Abstract
Electronic cigarette (ECIG) nicotine delivery and other effects may depend on liquid nicotine concentration and user experience. This study is the first to examine systematically the influence of ECIG liquid nicotine concentration and user experience on nicotine delivery, heart rate, puff topography, and subjective effects. Thirty-three ECIG-experienced individuals and 31 ECIG-aïve cigarette smokers completed four laboratory conditions that consisted of two, 10-puff bouts (30-second IPI) with a 3.3 volt ECIG battery attached to a 1.5 Ohm “cartomizer” (7.3 watts) filled with 1 ml ECIG liquid. Conditions differed by liquid nicotine concentration: 0, 8, 18, or 36 mg/ml. Participants’ plasma nicotine concentration was related directly to liquid nicotine concentration and dependent on user experience with significantly higher mean plasma nicotine increases observed in ECIG-experienced individuals relative to ECIG-naïve smokers in each active nicotine condition. When using 36 mg/ml, mean plasma nicotine increase for ECIG-experienced individuals was 17.9 ng/ml (SD = 17.2) and 6.9 (SD = 7.1; p < .05) for ECIG-naive. Between-group differences were likely due to longer puffs taken by experienced ECIG users: collapsed across condition, mean puff duration was 5.6 seconds (SD = 3.0) for ECIG-experienced and 2.9 (SD = 1.5) for ECIG-naive. ECIG-use also suppressed nicotine/tobacco abstinence symptoms in both groups; the magnitude of abstinence symptom suppression depended upon liquid nicotine concentration and user experience. These and other recent results suggest that effective policies intended to limit ECIG nicotine delivery will need to account for factors in addition to liquid nicotine concentration (e.g., device power and user behavior).
Keywords: electronic cigarettes, e-cigarettes, plasma nicotine, puff topography, subjective effects
Electronic cigarettes (ECIGs) are a class of products that use an electrically-powered heating element to aerosolize a liquid, that often contains nicotine, for user inhalation (Breland et al., 2016; Grana et al., 2014; Hajek et al., 2014). The prevalence of ECIG use has been increasing globally (Singh et al., 2016; Adkinson et al., 2013), but ECIG effects (e.g., nicotine delivery, user subjective experience, health outcomes) remain unclear. This lack of clarity may be due, in part, to the variability of design features and liquid constituents across this class of products. In August 2016, the US FDA began regulating ECIGs and their “parts and components” (81 FR 28973, 2016); the European Union also regulates ECIG liquids (i.e., they cannot exceed 20 mg/ml; European Union Directive 2014/40/EU). Systematic investigations regarding how characteristics of ECIG devices, liquids, and user puffing behaviors influence ECIG effects, including nicotine delivery, may be valuable in informing future evidence-based policy decisions regarding ECIGs.
ECIGs vary markedly, but typically consist of an electric power source (e.g., battery), a heating element (called an “atomizer”), and a reservoir that stores a liquid solution. The liquid generally contains solvents such as propylene glycol (PG) and/or vegetable glycerin (VG), flavorants, and nicotine in concentrations that range from 0 to 36 mg/ml or higher (Breland et al., 2016). Importantly, varying device features (e.g., power), liquid nicotine concentration, and puff duration can alter the amount of nicotine emitted from the device (Talih et al., 2015). Similarly, the ability of an ECIG to deliver nicotine to the user may be influenced by each of these factors. For example, with regard to device power, a non-systematic evaluation of nicotine delivery in a small sample of experienced ECIG users who provided their own device and liquid demonstrated that ECIGs with mean power of 71.6W delivered significantly more nicotine than ECIGs with mean power of 8.6W, despite the lower power devices being paired with a higher nicotine concentration liquid (Wagener et al., 2017). Additionally, higher liquid nicotine concentration increases user blood nicotine concentration (Dawkins et al., 2016; also see Ramôa et al., 2016; Lopez et al., 2016a). Furthermore, experienced ECIG users obtain more nicotine than ECIG-naïve cigarette smokers (Farsalinos et al., 2015), likely reflecting differences in puff topography: experienced ECIG users take longer puffs relative to ECIG-naïve cigarette smokers (e.g., Spindle et al., 2017; Farsalinos et al., 2015; Hua et al., 2013). Thus, understanding the nicotine delivery of ECIGs as a product class will require some method of accounting for the three factors known to influence nicotine delivery: device features, liquid constituents, and user behavior (e.g., Shihadeh & Eissenberg, 2015).
Because nicotine is psychoactive, its delivery to blood likely influences ECIG-induced subjective effects. In previous clinical laboratory studies, ∼12-hour nicotine abstinent ECIG-naïve cigarette smokers reported at least partial suppression of tobacco abstinence symptoms following ECIG use (Nides et al., 2014; Dawkins et al., 2012; Vansickel et al., 2010); ∼12-hour nicotine abstinent, experienced ECIG users reported similar effects (Dawkins & Corcoran, 2014; Spindle et al., 2017; Vansickel & Eissenberg, 2013). Evaluating tobacco abstinence symptom suppression, as well as other subjective effects in current tobacco cigarette smokers is important, as the ability of these products to suppress tobacco abstinence may underlie their potential capacity to serve as cigarette substitutes in this population (e.g., Hajek, 2014; Etter, 2013). Characterizing these subjective effects in ECIG users who are not current tobacco smokers is also important, as any such effects may reveal the extent to which ECIGs support nicotine dependence in that population (e.g., Eissenberg, 2004). Thus, in addition to understanding the nicotine delivery potential of this product class, regulators may also benefit from further understanding of the factors that influence ECIG subjective effects.
The present study expands on preliminary reports that examined the influence of liquid nicotine concentration on plasma nicotine concentration (Ramôa et al., 2016; Lopez et al., 2016a) by increasing sample size, comparing across experienced ECIG users and ECIG-naïve cigarette smokers, and reporting on heart rate (HR) and subjective effects. Notably, unlike prior evaluations of ECIGs, the present study seeks to evaluate these products systematically by holding constant several important device features (e.g., battery power, heater resistance) and liquid constituents (e.g., PG:VG ratio) while manipulating liquid nicotine concentration and user experience. We hypothesized that liquid nicotine concentration and user experience would influence directly outcomes such as plasma nicotine delivery, HR, puff topography and user subjective effects.
Method
Participants
This study was approved by Virginia Commonwealth University’s (VCU’s) institutional review board and community volunteers were recruited by advertisement and word of mouth. Of the 127 individuals who provided informed consent for this study, data from 63 were not included, 41 because they were ineligible at screening and thus never participated in any sessions, and 22 because their participation was discontinued for the following reasons: 10 failed to attend scheduled sessions, six lacked venous access, three were non-compliant with pre-session abstinence criteria, one experienced an adverse event (nausea), one exhibited elevated blood pressure, and one exhibited elevated HR. Of those 64 participants whose data were included in the analysis, 33 were ECIG-experienced individuals and 31 were ECIG-naïve cigarette smokers.
Individuals were eligible to participate if they reported being healthy and aged 18–55. ECIG-experienced individuals were eligible if they reported using their ECIG for ≥ 3 months, using ≥ 1 ml of ECIG solution daily, using an ECIG liquid with a nicotine concentration ≥ 8 mg/ml, currently using ≤ 5 conventional tobacco cigarettes daily, and if they provided an expired air carbon monoxide (CO) sample with a concentration ≤ 10 ppm at screening (BreathCO monitor; Vitalograph; Lenexa, KS). The criterion of ≥ 8 mg/ml liquid nicotine concentration was intended to ensure that ECIG users were experienced with nicotine-containing liquids prior to their participation in this study. ECIG-naïve cigarette smokers were eligible if they reported using ≥ 10 conventional tobacco cigarettes daily, < 5 ECIG uses in their lifetime, and if they provided an expired CO sample with a concentration ≥ 15 ppm at screening as an indicator of current smoking status. Individuals were excluded if they reported: history of chronic disease or psychiatric condition, regular use of a prescription medication (aside from birth control), marijuana use > 10 days and alcohol use > 25 days in the past 30, and any illicit drug use (e.g. cocaine, opioids, benzodiazepines, and methamphetamine) in the past 30 days. For women, a positive pregnancy test (by urinalysis) at screening was exclusionary.
During screening, demographic information was collected and two dependence measures were administered. The Fagerström Test for Nicotine Dependence was modified such that the word “e-cigarette” appeared for ECIG-experienced individuals (Heatherton et al., 1991). For the Penn State Dependence Index, the Electronic Cigarette Dependence Index was administered to ECIG-experienced individuals and the Cigarette Dependence Index was administered to ECIG-naïve cigarette smokers (Foulds et al., 2015).
Materials
During each session, participants used an “eGo” 3.3 volt, 1000 mAh battery with a 1.5 Ohm, dual-coil, 510-style “cartomizer” (7.3 watts; cartomizer produced by SmokTech; Shenzhen, China). These device components were selected after preliminary testing revealed that their nicotine emissions approached those of a tobacco cigarette under some conditions (see Talih et al., 2015; 2017). The cartomizer was pre-loaded with 1 ml of a flavored liquid (tobacco or menthol; chosen by participants at screening), that was comprised of 70% PG and 30% VG (AVAIL Vapor, Richmond, VA). Liquid nicotine concentration differed by session (0, 8, 18, or 36 mg/ml) and was verified prior to administration and, on average, actual nicotine content was ±2 mg of labeled nicotine content.
Using an ECIG topography instrument developed at the American University of Beirut (see Spindle et al., 2015), puff topography was measured throughout each ECIG-use bout. The device uses a mouthpiece, pressure transducer, and calibrated software to detect flow-induced pressure changes that were amplified, digitized, and sampled every 100 ms. The pressure transducer and the orifice dimensions of each mouthpiece allowed measurement at puff velocities as low as 3 ml/second. This instrument does not interfere with the nicotine delivery or subjective effects observed after ECIG use (see Spindle et al., 2017).
Procedures
Participants completed four, double-blind ∼2.5-hour sessions at VCU’s Clinical Behavioral Pharmacology Laboratory. Session order was randomized and sessions were separated by a minimum of 48 hours. Prior to each session, participants were instructed to abstain from nicotine/tobacco and/or ECIG use for ≥ 12 hours. Abstinence from combustible tobacco was verified via participants’ expired air CO (≤ 10 ppm) and abstinence from ECIGs was verified retrospectively using a criterion of plasma nicotine concentration ≤ 5 ng/ml (as in Spindle et al., 2017; see below). In each session, participants completed two, 10-puff ECIG-use bouts (with 30 second inter-puff interval; IPI), separated by 60 minutes (as in Lopez, Hiler, Maloney, Eissenberg, & Breland, 2016b; Vansickel et al., 2010). A venous catheter was used to sample 7 ml of blood 10 times per session (5 min prior to and 5, 15, 30, 45, and 55 minutes after the onset of bout 1, and 5, 15, 30, and 45 minutes after the onset of bout 2). Subjective questionnaires were administered immediately following each blood sample. Physiological recording of HR occurred throughout each session and blood pressure was monitored regularly for safety but was not included as an outcome measure.
Outcome Measures
Plasma nicotine and heart rate
All blood samples were centrifuged, stored at −70°C, and analyzed for nicotine concentration with a limit of quantitation (LOQ) of 2 ng/ml (see Breland, Kleykamp, & Eissenberg, 2006) by VCU’s Bioanalytical Analysis Core Laboratories. HR was monitored every 20 seconds (Criticare Systems model 507; Waukesha, Wisconsin).
Puff topography
During each of the two 10-puff ECIG-use bouts, puff topography measures included puff number, puff duration (measured in seconds), puff volume (measured in milliliters) flow rate (i.e., puff velocity measured in milliliters per second), and inter-puff interval (IPI, measured in seconds and defined as the time between the onset of one puff and the onset of a subsequent puff as in Spindle et al., 2017, Vansickel et al., 2010). While puff number and IPI were recorded, they were held constant to 10 puffs and 30 second IPI.
Subjective questionnaires
Five questionnaires were administered at ten separate time points; four of which were administered using a computerized visual analog scale (VAS) that consisted of a word or phrase centered on a horizontal line with “not at all” on the left and “extremely” on the right. To record responses, participants clicked a mouse at any point on a horizontal line and scores were expressed as a percentage of total line length (0–100). The VAS questionnaires included the modified Hughes-Hatsukami withdrawal scale (11 items, omitting two items from the original: “Increased eating” and “Insomnia/Disturbed sleep”; Hughes & Hatsukami, 1986), the Direct Effects of Nicotine scale (10 items; Evans et al., 2006), the Direct Effects of ECIG-use scale (10 items; Pickworth, Bunker, & Henningfield, 1994; Foulds et al., 1992), and an acceptability questionnaire used to assess the extent to which the topography mouthpiece interfered with normal ECIG-use behavior (six items; Spindle et al., 2015; Blank, Disharoon, & Eissenberg, 2009). The fifth questionnaire was the 10-item Tiffany-Drobes Questionnaire of Smoking Urges Brief. Scores from the questionnaire’s individual items form two factors: intention to smoke (0–30), and anticipation of relief from withdrawal symptoms (0–24; Cox, Tiffany, & Christen, 2001). Items on the Hughes-Hatsukami withdrawal scale and Tiffany-Drobes Questionnaire of Smoking Urges Brief were modified for ECIG-experienced individuals such that whenever the word “cigarette” appeared in the original, the word “e-cigarette” appeared instead.
Data Preparation and Analysis
As in other studies (Lopez et al., 2016a; Vansickel et al., 2010), plasma nicotine values below the LOQ were replaced with the LOQ (2 ng/ml) as this approach is more conservative than assuming values below the LOQ were zero. HR data were averaged for the five minutes prior to each blood sampling and the five minutes during each ECIG-use bout. For topography data, two data cleaning procedures were performed automatically in real-time by the device’s software to correct for transducer noise prior to analysis: any two puffs that were separated by 300 ms or less were combined into one puff and any puffs with duration less than 300 ms were deleted; no record of these automatic corrections was maintained. Remaining data for each topography variable were averaged for each participant within each bout, resulting in two values for each topography measure for each participant.
Mixed Analysis of Variance (ANOVAs) with group as the between-subject factor (2 levels; ECIG-experienced or -naïve) and liquid nicotine concentration (hereafter referred to as “condition”; 4 levels) and time as the within-subject factors were conducted for plasma nicotine (10 levels of time), HR (10 levels of time), and topography (2 levels of time). Subjective effect data also had 10 levels of time except for the Direct Effects of ECIG-use and Topography Acceptability questionnaires that had 9 levels; the baseline time point was omitted from analysis because participants had not sampled the product or used the topography equipment at baseline. Separate ANOVAs were conducted to examine each individual item from all subjective questionnaires. Huynh-Feldt corrections were used to adjust for potential violations of the sphericity assumption.
For all outcome measures, to test differences across conditions within a group (e.g., to test the effects of nicotine concentration for ECIG-experienced individuals), post-hoc comparisons were made using Tukey’s Honestly Significant Difference (HSD) test to control for overall Type I error rate (Tukey, 1949). To test differences within conditions and between groups (i.e., ECIG-experienced versus -naïve individuals), planned contrasts (i.e., independent t-tests) were used to make comparisons for the timepoints immediately after each ECIG-use bout only. Because these planned contrasts were orthogonal at each timepoint, no corrections were made to type I error rate (Keppel, 1992).
Prior to conducting the main study analyses for plasma nicotine, HR, topography, and subjective effects described above, plasma nicotine data were first inspected to determine if any participants were not abstinent from nicotine/tobacco for the required ≥ 12 hours prior to the onset of any session. A criterion level of < 5 ng/ml was used for this determination (i.e., individuals with baseline plasma nicotine concentration of 5 ng/ml or higher were considered to be not abstinent; Spindle et al., 2017). Ultimately, 18 of the 33 ECIG-experienced and 21 of the 31 ECIG-naïve individuals met this criterion and were considered to have abstained from nicotine prior to each of the four sessions.
Prior to conducting the analyses described above, data were analyzed to understand whether abstinence status influenced each outcome measure within each group. For each group (ECIG-experienced and ECIG-naïve) mixed ANOVAs with abstinence status as the between-subject factor (abstinent or non-abstinent) and condition (4 levels) and time (10 levels for plasma; 10 for HR; 2 for topography; 10 for subjective variables except two questionnaires with 9 timepoints) as the within-subject factors were conducted to test for abstinence effects. In the results below, this analysis by abstinence status preceded and informed the overall analysis.
Results
Participant Characteristics
Data from 33 ECIG-experienced individuals and 31 ECIG-naïve cigarette smokers were included in the final study analyses. Neither participant age nor race differed significantly across groups (see Table 1). Collapsed across group, mean (SD) age was 30.6 (9.1) years. Forty participants were Caucasian, 15 were Black/African-American, 2 were Hispanic/Latino, 1 was Hawaiian/other Pacific Islander, 1 reported more than one race, and 5 reported “other”. As anticipated, groups differed on several demographic characteristics pertaining to eligibility criteria. For example, ECIG-experienced individuals smoked significantly fewer cigarettes/day (M = 0.2; SD = 0.8) relative to ECIG-naïve cigarette smokers (M = 16.5; SD = 9.4). While eligibility criteria allowed for some daily cigarette use (≤ 5 cigarettes per day), 29 of 33 ECIG-experienced participants reported no current cigarette use. Also ECIG-experienced individuals had significantly lower expired air CO at screening (M = 3.0 ppm; SD = 2.1; consistent with CO of non-smokers; Perkins, Karelitz, & Jao, 2013; Cropsey, Eldridge, Weaver, Villalobos, & Stitzer, 2006) relative to ECIG-naïve (M = 19.9 ppm; SD = 5.6). Furthermore, ECIG-naïve cigarette smokers had significantly less experience with ECIGs and mean (SD) lifetime ECIG use reported was 2 (1.6) uses. Also, groups differed on some demographic characteristics that did not pertain to the differing eligibility criteria. For example, significantly fewer ECIG-experienced women completed the study (n = 6) relative to ECIG-naïve (n = 13) and ECIG-experienced individuals had significantly lower scores on the Penn State Dependence Questionnaire at screening (M = 9.9; SD = 3.4; Foulds et al., 2015) relative to ECIG-naïve (M = 12.2; SD = 4.0; all ps < .05). As Table 1 shows, ECIG-experienced individuals reported that they had been using ECIGs for almost 1.5 years, used a liquid nicotine concentration of approximately 18 mg/ml on average, and consumed approximately 3.3 ml of ECIG liquid daily.
Table 1.
Demographic Characteristics by Group
| ECIG-experienced N = 33 |
ECIG-naïve N = 31 |
|||||
|---|---|---|---|---|---|---|
| Mean or N | SD | Mean or N | SD | t-statistic | p value | |
| Number Women | 6 | 13 | −2.1a | <.05 | ||
| Caucasian | 24 | 16 | −1.8a | n.s. | ||
| Age (years) | 30.3 | 8.4 | 30.8 | 9.9 | −0.3a | n.s. |
| Screen CO | 3.0 | 2.1 | 19.9 | 5.6 | −16.2a | <.05 |
| Cigarettes per day | 0.2 | 0.8 | 16.5 | 9.4 | −10.0a | <.05 |
| Volume liquid used/ day (ml) | 3.3 | 3.7 | N/A | N/A | N/A | N/A |
| Liquid concentration (mg/ml) | 17.5 | 5.4 | N/A | N/A | N/A | N/A |
| Months ECIG/cigarette use | 17.1 | 9.9 | 110.3 | 113.9 | N/A | N/A |
| FTNDc | 4.3 | 2.0 | 4.7 | 1.9 | −0.8a | n.s. |
| Penn State Dependence Indexd | 9.9 | 3.4 | 12.2 | 4.0 | −2.0b | <.05 |
| Session Flavor Menthol | 12 | 19 | −2.0a | n.s. | ||
Note: n.s. = not significant; N/A = not applicable.
df = 62
df = 57
The Fagerström Test for Nicotine Dependence (Heatherton et al., 1991) was modified such that the word “e-cigarette” appeared for ECIG-experienced individuals.
Penn State Dependence Index (Foulds et al., 2015); Electronic Cigarette Dependence Index was administered to ECIG-experienced individuals and the Cigarette Dependence Index was administered to ECIG-naïve cigarette smokers.
Plasma nicotine
Plasma nicotine data were first used to evaluate potential effects of abstinence status for ECIG-experienced and -naïve individuals. A significant main effect of abstinence status was observed for ECIG-experienced [F (1, 31) = 9.8 p < .05, ηp2= .24] and ECIG-naïve individuals [F (1, 29) = 9.6, p < .05, ηp2= .25]. Given that the criterion for determining abstinence status was baseline plasma nicotine concentration, inspection of the data revealed that for both groups, baseline plasma nicotine concentration was higher in some conditions for non-abstinent participants. For example, for ECIG-experienced participants in the 36 mg/ml condition, non-abstinent participants had a mean baseline plasma nicotine concentration of 7.1 ng/ml (SD = 5.2) while abstinent participants had a mean baseline concentration of 2.0 ng/ml (SD = 0.5; n.s., Tukey’s HSD). Similarly, for ECIG-naïve smokers in the 36 mg/ml condition, non-abstinent participants had a mean baseline plasma nicotine concentration of 5.8 ng/ml (SD = 4.9) while abstinent participants had a mean baseline concentration of 2.4 ng/ml (SD = 0.8; n.s., Tukey’s HSD). In an attempt to control for these baseline differences in plasma nicotine concentration, the same analyses (i.e., mixed ANOVAs with abstinence status as the between subject factor) were conducted using plasma nicotine concentration change scores, referred to as ‘nicotine boost’ and calculated by subtracting baseline plasma nicotine from post ECIG-use plasma nicotine values. These analyses revealed no significant interactions or main effects involving abstinence status for either group, suggesting that the observed effects of abstinence status using the raw data were a result of baseline differences. Thus, the final analyses (presented below) were conducted using nicotine boost data and included all participants.
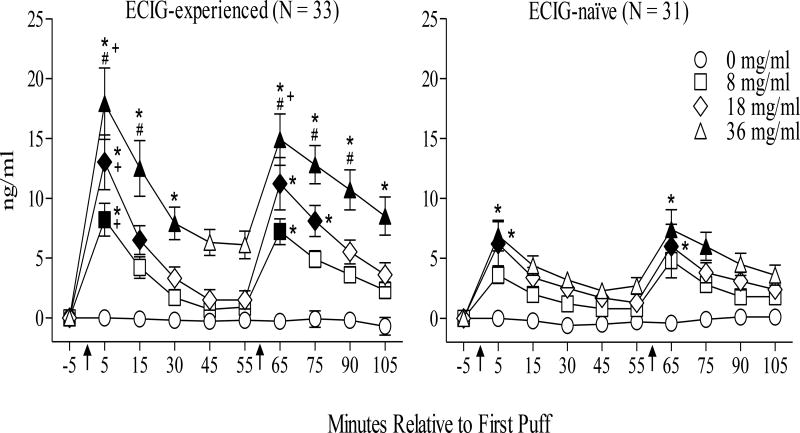
Table 2 shows the statistical analysis results for nicotine boost. There was a significant three-way condition by time by group interaction [F (27, 1674) = 2.6, p < .01, ηp2= .04] in addition to several other significant interactions and main effects (see Table 2). Figure 1 shows mean nicotine boost, over time, by condition for ECIG-experienced and -naïve individuals. For ECIG-experienced individuals, immediately after bout 1, mean (SD) nicotine boost increased significantly from baseline in all active liquid nicotine concentrations such that for 8 mg/ml plasma nicotine concentration was 8.2 ng/ml (7.8), for 18 mg/ml it was 13.0 ng/ml (6.2), and for 36 mg/ml it was 17.9 ng/ml (17.2; ps < .05, Tukey’s HSD). For ECIG-naïve cigarette smokers, immediately after bout 1, mean (SD) plasma nicotine boost increased significantly from baseline when using the 18 and 36 mg/ml conditions such that for 18 mg/ml it was 6.2 ng/ml (10.2), and for 36 mg/ml it was 6.8 ng/ml (7.1; ps < .05, Tukey’s HSD). Similar patterns in nicotine boost were observed for both groups immediately after bout 2.
Table 2.
Statistical Analyses Results for Plasma Nicotine, Heart Rate, and Subjective Measures by Group.
| Outcome Measure | Condition | Time | Group | Condition × Time | Time × Group | Condition × Group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | ηp2 | F | p | ηp2 | F | p | ηp2 | F | p | ηp2 | F | p | ηp2 | F | p | ηp2 | |
| Plasma Nicotinea | 40.3 | <.01 | .39 | 46.4 | <.01 | .43 | 10.6 | <.01 | .15 | 15.0 | <.01 | .19 | 6.7 | <.01 | .09 | 6.4 | <.01 | .09 |
| Heart Rateb | 7.9 | <.01 | .18 | 66.4 | <.01 | .64 | 0.0 | n.s. | .00 | 10.0 | <.01 | .21 | 3.3 | <.01 | .08 | 2.1 | n.s. | .06 |
| Subjective Measures Hughes-Hatsukamia | ||||||||||||||||||
| Anxious | 5.0 | <.01 | .07 | 10.0 | <.01 | .14 | 10.5 | <.01 | .15 | 1.4 | n.s. | .02 | 1.8 | n.s. | .03 | 0.6 | n.s. | .01 |
| Craving | 19.0 | <.01 | .23 | 24.1 | <.01 | .28 | 1.7 | n.s. | .03 | 4.8 | <.01 | .07 | 1.2 | n.s. | .02 | 3.6 | <.05 | .06 |
| Depression | 7.7 | <.01 | .11 | 2.1 | n.s. | .03 | 6.0 | <.05 | .09 | 1.2 | n.s. | .02 | 1.2 | n.s. | .02 | 4.7 | <.01 | .07 |
| Diff Con. | 8.6 | <.01 | .12 | 6.2 | <.01 | .09 | 3.3 | n.s. | .05 | 0.8 | n.s. | .01 | 0.6 | n.s. | .01 | 1.7 | n.s. | .03 |
| Drowsy | 6.8 | <.01 | .09 | 2.9 | <.05 | .04 | 0.8 | n.s. | .01 | 0.9 | n.s. | .02 | 1.5 | n.s. | .02 | 4.9 | <.01 | .07 |
| Hunger | 0.7 | n.s. | .01 | 15.5 | <.01 | .20 | 1.4 | n.s. | .02 | 1.2 | n.s. | .02 | 1.8 | n.s. | .03 | 1.7 | n.s. | .03 |
| Impatient | 6.2 | <.01 | .09 | 8.8 | <.01 | .12 | 8.4 | <.05 | .12 | 1.0 | n.s. | .02 | 0.4 | n.s. | .01 | 0.4 | n.s. | .01 |
| Irritable | 8.5 | <.01 | .12 | 7.6 | <.01 | .11 | 12.1 | <.01 | .16 | 0.9 | n.s. | .02 | 1.3 | n.s. | .02 | 0.0 | n.s. | .00 |
| Restless | 5.6 | <.01 | .08 | 5.7 | <.01 | .09 | 6.5 | <.05 | .09 | 1.3 | n.s. | .02 | 0.2 | n.s. | .00 | 0.2 | n.s. | .00 |
| Sweets | 0.4 | n.s. | .01 | 2.5 | <.05 | .04 | 1.4 | n.s. | .02 | 1.0 | n.s. | .02 | 1.4 | n.s. | .02 | 1.8 | n.s. | .03 |
| Urge | 20.8 | <.01 | .25 | 27.1 | <.01 | .30 | 1.7 | n.s | .03 | 4.4 | <.01 | .07 | 0.7 | n.s. | .01 | 4.4 | <.01 | .07 |
| Tiffany Drobes QSUa | ||||||||||||||||||
| Factor 1 | 17.5 | <.01 | .22 | 31.1 | <.01 | .33 | 0.74 | n.s. | .01 | 6.6 | <.01 | .09 | 1.1 | n.s. | .02 | 3.7 | <.05 | .06 |
| Factor 2 | 12.4 | <.01 | .17 | 15.7 | <.01 | .20 | 10.9 | <.01 | .15 | 1.7 | <.05 | .03 | 0.5 | n.s. | .01 | 0.8 | n.s. | .01 |
| Direct Effects of Nicotinea | ||||||||||||||||||
| Confused | 0.6 | n.s. | .01 | 1.3 | n.s. | .02 | 1.9 | n.s. | .03 | 0.7 | n.s. | .01 | 0.4 | n.s. | .01 | 0.7 | n.s. | .01 |
| Dizzy | 1.4 | n.s. | .02 | 2.0 | n.s. | .03 | 1.9 | n.s. | .03 | 1.3 | n.s. | .02 | 1.3 | n.s. | .02 | 2.0 | n.s. | .03 |
| Headache | 0.4 | n.s. | .01 | 1.2 | n.s. | .02 | 2.5 | n.s. | .04 | 0.9 | n.s. | .01 | 0.6 | n.s. | .01 | 0.3 | n.s. | .00 |
| Heart Pound | 0.3 | n.s. | .01 | 1.1 | n.s. | .02 | 3.5 | n.s. | .05 | 1.1 | n.s. | .02 | 1.2 | n.s. | .02 | 0.9 | n.s. | .02 |
| Light Headed | 1.6 | n.s. | .03 | 3.5 | <.01 | .05 | 1.7 | n.s. | .03 | 1.7 | n.s. | .03 | 0.8 | n.s. | .01 | 1.3 | n.s. | .02 |
| Nausea | 1.3 | n.s. | .02 | 1.3 | n.s. | .02 | 4.7 | <.05 | .07 | 1.5 | n.s. | .02 | 1.0 | n.s. | .02 | 0.1 | n.s. | .00 |
| Nervous | 1.0 | n.s. | .02 | 0.8 | n.s. | .01 | 8.4 | <.01 | .12 | 0.7 | n.s. | .01 | 0.7 | n.s. | .01 | 0.1 | n.s. | .00 |
| Salivate | 0.2 | n.s. | .00 | 1.1 | n.s. | .02 | 4.3 | <.05 | .07 | 0.6 | n.s. | .01 | 0.7 | n.s. | .01 | 1.4 | n.s. | .02 |
| Sweaty | 0.4 | n.s. | .01 | 0.8 | n.s. | .01 | 4.6 | <.05 | .07 | 1.2 | n.s. | .02 | 0.3 | n.s. | .01 | 3.7 | <.05 | .06 |
| Weak | 0.9 | n.s. | .01 | 0.6 | n.s. | .01 | 0.8 | n.s. | .01 | 0.8 | n.s. | .01 | 1.0 | n.s. | .02 | 1.4 | n.s. | .02 |
| Direct Effects of ECIG-Usec | ||||||||||||||||||
| Awake | 6.2 | <.01 | .09 | 2.0 | n.s. | .03 | 1.3 | n.s. | .02 | 1.5 | n.s. | .02 | 1.0 | n.s. | .02 | 3.0 | <.05 | .05 |
| Calm | 10.2 | <.01 | .14 | 7.7 | <.01 | .11 | 1.9 | n.s. | .03 | 1.7 | n.s. | .03 | 0.8 | n.s. | .01 | 2.9 | n.s. | .05 |
| Concentrate | 5.9 | <.01 | .09 | 1.2 | n.s. | .02 | 3.9 | n.s. | .06 | 1.0 | n.s. | .02 | 0.6 | n.s. | .01 | 1.7 | n.s. | .03 |
| Dizzy | 7.6 | <.01 | .11 | 4.5 | <.01 | .07 | 0.3 | n.s. | .01 | 1.2 | n.s. | .02 | 0.6 | n.s. | .01 | 0.7 | n.s. | .01 |
| Pleasant | 4.0 | <.05 | .06 | 4.7 | <.01 | .07 | 1.5 | n.s. | .02 | 0.8 | n.s. | .01 | 0.7 | n.s. | .01 | 3.7 | <.05 | .06 |
| Reduced Hunger | 6.4 | <.01 | .09 | 2.6 | <.05 | .04 | 1.0 | n.s. | .02 | 0.9 | n.s. | .01 | 0.5 | n.s. | .01 | 0.7 | n.s. | .01 |
| Right Now | 8.9 | <.01 | .13 | 9.6 | <.01 | .13 | 6.8 | <.01 | .09 | 4.2 | <.01 | .06 | 2.5 | <.05 | .04 | 2.4 | n.s. | .04 |
| Satisfy | 10.4 | <.01 | .14 | 8.8 | <.01 | .14 | 1.1 | n.s. | .02 | 1.1 | n.s. | .02 | 0.3 | n.s. | .01 | 5.9 | <.01 | .09 |
| Sick | 3.6 | <.05 | .06 | 1.3 | n.s. | .02 | 0.5 | n.s. | .01 | 0.5 | n.s. | .01 | 0.9 | n.s. | .02 | 0.3 | n.s. | .01 |
| Taste Good | 4.0 | <.01 | .06 | 5.7 | <.01 | .09 | 1.1 | n.s. | .02 | 1.0 | n.s. | .02 | 0.4 | n.s. | .01 | 1.4 | n.s. | .02 |
Note: Significant items are presented in bold; n.s = not significant; Condition*Time*Group interaction results are not displayed because they were significant for only one measure: plasma nicotine (see text for details).
df Condition (C) = (3,186); df Time (T) = (9,558); df Group (G) = (1,62); df C*T = (27,1674); df T*G = (9,558); df C*G = (3,186).
df C = (3,111); df T = (9,333); df G = (1,37); df C*T = (27,999); df T*G = (9,333); df C*G = (3,111); HR data were analyzed for abstinent participants only (see text for details).
df C = (3,186); df T = (8,496); df G = (1,62); df C*T = (24,1488); df T*G = (8,496); df C*G = (3,186).
Figure 1.
Mean ± plasma nicotine boost for 33 ECIG-experienced and 31 ECIG-naïve participants. Arrows indicate the onset of each 10-puff ECIG-use bout (30 sec IPI). Filled symbols indicate significant difference from baseline (−5 time point), asterisks (*) indicate significant differences from the 0 mg/ml condition at that time point and pound (#) symbols indicate significant differences between the 8 and 36 mg/ml condition at that timepoint (ps < .05; Tukey’s HSD). Plus signs (+) indicate significant between group differences at that time point for that concentration (only conducted on timepoints immediately post-bout; independent t-tests; ps < .05).
Planned contrasts, conducted for the timepoints immediately after bout 1 and 2, revealed significant between group differences (ECIG-experienced vs. -naïve) after bout 1 in the 8, 18, and 36 mg/ml conditions [ts (62) < −0.17; ps <05] but not in the 0 mg/ml condition. Overall, ECIG-experienced individuals obtained significantly higher plasma nicotine boost on average, immediately after bout 1 in all active nicotine conditions, and immediately after bout 2 in the 36 mg/ml condition (see Figure 1).
Heart rate
Potential effects of abstinence status on HR values were evaluated for ECIG-experienced and -naïve individuals. A significant three-way condition by time by abstinence status interaction was observed for ECIG-experienced [F (27, 837) = 1.9, p < .05, ηp2= .06], but no significant interactions or main effects of abstinence status were observed for ECIG-naïve individuals. To explore whether significant differences by abstinence status occurred due to baseline differences in HR (as seen above with plasma nicotine concentration), mixed ANOVAs with abstinence status as the between subject factor were conducted using HR difference scores and the same three-way interaction involving abstinence status remained significant [F (27, 837) = 1.9, p < .05, ηp2= .06]. Because abstinence status appeared to influence HR in ECIG-experienced participants, despite using difference scores to correct for baseline differences, final analyses were conducted using raw HR data and only abstinent participants for both groups (ECIG-experienced, N = 18; ECIG-naïve, N = 21).
As Table 2 shows, significant condition by time [F (27, 999) = 10.0, p < .01, ηp2= .21] and significant time by group [F (9, 333) = 3.3, p < .008, ηp2= .08] interactions were observed for HR, in addition to several significant main effects. Planned contrasts revealed no significant between group differences in HR at baseline or immediately after bout 1 or 2 [ts (37) > - 2.1, n.s]. For both groups, HR increased significantly from baseline immediately after bout 1 and 2 in all active liquid nicotine concentrations (8, 18, and 36 mg/ml), but not the 0 mg/ml condition. So, in the 0 mg/ml condition collapsed across group, mean (SD) HR increased (non-significantly) from 69.6 bpm (8.7) prior to bout 1 to 72.2 bpm (7.5) post-bout 1 and from 65.9 bpm (8.4) prior to bout 2 to 68.1 bpm (7.5) post-bout 2. However, in the 8 mg/ml condition, collapsed across group, mean (SD) HR increased from 66.4 bpm (6.5) prior to bout 1 to 73.0 bpm (7.4) post-bout 1 and from 65.1 bpm (6.5) prior to bout 2 to 69.9 bpm (7.8) post-bout 2 (both increases significant; ps < .05, Tukey’s HSD). In the 18 mg/ml condition, collapsed across group mean (SD) HR increased from 66.4 bpm (7.6) prior to bout 1 to 75.8 bpm (7.9) post-bout 1 and from 65.7 bpm (7.7) prior to bout 2 to 71.6 bpm (8.2) post-bout 2 (both increases significant; ps < .05, Tukey’s HSD). In the 36 mg/ml condition, collapsed across group mean (SD) HR increased from 66.6 bpm (7.1) prior to bout 1 to 77.0 bpm (8.6) post-bout 1 and from 67.3 bpm (7.8) prior to bout 2 to 72.7 bpm (9.3) post-bout 2 (both increases significant; ps < .05, Tukey’s HSD).
Puff topography
Raw data for puff topography variables of interest (i.e., puff duration, volume, and flow rate) were used to evaluate potential effects of abstinence status (because puff number was controlled at 10 puffs/bout and IPI was controlled at 30 seconds these variables are not analyzed as outcome measures). No significant interactions or main effects involving abstinence status were observed for any topography variables for either group, thus data from all participants were included in the analysis.
Mean (SD) puffing parameters for ECIG-experienced and -naïve individuals are displayed in Table 3. For puff duration a significant time by group interaction was observed [F (3, 186) = 5.42, p < .05, ηp2= .08] in addition to significant main effects of condition [F (3, 186) = 9.67, p < .01, ηp2= .14], time [F (1, 62) = 19.56, p < .001, ηp2= .24], and group [F (1, 62) = 28.28, p < .001, ηp2= .31]. Planned contrasts revealed significant between group differences indicating that ECIG-experienced individuals took significantly longer puffs relative to -naïve in all conditions and during each ECIG-use bout [ts (62) > 33, ps < .05].
Table 3.
Mean (SD) Puff Topography by Liquid Nicotine Concentration and Group for Bouts 1 and 2
| Liquid Nicotine Concentration (mg/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Bout 1 | Bout 2 | |||||||
| 0 | 8 | 18 | 36 | 0 | 8 | 18 | 36 | |
| Puff Duration (s) | ||||||||
| ECIG-experienced | 5.9+ | 5.7+ | 5.0+ | 4.7*+ | 6.2+ | 6.4+ | 5.8+ | 5.1*+ |
| (2.4) | (2.2) | (1.9) | (3.9) | (2.5) | (2.3) | (3.4) | (4.6) | |
| ECIG-naïve | 3.3 | 3.0 | 2.8* | 2.2* | 3.6 | 3.1* | 2.9* | 2.3* |
| (1.7) | (1.5) | (1.3) | (0.8) | (2.1) | (1.6) | (1.4) | (1.0) | |
| Volume (ml) | ||||||||
| ECIG-experienced | 175.7+ | 181.0+◆ | 127.0*+ | 123.3* | 206.0+ | 217.8+ | 137.4* | 123.7* |
| (149.7) | (139.6) | (80.8) | (168.1) | (202.9) | (177.3) | (87.6) | (150.0) | |
| ECIG-naïve | 100.0◆ | 101.5◆ | 86.5*◆ | 68.3* | 120.7 | 114.1 | 97.9* | 69.8* |
| (64.8) | (66.6) | (59.4) | (64.1) | (76.6) | (74.1) | (67.9) | (63.9) | |
| Flow Rate (ml/s) | ||||||||
| ECIG-experienced | 31.1 | 29.8 | 24.2 | 26.6 | 30.9 | 31.1 | 23.8+ | 27.4 |
| (24.9) | (16.7) | (11.0) | (21.3) | (20.6) | (18.2) | (10.8) | (21.1) | |
| ECIG-naïve | 32.5 | 34.1 | 29.8 | 31.2 | 36.4 | 36.7 | 34.6 | 30.7 |
| (21.7) | (18.9) | (17.9) | (28.8) | (22.7) | (20.0) | (22.6) | (25.4) | |
Note: asterisks indicate significant differences from the 0 mg/ml condition for that bout, diamond symbols
indicate across bout differences for that condition and group, and crosses
indicate significant differences between ECIG-experienced and -naïve individuals. Also, puff number (10) and IPI (30 sec) were controlled experimentally (see Method).
For puff volume a significant condition by time interaction was observed [F (3, 186) = 4.9, p < .01, ηp2 = .07] in addition to significant main effects of condition [F (3, 186) = 7.78, p < .001, ηp2= .11], time [F(1, 62) = 23.0, p < .001, ηp2= 0.27], and group [F (1, 62) = 8.7, p < .05, ηp2 = .12]. Planned contrasts revealed significant between group differences indicating that ECIG-experienced individuals took significantly larger puffs relative to -naïve during bout 1 in the 0, 8, and 18 mg/ml conditions and during bout 2 in the 0 and 8 mg/ml conditions [ts (62) > 3.1, ps < .05].
For flow rate a significant time by group interaction was observed [F (1, 62) = 4.4, p < .05, ηp2 = .07] in addition to a significant main effect of time [F (1, 62) = 7.56, p < .01, ηp2 = .11]. Planned contrasts revealed significant between group differences in the 18 mg/ml condition during bout 2 only [t (62) = -2.4, p < .03].
Subjective Measures
Raw data for each subjective questionnaire were used to evaluate potential effects of abstinence status for ECIG-experienced and -naïve individuals. No significant interactions or main effects involving abstinence status were observed for any subjective measures for either group, thus data from all participants were included in the analysis (see Table 2).
Hughes-Hatsukami Withdrawal Scale
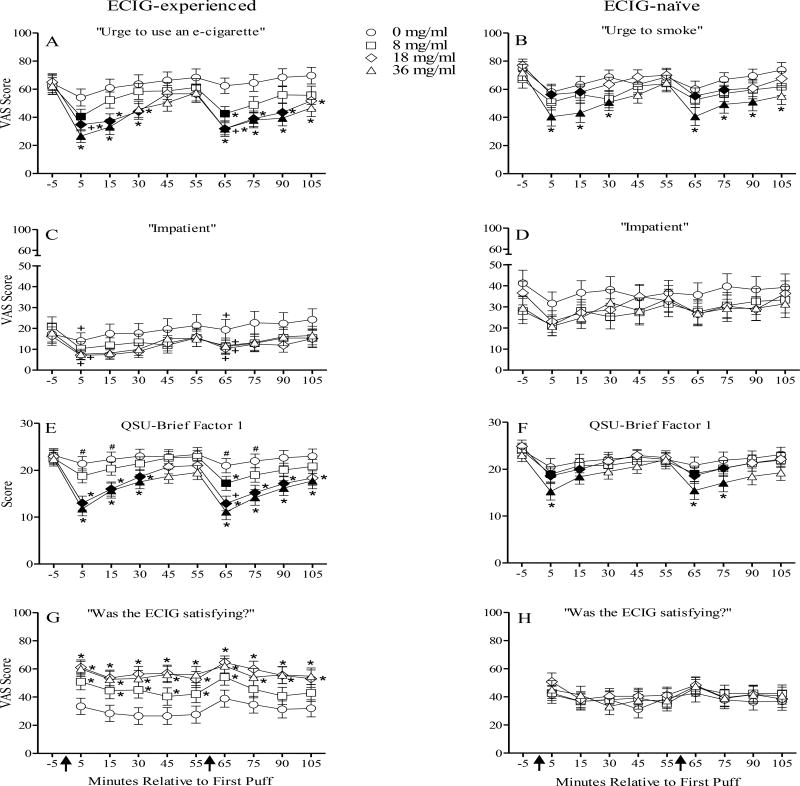
Significant condition by group interactions were observed for the items “Craving”, “Depression”, “Drowsy”, and “Urge” [Fs (3, 186) ≥ 3.6, ps < .05, ηp2s ≤ .07]; significant condition by time interactions were also observed for the items “Craving” and “Urge” [Fs (3, 186) ≥ 4.4, ps <.01, ηp2s ≤ .07]. Overall, for these items, the magnitude of the decrease in scores was dependent on condition and group such that the decrease in scores was more pronounced when using higher liquid nicotine concentrations and among ECIG-experienced individuals. For ECIG-experienced individuals, mean scores for “Urge” decreased significantly relative to baseline immediately after bout 1 and 2 in the 8, 18 and 36 mg/ml and for ECIG-naïve individuals, scores decreased significantly relative to baseline immediately after bout 1 and 2 in the 18 and 36 mg/ml conditions (Figure 2 panels A and B). For example, in the 36 mg/ml condition, mean (SD) scores decreased from 62.3 (33.4) prior to bout 1 to 26.6 (25.8) immediately after bout 1 for ECIG-experienced individuals and the scores decreased from 71.9 (32.3) immediately prior to bout 1 to 40.5 (36.4) immediately after bout 1 for ECIG-naïve individuals (ps < .05, Tukey’s HSD). A similar pattern was observed for “Craving.” Additionally, for the item “Depression”, planned contrasts revealed significant between group differences such that ECIG-naïve smokers had significantly higher scores relative to ECIG-experienced individuals at baseline and immediately after bout 1 and 2 in the 0 and 8 mg/ml conditions [ts (62) > −2.7, ps < .05]. For example, immediately after bout 1 in the 8 mg/ml condition, mean (SD) scores for “Depression” were 1.6 (3.5) for ECIG-experienced but 8.7 (15.1) for ECIG-naïve. A similar pattern was observed for the item “Drowsy”.
Figure 2.
Mean ± subjective ratings for 33 ECIG-experienced and 31 ECIG-naïve participants. Arrows and symbols are as indicated in Figure 1.
In addition to significant interactions, significant main effects of condition, time, and group were observed and are presented in Table 2. One item, “Impatient”, revealed significant main effects for condition, time and group and is presented in Figure 2 (panels C and D). Post hoc tests revealed no statistically significant differences across timepoints or conditions for either group. Planned contrasts revealed significant between group differences such that ECIG-naïve smokers had significantly higher scores for “Impatient” immediately after bout 1 in the 0, 18, and 36 mg/ml conditions and immediately after bout 2 in all conditions relative to ECIG-experienced individuals [ts (62) > −2.6, ps < .05]. For example, in the 36 mg/ml condition, mean (SD) scores for ECIG-experienced individuals were 7.6 (14.0) immediately after bout 1 and 11.6 (21.2) immediately after bout 2 whereas for ECIG-naïve individuals scores were 20.8 (25.7) immediately after bout 1 and 27.0 (32.9) immediately after bout 2 (ps < .05). A similar pattern was observed for the item “Irritable”.
Tiffany Drobes QSU-Brief
Significant condition by time interactions were observed for both QSU factors [Fs (27, 1647) ≥ 1.7, ps <.05, ηp2s ≤ .09] and also a condition by group interaction was observed for QSU Factor 1 [F (3, 186) = 3.7, p < .05, ηp2= .06], with each factor showing decreases immediately after bout 1 and 2 and with the magnitude of these decreases being more apparent when using higher liquid nicotine concentrations and among ECIG-experienced individuals. For example, Figure 2(panels E and F) displays data from QSU Factor 1, for which scores decreased significantly after bout 1, relative to baseline, for both groups in the 18 and 36 mg/ml conditions and in some instances, in the 8 mg/ml condition. For example, in the 36 mg/ml condition, mean (SD) scores decreased significantly from 22.4 (7.4) prior to bout 1 to 11.8 (8.6) immediately after bout 1 for ECIG-experienced individuals and scores decreased significantly from 23.0 (8.0) immediately prior to bout 1 to 15.2 (10.2) immediately after bout 1 for ECIG-naïve individuals (ps < .05, Tukey’s HSD). A similar pattern was observed for bout 2.
Direct Effects of Nicotine
A significant condition by group interaction was observed for the item “Sweaty” [F (3,186) = 3.7, p < .02, ηp2= .06]. Planned contrasts revealed significant between group differences for “Sweaty” immediately after bout 1 and 2 in the 8 mg/ml condition only [ts (62) > −2.6, ps < .05]. For example, immediately after bout 1 in the 8 mg/ml condition, mean (SD) scores for “Sweaty” were 1.5 (3.7) for ECIG-experienced and 9.0 (17.9) for ECIG-naïve. Also, significant main effects of group were observed for “Nausea”, “Nervous”, “Salivate”, and “Sweaty” [Fs (1, 62) ≥ 4.3, ps < .05, ηp2s≤ .12] with ECIG-naïve individuals reporting overall higher scores for these items.
Direct Effects of ECIG-Use
Significant condition by group interactions were observed for “Awake”, “Pleasant”, and “Satisfy” [Fs (3, 186) ≥ 3.0, ps < .05, ηp2s ≤ .09]. Figure 2 (panels G and H) displays data for the item “Satisfy” and overall patterns revealed that immediately after bout 1, significant differences between the 0 mg/ml condition and the 8, 18, and 36 mg/ml conditions were observed for ECIG-experienced individuals but not ECIG-naïve (ps < .05, Tukey’s HSD). For example, immediately after bout 1, scores for “Satisfy” for ECIG-experienced individuals were 33.4 (33.1) for 0 mg/ml condition, 50.8 (33.0) for 8 mg/ml, 61.0 (30.5) for 18 mg/ml and 60.0 (30.1) for 36 mg/ml (ps < .05, Tukey’s HSD). Conversely, immediately after bout 1, scores for “Satisfy” for ECIG-naïve individuals were 41.5 (33.9) for the 0 mg/ml condition, 42.7 (30.1) for 8 mg/ml, 50.7 (34.2) for 18 mg/ml and 45.1 (35.1) for 36 mg/ml. Planned contrasts revealed no significant between group differences for “Satisfy”.
For the item “Right Now” significant condition by time [F (24, 1488) = 4.2, p < .01, ηp2 = .06] and time by group [F (8, 496) = 2.5, p < .05, ηp2= .04] interactions were observed. Planned contrasts revealed significant between group differences with ECIG-experienced individuals reporting significantly higher scores relative to ECIG-naïve in the 0 and 8 mg/ml conditions immediately after bout 1, but not after bout 2 [ts (62) > 2.2, ps < .05]. For example, immediately after bout 1 in the 8 mg/ml condition, ECIG-experienced individuals mean (SD) score was 74.0 (32.2) and for ECIG-naïve the score was 47.0 (39.5). Finally, significant main effects of condition were observed for all ten items [Fs (3, 186) ≥ 3.6, ps < .05, ηp2s ≤ .14]; however, further post hoc testing revealed no significant differences across conditions.
Topography Acceptability Questionnaire
No significant interactions for any item were observed, however, significant main effects of time were observed for “Altered e-cigarette use behavior,” and “Increased awareness” [Fs (8, 496) ≥ 3.6, ps < .05, ηps≤ .06]. Post hoc tests revealed no statistically significant differences across timepoints for either group. A significant main effect of group was observed for the item “Made vaping less likely” [F (1, 62) = 5.7, p < .05, ηp2 = .08] with ECIG-naïve cigarette smokers reporting overall higher scores relative to ECIG-experienced individuals.
Discussion
This study was the first to evaluate systematically the extent to which liquid nicotine concentration and user experience influence plasma nicotine concentration, HR, puff topography, and subjective effects following acute ECIG use. Results from this study indicate that ECIG-associated nicotine delivery depends on liquid nicotine concentration and user experience, ECIG-delivered nicotine is physiologically active as indexed by HR increases, user puff topography differs as a function of user experience and liquid nicotine concentration, ECIG-use suppresses nicotine/tobacco abstinence symptoms in experienced ECIG users and ECIG-naïve cigarette smokers, and that the magnitude of abstinence symptom suppression depends upon liquid nicotine concentration, at least on some measures. Taken together, results from this study are relevant to efforts intended to regulate and/or predict ECIG effects.
The present study demonstrated that ECIG-associated nicotine delivery is related directly to liquid nicotine concentration (when device power and other liquid constituents are held constant), can meet or exceed the nicotine delivery of a tobacco cigarette, and depends on user experience. The direct relationship between liquid nicotine concentration and user plasma nicotine concentration is illustrated in Figure 1 and can be demonstrated more clearly when the mean nicotine boost data are collapsed across group: immediately after bout 1, mean (SD) nicotine boost was −0.1 ng/ml (1.5) in the 0 mg/ml condition, 6.0 ng/ml (6.6) in the 8 mg/ml condition, 10.0 ng/ml (12.2) in the 18 mg/ml condition, and 13.0 ng/ml (14.3) in the 36 mg/ml condition. Some participants in this study exceeded the nicotine boost typically observed after smoking a single tobacco cigarette (i.e., ∼15–20 ng/ml; Yan & D’Ruiz, 2015; Vansickel et al., 2010; Patterson et al., 2003). For example, while mean plasma nicotine boost observed among experienced ECIG users immediately after bout 1 in the 36 mg/ml condition was 17.9 ng/ml, 9 participants achieved a plasma nicotine boost twice as high (i.e., ≥ 36 ng/ml) suggesting that some experienced ECIG users can obtain nicotine from an ECIG with great efficiency. These nicotine delivery results are consistent with other studies that indicate that ECIGs can meet or exceed the nicotine delivery profile of combustible tobacco cigarettes (Spindle et al., 2017; Wagener et al., 2017; Dawkins et al., 2016). The plasma nicotine increases observed in the 8, 18, and 36 mg/ml condition were physiologically active, as indicated by accompanying significant increases in HR in those conditions, but not in the 0 mg/ml nicotine condition (where plasma nicotine did not increase significantly). Also, similar to a previous report (Farsalinos et al., 2015), the present study demonstrated that nicotine delivery differed across ECIG-experienced and -naïve individuals despite controlling for several characteristics that may influence nicotine delivery (e.g., device power, liquid PG:VG ratio, puff number) and these differences may be explained by differences in user puff topography.
Puff topography results were dependent on user experience and liquid nicotine concentration. The between-group puff topography results suggest a relationship between longer/larger puffs and higher plasma nicotine concentration (Farsalinos et al., 2015; see also Talih et al., 2015). In this study, ECIG-experienced individuals took significantly longer and larger puffs relative to ECIG-naïve smokers in every condition (see Table 3). For example, collapsed across condition and time (bout), mean (SD) puff duration was 5.6 s (3.0) for ECIG-experienced individuals and 2.9 s (1.5) for ECIG-naïve smokers and mean (SD) puff volume was 161.5 ml/s (152.1) for ECIG-experienced and 94.9 ml/s (68.7) for -naïve. The possibility of a relationship between longer puffs and higher plasma nicotine concentration is strengthened by the observation of significant positive correlations for puff duration and post-bout plasma nicotine concentration for the 8 (r =.53), 18 (r =.56), and 36 mg/ml conditions (r =.44, all ps < .01), and no such significant correlation for any other puff topography outcome. Puff topography was also related to liquid nicotine concentration. For example, during bout 1, collapsed across group, mean (SD) puff duration was 4.7 s (2.4) in the 0 mg/ml condition, 4.4 s (4.0) in the 8 mg/ml condition, 4.0 s (3.7) in the 18 mg/ml condition, and 3.5 s (2.8) in the 36 mg/ml condition. For puff volume, during bout 1, collapsed across group, mean (SD) puff volume was 140.1 ml/s (121.8) in the 0 mg/ml condition, 142.5 ml/s (116.7) in the 8 mg/ml condition, 107.4 ml/s (73.5) in the 18 mg/ml condition, and 96.7 ml/s (130.7) in the 36 mg/ml condition. These longer/ larger puffs when using lower liquid nicotine concentrations may be analogous to self-titration observed among cigarette smokers (Ashton et al., 1979). Indeed, compensatory puffing behavior was observed in a recent study in which experienced ECIG users increased their puff number and duration as well as volume of liquid consumed when using a 6 mg/ml liquid nicotine concentration relative to a 24 mg/ml under ad libitum puffing conditions (Dawkins et al., 2016). However, in the present study, without an “own brand” control condition (e.g., own brand ECIG or cigarette control) or an ad libitum puffing period, the extent to which the observed differences in puff duration/volume reflect compensatory behavior (either to get more nicotine from lower concentration liquids or less nicotine from higher concentration liquids) is unclear.
ECIG-use suppressed nicotine/tobacco abstinence symptoms in ECIG-experienced individuals and ECIG-naïve smokers; for some items, the magnitude of abstinence symptom suppression was dependent on liquid nicotine concentration and user experience. For example, for the Hughes-Hatsukami item “Urge”, reductions for both groups were more pronounced as liquid nicotine concentration increased. For example, collapsed across group, mean (SD) scores for “Urge” immediately after bout 1 were 56.0 (33.0) in the 0 mg/ml condition, 46.0 (30.4) in the 8 mg/ml condition, 45.2 (32.0) in the 18 mg/ml condition, and 33.3 (31.9) in the 36 mg/ml condition. A similar pattern was observed for the item “Craving” and QSU-brief Factors 1 and 2, highlighting the importance of liquid nicotine concentration in abstinence symptom suppression. In cigarette smokers, suppression of tobacco/nicotine abstinence symptoms after nicotine administration is considered one indicator of tobacco/nicotine dependence (e.g., Carter and Griffiths, 2009; Eissenberg, 2004), so these results suggest that the ECIGs used by the ECIG-experienced participants in this study were capable of supporting tobacco/nicotine dependence. This conclusion is supported by the observation of similar FTND scores in experienced ECIG users, relative to ECIG-naïve cigarette smokers, though scores were lower on the Penn State Dependence Index (see Table 1).
In addition to suppressing nicotine/tobacco abstinence symptoms, ECIG-use also produced other subjective effects, some of which depended upon ECIG experience. For example, experienced ECIG users rated “Satisfy” in the 8, 18, and 36 mg/ml conditions significantly higher relative to the 0 mg/ml condition and as liquid nicotine concentration increased so did “Satisfy” ratings, indicating a preference for an ECIG containing more rather than less nicotine. Conversely, ECIG-naïve cigarette smokers rated “Satisfy” similarly across liquid nicotine concentrations, including 0 mg/ml, suggesting that unlike experienced ECIG users, ECIG-naïve smokers may have been unable to differentiate across liquid nicotine concentrations or did not consider ECIGs with higher liquid nicotine concentration to be more satisfying. Alternatively, the similar scores across liquid nicotine concentration for ECIG-naïve smokers on this measure may reflect their overall inexperience with these products.
Understanding the factors that influence the subjective effects of ECIG-use will be important for understanding their abuse liability generally, and perhaps particularly important if ECIGs are to substitute completely for tobacco cigarettes among cigarette smoking populations (e.g., Hajek, 2014; Etter, 2013).
This study had several important limitations. First, the results obtained from this study’s directed puffing protocol (10 puffs with 30 s IPI) may differ from ad libitum puffing. While there are several advantages to controlling puff topography parameters (e.g., ability to compare across studies and products, reducing confounds associated with participants taking different puff number) future studies may seek to evaluate ECIG nicotine delivery and puff topography in a more naturalistic manner with an ad libitum puffing protocol (e.g., Spindle et al., 2017; Yan & D’Ruiz et al., 2015). Second, this study did not include an own brand control condition (e.g., own brand ECIG for experienced ECIG users and combustible tobacco cigarette for ECIG-naive cigarette smokers) that would have allowed for more direct comparison of participants’ usual topography and nicotine delivery. However, several evaluations of nicotine delivery and puff topography when using own brand ECIG or tobacco cigarette have been conducted to allow for cross-study comparisons (Spindle et al., 2017; Yan & D’Ruiz, 2015; Vansickel et al., 2010). Third, although the use of a single cartomizer may not be representative of the cartomizers and tanks typically used by experienced ECIG-users, standardization of the cartomizer was necessary to accommodate the topography mouthpiece used and ensures accurate measurement of puff topography; there is an increasing need for topography measurement tools that can be used with advanced-generation ECIGs. Fourth, some participants likely did not comply with protocol-mandated ≥ 12 hours nicotine/ tobacco abstinence prior to the onset of the study session, highlighting the challenge of studying non-combustible tobacco products for which short-term abstinence cannot be verified immediately prior to the start of the study session (Blank, Breland, Cobb, Spindle, Ramôa, & Eissenberg, 2016). Future clinical laboratory research addressing the acute effects of ECIGs and other non-combustible tobacco products will continue to meet such challenges until a reliable and cost-effective method for verifying abstinence from non-combustible tobacco products is discovered. Fifth, some “ECIG-naïve” cigarette smokers had tried ECIGs previously, and the extent to which these participants’ previous encounters with ECIGs may have influenced results of the present study is unclear. However, most cigarette smokers in the present study reported little to no experience with ECIGs (M = 2 uses; SD = 1.6) prior to study enrollment.
The findings from the present study have important regulatory implications. The US FDA has begun a process of regulating ECIGs and their “parts and components” (81 FR 28973, 2016). Effective regulation of ECIGs will require systematic evaluation of these products and the effects they produce, including their ability to deliver nicotine to the user. In this study, some ECIG-experienced individuals received more nicotine from 10 ECIG puffs in the 36 mg/ml condition than has been reported for 10 puffs from a tobacco cigarette (e.g., Yan & D’Ruiz, 2015; Vansickel et al., 2010). The rationale for ECIG device/liquid combinations that exceed the nicotine delivery profile of a combustible tobacco cigarette is uncertain, and such products may increase ECIG abuse and dependence (e.g., Cobb, Hendricks, & Eissenberg, 2015), a particular concern for youth and young adults in the US and elsewhere (USDHHS, 2016). For this reason, some jurisdictions have begun to regulate ECIG liquids in an attempt to control ECIG nicotine delivery to the user (e.g., European Union Directive 2014/40/EU limits ECIG liquid nicotine concentration such that it does not exceed 20 mg/ml). The results presented here highlight liquid nicotine concentration as one factor that influences nicotine delivery, and also indicate that puff topography is another. Device power is a third potential factor (e.g., Talih et al., 2015) and recent results indicate that ECIG liquids that are much lower than 20 mg/ml (e.g. approximately 4 mg/ml), when paired with high powered ECIGs (e.g., 70W), can meet and in some cases exceed the nicotine delivery profile of a combustible tobacco cigarette (Wagener et al., 2017). In light of results from the present study and other results (Wagener et al., 2017), policies aimed at limiting ECIG nicotine delivery to the user likely will need to account for more factors than liquid nicotine concentration alone: user behavior and device power are also relevant. A mathematical model is available that predicts the rate of ECIG nicotine emissions (e.g., nicotine flux; Shihadeh & Eissenberg, 2015) and it takes into account many factors, including liquid nicotine concentration, user behavior, and device power (Talih et al., 2017). Such models are potentially valuable regulatory tools.
Taken together, the results of this study demonstrate that liquid nicotine concentration is related directly to plasma nicotine concentration and that 10 puffs from a 7.3W ECIG loaded with 8, 18, or 36 mg/ml liquid can deliver physiologically active nicotine concentrations to ECIG-experienced and ECIG-naïve individuals. Generally, ECIG-experienced individuals obtained significantly more nicotine relative to ECIG-naïve smokers and this difference can be explained, in part, by differences in puff topography (i.e., longer, larger puffs). Additionally, ECIG-use suppresses nicotine/tobacco abstinence symptoms in ECIG-experienced individuals and ECIG-naïve smokers, again depending on liquid nicotine concentration. Findings from the present study are relevant for policymakers seeking to limit ECIG nicotine delivery to the user as well as researchers who are evaluating these products as a means for combustible tobacco cigarette replacement.
Public Significance Statement.
In the present study, ECIG effects, including nicotine delivery to the user and suppression of tobacco/nicotine abstinence symptoms, were related to liquid nicotine concentration and depended on user experience. Experienced ECIG users obtained more nicotine and also took longer and larger puffs than cigarette smokers with little or no ECIG experience. These results suggest that liquid nicotine concentration and user behavior are both important factors in predicting and controlling ECIG effects. Future policies intended to limit the nicotine delivery and other effects of ECIGs likely will need to account for many factors, including liquid nicotine concentration, user behavior, and based on other results, device power.
Acknowledgments
Disclosures and Acknowledgements: Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number P50DA036105. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Footnotes
All authors have contributed to this manuscript and have read and approved the final manuscript.
Drs. Eissenberg and Shihadeh are paid consultants in litigation against the tobacco industry and are named on a patent application for a device that measures the puffing behavior of electronic cigarette users. Other authors have no conflicts to report.
This manuscript is based on research performed for a Master’s thesis that was conducted, completed, and defended at Virginia Commonwealth University (VCU). The thesis is published in its entirety on VCU’s Scholars Compass website. Portions of this work were presented at the 20th 21st and 22nd annual meetings of the Society for Research on Nicotine and Tobacco. The authors would like to thank Barbara Kilgalen, Janet Austin, Hannah Mayberry, and Caroline Smith for their assistance in data collection and management.
References
- Adkison SE, O’Connor RJ, Bansal-Travers M, Hyland A, Borland R, Yong HH, Cummings M, McNeil A, Thrasher JF, Hammond D, Fong GT. Electronic nicotine delivery systems: international tobacco control four-country survey. American Journal of Preventive Medicine. 2013;44(3):207–215. doi: 10.1016/j.amepre.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton H, Stepney R, Thompson JW. Self-titration by cigarette smokers. BMJ. 1979;2(6186):357–360. doi: 10.1136/bmj.2.6186.357. doi: https://doi.org/10.1136/bmj.2.6186.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank MD, Breland AB, Cobb CO, Spindle T, Ramôa C, Eissenberg T. Clinical laboratory evaluation of e-cigarettes: methodological challenges. Tobacco Regulatory Science. 2016;2(4):426–439. doi: 10.18001/TRS.2.4.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank MD, Disharoon S, Eissenberg T. Comparison of methods for measurement of smoking behavior: mouthpiece-based computerized devices versus direct observation. Nicotine & Tobacco Research. 2009;11(7):96–903. doi: 10.1093/ntr/ntp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breland A, Soule E, Lopez A, Ramôa C, El-Hellani A, Eissenberg T. Electronic cigarettes: what are they and what do they do? Annals of the New York Academy of Sciences; 2016. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breland AB, Kleykamp BA, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine & Tobacco Research. 2006;8(6):727–738. doi: 10.1080/14622200600789585. [DOI] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug and Alcohol Dependence. 2009;105(S1):S14–S25. doi: 10.1016/j.drugalcdep.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb CO, Hendricks PS, Eissenberg T. Electronic cigarettes and nicotine dependence: evolving products, evolving problems. BMC medicine. 2015;13:119. doi: 10.1186/s12916-015-0355-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3(1):7–16. doi: 10.1080/14622200124218. [DOI] [PubMed] [Google Scholar]
- Cropsey KL, Eldridge GD, Weaver MF, Villalobos GC, Stitzer ML. Expired carbon monoxide levels in self-reported smokers and nonsmokers in prison. Nicotine & Tobacco Research. 2006;8(5):653–659. doi: 10.1080/14622200600789684. [DOI] [PubMed] [Google Scholar]
- Dawkins LE, Kimber CF, Doig M, Feyerabend C, Corcoran O. Self-titration by experienced e-cigarette users: blood nicotine delivery and subjective effects. Psychopharmacology. 2016;233:2933–2941. doi: 10.1007/s00213-016-4338-2. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology. 2014;231(2):401–407. doi: 10.1007/s00213-013-3249-8. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Turner J, Hasna S, Soar K. The electronic-cigarette: effects on desire to smoke, withdrawal symptoms and cognition. Addictive Behaviors. 2012;37(8):970–973. doi: 10.1016/j.addbeh.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act, 2016. 81 FR 28973 (May 10, 2016). Docket No. FDA-2014-N-0189.
- Eissenberg T. Measuring the emergence of tobacco dependence: the contribution of negative reinforcement models. Addiction. 2004;99(s1):5–29. doi: 10.1111/j.1360-0443.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- Etter JF. Should electronic cigarettes be as freely available as tobacco? Yes. British Medical Journal (Online) 2013:346. doi: 10.1136/bmj.f3845. [DOI] [PubMed] [Google Scholar]
- Evans SE, Blank M, Sams C, Weaver MF, Eissenberg T. Transdermal nicotine-induced tobacco abstinence symptom suppression: nicotine dose and smokers’ gender. Experimental and Clinical Psychopharmacology. 2006;14(2):121–135. doi: 10.1037/1064-1297.14.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Spyrou A, Stefopoulos C, Tsimopoulou K, Kourkoveli P, Tsiapras D, Kyrzopoulos S, Poulas K, Voudris V. Nicotine absorption from electronic cigarette use: comparison between experienced consumers (vapers) and naïve users (smokers) Scientific Reports. 2015:5. doi: 10.1038/srep11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J, Veldheer S, Yingst J, Hrabovsky S, Wilson SJ, Nichols TT, Eissenberg T. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking e-cigarette users. Nicotine & Tobacco Research. 2015;17(2):186–192. doi: 10.1093/ntr/ntu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J, Stapleton J, Feyerabend C, Vesey C, Jarvis M, Russell MA. Effect of transdermal nicotine patches on cigarette smoking: a double-blind crossover study. Psychopharmacology. 1992;106(3):421–427. doi: 10.1007/BF02245429. [DOI] [PubMed] [Google Scholar]
- Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972–1986. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P. Electronic cigarettes have a potential for huge public health benefit. BMC Medicine. 2014;12(1):225. doi: 10.1186/s12916-014-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hua M, Yip H, Talbot P. Mining data on usage of electronic nicotine delivery systems (ENDS) from YouTube videos. Tobacco Control. 2013;22(2):103–106. doi: 10.1136/tobaccocontrol-2011-050226. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Keppel G, Saufley WH, Tokunaga H. Introduction to design and analysis: A student’s handbook. Englewood Cliffs; New Jersey: Prentice Hall: 1992. [Google Scholar]
- Lopez AA, Hiler MM, Soule EK, Ramôa CP, Karaoghlanian NV, Lipato T, Breland AB, Shihadeh AL, Eissenberg T. Effects of electronic cigarette liquid nicotine concentration on plasma nicotine and puff topography in tobacco cigarette smokers: a preliminary report. Nicotine & Tobacco Research. 2016a;18(5):720–723. doi: 10.1093/ntr/ntv182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AA, Hiler M, Maloney S, Eissenberg T, Breland AB. Expanding clinical laboratory tobacco product evaluation methods to loose-leaf tobacco vaporizers. Drug and Alcohol Dependence. 2016b;169:33–40. doi: 10.1016/j.drugalcdep.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nides MA, Leischow SJ, Bhatter M, Simmons M. Nicotine blood levels and short-term smoking reduction with an electronic nicotine delivery system. American Journal of Health Behavior. 2014;38(2):265–274. doi: 10.5993/AJHB.38.2.12. [DOI] [PubMed] [Google Scholar]
- Patterson F, Benowitz N, Shields P, Kaufmann V, Jepson C, Wileyto P, Kucharski S, Lerman C. Individual differences in nicotine intake per cigarette. Cancer Epidemiology Biomarkers & Prevention. 2003;12(5):468–471. [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Jao NC. Optimal carbon monoxide criteria to confirm 4-hr smoking abstinence. Nicotine & Tobacco Research. 2013;15(15):978–982. doi: 10.1093/ntr/nts205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickworth WB, Bunker EB, Henningfield JE. Transdermal nicotine: reduction of smoking with minimal abuse liability. Psychopharmacology. 1994;115(1):9–14. doi: 10.1007/BF02244745. [DOI] [PubMed] [Google Scholar]
- Ramôa CP, Hiler MM, Spindle TR, Lopez AA, Karaoghlanian N, Lipato T, Breland A, Eissenberg T. Electronic cigarette nicotine delivery can exceed that of combustible cigarettes: a preliminary report. Tobacco Control. 2016;25(e1):e6–9. doi: 10.1136/tobaccocontrol-2015-052447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T, Marynak K, Arrazola RA, Cox S, Rolle IV, King BA. Vital signs: exposure to electronic cigarette advertising among middle school and high school students—United States, 2014. MMWR Morbidity and Mortal Weekly Report. 2016;64(52):1403–8. doi: 10.15585/mmwr.mm6452a3. [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Eissenberg T. Electronic cigarette effectiveness and abuse liability: predicting and regulating “nicotine flux”. Nicotine & Tobacco Research. 2015;17(2):158–162. doi: 10.1093/ntr/ntu175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle T, Hiler M, Breland A, Karaoghlanian N, Shihadeh A, Eissenberg T. The influence of a mouthpiece-based topography measurement device on electronic cigarette user’s plasma nicotine concentration, heart rate, and subjective effects under directed and ad libitum use conditions. Nicotine & Tobacco Research. 2017;19(4):469–476. doi: 10.1093/ntr/ntw174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle TR, Breland AB, Karaoghlanian NV, Shihadeh AL, Eissenberg T. Preliminary results of an examination of electronic cigarette user puff topography: the effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine & Tobacco Research. 2015;17(2):142–149. doi: 10.1093/ntr/ntu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talih S, Balhas Z, Salman R, El-Hage R, Karaoghlanian N, El-Hellani A, Baassiri M, Jaroudi E, Eissenberg T, Saliba N, Shihadeh A. Transport phenomena governing nicotine emissions from electronic cigarettes: Model formulation and experimental investigation. Aerosol Science and Technology. 2017;51(1):1–11. doi: 10.1080/02786826.2016.1257853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talih S, Balhas Z, Eissenberg T, Salman R, Karaoghlanian N, El Hellani A, Baalbaki R, Saliba N, Shihadeh A. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine & Tobacco Research. 2015;17(2):150–157. doi: 10.1093/ntr/ntu174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey JW. Comparing individual means in the analysis of variance. Biometrics. 1949;5(2):99–114. doi: 10.2307/3001913. [DOI] [PubMed] [Google Scholar]
- USDHHS. E-cigarette Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: USDHHS, CDC, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2016. [Google Scholar]
- Vansickel AR, Eissenberg T. Electronic cigarettes: Effective nicotine delivery after acute administration. Nicotine & Tobacco Research. 2013;15(1):267–270. doi: 10.1093/ntr/ntr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiology Biomarkers & Prevention. 2010;19(8):1945–1953. doi: 10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagener TL, Floyd EL, Stepanov I, Driskill LM, Frank SG, Meier E, Leavens EL, Tackett AP, Molina N, Queimado L. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tobacco Control. 2017;26:e23–e28. doi: 10.1136/tobaccocontrol-2016-053041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XS, D’Ruiz C. Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regulatory Toxicology & Pharmacology. 2015;71(1):24–34. doi: 10.1016/j.yrtph.2014.11.004. [DOI] [PubMed] [Google Scholar]