Introduction
There is a pressing need for the development of advanced heart failure therapeutics. Current state-of-the-art is protection from neurohumoral overstimulation, which fails to address the underlying cause of heart failure, namely loss of cardiomyocytes. Implantation of stem cell-derived cardiomyocytes via tissue engineered myocardium is being advanced to realize the remuscularization of the failing heart (1). Here, we discuss pharmacological challenges pertaining to the clinical translation of tissue engineered heart repair with a focus on engineered heart muscle.
Engineered Heart Muscle
Engineered heart muscle (EHM) for myocardial remuscularization is comprised of cardiomyocytes and non-contractile support cells, i.e., mostly fibroblasts (Figure 1). Self-organization of these cells into anisotropically structured functional syncytia is facilitated in a collagen hydrogel under defined pharmacological and biophysical stimulation. Early studies used the addition of Matrigel™ - a mixture of laminin, matricellular proteins, and growth factors - secreted from the Engelbreth-Holm-Swarm tumor to provide cardio-instructive signals (2). Most recently, we demonstrated that engineered human myocardium (EHM) can be developed under serum-free, defined conditions devoid of components that would preclude clinical applications (3).
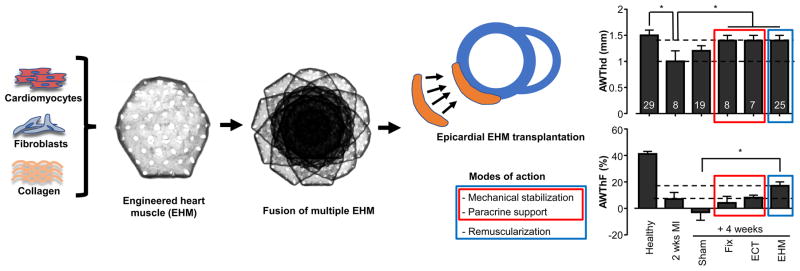
Figure 1. Tissue engineered heart repair by EHM implantation.
Self-organization of cardiomyocytes and stroma cells (in case of EHM fibroblasts) is supported by defined stimuli in a collagen-hydrogel (refer to Tiburcy et al. 2017 for details). Cast-molding and tissue fusion are applied to scale tissue according to clinical needs. Testing of EHM allografts confirmed a multimodal mode of action, with a dominant therapeutic effect by electromechanical integration of contractile EHM as well as ancillary effects by paracrine support and mechanical stabilization (refer to Zimmermann et al. 2006); note that all tested tissue formats (Fix, ECT, EHM) resulted in augmentation of the anterior heart wall thickness (AWThd), whereas only the implantation of EHM resulted in enhanced systolic thickening of the anterior heart wall (AWThF). Fix: formaldehyde fixed EHM (mechanical stabilization); ECT: tissue composed of viable non-myocytes (paracrine effect and mechanical stabilization); EHM: engineered heart muscle (remuscularization). AWThd and AWThF were measured by MRI. Investigated animals are indicated in the top bars. Graphs adapted from Zimmermann et al. 2006.
Human cardiomyocytes can be derived at scalable quantities from embryonic and induced pluripotent stem cells using directed differentiation (4); another attractive source for cardiomyocytes, mainly from a transplant immunology perspective, are MHC-haploidentical parthenogenetic stem cells (5). Tissue scale can be enhanced by cast molding and tissue fusion technologies (Figure 1), whilst accounting for sufficient nutrient and oxygen provision (2, 3). Function of tissue engineered myocardium must be verifiable by force measurements and should closely resemble the contractile properties of the bona fide human heart with its characteristic physiological responses (e.g., Frank-Starling mechanism; Bowditch phenomenon; canonical inotropic, chronotropic, and lusitropic responses to pharmacological stimulation). Alternative morphological (e.g., M-bands; t-tubule formation) and molecular (e.g., myosin heavy chain, myosin light chain, troponin isoform switch) parameters may also be used for benchmarking.
Pharmacological considerations
The development of first-in-class therapeutics poses a considerable challenge on researchers and regulatory authorities alike. Tissue engineered heart muscle are classified as Advanced Therapy Medicinal Products (ATMPs) and their authorization is regulated by national competent authorities, such as the Paul-Ehrlich-Institute (PEI) in Germany, the European Medicines Agency (EMA) for applications across Europe, or the U.S. Food and Drug Administration (FDA). A key challenge for the translation of experimental therapies into the clinic is that their production has to be according to current good manufacturing practice (cGMP) to obtain a manufacturing authorization for the production of an investigational medicinal product (IMP). This requires a full definition of cellular and non-cellular components as well as tissue reconstitution and culture conditions with meticulous quality control. Procedural challenges as to the realization of a cGMP tissue engineered product (TEP) have to be addressed early on and be guided by advice obtained from competent regulatory authorities to not face insurmountable issues at the stage of the transition from late preclinical to early clinical studies.
To advance TEPs towards clinical trials it is essential to propose a mode of action based on evidence obtained in preclinical models. The primary mode of action of EHM is functional myocardial remuscularization by its electromechanical integration into the recipient heart. Secondary effects may be mediated by the release of proteins or RNA (e.g., miR) from viable EHM and related biological modulation of the implant environment (paracrine effects) as well as by mechanical stabilization of the myocardial wall with a reduction of wall stress according to the law of Laplace. Previous studies with EHM allografts in rodent models have confirmed this multimodal mode of action, with a dominant effect by electromechanical integration and a lesser contribution of paracrine effects and mechanical stabilization (Figure 1; (2, 5)). This will also have to be considered when defining potency assays for quality control and release of EHM for clinical applications. Accordingly, as primary potency assays direct (destructive) and indirect (non-destructive) measurements of force of contraction by classical isometric force measurements and video-optic assessments of tissue contraction appear instrumental (3).
The therapeutic efficacy according to the anticipated primary mode of action will depend on the amount and maturity of electromechanically integrated cardiomyocytes. Given an estimated loss of approximately 1 billion cardiomyocytes in hemodynamically relevant myocardial insults it seems plausible that the same number of cardiomyocytes must be implanted for optimal therapeutic effects. A so far unresolved question is, however, how many cardiomyocytes are needed to achieve minimal therapeutic effects? This dose would likely be chosen at the start of a dose escalation study. Moreover, a minimal toxic dose should be established experimentally to estimate the therapeutic window.
What degree of functional maturation or sarcomere content per cardiomyocyte will be ideal for optimal results remains an open question. It seems that metabolic immaturity is supportive as it seems to ensure survival of EHM even under hostile in vitro and in vivo conditions. The capacity of cardiomyocyte grafts to fully mature in an appropriate environment has been demonstrated by several groups including ours (5). Similarly, there is evidence for maturation in extended EHM cultures (3) as well as in EHM implants (4, 5).
Pharmacodynamic (PD) and pharmacokinetic (PK) evaluation of tissue engineered products will have to address contractility as a function of the cell/tissue dose and tissue retention as well as biodistribution of the EHM and its components. A critical question is how to perform PD and PK preclinically, keeping in mind that the integration of human cells in animal models will be confounded by immunological responses to the xenograft and the physiological mismatch between human and animal models. The testing of animal surrogate tissue developed in accordance with human protocols and palpable bioequivalence, as determined by standardized potency assays, will, in our view, be highly informative. As to this end, rodent allograft studies have helped to define the multimodal mode of action of EHM (Figure 1).
Testing clinically relevant doses in rodents as well as the concept of allometric scaling appear less applicable in the evaluation of safety concerns (tumor formation and arrhythmia) associated with tissue engineered heart repair. Thus, large animal experiments that can accommodate clinically relevant doses under clinically relevant conditions are required. Pig and macaque models appear to be best suited. It is however mandatory for proper PD assessments to perform studies not confounded by immune reactions and physiological tissue mismatches. Preclinically this caveat may be best addressed by making use of surrogate tissues developed for auto- or allografting.
The lack of protocols allowing for stable derivation, propagation, and differentiation of pluripotent stem cells from pig and the possibility to derived pluripotent stem cells from different macaque species gives more clinical relevance to the latter. The possibility to reprogram somatic cells into induced pluripotent stem cells for subsequent use in cardiomyocyte derivation and tissue engineering could provide autograft material und thus may make immune suppression dispensable. However, there is evidence for acquired autoimmunogenicity, which may offset this advantage. In addition, the preparation of personalized autografts for applications in large patient cohorts as well as a high risk for production failure for example in case of acquired mutations make autografting unlikely in the near future. Thus, preclinical PD/PK studies would clearly benefit from macaque allograft studies under clinically acceptable immune suppression.
Conclusion
Hundreds of patients are presently being enrolled in clinical trials attempting organ repair by the implantation of embryonic, induced, and parthenogenetic human stem cell derivatives. Attempts to remuscularize the failing heart with cardiomyocyte allografts will follow. For optimal efficacy, cardiomyocytes will have to be electromechanically and structurally integrated to contribute to the recipient’s heart function. Clinical trials would benefit from PD/PK studies performed in preclinical allograft models with clinically relevant immune suppression and allograft dosing. The macaque model is used widely in transplant immunology studies and will be instrumental to inform clinical trials on tissue engineered heart repair. In may in fact serve as a surrogate model for Phase I trials, which are performed to test an IMP in healthy individuals before proof-of-concept studies are performed in patients with heart failure (Phase II) to gain insight as to the efficacy of tissue engineered heart repair.
Acknowledgments
B.F. is supported by an adumed research stipend. W.H.Z. is supported by the DZHK (German Center for Cardiovascular Research), the German Federal Ministry for Science and Education (BMBF FKZ 13GW0007A [CIRM-ET3]), the German Research Foundation (DFG ZI 708/10-1; SFB 937 TP18, SFB 1002 TPs C04, S1; IRTG 1618 RP12), the European Union FP7 CARE-MI, the Foundation Leducq, and the NIH (U01HL099997).
References
- 1.Ogle BM, et al. Distilling complexity to advance cardiac tissue engineering. Sci Transl Med. 2016;8:342ps13. doi: 10.1126/scitranslmed.aad2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmermann WH, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–8. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 3.Tiburcy M, et al. Defined Engineered Human Myocardium with Advanced Maturation for Applications in Heart Failure Modelling and Repair. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.116.024145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riegler J, et al. Human Engineered Heart Muscles Engraft and Survive Long Term in a Rodent Myocardial Infarction Model. Circ Res. 2015;117:720–30. doi: 10.1161/CIRCRESAHA.115.306985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Didie M, et al. Parthenogenetic stem cells for tissue-engineered heart repair. J Clin Invest. 2013;123:1285–98. doi: 10.1172/JCI66854. [DOI] [PMC free article] [PubMed] [Google Scholar]