopsych.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM. Psychoneuroendocrinology. Elsevier Ltd; 2013. Sex differences in methamphetamine seeking in rats: Impact of oxytocin; pp. 2343–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Clark JL, Hart SP, Pinckney MK. Sex differences in locomotor effects of morphine in the rat. Pharmacology, Biochemistry, and Behavior. 2006;85:850–8. doi: 10.1016/j.pbb.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Scala-Guenot D, Mouginot D, Strosser MT. Increase of intracellular calcium induced by oxytocin in hypothalamic cultured astrocytes. Glia. 1994:269–276. doi: 10.1002/glia.440110308. [DOI] [PubMed] [Google Scholar]
- Di Scala-Guenot D, Strosser MT. Biochem. J. Portland Press Ltd; 1992. Oxytocin receptors on cultured astroglial cells. Kinetic and pharmacological characterization of oxytocin-binding sites on intact hypothalamic and hippocampic cells from foetal rat brain; pp. 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013:179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermisch A, Barth T, Ruhle HJ, Skopkova J, Hrbas P, Landgraf R. On the blood-brain barrier to peptides: accumulation of labelled vasopressin, DesGlyNH2-vasopressin and oxytocin by brain regions. Endocrinol Exp. 1985;19:29–37. [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology (Berl) 2011:53–62. doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Samineni S, Allen PC, Stockinger D, Bales Kl, Hwa GG, Roberts JA. Psychoneuroendocrinology. 2016;66:185–94. doi: 10.1016/j.psyneuen.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2005:662–672. doi: 10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci. 2010;11:227–238. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Domes G. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Prog Brain Res. 2008;170:337–350. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- Ibragimov R, Kovacs GL, Szabo G, Telegdy G. Microinjection of oxytocin into limbic-mesolimbic brain structures disrupts heroin self-administration behavior: a receptor-mediated event? Life Sci. 1987;41:1265–71. doi: 10.1016/0024-3205(87)90205-0. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology (Berl) 2005;182:245–252. doi: 10.1007/s00213-005-0071-y. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Watterson LR, Nemirovsky NE, Hood LE, Villa A, Halstengard C, Zautra N, Olive MF. Attenuation of methamphetamine seeking by the mGluR2/3 agonist LY379268 in rats with histories of restricted and escalated self-administration. Neuropharmacology. 2013:290–301. doi: 10.1016/j.neuropharm.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Ermisch A, Hess J. Indications for a brain uptake of labelled vasopressin and ocytocin and the problem of the blood-brain barrier. Endokrinologie. 1979;73:77–81. [PubMed] [Google Scholar]
- Lee MR, Glassman M, King-Casas B, Kelly DL, Stein EA, Schroeder J, Salmeron BJ. European Neuropsychopharmacology. Elsevier; 2014. Complexity of oxytocin׳s effects in a chronic cocaine dependent population; pp. 1483–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Weerts EM. Oxytocin for the treatment of drug and alcohol use disorders. Behav Pharmacol. 2016;27:640–648. doi: 10.1097/FBP.0000000000000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Scheidweiler KB, Diao XX, Akhlaghi F, Cummins A, Huestis MA, Leggio L, Averbeck BB. Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: determination using a novel oxytocin assay. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.27. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong KC, Freeman LR, Berini CR, Ghee SM, See RE, Reichel CM. Oxytocin reduced cocaine cued fos expression in a regionally specific manner. International Journal of Neuropsychopharmacology. doi: 10.1093/ijnp/pyx058. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong KC, Zhou L, Ghee SM, See RE, Reichel CM. Oxytocin decreases cocaine taking, cocaine seeking, and locomotor activity in female rats. Exp Clin Psychopharmacol. 2016;24:55–64. doi: 10.1037/pha0000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque JC, Parent A. GABAergic interneurons in human subthalamic nucleus. Mov Disord. 2005;20:574–584. doi: 10.1002/mds.20374. [DOI] [PubMed] [Google Scholar]
- Lominac KD, Sacramento AD, Szumlinski KK, Kippin TE. Distinct neurochemical adaptations within the nucleus accumbens produced by a history of self-administered vs noncontingently administered intravenous methamphetamine. Neuropsychopharmacology. 2012:707–722. doi: 10.1038/npp.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Sabatier N, Dayanithi G, Russell JA, Leng G. The active role of dendrites in the regulation of magnocellular neurosecretory cell behavior. Prog Brain Res. 2002;139:247–256. doi: 10.1016/s0079-6123(02)39021-6. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology (Berl) 2000;148:196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- McRae-Clark AL, Baker NL, Maria MMS, Brady KT. Effect of oxytocin on craving and stress response in marijuana-dependent individuals: a pilot study. Psychopharmacology (Berl) 2013:623–631. doi: 10.1007/s00213-013-3062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mens WB, Witter A, van Wimersma Greidanus TB. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 1983;262:143–149. doi: 10.1016/0006-8993(83)90478-x. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Arcuni PA, Weinstein D, Woolley JD. Intranasal Oxytocin Selectively Modulates Social Perception, Craving, and Approach Behavior in Subjects With Alcohol Use Disorder. J Addict Med. 2016;10:182–189. doi: 10.1097/ADM.0000000000000213. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Kalivas PW. Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. Eur J Pharmacol. 2010;639:115–122. doi: 10.1016/j.ejphar.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myal S, O'Donnell P, Counotte DS. Nucleus accumbens injections of the mGluR2/3 agonist LY379268 increase cue-induced sucrose seeking following adult, but not adolescent sucrose self-administration. Neuroscience. 2015;305:309–315. doi: 10.1016/j.neuroscience.2015.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.) Guide for the care and use of laboratory animals. National Academies Press; Washington, D.C: 2011. [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–93. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Parsegian A, See RE. Nature. Publishing Group; 2014. Dysregulation of Dopamine and Glutamate Release in the Prefrontal Cortex and Nucleus Accumbens Following Methamphetamine Self-Administration and During Reinstatement in Rats; pp. 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press Inc.; San Diego, CA: 1998. [Google Scholar]
- Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov-Polevoi A, Casey RL, Fender T, Garbutt JC. Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcohol Clin Exp Res. 2013;37:484–489. doi: 10.1111/j.1530-0277.2012.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Ghee SM, Chan C, See RE. Sex differences in escalation of methamphetamine self-administration: Cognitive and motivational consequences. Psychopharmacology. 2012;223:371–380. doi: 10.1007/s00213-012-2727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Piloni G, Lopez-Hidalgo M, Martinez-Lorenzana G, Rodriguez-Jimenez J, Condes-Lara M. GABA-mediated oxytocinergic inhibition in dorsal horn neurons by hypothalamic paraventricular nucleus stimulation. Brain Res. 2007;1137:69–77. doi: 10.1016/j.brainres.2006.12.045. [DOI] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossoni E, Feng J, Tirozzi B, Brown D, Leng G, Moos F. Emergent synchronous bursting of oxytocin neuronal network. PLoS Comput Biol. 2008;4:e1000123. doi: 10.1371/journal.pcbi.1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z, Babarczy E, Krivan M, Szabo G, Kovacs GL, Barth T, Telegdy G. Selective attenuation of cocaine-induced stereotyped behaviour by oxytocin: putative role of basal forebrain target sites. Neuropeptides. 1991;19:51–56. doi: 10.1016/0143-4179(91)90073-r. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Kovacs GL. Pharmacology Biochemistry and Behavior. Elsevier Inc.; 2014. Oxytocin in learning and addiction: From early discoveries to the present; pp. 3–9. [DOI] [PubMed] [Google Scholar]
- Scofield MD, Boger HA, Smith RJ, Li H, Haydon PG, Kalivas PW. Gq-DREADD Selectively Initiates Glial Glutamate Release and Inhibits Cue-induced Cocaine Seeking. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, Roberts-Wolfe D, Kalivas PW. The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacol Rev. 2016;68:816–871. doi: 10.1124/pr.116.012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kupchik YM, Kalivas PW. Optogenetic inhibition of cortical afferents in the nucleus accumbens simultaneously prevents cue-induced transient synaptic potentiation and cocaine-seeking behavior. Brain Struct Funct. 2016;221:1681–1689. doi: 10.1007/s00429-015-0997-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76:142–159. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Vargas-Martinez F, Uvnas-Moberg K, Petersson M, Olausson HA, Jimenez-Estrada I. Neuropeptides as neuroprotective agents: Oxytocin a forefront developmental player in the mammalian brain. Prog Neurobiol. 2014;123c:37–78. doi: 10.1016/j.pneurobio.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Wooters TE, Dwoskin LP, Bardo MT. Age and sex differences in the locomotor effect of repeated methylphenidate in rats classified as high or low novelty responders. Psychopharmacology. 2006;188:18–27. doi: 10.1007/s00213-006-0445-9. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, Nishimori K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neurosci. 2009;29:2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaninetti M, Raggenbass M. Oxytocin receptor agonists enhance inhibitory synaptic transmission in the rat hippocampus by activating interneurons in stratum pyramidale. Eur J Neurosci. 2000;12:3975–3984. doi: 10.1046/j.1460-9568.2000.00290.x. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, See RE, Reichel CM. Oxytocin differentially affects sucrose taking and seeking in male and female rats. Behav Brain Res. 2015;283:184–90. doi: 10.1016/j.bbr.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Sun W-L, Young AB, Lee K, McGinty JF, See RE. Oxytocin reduces cocaine seeking and reverses chronic cocaine-induced changes in glutamate receptor function. Int J Neuropsychopharmacol. 2014;18(1) doi: 10.1093/ijnp/pyu009. [DOI] [PMC free article] [PubMed] [Google Scholar]