Synopsis
The etiologic agents of blastomycosis, Blastomyces dermatitidis and B. gilchristii, belong to a group of thermally dimorphic fungi that can infect healthy and immunocompromised individuals. Following inhalation of mycelial fragments and spores into the lungs, Blastomyces spp. convert into pathogenic yeast, which facilitates evasion of host immune defenses to cause pneumonia and disseminated disease. The clinical spectrum of pulmonary blastomycosis is diverse, ranging from subclinical infection, acute pneumonia resembling bacterial community-acquired pneumonia, chronic pneumonia mimicking tuberculosis or malignancy, and acute respiratory distress syndrome. The diagnosis of blastomycosis requires a high-degree of clinical suspicion and involves the use of culture and non-culture-based fungal diagnostic tests. The site and severity of infection, and the presence of underlying immunosuppression or pregnancy influence selection of antifungal therapy.
Keywords: Blastomycosis, Dimorphic fungi, Pneumonia, Acute respiratory distress syndrome
Introduction
Blastomyces dermatitidis and Blastomyces gilchristii are the etiologic agents of blastomycosis. Blastomyces spp. are thermally dimorphic fungi that grow as a filamentous mold in the environment and as a yeast in human tissues. Blastomycosis is endemic to North America, particularly states/provinces bordering the Mississippi, Ohio and St. Lawrence Rivers, and the Great Lakes. The clinical manifestations of blastomycosis are broad, ranging from asymptomatic infection to acute respiratory distress syndrome and death. Extrapulmonary dissemination to the skin, bone, and central nervous system can occur. While culture and non-culture diagnostic tests are available, a high index of clinical suspicion is essential for prompt diagnosis. Treatment guidelines published by the Infectious Disease Society of America and the American Thoracic Society recommend the use of polyene or azole antifungal agents, with selection influenced by disease severity, site of infection, immunosuppression and pregnancy.
Mycology
Recent phylogenic analysis has divided Blastomyces into two species, B. dermatitidis and B. gilchristii.1 Blastomyces spp. belong to a group of fungi that includes Histoplasma capsulatum, Coccidioides immitis and C. posadasii, Paracoccidioides brasiliensis and P. lutzii, Sporothrix schenckii, and Talaromyces marneffei (formerly Penicillium marneffei). Blastomyces undergoes a reversible, morphologic switch between hyphae at 22–25°C and yeast at 37°C. Blastomyces yeast forms (8–20 μm diameter) are characterized by a broad-based bud (4–10 μm) and doubly refractile cell wall (Figure 1).2 While this appearance is unique among dimorphic fungi, giant forms (28 to 40 μm diameter) have been described and can be confused with Coccidioides species.3 The mycelial form is characterized by septate hyphae (1 to 2 μm diameter) that produce asexual spores (4 to 5 μm diameter).2 In contrast to the yeast, hyphal morphology is not distinct and requires molecular confirmation or transition to yeast for identification.
Figure 1. Blastomyces dermatitidis yeast.
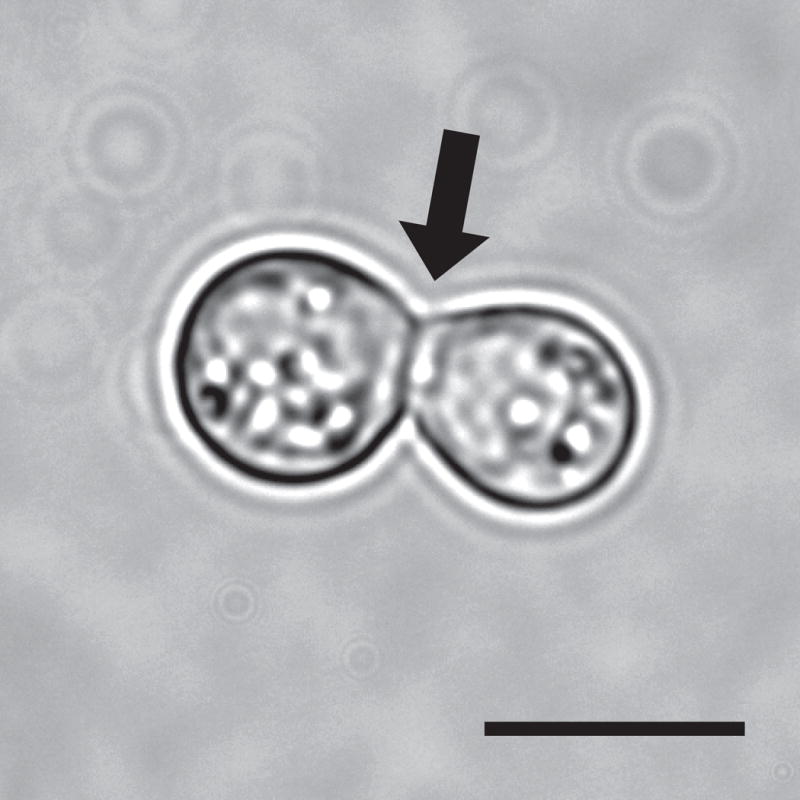
Broad-based budding yeast at 37°C. Arrow points to the broad-based bud between mother and daughter cell. Scale bar is 10 μm.
Geographic Distribution and Epidemiology
Knowledge about the geographic distribution and epidemiologic risks is important for including blastomycosis in the differential diagnosis in patients with pulmonary, cutaneous, bone, and CNS infections. In North America, Blastomyces is endemic to the midwestern, south-central, and southeastern regions of the United States and four Canadian provinces from Saskatchewan to Quebec (Figure 2). In the endemic region, Blastomyces is not uniformly distributed; rather it inhabits an ecologic niche that is characterized by forested, sandy soils with an acidic pH, decaying vegetation or organic material, and rotting wood located near water sources.4 Similar to H. capsulatum and Cryptococcus, Blastomyces can grow in bird guano. Although most infections are sporadic, occupational and recreational activities that disrupt soil (e.g., construction, exploration of beaver damns or underground forts, use of community compost pile, clearing brush or cutting trees, hunting, canoeing, boating, tubing, fishing) have all been associated with outbreaks of the disease (Table 1).4,5
Figure 2. Map of the distribution of endemic and epidemic blastomycosis in North America.
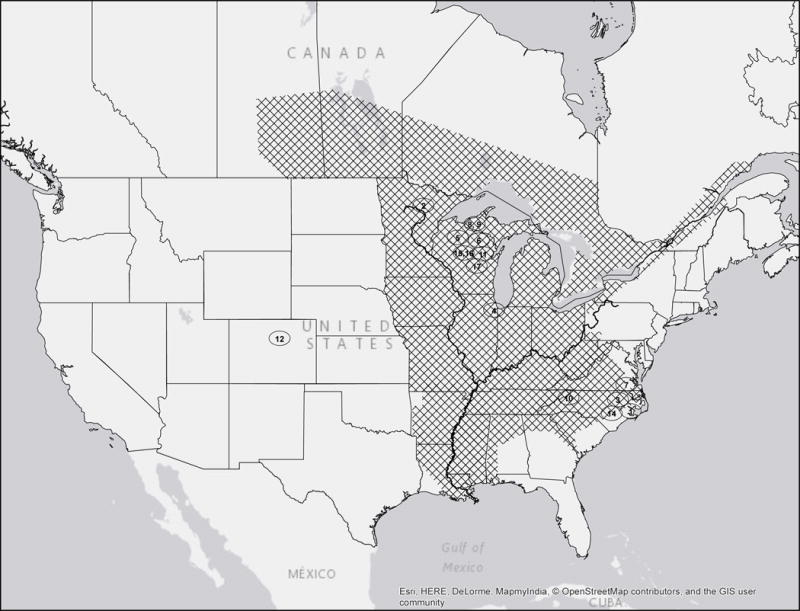
Cross-hatching denotes geographic distribution of cases. Circled numbers denote location of epidemics referred to in Table 1.
Table 1.
| Number | State | City or County | Year(s) | # Infected | Outbreak source |
|---|---|---|---|---|---|
| 1 | North Carolina | Pitt | 1953–1954 | 11 | Unknown |
| 2 | Minnesota | Bigfork | 1972 | 12 | Cabin construction |
| 3 | North Carolina | Enfield | 1975 | 5 | Harvest at peanut farm |
| 4 | Illinois | Westmont | 1974–1975 | 5 | Apartment complex construction |
| 5 | Wisconsin | Hayward | 1979 | 8 | Canoeing |
| 6 | Wisconsin | Eagle River | 1984 | 48 | Visiting an abandoned beaver lodge |
| 7 | Virginia | Southampton | 1984 | 4 | Raccoon hunting |
| 8 | Wisconsin | Portage & Waupaca | 1985 | 14 | Underground timber fort; fishing |
| 9 | Wisconsin | Vilas | 1988 | 32 | Hotel construction |
| 10 | Tennessee | Elizabethton | 1989 | 3 | Construction at rayon factory |
| 11 | Wisconsin | Oconto | 1989–1990 | 8 | Unknown |
| 12 | Colorado | Boulder | 1998 | 2 | Prairie dog relocation |
| 13 | Wisconsin | Indian Reservationt‡ | 1998–2000 | 9 | Likely related to construction/excavation |
| 14 | North Carolina | Duplin | 2001–2002 | 8 | Likely related to construction projects |
| 15 | Wisconsin | Merrill | 2006 | 21 | Community yard waste site |
| 16 | Wisconsin | Marathon | 2009–2010 | 55 | Unknown |
| 17 | Wisconsin | Waupaca | 2015 | 90 | Tubing on Little Wolf River |
references 5, 23, and www.dhs.wisconsin.gov/disease/bastomycosis.htm (accessed January 2016).
See Figure 2.
The specific Indian Reservation was not published and thus the location is not reflected in Figure 2
Rare autochthonous cases of culture-proven blastomycosis have been reported outside of North America. Approximately 100 cases have been described in 18 African nations, whereas less than 10 confirmed autochthonous cases have been reported in India.6,7 Blastomyces is not considered endemic to Central America, South America, Europe, Australia or Asia outside India.
The epidemiology of blastomycosis in North America is based mainly on retrospective studies and passive surveillance. Within endemic zones, six American states (Arkansas, Louisiana, Michigan, Minnesota, Missouri and Wisconsin) and two Canadian provinces (Manitoba and Ontario) require reporting of new cases. In North America, the annual incidence of blastomycosis ranges from 0.2 to 1.94 cases per 100,000 persons.8–11 Several hyperendemic regions exist including Kenora Ontario (117.2 human cases/100,000 population), Eagle River Wisconsin (101.3/100,000), Vilas County, Wisconsin (40.4/100,000), Washington Parish, Louisiana (6.8/100,000) and central/south central Mississippi (>5/100,000).12–15 The true incidence of blastomycosis is likely greater than the reported numbers. Reliable skin and serologic tests are not available. Moreover, approximately 50% of infected persons have subclinical or asymptomatic illness.4 Thus, epidemiologic data is limited to patients with clinically apparent infection that is diagnosed and reported. In the United States from 2007 to 2011 a total of 4,688 patients in 46 states were hospitalized for blastomycosis.16 The majority of the patients were hospitalized in the state which they resided; however, 8% of patients were admitted to hospitals outside of known endemic regions.16
The majority of blastomycosis cases occur in adults with less than 13% occurring in the pediatric population.5,17 Similar to histoplasmosis and coccidioidomycosis, blastomycosis epidemiologic studies of adults show a slight male predominance. Blastomyces is a primary fungal pathogen because it causes invasive disease in immunocompetent hosts. Indeed most patients with blastomycosis are immunocompetent. Immunocompromised by solid organ transplantation (SOT), tumor necrosis factor-α (TNF- α) inhibitors, malignancy or HIV/AIDS (human immunodeficiency virus/acquired immunodeficiency syndrome) can lead to more severe disease.18–24 The higher incidence of blastomycosis in ethnic groups including aboriginal ethnicity in Canada and Hmong populations in Wisconsin may signal genetic predisposition.9,23
Pathogenesis
The Phase Transition
The ability to convert from mold to yeast is an essential event in the pathogenesis of all dimorphic fungi including Blastomyces spp. This morphologic shift or phase transition is primarily influenced by a change in temperature and is a complex process involving global changes in transcription, metabolism, cell signaling, cell wall composition and plasma membrane lipid content.24 In the soil (22–25°C), B. dermatitidis and B. gilchristii grow as mold that produce infectious conidia (spores). After disruption of soil, often through human activity, aerosolized conidia and mold fragments inhaled into the lungs of a human host (37°C) convert to pathogenic yeast, which evade host immune defenses to cause infection. Moreover, conidia phagocytized by lung macrophages are able to survive and convert into yeast.25 This intracellular lifestyle is not unique to Blastomyces; other dimorphic pathogens including H. capsulatum, Coccidioides spp., and Paracoccidioides spp., exhibit similar intracellular preferences. For fungi such as H. capsulatum and Cryptococcus neoformans, survival in macrophages promotes dissemination; however, it is unknown if B. dermatitidis uses this “Trojan Horse” method for extrapulmonary dissemination.26
The development of molecular tools to genetically manipulate the dimorphic fungi has enabled the discovery of genes critical for the phase transition to yeast and virulence, including DRK1 (dimorphism-regulating kinase-1) and BAD1 (Blastomyces adhesion-1; formerly WI-1). DRK1 encodes a hybrid histidine kinase that is essential for the conversion of mold to yeast in B. dermatitidis, B. gilchristii, and H. capsulatum in response to a shift in temperature from 22 to 37°C.27 Deletion of DRK1 results in Blastomyces and Histoplasma cells that fail to convert to yeast and grow as hyphae at 37°C. DRK1 null mutants (DRK1Δ) also have altered distribution of cell wall carbohydrates such as α-(1,3)-glucan and chitin, and fail to express BAD1, an essential virulence factor.27 Blastomyces and Histoplasma cells with reduced transcription of DRK-1 are avirulent in a murine model of pulmonary infection.27 These findings offer genetic proof that the morphologic switch to yeast is essential for pathogenicity.
In the yeast phase, B. dermatitidis expresses BAD1, a 120-kDA protein that facilitates adhesion and immune evasion.28 BAD1 is secreted by B. dermatitidis yeast into the extracellular milieu and binds back to the cell surface via interactions with chitin in the cell wall. BAD1 functions as an adhesin that attaches yeast cells to host tissue by binding heparin sulfate.29 BAD1 enables immune evasion by repressing TNF-α production through transforming growth factor-β (TGF-β)-dependent and -independent mechanisms.30 TNF- α is an important cytokine that contributes to host defense against Blastomyces infection. In mice, neutralization of TNF- α results in progressive pulmonary blastomycosis.31 In addition to its effects on innate immunity, BAD1 alters adaptive immunity through inhibiting CD4+ T lymphocyte activation, which in turn, reduces production of interleukin-17 (IL-17) and interferon gamma (INF-γ).29 In a murine model of pulmonary infection, BAD1 null mutants (BAD1Δ) strains are avirulent.28 Moreover, the lungs of mice infected with BAD1Δ strains appear grossly normal and contain few granulomas.28 In addition to BAD-1, genes upregulated during pulmonary infection during pulmonary infection have been identified by in vivo transcriptional profiling of B. dermatitidis yeast.32
During phase transition to yeast, changes in cell wall carbohydrate composition may also contribute to virulence and immune evasion. During the transition from mold to yeast, the amount of cell wall α-(1,3)-glucan increases while β-(1,3)-glucan decreases from 40–50% in mycelia to less than 5% in yeast.33 The decreased β-(1,3)-glucan concentration in Blastomyces yeast cell walls has substantial diagnostic and therapeutic implications because it precludes the use of (1,3) β-glucan assays for diagnosis and renders echinocandins ineffective.
The transition in the opposite direction, yeast to mycelia, is important for environmental survival, mating to promote genetic diversity, and transmission to mammalian hosts. Recent genetic analyses identified a GATA transcription factor encoded by SREB that mediates the conversion from yeast to mycelia after a drop in temperature from 37 to 22°C.34 SREB null mutants (SREBΔ) exhibit a defect in the morphologic shift that corresponds to a reduction in neutral lipid (ergosterol, triacylglycerol) biosynthesis and lipid droplet formation. In B. dermatitidis and H. capsulatum, N-acetylglucosamine transporters NGT1 and NGT2 accelerate the transition to mycelia at 22°C.35
Host Response
Both the innate and adaptive immune responses are required to combat Blastomyces infection whereas humoral immunity is dispensable. Following inhalation of aerosolized conidia, alveolar macrophages and neutrophils phagocytize and kill conidia.36 However, conidia that survive phagocytosis germinate to yeast, which are more challenging for the host immune system to kill. B. dermatitidis yeast actively subvert host immune defenses by inhibiting host cell cytokine production, impairing CD4+ T lymphocyte activation, and suppressing nitric oxide production.29,30,37 Moreover, Blastomyces yeast are relatively resistant to reactive oxygen species produced by macrophages and neutrophils.37 Following recovery from blastomycosis, hosts develop cell-mediate immunity that lasts at least two years38, likely longer.
Clinical Manifestations
The clinical manifestations of Blastomycosis are heterogeneous and range from asymptomatic infection to pneumonia to acute respiratory distress syndrome (ARDS). Due to this clinical variability, Blastomycosis has been described as “the great pretender”. The lung is the primary portal of entry for aerosolized conidia following disruption of soil. Traumatic inoculation of skin (e.g., laboratory accidents) is rare but reported.40 Onset of symptoms occur 3 weeks to 3.5 months following inhalation of mycelial fragments or spores.4,41 When symptomatic, approximately 25–40% of patients will develop extrapulmonary dissemination.42 Common sites for disseminated disease are the skin, bone, genitourinary tract, and central nervous system (CNS); however, Blastomyces can infect nearly every organ in the body.5
Pulmonary Blastomycosis
Pulmonary infection is reported in more than 79% of patients with documented blastomycosis.5,8–10 The spectrum of pulmonary infection is broad and varies from subclinical pneumonia to ARDS.42,43 In both adult and pediatric populations, symptomatic pneumonia presents with fevers, chills, headache, productive or non-productive cough, dyspnea, chest pain and malaise.5,8,9,44 Acute pulmonary blastomycosis may be mild and can be mistaken for other lower respiratory tract infections including bacterial community acquired pneumonia (CAP); moreover, consolidation is the most common chest radiographic finding and is indistinguishable from CAP. Undiagnosed or untreated acute pulmonary blastomycosis can progress to ARDS or chronic pneumonia (Figures 3, 4). Symptoms and radiographic findings for chronic pulmonary blastomycosis are non-specific and can mimic other diagnoses such as lung neoplasm or tuberculosis.39 Symptoms can include fever, persistent cough, hemoptysis, night sweats, anorexia, weight loss and malaise.5,8,9,44 Chest radiography can show nodules, masses or cavitation (Figure 4). Because the clinical picture is nonspecific, blastomycosis is often not included in the differential diagnosis unless the patient has other findings such as skin lesions, fails to respond to antibacterial therapy, or has recognized risk factors for exposure to blastomycosis. Thus, symptoms may be present for several months prior to diagnosis.
Figure 3. Miliary blastomycosis and acute respiratory distress syndrome.
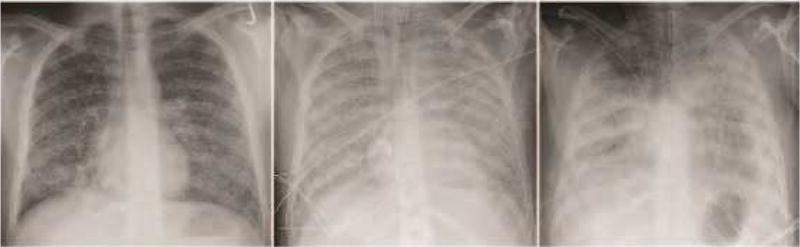
Chest radiographs demonstrating miliary blastomycosis that progressed to diffuse, dense consolidation in a patient with acute respiratory distress syndrome (ARDS).
Figure 4. Cavitary blastomycosis.
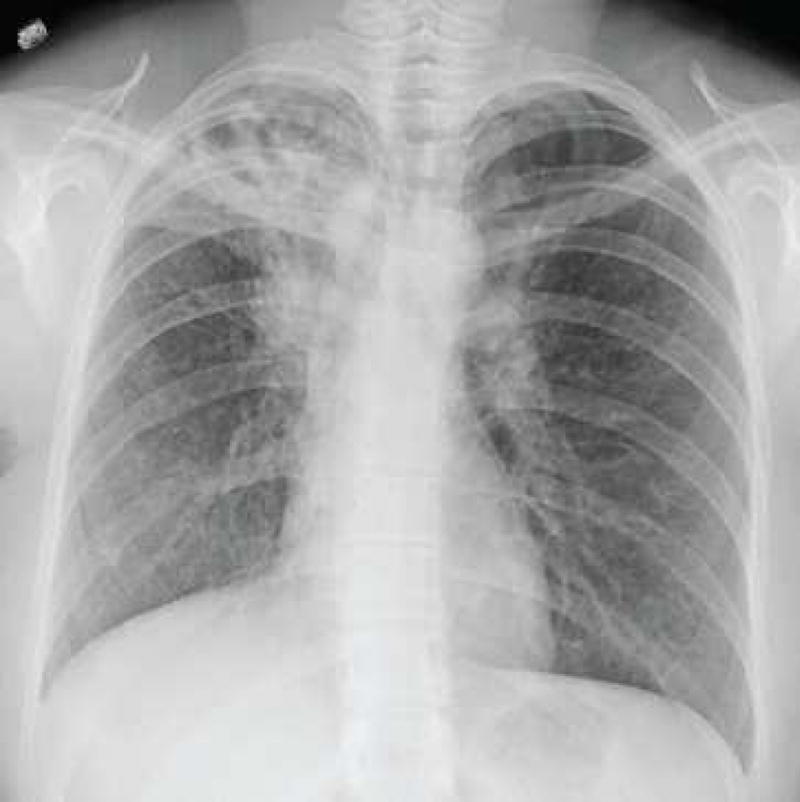
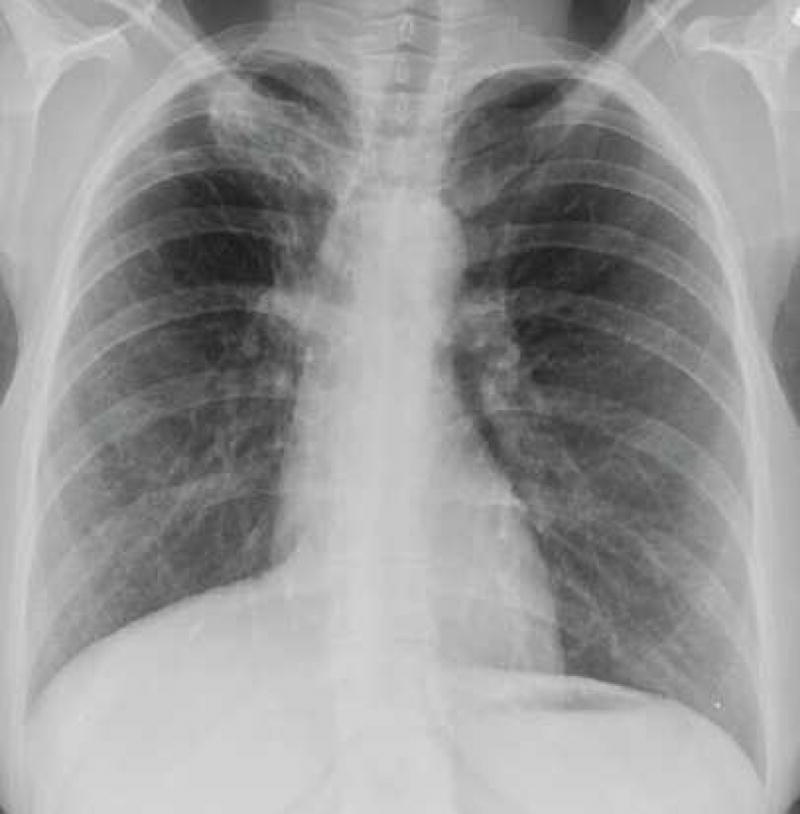
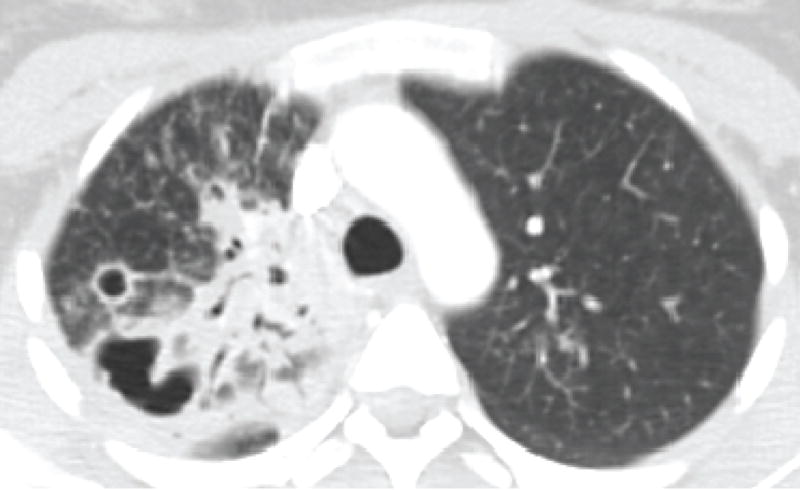
Chest radiograph (A) and corresponding chest computed tomography image (B) of a patient with multiple cavities and consolidation in the right upper lung at the time of initial clinical presentation. (C) Chest radiograph after completion of antifungal therapy demonstrates residual scarring and bronchiectasis.
A subset of patients with acute pulmonary blastomycosis can have a rapidly progressive infection resulting in respiratory failure or ARDS (Figure 3).42,43 In retrospective analyses of patients from Mississippi and Tennessee, ARDS was encountered in 8.4–14.8% of hospitalized patients with pulmonary blastomycosis.45,46 A delay in diagnosis of blastomycosis-induced ARDS is not uncommon with patients initially misdiagnosed with CAP that becomes fulminant in five to seven days or progressive CAP that fails to respond to multiple course of antibiotic therapy. Mortality due ARDS is high, often greater than 50%.45–47 In the majority of patients who die of blastomycosis-induced ARDS, the diagnosis was either not suspected or considered after the patient was moribund.44–46 Thus, early diagnosis of blastomycosis-induced ARDS is critical to decrease mortality.
Extrapulmonary and disseminated Blastomycosis
Blastomyces
Blastomyces can disseminate to any organ in the body. Evidence of dissemination will occur in approximately 25 to 40% of cases.42 Aside from rare cases of direct inoculation through penetrating trauma, accidental needle stick, or laboratory exposure, extrapulmonary blastomycosis represents disseminated disease and should be treated accordingly.
Cutaneous Blastomycosis
The skin is the most common extrapulmonary site of infection and cutaneous involvement occurs in up to 40–80% of patients with disseminated disease.48,49 Cutaneous disease often begin as papulopustular lesions that progress to ulcerative, verrucous, or crusted lesions (Figure 5). Other manifestations include violaceous nodules, plaques and abscesses.48 While erythema nodosum is common in patients with histoplasmosis or coccidioidomycosis, it is rarely described in Blastomyces infection. Cutaneous lesions can expand in an asymmetric fashion creating ulcerations and necrosis that can lead to disfigurement including permanent scarring.50 Less commonly, cutaneous blastomycosis can manifest as a draining sinus tract or ulcer from underlying osteomyelitis.48 Skin lesions can occur anywhere on the body but are often found on exposed areas including the head and extremities.48 Blastomycosis is much less likely than H. capsulatum or Paracoccidioides spp. to involve the mucous membranes; however, intra-oral, nasal and pharyngeal lesions have rarely been described.51 Although uncommon, cutaneous involvement of eyelid is the most common ophthalmologic finding.52 Endophthalmitis and orbital abscess are exceedingly rare.52 Involvement of the peri-orbital skin can be complicated by ectropion, which can require surgical correction following successful treatment of infection.50,52
Figure 5. Cutaneous ulcer.
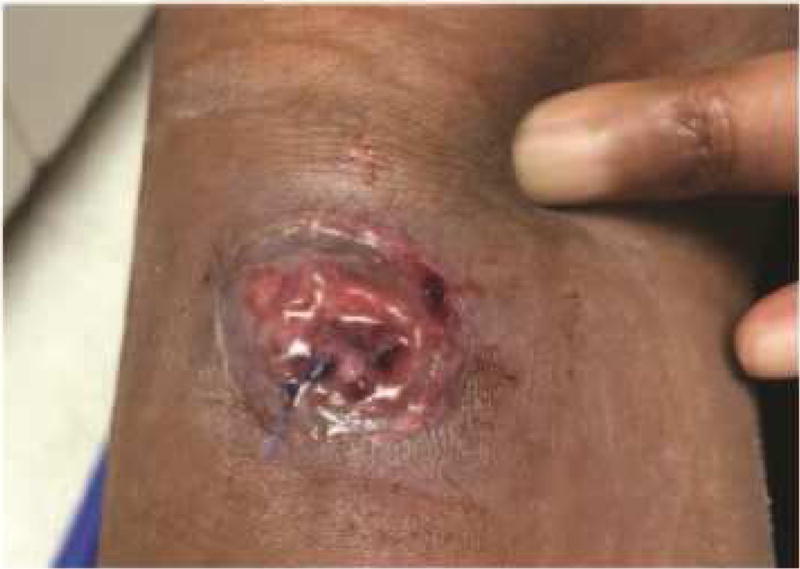
Cutaneous ulcer due to blastomycosis in a patient who received TNF-α inhibitor therapy.
Osseous Blastomycosis
The bone is the second most common site for dissemination of Blastomyces and occurs in approximately 5–25% of patients.8,44,46 Most patients with osteomyelitis have concomitant pulmonary blastomycosis. Osseous lesions are painful and can be associated with soft tissue abscess, draining sinus tracts, or cutaneous ulcers.53 Osseous invasion of Blastomyces is characterized by lytic destruction, periosteal reaction or sclerotic margins on radiography and granulomatous inflammation on histopathology.53,54 While any bone can be infected, the most common sites include the long bones, vertebrae, skull and ribs.44,53,54 Blastomycosis of the bone can mimic malignancy (e.g., sarcoma, giant cell tumor, metastases) and Pott’s disease (Mycobacterium tuberculosis).53,54 Through direct extension, infection can spread from bones to nearby joints and soft tissue resulting in septic arthritis and abscess, respectively.53,54 Progressive bone destruction can result in pathologic fracture (e.g., vertebral body collapse).54
Genitourinary Blastomycosis
Case series published in the 1950’s estimated a rate of GU dissemination to be as high as 20–30%; however, modern case series report prostate involvement in less than 10% of patients. In males, the most common sites of GU involvement are the prostate and epididymis. Symptoms of prostatitis include urinary obstruction, dysuria, perineal or suprapubic discomfort.55 Epididymitis presents with pain, scrotal swelling, testicular enlargement, and rarely, a draining sinus. In women, dissemination to the GU system can cause tubo-ovarian abscess, endometritis, and salpingitis. This can be complicated by extension to the peritoneum and omentum with or without new onset ascites.56 A single case of sexual transmission has been described following intercourse between a male with Blastomyces prostatitis and his wife who had endometrial adenocarcinoma.57
Central Nervous System Blastomycosis
CNS blastomycosis is estimated to occur in less than 5 to 10% of immunocompetent patients.58 Dissemination to the CNS results from either hematogenous seeding or direct invasion through untreated skull-based osteomyelitis and can manifest as meningitis, epidural abscess or brain abscess.58 Presenting symptoms can include headache, focal neurologic defects, confusion, visual disturbances and seizures. In patients with meningitis, cerebrospinal fluid (CSF) analysis reveals a lymphocytic or neutrophilic pleocytosis with elevated protein and hypoglycorrhachia.58 Blastomyces will grow about 45% of the time from CSF cultures; however a positive CSF Blastomyces antigen may facilitate diagnosis.58 A wide range of CNS complications have been reported including hydrocephalus, mass effect from edema, cerebral herniation, infarction, seizures, panhypopituitarism, weakness and impaired ability to function at school.5,58
Blastomycosis in Immunocompromised Hosts
HIV/AIDS
In contrast to histoplasmosis, blastomycosis is an uncommon infection in patients with HIV/AIDS. The majority of HIV patients with blastomycosis have a CD4+ T-lymphocyte count <200 cells/mm3 and two-thirds have a history of prior opportunistic infections.22 AIDS patients are more likely to have severe pulmonary disease (e.g. ARDS, miliary disease) and up to 40% have dissemination to the CNS.22 In one case series, approximately one quarter of AIDS-related blastomycosis was postulated to be caused by reactivation of latent infection.22 Prior to the era of modern antiretrovirals, mortality in patients with AIDS and blastomycosis exceeded 50%.22
Solid Organ Transplantation (SOT)
Blastomycosis is an uncommon infection in SOT recipients with a cumulative incidence of 0.13 – 0.14% in SOT patients from an endemic region.18,19 This rate is lower than the reported incidence of post-transplant histoplasmosis or coccidioidomycosis.18,19 The onset of disease post transplantation ranges from 12 days to 250 months.18,19 This variability may reflect different disease pathogenesis including (i) primary infection; (ii) reactivation of latent disease; (iii) and conversion of recently acquired, pre-transplant, asymptomatic infection to symptomatic disease.18 In contrast to histoplasmosis and coccidioidomycosis, donor-derived blastomycosis has not been reported. When compared to immunocompetent hosts, SOT patients have similar rates of disseminated disease (33–50%), but are at increased risk for severe pulmonary disease including respiratory failure and ARDS.18,19 Mortality for transplant-associated blastomycosis ranges from 33–38%, but increases to 67% in patients with ARDS. Life-long suppressive antifungal therapy is generally not required following appropriately treated blastomycosis.18
Anti-TNF-α therapy
TNF-α is a critical cytokine for host defense against blastomycosis. In murine models, antibody-mediated neutralization of TNF- α results in progressive pulmonary infection.31 Clinical data on blastomycosis in the setting of TNF- α exposure is sparse and is limited to case reports.20,59 Nevertheless, blastomycosis was listed in the 2008 warning issued by the Food and Drug Administration regarding increased risk of fulminant infections with endemic mycosis in patients receiving TNF inhibitor therapy.20
Blastomycosis in Pregnancy and Newborns
Blastomycosis in pregnancy and the newborn is rare and clinical information is limited to case reports.60–63 Women can be infected in any trimester but the disease is most frequently diagnosed in the second or third trimester.63 Case reports suggest disseminated disease (62%) is more common than isolated pulmonary infection (38%).63 Reliable data regarding the frequency of placental infection is lacking because examination by culture or histology has been conducted in only one third of clinical cases; however, placental involvement has been reported.60,63 Blastomycosis does not appear to increase risk for congenital malformations, but there is potential for transmission during the peripartum period. Neonatal pulmonary blastomycosis is rare and can be fatal.61,62 The underlying pathogenesis of neonatal blastomycosis is not well defined and may involve transplacental transmission or aspiration of infected vaginal secretions.
Diagnosis
The clinical presentation, physical exam, and the radiographic manifestations of blastomycosis are non-specific; therefore, a high index of suspicion is essential for prompt diagnosis. Delays in diagnosis are common, even in endemic areas, as few patients are correctly diagnosed at initial presentation and delays in diagnosis exceeding one month can occur in more than 40% of patients.15,39,46 A detailed history to identify possible exposures and at-risk hosts can facilitate a diagnosis. In patients with pneumonia, medical histories should include place of residence, travel, outdoor activities (e.g., fishing, canoeing, rafting), hobbies, recent home remodeling, exposure to road construction, and use of a wood burning stove or community compost pile. Blastomycosis in a household pet, such as a dog, suggests a common source of exposure and can serve as a harbinger of human infection.64 In patients with concomitant pulmonary and cutaneous disease, blastomycosis must be considered in the differential diagnosis.
Microscopic and Culture-Based Diagnostics
The most expeditious method to diagnose blastomycosis remains the examination of stained clinical specimens. While Blastomyces is not well visualized with Gram or hematoxylin and eosin (H&E) stains, sputum or tissue samples stained with 10% potassium hydroxide, calcofluor white, Gomori methenamine silver (GMS) or periodic acid-Schiff (PAS) can facilitate visualization of the characteristic Blastomyces yeast.49 The discovery of the characteristic yeast forms (8 to 20 μM) with broad-based budding and a doubly refractile cell wall can lead to a presumptive diagnosis of blastomycosis before the results of culture and non-culture tests are available. In one case series, the use of appropriately stained clinical specimens identified nearly 80% of culture-confirmed cases.65 Despite the effectiveness of fungal-specific stains in diagnosis, this technique is often underutilized.66 In tissue specimens, the presence of neutrophilic infiltration with noncaseating granulomas (i.e., pyogranulomatous inflammation) can suggest blastomycosis and thorough microscopic examination for Blastomyces yeast should be performed.
Culture of Blastomyces provides a definitive diagnosis. In the setting of pulmonary blastomycosis, the yield of culture from invasive bronchoscopy is excellent. One study demonstrated a 92% diagnostic yield for bronchoscopy.66 Even noninvasive methods including cultures from sputum, tracheal secretion or gastric washings yielded Blastomyces growth in 86% of samples.66 Specialized media including Sabouraud dextrose agar, potato dextrose agar, and brain-heart infusion media are required for growth.49 Incubator temperatures used in most clinical laboratories (25°C to 30°C) promote the growth of Blastomyces as a mold. Although highly specific, Blastomyces grows slow in culture. Fungal colonies take an average of 5 to 14 days to be visualized; however, when burden of infection is low, growth can take longer.49
Non-culture diagnostics
Classic antibody testing by complement fixation (CF) or immunodiffusion (ID) is not clinically useful for the diagnosis of blastomycosis due to poor sensitivity and specificity.41 A newer enzyme immunoassay (EIA) that uses microplates coated with BAD1 protein has enhanced sensitivity (87%) and specificity (94–99%); however, it is not yet commercially available.67 As BAD1 is unique to Blastomyces, BAD1 assays can distinguish between histoplasmosis and blastomycosis.67
An antigen assay that detects a galactomannan component in the cell wall of Blastomyces has supplanted CF and ID, and can be used to test urine, serum, BAL fluid, and CSF specimens.67–69 Sensitivity of antigenuria in patients with proven disease is 76.3 – 92.9% and specificity is 79.3%.69–71 False-positives can occur in the setting of other fungal infections such as histoplasmosis, paracoccidioidomycosis and penicilliosis (talaromycosis).67 The clinical impact of a false positive test is often minimal because paracoccidioidomycosis and penicilliosis (talaromycosis) can be removed from the differential diagnosis if the patient has not traveled to Central and South America (paracoccidioidomycosis), or Southeast Asia and China (talaromycosis). Moreover, the treatment of blastomycosis is similar to histoplasmosis. Serial urine antigen concentrations can be used to monitor response to treatment.71 Following initiation of therapy, a rise in antigenuria can occur (median of 11 days), which is followed by progressive decline in antigen titer with successful therapy.71 Initial post-treatment increase in titer may be reflect increased urinary excretion of antigen due to fungal cell death.71
Radiographic Manifestations
There are no pathognomonic radiographic patterns for pulmonary blastomycosis. Radiographic findings are nonspecific and may mimic bacterial pneumonia, tuberculosis or malignancy. Radiographic abnormalities may include diffuse airspace disease, consolidation, nodular masses, interstitial disease, cavitation or miliary disease (Figures 3, 4).72 Consolidation is the most common radiographic findings and may be present in the absence of pulmonary symptoms.72 Calcified lung lesions, hilar/mediastinal adenopathy, and pleural effusions are uncommon.72 MRI is the preferred imaging modality for CNS disease and is frequently abnormal in patients with CNS blastomycosis.58
Treatment
Guidelines for the diagnosis and treatment of blastomycosis are published by the Infectious Disease Society of America and the American Thoracic Society (Table 2).42,43 Treatment recommendations are based on the site and severity of infection, host immune status, and pregnancy. Antifungal treatment is recommended for all patients diagnosed with blastomycosis, including those with resolution of clinical symptoms before receiving therapy.42,43 Prior to the initiation of therapy, baseline evaluation of hematologic, hepatic and renal function should be obtained. Careful review of all medications is required to limit drug interactions commonly associated with azole antifungals. Itraconazole, voriconazole, posaconazole, and fluconazole can lengthen the QT interval, especially when administered with other medications that prolong the QT interval. In contrast, isavuconazole can shorten the QT interval and is contraindicated in patients with familial short QT syndrome. Itraconazole has a negative inotropic effect and the potential to exacerbate existing congestive heart failure (CHF) and should be used with caution in patients with ventricular dysfunction.73 Azole antifungals increase the serum concentration of HMG-CoA reductase inhibitors metabolized via cytochrome P450 3A4, which can increase the risk for statin-induced rhabdomyolysis. Pravastatin can be safely used with azoles because it is not metabolized by P450 3A4.42 Other significant azole drug-drug interactions include immunosuppressive medications, dihydropyridine calcium channel blockers, sulfonylureas, and anticonvulsants. Due to adverse effects of azole exposure on pregnancy including teratogenicity, all females of childbearing age should be screened for pregnancy.74,75
Table 2.
Summary of clinical practice guidelines for antifungal therapy against blastomycosis.
| Site of Infection | Disease severity | Initial therapy | Step-down therapy |
|---|---|---|---|
| Pulmonary blastomycosis | Mild to moderate | Oral itraconazole 200mg 3× daily for 3 days and then 1× or 2× daily for 6–12 monthsa | Not applicable |
| Moderately severe to severe | Lipid formulation of AmB 3–5mg/kg daily or AmB deoxycholate 0.7–1 mg/kg daily for 1–2 wks or until improvement is noted | Oral itraconazole 200mg 3× daily for 3 days and then 2× daily for 6–12 monthsa | |
| Disseminated or extrapulmonary blastomycosis | Mild to moderate | Oral itraconazole 200mg 3× daily for 3 days and then 1× or 2× daily for 6–12 monthsa,b | Not applicable |
| Moderately severe to severe | Lipid formulation of AmB 3–5mg/kg daily or AmB deoxycholate 0.7–1 mg/kg daily for 1–2 wks or until improvement is noted | Oral itraconazole 200mg 3× daily for 3 days and then 2× daily for at least 12 monthsa,b | |
| Central nervous system disease | Lipid formulation AmB 5 mg/kg per day for 4–6 wks | Options include: 1) Oral fluconazole 800mg daily 2) Oral itraconazole 200mg 2× or 3× daily 3) Voriconazole (200–400 mg 2× daily) Treatment should continue for at least 12 months and until resolution of CSF abnormalities |
|
| Immunocompromised patients | Lipid formulation of AmB 3–5mg/kg daily or AmB deoxycholate 0.7–1 mg/kg daily for 1–2 wks or until improvement is noted | Oral itraconazole 200mg 3× daily for 3 days and then 2× daily for at least 12 monthsa,c | |
| Pregnant women | All disease | Lipid formulation of AmB 3–5 mg/kg per day | Azoles should be avoided due to risks of teratogenicity and spontaneous abortion |
| Newborn | All disease | AmB deoxycholate 1.0 mg/kg per day | Not applicable |
| Children | Mild to moderate (non-meningeal) | Oral itraconazole 10 mg/kg per day (max of 400 mg/d) for 6–12 monthsa | Not applicable |
| Severe | AmB deoxycholate 0.7–1.0 mg/kg daily or lipid AmB at 3–5 mg/kg daily until improvement | Oral itraconazole 10 mg/kg per day (max of 400 mg/d) for 12 monthsa |
Therapeutic drug monitoring is required with goal serum levels (sum of itraconazole + hydroxy-itraconazole concentrations) of ≥ 1 μg/mL
Osteoarticular blastomycosis should be treated with at least 12 months total antifungal treatment
Life-long suppressive therapy with oral itraconazole, 200 mg/day may need to be considered in select patients including those with immunosuppression that cannot be reversed and in those who experience relapse despite appropriate therapy.
Amphotericin B (AmB)
Polyene AmB formulations are recommended for the treatment of patients with severe pulmonary infection, disseminated disease, CNS involvement, and underlying immunosuppression (eg., HIV/AIDS, SOT). AmB is also the first line agent for neonates and pregnant women.42,43 AmB deoxycholate has a long track record of clinical success with high cure rates.15,42 Despite well-demonstrated efficacy, the use of AmB is associated with significant cumulative toxicity. Nephrotoxicity is the most common treatment-limiting toxicity and occurs in more than 30% of treated patients.76 Other adverse effects include infusion reactions (e.g., fever, rigors, hypoxia, nausea, vomiting, hypertension, hypotension) and electrolyte disturbances (hypokalemia, hypomagnesemia).76 The risks of nephrotoxicity can be minimized by 0.9% normal saline infusions administered before and after AmB, and by avoidance of diuretics and nephrotoxic agents. Most patients require scheduled replacement potassium and magnesium to offset renal loss of these electrolytes. Frequent monitoring of electrolytes and creatinine is essential during AmB therapy (e.g., at least 2–3 times per week). Lipid AmB preparations (e.g. liposomal amphotericin, AmB lipid complex, and AmB colloidal dispersion) are preferred over AmB deoxycholate because these formulation have lower rates of nephrotoxicity. For CNS blastomycosis, liposomal amphotericin is the preferred polyene because it has the best penetration of the blood-brain barrier among the lipid formulations.42
Triazoles
In contrast to AmB, azole antifungal agents are fungistatic against Blastomyces. Itraconazole is the first-line agent for the treatment of mild to moderate, non-CNS blastomycosis, and for step-down therapy following induction treatment with AmB.42,43 Oral itraconazole can be prescribed as a solution or a capsule; however, administration of these formulations is not equivalent. Therapeutic drug monitoring (TDM) is important to optimize itraconazole dosing because serum concentrations are influenced by formulation, dosage, and interpatient variability in drug metabolism. Serum concentrations are approximately 30% higher with the use of solution than with capsule formulation.42 Itraconazole solution can be taken without regard to food and does not require gastric acidity for absorption. In contrast, itraconazole capsules must be taken with food and an acidic beverage to maximize absorption.42,43 Therefore, in patients who are taking H2-blockers or proton-pump inhibitors, itraconazole solution is the preferred formulation. Itraconazole levels should be obtained after two weeks of therapy when a steady state concentration is reached. Due to a long half-life of approximately 24-hours, serum specimens for TDM can be obtained at any time, independent of when the itraconazole dose was administered. Total itraconazole level is calculated by adding itraconazole and hydroxy-itraconazole concentrations with a goal level between 1 – 5.5 Hg/mL. Hydroxy-itraconazole, which is a metabolite of itraconazole, has antifungal activity. Serum levels of ≥ 10.0 μg/mL are unnecessary and associated with drug toxicity.42 Liver function tests should be obtained at baseline, 2 and 4 weeks into therapy and then every 3 months thereafter.42
Newer triazoles including voriconazole, posaconazole and isavuconazole have activity against B. dermatitidis77–79 Voriconazole should be taken in the absence food to optimize absorption. The goal serum trough concentration for voriconazole is between 1 and 5.5 μg/mL.80 The absorption of posaconazole solution is maximized by high-fat meals, whereas posaconazole delayed-release tablets are not affected by food or gastric acid inhibitors. The target posaconazole level is not well defined but most experts recommend levels greater than 0.5 to 1 μg/mL.80 Isavuconazole capsules can be administered without regard to food or stomach acidity and TDM is not needed. Parental formations are available for voriconazole, posaconazole, and isavuconazole. Voriconazole and posaconazole have been successfully used to treat blastomycosis including the use of voriconazole for CNS infection.77,78
Steroids and ARDS
Despite appropriate antifungal therapy, the mortality of blastomycosis-induced ARDS remains high.45–47 Case reports have suggested the potential for adjunctive steroids to improve survival; however, a recent retrospective analysis of 43 patients (1992–2014) with ARDS due to blastomycosis did not demonstrate reduced mortality in patients who received steroids.81–83 Nevertheless, additional research is needed regarding the dose, duration and efficacy of adjuvant steroids in ARDS.
Mortality
Large case series from Wisconsin and Manitoba report a case fatality rate between 4.3 and 6.3%.16,17 Mortality has been associated with a shorter duration of symptoms likely suggesting more fulminant presentation and a compromised immune status of the host. Blastomycosis-induced ARDS is associated with high mortality rate even in patients receiving appropriate antifungal treatment.18,45–47,83 The mortality of blastomycosis in AIDS patients in the absence of immune reconstitution is nearly 40% and most deaths occur within three weeks of diagnosis.22 Similarly, the mortality of patients immunosuppressed by solid organ transplantation is 33–38% and increases in the setting of respiratory failure.18,19
Conclusion
Blastomycosis is often a diagnostic and therapeutic challenge. Even in endemic areas, the non-specific clinical manifestations of blastomycosis will frequently lead to a delay in diagnosis. For physicians within areas of Blastomyces endemicity, certain clinical characteristics should trigger suspicion including (i) unresolving pneumonia despite appropriate CAP management; (ii) simultaneous pulmonary/cutaneous infection; (iii) ARDS; and (iv) illness following recognizable risk factors for Blastomyces exposure. The knowledge that Blastomyces spp. can infect, and disseminate, in both the immunocompromised and the immunocompetent is essential. An understanding of phase transition will remind clinicians that β-(1,3)-glucan assays and echinocandin antifungals have no role in diagnosis or therapy of blastomycosis. And ultimately, an awareness of common issues confronting clinicians using polyene and azole antifungals will decrease risks associated with treatment.
Key Points.
Blastomyces dermatitidis and B. gilchristii are the etiologic agents of blastomycosis.
Blastomyces spp. infect healthy and immunocompromised hosts.
Pulmonary manifestations of blastomycosis range from subclinical infection to acute respiratory distress syndrome.
Diagnosis of blastomycosis requires a high degree of clinical suspicion and involves the use of culture and non-culture diagnostic methods.
Blastomycosis should be considered in patients who live or visit regions where Blastomyces is endemic and have unresolving pneumonia despite antibiotic therapy, concomitant pulmonary and cutaneous infection, acute respiratory distress syndrome, or a compatible illness following recognizable risk factors for Blastomyces exposure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We have nothing to disclose.
References
- 1.Brown EM, McTaggart LR, Zhang SX, Low DE, Stevens DA, Richardson SE. Phylogenetic analysis reveals a cryptic species Blastomyces gilcristii, sp. nov. within the human pathogenic fungus. Blastomyces dermatitidis PLoS ONE. 2013;8(3):e59237. doi: 10.1371/journal.pone.0059237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf PL, Russel B, Shimoda A, editors. Section X: Identification of Dimorphic Fungi Causing Systemic Mycosis. New York: John Wiley & Sons; 1975. Practical Clinical Microbiology and Mycology: Techniques and Interpretations; pp. 486–488. [Google Scholar]
- 3.Wu SJ, Valyi-Nagy T, Engelhard HH, et al. Secondary intracerebral blastomycosis with giant yeast forms. Mycopathologia. 2005;160(3):253–725. doi: 10.1007/s11046-005-0147-6. [DOI] [PubMed] [Google Scholar]
- 4.Klein BS, Vergeront JM, Weeks RJ, et al. Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N Engl J Med. 1986;314(9):529–534. doi: 10.1056/NEJM198602273140901. [DOI] [PubMed] [Google Scholar]
- 5.Gauthier G, Klein BS. Chapter 198 – Blastomycosis. In: Cherry JD, Harrison GJ, editors. Feigin and Cherry’s Textbook of Pediatric Infectious Disease. Seventh. Philadelphia (PA): Elsevier Saunders; 2014. pp. 2723–2743. [Google Scholar]
- 6.Baily GG, Robertson VJ, Neill P, Garrido P, Levy LF. Blastomycosis in Africa: clinical features, diagnosis, and treatment. Rev Infect Dis. 1991;13(5):1005–1008. doi: 10.1093/clinids/13.5.1005. [DOI] [PubMed] [Google Scholar]
- 7.Randhawa HS, Chowdhary A, Kathuria S, et al. Blastomycosis in India; report of an imported case and current status. Med Mycol. 2013;51(2):185–192. doi: 10.3109/13693786.2012.685960. [DOI] [PubMed] [Google Scholar]
- 8.Carlos WG, Rose AS, Wheat LJ, et al. Blastomycosis in Indiana: digging up more cases. Chest. 2010;138(6):1377–1382. doi: 10.1378/chest.10-0627. [DOI] [PubMed] [Google Scholar]
- 9.Crampton TL, Light RB, Berg GM, et al. Epidemiology and clinical spectrum of blastomycosis diagnosed at Manitoba hospitals. Clin Infect Dis. 2002;34(10):1310–1316. doi: 10.1086/340049. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Blastomycosis – Wisconsin, 1986–1995. MMWR Morb Mortal Wkly Rep. 1996;45(28):601–603. [PubMed] [Google Scholar]
- 11.Furcolow ML, Chick EW, Busey JP, Menges RW. Prevalence and incidence studies of human and canine blastomycosis. 1. Cases in the United Stated, 1885–1968. Am Rev Respir Dis. 1970;102(1):60–67. doi: 10.1164/arrd.1970.102.1.60. [DOI] [PubMed] [Google Scholar]
- 12.Baumgardner DJ, Buggy BP, Mattson BJ, Burdick JS, Ludwig D. Epidemiology of blastomycosis in a region of high endemicity in north central Wisconsin. Clin Infect Dis. 1992;15(4):629–635. doi: 10.1093/clind/15.4.629. [DOI] [PubMed] [Google Scholar]
- 13.Dwight PJ, Naus M, Sarsfield P, Limerick B. An outbreak of human blastomycosis: the epidemiology of blastomycosis in the Kenora catchment region of Ontario, Canada. Can Commun Dis Rep. 2000;26(10):82–91. [PubMed] [Google Scholar]
- 14.Lowry PW, Kelso KY, McFarland LM. Blastomycosis in Washington Parish, Louisiana 1976–1985. Am J Epidemiology. 1989;130(1):151–159. doi: 10.1093/oxfordjournals.aje.a115307. [DOI] [PubMed] [Google Scholar]
- 15.Chapman SW, Lin AC, Hendricks KA, et al. Endemic blastomycosis in Mississippi: epidemiologic and clinical studies. Semin Respir Infect. 1997;12(3):219–228. [PubMed] [Google Scholar]
- 16.Seitz AE, Younes N, Steiner CA, Prevots DR. Incidence and trends of blastomycosis-associated hospitalizations in the United States. PLoS ONE. 2014;9(8):e105466. doi: 10.1371/journal.pone.0105466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris S, Brophy J, Richardson SE, et al. Blastomycosis in Ontario, 1994–2003. Emerg Infect Dis. 2006;12:274–279. doi: 10.3201/eid1202.050849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gauthier GM, Safdar N, Klein BS, Andes Dr. Blastomycosis in solid organ transplant recipients. Transpl Infect Dis. 2007;9(4):310–317. doi: 10.1111/j.1399-3062.2007.00227.x. [DOI] [PubMed] [Google Scholar]
- 19.Grim SA, Proria L, Miller R, et al. A multicenter study of histoplasmosis and blastomycosis after solid organ transplantation. Transpl Infect Dis. 2012;14(1):17–23. doi: 10.1111/j.1399-3062.2011.00658.x. [DOI] [PubMed] [Google Scholar]
- 20.Smith JA, Kauffman CA. Endemic fungal infections in patients receiving tumour necrosis factor-alpha inhibitor therapy. Drugs. 2009;69(11):1403–1415. doi: 10.2165/00003495-200969110-00002. [DOI] [PubMed] [Google Scholar]
- 21.Pappas PG. Blastomycosis in the immunocompromised patient. Semin Respir Infect. 1997;12(3):243–251. [PubMed] [Google Scholar]
- 22.Pappas PG, Pottage JC, Powderly WG, et al. Blastomycosis in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1992;116(10):847–853. doi: 10.7326/0003-4819-116-10-847. [DOI] [PubMed] [Google Scholar]
- 23.Roy M, Benedict K, Deak E, Kirby M, et al. A Large Community Outbreak of Blastomycosis in Wisconsin with Geographic and Ethnic Clustering. Clin Infect Dis. 2013;57(5):655–662. doi: 10.1093/cid/cit366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gauthier GM, Klein BS. Insights into fungal morphogenesis and immune evasion. Microbe. 2008;3:416–423. doi: 10.1128/microbe.3.416.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterkel AK, Mettelman R, Wüthrich M, Klein BS. The unappreciated intracellular lifestyle of Blastomyces dermatitidis. J Immunol. 2015;194(4):1796–1805. doi: 10.4049/jimmunol.1303089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charlier C, Nielsen K, Daou S, Brigitte M, Chretien F, Dromer F. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun. 2009;77(1):120–127. doi: 10.1128/IAI.01065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nemecek JC, Wüthrich M, Klein BS. Global control of dimorphism and virulence in fungi. Science. 2006;312(5773):583–588. doi: 10.1126/science.1124105. [DOI] [PubMed] [Google Scholar]
- 28.Brandhorst TT, Wüthrich M, Warner T, Klein B. Targeted gene disruption reveals an adhesion indispensable for pathogenicity of Blastomyces dermatitidis. J Exp Med. 1999;189(8):1207–1216. doi: 10.1084/jem.189.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandhorst TT, Roy R, Wüthrich M, et al. Structure and function of a fungal adhesion that binds heparin and mimics thrombospondin-1 by blocking T cell activation and effector function. PLoS Pathog. 2013;9(7):e1003464. doi: 10.1371/journal.ppat.1003464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finkel-Jimenez B, Wüthrich M, Klein BS. BAD1, an essential virulence factor of Blastomyces dermatitidis, suppress hosts TNF-α production through TGF-β-dependent and –independent mechanisms. J Immunol. 2002;168(11):5746–5755. doi: 10.4049/jimmunol.168.11.5746. [DOI] [PubMed] [Google Scholar]
- 31.Finkel-Jimenez, Wüthrich M, Brandhorst T, Klein BS. The WI-1 adhesin blocks phagocyte TNF-α production, imparting pathogenicity on Blastomyces dermatitidis. J Immunol. 2001;166(4):2665–2673. doi: 10.4049/jimmunol.166.4.2665. [DOI] [PubMed] [Google Scholar]
- 32.Muñoz JF, Gauthier GM, Desjardins CA, et al. The dynamic genome and transcriptome of the human fungal pathogen Blastomyces and close relative Emmonsia. PLoS Pathog. 2015;11(10):e1005493. doi: 10.1371/journal.pgen.1005493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanetsuna F, Carbonell LM. Cell wall composition of the yeast-like and mycelial forms of Blastomyces dermatitidis. J Bacteriol. 1971;106(3):946–948. doi: 10.1128/jb.106.3.946-948.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marty AJ, Broman AT, Zarnowski R, et al. Fungal morphology, iron homeostasis, and lipid metabolism regulated by a GATA transcription factor in Blastomyces dermatitidis. PLoS Pathog. 2015;11(6):31004959. doi: 10.1371/journal.ppat.1004959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilmore SA, Naseem S, Konopka JB, Sil A. N-acetylglucosamine (G1cNAc) triggers a rapid temperature-responsive morphogenetic program in thermally dimorphic fungi. PLoS Genet. 2013;9:e1003799. doi: 10.1371/journal.pgen.1003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugar AM, Picard M, Wagner R, Kornfeld H. Interactions between human bronchoalveolar macrophages and Blastomyces dermatitidis conidia: demonstration of fungicial and fungistatic effects. J Infect Dis. 1995;171(6):1559–1562. doi: 10.1093/infdis/171.6.1559. [DOI] [PubMed] [Google Scholar]
- 37.Rocco NM, Carmen JC, Klein BS. Blastomyces dermatitidis yeast cells inhibit nitric oxide production by alveolar macrophage inducible nitric oxide synthase. Infect Immun. 2001;79(6):2385–95. doi: 10.1128/IAI.01249-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein BS, Bradsher RW, Vergeront JM, Davis JP. Development of long-term specific cellular immunity after acute Blastomyces dermatitidis infection: assessments following a large point source outbreak in Wisconsin. J Infect Dis. 1990;151(1):97–101. doi: 10.1093/infdis/161.1.97. [DOI] [PubMed] [Google Scholar]
- 39.Lemos LB, Baliga M, Guo M. Blastomycosis: The great pretender can also be an opportunist. Initial clinical diagnosis and underlying disease in 123 patients. Ann Diagn Pathol. 2002;6(3):194–203. doi: 10.1053/adpa.2002.34575. [DOI] [PubMed] [Google Scholar]
- 40.Larson DM, Eckman MR, Alber RL, Goldschmidt VG. Primary cutaneous (inoculation) blastomycosis: an occupational hazard to pathologist. Am J Clinc Pathol. 1983;79(2):253–255. doi: 10.1093/ajcp/79.2.253. [DOI] [PubMed] [Google Scholar]
- 41.Klein BS, Vergeront JM, Kaufman L, et al. Serologic tests for blastomycosis: assessments during a large point-source outbreak in Wisconsin. J Infect Dis. 1987;155(2):262–268. doi: 10.1093/infdis/155.2.262. [DOI] [PubMed] [Google Scholar]
- 42.Chapman SW, Dismukes WI, Proia LA, et al. Infectious Diseases Society of America Clinical practice guidelines for the management of blastomycosis: 2008 updated by the Infectious Disease Society of America. Clin Infect Dis. 2008;46(12):1801–1811. doi: 10.1086/588300. [DOI] [PubMed] [Google Scholar]
- 43.Limper AH, Knox KS, Sarosi GA, et al. An Official American Thoracic Society Statement: Treatment of Fungal Infections in Adult Pulmonary and Critical Care patients. Am J Respir Crit Care Med. 2011;183:96–128. doi: 10.1164/rccm.2008-740ST. [DOI] [PubMed] [Google Scholar]
- 44.Fanella S, Skinner S, Trepnman E, Embil JM. Blastomycosis in children and adolescents: a 30-year experience from Manitoba. Med Mycol. 2011;49(6):627–32. doi: 10.3109/13693786.2010.547994. [DOI] [PubMed] [Google Scholar]
- 45.Lemos LB, Baliga M, Guo M. Acute respiratory distress syndrome and blastomycosis: presentation of nine cases and review of the literature. Ann Diagn Pathol. 2001;5(1):1–9. doi: 10.1053/adpa.2001.21473. [DOI] [PubMed] [Google Scholar]
- 46.Vasquez JE, Mehta JB, Agrawal R, et al. Chest. 1998;114(2):436–443. doi: 10.1378/chest.114.2.436. [DOI] [PubMed] [Google Scholar]
- 47.Meyer KC, McManus EJ, Maki DG. Overwhelming pulmonary blastomycosis associated with the adult respiratory distress syndrome. N Engl J Med. 1993;329(17):1231–1236. doi: 10.1056/NEJM199310213291704. [DOI] [PubMed] [Google Scholar]
- 48.Smith JA, Riddell J, IV, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15(5):440–449. doi: 10.1007/s11908-013-0352-2. [DOI] [PubMed] [Google Scholar]
- 49.Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23(2):367–381. doi: 10.1128/CMR.00056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saucier J, Gauthier G. Photo quiz. Verrucous lesions and ectropion in an immunocompetent individual. Clin Infect Dis. 2012;55(10):1390–1391. 1426–1428. doi: 10.1093/cid/cis661. [DOI] [PubMed] [Google Scholar]
- 51.Reder PA, Neel HB. Blastomycosis in otolaryngology: review of a large series. Laryngoscope. 1993;103(1 Pt 1):53–58. doi: 10.1288/00005537-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 52.Pariseau B, Lucarelli MJ, Appen RE. A concise history of ophthalmic blastomycosis. Ophthalmology. 2007;114(11):e27–32. doi: 10.1016/j.ophtha.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 53.Jain R, Sing K, Lamzabi I, Harbhajanka A, Gattuso P, Reddy BV. Blastomycosis of the bone: a clinicopathologic study. Am J Clin Pathol. 2014;142(5):609–616. doi: 10.1309/AJCPG2CFGHZ4URLN. [DOI] [PubMed] [Google Scholar]
- 54.Saccente M, Abernathy RS, Pappas PG, Shah HR, Bradsher RW. Vertebral blastomycosis with paravertebral abscess: report of eight cases and review of the literature. Clinc Infect Dis. 1998;26(2):413–418. doi: 10.1086/clinids/26.2.413. [DOI] [PubMed] [Google Scholar]
- 55.Seo R, Oyasu R, Schaeffer A. Blastomycosis of the epididymis and prostate. Urology. 1997;50(6):980–982. doi: 10.1016/S0090-4295(97)00406-8. [DOI] [PubMed] [Google Scholar]
- 56.Barocas JA, Gauthier GM. Peritonitis caused by Blastomyces dermatitidis in a kidney transplant recipient: case report and literature review. Transpl Infect Dis. 2014;16(4):634–641. doi: 10.1111/tid.12234. [DOI] [PubMed] [Google Scholar]
- 57.Farber E, Leahy M, Meadows T. Endometrial Blastomycosis Acquired by Sexual Contact. Obstetrics & Gynecology. 1968;32(2):195–199. [PubMed] [Google Scholar]
- 58.Bariola JR, Perry P, Pappas PG, et al. Blastomycosis of the central nervous system: a multicenter review of diagnosis and treatment in the modern era. Clin Infect Dis. 2010;50(6):797–804. doi: 10.1086/650579. [DOI] [PubMed] [Google Scholar]
- 59.Smith RJ, Boos MD, Burnham JM, McKay EM, Kim J, et al. Atypical cutaneous blastomycosis in a child with juvenile idiopathic arthritis on infliximab. Pediatrics. 2015;136(5):e1386–1389. doi: 10.1542/peds.2015-1675. [DOI] [PubMed] [Google Scholar]
- 60.MacDonald D, Alguire PC. Adult respiratory distress syndrome due to blastomycosis during pregnancy. Chest. 1990;98(6):1527–1528. doi: 10.1378/chest.98.6.1527. [DOI] [PubMed] [Google Scholar]
- 61.Maxson S, Miller SF, Tryka AF, et al. Perinatal blastomycosis: a review. Pediatr Infect Dis J. 1992;11(9):760–763. doi: 10.1097/00006454-199209000-00016. [DOI] [PubMed] [Google Scholar]
- 62.Watts EA, Gard PD, Tuthill SW. First reported case of intrauterine transmission of blastomycosis. Pediatr Infect Dis J. 1983;2(4):308–310. doi: 10.1097/00006454-198307000-00012. [DOI] [PubMed] [Google Scholar]
- 63.Lemos LB, Soofi M, Amir E. Blastomycosis and pregnancy. Ann Diagn Pathol. 2002;6(4):211–215. doi: 10.1053/adpa.2002.34729. [DOI] [PubMed] [Google Scholar]
- 64.Sarosi GA, Eckman MR, Davies SF, et al. Canine blastomycosis as a harbinger of human disease. Ann Intern Med. 1979;91(5):733–735. doi: 10.7326/0003-4819-91-5-733. [DOI] [PubMed] [Google Scholar]
- 65.Patel AJ, Gattuso P, Reddy VB. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am J Surg Pathol. 2010;34(2):256–261. doi: 10.1097/PAS.0b013e3181ca48a5. [DOI] [PubMed] [Google Scholar]
- 66.Martynowicz MA, Prakash UB. Pulmonary blastomycosis: an appraisal of diagnostic techniques. Chest. 2002;121(3):768–773. doi: 10.1378/chest.121.3.768. [DOI] [PubMed] [Google Scholar]
- 67.Richer SM, Smedema ML, Durkin MM, et al. Development of a highly sensitive and specific blastomycosis antibody enzyme immunoassay using Blastomyces dermatitidis surface protein BAD-1. Clin Vaccine Immunol. 2014;21(2):143–146. doi: 10.1128/CVI.00597-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Durkin M, Witt J, Lemonte A, Wehat B, Connolly P. Antigen assay with the potential to aid in diagnosis of blastomycosis. J Clin Microbiol. 2004;42:4873–4875. doi: 10.1128/JCM.42.10.4873-4875.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bariola JR, Hage CA, Durkin M, et al. Detection of Blastomyces dermatitidis antigen in patients with newly diagnosed blastomycosis. Diagn Msicrobiol Infect Dis. 2011;69(2):143–146. doi: 10.1016/j.diagmicrobio.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 70.Connolly P, Hage CA, Bariola JR, et al. Blastomyces dermatitidis antigen detection by quantitative enzyme immunoassay. Clin Vaccine Immunol. 2012;19(1):53–56. doi: 10.1128/CVI.05248-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frost HM, Novicki TJ. Blastomyces antigen detection for diagnosis and management of blastomycosis. J Clin Microbiol. 2015;53(11):3660–3662. doi: 10.1128/JCM.02352-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fang W, Washington L, Kumar N. Imaging manifestations of blastomycosis: a pulmonary infection with potential dissemination. Radiographics. 2007;27(3):641–655. doi: 10.1148/rg.273065122. [DOI] [PubMed] [Google Scholar]
- 73.Ahmad SR, Singer SJ, Leissa BG. Congestive heart failure associated with itraconazole. Lancet. 2001;357(9270):1766–1777. doi: 10.1016/S0140-6736(00)04891-1. [DOI] [PubMed] [Google Scholar]
- 74.De Santis M, Di Gianantonio E, Cesari E, Ambrosini G, Straface G, Clementi M. First-trimester itraconazole exposure and pregnancy outcome: a prospective cohort study of women contacting teratology information services in Italy. Drug Saf. 2009;32(3):239–244. doi: 10.2165/00002018-200932030-00006. [DOI] [PubMed] [Google Scholar]
- 75.Pursley TJ, Blomquist IK, Abraham, Andersen HF, Bartley JA. Fluconazole-induced congential anomalies in three infants. Clin Infect Dis. 1996;22(2):336–340. doi: 10.1093/clinids/22.2.336. [DOI] [PubMed] [Google Scholar]
- 76.Girois SB, Chapuis F, Decullier E, Revol GB. Adverse effects of antifungal therapies in invasive fungal infections: review and meta-analysis. Eur J Clinc Microbiol Infect Dis. 2005;24(2):119–130. doi: 10.1007/s10096-005-1281-2. [DOI] [PubMed] [Google Scholar]
- 77.Ta M, Flowers SA, Rogers PD. The role of voriconazole in the treatment of central nervous system blastomycosis. Ann Pharmacother. 2009;43(10):1696–1700. doi: 10.1345/aph.1M010. [DOI] [PubMed] [Google Scholar]
- 78.Proia LA, Harnisch DO. Successful use of posaconazole for treatment of blastomycosis. Antimicrob Agents Chemother. 2012;56(7):4029. doi: 10.1128/AAC.00359-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gonzalez GM. In vitro activities of isavuconazole against opportunistic filamentous and dimorphic fungi. Med Mycol. 2009;47(1):71–76. doi: 10.1080/13693780802562969. [DOI] [PubMed] [Google Scholar]
- 80.Andes D, Pascual A, Marchetti O. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrobial Agents Chemother. 2009;53(1):24–34. doi: 10.1128/AAC.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lahm T, Neese S, Thornburg AT, Ober MD, Sarosi GA, Hage CA. Corticosteroids for blastomycosis-induced ARDS: a report of two patients and review of the literature. Chest. 2008;133(6):1478–1480. doi: 10.1378/chest.07-2778. [DOI] [PubMed] [Google Scholar]
- 82.Plamondon M, Lamontagne F, Allard C, Pepin J. Corticosteroids as adjunctive therapy in severe blastomycosis-induced acute respiratory distress syndrome in an immunosuppressed patient. Clin Infect Dis. 2010;51(1):e1–e3. doi: 10.1086/653429. [DOI] [PubMed] [Google Scholar]
- 83.Schwartz IS, Embil JM, Sharma A, Goulet S, Light RB. Management and outcome of acute respiratory distress syndrome caused by blastomyosis: a retrospective case series. Medicine. 2016;95(18):e3538. doi: 10.1097/MD.0000000000003538. [DOI] [PMC free article] [PubMed] [Google Scholar]


