Abstract
Background: Prepregnancy body mass index [BMI (in kg/m2)], gestational weight gain, and postpartum weight retention may have distinct effects on the development of child obesity, but their combined effect is currently unknown.
Objective: We described longitudinal trajectories of maternal weight from before pregnancy through the postpartum period and assessed the relations between maternal weight trajectories and offspring obesity in childhood.
Design: We analyzed data from 4436 pairs of mothers and their children in the National Longitudinal Survey of Youth 1979 (1981–2014). We used latent-class growth modeling in addition to national recommendations for prepregnancy BMI, gestational weight gain, and postpartum weight retention to create maternal weight trajectory groups. We used modified Poisson regression models to assess the associations between maternal weight trajectory group and offspring obesity at 3 age periods (2–5, 6–11, and 12–19 y).
Results: Our analysis using maternal weight trajectories based on either latent-class results or recommendations showed that the risk of child obesity was lowest in the lowest maternal weight trajectory group. The differences in obesity risk were largest after 5 y of age and persisted into adolescence. In the latent-class analysis, the highest-order maternal weight trajectory group consisted almost entirely of women who were obese before pregnancy and was associated with a >2-fold increase in the risk of offspring obesity at ages 6–11 y (adjusted RR: 2.39; 95% CI: 1.97, 2.89) and 12–19 y (adjusted RR: 2.74; 95% CI: 2.13, 3.52). In the analysis with maternal weight trajectory groups based on recommendations, the risk of child obesity was consistently highest for women who were overweight or obese at the beginning of pregnancy.
Conclusion: These findings suggest that high maternal weight across the childbearing period increases the risk of obesity in offspring during childhood, but high prepregnancy BMI has a stronger influence than either gestational weight gain or postpartum weight retention.
Keywords: obesity etiology, pediatric obesity, pregnancy, weight gain, epidemiology
INTRODUCTION
Obesity affects children’s physical, social, and emotional health and puts them at higher risk of obesity and its related diseases in adulthood (1). For the past 15 y, obesity [BMI (in kg/m2) ≥95th percentile for age and sex] has affected ∼1 in 6 American children and adolescents (2). Child obesity prevention efforts have been predominantly school based, but many children are already obese when they begin school and a growing body of evidence suggests that gestation and early life are critical periods for child obesity development (1–7). In their recent systematic review of possible causes of child obesity occurring in the “first 1000 d” (conception through age 2 y), Woo Baidal et al. (3) identified high prepregnancy BMI and excessive gestational weight gain, along with prenatal tobacco use, high infant birth weight, and high infant weight gain, as leading risk factors. The possible association between postpartum weight and child obesity has also been investigated in a few recent studies (8–10), which reported increased risks of child obesity with high postpartum weight retention or gain. Maternal weight and weight change are thought to affect child obesity through shared genetic predispositions, intrauterine effects (particularly of increased insulin concentrations on metabolic regulation), and environmental factors such as shared diet quality, feeding behaviors, and obesogenic home and community environments (4–7). The interplay of these pathways, however, is complex and not yet understood (4–6).
Prepregnancy BMI, gestational weight gain, and postpartum weight retention have generally been studied as distinct causes of child health outcomes (3, 11–13). This approach, however, fails to consider the combined effects of these closely related maternal weight measures (14–16). Studying maternal weight as a trajectory from before pregnancy through the postpartum period could enable a more comprehensive understanding of maternal weight measures and how they may jointly influence the development of child obesity. This approach has provided new insights into child growth and development (10, 17, 18) but, to our knowledge, has not been applied to maternal weight across the childbearing period. The identification of high-risk maternal weight trajectories could improve the understanding of the relation between maternal and child weight, inform targeted interventions in mothers, and help determine which children are at the highest risk of obesity.
The goals of this study were to 1) describe longitudinal trajectories of maternal weight from before pregnancy through the postpartum period using national, longitudinal data from 1981 to 2014 and 2) assess the relations between maternal weight trajectories and offspring obesity in childhood and adolescence.
METHODS
Study design and population
The data used in this research were from the National Longitudinal Survey of Youth 1979 (NLSY79), which is an ongoing, nationally representative cohort study that enrolled adolescents and young adults aged 14–21 y in 1979 and followed them annually until 1994 and biennially thereafter. The NLSY79 began collecting data on weight and height in 1981. In 1986, the study began to enroll female participants’ children in the National Longitudinal Survey of Youth 1979 Children and Young Adults (NLSY79-CYA) subcohort and conducted biennial assessments and interviews thereafter. These studies are described in detail elsewhere (19, 20). This study draws from the NLSY79-CYA mothers and their children through 2014 (4934 women and 11,521 children). The study population was restricted to full-term (≥37 wk of gestation) singletons born after 1981 (n = 8047). By restricting the sample to full-term, singleton births, the Institute of Medicine (IOM) gestational weight gain recommendations (16) could be applied appropriately. Maternal weight and height were only collected after 1981. We excluded participants because of missing data for the following variables: maternal prepregnancy, delivery, or postpartum BMI (n = 2184; 27%), gestational age (n = 105; 2%), child BMI (if missing all measurements during ages 2–19 y) (n = 480; 8%), or covariates (n = 842; 15%). The final sample included 4436 mother-child pairs. Further sample selection details are shown in Supplemental Figure 1. The University of California, Berkeley Committee for the Protection of Human Subjects approved this study.
Maternal weight status and characteristics
NLSY79 participants self-reported their current weight in each survey wave beginning in 1981, and prepregnancy and delivery weights were self-reported in the first survey wave of the NLSY79-CYA after delivery beginning in 1986. Maternal height was self-reported in 1981, 1982, 1985, 2006, and 2008; the first height measurement preceding the delivery year was used as the prepregnancy height. The reliability of prepregnancy weight, recalled postpartum, was assessed by comparing the prepregnancy weight to the weight reported at the closest survey before that pregnancy, which was found to be highly correlated (r = 0.9) across delivery years. Prepregnancy BMI was calculated from prepregnancy weight and height and gestational weight gain was the difference between delivery weight and prepregnancy weight (in kilograms). Postpartum weight was defined as women’s first self-reported current weight between 6 and 24 mo after delivery (mean ± SD: 12 ± 4 mo). If a subsequent pregnancy began during this time, the prepregnancy weight reported for that pregnancy was used as the postpartum weight measurement corresponding to the first pregnancy (n = 441; 10%). Postpartum weight retention was defined as the difference between postpartum weight and prepregnancy weight (in kilograms), which could include weight gain or loss. The mean postpartum weight retention was 2.9 ± 6.0 kg 6–12 mo postpartum and 2.1 ± 6.2 kg 18–24 mo postpartum.
We used latent-class growth modeling to create maternal weight trajectory groups from before pregnancy through the postpartum period. This method allows longitudinal data to inform groupings of individuals following distinct patterns of change over time (21, 22). This method also can account for varying gestational durations and postpartum follow-up times and is further described below in the section on statistical analysis.
To investigate whether our results were robust to different classification schemes for maternal weight across the childbearing period and relevant to current public health recommendations, we assessed 3 additional trajectory classifications in sensitivity analyses. For the first sensitivity analysis, we used national recommendations and prior studies (14, 16, 23) to dichotomize each woman’s prepregnancy BMI (overweight or obese: ≥25), gestational weight gain (excessive based on the IOM guidelines: ≥18 kg if underweight, ≥16 kg if normal weight, ≥11.5 kg if overweight, or ≥9 kg if obese), and postpartum weight retention (high: ≥5 kg). Thus, each woman was classified as “above” or “below” the cutoff for each time point and these classifications were combined into 1 of 8 maternal weight trajectory groups: below-below-below (reference group), below-below-above, below-above-below, below-above-above, above-below-below, above-below-above, above-above-below, and above-above-above. For the second sensitivity analysis, we used 3 categories of gestational weight gain (inadequate, adequate, or excessive) instead of 2 categories (not excessive or excessive) to determine whether there were differences between inadequate and adequate weight gain, as defined by the IOM (16), in relation to child obesity. For the third sensitivity analysis, we used the median gestational weight gain (13.6 kg; 30.0 lb) instead of IOM recommendations to dichotomize gestational weight gain. Gestational weight gain defined according to the IOM recommendations is a relative measure; to qualify as excessive weight gain, less weight must be gained as prepregnancy BMI group increases.
We used causal diagrams based on prior evidence and knowledge to select covariates a priori from available data (24). Confounders were self-reported and included maternal education level at delivery (less than high school, high school completion, or some college or more), employment status at delivery (unemployed, part-time, or full-time), race/ethnicity (set by NLSY79 as Hispanic, non-Hispanic black, or non-Hispanic nonblack), marital status (married or unmarried), equivalized household income (inflation-adjusted dollars), smoking during pregnancy (yes or no), delivery year, child sex (female or male), child weight-for-gestational age (small-for-gestational age <10th percentile and large-for-gestational age >90th percentile) (25), and ever breastfed (1–3, 12, 16, 26, 27).
Child obesity
Child weight and height data were collected in biennial surveys from age 2 to 19 y. Trained study personnel measured 65% of the child weights and 69% of the child heights in the final sample; the remaining data were self-reported by the mothers or children (at older ages). We used the SAS program for the 2000 CDC growth charts to calculate age- and sex-specific BMI percentiles and biologically implausible values, which were then excluded (28). We assessed whether a child was ever obese (BMI ≥95th percentile) in early, middle, or late childhood (ages 2–5, 6–11, and 12–19 y, respectively), in accord with the age groups used by national child BMI surveillance methods (2). We also used overweight (BMI ≥85th and <95th percentile) as the outcome in a secondary analysis.
Statistical analysis
We used latent-class growth modeling to create maternal weight trajectory groups based on maternal BMI at the start of pregnancy, at delivery, and 6–24 mo postpartum, in addition to gestational duration and postpartum follow-up time. The modeling was conducted with the “traj” statistical software plugin for Stata (Stata Corporation, College Station, TX) and 3 trajectory groups were selected using the Bayesian information criterion (21, 22). We tested the associations between the trajectory groups and child obesity in each age group (2–5, 6–11, or 12–19 y) using Poisson regression models with robust standard errors, which enable the estimation of RRs as opposed to ORs. The models accounted for the complex survey design of the NLSY79 studies and family-level clustering. We adjusted the regression models for education, employment, race/ethnicity, marital status, household income, smoking, delivery year, child sex, child weight-for-gestational age, and ever breastfed, as detailed above. The multiple regression models were used to predict the adjusted probability of child obesity in each maternal weight trajectory group.
We used the same analytic procedure in several secondary analyses. First, child overweight was used as the outcome instead of child obesity. We then replicated the regression models with missing covariate data multiply imputed to assess bias from missing data. The multiple imputation procedure used chained equations to create 50 datasets. The percentage of missing observations for each covariate ranged from 0% to 9%, with a median of 2%. Third, only child weight and height measurements made by trained study personnel were used. Finally, we replicated analyses using the alternative trajectory groups: 1) recommendation-based categories, 2) 3 categories of gestational weight gain adequacy, and 3) median gestational weight gain.
All analyses were conducted in Stata MP (version 14).
RESULTS
Sixty percent of women in the study sample started their pregnancy overweight or obese, gained gestational weight in excess of the IOM guidelines, or retained ≥5 kg postpartum. Three maternal weight trajectory groups were identified through the use of latent-class growth modeling. The first trajectory group included nearly two-thirds of women and had the lowest proportions of prepregnancy overweight or obesity, excessive gestational weight gain, and high postpartum weight retention (Table 1). In the second trajectory group, over half of women were overweight before pregnancy and gained excessive gestational weight, and one-third retained high postpartum weight. Nearly all women in the third trajectory group were obese before pregnancy, more than half gained excess gestational weight, and one-third retained high postpartum weight. Additional characteristics of the mother-child pairs varied across the trajectory groups. The proportion of women who were not college educated, were non-Hispanic black, and delivered in later years at older ages increased as the trajectory group order increased. Supplemental Table 1 provides the characteristics of mother-child pairs that were included in the final sample, were eligible but excluded from the final sample, and were ineligible for the study. Within the eligible study population, there were minimal differences between included and excluded subjects.
TABLE 1.
Distribution of characteristics within each maternal weight latent-class trajectory group in the study sample (n = 4436)1
| Trajectory group |
||||
| Characteristic | Sample size,2 n | 1 (n = 2799) | 2 (n = 1369) | 3 (n = 268) |
| Prepregnancy BMI, kg/m2 | ||||
| Underweight, <18.5 | 284 | 9.9 | 0.0 | 0.0 |
| Normal weight, 18.5–24.9 | 2897 | 88.3 | 31.9 | 0.0 |
| Overweight, 25–29.9 | 829 | 1.8 | 53.9 | 3.0 |
| Class 1 obese, 30–34.9 | 282 | 0 | 13.9 | 42.2 |
| Class 2 obese, 35–39.9 | 102 | 0 | 0.3 | 37.3 |
| Class 3 obese, ≥40 | 42 | 0 | 0 | 17.5 |
| Prepregnancy BMI | 20.9 ± 1.9 | 26.6 ± 2.9 | 36.4 ± 4.7 | |
| Gestational weight gain, kg | 14.4 ± 5.4 | 15.5 ± 7.1 | 11.0 ± 8.6 | |
| Inadequate | 1202 | 32.4 | 11.7 | 23.9 |
| Adequate | 1349 | 37.1 | 23.0 | 15.5 |
| Excessive | 1841 | 30.5 | 65.3 | 60.6 |
| Postpartum weight retention, kg | 1.5 ± 4.2 | 3.4 ± 7.2 | 3.4 ± 12.7 | |
| ≥5 | 1092 | 14.6 | 33.9 | 34.7 |
| Education at birth | ||||
| Less than high school | 852 | 13.4 | 12.5 | 16.4 |
| High school | 2941 | 65.1 | 68.7 | 69.1 |
| College | 643 | 21.5 | 18.8 | 14.5 |
| Employment at birth | ||||
| Unemployed | 1486 | 29.0 | 29.0 | 32.5 |
| Part-time | 1345 | 31.7 | 27.1 | 22.7 |
| Full-time | 1605 | 39.3 | 43.9 | 44.8 |
| Race/ethnicity | ||||
| Non-Hispanic nonblack | 2614 | 82.5 | 74.4 | 71.9 |
| Non-Hispanic black | 1099 | 12.1 | 17.1 | 23.2 |
| Hispanic | 723 | 5.5 | 8.5 | 4.9 |
| Married at birth | 3124 | 78.9 | 78.1 | 69.3 |
| Equivalized household income at birth | ||||
| Lowest tertile | 1479 | 23.4 | 23.1 | 27.4 |
| Middle tertile | 1479 | 31.0 | 33.4 | 35.1 |
| Highest tertile | 1478 | 45.6 | 43.5 | 37.5 |
| Age at birth, y | ||||
| <20 | 229 | 3.8 | 2.5 | 1.3 |
| 20–30 | 3206 | 69.0 | 62.9 | 54.5 |
| ≥30 | 1001 | 27.3 | 34.6 | 44.2 |
| Smoked during pregnancy | 1186 | 28.9 | 23.8 | 20.5 |
| Child birth year | ||||
| Before 1985 | 1434 | 28.0 | 18.5 | 10.7 |
| 1985 until 1995 | 2617 | 63.7 | 66.9 | 62.7 |
| During or after 1995 | 385 | 8.3 | 14.6 | 26.6 |
| Child sex | ||||
| Female | 2151 | 48.7 | 45.9 | 50.8 |
| Male | 2285 | 51.3 | 54.1 | 49.2 |
| Child weight for gestational age | ||||
| Small for gestational | 501 | 11.3 | 7.4 | 8.3 |
| Appropriate for gestational | 3437 | 78.1 | 76.1 | 74.5 |
| Large for gestational age | 498 | 10.6 | 16.5 | 17.2 |
| Child ever breastfed | 2291 | 60.1 | 56.6 | 47.7 |
Values are percentages or means ± SDs unless otherwise indicated. Percentages are weighted for the survey sampling design.
Sample sizes are not weighted and refer to the number of mother-child pairs.
The overall prevalence of child obesity was 18% in 2- to 5-y-olds, 24% in 6- to 11-y-olds, and 21% in 12- to 19-y-olds. In unadjusted and adjusted regression analyses, the higher-order maternal weight trajectory groups were associated with an increased risk of child obesity compared with the first trajectory group in all child age groups (Table 2, Supplemental Table 2). Adjustment for covariates attenuated association estimates but did not change the statistical significance of the results. Among women in the second trajectory group, the adjusted risk of child obesity was 45% higher in 2- to 5-y-olds, 64% higher in 6- to 11-y-olds, and 73% higher in 12- to 19-y-olds compared with women in the first trajectory group. Among women in the third trajectory group, the adjusted risk of child obesity was 61% higher in 2- to 5-y-olds, 139% higher in 6- to 11-y-olds, and 174% higher in 12- to 19-y-olds compared with women in the first trajectory group.
TABLE 2.
Adjusted associations between maternal weight latent-class trajectory groups and child obesity status in 3 age groups (n = 4436)1
| Age group, y |
||||
| Trajectory group | 2–5 | Sample size,2 n (%) | 6–11 | 12–19 |
| 1 | 2799 (64.1) | Reference | Reference | Reference |
| 2 | 1369 (29.9) | 1.45 (1.23, 1.72) | 1.64 (1.42, 1.89) | 1.73 (1.46, 2.05) |
| 3 | 268 (6.0) | 1.61 (1.19, 2.18) | 2.39 (1.97, 2.89) | 2.74 (2.13, 3.52) |
Values are adjusted RRs (95% CIs) unless otherwise indicated. Modified Poisson regression models were adjusted for maternal education, employment, race/ethnicity, marital status, equivalized household income, smoking during pregnancy, delivery year, child sex, weight-for-gestational age status, and ever breastfed.
Percentages are weighted for the survey sampling design. Sample sizes are not weighted and refer to the number of mother-child pairs.
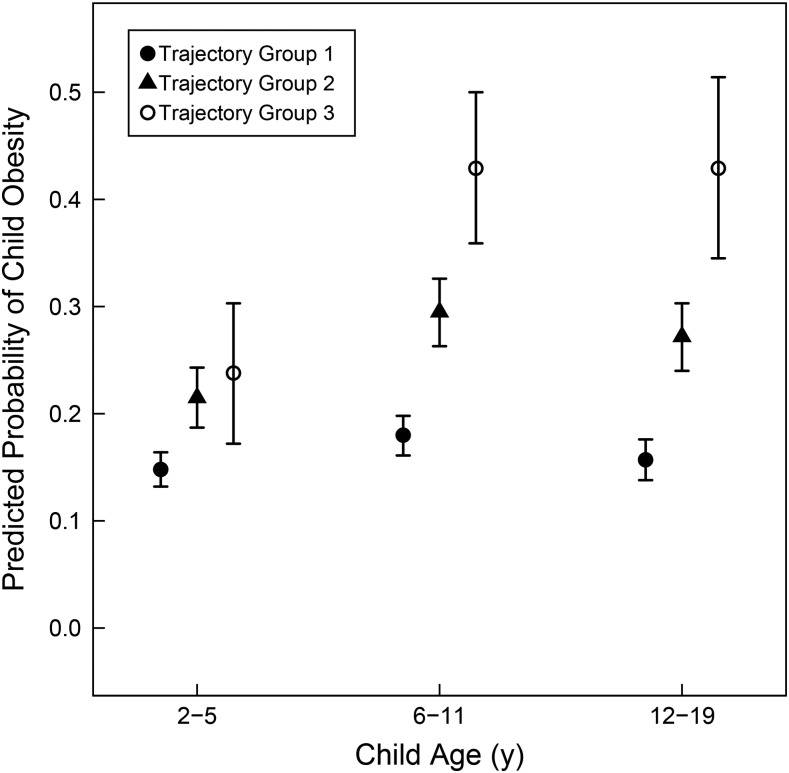
Predicted probabilities of child obesity adjusted for covariates also varied by maternal weight trajectory group (Figure 1). Differences between the trajectory groups in the predicted probability of child obesity were largest and most consistent for ages 6–11 and 12–19 y. In those age groups, the predicted probability of child obesity was ∼17% for the first maternal weight trajectory group, 28% for the second trajectory group, and 43% for the third trajectory group.
FIGURE 1.
Adjusted predicted probabilities of child obesity across maternal weight latent-class trajectory groups in 3 child age groups. Markers indicate predicted probabilities of child obesity and lines indicate 95% CIs, adjusted for covariates.
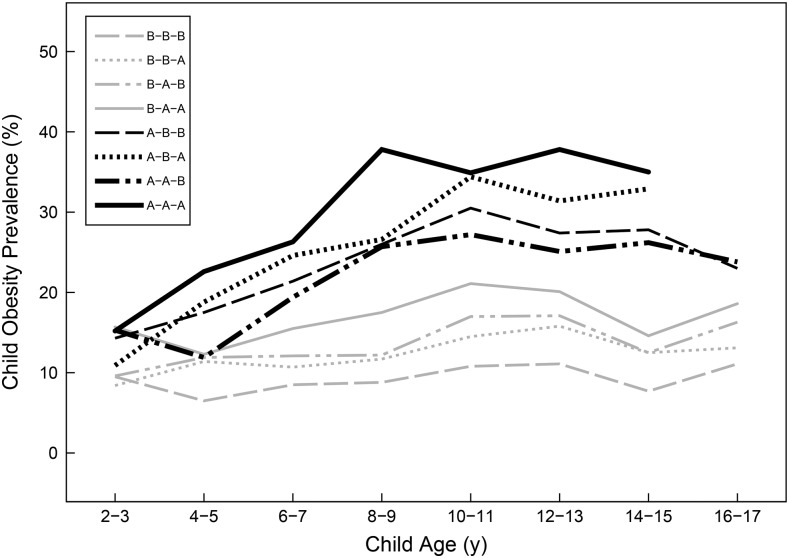
When maternal weight recommendations were used to classify trajectory groups, the prevalence of child obesity was highest among women who were overweight or obese before pregnancy and lowest in the reference trajectory group (below-below-below) across all child ages (Figure 2). Among women who were overweight or obese before pregnancy, the adjusted risks of child obesity were ∼50–200% higher than in the reference group (Table 3). Among women who were normal weight or underweight before pregnancy, the adjusted risk of child obesity was only higher in the trajectory group with both excessive gestational weight gain and high postpartum weight retention (below-above-above).
FIGURE 2.
Prevalence of child obesity across ages and stratified by recommendation-based maternal weight trajectory groups. The key indicates women who were classified as “below” or “above” (denoted as B or A, respectively) the cutoff at each maternal weight time point (prepregnancy, delivery, and postpartum). “Above” status was defined as follows: prepregnancy, BMI (in kg/m2) ≥25; at delivery, gestational weight gain in excess of the Institute of Medicine recommendations; and postpartum, weight retention ≥5 kg. Data are not shown for cell sizes <30.
TABLE 3.
Adjusted associations between recommendation-based maternal weight trajectory groups and child obesity status in 3 age groups (n = 4436)1
| Age group, y |
||||
| Maternal weight trajectory group | Sample size,2 n (%) | 2–5 | 6–11 | 12–19 |
| Below-below-below | 1748 (40.1) | Reference | Reference | Reference |
| Below-below-above | 324 (6.2) | 1.08 (0.69, 1.69) | 1.13 (0.77, 1.65) | 0.85 (0.44, 1.62) |
| Below-above-below | 715 (17.9) | 1.24 (0.90, 1.69) | 1.15 (0.85, 1.55) | 1.16 (0.74, 1.83) |
| Below-above-above | 385 (8.0) | 1.55 (1.08, 2.23) | 1.45 (1.00, 2.09) | 1.48 (0.93, 2.38) |
| Above-below-below | 377 (8.4) | 1.90 (1.36, 2.63) | 2.32 (1.76, 3.05) | 1.91 (1.28, 2.85) |
| Above-below-above | 102 (2.0) | 1.82 (1.06, 3.12) | 2.31 (1.43, 3.73) | 1.80 (0.93, 3.46) |
| Above-above-below | 473 (11.5) | 1.48 (1.00, 2.22) | 2.12 (1.56, 2.88) | 2.04 (1.36, 3.04) |
| Above-above-above | 268 (5.3) | 2.09 (1.52, 2.87) | 2.71 (1.98, 3.69) | 3.06 (2.15, 4.35) |
Values are adjusted RRs (95% CIs) unless otherwise indicated. Modified Poisson regression models adjusted for maternal education, employment, race/ethnicity, marital status, equivalized household income, smoking during pregnancy, delivery year, child sex, weight-for-gestational age status, and ever breastfed.
Percentages are weighted for the survey sampling design. Sample sizes are not weighted and refer to the number of mother-child pairs.
The overall findings did not change for the additional sets of analyses: using child overweight instead of child obesity as the outcome, in models in which covariates were multiply imputed, in a subset of only measured child BMI values, and using different maternal weight trajectory classifications (Supplemental Tables 3–7).
DISCUSSION
In this nationally representative longitudinal study, the trajectory of maternal weight from the beginning of pregnancy through the postpartum period was associated with the risk of obesity in childhood. Children of women in the lowest maternal weight trajectory group had the lowest risk of obesity from early childhood into adolescence. The differences in child obesity risk among maternal weight trajectory groups, however, were largest for age groups 6–11 and 12–19 y. In addition, the absolute risk of child obesity was still ∼16% in the lowest trajectory group. Beginning pregnancy at an obese BMI appeared to confer the greatest risk of offspring obesity. The novel trajectory approach used here provides new insights into the complex relation between maternal and child weight, which may be useful in informing efforts to prevent obesity in childhood.
The ideal pattern of weight change during the childbearing period is to begin pregnancy at a healthy weight, gain moderately during pregnancy, and return to a healthy BMI within a year. There is consistent evidence that beginning pregnancy at a high BMI and, to a lesser extent, gaining excessive weight during pregnancy both increase the risk of child obesity (12, 26, 27). Recent studies from our group (8) and others (9, 10) suggest that weight retention or gain postpartum may also increase this risk. For example, in a Dutch birth cohort, van Rossem et al. (9) found that BMI and risk of overweight were highest in children whose mothers gained gestational weight in excess of the IOM recommendations and had a high rate of weight gain between 1 and 14 y postpartum, adjusting for prepregnancy BMI. Although our current study is consistent with prior evidence suggesting that high gestational weight gain and high postpartum weight retention or postpartum gain may have compounded effects on child BMI, we found that the risk of obesity was highest in children whose mothers had the highest BMI values before pregnancy. Nearly all women in the highest-risk latent-class trajectory group had prepregnancy obesity. We also note that child obesity risk was higher in children with overweight or obese mothers without excessive gestational weight gain or high postpartum weight retention than in children with normal-weight or underweight mothers who had both excessive gestational weight gain and high postpartum weight retention.
Recently, a number of intervention studies have concentrated on reducing excessive gestational weight gain after the first trimester or promoting postpartum weight loss in women who begin pregnancy overweight or obese (5, 29–31). Although some interventions have modestly reduced excessive weight gain, they have had little to no effect on infant health outcomes and have not had sufficient follow-up time to assess the impact on child obesity (5, 29–31). Interventions that focus on reducing prepregnancy obesity are an important area for future work. High prepregnancy BMI may have the strongest effect on offspring obesity by exposing children from the earliest embryonic stages to the physiologic environment created by excess maternal weight, which may have long-lasting effects on their own metabolic regulation (4–7). This exposure is amplified by further differences associated with genetic and epigenetic factors, infant feeding, and the family food environment that likely contributed to their mothers’ excess weight (4–6). Interventions that help women lose excess weight after pregnancy are another strategy to improve prepregnancy BMI and thus offspring health in subsequent pregnancies.
The strengths of this study include the use of data collected prospectively from 1981 to 2014 in a nationally representative sample of women and their children. The sample included various racial-ethnic groups, socioeconomic backgrounds, and geographic regions of the United States. It is unusual to have data on offspring weight and height collected from birth into adolescence, which allowed us to observe differences in child obesity across ages. Most child weight and height values were also measured by trained study personnel, and results did not differ by type of measurement. In addition, maternal weight measurements were collected prospectively after 6 mo postpartum, which allowed us to study postpartum weight after most gestational weight is lost (14). To our knowledge, longitudinal trajectories are a novel approach to classifying maternal weight change and enabled a more comprehensive assessment of prepregnancy BMI, gestational weight gain, and postpartum weight retention in this study than in previous studies.
There are also several limitations in this study. Maternal weights and heights were self-reported, which could have caused misclassification of the maternal BMI and weight measures and thus the maternal weight trajectories. We assessed the reliability of the prepregnancy weights but were unable to assess validity. However, in their recent systematic review, Headen et al. (32) concluded that the misclassification of pregnancy-related weight caused by self-report does not meaningfully bias associations with perinatal health outcomes. We defined postpartum weight retention as the difference between weight 6–24 mo postpartum and weight prepregnancy. We recognize that 6 mo may not have allowed adequate time for women to lose their gestational weight gain and 2 y may have allowed women to gain weight unrelated to pregnancy. Unfortunately, the design of the cohort did not allow an adequate sample size to assess postpartum weight retention at 1 y, as has been suggested (33–35). Nonetheless, the time frame we used is consistent with the “intermediate postpartum weight retention” definition (3 mo to 3 y) used by the Agency for Healthcare Research (36) and the IOM (16). Future studies that measure maternal weight prospectively from before pregnancy through 1 y postpartum and then follow their children for obesity are needed. Missing data were an additional limitation of our study, causing the exclusion of one-third of eligible subjects; however, analyses with multiply imputed covariate data and comparison of the included and excluded samples did not indicate differences that would meaningfully bias the results. Although many covariates were available in the dataset to adjust statistical analyses, they were self-reported and we were unable to assess several covariates, particularly dietary intake, physical activity, body composition, paternal weight, and detailed breastfeeding and child feeding practices, which should be addressed in future studies.
The generalizability of these results to other populations is unknown. Women in the study sample delivered between 1981 and 2006, 28% of women were overweight or obese before pregnancy, 43% gained excessive weight in pregnancy, and 22% retained ≥5 kg postpartum. Contemporary national estimates for prepregnancy overweight or obesity, excessive gestational weight gain, and postpartum weight retention ≥5 kg are ∼50% for each indicator (16, 37, 38). We limited our analysis to full-term singletons. Additional research in contemporary birth cohorts and in preterm and twin births would be natural next steps to expand the findings of this study.
Maternal weight trajectories from before pregnancy through the postpartum period were identified and associated with offspring obesity risk in childhood and adolescence in this nationally representative cohort study. The results suggest that studying prepregnancy BMI, gestational weight gain, and postpartum weight retention in combination may further our understanding of the connection between maternal and child weight. In addition, high maternal weight across the childbearing period may compound the risk of child obesity, but we found that a high prepregnancy BMI has the strongest influence.
Acknowledgments
The authors’ responsibilities were as follows—SAL: designed the study, performed the data analysis, wrote the manuscript, and had primary responsibility for the final content; KMR and JCK: provided study oversight; BA: designed the study and provided study oversight; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: IOM, Institute of Medicine; NLSY79, National Longitudinal Survey of Youth 1979; NLSY79-CYA, National Longitudinal Survey of Youth 1979 Children and Young Adults.
REFERENCES
- 1.Koplan JP, Liverman C, Kraak V, editors; Institute of Medicine. Preventing childhood obesity: health in the balance. Washington (DC): The National Academies Press; 2005. [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, Flegal KM. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA 2016;315:2292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM. Risk factors for childhood obesity in the first 1,000 days: a systematic review. Am J Prev Med 2016;50:761–79. [DOI] [PubMed] [Google Scholar]
- 4.Poston L. Maternal obesity, gestational weight gain and diet as determinants of offspring long term health. Best Pract Res Clin Endocrinol Metab 2012;26:627–39. [DOI] [PubMed] [Google Scholar]
- 5.Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017;356:j1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oken E. Maternal and child obesity: the causal link. Obstet Gynecol Clin North Am 2009;36:361–77. [DOI] [PubMed] [Google Scholar]
- 7.Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction 2010;140:387–98. [DOI] [PubMed] [Google Scholar]
- 8.Robinson CA, Cohen AK, Rehkopf DH, Deardorff J, Ritchie L, Jayaweera RT, Coyle JR, Abrams B. Pregnancy and post-delivery maternal weight changes and overweight in preschool children. Prev Med 2014;60:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Rossem L, Wijga AH, Gehring U, Koppelman GH, Smit HA. Maternal gestational and postdelivery weight gain and child weight. Pediatrics 2015;136:e1294–301. [DOI] [PubMed] [Google Scholar]
- 10.Musaad SM, Donovan SM, Fiese BH. The independent and cumulative effect of early life risk factors on child growth: a preliminary report. Child Obes 2016;12:193–201. [DOI] [PubMed] [Google Scholar]
- 11.Olson CM, Demment MM, Carling SJ, Strawderman MS. Associations between mothers’ and their children’s weights at 4 years of age. Child Obes 2010;6:201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrenthal DB, Maiden K, Rao A, West DW, Gidding SS, Bartoshesky L, Carterette B, Ross J, Strobino D. Independent relation of maternal prenatal factors to early childhood obesity in the offspring. Obstet Gynecol 2013;121:115–21. [DOI] [PubMed] [Google Scholar]
- 13.Leonard SA, Petito LC, Rehkopf DH, Ritchie LD, Abrams B. Weight gain in pregnancy and child weight status from birth to adulthood in the United States. Pediatr Obes 2016. Jun 28 (Epub ahead of print; DOI: 10.1111/ijpo.12163). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunderson EP, Abrams B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiol Rev 2000;22:261–74. [DOI] [PubMed] [Google Scholar]
- 15.Endres LK, Straub H, McKinney C, Plunkett B, Minkovitz CS, Schetter CD, Ramey S, Wang C, Hobel C, Raju T, et al. Postpartum weight retention risk factors and relationship to obesity at 1 year. Obstet Gynecol 2015;125:144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute of Medicine and National Research Council Committee to Reexamine IOM Pregnancy Weight Guidelines; Rasmussen KM, Yaktine AL, editors. Weight gain during pregnancy: reexamining the guidelines. Washington (DC): The National Academies Press; 2009. [PubMed] [Google Scholar]
- 17.Nonnemaker JM, Morgan-Lopez AA, Pais JM, Finkelstein EA. Youth BMI trajectories: evidence from the NLSY97. Obesity (Silver Spring) 2009;17:1274–80. [DOI] [PubMed] [Google Scholar]
- 18.Tremblay RE, Nagin DS, Séguin JR, Zoccolillo M, Zelazo PF, Boivin M, Pérusse D, Japel C. Physical aggression during early childhood: trajectories and predictors. Pediatrics 2004;114:e43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Center for Human Resource Research. NLSY79 user’s guide [Internet]. Columbus (OH): The Ohio State University; 2001. [cited 2017 Apr 21]. Available from: https://www.bls.gov/nls/79guide/2001/nls79g0.pdf. [Google Scholar]
- 20.Center for Human Resource Research. NLSY79 child and young adult data users guide [Internet]. Columbus (OH): The Ohio State University; 2009. [cited 2017 Apr 21]. Available from: https://www.nlsinfo.org/pub/usersvc/Child-Young-Adult/2006ChildYA-DataUsersGuide.pdf. [Google Scholar]
- 21.Andruff H, Carraro N, Thompson A, Gaudreau P, Louvet B. Latent class growth modelling: a tutorial. Tutor Quant Methods Psychol 2009;5:11–24. [Google Scholar]
- 22.Jones BL, Nagin DS. A note on a Stata plugin for estimating group-based trajectory models. Sociol Methods Res 2013;42:608–13. [Google Scholar]
- 23.Pedersen P, Baker JL, Henriksen TB, Lissner L, Heitmann BL, Sorensen TI, Nohr EA. Influence of psychosocial factors on postpartum weight retention. Obesity (Silver Spring) 2011;19:639–46. [DOI] [PubMed] [Google Scholar]
- 24.Hernán MA, Hernández-Díaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol 2002;155:176–84. [DOI] [PubMed] [Google Scholar]
- 25.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salsberry PJ, Reagan PB. Dynamics of early childhood overweight. Pediatrics 2005;116:1329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rooney BL, Mathiason MA, Schauberger CW. Predictors of obesity in childhood, adolescence, and adulthood in a birth cohort. Matern Child Health J 2011;15:1166–75. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. A SAS program for the 2000 CDC growth charts (ages 0 to <20 years) [Internet]. 2016. [cited 2017 Apr 7]. Available from: https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm.
- 29.Hanson M, Barker M, Dodd JM, Kumanyika S, Norris S, Steegers E, Stephenson J, Thangaratinam S, Yang H. Interventions to prevent maternal obesity before conception, during pregnancy, and post partum. Lancet Diabetes Endocrinol 2017;5:65–76. [DOI] [PubMed] [Google Scholar]
- 30.Flynn AC, Dalrymple K, Barr S, Poston L, Goff LM, Rogozinska E, van Poppel MN, Rayanagoudar G, Yeo S, Barakat Carballo R, et al. Dietary interventions in overweight and obese pregnant women: a systematic review of the content, delivery, and outcomes of randomized controlled trials. Nutr Rev 2016;74:312–28. [DOI] [PubMed] [Google Scholar]
- 31.International Weight Management in Pregnancy Collaborative Group. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomised trials. BMJ 2017;358:j3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Headen I, Cohen AK, Mujahid M, Abrams B. The accuracy of self-reported pregnancy-related weight: a systematic review. Obes Rev 2017;18:350–69. [DOI] [PubMed] [Google Scholar]
- 33.Lipsky LM, Strawderman MS, Olson CM. Maternal weight change between 1 and 2 years postpartum: the importance of 1 year weight retention. Obesity (Silver Spring) 2012;20:1496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmitt NM, Nicholson WK, Schmitt J. The association of pregnancy and the development of obesity - results of a systematic review and meta-analysis on the natural history of postpartum weight retention. Int J Obes (Lond) 2007;31:1642–51. [DOI] [PubMed] [Google Scholar]
- 35.Abebe DS, Von Soest T, Von Holle A, Zerwas SC, Torgersen L, Bulik CM. Developmental trajectories of postpartum weight 3 years after birth: Norwegian Mother and Child Cohort study. Matern Child Health J 2015;19:917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Agency for Healthcare Research and Quality. Outcomes of maternal weight gain. AHRQ publication no. 08-E009. Rockville (MD): Agency for Healthcare Research and Quality; 2008. [Google Scholar]
- 37.Robbins CL, Zapata LB, Farr SL, Kroelinger CD, Morrow B, Ahluwalia I, D’Angelo DV, Barradas D, Cox S, Goodman D, et al. Core state preconception health indicators–pregnancy risk assessment monitoring system and behavioral risk factor surveillance system, 2009. MMWR Surveill Summ 2014;63:1–62. [PubMed] [Google Scholar]
- 38.Deputy NP, Sharma AJ, Kim SY. Gestational weight gain–United States, 2012 and 2013. MMWR Morb Mortal Wkly Rep 2015;64:1215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]