Abstract
Repetitive administration is routinely used to maintain therapeutic drug levels, but previous studies have documented an accelerated blood clearance of some lipid-based delivery systems under these conditions. To assess the effect of repetitive administration, non-PEGylated lipoplexes (+/− = 0.5) were administered four times via tail vein injection at 3-day intervals to immunocompetent Balb/c mice bearing 4T1 tumors. This study measured the effect of repeat administration of non-targeted lipoplexes on clearance, cytokine/chemokine response, plasmid distribution, reporter gene expression, and liver toxicity. We do not observe a refractory period or a statistically significant difference in blood clearance between the first administration and subsequent injections of this lipoplex formulation, consistent with the absence of a cytokine/chemokine response. However, we do see a significant effect on both plasmid accumulation and expression; an enhancement of 26-fold and 10-fold in tumor plasmid levels and expression, respectively, after 4 injections as compared to that after a single injection. In addition, in vivo imaging suggests that expression in other organs had diminished rapidly 72 h after each administration, in contrast to relatively constant expression in the tumor. Taken together, the findings indicate that gene delivery to tumors can be dramatically enhanced by employing repetitive administration.
Keywords: mmunocompetent, gene delivery, lipoplex, biodistribution, refractory period, liver toxicity, cholesterol nanodomain, sphingosine, repeat dosing, clearance
Introduction
Hereditary diseases were thought to be incurable prior to the demonstration that DNA could be successfully delivered to cells. While the use of calcium phosphate for transfection was utilized for early laboratory experiments, the demonstration that cationic lipids were capable of delivering DNA much more efficiently brought hopes that similar approaches might one day allow genetic diseases to be corrected[1–3]. This hope has recently been bolstered by the demonstration of specific gene splicing by the CRISPR system[4]. It is now clear that RNA (miRNA, mRNA, siRNA) can also be used to alter gene expression, but effective treatment still depends upon developing delivery approaches that are sufficiently targeted and efficient such that expression/silencing can be adequately regulated to impart a therapeutic effect. Although many different nucleic acid-based treatments have been tested in clinical trials, very few have been approved for use as pharmaceutical products. However, new approaches to nucleic acid-based therapy continue to evolve and are currently being tested in clinical trials.
With the exception of Glybera®, which is purported to be curative after a single treatment, it is thought that current nucleic acid-based medicines will need to be administered repetitively in order to achieve and maintain therapeutic effects. Prior to clinical trials, extensive animal testing is performed, and dosing studies typically employ repetitive administration to simulate dosing that would likely be employed in the clinic[5–7]. However, initial animal studies designed to assess pharmacokinetic parameters, delivery efficiency, and toxicity typically employ a single administration to evaluate the merits of a particular approach/strategy before continuing with further animal testing[8–11].
Decades of work with soluble small molecule therapeutics has led to our conventional understanding of pharmacokinetic profiles, i.e., adsorption, distribution, metabolism, excretion. According to this classic paradigm, bioavailability is correlated with drug levels in the blood, and the therapeutic effect is abolished after clearance from the blood. In this scenario, repeat administration is utilized to maintain blood levels of the drug within the range that corresponds with the therapeutic effect, i.e., the “therapeutic window”[5]. However, it is clear that gene-based therapies will need to be taken up into cells prior to having a therapeutic effect, and therefore a significant hysteresis between blood levels and gene expression is expected. The situation is further complicated by the fact that delivery vehicles administered intravenously will initially encounter the vasculature endothelium, then be translocated to (and subsequently taken up by) the target cell, and exogenous genes must ultimately access the nucleus after uptake[12–16]. Considering the time needed after transcription for mRNA to be transported into the cytoplasm as well as the residence time of mRNA and protein within the cell, the exogenous gene may be degraded and/or silenced while the encoded protein responsible for the therapeutic effect continues to be active. It follows that blood levels at any point in time may not accurately reflect the biological activity of nucleic acid-based therapeutics.
Similarly, because the effects of most nucleic acid-based therapeutics will not depend on maintaining high blood levels, repetitive administration is used to progressively enhance deposition in the target tissue with the hope that sufficient levels of nucleic acid accumulate to elicit a therapeutic effect within the target cell[6, 17, 18]. Indeed, previous studies have utilized repetitive administration of lipoplexes to extend transgene expression [17, 18] and achieve enhanced levels of siRNA-induced silencing[12, 14]. Under this scenario, it may be expected that each administration results in a constant amount of deposition in tissues. This deposition process is presumably arrested after the nucleic acid is cleared from the blood. As described above, it may be expected that the therapeutic effect resulting from gene expression and/or silencing may be appreciably delayed as compared to blood clearance; however, it is typically presumed that a single administration should result in a finite level of deposition/distribution in tissues. Assuming that blood clearance is complete before subsequent administration of a repeat dose, it follows that successive dosing would be additive. However, one must account for degradation of the therapeutic nucleic acid that occurs in the tissues prior to deposition of the subsequent dose. Accordingly, two doses of a gene delivery system should result in a maximum of twice the deposition and expression of a single dose, three doses result in a maximum of three times that of a single dose, etc. In this way, the effects of the therapeutic nucleic acid should be progressively enhanced by repetitive administrations.
It is well recognized that nonviral gene delivery systems suffer from inefficient delivery as compared to their viral counterparts. In particular, synthetic delivery systems have the potential for repeat administration due to the lack of a specific immune response to the vector, and this strategy allows delivery from nonviral systems to be greatly enhanced[6, 12, 14]. However, gene delivery studies with nonviral systems have demonstrated that repeat transfection requires a “refractory period” (e.g., two weeks) in order to obtain expression from a subsequent dose, and typically the goal of repetitive dosing is simply to maintain expression levels, not increase them[18]. Furthermore, it is generally recognized that both the nucleic acid component as well as the nonviral delivery system can be immunostimulatory, and this can affect delivery after successive administrations [18]. Therefore, we have taken great lengths to diminish the adverse response to our delivery system by utilizing minimal amounts of naturally-occurring lipids, reducing the CpG content of the plasmid, and avoiding the use of PEGylation [19–23]. The effects of these particle alterations on the cytokine/chemokine response after repetitive injection was determined, and we also characterize the effects of repeat administration of a lipoplex formulation on clearance, organ accumulation, and liver toxicity. We observe effects on each of these parameters that are not additive and are thereby inconsistent with a conventional pharmacokinetic profile. We feel that these results demonstrate that the correlation between clearance, tissue accumulation, reporter gene expression, and liver toxicity are not straightforward, and are worthy of further investigation. In addition, our in vivo imaging demonstrates that reporter gene expression is widely distributed throughout the mouse 24 h after each tail vein injection, but predominantly confined to the tumor at later times (72 h).
Materials and Methods
Lipoplex preparation and luciferase expression
Sphingosine, cholesterol, and 1,2-diarachidoyl-sn-glycero-3-phosphocholine (DAPC) were purchased from Avanti Polar Lipids (Alabaster, AL) and used to prepare liposomes at a 3:2:5 mole ratio (respectively) as previously described[20]. Liposomes were then mixed with a modified (CMV removed, ROSA26 added, based upon Watcharanurak et al.[24]) pSelect-LucSh (Invivogen, San Diego, CA) plasmid encoding luciferase at a charge ratio of 0.5[19, 20]. These modifications to the plasmid have been shown to prolong expression for weeks to months[24]. The resulting lipoplexes have a diameter of 280.9 ± 10.8 nm, a zeta potential of −24.4 ± 2.9 mV, and were diluted 1:1 (v/v) with 12% hydroxyl ethyl starch (MW 250,000, Fresenius; Linz, Austria) prior to administration[19]. The use of hydroxyl ethyl starch at a final concentration of 6% (w/v) serves to adjust the tonicity and results in more consistent, but not increased, delivery (unpublished observations). Fifty micrograms of DNA complexed with 0.25 μmoles lipid was injected via tail vein as previously described [19]. Each mouse received a series of four injections at three-day intervals. Prior to treatment with lipoplexes, female immunocompetent Balb/c 6–10 weeks old were acquired from Jackson labs (Bar Harbor, ME) and inoculated in each shoulder with one million 4T1 murine mammary carcinoma cells (ATCC #CRL-2539). Luciferase expression was monitored in extracted tissues with Promega Luciferase Assay Reagents (Madison, WI) as previously described[23]. All animal procedures were approved by the University of Colorado Institute for Animal Care and Use Committee in accordance with guidelines from the National Institutes of Health.
Cytokine/Chemokine Response
A separate set of tumor-bearing mice were used to quantify levels of specific cytokines and chemokines after repetitive injection of lipoplexes. In addition to the lipoplex formulation described above, mice were treated with phosphate buffered saline (negative control), lipoplexes formulated with 5% DSPE-PEG2000 (Avanti), CpG-containing plasmid (Valentis, Inc.), 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP; Avanti) instead of sphingosine, or Lipofectamine 2000 (Invitrogen, Carlsbad, CA) as previously described [19, 20, 22, 25]. In these studies, a single mouse was treated with each formulation as described above, and blood was collected in Eppendorf tubes 2 h after the second injection of lipoplexes. Serum samples were obtained per manufacturer’s instructions: samples were allowed to clot for 30 minutes, spun at 2,000g for 15 minutes, and serum was recovered (R&D Systems, Minneapolis MN). Samples were assayed for cytokine/chemokine activity via manufacturer’s instructions using the mouse cytokine array panel A (#ARY006 from R&D Systems, Minneapolis MN). The 2 h time point after the second injection was chosen because that is when cytokine/chemokine response was shown to be maximized in previous studies[26].
Determination of Plasmid Levels in Tissues
To determine delivery of plasmid DNA to mouse tissues, animals were sacrificed 24 h after the first and fourth intravenous administration of lipoplexes, and their organs were harvested and flash frozen in liquid nitrogen. Organs were then thawed, weighed, and DNA was extracted using the Qiagen DNeasy Blood and Tissue Kit (Qiagen, Germantown, MD). Quantitative PCR (qPCR) was then performed on the samples using QuantiTech RTPCR Kit (Qiagen, Germantown, MD) on an Applied Biosystems 7500 RTPCR instrument (Grand Island, NY). A standard curve of pure plasmid was used for quantification as well as amplicon efficiency factors that account for amplification that is not perfectly efficient (as suggested by the Applied Biosystems 7500 Manual referencing Fenster et al. “Real-Time PCR.” Current Protocols Essential Laboratory Techniques, 2009: 10.3.1–10.3.33).
Blood Levels
To determine blood levels of the lipoplexes, individuals were bled at 5, 30, 60, 240, and 1440 minutes after each injection using their submandibular veins. Briefly, mice were anesthetized using isoflurane and then bled by lancing the submandibular vein on the cheek per standard protocol. Blood was collected in tubes containing sodium citrate (anticoagulant) and spun (2,000 x g for 10 minutes) to remove red blood cells, and the resulting plasma was then prepped for qPCR using Qiagen DNeasy Blood and Tissue Kit. qPCR was performed as previously described using the QuantiTech RTPCR Kit (both Qiagen, Germantown, MD) and a standard curve of pure plasmid[19, 22].
Extraction Efficiency
In order to determine the extraction efficiency of the Qiagen DNeasy Blood and Tissue Kit (Qiagen, Germantown, MD), plasmid DNA (50 μg) was injected into organs freshly harvested from Balb/c mice. Each organ was processed per the Qiagen DNeasy protocol, and plasmid quantified by qPCR. A standard curve of pure plasmid was used, and the calculated extraction efficiencies were used to adjust DNA recoveries in our experiments.
In vivo Imaging
Luciferase expression in Balb/c mice was imaged at different times (see Fig. 5) using the IVIS imaging system (Xenogen Corp., Alameda, CA) in the University of Colorado Animal Facility as previously described[22]. Briefly, tumor-bearing mice were injected intraperitoneally with D-firefly luciferin substrate (150 mg/kg; Xenogen Corp.) ten minutes prior to anesthetizing mice (2.5% isoflurane in 5 L O2/min). At each timepoint, anesthetized mice were placed in a light-tight chamber and imaging is performed. Images were processed with Living Image software, and representative images were selected for the panels in Figure 5. After the final timepoint (240 h, four injections), tumor volumes in mice were 200–500 mm3, and tissues were extracted as described above.
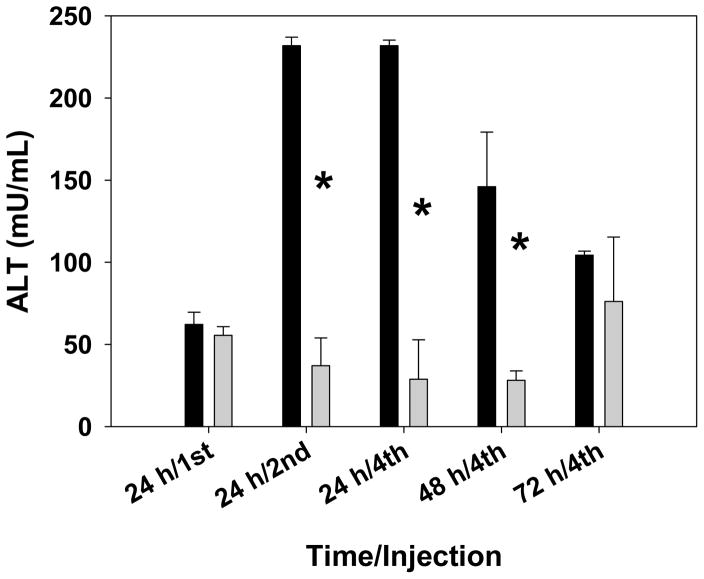
Figure 5.
Blood levels of ALT were measured after intravenous injection of lipoplexes (dark bars) or PBS (light bars). The bars represent the mean and one standard error of ALT levels in blood extracted at each timepoint from three mice. Asterisks indicate statistically significant differences (p < 0.004).
Liver Toxicity
A separate set of mice subjected to the same treatment protocol were used for ALT measurements, and liver toxicity was assessed by monitoring the levels of alanine aminotransferase (ALT) after the first, second, and fourth injections of either lipoplexes or saline. Blood was collected from the submandibular vein of experimental animals as described above, and enzyme levels were assessed with an Alanine Transaminase Activity Assay from Abcam PLC (Cambridge, MA) as previously described[19].
Results
Blood Levels
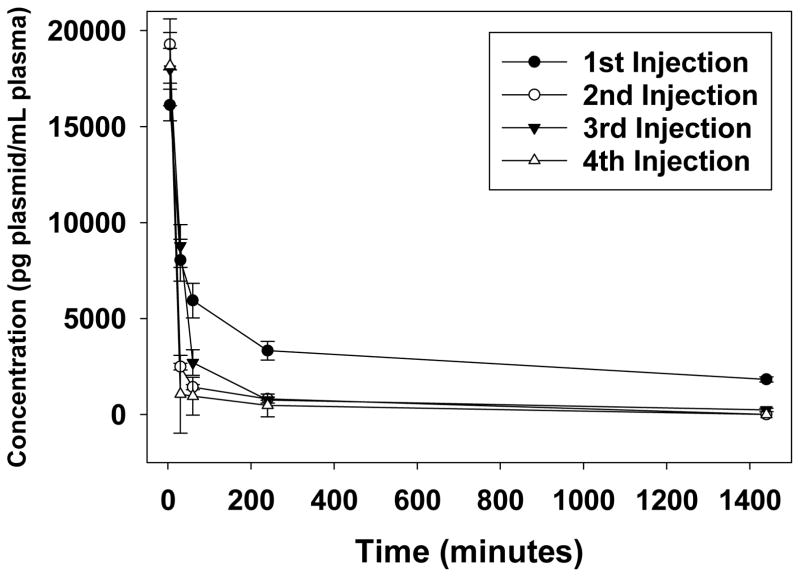
Considering the accelerated blood clearance (ABC) phenomenon in which subsequent doses of PEGylated liposomes are cleared more rapidly than the initial dose [27, 28], we assessed blood levels of lipoplexes lacking PEG after four intravenous administrations in immunocompetent Balb/c mice bearing 4T1 tumors. Tumor-bearing mice were administered four doses of lipoplexes via tail vein injection (one dose every three days), and blood samples were collected after each injection as described above. Consistent with our previous studies in immunocompetent mice[19], lipoplexes were cleared rapidly from the plasma (Figure 1). Plasmid was detected at very low levels in the plasma for 24 h after the initial dose, in contrast to subsequent doses (Fig. 1). However, no statistical difference between the AUC of the first dose and that of subsequent doses was observed (two-tailed t test; p > 0.14). We point out that the majority of the injected dose is cleared almost immediately (within 5 min), consistent with other pharmacokinetic studies of lipoplexes[12, 15, 29–31]. It should be noted that the inherent variability among mice combined with the low number of animals used in our study (n = 3) may prevent us from statistically resolving small differences in blood profiles. Although a larger cohort of mice might reveal some differences in blood clearance, our results indicate that any differences are likely minimal.
Figure 1.
Plasma was collected from mice 5, 30, 60, 240, and 1440 minutes after each intravenous administration of lipoplexes, and plasmid was subsequently quantified by qPCR. Symbols and error bars represent the mean and one standard error of samples taken from three mice.
Cytokine/Chemokine Response
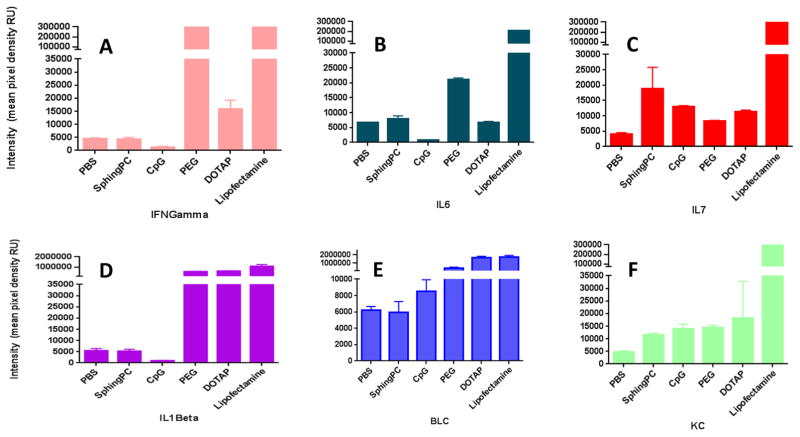
Previous studies have demonstrated that immunogenicity and the corresponding cytokine/chemokine response can alter the pharmacokinetics upon repetitive administration[7, 18, 32, 33]. Accordingly, we investigated the effects of specific alterations in our lipoplex formulations on the cytokine/chemokine response elicited after repetitive administration. Our experiments quantifying relative levels of 37 different cytokines and chemokines show that Keratinocyte Chemoattractant (KC) was the only chemokine that was elevated above that observed with PBS upon repetitive intravenous administration of the sphingosine-based formulation (Figure 2, Table I). Formulation of this lipoplex with a plasmid containing extensive CpG sequences elicited elevated levels of IL-1alpha, IL-7, and IL-13 in addition to KC. In contrast to these relatively specific changes due to CpG sequences, PEGylation of the sphingosine-based lipoplex elicited elevation in 30 of the 37 measured cytokines and chemokines (Table I). While lipofectamine-based lipoplexes elicited elevated levels in every cytokine, substitution of DOTAP for sphingosine caused elevation in 11 cytokines. With the exception of the lipofectamine-based lipoplexes, most cytokine and chemokine responses were only mildly elevated, and specific values for selected cytokines and chemokines are depicted in Figure 2.
Figure 2.
Relative levels of specific cytokines/chemokines elicited 2 h after a second intravenous administration of PBS or lipoplexes formulated with sphingosine, CpG-containing plasmid, 5% DSPE-PEG 2000, DOTAP, or lipofectamine. Individual cytokines/chemokines are shown in separate panels.
Table I. Cytokine and Chemokine Response to Lipoplex Formulations.
Cytokine and chemokine levels were measured in blood from mice repeatedly administered different lipoplex formulations as described in the methods. Levels that were significantly higher than that in mice administered PBS (t-test, p > 0.05) are indicated with an asterisk.
| Sphingosinebased | CpG – rich Plasmid | PEG-DSPE | DOTAP | Lipofectamine | |
|---|---|---|---|---|---|
| BLC | * | * | * | ||
| C5 | * | * | * | ||
| G-CSF | * | * | * | ||
| GM-CSF | * | * | |||
| I-309 | * | * | |||
| Eotaxin | * | * | * | ||
| sICAM-1 | * | ||||
| IFN-gamma | * | * | |||
| IL-1alpha | * | * | * | ||
| IL-1beta | * | * | * | ||
| IL-1ra | * | * | |||
| IL-2 | * | * | |||
| IL-3 | * | * | |||
| IL-4 | * | ||||
| IL-5 | * | * | * | ||
| IL-6 | * | * | |||
| IL-7 | * | * | * | * | |
| IL-10 | * | * | |||
| IL-13 | * | * | * | ||
| IL-12p70 | * | ||||
| IL-16 | * | * | |||
| IL-17 | * | * | |||
| IL-23 | * | * | * | ||
| IL-27 | * | * | * | ||
| IP-10 | * | * | * | ||
| I-TAC | * | ||||
| KC | * | * | * | * | |
| M-CSF | * | * | * | ||
| JE | * | * | |||
| MCP-5 | * | * | |||
| MIG | * | * | |||
| MIP-1alpha | * | * | |||
| MIP-1beta | * | * | |||
| MIP-2 | * | * | |||
| RANTES | * | ||||
| SDF-1 | * | ||||
| TNF-alpha | * | * |
Tumor Accumulation and Expression
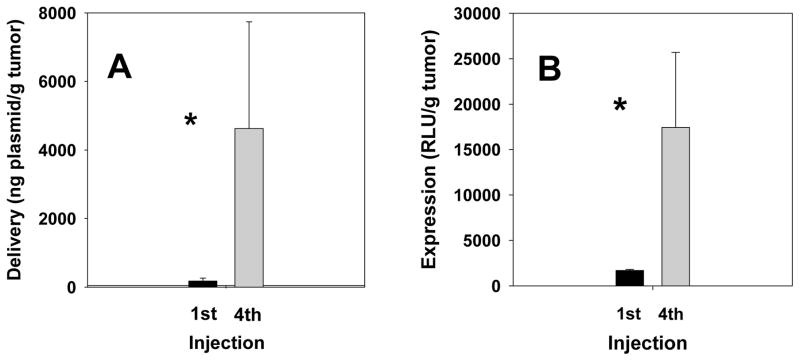
As described above, our dosing protocol involved four doses administered at three-day intervals. Because the lipoplexes are rapidly cleared from the blood, there should be minimal potential for any dose to contribute to additional organ accumulation in the subsequent dosing (i.e., three days after injection). Accordingly, each injection of lipoplexes would be expected to result in similar accumulation in the tumor. In fact, considering the more prolonged circulation of a small portion of lipoplexes from the first dose, one might expect that subsequent doses would contribute less to tumor accumulation. Furthermore, any plasmid that accumulates in the tumor (or any organ) would be expected to be degraded in and/or cleared from that tissue, and therefore we predicted that plasmid levels in the tumor after the fourth injection could only be a maximum of four-fold that observed after a single dose. However, our data indicate that both plasmid levels (26-fold) and luciferase expression (10-fold) in the tumor are enhanced by more than four-fold after the fourth dose as compared to that after a single administration (Fig. 3). We suggest that the lower enhancement of expression as compared to plasmid levels may be due to plasmid accumulated in the tumor that may still be in the process of being expressed, e.g., has yet to be internalized, dissociated from the lipid carrier, or gain access to the nucleus. This same phenomenon could potentially explain why luciferase expression is enhanced by more than fourfold after a series of four injections, i.e., some plasmids delivered after a single injection have yet to be expressed at 24 h, but may be available for expression at later timepoints. While such an effect could potentially account for the boost in luciferase activity, this explanation is not applicable to the 26-fold enhancement in plasmid accumulation that we observe.
Figure 3.
Plasmid levels (A) and luciferase expression (B) were quantified in tumors extracted from mice 24 h after the first and fourth administration of lipoplexes. The bars represent the mean and one standard error of six tumors extracted from three mice. Asterisks indicate statistically significant differences (p < 0.0001).
Accumulation and Expression in Organs
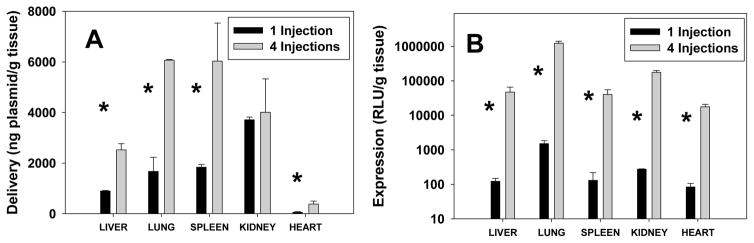
The effect of repetitive administration on the accumulation of plasmid in different organs was more consistent with the expectation that enhancement after four administrations would be somewhat less than four-fold that observed after a single injection. The enhancement in plasmid levels in the liver, lung, spleen, and kidney ranged from 1.1- to 3.6-fold, with the heart exhibiting much lower levels that were enhanced 7.1-fold, as compared to a single injection (Fig. 4A). Although expression often does not correlate strongly with plasmid levels, luciferase activity was enhanced by greater than two orders of magnitude in each of the organs, varying between 210- and 815-fold as compared to that seen after a single injection (Fig. 4B). It is surprising that the modest increases in plasmid accumulation after four doses resulted in such dramatic increases in expression in each organ. This effect is especially perplexing considering that the exact opposite trend was observed in the tumor, i.e., plasmid levels were enhanced to a greater extent than expression. Although we cannot reach definitive conclusions regarding the mechanisms involved, these data clearly suggest that the process by which plasmid is internalized and expressed is fundamentally different in the tumor as compared to other organs.
Figure 4.
Plasmid levels (A) and luciferase expression (B) were quantified in tissues extracted from mice 24 h after the first and fourth administration of lipoplexes. The bars represent the mean and one standard error of tissues extracted from three mice. Note log scale in B. Asterisks indicate statistically significant differences (p < 0.05).
Liver Toxicity
Liver toxicity was assessed by measuring the levels of alanine aminotransferase (ALT) in the blood 24 h after the first, second, and fourth injection. In addition, blood samples were also collected at 48 h and 72 h after a fourth injection to monitor the ability of the liver to recover from repetitive injections. A separate set of tumor-bearing mice was also administered PBS as a control to determine the extent to which ALT levels are altered in response to repetitive injections. As shown in Figure 5, ALT levels 24 h after a single dose of lipoplexes were comparable to that exhibited by mice administered PBS. However, ALT levels were elevated by almost four-fold 24 h after the second and fourth dose, as compared to that observed after a single dose of lipoplexes. ALT levels gradually receded after the fourth dose eventually reaching levels that were elevated less than two-fold after 72 h, as compared to that seen 24 h after a single injection of lipoplexes (Fig. 5).
In vivo Imaging
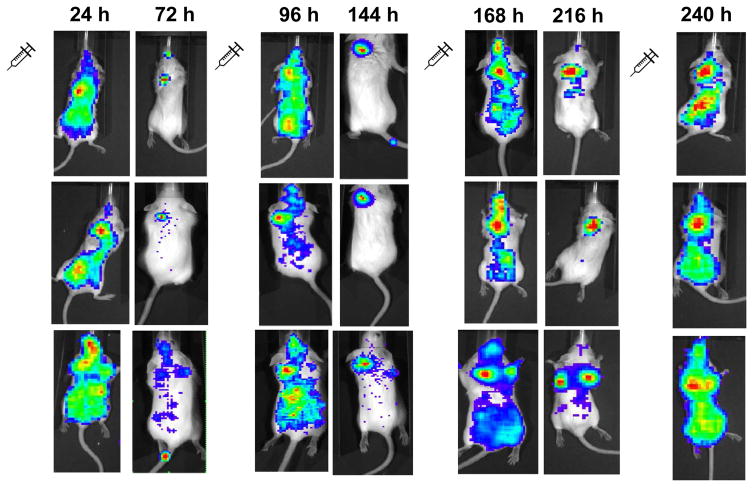
Luciferase expression in live mice was imaged 24 h and 72 h after the first three injections, and 24 h after the fourth injection. Figure 6 shows representative images at each time point from three different mice. The most striking result is the wide distribution of expression 24 h after each injection as compared to expression 72 h after each injection which is largely confined to the tumor. It is important to remember that the imaging of luciferase expression is highly depth-dependent, and therefore images that depict luciferase activity that is limited to the tumor should not be interpreted as evidence that expression occurs only in the tumor. Furthermore, organs were only extracted 24 h after the first and fourth injection, therefore reliable quantification of plasmid levels and luciferase expression at timepoints where imaging depicts expression primarily localized to the tumor (i.e., 72 h after each injection) was not possible. Attempts to quantitatively evaluate images from these experiments did not yield consistent results. However, successive images in each mouse show a general trend of progressively increasing luciferase expression in tumors after each dose of lipoplexes.
Figure 6.
Luciferase activity was imaged in mice 24 and 72 h after repetitive injections of lipoplexes. Each row of images was taken from an individual mouse at the indicated times.
Discussion
The goal of repetitive dosing of traditional pharmaceuticals is to maintain blood levels at therapeutic levels. Accordingly, a subsequent dose is timed such that the declining blood levels from the previous dose are boosted to maintain the minimum therapeutic drug concentration. This conventional paradigm assumes that each dose displays similar pharmacokinetics, which is typical for small molecule drugs. However, macromolecular therapeutics and particulate delivery systems administered intravenously can elicit immune responses that alter the clearance of subsequent doses[27, 28, 34–40]. This effect has been extensively characterized for PEGylated liposomes due to the concerns over immune responses to PEG conjugates; we and others have questioned the advantages of PEGylation when it comes to drug delivery, especially when pursuing intracellular delivery[5, 9, 11, 38, 41]. In contrast, it has been reported that liposomes lacking PEG elicit minimal, if any, immune response that would result in accelerated blood clearance[28, 36, 37]. Our data with lipoplexes lacking PEG are consistent with these latter reports, and no significant differences between injections were observed. Admittedly, the small number of animals used in our study could have obscured differences that might be observed in a larger cohort. However, the lipid doses administered in our study (12.6 μmole/kg) are considerably higher than those of PEGylated liposomes employed in studies reporting an ABC effect (0.001 μmole/kg priming dose, 5 μmole/kg subsequent dose)[28, 42]. Moreover, a three-day interval between injections of PEGylated liposomes was found to cause dramatic changes in blood levels (p < 0.005) [28, 42], further indicating that our formulation exhibits minimal, if any, accelerated blood clearance.
In addition to PEGylation, it is important to recognize that the DNA in delivery systems can also elicit an immune response that could potentially contribute to accelerated blood clearance [20, 24, 43]. In order to reduce the potential immune response to the plasmid used in our experiments, we have minimized the number of CpG motifs. Also, the lipoplexes employed here are comprised of naturally-occurring lipids (i.e., phosphatidylcholine, cholesterol, sphingosine) which greatly reduce their toxicity and potential immunogenicity, as compared to other gene delivery vehicles[20]. Consistent with the absence of accelerated blood clearance, the cytokine/chemokine response to repeat administration of our formulation is comparable to that for saline, with the exception of KC (Figure 4, Table I). Although incorporation of a plasmid containing extensive CpG sequences caused mild elevation of three additional cytokines, formulations containing DOTAP, PEG or lipofectamine elicited elevation of a large number of cytokines and chemokines. These findings are consistent with previous studies showing a correlation between cytokine/chemokine responses and the IgM production responsible for accelerated blood clearance[32, 33, 44, 45]. In particular, IL-6 and IFN-gamma are related to IgM production in B cells, but neither of these were elevated when the sphingosine-based formulation was administered, which likely contributes to the lack of accelerated blood clearance we observe[33, 46]. We conclude that the lipoplex modifications we have employed appear to make it unnecessary to co-administer liposomal chemotherapeutics in order achieve safe sequential administration as reported by Kiwada and colleagues [7, 32, 47].
Curiously, repeat administration of the sphingosine-based formulation did elicit elevated levels of KC; a chemoattractant that recruits leukocytes. While immune cell recruitment is characteristic of inflammatory responses, recruitment alone is not sufficient for inflammation[48]. Previous studies have shown elevated KC in response to liver injury[49], consistent with the higher ALT measurements we observe (Fig. 5). More specifically, elevation of KC is associated with a damage response, as opposed to the danger response that would be elicited upon invasion by exogenous (“non-self”) entities[48]. We believe that the ability of our particles to avoid triggering the foreign body response likely allows us to elude the specific IgM production responsible for accelerated blood clearance, thereby enabling safe, repetitive administration.
Our observation that repetitive administration enhances delivery is consistent with previous studies with both plasmid DNA and siRNA[6, 12, 14, 17]. In contrast, a recent study by Lindberg et al. reported that repeat administration of lipoplexes was only able to regain (i.e., not increase) expression levels observed after an initial dose[18]. Furthermore, these authors clearly demonstrated that a refractory period of several weeks was required between administrations in order to achieve transgene expression comparable to the initial dose, consistent with other studies[50, 51]. Our results differ sharply from these observations, and suggest that delivery is increased beyond that seen after the initial administration, and this was achieved by successive injections that were just three days apart. Consistent with the lack of a refractory period with our lipoplexes, we have observed similar increases in expression when four injections were administered at only one day intervals (data not shown). The refractory period observed in previous studies is thought to be closely related to immunostimulatory effects of both the nucleic acid and the delivery vehicle[18, 51, 52]. As described above, we have utilized a plasmid with minimal CpG sequences, avoided the use of PEGylation, and employed naturally-occurring lipids, and we propose that these factors are responsible for the lack of cytokine/cytokine response and refractory period observed in our study. However, our study only evaluated a single dose of lipoplexes, and additional studies would be required to determine if a refractory period would be observed at different doses and/or dosing intervals.
Curiously, the enhancement of both plasmid accumulation and expression after repetitive administration showed opposite trends, i.e., the increase in plasmid accumulation exceeded that of expression in the tumor whereas the enhancement in plasmid expression greatly exceeded that of accumulation in the other organs (compare Figs 3+4). These distinctly different trends suggest that the timing of plasmid delivery relative to expression is fundamentally different in tumors as compared to other organs. More specifically, plasmid levels in the tumor at 24 h were > 5-fold lower than that in the liver, lung, spleen or kidney, yet luciferase activity in the tumor was higher than any of these organs. Moreover, luciferase activity at 24 h was 11% higher in the tumor than in the lung, despite plasmid levels that were 90% lower! These results indicate that plasmid delivered to the tumor is more readily available for expression, in agreement with previous findings [53, 54]. This is consistent with the lower degree of expression enhancement in the tumor (10-fold) after repetitive injections as compared to that observed in the other organs (> 200-fold) due to more rapid expression of delivered plasmids in the tumor at 24 h. This effect could potentially be due to the greater rate of cell division in tumors that allow plasmids to more readily access the nucleus as compared to cells associated with other tissues that divide more slowly[55–58]. According to this hypothesis, plasmid delivered to non-tumor cells exhibit delayed expression due to reduced rates of cell division, and this contributes to the much larger enhancement of expression observed at later times (i.e., after 4 injections). Considering that the lifetime of free plasmids in the cytoplasm is only 50–90 minutes[59, 60], the rate of lipoplex dissociation likely plays a role in maintaining plasmid integrity prior to mitosis. Alternatively, differences in cell type between tumors and other tissues (e.g., macrophages in lung, liver, and spleen) might result in dissimilar trafficking and/or more rapid degradation of internalized plasmid.
In addition to the very different trends in luciferase activity noted above, repetitive administration was able to enhance plasmid delivery to the tumor to a much greater extent than in other organs (compare Figs. 3A and 4A). This curious result is consistent with the enhanced delivery to tumors observed in previous studies employing repetitive injections[6, 12, 14], but the precise mechanism responsible for this effect is unclear. Previous studies have suggested that cell killing due to co-administration of liposomal chemotherapeutics reduces interstitial pressure and increases interstitial space within the tumor, which is proposed to facilitate accumulation from subsequent injections[7, 32, 47]. While this may be an effective strategy for enhancing tumor accumulation, our experiments did not employ chemotherapeutics, and thus we do not feel that these mechanisms are relevant to our observations. However, the vasculature of tumors, in contrast to established organs, is rapidly changing, and thus it is possible that particle deposition alters tumor vascularization. Such an effect has recently been described by Sabnani et al.[61], and earlier studies have demonstrated that particulate delivery systems primarily deposit on the tumor vasculature[14, 15, 62]. Therefore, it is possible that particle deposition selectively stimulates greater vascularization in the tumor, which thereby enhances delivery via subsequent injections. Alternatively, the rapid growth rate of 4T1 cells can cause a significant difference in tumor vascularity and size between the first and fourth injections that might alter particle deposition[63]. Further studies would need to be conducted to test these hypotheses.
The images in figure 6 show a broad initial distribution throughout the body after 24 h that is predominantly confined to the tumor 72 h after each injection. Similar images have been generated after administration of fluorescently-labelled macromolecules, and the localization to the tumor in later images has been presented as evidence for reduced lymphatic clearance according to the enhanced permeation and retention effect[64]. But it is important to realize that our images are fundamentally different because they depict expression of a delivered plasmid by recipient cells as opposed to distribution of the administered particles. As discussed above, previous studies have shown that efficient expression of plasmid requires cell division[55–58], and thus our images depict the presence of dividing cells throughout the body. This finding is consistent with studies showing that lipoplexes administered intravenously predominantly transfect the vascular endothelium, which is known to possess rapidly-proliferating cells [14, 15, 65, 66]. However, the images taken 72 h after each injection indicate that luciferase expression as detected by the IVIS imaging system is dramatically reduced in the majority of the body. The depth dependence of luminescence detection complicates interpretations of the images in figure 5[67], but these findings suggest that a large fraction of the luciferase expression present in tissues at 24 h is not observed at 72 h. Furthermore, this effect was clearly evident after each of the first three injections, and we speculate that transfected cells of the rapidly dividing vascular endothelium are routinely sloughed off, resulting in the apparent disappearance of expression after 72 h. This suggestion is consistent with studies showing that cell proliferation in the vascular endothelium occurs continuously due to injury from normal blood flow, and that damaged cells are sloughed into the bloodstream and eliminated [68, 69]. It is possible that additional injury is produced during intravenous injection (especially in animal models), which could further contribute to our observations. Although quantification of the extent to which plasmid accumulates in non-vascular tissues after repetitive injection would require more sophisticated experimentation, we hypothesize that characterization at later timepoints (i.e., ≥ 72 h) might provide results that more accurately reflect delivery to cells outside of the vasculature. The fact that the images do not depict dramatic reductions in luciferase activity within the tumors suggests that lipoplexes were able to more readily access longer-lived cells within the tumor, consistent with the altered vasculature associated with tumors[12, 64, 70–73].
In conclusion, our data demonstrate that our lipoplex formulation is rapidly cleared from the plasma, and we do not observe the ABC phenomenon described for PEGylated liposomes. While the lack of PEGylation in our delivery system may be responsible for the rapid blood clearance, this may also explain why the ABC effect was not evident. Furthermore, we suggest that the low CpG content and less aggressive promoter in our plasmid combined with the low amounts of naturally-occurring lipids in our delivery vehicle result in minimal immunostimulation that allows effective repetitive administration without the ABC or refractory period observed in other studies. This suggestion is consistent with the low cytokine/chemokine response we observe after repetitive administration of our sphingosine-based lipoplex. Plasmid levels in the tumor after a single injection were relatively low compared to other organs, but accumulation was comparable on a per gram tissue basis after four injections administered at three-day intervals. In contrast, luciferase expression was higher in the tumor 24 h after the initial injection, but lower than lung, liver, and spleen 24 h after the fourth injection. Liver enzyme (ALT) levels were slightly elevated (2X that of saline) after the first injection, and became more highly elevated after subsequent administrations. In vivo imaging revealed a wide distribution of expression throughout the body 24 h after each injection that was predominantly confined to the tumor after 72 h. Most importantly, these results demonstrate that plasmid levels and reporter gene expression in the tumor are greatly enhanced after repetitive administration. The observation that delivery is enhanced by much greater than four-fold after four injections suggests that an administration of lipoplexes may preferentially affect the tumor environment in a way that promotes tumor uptake and/or retention of subsequent doses. Future studies will investigate the extent to which these observations are applicable to other lipoplex formulations and/or tumor models.
Acknowledgments
This work was supported by grant #1RO1GM093287 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xu L, Anchordoquy T. Drug delivery trends in clinical trials and translational medicine: challenges and opportunities in the delivery of nucleic acid-based therapeutics. Journal of pharmaceutical sciences. 2011;100:38–52. doi: 10.1002/jps.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, et al. Lipofection - a Highly Efficient, Lipid-Mediated DNA-Transfection Procedure. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:7413–7. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felgner PL, Ringold GM. Cationic Liposome-Mediated Transfection. Nature. 1989;337:387–8. doi: 10.1038/337387a0. [DOI] [PubMed] [Google Scholar]
- 4.Xue W, Chen SD, Yin H, Tammela T, Papagiannakopoulos T, Joshi NS, et al. CRISPR-mediated direct mutation of cancer genes in the mouse liver. Nature. 2014;514:380. doi: 10.1038/nature13589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charrois GJR, Allen TM. Multiple injections of pegylated liposomal doxorubicin: Pharmacokinetics and therapeutic activity. Journal of Pharmacology and Experimental Therapeutics. 2003;306:1058–67. doi: 10.1124/jpet.103.053413. [DOI] [PubMed] [Google Scholar]
- 6.Meyer O, Schughart K, Pavirani A, Kolbe HVJ. Multiple systemic expression of human inferferon-beta in mice can be achieved upon repeated administration of optimized pcTG90-lipoplex. Gene therapy. 2000;7:1606–11. doi: 10.1038/sj.gt.3301289. [DOI] [PubMed] [Google Scholar]
- 7.Abu Lila AS, Eldin NE, Ichihara M, Ishida T, Kiwada H. Multiple administration of PEG-coated liposomal oxaliplatin enhances its therapeutic efficacy: A possible mechanism and the potential for clinical application. Int J Pharmaceut. 2012;438:176–83. doi: 10.1016/j.ijpharm.2012.08.030. [DOI] [PubMed] [Google Scholar]
- 8.Harasym TO, Cullis PR, Bally MB. Intratumor distribution of doxorubicin following i.v. administration of drug encapsulated in egg phosphatidylcholine/cholesterol liposomes. Cancer chemotherapy and pharmacology. 1997;40:309–17. doi: 10.1007/s002800050662. [DOI] [PubMed] [Google Scholar]
- 9.Hong RL, Huang CJ, Tseng YL, Pang VF, Chen ST, Liu JJ, et al. Direct comparison of liposomal doxorubicin with or without polyethylene glycol coating in C-26 tumor-bearing mice: Is surface coating with polyethylene glycol beneficial? Clinical Cancer Research. 1999;5:3645–52. [PubMed] [Google Scholar]
- 10.Mayer LD, Dougherty G, Harasym TO, Bally MB. The role of tumor-associated macrophages in the delivery of liposomal doxorubicin to solid murine fibrosarcoma tumors. Journal of Pharmacology and Experimental Therapeutics. 1997;280:1406–14. [PubMed] [Google Scholar]
- 11.Parr MJ, Masin D, Cullis PR, Bally MB. Accumulation of liposomal lipid and encapsulated doxorubicin in murine Lewis lung carcinoma: The lack of beneficial effects by coating liposomes with poly(ethylene glycol) Journal of Pharmacology and Experimental Therapeutics. 1997;280:1319–27. [PubMed] [Google Scholar]
- 12.Aleku M, Fisch G, Mopert K, Keil O, Arnold W, Kaufmann J, et al. Intracellular localization of lipoplexed siRNA in vascular endothelial cells of different mouse tissues. Microvasc Res. 2008;76:31–41. doi: 10.1016/j.mvr.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Muroski ME, Carnevale KJF, Riskowski RA, Strouse GF. Plasmid Transfection in Mammalian Cells Spatiotemporally Tracked by a Gold Nanoparticle. Acs Nano. 2015;9:124–33. doi: 10.1021/nn5060305. [DOI] [PubMed] [Google Scholar]
- 14.Santel A, Aleku M, Keil O, Endruschat J, Esche V, Fisch G, et al. A novel siRNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gene therapy. 2006;13:1222–34. doi: 10.1038/sj.gt.3302777. [DOI] [PubMed] [Google Scholar]
- 15.Thurston G, McLean JW, Rizen M, Baluk P, Haskell A, Murphy TJ, et al. Cationic liposomes target angiogenic endothelial cells in tumors and chronic inflammation in mice. J Clin Invest. 1998;101:1401–13. doi: 10.1172/JCI965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uyechi LS, Gagne L, Thurston G, Szoka FC. Mechanism of lipoplex gene delivery in mouse lung: binding and internalization of fluorescent lipid and DNA components. Gene therapy. 2001;8:828–36. doi: 10.1038/sj.gt.3301461. [DOI] [PubMed] [Google Scholar]
- 17.Alton EWFW, Armstrong DK, Ashby D, Bayfield KJ, Bilton D, Bloomfield EV, et al. Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Resp Med. 2015;3:684–91. doi: 10.1016/S2213-2600(15)00245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindberg MF, Le Gall T, Carmoy N, Berchel M, Hyde SC, Gill DR, et al. Efficient in vivo transfection and safety profile of a CpG-free and codon optimized luciferase plasmid using a cationic lipophosphoramidate in a multiple intravenous administration procedure. Biomaterials. 2015;59:1–11. doi: 10.1016/j.biomaterials.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 19.Betker JL, Anchordoquy TJ. Effect of charge ratio on lipoplex-mediated gene delivery and liver toxicity. Therapeutic delivery. 2015;6:1243–53. doi: 10.4155/tde.15.77. [DOI] [PubMed] [Google Scholar]
- 20.Betker JL, Anchordoquy TJ. Relating toxicity to transfection: using sphingosine to maintain prolonged expression in vitro. Molecular pharmaceutics. 2015;12:264–73. doi: 10.1021/mp500604r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu L, Anchordoquy TJ. Cholesterol domains in cationic lipid/DNA complexes improve transfection. Biochimica et biophysica acta. 2008;1778:2177–81. doi: 10.1016/j.bbamem.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Bradshaw-Pierce EL, Delille A, Gustafson DL, Anchordoquy TJ. In vivo comparative study of lipid/DNA complexes with different in vitro serum stability: effects on biodistribution and tumor accumulation. Journal of pharmaceutical sciences. 2008;97:237–50. doi: 10.1002/jps.21076. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Anchordoquy TJ. The role of lipid charge density in the serum stability of cationic lipid/DNA complexes. Biochimica et biophysica acta. 2004;1663:143–57. doi: 10.1016/j.bbamem.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Watcharanurak K, Nishikawa M, Takahashi Y, Takakura Y. Controlling the kinetics of interferon transgene expression for improved gene therapy. Journal of drug targeting. 2012;20:764–9. doi: 10.3109/1061186X.2012.716848. [DOI] [PubMed] [Google Scholar]
- 25.Betker JL, Gomez J, Anchordoquy TJ. The effects of lipoplex formulation variables on the protein corona and comparisons with in vitro transfection efficiency. Journal of controlled release : official journal of the Controlled Release Society. 2013;171:261–8. doi: 10.1016/j.jconrel.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abrams MT, Koser ML, Seitzer J, Williams SC, DiPietro MA, Wang W, et al. Evaluation of efficacy, biodistribution, and inflammation for a potent siRNA nanoparticle: effect of dexamethasone co-treatment. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18:171–80. doi: 10.1038/mt.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dams ET, Laverman P, Oyen WJ, Storm G, Scherphof GL, van Der Meer JW, et al. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. The Journal of pharmacology and experimental therapeutics. 2000;292:1071–9. [PubMed] [Google Scholar]
- 28.Ishida T, Ichihara M, Wang X, Yamamoto K, Kimura J, Majima E, et al. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. Journal of controlled release : official journal of the Controlled Release Society. 2006;112:15–25. doi: 10.1016/j.jconrel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Sakurai F, Nishioka T, Yamashita F, Takakura Y, Hashida M. Effects of erythrocytes and serum proteins on lung accumulation of lipoplexes containing cholesterol or DOPE as a helper lipid in the single-pass rat lung perfusion system. Eur J Pharm Biopharm. 2001;52:165–72. doi: 10.1016/s0939-6411(01)00165-5. [DOI] [PubMed] [Google Scholar]
- 30.Simberg D, Weisman S, Talmon Y, Faerman A, Shoshani T, Barenholz Y. The role of organ vascularization and lipoplex-serum initial contact in intravenous murine lipofection. J Biol Chem. 2003;278:39858–65. doi: 10.1074/jbc.M302232200. [DOI] [PubMed] [Google Scholar]
- 31.Betker JL, Anchordoquy TJ. Assessing the effect of a nude mouse model on nanparticle-mediated gene delivery. Drug Deliv Transl Re. doi: 10.1007/s13346-016-0327-6. in press. [DOI] [PubMed] [Google Scholar]
- 32.Alaaeldin E, Abu Lila AS, Moriyoshi N, Sarhan HA, Ishida T, Khaled KA, et al. The Co-Delivery of Oxaliplatin Abrogates the Immunogenic Response to PEGylated siRNA-Lipoplex. Pharm Res-Dordr. 2013;30:2344–54. doi: 10.1007/s11095-013-1078-4. [DOI] [PubMed] [Google Scholar]
- 33.Tagami T, Uehara Y, Moriyoshi N, Ishida T, Kiwada H. Anti-PEG IgM production by siRNA encapsulated in a PEGylated lipid nanocarrier is dependent on the sequence of the siRNA. Journal of Controlled Release. 2011;151:149–54. doi: 10.1016/j.jconrel.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 34.Moghimi SM, Hamad I, Bunger R, Andresen TL, Jorgensen K, Hunter AC, et al. Activation of the human complement system by cholesterol-rich and pegylated liposomes - Modulation of cholesterol-rich liposome-mediated complement activation by elevated serum LDL and HDL levels. J Liposome Res. 2006;16:167–74. doi: 10.1080/08982100600848801. [DOI] [PubMed] [Google Scholar]
- 35.Moghimi SM, Hunter AC, Andresen TL. Factors Controlling Nanoparticle Pharmacokinetics: An Integrated Analysis and Perspective. Annu Rev Pharmacol. 2012;52:481–503. doi: 10.1146/annurev-pharmtox-010611-134623. [DOI] [PubMed] [Google Scholar]
- 36.Saadati R, Dadashzadeh S, Abbasian Z, Soleimanjahi H. Accelerated Blood Clearance of PEGylated PLGA Nanoparticles Following Repeated Injections: Effects of Polymer Dose, PEG Coating, and Encapsulated Anticancer Drug. Pharm Res-Dordr. 2013;30:985–95. doi: 10.1007/s11095-012-0934-y. [DOI] [PubMed] [Google Scholar]
- 37.Semple SC, Harasym TO, Clow KA, Ansell SM, Klimuk SK, Hope MJ. Immunogenicity and rapid blood clearance of liposomes containing polyethylene glycol-lipid conjugates and nucleic acid. Journal of Pharmacology and Experimental Therapeutics. 2005;312:1020–6. doi: 10.1124/jpet.104.078113. [DOI] [PubMed] [Google Scholar]
- 38.Verhoef JJF, Anchordoquy TJ. Questioning the use of PEGylation for drug delivery. Drug Deliv Transl Re. 2013;3:499–503. doi: 10.1007/s13346-013-0176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verhoef JJF, Carpenter JF, Anchordoquy TJ, Schellekens H. Potential induction of anti-PEG antibodies and complement activation toward PEGylated therapeutics. Drug Discov Today. 2014;19:1945–52. doi: 10.1016/j.drudis.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Ishida T, Kiwada H. Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. Journal of controlled release : official journal of the Controlled Release Society. 2007;119:236–44. doi: 10.1016/j.jconrel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 41.Waterhouse DNT, PG, Mayer LD, Bally MB. A comparison of liposomal formulations of doxorubicin with drug administered in free form. Drug Safety. 2001;24:903–20. doi: 10.2165/00002018-200124120-00004. [DOI] [PubMed] [Google Scholar]
- 42.Ishida T, Kiwada H. Accelerated blood clearance (ABC) phenomenon upon repeated injection of PEGylated liposomes. Int J Pharmaceut. 2008;354:56–62. doi: 10.1016/j.ijpharm.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Tagami T, Nakamura K, Shimizu T, Yamazaki N, Ishida T, Kiwada H. CpG motifs in pDNA-sequences increase anti-PEG IgM production induced by PEG-coated pDNA-lipoplexes. Journal of Controlled Release. 2010;142:160–6. doi: 10.1016/j.jconrel.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 44.Glaum MC, Narula S, Song D, Zheng Y, Anderson AL, Pletcher CH, et al. Toll-like receptor 7-induced naive human B-cell differentiation and immunoglobulin production. J Allergy Clin Immun. 2009;123:224–30. doi: 10.1016/j.jaci.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 45.Hanten JA, Vasilakos JP, Riter CL, Neys L, Lipson KE, Alkan SS, et al. Comparison of human B cell activation by TLR7 and TLR9 agonists. Bmc Immunol. 2008;9 doi: 10.1186/1471-2172-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yi AK, Chace JH, Cowdery JS, Krieg AM. IFN-gamma promotes IL-6 and IgM secretion in response to CpG motifs in bacterial DNA and oligodeoxynucleotides. J Immunol. 1996;156:558–64. [PubMed] [Google Scholar]
- 47.Abu Lila AS, Doi Y, Nakamura K, Ishida T, Kiwada H. Sequential administration with oxaliplatin-containing PEG-coated cationic liposomes promotes a significant delivery of subsequent dose into murine solid tumor. Journal of Controlled Release. 2010;142:167–73. doi: 10.1016/j.jconrel.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 48.Matzinger P. Tolerance, Danger, and the Extended Family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 49.Son DS, Parl AK, Rice VM, Khabele D. Keratinocyte chemoattractant (KC)/human growth-regulated oncogene (GRO) chemokines and pro-inflammatory chemokine networks in mouse and human ovarian epithelial cancer cells. Cancer Biol Ther. 2007;6:1302–12. doi: 10.4161/cbt.6.8.4506. [DOI] [PubMed] [Google Scholar]
- 50.Li S, Huang L. In vivo gene transfer via intravenous administration of cationic lipid-protamine-DNA (LPD) complexes. Gene therapy. 1997;4:891–900. doi: 10.1038/sj.gt.3300482. [DOI] [PubMed] [Google Scholar]
- 51.Song YK, Liu F, Chu SY, Liu DX. Characterization of cationic liposome-mediated gene transfer in vivo by intravenous administration. Hum Gene Ther. 1997;8:1585–94. doi: 10.1089/hum.1997.8.13-1585. [DOI] [PubMed] [Google Scholar]
- 52.Tan YD, Li S, Pitt BR, Huang L. The inhibitory role of CpG immunostimulatory motifs in cationic lipid vector-mediated transgene expression in vivo. Hum Gene Ther. 1999;10:2153–61. doi: 10.1089/10430349950017149. [DOI] [PubMed] [Google Scholar]
- 53.Heyes J, Palmer L, Chan K, Giesbrecht C, Jeffs L, MacLachlan I. Lipid encapsulation enables the effective systemic delivery of polyplex plasmid DNA. Molecular Therapy. 2007;15:713–20. doi: 10.1038/sj.mt.6300101. [DOI] [PubMed] [Google Scholar]
- 54.Ambegia E, Ansell S, Cullis P, Heyes J, Palmer L, MacLachlan I. Stabilized plasmid-lipid particles containing PEG-diacylglycerols exhibit extended circulation lifetimes and tumor selective gene expression. Bba-Biomembranes. 2005;1669:155–63. doi: 10.1016/j.bbamem.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Brunner S, Sauer T, Carotta S, Cotten M, Saltik M, Wagner E. Cell cycle dependence of gene transfer by lipoplex polyplex and recombinant adenovirus. Gene therapy. 2000;7:401–7. doi: 10.1038/sj.gt.3301102. [DOI] [PubMed] [Google Scholar]
- 56.Fasbender A, Zabner J, Zeiher BG, Welsh MJ. A low rate of cell proliferation and reduced DNA uptake limit cationic lipid-mediated gene transfer to primary cultures of ciliated human airway epithelia. Gene therapy. 1997;4:1173–80. doi: 10.1038/sj.gt.3300524. [DOI] [PubMed] [Google Scholar]
- 57.Mortimer I, Tam P, MacLachlan I, Graham RW, Saravolac EG, Joshi PB. Cationic lipid-mediated transfection of cells in culture requires mitotic activity. Gene therapy. 1999;6:403–11. doi: 10.1038/sj.gt.3300837. [DOI] [PubMed] [Google Scholar]
- 58.Tseng WC, Haselton FR, Giorgio TD. Mitosis enhances transgene expression of plasmid delivered by cationic liposomes. Bba-Gene Struct Expr. 1999;1445:53–64. doi: 10.1016/s0167-4781(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 59.Pollard H, Toumaniantz G, Amos JL, Avet-Loiseau H, Guihard G, Behr JP, et al. Ca2+-sensitive cytosolic nucleases prevent efficient delivery to the nucleus of injected plasmids. Journal of Gene Medicine. 2001;3:153–64. doi: 10.1002/jgm.160. [DOI] [PubMed] [Google Scholar]
- 60.Lechardeur D, Sohn KJ, Haardt M, Joshi PB, Monck M, Graham RW, et al. Metabolic instability of plasmid DNA in the cytosol: a potential barrier to gene transfer. Gene therapy. 1999;6:482–97. doi: 10.1038/sj.gt.3300867. [DOI] [PubMed] [Google Scholar]
- 61.Sabnani MK, Rajan R, Rowland B, Mavinkurve V, Wood LM, Gabizon AA, et al. Liposome promotion of tumor growth is associated with angiogenesis and inhibition of antitumor immune responses. Nanomedicine : nanotechnology, biology, and medicine. 2015;11:259–62. doi: 10.1016/j.nano.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 62.Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WC. Mediating tumor targeting efficiency of nanoparticles through design. Nano letters. 2009;9:1909–15. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 63.Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 Breast Tumor Model. Current Protocols in Immunology. 2000:20.2.1–.2.16. doi: 10.1002/0471142735.im2002s39. [DOI] [PubMed] [Google Scholar]
- 64.Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Advanced drug delivery reviews. 2013;65:71–9. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 65.Schwartz SM, Benditt EP. Clustering of Replicating Cells in Aortic Endothelium. Proceedings of the National Academy of Sciences of the United States of America. 1976;73:651–3. doi: 10.1073/pnas.73.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wright HP. Mitosis patterns in aortic endothelium. Atherosclerosis. 1972;15:93–100. doi: 10.1016/0021-9150(72)90042-1. [DOI] [PubMed] [Google Scholar]
- 67.Zinn KR, Chaudhuri TR, Szafran AA, O’Quinn D, Weaver C, Dugger K, et al. Noninvasive bioluminescence imaging in small animals. Ilar J. 2008;49:103–15. doi: 10.1093/ilar.49.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caplan BA, Schwatrz CJ. increased endothelial cel turnover in areas of in vivo evans blue uptake in the pig aorta. Atherosclerosis. 1973;17:401–17. doi: 10.1016/0021-9150(73)90031-2. [DOI] [PubMed] [Google Scholar]
- 69.Yoder MC. Is Endothelium the Origin of Endothelial Progenitor Cells? Arterioscl Throm Vas. 2010;30:1094–103. doi: 10.1161/ATVBAHA.109.191635. [DOI] [PubMed] [Google Scholar]
- 70.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, et al. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4607–12. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu NZ, Da D, Rudoll TL, Needham D, Whorton AR, Dewhirst MW. Increased Microvascular Permeability Contributes to Preferential Accumulation of Stealth Liposomes in Tumor-Tissue. Cancer research. 1993;53:3765–70. [PubMed] [Google Scholar]
- 72.Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156:1363–80. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nagy JA, Chang SH, Shih SC, Dvorak AM, Dvorak HF. Heterogeneity of the Tumor Vasculature. Semin Thromb Hemost. 2010;36:321–31. doi: 10.1055/s-0030-1253454. [DOI] [PMC free article] [PubMed] [Google Scholar]