Abstract
The basis for neuronal dysfunction following inflammatory demyelination of the central nervous system (CNS) remains poorly understood. We characterized the network response to white matter injury in the anterior visual pathway using an experimental model of optic neuritis (ON), as ON is often an early manifestation of immune-mediated CNS demyelination in multiple sclerosis (MS). Optical intrinsic signal imaging was performed before and after the induction of ON in mice to measure changes in cortical network functional connectivity. We observed a greater loss of connectivity between homotopic visual cortices in ON mice compared to controls. Further, decreases in homotopic visual cortex connectivity were associated with visual acuity loss in ON mice. These results demonstrate that network connectivity changes resulting from ON can be modeled in an experimental murine system. Future studies will identify the mechanisms that cause neuronal dysfunction due to white matter injury seen in MS.
Keywords: Functional Connectivity, Optic Neuritis, Multiple Sclerosis, Demyelination, Optical Imaging, Visual Cortex
Introduction
Murine models such as experimental autoimmune encephalomyelitis (EAE) have been instrumental in the identification of immune mechanisms involved in the hallmark myelin and nerve damage of multiple sclerosis (MS) (Wu, et al., 2011). However, it remains unclear how inflammatory demyelination of the central nervous system (CNS) produces acute as well as lasting neurologic disability. Examining neuronal changes that occur with white matter injury in EAE provides tremendous potential for uncovering the mechanisms of disability seen in MS.
Changes in visual processing are often a heralding sign of MS. The visual system is therefore an excellent candidate for assessing progression and accrual of disability in MS. Similar to MS, mice with EAE often develop optic neuritis (ON) (Bettelli, et al., 2003). In fact, ON can be an isolated feature in murine models of inflammatory demyelination, with spontaneous development occurring in over 40% of mice that express the transgenic T cell receptor (TCR) specific for myelin oligodendrocyte glycoprotein (MOG) (Bettelli, et al., 2003).
In humans, functional MRI (fMRI) has been used to study the presence of functional connections across brain networks, rooted in low frequency (0.008–0.09 Hz) oscillations, known as functional connectivity (FC) (Raichle, 2011). The blood oxygen level dependent (BOLD) signal typically used in fMRI is based on oscillations in local concentrations of oxy- and deoxy-hemoglobin. These changes are a proxy for neural activity and have been used to identify network structure (Raichle, 2011).
Alternatively, optical neuroimaging methods can generate hemodynamic FC mapping in animal models that do not require high-field small-animal MRI scanners and are less costly (White, et al., 2011). One such technique, optical intrinsic signal (OIS) imaging, relies on changes in reflectance of incident light at various wavelengths due to absorption by hemoglobin in a manner analogous to BOLD fMRI (White, et al., 2011). Transcranial FC OIS (fcOIS) imaging has already been used to reveal resting state FC networks in a murine system (White, et al., 2011). Mouse fcOIS has been shown to be a sensitive assay for several neurological diseases, including ischemic stroke and Alzheimer’s disease (Bauer, et al., 2014, Bergonzi, et al., 2015).
We recently reported connectivity changes in patients with acute ON that included loss of homotopic connectivity between the left and right primary visual cortices and a loss of anti-correlation between V1 and extra-visual regions compared to healthy subjects (Wu, et al., 2015). Notably, these changes were significantly correlated with visual outcome measures, suggesting that meaningful alterations of cortical network connectivity can be detected relatively early on in the disease course. However, identifying the underpinnings of these alterations in humans is difficult. Hence, interrogating well-controlled animal model systems of inflammatory CNS demyelination with fcOIS could aid in the elucidation of the mechanisms underlying changes in neurologic function during MS.
The goal of this study was to determine whether connectivity changes from ON could be modeled in a murine system using fcOIS. Using a mouse model of ON, we collected behavioral measures of visual acuity (VA) and optical imaging of spontaneous hemodynamic activity both before and after inducing ON. We observed a significant decline in VA following the induction of ON. Using fcOIS, we observed reductions in bilateral FC in the visual cortex in mice with ON. These results provide technical and mechanistic insight into changes of cortical function due to ON in an experimental animal model system. As such, these results could facilitate the testing of potential interventional strategies for inflammatory demyelinating diseases of the CNS.
Materials and Methods
Mice
Male and female C57BL/6 (B6) and 2D2 mice (Bettelli, et al., 2003) were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were housed in specific pathogen-free conditions. All animal experiments were performed in compliance with regulations specified by the Washington University in St. Louis Animal Studies Committee.
Optic neuritis induction and evaluation
To determine whether connectivity alterations are observed in murine ON, we examined 2D2 mice which are highly susceptible to ON (Bettelli, et al., 2003). Because spontaneous demyelination in rodents is quite variable, the frequency of ON in 2D2 mice was enhanced by immunization of a cohort of 16 2D2 mice with a low dose of the 35–55 peptide fragment of MOG (MOG35–55) in Complete Freunds Adjuvant (CFA) (Bettelli, et al., 2003). Approximately four weeks later (range: 24–28 days), ON was assessed histologically and detected in nine 2D2 mice (“ON+”). Optic nerves from all mice were dissected and frozen in OCT (Tissue-Tek) before sectioning at 4 μm. Tissue was stained with Hematoxylin and Eosin and scored for inflammatory infiltrates according to an established protocol (Wu, et al., 2011). Spatial visual acuity was performed using a virtual optomotor system (OptoMotry; CerebralMechanics, Lethbride, Alberta, Canada) as described (Douglas, et al., 2005). All mice were tested with both clockwise and counter-clockwise variable spatial frequency sine wave patterns. Visual thresholds for each animal were defined as the highest spatial frequency the animal could track as determined by the same observer masked to genotype. A cohort of seven 2D2 mice that did not develop ON served as controls. Additionally, because naive 2D2 mice are genetically identical to B6 mice except for the T cell receptor transgene directing T cell recognition of MOG, four B6 mice immunized with low-dose MOG35–55 that did not develop ON were added to the 2D2 cohort without ON, producing a total of 11 mice without ON (“ON−“), (Fig. 1A).
Figure 1. Altered FC over time in a murine model of ON.
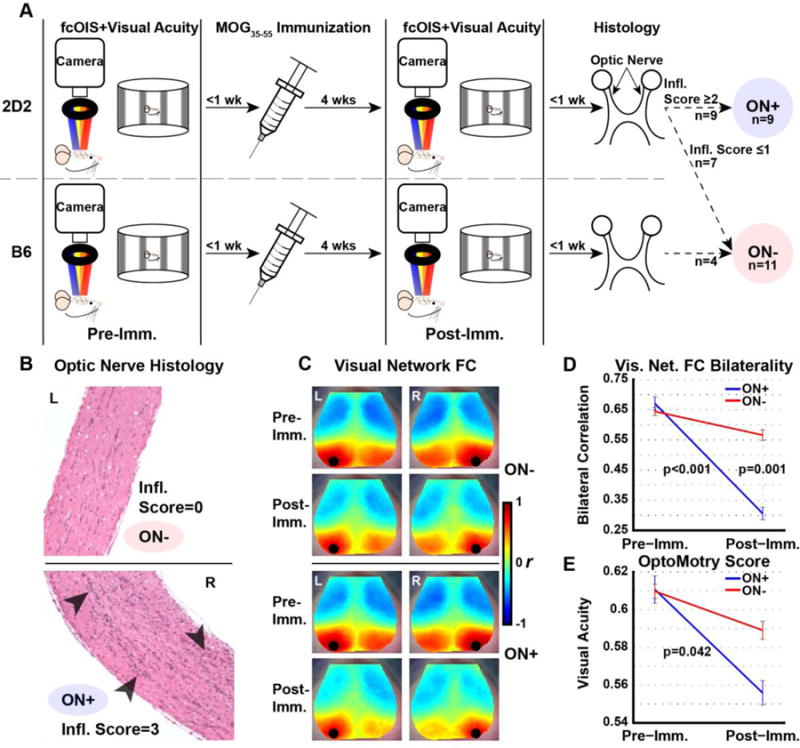
(A) Experimental design consisting of a baseline OptoMotry and fcOIS session, immunization with MOG35–55, and a second OptoMotry and fcOIS session for all 2D2 and B6 mice included in the study. Subsequently, optic nerves were harvested and scored for extent of inflammation to determine ON status. (B) Representative images from an unaffected optic nerve (inflammation score = 0, top) and with inflammatory infiltrates categorized as ON (inflammation score = 3, bottom). (C) Seed-based homotopic FC maps of visual, motor, and retrosplenial networks for the unaffected (WT+2D2) and ON groups at pre- and post-immunization time points. (D) Significant longitudinal reduction (p<0.001) in FC bilaterality in the visual network that was also significantly lower (p = 0.001) cross-sectionally in ON+ compared to ON− at the post-immunization timepoint. (E) Longitudinal group-wise OptoMotry performance scores are significantly reduced (p<0.05) in the ON+ group (n=9) but not in ON− mice (n=11).
Connectivity imaging
FcOIS acquisition was carried out using a previously described imaging system and approach (White, et al., 2011). Following intraperitoneal injection of 86.9 mg/kg of Ketamine and 13.4 mg/kg of Xylazine for anesthesia along with local Lidocaine injection and resection of the scalp, mice were placed on a heating pad maintained at 37°C (mTCII, Cell Microcontrols) while their heads were secured in a stereotactic frame. Sequential illumination was provided by LEDs at four wavelengths (LEDs; 478 nm, 588 nm, 610 nm, and 625 nm; RLS-5B475-S, B5B-4343-TY, B5B435-30S, and OSCR5111A-WY, respectively, Roithner Lasertechnik) and a cooled, frame-transfer EMCCD camera (iXon 897, Andor Technologies) was used for image detection with a field of view of approximately 1cm2, The LEDs and camera were time synchronized and externally triggered using custom software (MATLAB, Mathworks). A frame-rate of 120 Hz was used to acquire images at 30 Hz with four temporally encoded wavelengths. To minimize specular reflection from the surface of the mouse skull, crossed linear polarizers were placed immediately in front of the LEDs and the camera lens.
Image processing and FC analysis
Image processing and analysis approaches have been previously described (White, et al., 2011). In brief, images were spatially binned from full frame 512×512 pixels to 128×128 pixels. Changes in light reflectance were converted to differential changes in concentration of oxyhemoglobin and deoxyhemoglobin using the Modified Beer-Lambert Law. Images were then temporally filtered over the canonical FC frequency band (0.009Hz-0.08Hz), spatially smoothed with a 5×5 Gaussian kernel, and down-sampled from ~30Hz to 1Hz. Finally, the global signal (mean time series across the skull) was regressed from the time series at every pixel to remove global sources of shared variance. Using the Paxinos histological atlas as a reference, cortical fcOIS was measured based on 0.5 mm diameter circles. These seeds sampled the time series from the left and right visual, motor, retrosplenial, somatosensory, parietal, cingulate, and auditory networks (White, et al., 2011). All time series within a seed were averaged and were correlated against the time series at every pixel over the brain to construct seed-based FC maps. FC maps from each imaging session were Fisher z-transformed before being averaged within and across mice and subsequently back-transformed for plotting.
Statistical analysis
Once determination of ON was histologically determined at the conclusion of the experiment, OptoMotry scores and imaging data were segregated based on extent of optic nerve inflammation. A given mouse was classified with ON if at least one optic nerve had an inflammation score ≥ 2 (Wu, et al., 2011). Post-hoc Welch’s unequal variances t-test and paired Student’s t-tests used to examine longitudinal changes in OptoMotry performance and bilateral FC scores. All FC correlation values were first Fisher z-transformed before all statistical analysis.
Results
ON induction in 2D2 mice
We examined 2D2 mice with high susceptibility of ON for FC alterations. In total, we immunized 16 2D2 mice (Fig. 1A), of which nine exhibited evidence of ON (“ON+”) by histologic assessment four weeks following immunization as reflected by inflammatory infiltrates and perivascular cuffing (Fig. 1B). Seven 2D2 mice along with four B6 mice also immunized with low-dose MOG peptide that did not develop ON served as controls, producing a total of 11 mice without ON (“ON−“), (Fig. 1A).
Connectivity alterations in 2D2 mice with ON
Experimental mice were assessed by fcOIS imaging in longitudinal fashion: before and after immunization with MOG35–55 (Fig. 1A). Both the ON− and ON+ groups were imaged in parallel using fcOIS. Comparison of all seven seed-based networks between WT (n=4) and 2D2 (n=7) mice without ON at both the pre- and post-immunization time points are shown in Supplementary Table 1. Because no significant differences were observed between these groups for any network at either time point, we pooled WT and 2D2 mice without ON to serve as the control cohort. At baseline, FC maps (Fig. 1C) were similar in the visual network (Fig. 1D) and other networks for the control and ON+ mice (Table 1). Additionally, OptoMotry was performed to assess the clinical outcome of ON (Douglas, et al., 2005) and revealed similar visual function for ON− and ON+ mice at baseline (Fig. 1E).
Table 1.
Mean ± SD bilateral FC scores for seven networks identified by fcOIS.
| ON− | ON+ | ||
|---|---|---|---|
| Pre-immunization | p value* | ||
| Cingulate | 0.76 ± 0.25 | 0.74 ± 0.17 | 0.642 |
| Motor | 0.83 ± 0.26 | 0.79 ± 0.10 | 0.239 |
| Somatosensory | 0.47 ± 0.24 | 0.36 ± 0.25 | 0.303 |
| Retrosplenial | 0.87 ± 0.26 | 0.87 ± 0.20 | 0.997 |
| Parietal | 0.49 ± 0.26 | 0.50 ± 0.14 | 0.836 |
| Auditory | 0.53 ± 0.23 | 0.53 ± 0.25 | 0.973 |
| Visual | 0.64 ± 0.14 | 0.67 ± 0.20 | 0.571 |
| Post-immunization | |||
| Cingulate | 0.63 ± 0.27 | 0.49 ± 0.28 | 0.115 |
| Motor | 0.65 ± 0.34 | 0.55 ± 0.40 | 0.405 |
| Somatosensory | 0.46 ± 0.19 | 0.38 ± 0.29 | 0.416 |
| Retrosplenial | 0.80 ± 0.31 | 0.69 ± 0.29 | 0.084 |
| Parietal | 0.50 ± 0.22 | 0.32 ± 0.29 | 0.082 |
| Auditory | 0.45 ± 0.26 | 0.33 ± 0.18 | 0.200 |
| Visual | 0.57 ± 0.20 | 0.31 ± 0.19 | 0.001 |
| Longitudinal p value** | ON− → ON− | ON+ →ON+ | |
| Cingulate | 0.047 | 0.001 | |
| Motor | 0.016 | 0.008 | |
| Somatosensory | 0.946 | 0.858 | |
| Retrosplenial | 0.092 | 0.001 | |
| Parietal | 0.909 | 0.049 | |
| Auditory | 0.355 | 0.011 | |
| Visual | 0.130 | 3.74E-04 | |
Welch’s t-test
Paired t-test
Mice were longitudinally studied approximately four weeks after induction of ON (immediately prior to harvesting their optic nerves for inflammation scoring). A reduction in FC within the cingulate and motor networks was observed in mice without ON (Table 1). ON+ mice also exhibited declines in FC in all networks except for the somatosensory network (Table 1). Notably, the visual network demonstrated a decline in FC over time in ON+ mice compared to ON− mice (p=0.0004; Fig. 1D; Table 1). The visual cortex was the only region examined that provided a significant reduction in homotopic bilateral FC in the ON+ group compared to ON− (p=0.001, Table 1; Fig. 1D). Qualitatively, this reduction can be seen in the diminished area of high-magnitude positive correlation in contralateral visual cortex in FC maps constructed using both left and right visual seeds (Fig. 1C, top). Additionally, within the visual cortex, a significant interaction (p=0.008) between timepoint and disease state was observed (Fig. 1D).
The histologically-confirmed ON mice also had a significant decline in OptoMotry score (p=.042; Fig. 1E) compared to mice without ON. However, there was no group-wise cross-sectional difference in performance at either time point. Thus, our cohort with optic nerve inflammatory demyelination (ON+) showed a disease-related decline in performance despite absolute cross-sectional performance being in parity with the ON− group.
Discussion
Using an in vivo optical imaging technique to measure hemodynamics, we observed a decrease in bilateral FC within cortical visual areas in ON+ compared to ON− mice. There was also a highly significant longitudinal reduction in visual bilaterality and a significant interaction between disease status and time in this score, suggesting a possible time-dependent deficit in visual connectivity associated with ON.
What factors contribute to cortical network alterations after disruption of the anterior visual pathway? Though multiple networks showed significant longitudinal reductions in bilateral FC, only visual cortex was significantly lower in ON+ than ON− at the post-immunization timepoint. It is possible that direct trans-synaptic loss of input results in alterations of cortical synaptic connections. This idea is supported in part by work by Gabilondo and colleagues using structural MRI and voxel-based morphometry and OCT to show that in MS patients without a history of ON, visual cortex volume was associated with retinal nerve fiber layer thickness, suggesting retrograde (posterior visual pathway to anterior visual pathway) degeneration in the retina. However, in patients with ON, visual cortex volume was more atrophied compared to MS patients without prior ON, suggesting anterograde trans-synaptic degeneration, an effect that could be associated with the ON− dependent visual FC results observed in this study (Gabilondo, et al., 2014). It is not clear whether cell loss is required for this effect or if the reduction of overall visual signal resulting from ON is sufficient to alter the dynamics of homotopic bilateral FC (Gallo, et al., 2012). Imaging modalities, including DTI and DBSI, are well suited to address the degree to which structural alterations drive cortical adaptations in vivo using murine ON models.
Limitations of our study include the use of immunization to induce ON. While MOG is expressed most highly in the optic nerve relative to other structures within the CNS (Bettelli, et al., 2003), potential for immune responses targeting white matter more diffusely in our model cannot be excluded. While various FC changes were seen in several regions other than the visual system after immunization (Fig. 1C), only the visual network provided a significant group-wise reduction in ON+ mice compared to controls. A general decline in connectivity was observed in mice with and without ON. This may be a result of multiple factors, including residual effects of repeated invasive surgeries and/or a mild, diffuse inflammatory response to immunization in both cohorts. We also suspect age is another variable contributing to the overall decline in connectivity observed in our study. For example, Bero et al. (Bero, et al., 2012) showed significant age-related decline in retrosplenial cortex bilaterality in wild-type B6C3 mice using the fcOIS system utilized in our experiments. Also, this study did not include sham-immunized B6 mice. However, a number of previous studies using the same fcOIS system described here have established baseline FC topography in both wild-type B6 (Bero, et al., 2012) and Swiss-Webster mice (e.g. (Bauer, et al., 2014, Bergonzi, et al., 2015)). Because the scope of this study was to examine the ability of the 2D2 mouse model and fcOIS imaging system to recapitulate previously-reported ON-specific effects on FC, especially in the visual cortex (Wu et al 2015), the immunized pooled WT and 2D2 group with negligible optic nerve inflammation used here serves as an appropriate direct control. Importantly, no evidence of spinal cord involvement reminiscent of EAE was observed in any of the immunized animals (data not shown). It should be noted that the planar fcOIS imaging system used in the present study only provides hemodynamic information from superficial cortical layers (approximately I-IV/V). Given the extent of pervasive white matter damage that is associated with MS, a more direct probe of cortical white matter and subcortical regions would enable more extensive characterization of disease-associated effects on FC. Finally, the limitations of OptoMotry stem from its limited dynamic range to detect visual changes (Kretschmer, et al., 2015).
Surprisingly, we did not observe a statistically significant correlation between visual bilateral FC and VA. However, Douglas and colleagues reported that neither unitlateral nor bilateral cortical lesions in primary visual cortex had any negative impact on VA as measured using OptoMotry (Douglas, et al., 2005). Thus, it is possible that a mechanism that damages V1 (which would likely be detectable by our fcOIS system) would not manifest itself in reduced VA performance. Techniques to assess graded changes in visual function, such as optical coherence tomography or low contrast letter acuity testing that are used in human disease (Balcer, et al., 2015), are not optimal for mice lacking high level processing of vision. Newer techniques combining optomotor responses with optokinetic ocular movements in rodents may prove to be more sensitive to incremental changes in afferent pathway function than optometry alone (Kretschmer, et al., 2015).
Nonetheless, using the visual system to study the neuronal consequence of immune-mediated white matter dysfunction offers several advantages, including a well-characterized anatomy and physiology, readily available tools for the quantification of function, and wide representation throughout the brain that lends to frequent direct or indirect involvement in MS and other neurologic disorders. Of particular benefit is the ability to tie connectivity changes resulting from inflammatory demyelination of the CNS directly to immune mediators. Readily available experimental murine tools, including knockouts and transgenics, should facilitate exploration of immune and/or neural mechanisms underlying connectivity alterations in EAE and MS. Hence, taking advantage of the animal model given the transgenic resources available is highly feasible and extremely promising for uncovering the cellular and molecular foundations of neurologic dysfunction in ON and MS. In the future, an extended longitudinal approach would be useful in determining the dynamics of connectivity alterations following ON during later, non-inflammatory recovery stages not captured in this study.
Supplementary Material
Acknowledgments
We thank Kenneth Shindler, MD, PhD (The University of Pennsylvania) for input on ON assessment and Grant Baxter at Washington University in St. Louis for technical assistance. Funding: this work was supported by the National Institutes of Health (grant numbers R01NS083678 (GFW), R01NS0836780 (BMA), R01NR014449 (BMA), R01NR012907 (BMA), and R01NR012657 (BMA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balcer LJ, Miller DH, Reingold SC, Cohen JA. Vision and vision-related outcome measures in multiple sclerosis. Brain. 2015;138:11–27. doi: 10.1093/brain/awu335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer AQ, Kraft AW, Wright PW, Snyder AZ, Lee JM, Culver JP. Optical imaging of disrupted functional connectivity following ischemic stroke in mice. Neuroimage. 2014;99:388–401. doi: 10.1016/j.neuroimage.2014.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergonzi KM, Bauer AQ, Wright PW, Culver JP. Mapping functional connectivity using cerebral blood flow in the mouse brain. J Cereb Blood Flow Metab. 2015;35:367–370. doi: 10.1038/jcbfm.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bero AW, Bauer AQ, Stewart FR, White BR, Cirrito JR, Raichle ME, Culver JP, Holtzman DM. Bidirectional relationship between functional connectivity and amyloid-beta deposition in mouse brain. J Neurosci. 2012;32:4334–4340. doi: 10.1523/JNEUROSCI.5845-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RM, Alam NM, Silver BD, McGill TJ, Tschetter WW, Prusky GT. Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis Neurosci. 2005;22:677–684. doi: 10.1017/S0952523805225166. [DOI] [PubMed] [Google Scholar]
- Gabilondo I, Martinez-Lapiscina EH, Martinez-Heras E, Fraga-Pumar E, Llufriu S, Ortiz S, Bullich S, Sepulveda M, Falcon C, Berenguer J, Saiz A, Sanchez-Dalmau B, Villoslada P. Trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. Ann Neurol. 2014;75:98–107. doi: 10.1002/ana.24030. [DOI] [PubMed] [Google Scholar]
- Gallo A, Esposito F, Sacco R, Docimo R, Bisecco A, Della Corte M, D’Ambrosio A, Corbo D, Rosa N, Lanza M, Cirillo S, Bonavita S, Tedeschi G. Visual resting-state network in relapsing-remitting MS with and without previous optic neuritis. Neurology. 2012;79:1458–1465. doi: 10.1212/WNL.0b013e31826d5eea. [DOI] [PubMed] [Google Scholar]
- Kretschmer F, Sajgo S, Kretschmer V, Badea TC. A system to measure the Optokinetic and Optomotor response in mice. J Neurosci Methods. 2015;256:91–105. doi: 10.1016/j.jneumeth.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. The restless brain. Brain Connect. 2011;1:3–12. doi: 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BR, Bauer AQ, Snyder AZ, Schlaggar BL, Lee JM, Culver JP. Imaging of functional connectivity in the mouse brain. PLoS One. 2011;6:e16322. doi: 10.1371/journal.pone.0016322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GF, Brier MR, Parks CA, Ances BM, Van Stavern GP. An Eye on Brain Integrity: Acute Optic Neuritis Affects Resting State Functional Connectivity. Invest Ophthalmol Vis Sci. 2015;56:2541–2546. doi: 10.1167/iovs.14-16315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GF, Shindler KS, Allenspach EJ, Stephen TL, Thomas HL, Mikesell RJ, Cross AH, Laufer TM. Limited sufficiency of antigen presentation by dendritic cells in models of central nervous system autoimmunity. J Autoimmun. 2011;36:56–64. doi: 10.1016/j.jaut.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


