Abstract
The central nervous system (CNS) and its meningeal coverings accommodate a diverse myeloid compartment that includes parenchymal microglia and perivascular macrophages as well as choroid plexus and meningeal macrophages, dendritic cells, and granulocytes. These myeloid populations enjoy an intimate relationship with the CNS, playing an essential role in both health and disease. Although the importance of these cells is clearly recognized, their exact function in the CNS continues to be explored. Here we review the subsets of myeloid cells that inhabit the parenchyma, meninges, and choroid plexus, and discuss their roles in CNS homeostasis. We also discuss the role of these cells in various neurological pathologies, such as autoimmunity, mechanical injury, neurodegeneration, and infection. We highlight the neuroprotective nature of certain myeloid cells, emphasizing their therapeutic potential for the treatment of neurological conditions.
Introduction
The complex structure of the CNS and its covering meninges accounts for a number of major differences in its immune responses compared to other peripheral tissues (Ransohoff and Brown, 2012). The CNS has long been regarded as a site of immune privilege due to the concept of a blood-brain barrier and the lack of lymphatic drainage that allows the transport of metabolic waste and CNS-derived antigen (Louveau et al., 2015a). It has become clear that continuous immune surveillance of the CNS does exist with certain limitations and is depending in part upon specialized myeloid cells within anatomical niches (Goldmann et al., 2016b; Kierdorf et al., 2015; Prinz and Priller, 2014). By orchestrating these immune sentinels, namely microglia, in the CNS parenchyma and macrophages and DC in the meninges (dura, arachnoid and pia matter), choroid plexus and perivascular spaces, this tissue can mount a robust protective and restorative response when necessary. In addition to their described inflammatory role, communication between neurons and myeloid cells is critical for proper brain function. Mutations in key myeloid genes are associated with numerous neurological disorders (Naj et al., 2011; Paloneva et al., 2002; Rademakers et al., 2011). Disrupting the interactions (in mouse experiments) between neuronal and myeloid cells has devastating effects on memory, sociability, anxiety and other behavioral domains, demonstrating the importance of myeloid cells in normal brain physiology (Nautiyal et al., 2008; Parkhurst et al., 2013; Zhan et al., 2014).
The myeloid compartment contains a diverse set of immune cells that participate in the response to tissue damage and pathogens, in addition to performing specialized functions pertinent to specific tissues. Most studies investigating effects of myeloid cells on neurons focus on microglia, the brain’s most prominent immune cells, which are situated in the parenchyma and serve as the tissue-resident macrophages of the central nervous system (CNS). Microglia make intimate contacts with synapses (Tremblay et al., 2010) and have been implicated, ironically, both in the construction of neural circuits in development (Schafer et al., 2012) and in the degeneration of synapses in neurodegenerative diseases (Hong et al., 2016). Other macrophages that influence the CNS include perivascular macrophages along the blood vessels of the brain, macrophages within the choroid plexus, and meningeal macrophages in the leptomeninges (Goldmann et al., 2016a). The meninges host additional myeloid cells including dendritic cells (DCs), monocytes, and granulocytes, which reportedly influence the brain mostly during or after an insult or other pathology (Chinnery et al., 2010) (Figure 1). Since recent reviews have discussed CNS myeloid cell origin and development (Prinz et al., 2017), this review will focus primarily on how myeloid cells function in the healthy CNS and their responses to autoimmunity, degenerative diseases, injury and infection.
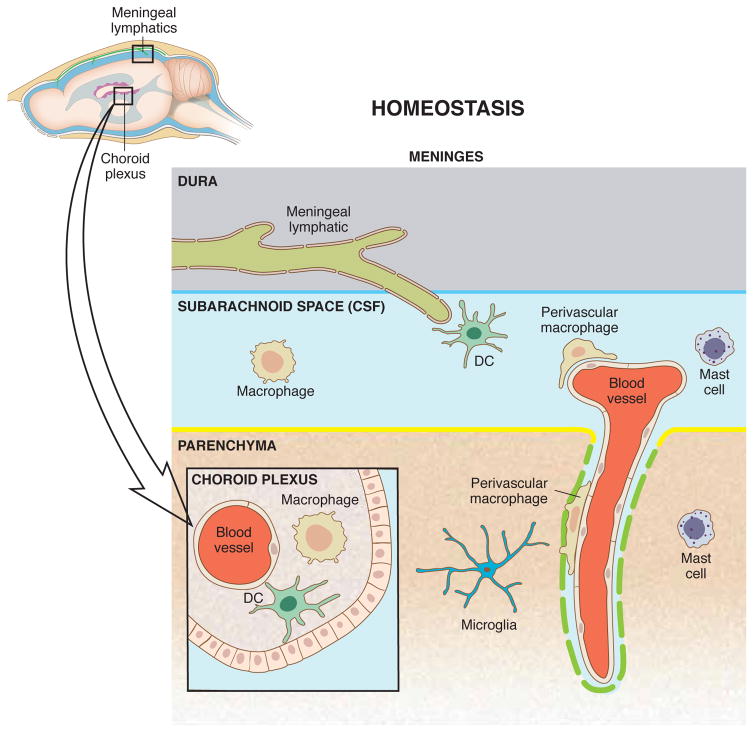
Figure 1. CNS-associated myeloid cells in homeostasis.
Within the complex anatomy of the brain and spinal cord, myeloid cells are strategically positioned in the parenchyma, nearby its blood vessels and within the meninges, where they fulfill homeostatic and surveillance tasks. While microglia are situated in the parenchyma and actively scan intraneuronal space, macrophages, dendritic cells and mast cells reside in the linings such as the meninges and choroid plexus and sample local debris and intruders from the blood and cerebrospinal fluid (Goldmann et al., 2016a). The communication with the peripheral immune system is presumably happening through meningeal lymphatic vessels, through which meningeal immune cells and soluble molecules drain to the deep cervical lymph nodes. It is important to note that depletion of myeloid cells in the CNS compartments leads to disturbed neurogenesis, impaired blood-brain barrier integrity and neuronal dysfunction.
Microglia
Microglia are tissue-resident macrophages that participate in the development of neuronal circuits, maintenance of synapses, and neurogenesis. Derived from erythomyeloid precursors in the extra-embryonic yolk sac (Gomez Perdiguero et al., 2015; Kierdorf et al., 2013), they seed the brain early in embryonic development (Ginhoux et al., 2010) and maintain a tiled pattern in the parenchyma through coupled apoptosis and local proliferation (Askew et al., 2017). Under physiological conditions, monocytes do not contribute to the pool of microglia, as in some peripheral tissues (Ajami et al., 2007). Whether or not they contribute in pathology is still debated, and will be discussed below.
Microglia play an important part in pruning synapses of the developing lateral geniculate nucleus, a hub for relaying neurons of the visual circuit by means of a complement-mediated process (Schafer et al., 2012). Therefore, mice lacking the complement protein C1q, C3, or complement receptor C3R demonstrate impaired synaptic pruning and connectivity (Schafer et al., 2012; Stevens et al., 2007). Synaptic markers can also be detected in microglia of other brain regions besides the lateral geniculate nucleus, and acute depletion of microglia in development leads to long-lasting changes in adult behavior (Nelson and Lenz, 2017; VanRyzin et al., 2016). Synaptic pruning can also be mediated by the CX3C chemokine receptor 1 (CX3CR1), whose ligand, CX3CL1, is expressed on developing neurons (Paolicelli et al., 2011). As with C1q-, C3-, or C3R-deficient mice, the brains of CX3CR1-deficient mice accumulate apoptotic neurons and synapses, thus affecting synaptic activity (Paolicelli et al., 2011). Functionally, CX3CR1-deficient mice exhibit decreased synaptic transmission, reduced functional connectivity, and behavioral deficits that are used in tests devised to model symptoms of autism spectrum disorder (Zhan et al., 2014). Microglial processes are dynamic and continually sample the environment (Nimmerjahn et al., 2005), allowing them to detect changes in pH, purines, cytokines, chemokines, amino acids, and inorganic compounds (Prinz et al., 2011). Given their vital role for normal brain function, it is not surprising that microglia dysfunction can be linked to some neurological disorders. Mutation in the homeobox B8 gene locus results in obsessive-compulsive grooming and could be mapped to primary dysfunction in microglia (Chen et al., 2010). Rett syndrome, a severe neurological disorder, is caused by a mutation in the gene encoding methyl-CpG-binding protein-2 and results not only from a defect in neurons (Chen et al., 2003) but also astrocytes (Lioy et al., 2011) and microglia (Cronk et al., 2015; Derecki et al., 2012). In both disease models, the abnormal behavior and phenotype could be partially corrected by reconstitution of the myeloid compartment with healthy bone-marrow derived progenitors, further supporting the role for microglia in maintaining neuronal function. These characteristics suggest that microglia are critical for proper neuronal function, and hence their elimination would be devastating to the brain. Recent data, however, have challenged this notion (Elmore et al., 2014), and how microglia influence neural development and physiology is still unclear (Fig. 2).
Figure 2. CNS modulating roles of microglia.
Microglia contribute to neuronal health but also participate in neuronal dysfunction and morphologic abnormalities. Left: The neuro-supportive role of microglia is mediated by secretion of trophic factors, such as BDNF (brain-derived neurotrophic factor) (Parkhurst et al., 2013) and participation of pruning as part of the normal developmental program (Paolicelli et al., 2011; Stevens et al., 2007). Their phagocytic activity is crucial for the cleanup of senescent cells and debris (Takahashi et al., 2005) and slowing the toxic effect of amyloid-β (Farfara et al., 2011). Right: Without vital microglia function, the CNS loses an essential facilitator of neuronal synaptic function (Parkhurst et al., 2013). Interactions with neurons promote plasticity but aberrant activity may facilitate intense inflammatory reactions that result in CNS pathology (Zhang et al., 2011). Phagocytic pursuit declines with age and can promote the progression of pathology and lead to cognitive regression (Hickman et al., 2008).
Depletion of microglia from the adult brain has yielded conflicting results. Parkhurst et al. used an inducible Cre-recombinase system to drive expression of the diphtheria toxin receptor in CX3CR1-expressing cells (CX3CR1CreER/+::R26iDTR/+), including microglia. Depletion of microglia with diphtheria toxin caused reduced spine dynamics and learning deficits (Parkhurst et al., 2013). Similar results were found in the retina, where long-term microglial depletion resulted in synapse degeneration and visual deficits (Wang et al., 2016a). Using an alternative approach, Elmore et al. used a colony stimulating factor 1 receptor (CSF1R) antagonist to effectively deplete microglia (Elmore et al., 2014). Surprisingly, this method of depleting microglia did not produce deficits in multiple behavioral assays, including assays for learning (Elmore et al., 2014). In attempting to reconcile these differences, one possibility to consider is that the systems were studying neuronal function under two different scenarios, namely microglial depletion and microglial proliferation. In the study by Elmore et al., microglia were depleted throughout the testing protocol, whereas in the study by Parkhurst at al., the mice were tested 2–4 days after depletion, when microglia are already aggressively repopulating the brain (Bruttger et al., 2015). It is also worth considering that this difference might be due to unresolved issues related to target cell specificity since all myeloid cells depend on the growth factor receptor CSF1R and its ligands CSF1 and interleukin (IL) 34. As a consequence, survival, proliferation and differentiation of CNS myeloid cell populations such as monocytes and perivascular macrophages are affected, which might also influence neuronal conditions. Unfortunately, true microglia-specific drugs are currently not available. Further studies utilizing genetic approaches are needed to clarify the microglia-dependent effects on neuronal function under these conditions (Figure 2).
Non-parenchymal macrophages: Perivascular, choroid plexus, and meningeal macrophages
Perivascular macrophages, as their name suggests, are located in the perivascular spaces, where they are positioned between blood vessels and astrocytic end-feet. Interestingly, unlike peripheral monocyte and macrophage populations, perivascular macrophages and microglia are transcriptionally related (Goldmann et al., 2016a). In terms of their functions the perivascular macrophages, like macrophages in the meninges and the choroid plexus, appear to play important roles in sampling local debris and dying cells as well as in communicating with local cells (Brendecke and Prinz, 2015; Mendes-Jorge et al., 2009). Perivascular macrophages clear amyloid-β from the CNS, suggesting they are important players in aging and in preventing neurodegeneration (Aguzzi et al., 2013; Nigro et al., 2016). Not less importantly, perivascular macrophages preserve the health of endothelial cells, promote capillary stability, regulate vascular constriction, and maintain blood–brain barrier (BBB) integrity (He et al., 2016; Mendes-Jorge et al., 2009). The location of perivascular macrophages uniquely positions them to sample blood and brain interstitial fluid at the same time. This might imply that they are eminently well situated to monitor both CNS and peripheral homeostasis. If this is indeed the case, it might enable us to gain important insights into the function of perivascular macrophages and their possible role in communication between the periphery and the CNS.
The choroid plexus, which lines the four ventricles of the brain, is a highly vascularized villous structure that produces two-thirds of the cerebrospinal fluid (CSF) filling the ventricles and subarachnoid spaces (Sakka et al., 2011). It is home to its own tissue-resident macrophage, the epiplexus cell, as well as to monocytes, dendritic cells (DCs), and mast cells (Allen, 1975; Kappers et al., 1958; Quintana et al., 2015; Shechter et al., 2013a). The choroid plexus has been viewed as an “educative gate” that skews immune cells towards a more anti-inflammatory phenotype. This environment is thought to contribute to the health of the CNS and to promote recovery after injury (Shechter et al., 2013a; Shechter et al., 2013b).
Meningeal macrophages are derived from early embryonic precursors without a monocyte intermediate and stay in the meninges until adulthood (Goldmann et al., 2016a). Although macrophages have similar characteristics across various tissues, they also have highly specific functions depending on their location (Okabe and Medzhitov, 2014). Therefore, their distinct position in the subdural meninges predisposes them to survey the cerebrospinal fluid and the extracellular lumen of meningeal blood vessels and act as a sentinel for infection and tissue damage (Roth et al., 2014). Most notably, meningeal macrophages are sensitive to the health of their environment (Cohen et al., 2014; Ginhoux et al., 2016; Hoeffel and Ginhoux, 2015; Kierdorf et al., 2015) and can acquire either anti-inflammatory or pro-inflammatory phenotypes (Kigerl et al., 2009) to regulate subsequent immune responses (Brendecke and Prinz, 2015).
Besides maintaining meningeal health, macrophages can also influence the health of the CNS. Mice trained in a Morris water maze (an assay of spatial memory) showed increased numbers of interleukin 4 (IL-4)-producing T cells in their meninges (Derecki et al., 2010). Moreover, the use of T cell-deficient mice or the deletion of IL-4 from T cells was shown to impair spatial memory and lead to a pro-inflammatory skew in meningeal macrophages, pointing to an important role for macrophages in learning and memory. Injection of anti-inflammatory macrophages (skewed ex vivo with IL-4) indeed enhanced water maze performance (Derecki et al., 2011).
Altogether, the above findings suggested that macrophages can play an important role in CNS health, at least with regard to spatial memory. Many questions remain unanswered: Which other behavioral domains are influenced by meningeal macrophages? How do macrophages communicate with CNS neurons? Do the functions of macrophages in the choroid plexus differ from those of meningeal macrophages? How do these cells influence the development of the CNS? Finding answers to these questions should provide a comprehensive understanding of the specialized interactions of CNS macrophages with their local milieu and expand our strategies on how to therapeutically target these cell types to benefit the brain.
Monocytes
Under steady-state conditions, monocytes are not detectable in brain or spinal cord parenchyma, however, they are, observed in the meninges (Mildner et al., 2008). More definitive evidence is needed to demonstrate that they are located in the meningeal space and elegant approaches to direct label circulating monocytes with fluorescent antibodies would permit in vivo fate mapping and positioning. In the periphery, monocytes can phagocytose debris, degrade and rearrange extracellular matrix, communicate with local cells, present antigens to lymphocytes, regulate inflammation, recruit other immune cells, promote angiogenesis, and help to repair wounds (Ginhoux and Jung, 2014). These functions may be particularly useful around the CNS, where immune surveillance is important for homeostasis (Shechter et al., 2013a).
Monocyte functions have been examined largely under pathological conditions, since these cells play a key role in regulating inflammation as well as in combating infection. As discussed later, monocytes and their derived macrophages and DCs play an essential role in recovery after spinal cord injury (Gadani et al., 2015b; Shechter et al., 2009; Shechter et al., 2013b). The homeostatic functions of monocytes, however, have seldom been discussed.
Dendritic cells
DCs are found in the human and rodent choroid plexus and meninges (Anandasabapathy et al., 2011; Chinnery et al., 2010; McMenamin, 1999; Serot et al., 1997). Three morphologically distinct subsets within the CNS borders were identified (Nayak et al., 2012) that are derived from bone marrow and all responsive to fms-like tyrosine kinase 3 ligand (FLT3L) (Anandasabapathy et al., 2011). Global deletion of integrin αx (CD11c) positive cells was found to result in a myeloproliferative disorder and loss of peripheral tolerance (Birnberg et al., 2008; Ohnmacht et al., 2009), but was found insufficient to promote CNS inflammation. However, at the time of the two last-mentioned studies, DCs were poorly defined phenotypically, and since then a standardized nomenclature based on developmental and transcriptional attributes has been proposed (Guilliams et al., 2014, Murphy, 2016 #300). This is likely to help future studies, since the marker used most frequently to examine DCs has been CD11c, which can be expressed also by other immune cells, including developing or activated microglia, macrophages, and monocyte-derived dendritic cells (moDCs) (Merad et al., 2013). The accumulation of numerous antigen-presenting cells (APCs) around lymphatic vessels in the meninges (Louveau et al., 2015b) strongly suggests that these vessels might serve as important pathways for DC migration in inflammatory diseases. Their function under homeostatic conditions, however, is not understood.
Granulocytes
Granulocytes, consisting of neutrophils, mast cells, eosinophils, and basophils, are all found within meningeal spaces (mast cells also reside within the CNS parenchyma). Granulocytes are particularly useful in combating pathogen invasion and in responding to tissue damage.
Unlike many other immune cells, neutrophils exit the bone marrow in a fully mature state. In general, neutrophils are thought to have very short lives of not more than a few hours (Kolaczkowska and Kubes, 2013). However, reservoirs of mature neutrophils can be found in the lung, spleen, liver and bone marrow, suggesting that these cells may nevertheless persist for more than a few hours at a time (Kolaczkowska and Kubes, 2013). Neutrophils are largely considered to function only during times of disease and injury. This assumption is based on their ability to mobilize quickly and being among the first cells to respond to tissue damage. A small population of neutrophils resides in the steady-state meninges (Cronk et al., 2015), but their function under homeostatic conditions is not understood.
Mast cells and their secreted mediators are best known for their supportive role in allergic reactions such as asthma and anaphylaxis. We now know that mast cells not only function as pathogen sensors and modulators of inflammation (reviewed by (Abraham and St John, 2010), but also act as important regulators in the normal physiology of the skin, the digestive tract, and other sites in the body. Their response is multifaceted, and ranges from long-distance communication via lymphatics, through particle-packaged cytokines to angiogenesis modulation and barrier function. The brain has been shown to harbor resident mast cells in the parenchyma, choroid plexus, perivascular space, and brain lining (Hough, 1988) in many mammalian and avian species (Theoharides, 1990). Their potential role in normal brain function and their participation in maintenance of equilibrium has long been controversial, owing to the considerable variability in their numbers and distribution among species, individuals, and sexes (Dines and Powell, 1997; Theoharides, 1990).
In the brain, mast cells localized near the hippocampus influence neurons through the secretion of histamine, serotonin, and other neuromodulators to affect learning, memory, and anxiety (Chikahisa et al., 2013; Nautiyal et al., 2008). Mast cell-deficient (KitW-sh) mice exhibit hippocampus-dependent behavioral deficits that can be ameliorated by treatment with a selective serotonin-reuptake inhibitor, indicating that these cells make a significant contribution as a source of serotonin (Nautiyal et al., 2012). Intriguingly, migration of mast cells into the parenchyma can be induced. Studies in animal models have shown that mature mast cells increase in number in the brain during prolonged phototherapy (Silverman et al., 2002). Since mast cells seem to be among the few immune cells that migrate into the CNS under physiological conditions, an understanding of how they can affect neuronal function may open new therapeutic targets for cognitive disorders.
Alterations in mast-cell interactions, numbers, and functioning in the brains of aged individuals have not been extensively investigated. An interesting goal for future studies will be to understand what dictates the specificity of mast-cell action on neurons, microglia and endothelial cells, and whether their manipulation might allow targeted delivery of neuromodulators to specific brain regions. Because of their location at the host environment interface of the brain, as well as their ability to communicate via lymphatics, it seems reasonable to speculate that mast cells serve a unique duty of long-distance delivery of small quantities of mediators that they sense in the blood and the CSF. Mast cells are also known for their ability to recruit other immune cells to sites of action, and have been proposed as major immune regulators in multiple sclerosis, seizure induction, and CNS infection (Sayed et al., 2010; Skaper et al., 2013; Yillar and Kucukhuseyin, 2008).
Autoimmunity
Multiple sclerosis (MS) is the most common autoimmune disorder affecting the brain and spinal cord. While the cause of this disabling disease is not entirely clear, the underlying mechanisms are considered to be attacks on the nervous system by infiltrating immune cells, especially auto-aggressive T cells. In recent years many studies have demonstrated that the cooperation between myeloid cells and activated myelin-reactive T cells contributes to lesion formation in the white matter of the brain (Brendecke and Prinz, 2015; Prinz and Priller, 2017). Other reports suggest, however, that myeloid cells can aid recovery by promoting remyelination (Miron et al., 2013), secreting neuroprotective mediators (Butovsky et al., 2006), and promoting phagocytosis of growth-inhibitory myelin debris (Piccio et al., 2007). Thus, their role in pathological mechanisms of CNS autoimmunity remains controversial.
Myeloid cells are among the most prominent of the immune infiltrates in MS, and large numbers of macrophages, monocytes, and DCs have been identified in actively demyelinating lesions (Bruck et al., 1996; Henderson et al., 2009). In experimental autoimmune encephalomyelitis (EAE), an animal model for MS, myeloid cells act in concert with T cells, and strategies for myeloid cell depletion using genetic tools and chemokine-receptor-deficient mice, or pharmacological treatments with clodronate liposomes, silica dust, or minocycline, have been found to alleviate or even prevent neurological symptoms (Mildner et al., 2009). Whereas tissue-resident microglia seem to clear tissue debris, contribute to recovery, and aim to protect the CNS, monocyte-derived phagocytes instruct demyelination (Goldmann and Prinz, 2013; Lewis et al., 2014; Yamasaki et al., 2014). The pathological role of circulating Ly6ChiCCR2+ monocytes has been well described in neuroinflammatory conditions (Ji et al., 2013). Notably, T cell-derived granulocyte macrophage colony-stimulating factor (GM-CSF) is the major driver of C-C chemokine receptor (CCR) 2 positive monocyte-mediated activities (Croxford et al., 2015) and is sufficient to induce EAE (Spath et al., 2017). Once in the CNS, infiltrating myeloid cells not only exert effector function by attracting lymphocytes, but also generate oxidative stress and cytotoxic factors (Fischer et al., 2012). Due to their phenotypic similarities, the behavioral responses and the fate of monocytes and microglia have not been completely resolved. It was suggested that they do not integrate into the CNS on a permanent basis (Ajami et al., 2011). The use of new genetic systems, such as CX3CR1ERT2Cre transgenic mice (which allows specific manipulation of microglia function (Parkhurst 2013)) will presumably enable us to more directly study and manipulate microglia or monocytes separately.
Before T cells cross the glia limitans, they exit the vascular lumen and gather in perivascular and meningeal spaces. Recent research has shed light on perivascular and meningeal myeloid cells that are strategically placed at CNS interfaces that are believed to reactivate infiltrating autoreactive T cells to undergo expansion and enter the parenchyma (Bartholomaus et al., 2009; Pesic et al., 2013; Tran et al., 1998). Blocking the adhesion molecule very late antigen-4 (VLA-4) with monoclonal antibodies strongly impairs the migration of T cells into the brain parenchyma (Polman et al., 2006). Moreover, local injection of the chemokine blocker anti-CXCR3 mAb, but also of anti-LFA-1 (lymphocyte function-associated antigen 1) and VLA-4, into the CSF results in detachment of T cells from the meninges and decreased T cell invasion of the spinal cord parenchyma (Schlager et al., 2016). Meningeal phagocytes produce the appropriate ligands required to enforce T cell adhesiveness (Schlager et al., 2016). The exact anatomical location (periphery, secondary lymphoid tissue, or meningeal spaces) of these T cells, and the identity of the agents that activate them to become encephalitogenic, is still a subject of dispute.
DCs, under autoimmune disease conditions, perform five known major functions: (i) they sample and process CNS antigens; (ii) they shape the anti-myelin T cell response in draining lymph nodes; (iii) they maintain and overcome tolerance to CNS antigens; (iv) they reactivate T cells in the meninges; and (v) they directly induce tissue damage within the CNS (Sie and Korn, 2017). Importantly, autoreactive T cells encounter their cognate antigen on an APC at least twice over the course of disease: at the initial priming and again during reactivation in the CNS (Greter et al., 2005). Compared to healthy subjects, patients with MS demonstrate an abundance of DCs (both classical DCs (cDC) and plasmacytoid DCs (pDC)) in the CSF and around MS lesions (Lande et al., 2008; Longhini et al., 2011). pDCs (but not cDCs) from MS patients exhibit pronounced upregulation of CCR5 and CCR7, which is thought to alter their trafficking behavior and direct their migration to the CNS-draining lymph nodes in order to activate the autoimmune T-cell repertoire (Thewissen et al., 2014).
Most concepts of DC biology, and specifically of how they orchestrate T-cell differentiation at different stages of CNS autoimmune diseases, are derived from research findings on EAE in mice and rats. Strikingly, DCs isolated from EAE models effectively transfer the disease to naïve recipients (Knight et al., 1983). Although DCs are important for the priming of autoreactive lymphocytes, several models of DC lineage ablation show that these cells are not strictly required and that other APCs can substitute for them (Isaksson et al., 2012; Yogev et al., 2012). While the type and source of antigens that initiate MS are not known, experimental evidence suggests that both antigens and APCs from the meninges can reach the deep cervical lymph nodes and potentiate an adaptive immune response against myelin (Karman et al., 2004). The deep cervical lymph nodes of MS patients have indeed been found to harbor neuronal antigens in non-inflammatory major histocompatibility complex (MHC) II+ cells (van Zwam et al., 2009). It is not yet known, however, which of the known routes (nasal, perineural, or meningeal lymphatics) serve as the major conduit for sampling and processing of antigens and for APCs to exit the CNS (Engelhardt et al., 2017; Kipnis, 2016). In addition to initiating immune responses, DCs also promote and maintain peripheral T-cell tolerance by inducing Treg, and mice lacking CD11c+ cells are hypersensitive to EAE (Yogev et al., 2012). Despite some major advances in the characterization of human DCs, however, the full functional range of subsets and roles, both for the promotion and for the inhibition of pathology, remains to be determined.
Given their secretory nature and their residence in the CNS and meninges, it is not surprising that mast cells are involved in the initiation and progression of MS. These cells were identified in the CNS in the vicinity of MS lesions (Theoharides, 1990; Toms et al., 1990), and the proportion undergoing degranulation was significantly increased in EAE brains (Bo et al., 1991; Brenner et al., 1994). Mice deficient in mast cells showed profoundly less susceptibility than normal mice to EAE because of their diminished accumulation of autoreactive T cells in the meninges (Christy et al., 2013). Prior to disease onset, these cells secrete several inflammatory mediators that induce neutrophil influx and alter BBB integrity at early stages of the disease (Zhuang et al., 1996). Notably, mast cells appear to greatly facilitate T-cell recruitment and amplify their effector function through the action of IL-1β and GM-CSF (Russi and Brown, 2015).
Despite some convincing concepts of adaptive immunity, derived from the vast amount of research done over the last half-century, drugs targeting putative mediators for T-cell adhesion and penetration into the CNS have adverse side effects, Thus, understanding MS and stopping it in its tracks also clearly requires keener insight into the workings of the immune system’s myeloid compartment, comprising the cellular players of innate immunity.
Degeneration
The continuing rise in human life expectancy is accompanied, not surprisingly, by a dramatic increase in the frequency of neurodegenerative disorders. Neurodegeneration can manifest in many different diseases. Some of the more common diseases are Parkinson’s disease (PD), Alzheimer’s disease (AD), frontotemporal dementia (FTD), amyotrophic lateral sclerosis (ALS; also known as Lou Gehrig’s disease), and Huntington’s disease (HD). All are characterized by a progressive degeneration of neurons, with associated motor dysfunction, sensory impairment, memory loss, and personality changes. The role of the immune system in neurodegenerative diseases is complex, being protective in some cases and detrimental in others (Donnelly et al., 2011; Heppner et al., 2015; Prokop et al., 2013; Selkoe and Hardy, 2016; Shechter and Schwartz, 2013; Vinet et al., 2012; Walsh and Kipnis, 2011). Here we will discuss myeloid cells and their impact on neurodegeneration.
AD is characterized by gradual memory loss as a result of progressive brain atrophy accompanied by hallmark amyloid plaques and neurofibrillary tangles (Bertram et al., 2010; Sweeney et al., 2016; Zhao et al., 2015). While the participation of myeloid cells (microglia as well as peripheral monocytes and their derived macrophages) in AD has been extensively researched, their contributions to disease pathology and repair are still a matter of debate (Hong et al., 2016; Prokop et al., 2015; Simard et al., 2006; Yamasaki et al., 2014). Simard et al. have shown that bone marrow-derived mononuclear phagocytes infiltrate the AD mouse brain and accumulate around amyloid-β plaques. Transplantation of wild-type bone marrow into AD mice, was found to result in reduced AD pathology, supposedly owing to phagocytic actions of the bone marrow-derived cells (Simard et al., 2006). Disease pathology was reported to be further ameliorated upon treatment of mice with macrophage colony-stimulating factor (M-CSF) after bone-marrow transplantation (Boissonneault et al., 2009). Supporting evidence for the role of peripheral blood-borne macrophages came from studies in which CCR2 knockout mice were crossed with APP mice (Tg-2576) (El Khoury et al., 2007). CCR2 is required for monocyte infiltration into the CNS (Naert and Rivest, 2011); the worsened AD pathology in these mice was explained as due in part to inability of peripheral monocytes to enter the CNS parenchyma.
Findings on monocyte-derived macrophages in AD are inconclusive (Prokop et al., 2015). It is commonly believed that monocytes do not engraft CNS unless it is preconditioned by irradiation (Mildner et al., 2007). Indeed microglia, unlike all other tissue-resident macrophages, are not replaced by peripheral cells throughout life, but maintain their pool through cell proliferation (Ginhoux et al., 2010; Goldmann et al., 2016a). Studies of parabiotic mice showed that a CNS disease state (such as ALS, for example) is insufficient to allow monocyte recruitment (Ajami et al., 2007). Moreover, those studies suggested that only unique bone marrow-derived progenitors, which are not normally found in the peripheral blood, are capable of engrafting the brain parenchyma (Ajami et al., 2011; Ajami et al., 2007). The situation is different in severe inflammatory conditions such as EAE. Peripheral monocytes in EAE do enter the CNS parenchyma, but are believed to be short-lived (Ajami et al., 2011; Mildner et al., 2007), and their role in EAE pathogenesis is supposedly detrimental. It is therefore still a matter of debate whether peripheral monocytes contribute to disease pathology, ameliorate it, or do not even enter the CNS parenchyma (Figure 3).
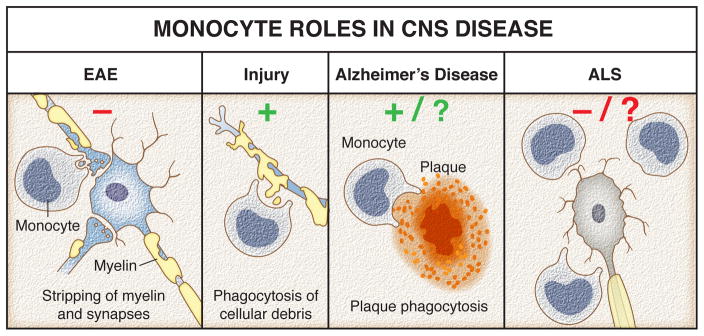
Figure 3. Monocytes roles in CNS diseases.
In EAE, monocytes apply cytotoxic effects on neurons and trigger disease progression by stripping myelin. Contrary, after traumatic injury peripherally derived monocytes are required to remove cellular debris and ultimately promote regeneration. In Alzheimer’s disease, monocytes potentially reduce amyloid loads in the brain through enhanced clearance of amyloid plaques. However, most recent data argue against the possibility that monocytes infiltrate the CNS so their involvement is questionable. Targeting circulating Ly6C+ monocytes slowed amyotrophic lateral sclerosis (ALS) progression and attenuated neuronal damage. Their presence and potentially damaging actions within the CNS remain controversial.
During development, C1q and C3 mark synapses for elimination by microglia (Schafer et al., 2012). A similar mechanism was recently suggested to participate in early stages of AD (Hong et al., 2016). This process is augmented by the presence of oligomeric amyloid-β, and amyloid-β-dependent synapse loss can be prevented in mice lacking C1q, C3, or CR3 (Hong et al., 2016). Overall, these data suggest that microglia contribute to AD pathogenesis. However, mutations in TREM2 (a phagocytic receptor expressed by microglia) have been associated with AD, and studies of AD mouse models suggest that signaling through TREM2 is required for microglial phagocytosis. Thus, TREM2 deletion results in accelerated AD pathology (Wang et al., 2016b). This view is challenged, however, by studies in another mouse model of AD, where TREM2 deletion results in ameliorated pathology (Jay et al., 2015). In this later work, interestingly, the proposed mechanism operates through elimination of peripheral monocyte engraftment infiltration into the CNS of TREM2-knockout mice. However, this study was based on phenotypic markers of monocytes that did not discriminate them well from microglia (Butovsky et al., 2012). More convincing parabiosis experiments indicate that peripheral blood monocytes are not recruited to the diseased CNS, which argues against the hypothesis that monocytes engraft during AD (Wang et al., 2016b). It is indeed questionable whether microglia play only a detrimental role in AD. Why would CNS-resident cells contribute to pathology of their own site of residence? A more plausible scenario might be that aging imposes wear and tear on microglia, making them incapable of coping with the challenges wrought by processes in the aging brain. From an evolutionary point of view, the failure of microglia to support the brain in its hour of need rather than their active contribution to the damage would be an easier hypothesis to entertain.
The role of myeloid cells has also been actively studied in other forms of neurodegeneration, such as FTD, PD, and ALS. FTD is a collection of diseases that are associated with tau or TAR DNA-binding protein 43 (TDP-43) pathology and result in degeneration of the frontal and temporal lobes of the brain, accompanied by progressive changes in personality, memory, speech, and motor function. FTD is modeled in mice through loss of the progranulin gene (Grn) (Filiano et al., 2013; Lui et al., 2016). In Grn-knockout mice, microglia phagocytose important inhibitory synapses in the ventral thalamus. The resulting hyperexcitation of thalamocortical circuits is manifested in obsessive-compulsive grooming behaviors. This disease pathogenesis is attenuated in Grn-knockout mice that also lack C1qα, suggesting that the microglia affect FTD progression in a complement-dependent manner (Lui et al., 2016).
Patients with ALS experience progressive degeneration of the motor neurons of the spinal cord, resulting in motor dysfunction, muscular atrophy, paralysis, and death. ALS pathogenesis is often associated with aggregates of superoxide dismutase 1 (SOD1), FUS, or TDP-43 proteins. SOD1 mutants are often used to model ALS in mice. Initial studies of ALS in mouse models suggested that myeloid cells play an important role in slowing down progression of the disease (Appel et al., 2011; Beers et al., 2006). In later works, it was debated whether the cells governing the detrimental versus beneficial effects on disease progression are microglia or peripheral monocytes (Butovsky et al., 2012). Several studies suggested that accumulation of monocytes in the CNS parenchyma during later stages of the disease is detrimental. This notion was based on downregulation of the microglial genetic signature and appearance of the monocyte signature in myeloid-cell transcriptomes isolated from the spinal cords of these mice (Butovsky et al., 2012). Those studies were subsequently challenged, as CCR2+ cells could not be detected in the spinal cords of ALS mice (Chiu et al., 2013). In any case, it seems that the presence of CCR2 would not be sufficient as evidence either for or against engraftment of monocytes, because shortly after infiltration into the intact CNS parenchyma, monocytes downregulate their CCR2 and upregulate their CX3CR1, making them indistinguishable from microglia. These discrepancies emphasize the urgent need for better genetic signatures and more effective tools to distinguish microglia from monocyte-derived macrophages. Lack of these tools precludes our understanding of the differential contributions of myeloid-cell subsets in the CNS, and often leads to misinterpretation.
CNS injury
Myeloid involvement is particularly important for the wound-healing process after traumatic CNS injury (Fig. 3). Whether the effects of myeloid cells on recovery are beneficial or detrimental (or both) is still largely a matter of debate, and may depend on the type of injury (Donnelly et al., 2011; Greenhalgh and David, 2014; Kroner et al., 2014; Popovich, 2014; Ransohoff, 2016; Rolls et al., 2008; Shechter et al., 2013c). Whereas in some types of injury myeloid cells play an important beneficial role in promoting angiogenesis and axon regeneration, in other injury models these cells apparently contribute to secondary degeneration. In many instances the myeloid involvement is finely nuanced, with the extent of recovery being critically influenced by the timing and the route of myeloid cells infiltrating post injury. Below we discuss the participation of myeloid cells in several types of traumatic CNS injury.
Labeling for markers of microglia and astrocytes in the injured CNS nicely demonstrates mutually exclusive coverage by these two types of CNS-resident cells. Whereas the site of injury is occupied by microglia, astrocytes demarcate its borders by generating a glial scar (Hauben et al., 2000; Sofroniew, 2005). Macrophages derived from peripheral monocytes are also found at injury sites, where they intermingle with microglia and also interact with astrocytes at the borders of the injury epicenter (Shechter et al., 2009; Shechter et al., 2013c).
Historically, macrophages and other immune cells were considered to be detrimental to recovery from injuries to nervous tissue. This view was initially challenged by research in the peripheral nervous system, where macrophages play important roles in clearing myelin debris and supporting neural regeneration after injury (Hirata et al., 1999; Keilhoff et al., 2007; Venezie et al., 1995). Later, beneficial roles were attributed to peripheral macrophages also in rodent injuries to the CNS (Benowitz and Yin, 2007; Shechter et al., 2013c), by inducing recovery of severely damaged axons optic nerve crush injury models and regeneration of severed axons in models of spinal cord transection (Rapalino et al., 1998). These activated macrophages were suggested to contribute to axonal regeneration through production of growth factors and clearance of myelin debris, but in reality the mechanisms underlying those beneficial effects were not fully understood. Future studies should be aimed at gaining a better understanding of the differential roles of various myeloid cell subtypes operating at different times after CNS injury and their specific mechanistic contributions to the acceleration or amelioration of damage.
There is overall consensus that peripheral monocytes migrate into the injured CNS tissue, although the exact routes of entry and the types of infiltrating myeloid precursors remain to be more precisely clarified (Shechter et al., 2013c). Some studies have claimed that potentially beneficial monocyte responses cannot be fully expressed in the injured CNS because the monocytes are trapped within the fibrotic glial scar (Shechter et al., 2009). Dissociation of the extracellular matrix around the injured area, thereby allowing monocytes to populate a site otherwise predominantly occupied by microglia, improved neuronal survival.
There is also argument over the types of monocytes that enter the site of injury. Progenitors of protective tissue-building macrophages are suggested to enter from the choroid plexus and migrate to the injury site through the central canal, whereas detrimental progenitors of classically activated macrophages supposedly enter through the blood vasculature (Roth et al., 2014; Russo and McGavern, 2015; Shechter et al., 2013c). The hypothesis of differential routes of entry for different macrophage precursors, while interesting, raises numerous questions. For example, how do the different precursors know which path to take? What chemokines guide the two different monocyte populations? Why would detrimental monocytes be needed at the site of injury at all?
We have demonstrated that CNS injury results in generation of an alarm signal, interleukin 33 (IL-33), which is secreted by damaged oligodendrocytes (Gadani et al., 2015a; Gadani et al., 2015b). In astrocytes, IL-33 induces the secretion of various chemokines, among them CCL2, which in turn recruits peripheral blood monocytes (supposedly via the meningeal blood vasculature, although this requires further substantiation). Elimination of IL-33, or lack of CCR2 expression on monocytes, results in impaired outcome of spinal cord injury. The mechanism underlying acquisition of a beneficial phenotype by CNS-engrafting monocytes has yet to be identified.
We have recently demonstrated the presence of innate lymphoid cells type-2 (ILC2) cells within meningeal tissue (Gadani et al., 2017). In response to spinal cord injury these cells become activated via IL-33 signaling and produce IL-5 and IL-13, which in turn may trigger an alternative activation phenotype of engrafting monocytes, thereby dictating a beneficial response. It is plausible that in the absence of IL-33 or ILC2 cells the infiltrating myeloid and lymphoid cells will acquire a more pro-inflammatory phenotype, thus damaging rather than supporting the injured spinal cord tissue. Further work is needed in order to address these hypotheses and test them in animal models.
Not only monocytes but also neutrophils play beneficial roles in CNS injury, primarily in models of optic nerve crush. For example, the addition of zymosan after optic nerve crush results in severe neuroinflammation and in recruitment of neutrophils that potentiate neuronal outgrowth (Baldwin et al., 2015; de Lima et al., 2012; Yin et al., 2003).
The post-injury role of microglia is possibly even less well understood than that of peripheral monocytes. Because the post-injury response of activated microglia is indistinguishable from that of monocyte-derived macrophages, there are no tools for depleting microglia without also affecting the macrophages. Thus, their roles in recovery, when taken together, may run the gamut from destructors to protectors (or simply bystander observers), and there is literature to collectively support all of them (Gadani et al., 2015a) (Figure 3). Further work and more sophisticated tools will be needed to prise apart the contributions of microglia and those of monocytes to neural recovery after CNS injury. It is also important to note that in models of traumatic brain injury and of stroke, the role of inflammation is more generally referred to as detrimental. These views may also sharpen as better tools are developed for interpreting the ways in which cells during different injury paradigms might convey their “reports” on the state of the damaged tissue to the peripheral immune cells. It is possible, for example, that interference with glymphatic function (a proposed mechanism how extracellular solutes clear out of the brain parenchyma) as a result of head trauma or a stroke would delay the delivery of danger signals to the lymph nodes, resulting in delayed and altered immune responses—an outcome that emphatically is not the case after injury to the spinal cord. It is also possible that impaired glymphatic function might result in the accumulation and persistence of toxic immune mediators within the site of injury, triggering irreversible destructive processes. Future studies should adopt a more global approach when investigating immune responses to CNS injury and take into account not only the possibility of interference with glymphatic function (Xie et al., 2013), but also the possibly disrupted function of meningeal lymphatic vessels (Louveau et al., 2015b) that communicate signals from the CNS to the peripheral immune system.
Infection
Myeloid cells are uniquely positioned and endowed to respond quickly to CNS-invading pathogens, including bacteria, viruses, fungi, and parasites. Pathogenic invasion occurs through paracellular or transcellular routes (Coureuil et al., 2009; Konradt et al., 2016), through retro- and anterograde migration on neuronal projections, and through the ability to hijack a migratory cell (often a monocyte) in order to cross the BBB (Drevets et al., 2004). Once the trespassers have gained entry into meningeal spaces, the macrophages that reside there—as well as those in perivascular spaces—will take a particular interest in eliminating the invaders, and will thereby ultimately protect the CNS against inflammation (Bauler et al., 2017; Polfliet et al., 2001). Following this disciplinary action, the macrophages release a variety of chemoattractants into the CSF to recruit neutrophils and monocytes for additional support (Klein et al., 2017; Mildner et al., 2008). Unfortunately, this process is not always beneficial, and the pathological potential of the recruited monocytes has been actualized into several CNS disease models. Release of hostile players such as proteolytic enzymes, barrier-disrupting cytokines including TNFα, IL-6 and IL1β, as well as reactive oxygen species, promote vascular and basement-membrane breakdown. In meninges infected by lymphocytic choriomeningitis virus, disease develops through the release of chemoattractants by CD8 T cells, thus demonstrating an underlying role for adaptive immune cells in setting the stage for immunopathology. Numerous monocytes extravasate from the vascular lumen in a synchronized event that leads to global micro-hemorrhages in the meninges and induction of fatal seizures (Kim et al., 2009). The impact on the BBB might derive from monocyte adherence to the blood vasculature (Wedmore and Williams, 1981) or chemokine release (Stamatovic et al., 2005). In line with these observations in animal models, a minimal presence of T-cell responses in the CSF of patients with acute bacterial meningitis correlated with a larger number of monocytes and increased risk of mortality and inflammatory syndrome (Jarvis et al., 2015). Therapies aimed at reducing neutrophil and monocyte activation are challenging because of the rapid turnover of these cells and adverse side effects on otherwise beneficial myeloid cells. However, peripheral sampling of CNS-released antigens is an important pathway for initiating immune responses to pathogens, and drainage routes might be accessible to treatment. Homing of DCs to CNS meninges (Anandasabapathy et al., 2011) indicated that antigens can be processed and loaded on meningeal APCs. Using intracerebral injection of antigen-loaded DCs it was revealed that these DCs migrate from the CNS into draining lymph nodes (Karman et al., 2004) and are retained if CCR7 is deficient (Clarkson et al., 2017). Upon reaching the lymph nodes, DCs might be implicated in activating T cells and potentially leading to recruitment of activated T cells back to the brain; this, however, has not been shown under either physiological or inflammatory conditions.
The route of entry into the CNS is a key factor for further dissemination of the pathogen from the meninges to the parenchyma. The ability to leave the vascular compartment without serious detection and with limited bystander damage might have evolved as a smart mechanism to facilitate the transmission of parasites such as Toxoplasma gondii and plasmodium. Although the detection machinery of the CNS is composed of pattern recognition molecules along all routes of invasion, microbial pathogens nevertheless escape or are not noticed. Microglia can contribute to the phagocytosis of pathogens (Goldman et al., 2001) or to the recognition of acute bacterial compounds through Toll-like receptors. These responses might enhance pathogen clearance, but they might also affect neurogenesis and consequently induce neurotoxicity through the release of oxidants that activate the inflammasome (Braun et al., 1999). In persistently infected mice, microglia can be a reservoir of viral infection and be prompted to present antigens and support CD8 T-cell-mediated viral clearance (Herz et al., 2015). A key challenge for neuroimmunologists, then, will be to understand how to promote local antimicrobial immune responses without causing pathology. Differential targeting of the local immune systems and molecular tactics in the CNS may therefore be a suitable goal.
Concluding remarks
An accumulation of exciting evidence points to the likelihood of extensive crosstalk between myeloid and CNS-resident cells, even in the absence of overt immunopathology. Their exact function might differ according to distinct subsets and locations, but it appears that myeloid cells confer an overall beneficial effect on CNS health. Steady-state cellular actions are fundamental for the guidance of neurons, the refinement of synapses, and the ability to generate memories. If communication is interrupted by protein deposits like amyloid-β, physical and mental abilities will falter. Pathologies such as PD and AD evidently possess some degree of maladaptive myeloid cells as contributing factors, raising the possibility of developing a new angle of therapeutic intervention.
Myeloid cells closely observe the vasculature, scavenge the material that enters tissue fluids, and participate in basic tissue cleanup. Key cellular components of myeloid immunity in the perivascular spaces, choroid plexus, and meningeal lining dutifully shield the CNS parenchyma from external influences and invaders. Their activities as bodyguards experience a fundamental change of scene in the inflamed CNS, once the BBB is disrupted and peripheral cells and compounds gain access to it. Microglia and other brain macrophages are now subjected to a new and unfriendly environment, with peripheral cues to which they may respond by malfunctioning. Despite major advances, several key areas still demand investigation. These include, for example, the capacity of myeloid cells to self-renew, the functional fulfillment of stem-cell therapy or bone-marrow transplantation to replace endogenous populations, and the molecular tactics employed by myeloid cells to communicate with CNS neurons.
Acknowledgments
We would like to thank S. Smith for editing the manuscript and A. Impagliazzo for the artwork. We would also like to thank all the members of the Kipnis lab for their valuable comments during multiple discussions of this work. This work was supported by a grant from the National Institutes of Health (NS096967 to J.K) and the German Research Council (DFG) (CRC-TR-128, B11 to J.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguzzi A, Barres BA, Bennett ML. Microglia: Scapegoat, Saboteur, or Something Else? Science (New York, NY) 2013;339:156–161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FMV. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Allen DJ. Electron microscopy of epiplexus macrophages (Kolmer cells) in the dog. Journal of Comparative Neurology. 1975;161:197–214. doi: 10.1002/cne.901610205. [DOI] [PubMed] [Google Scholar]
- Anandasabapathy N, Victora GD, Meredith M, Feder R, Dong B, Kluger C, Yao K, Dustin ML, Nussenzweig MC, Steinman RM, Liu K. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J Exp Med. 2011;208:1695–1705. doi: 10.1084/jem.20102657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel SH, Zhao W, Beers DR, Henkel JS. The microglial-motoneuron dialogue in ALS. Acta Myol. 2011;30:4–8. [PMC free article] [PubMed] [Google Scholar]
- Askew K, Li K, Olmos-Alonso A, Garcia-Moreno F, Liang Y, Richardson P, Tipton T, Chapman MA, Riecken K, Beccari S, et al. Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain. Cell reports. 2017;18:391–405. doi: 10.1016/j.celrep.2016.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin KT, Carbajal KS, Segal BM, Giger RJ. Neuroinflammation triggered by beta-glucan/dectin-1 signaling enables CNS axon regeneration. Proc Natl Acad Sci U S A. 2015;112:2581–2586. doi: 10.1073/pnas.1423221112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomaus I, Kawakami N, Odoardi F, Schlager C, Miljkovic D, Ellwart JW, Klinkert WE, Flugel-Koch C, Issekutz TB, Wekerle H, Flugel A. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- Bauler TJ, Starr T, Nagy TA, Sridhar S, Scott D, Winkler CW, Steele-Mortimer O, Detweiler CS, Peterson KE. Salmonella Meningitis Associated with Monocyte Infiltration in Mice. Am J Pathol. 2017;187:187–199. doi: 10.1016/j.ajpath.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, Siklos L, McKercher SR, Appel SH. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz L, Yin Y. Rewiring the injured CNS: Lessons from the optic nerve. Exp Neurol. 2007 doi: 10.1016/j.expneurol.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Birnberg T, Bar-On L, Sapoznikov A, Caton ML, Cervantes-Barragan L, Makia D, Krauthgamer R, Brenner O, Ludewig B, Brockschnieder D, et al. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 2008;29:986–997. doi: 10.1016/j.immuni.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Bo L, Olsson T, Nyland H, Kruger PG, Taule A, Mork S. Mast cells in brains during experimental allergic encephalomyelitis in Lewis rats. J Neurol Sci. 1991;105:135–142. doi: 10.1016/0022-510x(91)90136-u. [DOI] [PubMed] [Google Scholar]
- Boissonneault V, Filali M, Lessard M, Relton J, Wong G, Rivest S. Powerful beneficial effects of macrophage colony-stimulating factor on beta-amyloid deposition and cognitive impairment in Alzheimer’s disease. Brain. 2009;132:1078–1092. doi: 10.1093/brain/awn331. [DOI] [PubMed] [Google Scholar]
- Braun JS, Novak R, Herzog KH, Bodner SM, Cleveland JL, Tuomanen EI. Neuroprotection by a caspase inhibitor in acute bacterial meningitis. Nat Med. 1999;5:298–302. doi: 10.1038/6514. [DOI] [PubMed] [Google Scholar]
- Brendecke SM, Prinz M. Do not judge a cell by its cover—diversity of CNS resident, adjoining and infiltrating myeloid cells in inflammation. Semin Immunopathol. 2015;37:591–605. doi: 10.1007/s00281-015-0520-6. [DOI] [PubMed] [Google Scholar]
- Brenner T, Soffer D, Shalit M, Levi-Schaffer F. Mast cells in experimental allergic encephalomyelitis: characterization, distribution in the CNS and in vitro activation by myelin basic protein and neuropeptides. J Neurol Sci. 1994;122:210–213. doi: 10.1016/0022-510x(94)90300-x. [DOI] [PubMed] [Google Scholar]
- Bruck W, Sommermeier N, Bergmann M, Zettl U, Goebel HH, Kretzschmar HA, Lassmann H. Macrophages in multiple sclerosis. Immunobiology. 1996;195:588–600. doi: 10.1016/S0171-2985(96)80024-6. [DOI] [PubMed] [Google Scholar]
- Bruttger J, Karram K, Wortge S, Regen T, Marini F, Hoppmann N, Klein M, Blank T, Yona S, Wolf Y, et al. Genetic Cell Ablation Reveals Clusters of Local Self-Renewing Microglia in the Mammalian Central Nervous System. Immunity. 2015;43:92–106. doi: 10.1016/j.immuni.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Siddiqui S, Gabriely G, Lanser AJ, Dake B, Murugaiyan G, Doykan CE, Wu PM, Gali RR, Iyer LK, et al. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest. 2012;122:3063–3087. doi: 10.1172/JCI62636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Chen SK, Tvrdik P, Peden E, Cho S, Wu S, Spangrude G, Capecchi MR. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141:775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Chikahisa S, Kodama T, Soya A, Sagawa Y, Ishimaru Y, Sei H, Nishino S. Histamine from brain resident MAST cells promotes wakefulness and modulates behavioral states. PLoS One. 2013;8:e78434. doi: 10.1371/journal.pone.0078434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnery HR, Ruitenberg MJ, McMenamin PG. Novel characterization of monocyte-derived cell populations in the meninges and choroid plexus and their rates of replenishment in bone marrow chimeric mice. J Neuropathol Exp Neurol. 2010;69:896–909. doi: 10.1097/NEN.0b013e3181edbc1a. [DOI] [PubMed] [Google Scholar]
- Chiu IM, Morimoto ET, Goodarzi H, Liao JT, O’Keeffe S, Phatnani HP, Muratet M, Carroll MC, Levy S, Tavazoie S, et al. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep. 2013;4:385–401. doi: 10.1016/j.celrep.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy AL, Walker ME, Hessner MJ, Brown MA. Mast cell activation and neutrophil recruitment promotes early and robust inflammation in the meninges in EAE. J Autoimmun. 2013;42:50–61. doi: 10.1016/j.jaut.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Clarkson BD, Walker A, Harris MG, Rayasam A, Hsu M, Sandor M, Fabry Z. CCR7 deficient inflammatory Dendritic Cells are retained in the Central Nervous System. Sci Rep. 2017;7:42856. doi: 10.1038/srep42856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Matcovitch O, David E, Barnett-Itzhaki Z, Keren-Shaul H, Blecher-Gonen R, Jaitin DA, Sica A, Amit I, Schwartz M. Chronic exposure to TGFβ1 regulates myeloid cell inflammatory response in an IRF7-dependent manner. The EMBO Journal. 2014;33:2906–2921. doi: 10.15252/embj.201489293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coureuil M, Mikaty G, Miller F, Lecuyer H, Bernard C, Bourdoulous S, Dumenil G, Mege RM, Weksler BB, Romero IA, et al. Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science. 2009;325:83–87. doi: 10.1126/science.1173196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronk JC, Derecki NC, Ji E, Xu Y, Lampano AE, Smirnov I, Baker W, Norris GT, Marin I, Coddington N, et al. Methyl-CpG binding protein 2 regulates microglia and macrophage gene expression in response to inflammatory stimuli. Immunity. 2015;42:679–691. doi: 10.1016/j.immuni.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxford AL, Lanzinger M, Hartmann FJ, Schreiner B, Mair F, Pelczar P, Clausen BE, Jung S, Greter M, Becher B. The Cytokine GM-CSF Drives the Inflammatory Signature of CCR2+ Monocytes and Licenses Autoimmunity. Immunity. 2015;43:502–514. doi: 10.1016/j.immuni.2015.08.010. [DOI] [PubMed] [Google Scholar]
- de Lima S, Koriyama Y, Kurimoto T, Oliveira JT, Yin Y, Li Y, Gilbert HY, Fagiolini M, Martinez AM, Benowitz L. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc Natl Acad Sci U S A. 2012;109:9149–9154. doi: 10.1073/pnas.1119449109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. The Journal of Experimental Medicine. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Cronk JC, Lu Z, Xu E, Abbott SB, Guyenet PG, Kipnis J. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484:105–109. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Quinnies KM, Kipnis J. Alternatively activated myeloid (M2) cells enhance cognitive function in immune compromised mice. Brain, behavior, and immunity. 2011;25:379–385. doi: 10.1016/j.bbi.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dines KC, Powell HC. Mast cell interactions with the nervous system: relationship to mechanisms of disease. J Neuropathol Exp Neurol. 1997;56:627–640. [PubMed] [Google Scholar]
- Donnelly DJ, Longbrake EE, Shawler TM, Kigerl KA, Lai W, Tovar CA, Ransohoff RM, Popovich PG. Deficient CX3CR1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6Clo/iNOS+ macrophages. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:9910–9922. doi: 10.1523/JNEUROSCI.2114-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets DA, Dillon MJ, Schawang JS, Van Rooijen N, Ehrchen J, Sunderkotter C, Leenen PJ. The Ly-6Chigh monocyte subpopulation transports Listeria monocytogenes into the brain during systemic infection of mice. J Immunol. 2004;172:4418–4424. doi: 10.4049/jimmunol.172.7.4418. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol. 2017;18:123–131. doi: 10.1038/ni.3666. [DOI] [PubMed] [Google Scholar]
- Farfara D, Trudler D, Segev-Amzaleg N, Galron R, Stein R, Frenkel D. gamma-Secretase component presenilin is important for microglia beta-amyloid clearance. Ann Neurol. 2011;69:170–180. doi: 10.1002/ana.22191. [DOI] [PubMed] [Google Scholar]
- Filiano AJ, Martens LH, Young AH, Warmus BA, Zhou P, Diaz-Ramirez G, Jiao J, Zhang Z, Huang EJ, Gao FB, et al. Dissociation of frontotemporal dementia-related deficits and neuroinflammation in progranulin haploinsufficient mice. J Neurosci. 2013;33:5352–5361. doi: 10.1523/JNEUROSCI.6103-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer MT, Sharma R, Lim JL, Haider L, Frischer JM, Drexhage J, Mahad D, Bradl M, van Horssen J, Lassmann H. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain. 2012;135:886–899. doi: 10.1093/brain/aws012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani SP, Smirnov I, Smith AT, Overall CC, Kipnis J. Characterization of meningeal type 2 innate lymphocytes and their response to CNS injury. J Exp Med. 2017;214:285–296. doi: 10.1084/jem.20161982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani SP, Walsh JT, Lukens JR, Kipnis J. Dealing with Danger in the CNS: The Response of the Immune System to Injury. Neuron. 2015a;87:47–62. doi: 10.1016/j.neuron.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani SP, Walsh JT, Smirnov I, Zheng J, Kipnis J. The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron. 2015b;85:703–709. doi: 10.1016/j.neuron.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas SK. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol. 2016;17:34–40. doi: 10.1038/ni.3324. [DOI] [PubMed] [Google Scholar]
- Goldman D, Song X, Kitai R, Casadevall A, Zhao ML, Lee SC. Cryptococcus neoformans induces macrophage inflammatory protein 1alpha (MIP-1alpha) and MIP-1beta in human microglia: role of specific antibody and soluble capsular polysaccharide. Infect Immun. 2001;69:1808–1815. doi: 10.1128/IAI.69.3.1808-1815.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann T, Prinz M. Role of microglia in CNS autoimmunity. Clin Dev Immunol. 2013;2013:208093. doi: 10.1155/2013/208093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann T, Wieghofer P, Jordao MJ, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, Krueger M, et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016a;17:797–805. doi: 10.1038/ni.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann T, Wieghofer P, Jordao MJC, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, Krueger M, et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016b;17:797–805. doi: 10.1038/ni.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh AD, David S. Differences in the phagocytic response of microglia and peripheral macrophages after spinal cord injury and its effects on cell death. J Neurosci. 2014;34:6316–6322. doi: 10.1523/JNEUROSCI.4912-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, Yona S. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14:571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauben E, Butovsky O, Nevo U, Yoles E, Moalem G, Agranov E, Mor F, Leibowitz-Amit R, Pevsner E, Akselrod S, et al. Passive or active immunization with myelin basic protein promotes recovery from spinal cord contusion. J Neurosci. 2000;20:6421–6430. doi: 10.1523/JNEUROSCI.20-17-06421.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Mack JJ, Güç E, Warren CM, Squadrito ML, Kilarski WW, Baer C, Freshman RD, McDonald AI, Ziyad S, et al. Perivascular Macrophages Limit Permeability. Arteriosclerosis, Thrombosis, and Vascular Biology. 2016 doi: 10.1161/ATVBAHA.116.307592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson AP, Barnett MH, Parratt JD, Prineas JW. Multiple sclerosis: distribution of inflammatory cells in newly forming lesions. Ann Neurol. 2009;66:739–753. doi: 10.1002/ana.21800. [DOI] [PubMed] [Google Scholar]
- Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- Herz J, Johnson KR, McGavern DB. Therapeutic antiviral T cells noncytopathically clear persistently infected microglia after conversion into antigen-presenting cells. J Exp Med. 2015;212:1153–1169. doi: 10.1084/jem.20142047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata K, Mitoma H, Ueno N, He JW, Kawabuchi M. Differential response of macrophage subpopulations to myelin degradation in the injured rat sciatic nerve. J Neurocytol. 1999;28:685–695. doi: 10.1023/a:1007012916530. [DOI] [PubMed] [Google Scholar]
- Hoeffel G, Ginhoux F. Ontogeny of Tissue-Resident Macrophages. Frontiers in Immunology. 2015;6:486. doi: 10.3389/fimmu.2015.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough LB. Cellular localization and possible functions for brain histamine: recent progress. Prog Neurobiol. 1988;30:469–505. doi: 10.1016/0301-0082(88)90032-9. [DOI] [PubMed] [Google Scholar]
- Isaksson M, Lundgren BA, Ahlgren KM, Kampe O, Lobell A. Conditional DC depletion does not affect priming of encephalitogenic Th cells in EAE. Eur J Immunol. 2012;42:2555–2563. doi: 10.1002/eji.201142239. [DOI] [PubMed] [Google Scholar]
- Jarvis JN, Meintjes G, Bicanic T, Buffa V, Hogan L, Mo S, Tomlinson G, Kropf P, Noursadeghi M, Harrison TS. Cerebrospinal fluid cytokine profiles predict risk of early mortality and immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis. PLoS Pathog. 2015;11:e1004754. doi: 10.1371/journal.ppat.1004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TR, Miller CM, Cheng PJ, Graham LC, Bemiller S, Broihier ML, Xu G, Margevicius D, Karlo JC, Sousa GL, et al. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. J Exp Med. 2015;212:287–295. doi: 10.1084/jem.20142322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Q, Castelli L, Goverman JM. MHC class I-restricted myelin epitopes are cross-presented by Tip-DCs that promote determinant spreading to CD8(+) T cells. Nat Immunol. 2013;14:254–261. doi: 10.1038/ni.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappers JA, ten Kate IB, de Bruyn HJ. On mast cells in the choroid plexus of the axolotl (Ambystoma Mex.) Zeitschrift für Zellforschung und Mikroskopische Anatomie. 1958;48:617–634. doi: 10.1007/BF00398650. [DOI] [PubMed] [Google Scholar]
- Karman J, Ling C, Sandor M, Fabry Z. Dendritic cells in the initiation of immune responses against central nervous system-derived antigens. Immunol Lett. 2004;92:107–115. doi: 10.1016/j.imlet.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Keilhoff G, Langnaese K, Wolf G, Fansa H. Inhibiting effect of minocycline on the regeneration of peripheral nerves. Dev Neurobiol. 2007;67:1382–1395. doi: 10.1002/dneu.20384. [DOI] [PubMed] [Google Scholar]
- Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, Wieghofer P, Heinrich A, Riemke P, Holscher C, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci. 2013;16:273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- Kierdorf K, Prinz M, Geissmann F, Gomez Perdiguero E. Development and function of tissue resident macrophages in mice. Seminars in Immunology. 2015;27:369–378. doi: 10.1016/j.smim.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JV, Kang SS, Dustin ML, McGavern DB. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature. 2009;457:191–195. doi: 10.1038/nature07591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J. Multifaceted interactions between adaptive immunity and the central nervous system. Science. 2016;353:766–771. doi: 10.1126/science.aag2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RS, Garber C, Howard N. Infectious immunity in the central nervous system and brain function. Nat Immunol. 2017;18:132–141. doi: 10.1038/ni.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight SC, Mertin J, Stackpoole A, Clark J. Induction of immune responses in vivo with small numbers of veiled (dendritic) cells. Proc Natl Acad Sci U S A. 1983;80:6032–6035. doi: 10.1073/pnas.80.19.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- Konradt C, Ueno N, Christian DA, Delong JH, Pritchard GH, Herz J, Bzik DJ, Koshy AA, McGavern DB, Lodoen MB, Hunter CA. Endothelial cells are a replicative niche for entry of Toxoplasma gondii to the central nervous system. Nat Microbiol. 2016;1:16001. doi: 10.1038/nmicrobiol.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M, David S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron. 2014;83:1098–1116. doi: 10.1016/j.neuron.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Lande R, Gafa V, Serafini B, Giacomini E, Visconti A, Remoli ME, Severa M, Parmentier M, Ristori G, Salvetti M, et al. Plasmacytoid dendritic cells in multiple sclerosis: intracerebral recruitment and impaired maturation in response to interferon-beta. J Neuropathol Exp Neurol. 2008;67:388–401. doi: 10.1097/NEN.0b013e31816fc975. [DOI] [PubMed] [Google Scholar]
- Lewis ND, Hill JD, Juchem KW, Stefanopoulos DE, Modis LK. RNA sequencing of microglia and monocyte-derived macrophages from mice with experimental autoimmune encephalomyelitis illustrates a changing phenotype with disease course. J Neuroimmunol. 2014;277:26–38. doi: 10.1016/j.jneuroim.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kaspar BK, Hirrlinger PG, Kirchhoff F, Bissonnette JM, Ballas N, Mandel G. A role for glia in the progression of Rett’s syndrome. Nature. 2011;475:497–500. doi: 10.1038/nature10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhini AL, von Glehn F, Brandao CO, de Paula RF, Pradella F, Moraes AS, Farias AS, Oliveira EC, Quispe-Cabanillas JG, Abreu CH, et al. Plasmacytoid dendritic cells are increased in cerebrospinal fluid of untreated patients during multiple sclerosis relapse. J Neuroinflammation. 2011;8:2. doi: 10.1186/1742-2094-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Harris TH, Kipnis J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 2015a;36:569–577. doi: 10.1016/j.it.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015b;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui H, Zhang J, Makinson Stefanie R, Cahill Michelle K, Kelley Kevin W, Huang HY, Shang Y, Oldham Michael C, Martens Lauren H, Gao F, et al. Progranulin Deficiency Promotes Circuit-Specific Synaptic Pruning by Microglia via Complement Activation. Cell. 2016;165:921–935. doi: 10.1016/j.cell.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol. 1999;405:553–562. [PubMed] [Google Scholar]
- Mendes-Jorge L, Ramos D, Luppo M, Llombart C, Alexandre-Pires G, Nacher V, Melgarejo V, Correia M, Navarro M, Carretero A, et al. Scavenger Function of Resident Autofluorescent Perivascular Macrophages and Their Contribution to the Maintenance of the Blood–Retinal Barrier. Investigative Ophthalmology & Visual Science. 2009;50:5997–6005. doi: 10.1167/iovs.09-3515. [DOI] [PubMed] [Google Scholar]
- Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A, Djukic M, Garbe D, Wellmer A, Kuziel WA, Mack M, Nau R, Prinz M. Ly-6G+CCR2- myeloid cells rather than Ly-6ChighCCR2+ monocytes are required for the control of bacterial infection in the central nervous system. J Immunol. 2008;181:2713–2722. doi: 10.4049/jimmunol.181.4.2713. [DOI] [PubMed] [Google Scholar]
- Mildner A, Mack M, Schmidt H, Bruck W, Djukic M, Zabel MD, Hille A, Priller J, Prinz M. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain. 2009;132:2487–2500. doi: 10.1093/brain/awp144. [DOI] [PubMed] [Google Scholar]
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJ, ffrench-Constant C. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naert G, Rivest S. CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2011;31:6208–6220. doi: 10.1523/JNEUROSCI.0299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, Gallins PJ, Buxbaum JD, Jarvik GP, Crane PK, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43:436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nautiyal KM, Dailey CA, Jahn JL, Rodriquez E, Son NH, Sweedler JV, Silver R. Serotonin of mast cell origin contributes to hippocampal function. Eur J Neurosci. 2012;36:2347–2359. doi: 10.1111/j.1460-9568.2012.08138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nautiyal KM, Ribeiro AC, Pfaff DW, Silver R. Brain mast cells link the immune system to anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:18053–18057. doi: 10.1073/pnas.0809479105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D, Zinselmeyer BH, Corps KN, McGavern DB. In vivo dynamics of innate immune sentinels in the CNS. Intravital. 2012;1:95–106. doi: 10.4161/intv.22823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LH, Lenz KM. Microglia depletion in early life programs persistent changes in social, mood-related, and locomotor behavior in male and female rats. Behav Brain Res. 2017;316:279–293. doi: 10.1016/j.bbr.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro A, Colombo F, Casella G, Finardi A, Verderio C, Furlan R. Myeloid Extracellular Vesicles: Messengers from the Demented Brain. Frontiers in Immunology. 2016;7 doi: 10.3389/fimmu.2016.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, Bianchin M, Bird T, Miranda R, Salmaggi A, et al. Mutations in Two Genes Encoding Different Subunits of a Receptor Signaling Complex Result in an Identical Disease Phenotype. The American Journal of Human Genetics. 2002;71:656–662. doi: 10.1086/342259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesic M, Bartholomaus I, Kyratsous NI, Heissmeyer V, Wekerle H, Kawakami N. 2-photon imaging of phagocyte-mediated T cell activation in the CNS. J Clin Invest. 2013;123:1192–1201. doi: 10.1172/JCI67233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccio L, Buonsanti C, Mariani M, Cella M, Gilfillan S, Cross AH, Colonna M, Panina-Bordignon P. Blockade of TREM-2 exacerbates experimental autoimmune encephalomyelitis. Eur J Immunol. 2007;37:1290–1301. doi: 10.1002/eji.200636837. [DOI] [PubMed] [Google Scholar]
- Polfliet MM, Zwijnenburg PJ, van Furth AM, van der Poll T, Dopp EA, Renardel de Lavalette C, van Kesteren-Hendrikx EM, van Rooijen N, Dijkstra CD, van den Berg TK. Meningeal and perivascular macrophages of the central nervous system play a protective role during bacterial meningitis. J Immunol. 2001;167:4644–4650. doi: 10.4049/jimmunol.167.8.4644. [DOI] [PubMed] [Google Scholar]
- Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- Popovich PG. Neuroimmunology of traumatic spinal cord injury: A brief history and overview. Experimental Neurology. 2014;258:1–4. doi: 10.1016/j.expneurol.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Prinz M, Erny D, Hagemeyer N. Ontogeny and homeostasis of CNS myeloid cells. Nat Immunol. 2017;18:385–392. doi: 10.1038/ni.3703. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci. 2017;20:136–144. doi: 10.1038/nn.4475. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci. 2011;14:1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- Prokop S, Miller KR, Drost N, Handrick S, Mathur V, Luo J, Wegner A, Wyss-Coray T, Heppner FL. Impact of peripheral myeloid cells on amyloid-beta pathology in Alzheimer’s disease-like mice. J Exp Med. 2015;212:1811–1818. doi: 10.1084/jem.20150479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop S, Miller KR, Heppner FL. Microglia actions in Alzheimer’s disease. Acta Neuropathol. 2013;126:461–477. doi: 10.1007/s00401-013-1182-x. [DOI] [PubMed] [Google Scholar]
- Quintana E, Fernández A, Velasco P, de Andrés B, Liste I, Sancho D, Gaspar ML, Cano E. DNGR-1+ dendritic cells are located in meningeal membrane and choroid plexus of the noninjured brain. Glia. 2015;63:2231–2248. doi: 10.1002/glia.22889. [DOI] [PubMed] [Google Scholar]
- Rademakers R, Baker M, Nicholson AM, Rutherford NJ, Finch N, Soto-Ortolaza A, Lash J, Wider C, Wojtas A, DeJesus-Hernandez M, et al. Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat Genet. 2011;44:200–205. doi: 10.1038/ng.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353:777–783. doi: 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapalino O, Lazarov-Spiegler O, Agranov E, Velan GJ, Yoles E, Fraidakis M, Solomon A, Gepstein R, Katz A, Belkin M, et al. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat Med. 1998;4:814–821. doi: 10.1038/nm0798-814. [DOI] [PubMed] [Google Scholar]
- Rolls A, Shechter R, London A, Segev Y, Jacob-Hirsch J, Amariglio N, Rechavi G, Schwartz M. Two faces of chondroitin sulfate proteoglycan in spinal cord repair: a role in microglia/macrophage activation. PLoS Med. 2008;5:e171. doi: 10.1371/journal.pmed.0050171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505:223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russi AE, Brown MA. The meninges: new therapeutic targets for multiple sclerosis. Translational research: the journal of laboratory and clinical medicine. 2015;165:255–269. doi: 10.1016/j.trsl.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo MV, McGavern DB. Immune Surveillance of the CNS following Infection and Injury. Trends Immunol. 2015;36:637–650. doi: 10.1016/j.it.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:309–316. doi: 10.1016/j.anorl.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Sayed BA, Christy AL, Walker ME, Brown MA. Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: a role for neutrophil recruitment? J Immunol. 2010;184:6891–6900. doi: 10.4049/jimmunol.1000126. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlager C, Korner H, Krueger M, Vidoli S, Haberl M, Mielke D, Brylla E, Issekutz T, Cabanas C, Nelson PJ, et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature. 2016;530:349–353. doi: 10.1038/nature16939. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serot JM, Foliguet B, Bene MC, Faure GC. Ultrastructural and immunohistological evidence for dendritic-like cells within human choroid plexus epithelium. Neuroreport. 1997;8:1995–1998. doi: 10.1097/00001756-199705260-00039. [DOI] [PubMed] [Google Scholar]
- Shechter R, London A, Schwartz M. Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat Rev Immunol. 2013a;13:206–218. doi: 10.1038/nri3391. [DOI] [PubMed] [Google Scholar]
- Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, Rolls A, Mack M, Pluchino S, Martino G, et al. Infiltrating Blood-Derived Macrophages Are Vital Cells Playing an Anti-inflammatory Role in Recovery from Spinal Cord Injury in Mice. PLoS Med. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, Kim KW, Klein E, Kalchenko V, Bendel P, et al. Recruitment of Beneficial M2 Macrophages to Injured Spinal Cord Is Orchestrated by Remote Brain Choroid Plexus. Immunity. 2013b;38:555–569. doi: 10.1016/j.immuni.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, Kim KW, Klein E, Kalchenko V, Bendel P, et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013c;38:555–569. doi: 10.1016/j.immuni.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, Schwartz M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: no longer ‘if’ but ‘how’. J Pathol. 2013;229:332–346. doi: 10.1002/path.4106. [DOI] [PubMed] [Google Scholar]
- Sie C, Korn T. Dendritic cells in central nervous system autoimmunity. Semin Immunopathol. 2017;39:99–111. doi: 10.1007/s00281-016-0608-7. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Asarian L, Khalil M, Silver R. GnRH, brain mast cells and behavior. Prog Brain Res. 2002;141:315–325. doi: 10.1016/S0079-6123(02)41102-8. [DOI] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Facci L, Giusti P. Glia and mast cells as targets for palmitoylethanolamide, an anti-inflammatory and neuroprotective lipid mediator. Mol Neurobiol. 2013;48:340–352. doi: 10.1007/s12035-013-8487-6. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- Spath S, Komuczki J, Hermann M, Pelczar P, Mair F, Schreiner B, Becher B. Dysregulation of the Cytokine GM-CSF Induces Spontaneous Phagocyte Invasion and Immunopathology in the Central Nervous System. Immunity. 2017;46:245–260. doi: 10.1016/j.immuni.2017.01.007. [DOI] [PubMed] [Google Scholar]
- Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, Andjelkovic AV. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci. 2016;19:771–783. doi: 10.1038/nn.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC. Mast cells: the immune gate to the brain. Life Sci. 1990;46:607–617. doi: 10.1016/0024-3205(90)90129-f. [DOI] [PubMed] [Google Scholar]
- Thewissen K, Nuyts AH, Deckx N, Van Wijmeersch B, Nagels G, D’Hooghe M, Willekens B, Cras P, Eijnde BO, Goossens H, et al. Circulating dendritic cells of multiple sclerosis patients are proinflammatory and their frequency is correlated with MS-associated genetic risk factors. Mult Scler. 2014;20:548–557. doi: 10.1177/1352458513505352. [DOI] [PubMed] [Google Scholar]
- Toms R, Weiner HL, Johnson D. Identification of IgE-positive cells and mast cells in frozen sections of multiple sclerosis brains. J Neuroimmunol. 1990;30:169–177. doi: 10.1016/0165-5728(90)90101-r. [DOI] [PubMed] [Google Scholar]
- Tran EH, Hoekstra K, van Rooijen N, Dijkstra CD, Owens T. Immune invasion of the central nervous system parenchyma and experimental allergic encephalomyelitis, but not leukocyte extravasation from blood, are prevented in macrophage-depleted mice. J Immunol. 1998;161:3767–3775. [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zwam M, Huizinga R, Melief MJ, Wierenga-Wolf AF, van Meurs M, Voerman JS, Biber KP, Boddeke HW, Hopken UE, Meisel C, et al. Brain antigens in functionally distinct antigen-presenting cell populations in cervical lymph nodes in MS and EAE. J Mol Med (Berl) 2009;87:273–286. doi: 10.1007/s00109-008-0421-4. [DOI] [PubMed] [Google Scholar]
- VanRyzin JW, Yu SJ, Perez-Pouchoulen M, McCarthy MM. Temporary Depletion of Microglia during the Early Postnatal Period Induces Lasting Sex-Dependent and Sex-Independent Effects on Behavior in Rats. eNeuro. 2016;3 doi: 10.1523/ENEURO.0297-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venezie RD, Toews AD, Morell P. Macrophage recruitment in different models of nerve injury: lysozyme as a marker for active phagocytosis. J Neurosci Res. 1995;40:99–107. doi: 10.1002/jnr.490400111. [DOI] [PubMed] [Google Scholar]
- Vinet J, Weering HR, Heinrich A, Kalin RE, Wegner A, Brouwer N, Heppner FL, Rooijen N, Boddeke HW, Biber K. Neuroprotective function for ramified microglia in hippocampal excitotoxicity. Journal of neuroinflammation. 2012;9:27. doi: 10.1186/1742-2094-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JT, Kipnis J. Regulatory T cells in CNS injury: the simple, the complex and the confused. Trends in Molecular Medicine. 2011;17:541–547. doi: 10.1016/j.molmed.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhao L, Zhang J, Fariss RN, Ma W, Kretschmer F, Wang M, Qian HH, Badea TC, Diamond JS, et al. Requirement for Microglia for the Maintenance of Synaptic Function and Integrity in the Mature Retina. J Neurosci. 2016a;36:2827–2842. doi: 10.1523/JNEUROSCI.3575-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ulland TK, Ulrich JD, Song W, Tzaferis JA, Hole JT, Yuan P, Mahan TE, Shi Y, Gilfillan S, et al. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J Exp Med. 2016b;213:667–675. doi: 10.1084/jem.20151948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedmore CV, Williams TJ. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981;289:646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R, Wu PM, Doykan CE, Lin J, Cotleur AC, et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med. 2014;211:1533–1549. doi: 10.1084/jem.20132477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yillar DO, Kucukhuseyin C. The effects of compound 48/80, morphine, and mast cell depletion on electroshock seizure in mice. J Basic Clin Physiol Pharmacol. 2008;19:1–14. doi: 10.1515/jbcpp.2008.19.1.1. [DOI] [PubMed] [Google Scholar]
- Yin Y, Cui Q, Li Y, Irwin N, Fischer D, Harvey AR, Benowitz LI. Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci. 2003;23:2284–2293. doi: 10.1523/JNEUROSCI.23-06-02284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev N, Frommer F, Lukas D, Kautz-Neu K, Karram K, Ielo D, von Stebut E, Probst HC, van den Broek M, Riethmacher D, et al. Dendritic cells ameliorate autoimmunity in the CNS by controlling the homeostasis of PD-1 receptor(+) regulatory T cells. Immunity. 2012;37:264–275. doi: 10.1016/j.immuni.2012.05.025. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D, Gross CT. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. 2014;17:400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wang XJ, Tian LP, Pan J, Lu GQ, Zhang YJ, Ding JQ, Chen SD. CD200-CD200R dysfunction exacerbates microglial activation and dopaminergic neurodegeneration in a rat model of Parkinson’s disease. J Neuroinflammation. 2011;8:154. doi: 10.1186/1742-2094-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and Dysfunction of the Blood-Brain Barrier. Cell. 2015;163:1064–1078. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Silverman AJ, Silver R. Brain mast cell degranulation regulates blood-brain barrier. J Neurobiol. 1996;31:393–403. doi: 10.1002/(SICI)1097-4695(199612)31:4<393::AID-NEU1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]