Abstract
Background: Emerging research suggests that redistributing total protein intake from 1 high-protein meal/d to multiple moderately high-protein meals improves 24-h muscle protein synthesis. Over time, this may promote positive changes in body composition.
Objective: We sought to assess the effects of within-day protein intake distribution on changes in body composition during dietary energy restriction and resistance training.
Design: In a randomized parallel-design study, 41 men and women [mean ± SEM age: 35 ± 2 y; body mass index (in kg/m2): 31.5 ± 0.5] consumed an energy-restricted diet (750 kcal/d below the requirement) for 16 wk while performing resistance training 3 d/wk. Subjects consumed 90 g protein/d (1.0 ± 0.03 g · kg−1 · d−1, 125% of the Recommended Dietary Allowance, at intervention week 1) in either a skewed (10 g at breakfast, 20 g at lunch, and 60 g at dinner; n = 20) or even (30 g each at breakfast, lunch, and dinner; n = 21) distribution pattern. Body composition was measured pre- and postintervention.
Results: Over time, whole-body mass (least-squares mean ± SE: −7.9 ± 0.6 kg), whole-body lean mass (−1.0 ± 0.2 kg), whole-body fat mass (−6.9 ± 0.5 kg), appendicular lean mass (−0.7 ± 0.1 kg), and appendicular fat mass (−2.6 ± 0.2 kg) each decreased. The midthigh muscle area (0 ± 1 cm2) did not change over time, whereas the midcalf muscle area decreased (−3 ± 1 cm2). Within-day protein distribution did not differentially affect these body-composition responses.
Conclusion: The effectiveness of dietary energy restriction combined with resistance training to improve body composition is not influenced by the within-day distribution of protein when adequate total protein is consumed. This trial was registered at clinicaltrials.gov as NCT02066948.
Keywords: exercise, weight loss, muscle mass, heart health, protein patterning
INTRODUCTION
Health-promoting weight loss strategies typically are designed to produce positive changes in body composition, including fat mass loss and the preservation of lean body mass (1). Higher total dietary protein intakes (1.2–1.5 g · kg−1 · d−1) help preserve lean mass and improve body composition during weight loss in young, middle-aged, and older adults (2–6). During the past decade, within-day distribution of dietary protein has emerged as a possible modifier of body composition and skeletal muscle size (7–9). The concept is to redistribute daily total protein intake from mostly being consumed at 1 high-protein meal (skewed distribution) to being evenly consumed at 3 moderate-protein meals (7). One specific within-day protein distribution strategy promoted in the scientific (10), clinical (11), and lay-public literature is to evenly divide 90 g protein/d between three 30-g protein meals. Currently, the scientific foundation for recommendations to evenly distribute daily protein intake between 3 meals comes mainly from short-term feeding studies utilizing protein supplements (12) or lean beef (10), which included measurements of muscle protein synthesis (MPS) rates. The initial study reported that evenly redistributing daily protein into multiple moderately high-protein meals resulted in a 25% greater (faster) 24-h MPS rate than a skewed protein distribution (10). Subsequent research also reported a 19% greater MPS rate after a 13-h period with even or skewed protein intake (12). Collectively, these early results suggest that daily MPS rates are greater when meal-to-meal protein intake is evenly distributed, compared with a typical skewed protein intake that contains only 1 much larger-protein meal/d.
Theoretically, a greater daily MPS rate will positively affect body composition over time (13), particularly in situations in which there may be an increased risk of a reduction in lean body mass. Dietary energy restriction is a robust catabolic stimulus that reduces fat mass and usually reduces lean mass and muscle size (14–16). Alternatively, resistance training is an anabolic stimulus that increases the rate of MPS (17–20) and promotes increases in lean mass, including skeletal muscle (21). Currently, a paucity of longitudinal research studies exist that critically assess whether within-day protein distribution helps retain lean body mass during periods of purposeful weight loss when consumed within the context of practical dietary patterns utilizing whole foods. The aim of this study was to assess the effects of within-day protein intake distribution during dietary energy restriction on changes in lean body mass and midthigh muscle area while resistance training. We hypothesized that evenly distributing daily protein intake with concurrent resistance training would result in the retention of lean body mass during periods of energy restriction compared with skewing protein intake. The even within-day protein distribution may offer adults who are overweight or obese another dietary strategy to improve their body composition during intentional weight loss without altering total protein intake.
METHODS
Experimental design
This 18-wk study included a 2-wk baseline testing period, followed by a 16-wk randomized controlled intervention period (Supplemental Table 1). During the intervention period, all subjects consumed a controlled, energy-restricted diet and participated in a progressive overload resistance training program. Each subject was randomly assigned (using an online randomization plan generator; http://www.randomization.com/) to 1 of 2 dietary groups and was instructed to consume meal-specific foods and beverages to achieve an even or skewed within-day protein distribution (EVEN or SKEW, respectively). The clinical laboratory manager, J Green, who was not involved in data collection or analysis, generated the random allocation sequence and assigned subjects to the intervention. Postintervention testing was completed during intervention week 16. All subjects were instructed to maintain their habitual types and levels of physical activities aside from the prescribed resistance training.
Subjects
Fifty-eight adults recruited from the greater Lafayette, Indiana, community provided written consent before participation. This study was conducted between January 2014 and November 2015 and was stopped after recruitment goals were met. Study inclusion criteria were as follows: age 19–50 y, BMI (in kg/m2) of 27.0–34.9, stable weight (±4.5 kg during previous 3 mo), nonsmoking status, not diabetic, no acute illness, not pregnant or lactating, ability to exercise, not claustrophobic and able to complete MRI testing, and willing and able to travel to testing facilities. The study protocol and all study documents were approved by the Purdue University Biomedical Institutional Review Board. This study was registered at clinicaltrials.gov as NCT02066948.
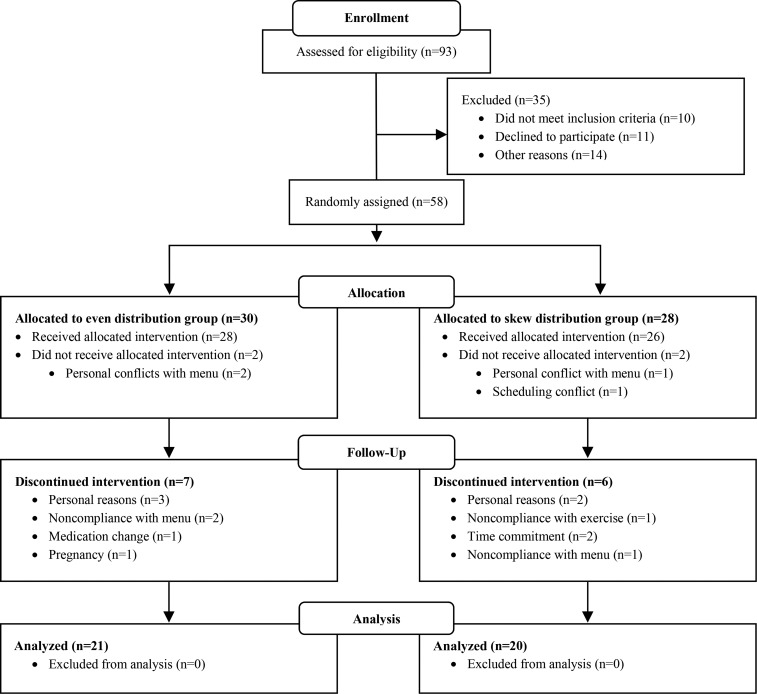
Fifty-four subjects completed all baseline testing and started the intervention, whereas 4 subjects (EVEN, n = 2; SKEW, n = 2) dropped out before starting the intervention because of personal conflicts with the menu (n = 3) or a scheduling conflict (n = 1). Thirteen subjects left the study during the intervention period (EVEN, n = 7; SKEW, n = 6) for personal reasons (n = 5), time commitment (n = 2), noncompliance with the menu (n = 3), noncompliance with exercise (n = 1), medication change (n = 1), or pregnancy (n = 1). Forty-one subjects [EVEN: n = 21 (6 men and 15 women); SKEW: n = 20 (9 men and 11 women); race-ethnicity: African American, n = 1; Asian/Pacific Islander, n = 2; and Caucasian, n = 38] completed all study procedures and their data were analyzed (Figure 1).
FIGURE 1.
Study recruitment flow diagram.
Diet intervention
Each subject’s total energy requirement was estimated before the study through the use of sex-specific equations for overweight or obese adults with a low activity level (physical activity coefficients were 1.12 for men and 1.16 for women) (22). Throughout the intervention period, each subject consumed a diet providing 750 kcal/d less than their estimated energy requirement. The diets consisted of a 1400 kcal/d base diet that contained 90 g protein, 40 g fat, and 170 g carbohydrate (i.e., a 35–65% ratio of nonprotein energy intake from fat and carbohydrate) and were designed so that all foods were consumed at breakfast, lunch, and dinner. Additional fat and carbohydrate was added to each subject’s daily intakes, as necessary, to achieve his or her individualized energy allowance while maintaining the 35–65% fat-to-carbohydrate ratio. For all subjects, the prescribed within-day distribution of total energy intake was ∼20% breakfast, 30% lunch, 36% dinner, and 14% snacks, respectively. The prescribed within-day protein distributions of the 2 groups were as follows: 1) EVEN: 30, 30, and 30 g protein/meal consumed at breakfast, lunch, and dinner, respectively, and 2) SKEW: 10, 20, and 60 g protein/meal, respectively. Foods consumed as snacks contained minimal protein. Dietary protein sources are listed in Supplemental Table 2. The individualized menus were developed by A Wright, a registered dietitian, using ProNutra software (Viocare Inc.). The within-day energy and protein distributions of the SKEW pattern were consistent with NHANES distributions (8).
A digital platform scale (model ES200L; Ohaus Corporation) was used to measure body mass at baseline and once weekly during the intervention period. If body mass loss was <0.5 kg/wk for 2 consecutive weeks, the subject’s energy intake was lowered by reducing nonprotein energy intake. At baseline, each subject’s 24-h food intakes were assessed for energy and macronutrient contents using a 3-d food record (Nutrition Data System for Research software, version 2014; Nutrition Coordinating Center, University of Minnesota). Salting and herbal seasoning of food, water, and non–energy, caffeine-containing beverages were allowed ad libitum during the intervention. Subjects were provided with a digital food scale (Salter Microtronic Electronic Kitchen Scale) to aid in measuring portion sizes. All diet-related activities and assessments were performed in conjunction with the Indiana Clinical Research Center Bionutrition Facility at Purdue University.
Dietary compliance
During the intervention, dietary compliance was assessed through the use of daily menu checklists and periodic pre- and postmeal date- and time-identified photography. The study dietitian and other research staff members also contacted subjects weekly in person, by E-mail, and by phone to encourage compliance with the prescribed menus. See Supplemental Table 3 for more information about dietary compliance measures.
Resistance training
All subjects performed 3 sets of resistance exercises on 3 nonconsecutive days per week. The exercises included the seated chest press, seated upper back row, seated bilateral leg extension, seated bilateral angled leg presses and seated bilateral leg curl (core exercises) and 2 additional auxiliary exercises (Technogym). The hip abductor and seated shoulder press exercises were alternated every other training session with the latissimus dorsi pull down and hip adductor exercises. The first set for each exercise was completed in 8–10 repetitions, with the last 2 sets completed to volitional fatigue. In weeks 1, 2, and 3–16 of the intervention, subjects performed each exercise at 60%, 70%, and 80% of their most recently measured 1-repetition maximum, respectively. Each training session was supervised, lasted ∼1 h, and included 10-min warm-up and cool-down periods consisting of low-intensity aerobic exercise and stretching.
During baseline and every fourth week, 1-repetition maximum testing was performed to measure subjects’ maximal strength on the 5 core exercises. Measurements were taken on the same machines used for training. Whole body strength was considered the sum of the 1 repetition maximums for 4 core exercises. The seated bilateral angled leg press core exercise was removed from the analysis of composite whole body strength because the subject's strength often exceeded the machine's available resistance. Further details regarding the resistance training protocol are listed in Supplemental Table 3.
Resistance training compliance
Resistance training attendance was documented to assess compliance with the intervention procedures. Only one subject was removed from the intervention for continued absence from resistance training sessions. Resistance training compliance was calculated as the percentage of resistance training sessions attended out of 48 total sessions.
Body composition
At baseline and intervention week 16, fasting state whole body mass, fat mass, and soft tissue lean mass were measured with dual-energy X-ray absorptiometry (GE Lunar Prodigy with version 11.1 enCORE iDXA software). Procedures are detailed in Supplemental Table 2. We used MRI (3T General Electric Signa HDx system) to measure the right midthigh and calf muscle areas for cross-sectional area, muscle area, subcutaneous fat area, and intramuscular adipose tissue (IMAT) area. See Supplemental Table 3 for more information on MRI procedures and image processing.
Statistical analysis
Analyses were completed with data from the 41 subjects who finished the intervention. To reflect that subject age and sex were used as covariates, all results are presented as least-squares means ± SEs unless otherwise stated. The main effect of time and group-by-time interactions were assessed with a 2 × 2 factor repeated-measures ANOVA (SAS MIXED procedure; group: EVEN, SKEW; time: pre- and postintervention). Group-by-time interactions were the effect of within-day dietary protein distribution (group) from pre- to postintervention (time). We used an unpaired, 2-tailed t test (SAS TTEST procedure) to test for group differences in preintervention age and height and in the percentages of postintervention menu checklist compliance, meal picture compliance, and resistance training compliance. Statistical significance was determined at P < 0.05. Statistical analysis was performed with SAS software (version 9.3; SAS Institute).
RESULTS
Subjects
At baseline, age, body proportions, body composition, and maximal muscle strength characteristics were not significantly different between the EVEN and SKEW groups (Table 1). There was only one adverse event: one subject fainted during resistance training. This adverse event was medically and administratively resolved.
TABLE 1.
Changes in anthropometrics, body composition, and whole body strength in the EVEN and SKEW groups after consuming an energy-restricted diet and performing resistance training for 16 wk1
| EVEN |
SKEW |
P value |
||||||
| Parameter | Preintervention | Postintervention | Change | Preintervention | Postintervention | Change | Time | G × T |
| Age,2,3 y | 33 ± 2 | — | — | 36 ± 2 | — | — | — | — |
| Height,2,3 cm | 170.5 ± 1.7 | — | — | 173.1 ± 1.9 | — | — | — | — |
| Body mass,3,4 kg | 95.9 ± 2.3 | 87.2 ± 2.3 | −8.6 ± 0.9 | 92.8 ± 2.3 | 85.5 ± 2.3 | −7.3 ± 0.9 | <0.001 | 0.320 |
| BMI,3,4 kg/m2 | 31.9 ± 0.5 | 29.0 ± 0.5 | −2.9 ± 0.3 | 30.8 ± 0.5 | 28.3 ± 0.5 | −2.4 ± 0.3 | <0.001 | 0.274 |
| Waist circumference,4,5 cm | 104.6 ± 1.8 | 95.4 ± 1.8 | −9.1 ± 1.0 | 102.6 ± 1.8 | 94.2 ± 1.8 | −8.4 ± 1.1 | <0.001 | 0.601 |
| Hip circumference,4,6 cm | 114.5 ± 1.5 | 107.6 ± 1.5 | −6.9 ± 0.8 | 112.5 ± 1.5 | 106.3 ± 1.5 | −6.3 ± 0.9 | <0.001 | 0.599 |
| Waist:hip4,5 | 0.91 ± 0.01 | 0.89 ± 0.01 | −0.03 ± 0.01 | 0.91 ± 0.01 | 0.89 ± 0.01 | −0.03 ± 0.01 | <0.001 | 0.996 |
| Whole body3,4 | ||||||||
| Lean mass, kg | 55.8 ± 1.2 | 54.2 ± 1.2 | −1.5 ± 0.4 | 54.1 ± 1.2 | 53.6 ± 1.2 | −0.5 ± 0.4 | <0.001 | 0.067 |
| Lean mass, % | 57.8 ± 1.0 | 61.9 ± 1.0 | 4.1 ± 0.5 | 58.1 ± 1.0 | 62.6 ± 1.0 | 4.5 ± 0.5 | <0.001 | 0.576 |
| Fat mass, kg | 36.9 ± 1.6 | 29.9 ± 1.6 | −7.1 ± 0.7 | 35.7 ± 1.6 | 28.9 ± 1.6 | −6.8 ± 0.7 | <0.001 | 0.789 |
| Fat mass, % | 38.8 ± 1.1 | 34.4 ± 1.1 | −4.4 ± 0.5 | 38.6 ± 1.1 | 33.9 ± 1.1 | −4.7 ± 0.5 | <0.001 | 0.640 |
| Lean mass index, kg/m2 | 18.4 ± 0.3 | 17.9 ± 0.3 | −0.5 ± 0.1 | 17.8 ± 0.3 | 17.6 ± 0.3 | −0.2 ± 0.1 | <0.001 | 0.066 |
| Fat mass index, kg/m2 | 12.4 ± 0.5 | 10.0 ± 0.5 | −2.4 ± 0.2 | 12.0 ± 0.5 | 9.7 ± 0.5 | −2.3 ± 0.2 | <0.001 | 0.695 |
| Lean mass:fat mass | 1.6 ± 0.1 | 1.9 ± 0.1 | 0.3 ± 0.1 | 1.6 ± 0.1 | 2.1 ± 0.1 | 0.4 ± 0.1 | <0.001 | 0.281 |
| Appendicular,3,4 kg | ||||||||
| Total mass | 43.3 ± 1.1 | 39.7 ± 1.1 | −3.5 ± 0.4 | 42.2 ± 1.1 | 39.0 ± 1.1 | −3.1 ± 0.4 | <0.001 | 0.498 |
| Lean mass | 26.4 ± 0.7 | 25.5 ± 0.7 | −1.0 ± 0.2 | 25.7 ± 0.7 | 25.1 ± 0.7 | −0.5 ± 0.2 | <0.001 | 0.169 |
| Fat mass | 15.3 ± 0.8 | 12.7 ± 0.8 | −2.6 ± 0.3 | 15.1 ± 0.7 | 12.4 ± 0.7 | −2.7 ± 0.3 | <0.001 | 0.917 |
| Right leg,3,4 kg | ||||||||
| Total mass | 16.3 ± 0.5 | 15.0 ± 0.5 | −1.3 ± 0.2 | 16.0 ± 0.4 | 14.8 ± 0.4 | −1.2 ± 0.2 | <0.001 | 0.594 |
| Lean mass | 10.0 ± 0.3 | 9.6 ± 0.3 | −0.4 ± 0.1 | 9.7 ± 0.2 | 9.5 ± 0.2 | −0.2 ± 0.1 | <0.001 | 0.186 |
| Fat mass | 5.7 ± 0.3 | 4.8 ± 0.3 | −1.0 ± 0.1 | 5.8 ± 0.3 | 4.7 ± 0.3 | −1.0 ± 0.1 | <0.001 | 0.691 |
| Right midthigh area,4,7 cm2 | ||||||||
| Total cross section | 308 ± 11 | 277 ± 11 | −31 ± 4 | 288 ± 11 | 263 ± 11 | −25 ± 4 | <0.001 | 0.253 |
| Muscle | 158 ± 5 | 155 ± 5 | −2 ± 2 | 146 ± 4 | 147 ± 4 | 2 ± 2 | 0.797 | 0.136 |
| Subcutaneous fat | 121 ± 11 | 104 ± 11 | −28 ± 3 | 124 ± 11 | 100 ± 11 | −25 ± 3 | <0.001 | 0.515 |
| IMAT | 10 ± 1 | 9 ± 1 | −1 ± 0 | 10 ± 1 | 9 ± 1 | −1 ± 0 | <0.001 | 0.829 |
| Right midcalf area,4,7 cm2 | ||||||||
| Cross section | 126 ± 3 | 118 ± 3 | −9 ± 1 | 123 ± 4 | 116 ± 4 | −7 ± 1 | <0.001 | 0.452 |
| Muscle | 79 ± 3 | 76 ± 3 | −4 ± 1 | 78 ± 3 | 76 ± 3 | −3 ± 1 | <0.001 | 0.444 |
| Subcutaneous fat | 32 ± 3 | 28 ± 3 | −4 ± 1 | 31 ± 3 | 277 ± 3 | −4 ± 1 | <0.001 | 0.806 |
| IMAT | 14 ± 1 | 10 ± 1 | −3 ± 1 | 9 ± 1 | 7 ± 1 | −2 ± 0.0 | <0.001 | 0.375 |
| Strength,4,8 kg | ||||||||
| Whole body | 511 ± 22 | 625 ± 24 | 114 ± 17 | 535 ± 22 | 630 ± 24 | 95 ± 19 | <0.001 | 0.470 |
EVEN, 30 g of protein consumed each at breakfast, lunch, and dinner; G × T, group-by-time interaction; IMAT, intramuscular adipose tissue; SKEW, 10 g of protein consumed at breakfast, 20 g at lunch, and 60 g at dinner.
Data are presented as means ± SEMs. An unpaired, 2-tailed t test (TTEST procedure, SAS version 9.3; SAS Institute) was used to test for differences between groups in preintervention age and height and were not different.
n = 6 men and 15 women (EVEN) and 9 men and 11 women (SKEW).
Data are presented as the least-squares means ± SEs. A repeated-measures ANOVA (MIXED procedure, SAS version 9.3) was used to test for main effects of time and G × T interaction. No significant G × T for the measured variables was observed between the EVEN and SKEW groups.
Preintervention: n = 6 men and 15 women (EVEN) and 9 men and 11 women (SKEW). Postintervention: n = 6 men and 15 women (EVEN) and 7 men and 11 women (SKEW).
Preintervention: n = 6 men and 15 women (EVEN) and 9 men and 11 women (SKEW). Postintervention: n = 6 men and 15 women (EVEN) and 8 men and 11 women (SKEW).
Preintervention: n = 6 men and 15 women (EVEN) and 9 men and 10 women (SKEW). Postintervention: n = 6 men and 14 women (EVEN) and 7 men and 10 women (SKEW).
Whole-body strength is the sum of the 4 core exercises. Preintervention: n = 5 men and 15 women (EVEN) and 7 men and 11 women (SKEW). Postintervention: n = 2 men and 12 women (EVEN) and 4 men and 8 women (SKEW).
Dietary intervention
At baseline, habitual protein intake in the EVEN (15 ± 4, 30 ± 3, and 31 ± 5 g of protein at breakfast, lunch, and dinner, respectively; total protein intake: 82 ± 4 g/d) and SKEW (15 ± 2, 31 ± 4, and 39 ± 5 g protein/meal; 90 ± 4 g/d) groups were not different. The 90 g protein/d intake prescribed throughout the 16-wk intervention period equated to 1.0 ± 0.04 and 1.0 ± 0.04 g · kg−1 · d−1 at intervention week 1 and 1.1 ± 0.04 and 1.1 ± 0.04 g · kg−1 · d−1 at intervention week 16 for the EVEN and SKEW groups, respectively (Supplemental Table 4). The apparent increase in protein intake at week 16 was attributable to differences in week 1 compared with week 16 body masses. Week 16 menu checklists indicated that the EVEN group consumed 31 ± 0, 29 ± 0, and 29 ± 0 g of protein at breakfast, lunch, and dinner, respectively (Supplemental Table 4). The SKEW group consumed 11 ± 0, 20 ± 0, and 59 ± 0 g of protein at breakfast, lunch, and dinner, respectively (Supplemental Table 5). Subjects in the EVEN and SKEW groups were deemed compliant to their respective diets 80 ± 4% and 82 ± 3%, respectively, of 54 meals visually assessed with photography and 92 ± 2% and 88 ± 2%, respectively, of 294 meals assessed with menu checklists (Supplemental Table 6).
Resistance training intervention
Resistance training compliance was >85% for both groups but was statistically lower for the EVEN group than the SKEW group, averaging 41 (86.0 ± 1.6%) and 43 (91.0 ± 1.5%) of 48 resistance training sessions, respectively (Supplemental Table 6). Whole body strength increased by ∼20% in each group, independent of protein distribution (Table 1).
Body composition
Within-day dietary protein distribution did not influence responses over time for the whole body and muscle-specific outcomes (Table 1). Over time, whole-body mass (−7.9 ± 0.6 kg), BMI (−2.7 ± 0.2), whole-body lean mass (−1.0 ± 0.2 kg), whole-body fat mass (−6.9 ± 0.5 kg), fat mass percentage (−4.6 ± 0.4%), lean mass index (−0.3 ± 0.1 kg/m2), fat mass index (−2.3 ± 0.2 kg/m2), appendicular lean mass (−0.7 ± 0.1 kg), appendicular fat mass (−2.6 ± 0.2 kg), waist circumference (−8.8 ± 0.7 cm), hip circumference (−6.6 ± 0.6 cm), and waist:hip ratio (−0.03 ± 0.01) each decreased and lean mass percentage (4.3 ± 0.4%) increased (main effects of time, P < 0.0001).
Total midthigh area (−28 ± 3 cm2), midthigh subcutaneous fat area (−27 ± 2 cm2), and IMAT area (−1 ± 0 cm2) decreased, independent of protein distribution (main effects of time, P < 0.0001). Midthigh muscle area (0 ± 1 cm2) did not change from pre- to postintervention (main effect of time, P = 0.797). Total midcalf area (−8 ± 1 cm2), midcalf muscle area (−3 ± 1 cm2), subcutaneous fat area (−4 ± 1 cm2), and IMAT area (−3 ± 1 cm2) decreased from pre- to postintervention (main effects of time, P < 0.0001).
DISCUSSION
To our knowledge, this study is the first randomized controlled trial to use strict dietary and resistance training controls to assess the efficacy of consuming an even or skewed protein distribution on changes in whole body lean mass during energy restriction while resistance training. Contrary to our hypothesis, distributing daily protein intake evenly between 3 meals (30 g at breakfast, lunch, and dinner) compared with a more typical skewed distribution pattern (10 g at breakfast, 20 g at lunch, and 60 g at dinner) does not influence body composition responses in adults undergoing purposeful weight loss and resistance training.
To our knowledge, before we initiated the current study, only one study had assessed the effects of within-day protein distribution on MPS (10). This study was completed in young adults in energy balance and demonstrated that evenly consuming protein (30 g at breakfast, lunch, and dinner) resulted in a 25% greater 24-h MPS rate than consuming a skewed distribution (10 g at breakfast, 15 g at lunch, and 60 g at dinner) with the same amount of total daily protein (10). Since we initiated the current study, subsequent research has shown inconsistent results regarding within-day protein distribution and MPS (12, 23). One study with older adults, also in energy balance, showed that consuming an even protein distribution did not have a differential effect on the daily whole body protein synthesis rate compared with consuming an uneven distribution (23). The authors (23) attributed the inconsistent results primarily to a difference in the study population (older adults compared with younger adults) and the age-associated blunting of the postprandial MPS response to protein ingestion (24). Other possibilities include the quantity of protein consumed per meal and whether the protein was contained in whole foods (23) or supplements (12). These factors are associated with alterations in protein digestion and amino acid absorption kinetics. It may be that even greater protein intakes per meal are required within the context of mixed-nutrient meals. A subsequent study also reported no effect of protein distribution on MPS rates in older adults in energy balance (12). The protein intake at each meal was ∼0.26 g/kg, well below the estimated requirement of 0.4 g · kg−1 · meal−1 for older adults to maximally stimulate MPS (25). More applicable to the current study, this same group (12) also measured daily MPS in energy restriction with and without resistance training. They reported that a balanced protein distribution (25 g at breakfast, lunch, and dinner) resulted in a 19% greater 13-h MPS rate than a skewed protein distribution (10 g at breakfast, 15 g at lunch, and 50 g at dinner) irrespective of resistance training (12). Although relating these acute MPS results to the current null lean mass result is problematic, one possibility is that measurements of MPS do not directly translate to changes in long-term lean mass homeostasis (26).
A second possibility for the null body composition results may be that the quantity of protein prescribed at each meal between the EVEN and SKEW groups was not sufficiently different to detect a measurable effect of protein distribution on lean body mass changes. High-quality protein sources including pork, egg, beef, and dairy were prescribed at breakfast, lunch, and dinner during the intervention. When these types of high-quality protein sources are consumed, the maximal MPS response is estimated to be reached at doses of 0.24 g/kg (25). According to the menu checklists, the SKEW group consumed ∼20 and ∼60 g of high-quality protein at lunch and dinner, respectively. This is equivalent to ∼0.24 g/kg at lunch and ∼0.71 g/kg at dinner, both of which are hypothesized to be adequate quantities of protein to maximize the MPS response in young adults (25). Consequently, the SKEW group may have consumed 2 meals (lunch and dinner) that provided sufficient protein to maximize MPS. It may be that a slightly lesser MPS rate at 1 meal (breakfast) does not substantially affect lean body mass enough to be detectable given our study design.
A third possibility for the null body composition results is that consuming an even protein distribution did not promote greater daily MPS. A recent analysis estimated that 0.24 g/kg is needed to maximally stimulate MPS in younger adults (25). In the present study, the per-meal dose for the EVEN distribution was 30 g within the context of mixed-nutrient meals. We provided more than enough protein to meet this threshold (∼0.3 g · kg−1 · meal−1) plus a “safety margin” of ∼0.1 g · kg−1 · meal−1. However, the 0.24 g · kg−1 · meal−1 estimates are based on studies that used isolated intact proteins. Perhaps the protein quantity within mixed-nutrient meal needs to be greater than when a protein supplement is consumed alone. Mixed-nutrient meals inherently contain a mix of protein qualities and have altered protein digestion kinetics and amino acid availabilities compared with isolated intact proteins from protein supplements. It may be that the EVEN group did not consume adequate protein at each meal to maximally stimulate MPS, whereas the SKEW group met the protein dose needed to maximally stimulate MPS at dinner. This may be evident in our lean body mass outcome showing a trend (P = 0.067) for greater lean body mass retention in the SKEW group than the EVEN group. Indeed, other studies showed that consuming ≥1 meal that theoretically maximizes MPS may be better for lean body mass retention than consuming 3 evenly distributed meals that are “protein insufficient” (27, 28).
Observational assessments of the influence of within-day protein distribution on lean mass in humans also contribute to the body of literature. In support of the even protein distribution concept, an analysis of >1000 adults aged 50–85 y from the 1999–2002 NHANES showed that more frequent consumption of meals with ≥30 g protein was associated with greater leg lean mass (8). Importantly, the reference group was consuming 0.64 g · kg−1 · d−1, which is less than the Recommended Dietary Allowance for protein (0.8 g · kg−1 · d−1). Conversely, the comparator groups (groups consuming 1 and 2 meals/d containing ≥30 g of protein) had a relative protein intake of 1.06 and 1.4 g · kg−1 · d−1, respectively. These results (8) may reflect the consequences of consuming less than the Recommended Dietary Allowance for protein on lean body mass quantity and not the benefits of evenly distributing protein intake. The ensuing NuAge study (Quebec Longitudinal Study on Nutrition as a Determinant of Successful Aging), a longitudinal cohort study, also characterized the effect of protein distribution on lean mass after a 2-y follow-up in both older men and women (13). The NuAge study showed that an even protein distribution was associated with greater lean mass in both older men and women at baseline and after follow-up. However, changes in lean body mass over the 2-y observational period were not different between the even and skewed distribution groups (13). Perhaps protein distribution does not affect lean body mass, which is in agreement with the results of the present study. However, questions regarding statistical power and group sample size for both the NuAge study and the current study underscore the importance of new research with larger sample sizes and longer durations to investigate potential influence of protein distribution on changes in lean body mass.
In summary, improvements in body composition may be achieved through dietary energy restriction combined with resistance training when adequate total protein is consumed in either an even or skewed distribution pattern.
Acknowledgments
We thank Jan Green, Amy Wright, Anne Wilcox, Steven Hulsey, Zach Powell, Susannah Gordon, and Michelle Wilson for assistance with clinical scheduling and assessments, menu creation, and data entry. We also thank Arthur Rosen, our study physician.
The authors’ responsibilities were as follows—JEK, DP-J, and WWC: designed the research project; JLH and JEK: were responsible for participant recruitment and conducting the research; JLH: compiled, processed, and analyzed the data; JLH and WWC: wrote the manuscript with editorial assistance from JEK and DP-J; and all authors: took responsibility for the final content and read and approved the final manuscript. DP-J participates on scientific advisory panels and has received research funds, travel expenses, and/or honoraria from the American Egg Board, Leprino Foods, National Dairy Council, National Cattlemen’s Beef Association, and US Dairy Export Council. WWC received research funds from the National Pork Board, American Egg Board-Egg Nutrition Center, National Dairy Council, and National Cattlemen’s Beef Association. WWC also served on the National Dairy Council’s Whey Protein Advisory Panel during the time this study was being conducted. None of the other authors reported a conflict of interest related to the study. Financial supporters of the study (American Egg Board-Egg Nutrition Center, National Dairy Council, Beef Checkoff, and Pork Checkoff) had no role in the design or conduct of the study; collection, analysis, or interpretation of the data; or writing of the manuscript.
Footnotes
Abbreviations used: EVEN, even within-day dietary protein distribution; IMAT, intramuscular adipose tissue; MPS, muscle protein synthesis; NuAge, Quebec Longitudinal Study on Nutrition as a Determinant of Successful Aging; SKEW, skewed within-day dietary protein distribution.
REFERENCES
- 1.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol 2014;63(Pt B):2985–3023. [DOI] [PubMed] [Google Scholar]
- 2.Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity (Silver Spring) 2007;15:421–9. [DOI] [PubMed] [Google Scholar]
- 3.Houston DK, Nicklas BJ, Ding JZ, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr 2008;87:150–5. [DOI] [PubMed] [Google Scholar]
- 4.Layman DK, Evans E, Baum JI, Seyler J, Erickson DJ, Boileau RA. Dietary protein and exercise have additive effects on body composition during weight loss in adult women. J Nutr 2005;135:1903–10. [DOI] [PubMed] [Google Scholar]
- 5.Kim JE, O’Connor LE, Sands LP, Slebodnik MB, Campbell WW. Effects of dietary protein intake on body composition changes after weight loss in older adults: a systematic review and meta-analysis. Nutr Rev 2016;74:210–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krieger JW, Sitren HS, Daniels MJ, Langkamp-Henken B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: a meta-regression. Am J Clin Nutr 2006;83:260–74. [DOI] [PubMed] [Google Scholar]
- 7.English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care 2010;13:34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loenneke JP, Loprinzi PD, Murphy CH, Phillips SM. Per meal dose and frequency of protein consumption is associated with lean mass and muscle performance. Clin Nutr 2016;35:1506–11. [DOI] [PubMed] [Google Scholar]
- 9.Dawson BM, Axford S. Nutrition as a part of healthy aging and reducing cardiovascular risk: improving functionality in later life using quality protein, with optimized timing and distribution. Semin Thromb Hemost 2014;40:695–703. [DOI] [PubMed] [Google Scholar]
- 10.Mamerow MM, Mettler JA, English KL, Casperson SL, Arentson-Lantz E, Sheffield-Moore M, Layman DK, Paddon-Jones D. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J Nutr 2014;144:876–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thalacker‐Mercer AE, Drummond MJ. The importance of dietary protein for muscle health in inactive, hospitalized older adults. Ann N Y Acad Sci 2014;1328:1–9. [DOI] [PubMed] [Google Scholar]
- 12.Murphy CH, Churchward-Venne TA, Mitchell CJ, Kolar NM, Kassis A, Karagounis LG, Burke LM, Hawley JA, Phillips SM. Hypoenergetic diet-induced reductions in myofibrillar protein synthesis are restored with resistance training and balanced daily protein ingestion in older men. Am J Physiol Endocrinol Metab 2015;308:E734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farsijani S, Morais JA, Payette H, Gaudreau P, Shatenstein B, Gray-Donald K, Chevalier S. Relation between mealtime distribution of protein intake and lean mass loss in free-living older adults of the NuAge study. Am J Clin Nutr 2016;104:694–703. [DOI] [PubMed] [Google Scholar]
- 14.Campbell WW, Haub MD, Wolfe RR, Ferrando AA, Sullivan DH, Apolzan JW, Iglay HB. Resistance training preserves fat-free mass without impacting changes in protein metabolism after weight loss in older women. Obesity (Silver Spring) 2009;17:1332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz ML, Watkins BA, Li Y, Anderson RA, Campbell WW. Chromium picolinate and conjugated linoleic acid do not synergistically influence diet- and exercise-induced changes in body composition and health indexes in overweight women. J Nutr Biochem 2008;19:61–8. [DOI] [PubMed] [Google Scholar]
- 16.Mojtahedi MC, Thorpe MP, Karampinos DC, Johnson CL, Layman DK, Georgiadis JG, Evans EM. The effects of a higher protein intake during energy restriction on changes in body composition and physical function in older women. J Gerontol A Biol Sci Med Sci 2011;66:1218–25. [DOI] [PubMed] [Google Scholar]
- 17.Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein-turnover and amino-acid-transport after resistance exercise in humans. Am J Physiol 1995;268:E514–20. [DOI] [PubMed] [Google Scholar]
- 18.Chesley A, MacDougall JD, Tarnopolsky MA, Atkinson SA, Smith K. Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol (1985) 1992;73:1383–8. [DOI] [PubMed] [Google Scholar]
- 19.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol 1997;273:E99–107. [DOI] [PubMed] [Google Scholar]
- 20.Phillips SM, Tipton KD, Ferrando AA, Wolfe RR. Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am J Physiol 1999;276:E118–24. [DOI] [PubMed] [Google Scholar]
- 21.Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol 2004;66:799–828. [DOI] [PubMed] [Google Scholar]
- 22.Institute of Medicine. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. Washington (DC): National Academies Press; 2005. [Google Scholar]
- 23.Kim IY, Schutzler S, Schrader A, Spencer H, Kortebein P, Deutz NE, Wolfe RR, Ferrando AA. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am J Physiol Endocrinol Metab 2015;308:E21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab 2000;85:4481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci 2015;70:57–62. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell CJ, Churchward-Venne TA, Cameron-Smith D, Phillips SM. What is the relationship between the acute muscle protein synthesis response and changes in muscle mass? J Appl Physiol 2015;118:495–7. [DOI] [PubMed] [Google Scholar]
- 27.Arnal MA, Mosoni L, Boirie Y, Houlier ML, Morin L, Verdier E, Ritz P, Antoine JM, Prugnaud J, Beaufrere B, et al. Protein pulse feeding improves protein retention in elderly women. Am J Clin Nutr 1999;69:1202–8. [DOI] [PubMed] [Google Scholar]
- 28.Bouillanne O, Curis E, Hamon-Vilcot B, Nicolis I, Chretien P, Schauer N, Vincent JP, Cynober L, Aussel C. Impact of protein pulse feeding on lean mass in malnourished and at-risk hospitalized elderly patients: a randomized controlled trial. Clin Nutr 2013;32:186–92. [DOI] [PubMed] [Google Scholar]