Abstract
Introduction
No study has examined structural brain changes specifically associated with chemotherapy in a lung cancer population. The aim of this cross-sectional study was to assess differences in brain structure between small-cell lung cancer patients (C+) following chemotherapy, non–small-cell lung cancer patients (C−) before chemotherapy and healthy controls (HC).
Methods
Twenty-eight small-cell lung cancer patients underwent a neuropsychological assessment and a structural magnetic resonance imaging, including T1-weighted and diffusion tensor imaging to examine gray matter density and white matter (WM) integrity, respectively, 1 month following completion of platinum-based chemotherapy. This group was compared with 20 age and education-matched non–small-cell lung cancer patients before receiving chemotherapy and 20 HC.
Results
Both C+ and C− groups exhibited cognitive impairment compared with the HC group. The C+ group performed significantly worse than HC in verbal fluency and visuospatial subtests; C− performed significantly worse than both C+ and HC in verbal memory. Voxel-based morphometry analysis revealed lower gray matter density in the insula and parahippocampal gyrus bilaterally, and left anterior cingulate cortex in C+ compared with HC. Diffusion tensor imaging indices showed focal decreased WM integrity in left cingulum and bilateral inferior longitudinal fasciculus in the C+ group and more widespread decreased integrity in the C− group compared with the HC group.
Conclusion
This study demonstrates that lung cancer patients exhibit cognitive impairment before and after chemotherapy. Before the treatment, C− showed verbal memory deficits as well as a widespread WM damage. Following treatment, the C+ group performed exhibited lower visuospatial and verbal fluency abilities, together with structural gray matter and WM differences in bilateral regions integrating the paralimbic system.
Keywords: Lung cancer, Chemotherapy, Cognitive impairment, Neuroimaging, Diffusion tensor imaging, Voxel-based morphometry
Chemotherapy-induced cognitive impairment or “chemobrain” is a well-recognized clinical syndrome, consisting of subtle to moderate cognitive changes across various domains.1 Although acute cognitive changes during chemotherapy are common,2 long-term cognitive changes post-treatment may persist in only a subgroup (17–34%) of cancer survivors.3 In recent years, several studies using neuroimaging (magnetic resonance imaging[MRI]) techniques have reported structural brain changes associated with chemotherapy.4 These changes consist of an early, diffuse gray matter (GM) decrease and white matter (WM) degeneration5–9 with some studies finding persistent structural alterations at longer intervals.5,6,10–13 Additionally, a widespread decrease in WM volume has been described in cancer patients before chemotherapy.14 This WM decrease is supported by several neuropsychological studies describing a lower cognitive performance in a subset of patients before adjuvant treatment,15,16 suggesting that cancer itself might have a negative effect processing.
To date, the majority of investigations on treatment and cancerrelated cognitive changes have focused on breast cancer patients. Cognitive effects in non–small-cell (NSCLC) and small-cell (SCLC) lung cancers, specifically, have not been extensively studied, potentially because of the fact that lung cancer is associated with shorter survival, has confounding co-morbidities and, for SCLC, additional treatments such as prophylactic cranial irradiation (PCI) are needed. Thus, the study of the near- or long-term effects of chemotherapy on cognition in this population remains challenging and under-represented in the literature.
The handful of studies that have focused on lung cancer patients found cognitive deficits early following chemotherapy treatment, with special emphasis on executive function, verbal fluency, and verbal memory.17–20 Early prospective studies examining neuropsychological performance in NSCLC patients found a marked cognitive decline 1 month post-chemotherapy with relative improvement at 7 months follow-up.20–22 Other studies, focusing on SCLC patients, found that nearly 60 to 90% of the patients were cognitively impaired 1 to 5 months after the end of chemotherapy.17–19,23,24
While the results of these studies are suggestive, little is known about the underlying structural or functional brain alterations following lung cancer chemotherapy treatment. We compared SCLC patients 1 month following chemotherapy (C+) with NSCLC patients before chemotherapy (C−) and healthy control (HC) groups in gray matter density (GMD) and WM integrity using structural MRI together with neuropsychological assessment.
PATIENTS AND METHODS
Patients
The patients were prospectively recruited from December 2010 to March 2013 from the Lung Cancer Unit of the ICO Duran i Reynals-Hospital Universitari de Bellvitge (n = 40) and from the Radiation Oncology Department of the ICO Badalona-Hospital Germans Trias i Pujol (n = 8). Patients were eligible if they had a histologically proven diagnosis of either NSCLC or SCLC, were between the ages of 40 and 70 years, had no severe concomitant systemic illness or psychiatric disorder with a negative impact on cognitive function, or had any contraindication to undergo MRI. The patients were excluded if they had an evidence of brain metastases on MRI. This cross-sectional analysis represents a part of an ongoing longitudinal study specifically designed to examine the effects of prophylactic cranial irradiation (PCI) on cognition in SCLC patients. SCLC patients (C+, n = 28) who were eligible to receive PCI and were anti-HU negative were enrolled 1 month following completion of chemotherapy and before PCI. However, to delineate the effects specific to chemotherapy over time, the SCLC group was contrasted with a NSCLC group, since SCLC patients receive PCI, thus confounding potential effects of PCI with chemotherapy. NSCLC group underwent the same platinum-based chemotherapy and did not receive PCI, facilitating the study of the long-term effects of chemotherapy in the longitudinal study. NSCLC patients (C−, n = 20) who were eligible to receive platinum-based chemotherapy were enrolled in the study before the initiation of chemotherapy. NSCLC was selected as the cancer control group because of its higher incidence, especially in comparison with SCLC. The recruitment of lung cancer patients before the initiation of treatment just after cancer diagnosis is very challenging. Patients are overwhelmed with several diagnostic tests and therefore less predisposed to collaborate in a trial. Thus, recruitment of the NSCLC group before chemotherapy facilitated the achievement of our designated sample size. Age and education-matched HC (n = 20) who met the same inclusion (except for cancer diagnosis) and exclusion criteria were recruited through community advertisements. Vascular risk factors were collected and classified in low-risk (if the patient had none or one risk factor) and high-risk (if the patient had two or more risk factors) groups.19 The study protocol was approved by the local Ethical Commission and informed consent was obtained from all participants. All statistical analysis was conducted in SPSS 18.0 (SPSS, Chicago, IL). One-way analysis of variance and Chi-square tests were used to test the group differences with a critical p-threshold of 0.05.
Neuropsychological Assessment
The patients were evaluated using the Mattis Dementia Rating Scale-2; selected subtests of the Spanish version of the Wechsler Adult Intelligence Scale-III (Vocabulary; Information; Similarities; Digit Span; Letter Number Sequencing; Block Design; Matrix Reasoning; Picture Completion); Rey Auditory Verbal Learning test; Wechsler Memory Scale–III Logical Memory I–II; Rey-Osterreith Complex Figure Test Copy, Immediate or First and Delayed; Spanish version of the Boston naming test; Verbal Fluency test (phonemic and semantic); Trail Making Test (A–B); and Beck Depression Inventory (BDI). Intelligence quotient was estimated using Vocabulary performance. Raw cognitive test scores were compared with the validated Spanish normative values, corrected for age and education, and converted into z-scores. Cognitive impairment was defined as a Mattis Dementia Rating Scale-2 raw score less than 123,25 one test greater than or equal to 2 or two tests greater than or equal to 1.5 SDs below the sample mean.26
Structural Neuroimaging
MRI scan acquisition
The participants were imaged on a 3-Tesla MRI (Siemens Magnetom Trio Tim SyngoB17; Siemens AG, Munich, Germany) with a 32-channel phased-array head coil. High-resolution structural images were obtained using magnetization-prepared, rapid-acquired gradient echo sequence (240 slices sagittal, TR (repetition = 2300 ms, TE = 2.98 ms, 1-mm isotropic voxels) and a whole-brain diffusion MRI sequence using diffusion tensor spin echo planar imaging was acquired (voxel size of 2.5 × 2.5 × 2.5 mm, matrix of 96 × 96, 55 slices with 2.5 mm-thick and no gap, TE = 98 ms, TR = 9600 ms, EPI factor = 96, field of view = 240 mm, bandwidth = 1022 Hz, echo-spacing = 1.08 ms, b-value = 1000 s/mm2). One single run of 64 diffusion-weighted directions with one nondiffusion-weighted volume was acquired. Finally, a fluid attenuated inversion recovery sequence was acquired (64 slices with 2.0 mm-thick, TE = 145 ms, TR = 9000 ms, voxel size 1.0 × 0.9 × 2.0 mm).
T1 Image Processing And Analysis
Morphometric analysis was carried out using voxel-based morphometry27 and processed using MATLAB version 7.8.0 (The MathWorks Inc., Natick, MA) and Statistical Parametric Mapping software (SPM8; The Welcome Department of Imaging Neuroscience, London). T1 images were segmented,28 the resulting Gray Matter (GM) tissue probability maps normalized into Montreal Neurological Institute (MNI) space using diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL)29,30 and smoothed using an isotropic spatial smoothing kernel (8 mm).
The individual smoothed GM density (GMD) images were entered into a second-level analysis using a one-way ANOVA model with the between subject variable of Group (HC; C−; C+). Four independent two-sample t tests were then conducted: C+ and C− versus HC, C+ versus HC, C− versus HC, C+ versus C−. For all the contrasts, a p lesser than or equal to 0.05 family-wise-error corrected at the cluster level was used, with an auxiliary p less than 0.001 uncorrected at the voxel level.
Diffusion-weighted imaging processing and analysis
Diffusion data processing was started by correcting for eddy current distortions and head motion using FMRIB’s Diffusion Toolbox (FDT)(FSL 5.0.1, www.fmrib.ox.ac.uk/fsl/).31 The gradient matrix was then rotated32 and brain extraction was performed using the brain extraction tool.33 The analysis continued with the reconstruction of the diffusion tensors using the linear least-squares algorithm included in the Diffusion Toolkit 0.6.2.2 (Ruopeng Wang, Van Wedeen, trackvis.org/dtk, Martinos Center for Biomedical Imaging, Massachusetts General Hospital). Finally, fractional anisotropy (FA), radial diffusivity (RD), and axial diffusivity (AD) maps for each subject were calculated using the eigenvalues (ʎ1, ʎ2, and ʎ3) extracted from the diffusion tensors.
FA, AD (ʎ1), and RD (ʎ2 + ʎ3/2) maps were registered to the MNI Functional Magnetic Resonance Imaging of the Brain or FMRIB’s high-resolution average of 58 well-aligned good quality FA images (FMRIB58_FA) template using FMRIB’s nonlinear image registration tool (FNIRT).34,35 The resulting FA, RD, and AD maps were fed into a second-level SPM8 analysis using a one-way ANOVA model with the between subject variable of Group (HC; C−; C+). Four independent two-sample t tests were then conducted: C+ and C− versus HC, C+ versus HC, C− versus HC, C+ versus C−. For all the contrasts, a p less than or equal to 0.05 uncorrected threshold at the cluster level was used, with an auxiliary p less than 0.001 uncorrected threshold at the voxel level.
RESULTS
Patient Characteristics
The study initially recruited 42 SCLC patients, 27 NSCLC patients, and 20 HC subjects. Fourteen SCLC patients were excluded (six had asymptomatic brain metastases in the baseline MRI; four did not tolerate the MRI procedure; three were excluded because of technical issues with the MRI; and one presented severe undiagnosed dementia). Seven NSCLC patients were excluded (five did not tolerate the MRI procedure; one did not complete all the examinations; and another patient was excluded because of technical issues with the MRI). The final groups consisted of 28 patients in the C+ group, 20 in the C− group, and 20 subjects in the HC group.
Characteristics of the entire cohort are described in Table 1. There were no significant differences between the groups in age, gender, education, or grouped vascular risk factors. When analyzed independently, smoking history showed a significant difference between lung cancer patients and HC (Fisher exact test, p < 0.0001), with no differences between both the cancer groups (C+ and C−, Fisher exact test, p > 0.34). Diabetes mellitus type II (DMII) showed a significantly higher incidence in lung cancer patients (χ2(2) = 6.86, p < 0.03) but did not differ between the lung cancer groups (C+ and C−, χ2 (1) = 3.02, p > 0.08). Disease and treatment-related characteristics of C+ and C− group are described in Table 2.
TABLE 1.
Baseline Demographics And Vascular Risk Factors Of The Entire Cohort
| C+ (n = 28) | C− (n = 20) | HC (n = 20) | p | |
|---|---|---|---|---|
| Age (years)a | 59.29 ± 5.58 | 60.30 ± 6.37 | 62.3 ± 8.08 | 0.30 |
| Genderb | ||||
| Male | 22 (79) | 18 (90) | 18 (90) | 0.42 |
| Female | 6 (21) | 2 (10) | 2 (10) | |
| Education (years)c | 7.50 (0.17) | 8.50 (0.16) | 8 (6.19) | 0.66 |
| Estimated verbal IQa | 10.96 (3.35) | 10.90 (3.13) | 12.20 (3.17) | 0.20 |
| Smokingb | 28 (100) | 19 (95) | 11 (55) | 0.0001 |
| Alcoholb | 4 (29) | 8 (20) | 10 (50) | 0.11 |
| HTb | 10 (36) | 10 (50) | 7 (35) | 0.53 |
| DM type IIb | 6 (21) | 9 (45) | 2 (10) | 0.03d |
| Dyslipidemiab | 9 (32) | 11 (55) | 11 (55) | 0.18 |
| Vascular risk factorsb | ||||
| Low-risk (0 or 1) | 13 (46) | 4 (20) | 8 (40) | 0.16 |
| High-risk (≥2) | 15 (54) | 16 (80) | 12 (60) | |
Mean ± SD.
n (%).
Median (range).
DM type II was not significant between C+ and C− (p > 0.08).
C+, chemotherapy-treated small-cell lung cancer group; C−, nonchemotherapy treated non–small-cell lung cancer group; HC, healthy control group; IQ, intelligence quotient; HT, hypertension; DM, diabetes mellitus.
TABLE 2.
Disease and Treatment-Related Characteristics of the Patients
| C+ (n = 28) | C− (n = 20) | p | |
|---|---|---|---|
| KPSa | 80 (70–100) | 90 (70–100) | 0.08 |
| Histologyb | |||
| SCLC | 28 (100) | ||
| NSCLC | |||
| Adenocarcinoma | 11 (55) | ||
| Squamous-cell carcinoma | 8 (40) | ||
| Nonclassified | 1 (5) | ||
| Tumor stageb | |||
| Limited disease | 22 (79) | ||
| Extensive disease | 6 (21) | ||
| IIB | 2 (10) | ||
| IIIA | 11 (55) | ||
| IIIB | 7(35) | ||
| Chemo typeb | |||
| CDDP-based | 21 (75) | ||
| CBDCA-based | 7 (25) | ||
| Number of chemo cyclesa | 4 (3–6) | ||
| Thoracic radiationb | 25 (89) | ||
median (range).
n (%).
C+, chemotherapy-treated small-cell lung cancer group; C−, nonchemotherapy treated non–small-cell lung cancer group; KPS, Karnosfky performance scale; SCLC, small-cell lung cancer; NSCLC, non–small-cell lung cancer; CDDP, cisplatin; CBDCA, carboplatin.
Neuropsychological Assessment
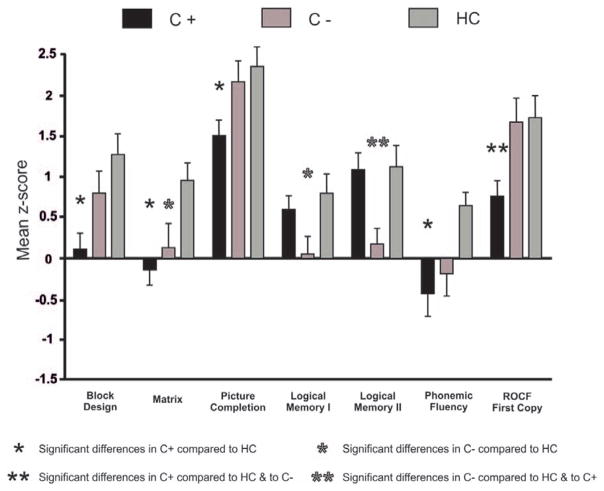
The neuropsychological assessment revealed that lung cancer patients performed significantly worse than the healthy controls in several subtests. Both the cancer groups exhibited a higher rate of cognitive impairment (39% of C+ and 30% of C−) compared with the healthy controls (5%) (χ2 (2) = 7.23, p < 0.027). However, there were no differences between the lung cancer groups (χ2 (1) = 0.44, p > 0.5). See Supplementary Table S1 (Supplemental Digital Content, http://links.lww.com/JTO/A702) and Figure 1.
FIGURE 1.
Neuropsychological results. Significant differences between the groups on neuropsychological testing. ROCF, Rey-Osterrieth complex figure.
Structural Neuroimaging
Voxel-based morphometry
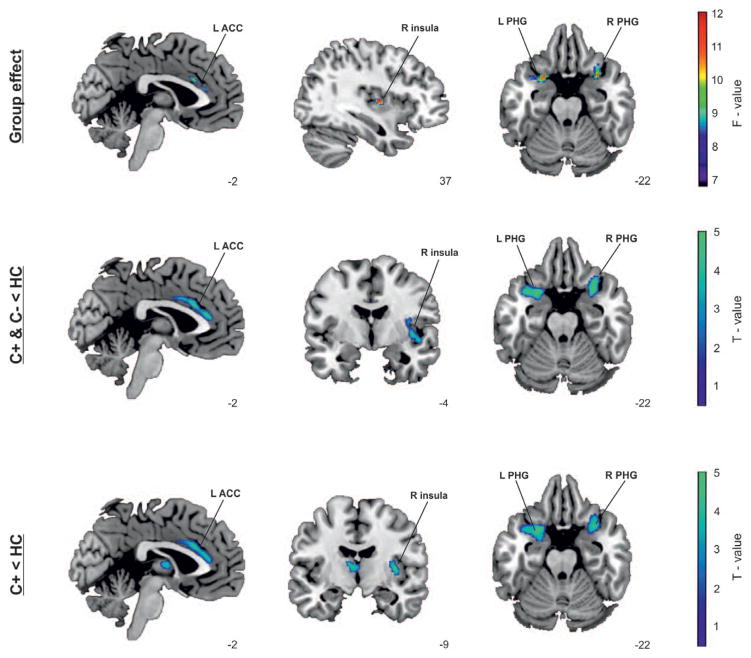
The analysis revealed differences in GMD between the groups, mainly in paralimbic regions (Fig. 2). Further pairwise t test analyses showed significant decrease in GMD for lung cancer patients (C+; C−) compared with the HC group in several brain regions including left insula, bilateral parahippocampal gyrus (PHG) and left anterior cingulate cortex (ACC). No regions exhibited less GMD in the HC group compared with either cancer group.
FIGURE 2.
Group differences for regional gray matter density (GMD) between groups. Group effect: The analysis revealed significant effects for group (C+, C−, HC [healthy controls]) in regional GMD in insula and parahippocampal gyrus (PHG) bilaterally and left anterior cingulate cortex (ACC). The results are displayed on an F-map and superimposed on an a-priori created T1 structural magnetic resonance imaging (MRI) template in standard stereotactic space. C+ and C− decreases compared with HC: The pairwise t test comparison between the cancer groups (C+ and C−) and HC group showed significant decreases in GMD of both the cancer groups in similar regions: left insula, bilateral PHG, and left ACC. The results are displayed on a T-map and superimposed on an a-priori created PHG, and left ACC. The results are displayed on a T-map and superimposed on an a-priori created T1 T1 structural MRI template in standard stereotactic space. C+ decreases compared with HC: The pairwise t test comparison between C+ and HC groups showed significant decreases of GMD in similar regions: right insula, bilateral structural MRI template in standard stereotactic space. L, left; R, right, PHG, parahippocampal gyrus; ACC, anterior cingulate cortex.
In the direct contrast between C+ and HC (Fig. 2), a significant decrease in GMD was exhibited in the right insula, bilateral PHG, and left ACC. No regions exhibited less GMD in the HC group compared with the C+ group. No significant differences were observed between the C+ and the C− groups or between the C− and the HC group. See Supplementary Table S2 (Supplemental Digital Content, http://links.lww.com/JTO/A702).
Diffusion tensor imaging-voxel based analysis
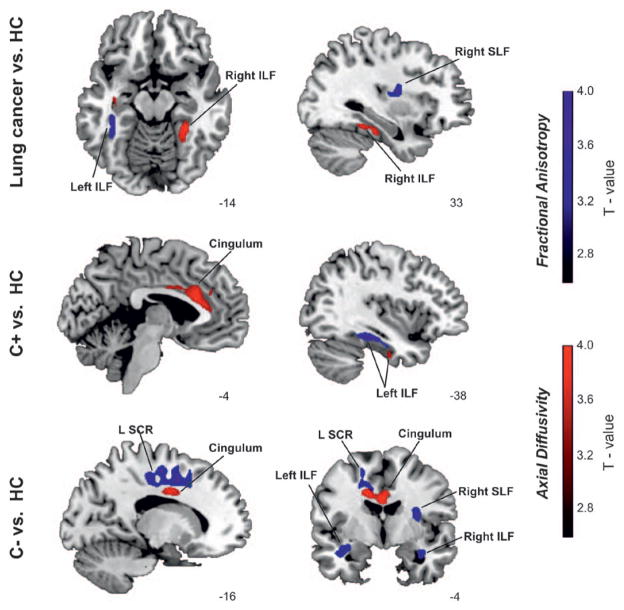
Between-group analysis found fractional anisotropy (FA) differences in the left cingulum and left inferior longitudinal fasciculus (ILF), right superior longitudinal fasciculus (SLF) and left superior corona radiata (SCR), and axial diffusivity (AD) differences especially focused in left cingulum. No significant differences were exhibited in RD between groups. FA is a highly sensitive but nonspecific biomarker of neuropathology and microstructural architecture,36 and AD represents the main diffusion direction aligned to the WM tracts and has been associated with several neuropathological changes especially related to axonal damage. Specifically, the increase of AD has been related to inflammatory processes and axonal atrophy.37
In the following pairwise t test analyses (C+ and C− versus HC, C+ versus HC, C− versus HC, C+ versus C−), lung cancer patients (C+ and C−) versus HC, C+ versus HC, and C− versus HC comparisons revealed significant differences. Lung cancer patients exhibited less WM integrity (lower FA and higher AD values) compared with HC in left ILF. Voxel-based t test analysis comparing C+ versus HC found significantly higher AD in left cingulum compared with HC. Additionally, the C− group exhibited lower WM integrity (lower FA and higher AD) in widespread regions, involving left cingulum and SCR, and right SLF. The comparison between C+ and C− found no significant differences. In brief, the DTI results were broadly concordant between the comparisons, even though the C− group pattern of effects was more extensive than that for the C+ group. See Supplementary Table S3 (Supplemental Digital Content, http://links.lww.com/JTO/A702).
DISCUSSION
This is the first study to document neuropsychological and structural neuroimaging changes in a cohort of small-cell lung cancer patients (SCLC, C+) treated with platinum-based chemotherapy. Our results revealed that patients in the C+ group exhibited cognitive impairment shortly after chemotherapy treatment, with special emphasis in visuospatial abilities and verbal fluency together with lower GMD in bilateral paralimbic regions and lower WM integrity in overlapping WM tracts. Additionally, before treatment, the C− group exhibited cognitive deficits in verbal working memory together with widespread WM decreases. These neuropsychological and structural imaging findings suggest that both cancer and cancer treatment may be associated with the development of central nervous system toxicity.
One-third of the patients in both lung cancer groups met the criteria for cognitive impairment; however, the neuropsychological profile was quite different. Following treatment, the C+ group performed worse than the HC group in visuospatial measures and verbal phonemic fluency. These cognitive deficits are similar to those previously described in breast cancer patients following chemotherapy.1 The NSCLC (C−) group performed worse than HC and C+ in long-term working verbal memory. Significantly, these cognitive deficits, especially in verbal working memory, have been described in cancer patients before the initiation of therapy.15,16 Although cognitive changes associated with either cancer or cancer treatment have been extensively recognized, the pathogenesis of these neurocognitive changes remains unclear. In this setting, several hypotheses have been proposed including the biology of cancer as well as common risk factors for the development of both cancer and mild cognitive changes in normal aging.3
Regarding our neuroimaging results, the C+ group presented a regional decrease in GMD in bilateral insula, bilateral PHG and left ACC 1 month following chemotherapy. The insula and PHG are paralimbic regions that play an important role in linking cognition and emotion as well as in extended episodic memory function.38 The cingulate cortex is usually considered part of the limbic cortex which lies immediately above the corpus callosum. The ACC is widely connected with diverse parts of the brain and is essential in problem solving, error recognition, and adaptive response to changing conditions.39 These structural differences found in SCLC confirm a subset of previous findings but also identify previously unreported regions that may be impacted following chemotherapy treatment. Structural neuroimaging findings in GM have been identified in medial and superior frontal gyri,5,6,13,26 parietal,11 medial temporal,5,13 and cerebellar regions.6,11,13 The DTI results showed an increase of AD in C+ group compared with HC in bilateral anterior temporal regions of the ILF and bilateral anterior cingulum. The ILF is an associate bundle connecting anterior-inferior temporal regions to parieto-occipital areas connecting visual areas to the amygdala and hippocampus.40 The cingulum is a medial associative bundle projecting from the cingulate gyrus around the corpus callosum to the entorhinal cortex that forms the WM core of the cingulate gyrus to the PHG and anterior temporal regions. Regarding the WM findings, our results are consistent with those described in the literature with the exception that our WM changes are less widespread and more focused on bilateral ILF and the cingulum.7,11 Significantly, for the C+ group, DTI results converge with GMD differences, and are concordant with observed neuropsychological deficits, providing strong support for acute structural and functional changes in bilateral paralimbic regions following chemotherapy treatment.
Although previous research has found lower WM integrity following chemotherapy treatment in cancer survivors,7,8,11 WM damage specifically associated with cancer patients before chemotherapy is still at present less clear.8,14 The C− group exhibited lower WM integrity in the same WM structures as the C+ group, with the addition of two complex projection systems, the left SCR and the right SLF. The SCR tract contains ascending and descending fibers that connect the subcortical nuclei and spinal cord with the cerebral cortex41 and the SLF forms an anatomical connection between the frontal and parietal regions.42 The WM findings and neuropsychological deficits exhibited by the C− group are in line with previous findings in cancer patients before chemotherapy.14
Specific differences between our study and previous neuroimaging studies include demographic, clinical, disease- and treatment-related factors that might explain the differences in the structural findings. First, patients included in our study were older (mean age 60 years) and less educated (mean education level 7 years) compared with patients in other studies.5,6,13,26 As a result, to control for these confounding factors, an age and education-matched healthy control group was included. In addition, the chemotherapy regimen used in lung cancer patients differs from those used in breast cancer. Although little is known about platinum-related brain neurotoxicity, an association with oxidative stress and brain morphological and molecular changes has been described.43
Concerning vascular risk factors, only smoking and DMII were significantly different between the groups. As expected, a higher proportion of lung cancer patients had a smoking history and in addition, DMII was more prevalent in lung cancer groups. Both factors have been associated with an increased risk of dementia and cognitive decline.44 Given these vulnerabilities, lung cancer survivors may be at higher risk of cognitive impairment, particularly compared with breast cancer survivors, who are typically younger and who generally have less medical co morbidities. Finally, in contrast to other cancer types, lung cancer is commonly associated with paraneoplastic syndromes. For this reason, only C+ patients with anti-HU negative antibodies were enrolled in our study.
Our study presents some limitations. The cross-sectional design of the study may have limited the possibility to clearly isolate the effect of chemotherapy from more general cancer-related changes. Similar to our findings, previous literature has found cognitive, structural and functional brain alterations in cancer-diagnosed individuals prior to treatment. Potential mechanisms include the biology of cancer, the inflammatory response triggering neurotoxic cytokines or common risk factors for the development of both cancer and mild cognitive changes,3,45 that may be associated with cognitive decline found in these patients. However, in addition to cognitive and structural changes prior to treatment, treatment with platinum-based chemotherapy agents may contribute to these cognitive changes, through DNA damage caused directly by the cytotoxic agents, or through increases in oxidative stress,3 resulting in damage to both WM and GM structures together with a different and specific cognitive impairment profile. The additive effect of cancer diagnosis and treatment with platinum based chemotherapy agents is suggested by our findings, given that the pattern of cognitive deficits and neuroanatomical findings were different between SCLC patients treated with chemotherapy versus NSCLC patients prior to treatment.
In conclusion, our study demonstrates that NSCLC (C−) patients exhibit cognitive impairment at baseline, before treatment, especially in verbal memory together with widespread WM decreases. One month following treatment, SCLC (C+) patients present distinct cognitive deficits especially in visuo-spatial abilities and verbal fluency, and structural GM and WM differences in bilateral regions integrating the paralimbic system. Further prospective studies are warranted to better delineate the effects of cancer and cancer treatment on cognition in lung cancer population.
Supplementary Material
FIGURE 3.
Group differences for regional diffusion tensor imaging (DTI) indexes (FA [fractional anisotropy] and AD [axial diffusivity]) between groups. Statistical maps of decreased FA (blue color) and increased AD (red color) are displayed at a p-value of 0.01. The pairwise t test comparison between the lung cancer groups (C+ and C−) and HC group found lower white matter (WM) integrity in bilateral inferior longitudinal fasciculus (ILF) and right superior longitudinal fasciculus (SLF) and left cingulum. The pairwise t test comparison between C+ and HC groups found lower WM integrity in bilateral ILF and left cingulum. The pairwise t test comparison between C− and HC groups found lower WM integrity in bilateral ILF, right SLF, left superior corona radiata (SCR) and left cingulum.
Acknowledgments
This work was supported by la Fundació Marató-TV3 (Acquired Spinal Cord and Brain Injuries Program [2012–2015] awarded to ARF) and the Catalan Government [Generalitat de Catalunya, 2009 SGR 93 to ARF]. Marta Simó was a recipient of a Rio Hortega research contract (code: CM11/00256) from the Carlos III National Health Institute (Spanish Government).
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Jim HS, Phillips KM, Chait S, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol. 2012;30:3578–3587. doi: 10.1200/JCO.2011.39.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahles TA, Saykin AJ, Furstenberg CT, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20:485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- 3.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simó M, Rifà-Ros X, Rodriguez-Fornells A, Bruna J. Chemobrain: a systematic review of structural and functional neuroimaging studies. Neurosci Biobehav Rev. 2013;37:1311–1321. doi: 10.1016/j.neubiorev.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Inagaki M, Yoshikawa E, Matsuoka Y, et al. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109:146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- 6.McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat. 2010;123:819–828. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deprez S, Amant F, Yigit R, et al. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum Brain Mapp. 2011;32:480–493. doi: 10.1002/hbm.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deprez S, Amant F, Smeets A, et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;30:274–281. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- 9.McDonald BC, Conroy SK, Smith DJ, West JD, Saykin AJ. Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: a replication and extension study. Brain Behav Immun. 2013;30(Suppl):117–125. doi: 10.1016/j.bbi.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Ruiter MB, Reneman L, Boogerd W, et al. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp. 2011;32:1206–1219. doi: 10.1002/hbm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Ruiter MB, Reneman L, Boogerd W, et al. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum Brain Mapp. 2012;33:2971–2983. doi: 10.1002/hbm.21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koppelmans V, de Ruiter MB, van der Lijn F, et al. Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Res Treat. 2012;132:1099–1106. doi: 10.1007/s10549-011-1888-1. [DOI] [PubMed] [Google Scholar]
- 13.Conroy SK, McDonald BC, Smith DJ, et al. Alterations in brain structure and function in breast cancer survivors: effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res Treat. 2013;137:493–502. doi: 10.1007/s10549-012-2385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scherling C, Collins B, Mackenzie J, et al. Structural brain differences in breast cancer patients compared to matched controls prior to chemotherapy. Int J Biol. 2012;4:23. [Google Scholar]
- 15.Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100:2292–2299. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- 16.Shilling V, Jenkins V, Morris R, Deutsch G, Bloomfield D. The effects of adjuvant chemotherapy on cognition in women with breast cancer—preliminary results of an observational longitudinal study. Breast. 2005;14:142–150. doi: 10.1016/j.breast.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Komaki R, Meyers CA, Shin DM, et al. Evaluation of cognitive function in patients with limited small cell lung cancer prior to and shortly following prophylactic cranial irradiation. Int J Radiat Oncol Biol Phys. 1995;33:179–182. doi: 10.1016/0360-3016(95)00026-U. [DOI] [PubMed] [Google Scholar]
- 18.Grosshans DR, Meyers CA, Allen PK, et al. Neurocognitive function in patients with small cell lung cancer: effect of prophylactic cranial irradiation. Cancer. 2008;112:589–595. doi: 10.1002/cncr.23222. [DOI] [PubMed] [Google Scholar]
- 19.Welzel T, Niethammer A, Mende U, et al. Diffusion tensor imaging screening of radiation-induced changes in the white matter after prophylactic cranial irradiation of patients with small cell lung cancer: first results of a prospective study. AJNR Am J Neuroradiol. 2008;29:379–383. doi: 10.3174/ajnr.A0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitney KA, Lysaker PH, Steiner AR, Hook JN, Estes DD, Hanna NH. Is “chemobrain” a transient state? A prospective pilot study among persons with non-small cell lung cancer. J Support Oncol. 2008;6:313–321. [PubMed] [Google Scholar]
- 21.Kaasa S, Olsnes BT, Thorud E, Høst H. Reduced short-term neuropsychological performance in patients with nonsmall-cell lung cancer treated with cisplatin and etoposide. Antibiot Chemother (1971) 1988;41:226–231. doi: 10.1159/000416209. [DOI] [PubMed] [Google Scholar]
- 22.Kaasa S, Olsnes BT, Mastekaasa A. Neuropsychological evaluation of patients with inoperable non-small cell lung cancer treated with combination chemotherapy or radiotherapy. Acta Oncol. 1988;27:241–246. doi: 10.3109/02841868809093532. [DOI] [PubMed] [Google Scholar]
- 23.Arriagada R, Le Chevalier T, Borie F, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst. 1995;87:183–190. doi: 10.1093/jnci/87.3.183. [DOI] [PubMed] [Google Scholar]
- 24.Gregor A, Drings P, Burghouts J, et al. Randomized trial of alternating versus sequential radiotherapy/chemotherapy in limited-disease patients with small-cell lung cancer: a European organization for research and treatment of cancer lung cancer cooperative group study. J Clin Oncol. 1997;15:2840–2849. doi: 10.1200/JCO.1997.15.8.2840. [DOI] [PubMed] [Google Scholar]
- 25.Mattis S. Dementia rating scale: professinal manual. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 26.Correa DD, Root JC, Baser R, et al. A prospective evaluation of changes in brain structure and cognitive functions in adult stem cell transplant recipients. Brain Imaging Behav. 2013;7:478–490. doi: 10.1007/s11682-013-9221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 28.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 29.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Ripollés P, Marco-Pallarés J, de Diego-Balaguer R, et al. Analysis of automated methods for spatial normalization of lesioned brains. Neuroimage. 2012;60:1296–1306. doi: 10.1016/j.neuroimage.2012.01.094. [DOI] [PubMed] [Google Scholar]
- 31.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 32.Leemans A, Jones DK. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med. 2009;61:1336–1349. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- 33.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersson JLR, Jenkinson M, Smith S. [Accessed September 2014];FMRIB Technical Report TR07JA1. 2007 available from http://www.fmrib.ox.ac.uk/analysis/techrep/tr07ja1/tr07ja1.pdf.
- 35.Andersson JLR, Jenkinson M, Smith S. [Accessed September 2014];FMRIB Technical Report TR07JA2. 2007 available from http://www.fmrib.ox.ac.uk/analysis/techrep/tr07ja2/tr07ja2.pdf.
- 36.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Acosta-Cabronero J, Alley S, Williams GB, Pengas G, Nestor PJ. Diffusion tensor metrics as biomarkers in Alzheimer’s disease. PLoS One. 2012;7:e49072. doi: 10.1371/journal.pone.0049072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann N Y Acad Sci. 2001;935:107–117. [PubMed] [Google Scholar]
- 40.Catani M, Jones DK, Donato R, Ffytche DH. Occipitotemporal connections in the human brain. Brain. 2003;126(Pt 9):2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- 41.Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Thiebaut de Schotten M, Dell’Acqua F, Forkel SJ, et al. A lateralized brain network for visuospatial attention. Nature neuroscience. 2011;14:1245–1246. doi: 10.1038/nn.2905. [DOI] [PubMed] [Google Scholar]
- 43.Bernocchi G, Bottone MG, Piccolini VM, et al. Developing central nervous system and vulnerability to platinum compounds. Chemother Res Pract. 2011;2011:315418. doi: 10.1155/2011/315418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol. 2012;30:3675–3686. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.