Abstract
Background: The potential impact of prior meal composition on the postprandial glycemic response and glycemic index (GI) and glycemic load (GL) value determinations remains unclear.
Objective: We determined the effect of meals that varied in macronutrient composition on the glycemic response and determination of GI and GL values of a subsequent standard test food.
Design: Twenty healthy participants underwent 6 test sessions within 12 wk. The subjects received each of 3 isocaloric breakfast meals (i.e., high carbohydrate, high fat, or high protein) on separate days in a random order, which was followed by a standard set of challenges (i.e., white bread and a glucose drink) that were tested on separate days in a random order 4 h thereafter. Each challenge provided 50 g available carbohydrate. Arterialized venous blood was sampled throughout the 2-h postchallenge period. GI, GL, and insulin index (II) values were calculated with the use of the incremental area under the curve (AUCi) method, and serum lipids were determined with the use of standard assays.
Results: The consumption of the high-protein breakfast before the white-bread challenge attenuated the rise in the postprandial serum glucose response (P < 0.0001) and resulted in lower glucose AUCi (P < 0.0001), GI (P = 0.0096), and GL (P = 0.0101) values than did the high-carbohydrate and high-fat breakfasts. The high-protein breakfast resulted in a lower insulin AUCi (P = 0.0146) for white bread than did the high-fat breakfast and a lower II value (P = 0.0285) than did the high-carbohydrate breakfast. The 3 breakfasts resulted in similar serum lipid responses to the white-bread challenge.
Conclusions: These data indicate that the macronutrient composition of the prior meal influences the glycemic response and the determination of GI and GL values for white bread. Future studies are needed to determine whether the background food macronutrient composition influences mean dietary GI and GL values that are calculated for eating patterns, which may alter the interpretation of the associations between these values and chronic disease risk. This trial was registered at clinicaltrials.gov as NCT01023646.
Keywords: glycemic index and glycemic load, glycemic response, healthy participants, macronutrient composition, nonesterified fatty acids
INTRODUCTION
The associations between average dietary glycemic index (GI) and glycemic load (GL) values that are calculated from dietary questionnaire–derived data and chronic disease risk have been investigated for a wide range of outcomes with equivocal results (1–14). Although some studies have reported that low-GI or low-GL diets are associated with reduced risk of cardiovascular disease (1–3) and diabetes (4–8), other studies have reported no significant association (9–14). The inconsistency in findings may have been due to methodologic and physiologic factors that were unaccounted for in the calculation of average dietary GI and GL values from dietary questionnaires (15–17). One potential factor is the variability in the GI value of individual foods that is used in the calculation (18–20). Individual GI values have sometimes been reported to accurately estimate meal or dietary GI and GL values (1, 4, 21–24) but not consistently (17, 25–30). For the calculation of meal or average dietary GI and GL values, GI values of individual foods were obtained from previously published data (31), which were measured after an overnight fast (4–6, 22). However, foods and meals that are recorded in dietary questionnaires are consumed at different times of the day and after the consumption of different foods preceding those reported, thus not in a fasting state. Concern has been raised that the GI values of individual foods may be influenced by the background meal or diet of study participants (16, 32–34).
One approach to explore this issue is to study the second meal effect, which is the effect of a prior meal’s composition on the glycemic response to a subsequent food or meal (35–40). Previous research has documented a second meal effect of carbohydrate-containing foods or meals with high GI compared with low GI in both healthy individuals and individuals with type 2 diabetes (35–37, 41–43). However, eating occasions usually contain multiple foods and beverages that differ in macronutrient compositions. A potential effect of the macronutrient composition of a prior meal on the glycemic response to a subsequent food or meal requires more attention.
The objective of this study was to assess the effect of breakfasts that varied in macronutrient composition on glycemic response and GI and GL value determinations to a subsequent standard test food (white bread containing 50 g available carbohydrate) consumed 4 h thereafter. Our hypothesis was that breakfasts that were high in carbohydrate, protein, or fat would have different effects on the postprandial glycemic response and, hence, alter GI and GL values of the test food (white bread) as well as serum insulin, HDL-cholesterol, LDL-cholesterol, triacylglycerol, and nonesterified fatty acid (NEFA) concentrations.
METHODS
Study population
Study participants [n = 20; women: 50% (all women were postmenopausal); 50–80 y of age; BMI (in kg/m2): 25–35] were recruited from the Greater Boston area. Exclusion criteria included fasting glucose concentrations ≥7 mmol/L; untreated hypertension; known chronic diseases (including cardiovascular disease, diabetes, kidney, thyroid, and liver diseases); malabsorptive disorder or irritable bowel syndrome; the use of medications known to affect glucose or lipid metabolism; tobacco use; alcohol consumption >7 drinks/wk; abnormal blood chemistry; weight gain or loss of ≥5 kg within the past 6 mo; poor venous access; and unwillingness to adhere to the study protocol. The present study was conducted according to the Declaration of Helsinki guidelines. All procedures were approved by the Institutional Review Board of Tufts University/Tufts Medical Center, and written informed consent was obtained from all study participants. The trial was registered at clinicaltrials.gov as NCT01023646 on 30 November 2009. The current study was conducted between 2012 and 2015.
Sample-size estimation
Based on our preliminary data (15), with the use of an SD of 22 for a mean white-bread GI value of 71, the estimated sample size that was required to provide 90% power and 80% power to detect differences in groups in mean GI values of 22.0 and 17.6, respectively, was 20. With the use of an SD as high as 31, a sample size of 20 participants had 80% power to detect differences of 25.0. All calculations were performed with α = 0.05.
Recruitment and screening
Volunteers who responded to the study advertisements via a telephone call or e-mail were contacted to determine if they had further interest in the study. They were provided with information about the study design. If they indicated an interest, a screening telephone questionnaire was administered to determine potential eligibility. If eligibility was established, a packet including a medical history and protocol-specific questionnaires was mailed to potential participants. For individuals who returned the packet and did not report an exclusion criterion, a prescreening in-person appointment was scheduled to acquaint them with the procedures in the Metabolic Research Unit (MRU) and to collect additional screening data. If the characteristics of a potential participant fell within the predetermined criteria, the individual was invited for a second in-person appointment to complete a full health screening and physical activity questionnaire. A participant flow diagram is presented in Supplemental Figure 1. A total of 53 volunteers were screened, and 21 participants were enrolled in the study. One participant’s participation was terminated before the end of the study because of noncompliance with study procedures.
Study design and interventions
Participants were challenged with each of the following 3 breakfast meals in a random order: high carbohydrate, high protein, and high fat. The total energy content of the test meals was equivalent to 33% of the participants’ estimated energy requirement, which was calculated according to the Harris-Benedict formula for women as follows:
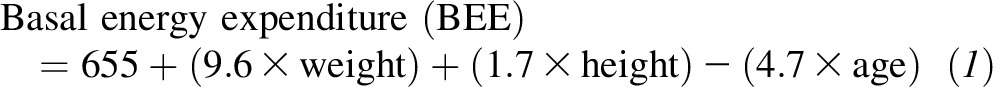 |
and for men as follows:
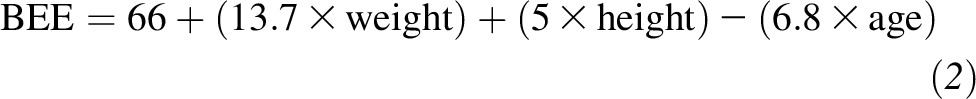 |
and the caloric requirement was estimated by multiplying the BEE by 1.5 for moderate activity (for most participants) and 1.7 for heavy activity. Detailed information about the test breakfast meals is presented in Supplemental Table 1. The high-carbohydrate breakfast included white bread, orange juice, peanut butter, 2% milk, and jelly. The percentages of energy from carbohydrate, protein, and fat were 65%, 14%, and 21%, respectively. The high-protein breakfast included white bread, orange juice, peanut butter, fat-free turkey, and fat-free egg substitute. The percentages of energy from carbohydrate, protein, and fat were 51%, 30%, and 19%, respectively. The high-fat breakfast included white bread, orange juice, whole milk, regular cream cheese, and regular cottage cheese. The percentages of energy from carbohydrate, protein, and fat were 39%, 15%, and 46%, respectively. The meal GI of each test breakfast was estimated on the basis of a formula using weighted sum GI values for each individual food item (22). These values were obtained from the International Table of Glycemic Index and Glycemic Load Values (31). If a food item was not listed in the International Table, the GI value of a similar food item was used. Meal GI values of the high-carbohydrate, high-protein, and high-fat breakfasts were 57.0, 56.8, and 53.3, respectively. The test breakfasts were consumed at 0800 within a 10-min time period under observation of an MRU staff member.
The GI value for white bread was determined 4 h after each of the 3 test breakfast meals. White bread (Pepperidge Farm Original White Bread; Pepperidge Farm Inc.) that contained 50 g available carbohydrate was used as the test food, and 500 mL of a glucose drink (100 g/L; 50 g available carbohydrate) was used as the reference food. The standard set of challenges (white bread and glucose drink) was tested on separate days after each of the 3 breakfast meals so that each participant underwent 6 sessions in the MRU. Each set of white bread and glucose drink was monitored over a 2-h period. Chemical analyses of foods were completed before the start of the studies (Covance Laboratories Inc.). The carbohydrate content was determined by difference. Before study initiation, these data were cross checked with the use of the USDA National Nutrient Database (https://ndb.nal.usda.gov/ndb/). The test breakfast meals and the white bread and glucose drink that were consumed after each test breakfast were fed in random order. The randomization sequence was generated by the statistician (LMA) before the start of the study using a block design as described earlier (16), and assignment was based on the enrollment date and time. HM, co-investigator NRM, principle investigator AHL, and all laboratory personnel were blinded to the random order.
Sessions took place 1 or 2 times/wk with a maximum of 12 wk to complete all sessions. During the study period, participants were requested to maintain their habitual diets and physical activity patterns. Subjects were instructed to not consume alcohol or engage in strenuous physical activities 72 h before the test day and were required to fast for 12 h before arrival at the MRU. Body weight and blood pressure were measured at each visit. Height and waist and hip circumferences were measured at the first and last visits. Fifteen minutes before the white-bread or glucose-drink challenge, a retrograde intravenous cannula was inserted into the lower cephalic or superficial dorsal veins of the hand for arterialized venous blood collection. During the course of the challenge, a continuous normal saline infusion was used to maintain the blood sampling line. In addition, volunteers were asked to place their hand in a moderately heated box (44–46°C) 15 min before each blood sampling time point. This technique avoided the inconsistencies that are associated with temperature control when heated pads are used. Immediately after the 4 h following the breakfast meal, a baseline blood sample was collected, and participants were provided with the white bread or glucose drink and were instructed to consume the food within 10 min under observation of an MRU staff member. Additional blood samples were collected at 15, 30, 45, 60, 90, and 120 min thereafter. During the 2-h test period, participants were requested to remain in the MRU under observation and were restricted to sedentary activities.
GI and GL calculations
GI values were calculated by dividing the incremental AUC (AUCi) for serum glucose that was obtained after consumption of white bread containing 50 g available carbohydrate by the glucose AUCi obtained in response to the glucose drink containing an equivalent amount of available carbohydrate (50 g glucose) over a 2-h period times 100 (44, 45). The AUCi was calculated via the geometric sums of the areas of the triangles and trapezoids above the fasting serum glucose concentration over a 2-h period as previously described (22). Per the recommended calculation method, the AUCi that fell beneath the baseline serum glucose concentration was excluded in the calculation. Similar calculations were done to obtain the insulin AUCi and insulin index (II). The GL was calculated by adjusting the GI value by the available carbohydrate content per serving (23, 31).
Biochemical measures
Serum concentrations of glucose, insulin, NEFA, triacylglycerol, HDL cholesterol, and LDL cholesterol were monitored throughout the 2-h test period. Blood samples were allowed to clot at room temperature for 30 min, and serum was immediately separated by centrifugation at 1500 × g at 4°C for 20 min. Blood samples for glucose determinations were collected in fluoride-containing tubes to inhibit glycolysis by blood cells. Serum glucose concentrations were measured with the use of an enzymatic method (assay CV <2%; Roche Diagnostics). Serum insulin concentrations were determined via an ELISA (assay CV <5%; ALPCO Diagnostics). Serum NEFA concentrations were determined according to an in vitro enzymatic method (Wako Chemicals), and serum triacylglycerol, HDL-cholesterol, and LDL-cholesterol concentrations were measured on a Hitachi 911 automated analyzer (Roche Diagnostics) with enzymatic reagents. The lipid assays were standardized through the Lipid Standardization Program of the CDC. High-sensitivity C-reactive protein concentrations were determined with the Tina-quant C-reactive protein (Latex) high-sensitivity immunoturbidimetric assay (Roche Diagnostics). Glycated hemoglobin was measured with an immunoturbidimetric assay (Roche Diagnostics). Body composition was measured with a Hologic QDR 4500 densitometer (Hologic Inc.). Percentages of total fat, lean muscle mass, and lean muscle mass plus bone mineral content in the whole-body, abdominal, and trunk regions were expressed as percentages of total weight.
Habitual physical activity assessment
Physical activity levels were assessed via a Community Healthy Activities Model Program for Seniors questionnaire. The questionnaire was scored with the use of the coding algorithm that was developed by the Community Healthy Activities Model Program for Seniors (46), and the average weekly physical activity energy expenditure was expressed as kcal/wk.
Statistical analyses
The data were analyzed with the use of SAS for Windows software (version 9.4; SAS Institute Inc.). Descriptive statistics and graphs (PROC UNIVARIATE; SAS Institute Inc.) were used to summarize the distributions of the outcome measures. A comparison of baseline characteristics between female and male participants was done with the use of an unpaired t test (PROC TTEST; SAS Institute Inc.). A 2-factor mixed ANOVA (PROC MIXED; SAS Institute Inc.) with the main effects of test breakfast and time and the test breakfast × time interaction with repeated measures for the participants was carried out to determine differences in serum glucose, insulin, NEFA, triacylglycerol, HDL-cholesterol, and LDL-cholesterol concentrations between test breakfasts over the 2-h study period after consumption of the white bread and glucose drink, respectively. When a test breakfast × time interaction was significant at P ≤ 0.05, multiple comparisons at each time point were carried out via the Tukey-Kramer method. The mixed-design ANOVA model (PROC MIXED) was used to test the differences in glucose and insulin AUCi, GI, GL, and II values between test breakfasts. Outcomes were modeled as repeated measures with a compound symmetry covariance matrix. Participant was designated as a random effect, and the test breakfast was designated as a fixed effect. For all outcomes, model selection was based on optimizing fit statistics (evaluated as the lowest Bayesian information criterion), and α was set at 0.05 for all tests. The Tukey-Kramer method was used for post hoc analyses. The data for all participants who completed all interventions are reported. The same analyses were also performed in female and male participants separately, and data are reported in the Supplemental Material and Supplemental Figures 2–5. Our study was not powered to determine a sex-specific analysis, and thus, data shown in Supplemental Figures 2–5 are only presented to consider whether there were any differences in trends of responses between female and male participants. Statistical significance was accepted at P ≤ 0.05. All data are presented as means ± SDs. Graphs were plotted with the use of GraphPad Prism 5 software (GraphPad Software).
RESULTS
Baseline characteristics of study participants
By design, the participants were healthy, older adults (mean age: 63 y), and 50% of subjects were women (Table 1). The mean BMI was in the overweight range. Blood pressure, waist circumference, high-sensitive C-reactive protein, and baseline serum glucose, insulin, glycated hemoglobin, NEFA, and lipoprotein concentrations were within the optimal or near optimal values. The mean weekly physical activity energy expenditure (which was based on self-reported responses) was estimated to be ∼4000 kcal/wk. Compared with male participants, female participants had a significantly lower diastolic blood pressure, waist circumference, waist-to-hip ratio, serum glucose concentrations, and percentages of lean mass and lean mass plus bone mineral content in whole-body and truck regions and higher percentages of total fat in whole-body and trunk regions and NEFA and HDL-cholesterol concentrations.
TABLE 1.
Baseline characteristics of study participants1
| Variable | All participants (n = 20) | Women (n = 10) | Men (n = 10) | P |
| Age, y | 63 ± 8 | 63 ± 9 | 64 ± 6 | 0.33 |
| Systolic blood pressure, mm Hg | 118 ± 15 | 114 ± 9 | 123 ± 6 | 0.23 |
| Diastolic blood pressure, mm Hg | 70 ± 8 | 66 ± 7 | 74 ± 6 | 0.0182 |
| BMI, kg/m2 | 27.1 ± 3.0 | 26.4 ± 3.3 | 27.7 ± 6 | 0.45 |
| Waist circumference, cm | 90.6 ± 10.2 | 84.4 ± 7.9 | 97.0 ± 6 | 0.0030 |
| Hip circumference, cm | 102.4 ± 5.8 | 103.4 ± 6.6 | 101.0 ± 6 | 0.46 |
| Waist-to-hip ratio | 0.9 ± 0.1 | 0.8 ± 0.0 | 1.0 ± 6 | 0.0004 |
| Body composition in whole body, % | ||||
| Total fat | 29.9 ± 7.8 | 35.7 ± 5.4 | 24.0 ± 6 | <0.0001 |
| Lean mass | 67.4 ± 7.9 | 61.5 ± 5.0 | 73.3 ± 6 | <0.0001 |
| Lean mass plus bone mineral content | 70.3 ± 8.2 | 64.3 ± 5.4 | 76.3 ± 6 | 0.0001 |
| Body composition in abdominal region, % | ||||
| Total fat | 29.0 ± 6.6 | 29.4 ± 7.0 | 28.5 ± 6 | 0.47 |
| Lean mass | 70.4 ± 6.5 | 70.0 ± 6.8 | 71.0 ± 6 | 0.45 |
| Lean mass plus bone mineral content | 71.0 ± 6.6 | 70.6 ± 7.0 | 71.5 ± 6 | 0.74 |
| Body composition in trunk region, % | ||||
| Total fat | 29.8 ± 7.9 | 36.0 ± 5.5 | 24.3 ± 6 | <0.0001 |
| Lean mass | 67.2 ± 8.3 | 61.0 ± 5.4 | 73.4 ± 6 | 0.0009 |
| Lean mass plus bone mineral content | 69.6 ± 8.6 | 63.3 ± 5.7 | 76.0 ± 6 | 0.0001 |
| hs-CRP,2 mg/L | 2.4 ± 2.2 | 2.6 ± 2.0 | 2.2 ± 6 | 0.55 |
| Glucose,2 mmol/L | 5.1 ± 0.4 | 4.9 ± 0.4 | 5.3 ± 6 | 0.0189 |
| Insulin,2 mU/L | 14.1 ± 7.6 | 13.5 ± 8.2 | 14.7 ± 6 | 0.72 |
| HbA1c,2 % | 5.5 ± 0.3 | 5.3 ± 0.1 | 5.6 ± 6 | 0.07 |
| NEFA,2 mmol/L | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 6 | 0.0301 |
| Serum lipids,2 mmol/L | ||||
| Total cholesterol | 4.4 ± 0.7 | 4.7 ± 0.6 | 4.2 ± 6 | 0.12 |
| Triacylglycerol | 1.5 ± 0.5 | 1.5 ± 0.6 | 1.5 ± 6 | 0.89 |
| HDL cholesterol | 1.1 ± 0.3 | 1.3 ± 0.3 | 1.0 ± 6 | 0.0130 |
| LDL cholesterol | 2.6 ± 0.6 | 2.7 ± 0.6 | 2.5 ± 6 | 0.55 |
| Physical activity, 1000 kcal/wk | 4.0 ± 2.2 | 4.3 ± 2.1 | 3.6 ± 6 | 0.44 |
All values are means ± SDs. A comparison of baseline characteristics between female and male participants was done with the use of an unpaired t test. HbA1c, glycated hemoglobin; hs-CRP, high-sensitivity C-reactive protein; NEFA, nonesterified fatty acid.
Measured at baseline (4 h postbreakfast) and immediately before the white-bread or glucose-drink challenge.
High-protein breakfast attenuated rise in postprandial glucose response to the white-bread challenge
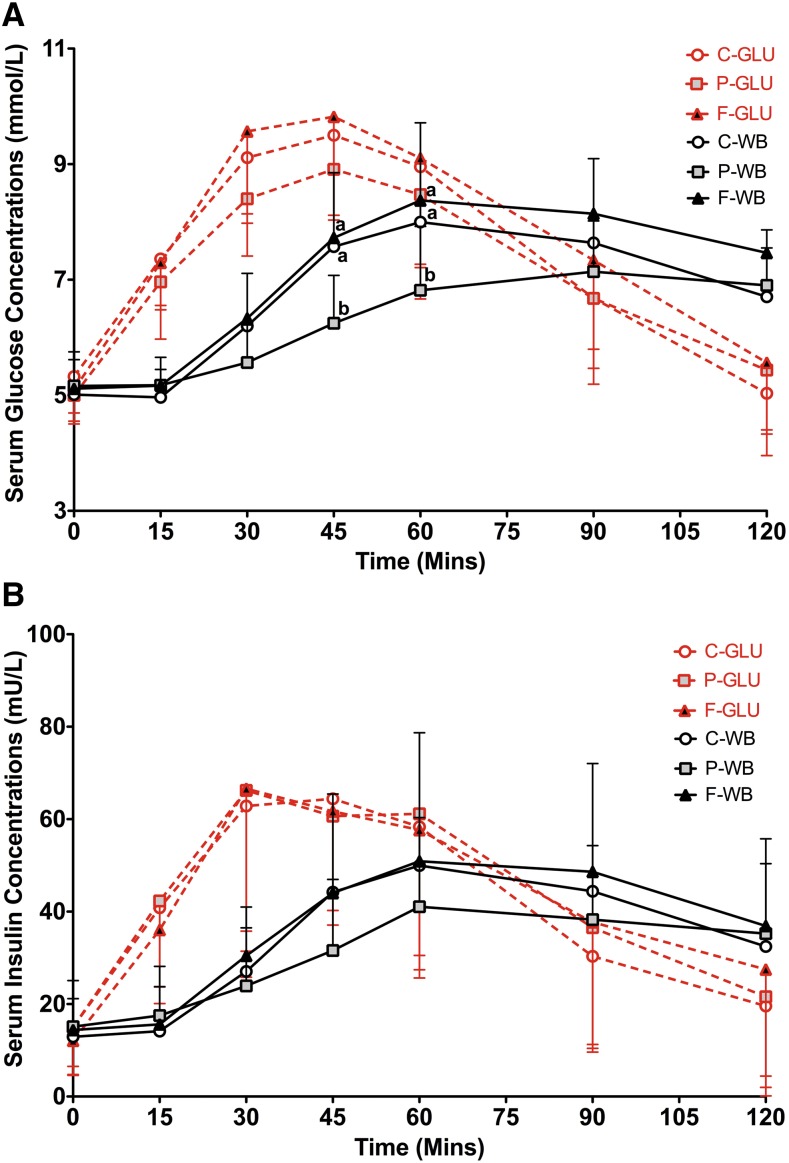
Consumption of the breakfasts that were high in carbohydrate, protein, or fat 4 h before intake of the glucose drink did not significantly affect serum glucose concentrations (P = 0.23) (Figure 1A, Table 2) or insulin concentrations (P = 0.34) (Figure 1B, Table 2) at any blood sampling time point. However, consumption of the high-protein breakfast 4 h before the white-bread challenge resulted in lower serum glucose concentrations than shown with consumption of the high-carbohydrate or high-fat breakfast at the latter time points (45 and 60 min; P < 0.0001), whereas the high-carbohydrate and high-fat breakfasts had similar effects (Figure 1A, Table 2). During the 2-h test period of the white-bread challenge, serum insulin concentrations were also influenced by the different breakfasts (P = 0.0312) (Figure 1B, Table 2). However, multiple comparisons between any 2 breakfasts were NS.
FIGURE 1.
Mean ± SD effects of different breakfasts on glycemic and insulin responses to the glucose-drink and white-bread challenges. Serum postprandial glucose (A) and insulin (B) concentrations from the glucose-drink and white-bread challenges that were preceded by breakfasts varying in macronutrient composition are presented. Differences in serum postprandial glucose or insulin concentrations in test breakfasts over a 2-h test period were determined with a 2-factor mixed ANOVA with the main effects of the test breakfast and time and test breakfast × time interaction with repeated measures for the participants after the glucose-drink and white-bread challenges, respectively. In the analysis of serum postprandial glucose concentrations, P values for the breakfast × time interaction after intakes of the glucose drink and white bread were P = 0.23 and P < 0.0001, respectively. In the analysis of serum postprandial insulin concentrations, P values of the breakfast × time interaction after intakes of the glucose drink and white bread were P = 0.34 and P = 0.0312, respectively. Because the breakfast × time interaction for white bread in both postprandial glucose and insulin analyses was significant at P ≤ 0.05, multiple comparisons at each time point were carried out with the use of the Tukey-Kramer method. Significance was accepted at P ≤ 0.05. Means with different letters are significantly different from each other at the same time point. n = 20. C-GLU, carbohydrate, glucose drink; C-WB, carbohydrate, white bread; F-GLU, fat, glucose drink; F-WB, fat, white bread; P-GLU, protein, glucose drink; P-WB, protein, white bread.
TABLE 2.
Effect of different breakfasts on glycemic and insulin responses after the glucose-drink and white-bread challenges1
| Time (min) |
||||||||
| 0 | 15 | 30 | 45 | 60 | 90 | 120 | P-breakfast × time | |
| Glucose response to glucose drink (n = 20), mmol/L | 0.23 | |||||||
| HC | 5.3 ± 0.8 | 7.4 ± 0.8 | 9.1 ± 1.1 | 9.5 ± 1.5 | 9.0 ± 1.7 | 6.7 ± 1.5 | 5.0 ± 1.1 | |
| HP | 5.0 ± 0.5 | 7.0 ± 1.0 | 8.4 ± 1.0 | 8.9 ± 1.4 | 8.5 ± 1.8 | 6.7 ± 1.2 | 5.4 ± 1.0 | |
| HF | 5.1 ± 0.4 | 7.3 ± 0.8 | 9.6 ± 1.4 | 9.8 ± 1.7 | 9.1 ± 1.9 | 7.3 ± 1.5 | 5.6 ± 1.2 | |
| Glucose response to white bread (n = 20), mmol/L | <0.0001 | |||||||
| HC | 5.0 ± 0.6 | 5.0 ± 0.5 | 6.2 ± 0.9 | 7.6 ± 1.3a | 8.0 ± 1.7a | 7.6 ± 1.5 | 6.7 ± 0.8 | |
| HP | 5.2 ± 0.6 | 5.2 ± 0.5 | 5.6 ± 0.6 | 6.2 ± 0.8b | 6.8 ± 1.1b | 7.1 ± 0.9 | 6.9 ± 1.0 | |
| HF | 5.1 ± 0.5 | 5.2 ± 0.4 | 6.3 ± 1.0 | 7.7 ± 1.4a | 8.4 ± 1.2a | 8.1 ± 1.1 | 7.5 ± 1.0 | |
| Insulin response to glucose drink (n = 20), mU/L | 0.34 | |||||||
| HC | 15.1 ± 8.5 | 40.9 ± 20.8 | 62.9 ± 31.4 | 64.4 ± 24.1 | 58.4 ± 32.7 | 30.3 ± 20.7 | 19.6 ± 19.4 | |
| HP | 15.0 ± 10.4 | 42.3 ± 18.6 | 66.2 ± 30.5 | 60.7 ± 28.1 | 61.2 ± 30.6 | 36.5 ± 26.1 | 21.6 ± 17.2 | |
| HF | 12.1 ± 7.3 | 36.1 ± 17.6 | 66.6 ± 40.8 | 61.7 ± 24.6 | 57.7 ± 30.2 | 37.7 ± 26.4 | 27.5 ± 25.5 | |
| Insulin response to white bread (n = 20), mU/L | 0.0312 | |||||||
| HC | 12.9 ± 8.3 | 14.1 ± 9.6 | 27.0 ± 14.0 | 44.2 ± 21.2 | 50.0 ± 28.7 | 44.4 ± 27.6 | 32.5 ± 17.9 | |
| HP | 15.1 ± 10.0 | 17.5 ± 10.6 | 23.9 ± 12.6 | 31.6 ± 15.4 | 41.1 ± 19.3 | 38.3 ± 16.0 | 35.3 ± 20.5 | |
| HF | 14.4 ± 8.9 | 15.6 ± 10.5 | 30.5 ± 16.9 | 44.0 ± 22.6 | 50.9 ± 25.4 | 48.6 ± 27.3 | 36.9 ± 24.9 | |
All values are means ± SDs. Serum glucose and insulin concentrations at different time points are presented. The statistical analysis was performed with the use of a 2-factor mixed ANOVA with the main effects of the test breakfast and time and test breakfast × time interaction with repeated measures for the participants to determine differences in serum glucose and insulin concentrations between test breakfasts over a 2-h test period after the glucose-drink or white-bread challenge, respectively. When a test breakfast × time interaction was significant at P ≤ 0.05, multiple comparisons at each time point were carried out via the Tukey-Kramer method. Means with different superscript letters were significantly different from each other at the same blood sampling time point. Differences in means between different blood sampling time points within each test breakfast group are not shown. HC, high carbohydrate; HF, high fat; HP, high protein.
High-protein breakfast resulted in lowest postprandial glucose AUCi, GI, and GL values for white bread
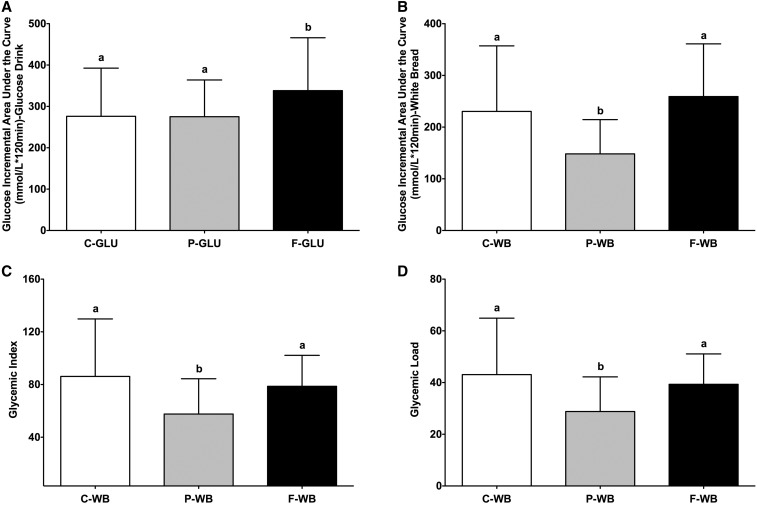
The glucose AUCi values after consumption of the glucose drink were 276 ± 116, 275 ± 89, and 338 ± 128 mmol/L (· 120 min for high-carbohydrate, high-protein, and high-fat breakfasts, respectively (Figure 2A). The glucose AUCi values after consumption of the white bread were 230 ± 127, 148 ± 66, and 259 ± 102 mmol/L (· 120 min for high-carbohydrate, high-protein, and high-fat breakfasts, respectively (Figure 2B). Consumption of the high-protein and high-carbohydrate breakfasts 4 h before intake of the glucose drink resulted in a lower glucose AUCi than was shown with consumption the high-fat breakfast (−19% and −18%, respectively; P = 0.0073) (Figure 2A). Similarly, consuming the high-protein breakfast 4 h before the white-bread challenge resulted in a lower glucose AUCi than was shown with consumption of the high-carbohydrate (−36%) and high-fat (−43%) breakfasts (P < 0.0001) (Figure 2B). The GI values for white bread were 86 ± 44, 58 ± 27, and 79 ± 24 for high-carbohydrate, high-protein, and high-fat breakfasts, respectively (Figure 2C). The GL values for white bread were 43 ± 22, 29 ± 13, and 39 ± 12 for high-carbohydrate, high-protein, and high-fat breakfasts, respectively (Figure 2D). Consequently, after participants consumed the high-protein breakfast compared with the high-carbohydrate and high-fat breakfasts, the GI values (−33% and −27%, respectively; P = 0.0096) (Figure 2C) and GL values (−33% and −27%, respectively; P = 0.0101) (Figure 2D) for white bread were lower. The high-carbohydrate and high-fat breakfasts had similar effects on glucose AUCi (Figure 2B), GI (Figure 2C) and GL (Figure 2D) values for white bread.
FIGURE 2.
Mean ± SD effects of different breakfasts on glucose AUCi, glycemic index, and glycemic load values. Glucose AUCi values for the glucose drink (A) and white bread (B) and glycemic index (C) and glycemic load (D) values for white bread after consumption of breakfasts varying in macronutrient compositions are presented. Differences in glucose AUCi, glycemic index, and glycemic load values between test breakfasts over a 2-h test period were determined with the use of a mixed-design ANOVA model with the participant as a random effect and the test breakfast as a fixed effect. The Tukey-Kramer method was used for post hoc analyses. Significance was accepted at P ≤ 0.05. Means with different letters are significantly different from each other. n = 20. AUCi, incremental AUC; C-GLU, carbohydrate, glucose drink; C-WB, carbohydrate, white bread; F-GLU, fat, glucose drink; F-WB, fat, white bread; P-GLU, protein, glucose drink; P-WB, protein, white bread.
High-protein breakfast resulted in lowest postprandial insulin AUCi and II values for white bread
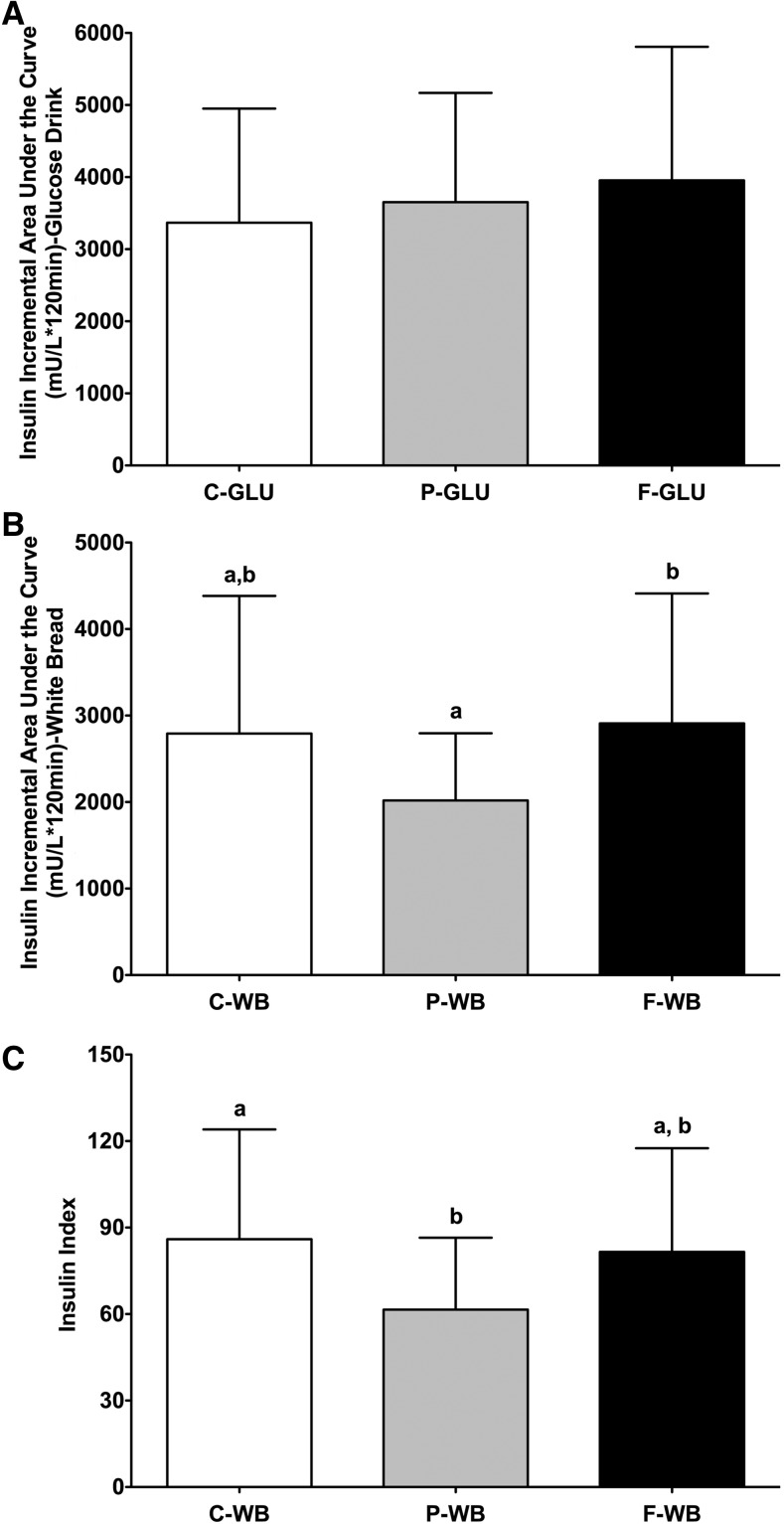
The insulin AUCi values after consumption of the glucose drink were 3365 ± 1584, 3653 ± 1516, and 3955 ± 1852 mU/L (· 120 min for high-carbohydrate, high-protein, and high-fat breakfasts, respectively (Figure 3A). The insulin AUCi values after consumption of the white bread were 2792 ± 1592, 2018 ± 778, and 2910 ± 1501 mU/L (· 120 min for high-carbohydrate, high-protein, and high-fat breakfasts, respectively (Figure 3B). The 3 breakfasts resulted in a similar insulin AUCi with the glucose drink (P = 0.29) (Figure 3A). However, consumption of the high-protein breakfast 4 h before the white-bread challenge resulted in a lower insulin AUCi than was shown with consumption of the high-fat breakfast (−31%) but not of high-carbohydrate breakfast (−28%) (P = 0.0146) (Figure 3B). The II values for white bread were 86 ± 38, 62 ± 25, and 82 ± 36 for high-carbohydrate, high-protein, and high-fat breakfasts, respectively (Figure 3C). The high-protein breakfast also resulted in a lower mean II value for white bread than for the high-carbohydrate breakfast (−28%) but not for the high-fat breakfast (−25%) (P = 0.0285) (Figure 3C). The high-carbohydrate and high-fat breakfasts had similar effects on the insulin AUCi (Figure 3B) and II (Figure 3C) values for white bread.
FIGURE 3.
Mean ± SD effects of different breakfasts on insulin AUCi and insulin index values. Insulin AUCi values for the glucose drink (A) and white bread (B) and insulin index values (C) for white bread after consumption of breakfasts varying in macronutrient composition are presented. Differences in insulin AUCi and insulin index values between test breakfasts over a 2-h test period were determined with the use of mixed-design ANOVA model with the participant as a random effect and the test breakfast as a fixed effect. The Tukey-Kramer method was used for post hoc analyses. Significance was accepted at P ≤ 0.05. Means with different letters are significantly different from each other. n = 20. AUCi, incremental AUC; C-GLU, carbohydrate, glucose drink; C-WB, carbohydrate, white bread; F-GLU, fat, glucose drink; F-WB, fat, white bread; P-GLU, protein, glucose drink; P-WB, protein, white bread.
Test breakfasts had similar effect on postprandial serum NEFA, triacylglycerol, HDL-cholesterol, and LDL-cholesterol responses to the white-bread challenge
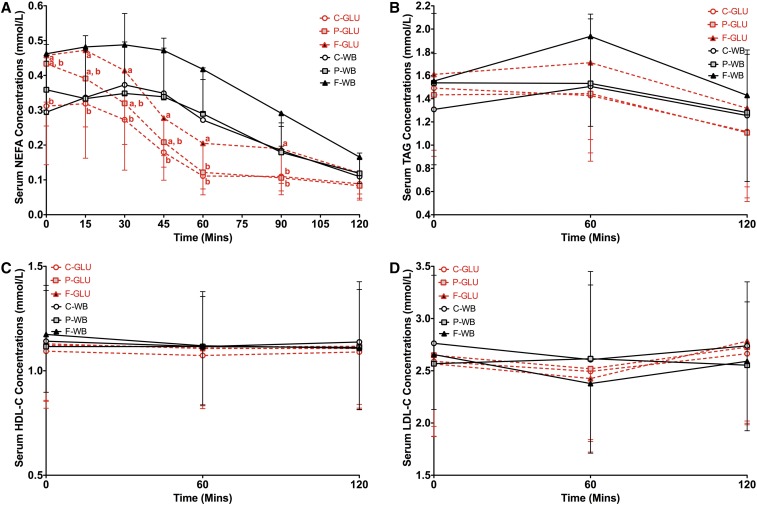
Consumption of the high-fat breakfast before the glucose-drink challenge resulted in higher serum NEFA concentrations than did consumption of the high-carbohydrate breakfast at the earlier time points (0, 15, 30, and 45 min) and consumption of high-carbohydrate and high-protein breakfasts at the later time points (60 and 90 min) (P = 0.0216) (Figure 4A). However, postprandial triacylglycerol (P = 0.91) (Figure 4B), HDL-cholesterol (P = 0.99) (Figure 4C), and LDL-cholesterol (P = 0.53) (Figure 4D) concentrations for the glucose drink were similar after consumption of the 3 breakfasts. Similarly, postprandial NEFA (P = 0.78) (Figure 4A), triacylglycerol (P = 0.30) (Figure 4B), HDL-cholesterol (P = 0.40) (Figure 4C), and LDL-cholesterol (P = 0.31) (Figure 4D) concentrations for white bread were similar after consumption of the 3 breakfasts.
FIGURE 4.
Mean ± SD effects of different breakfasts on postprandial serum NEFA, TAG, HDL-C, and LDL-C responses to the glucose-drink and white-bread challenges. Serum NEFA (A), TAG (B), HDL-C (C), and LDL-C (D) concentrations after glucose-drink and white-bread challenges preceded by breakfasts varying in macronutrient composition are presented. Differences in serum postprandial NEFA, TAG, HDL-cholesterol, and LDL-cholesterol concentrations between breakfasts over a 2-h test period were determined with the use of a 2-factor mixed ANOVA with the main effects of the test breakfast and time and test breakfast × time interaction with repeated measures for participants after intakes of the glucose drink and white bread, respectively. P values for the breakfast × time interaction after intake of the glucose drink were P = 0.0216, P = 0.91, P = 0.99, and P = 0.53 for NEFA, TAG, HDL-C, and LDL-C, respectively. P values for the breakfast × time interaction after white-bread intake were P = 0.78, P = 0.30, P = 0.40, and P = 0.31 for NEFA, TAG, HDL-C, and LDL-C, respectively. Because the breakfast × time interaction for NEFA after glucose-drink intake was significant at P ≤ 0.05, multiple comparisons at each time point were carried out via the Tukey-Kramer method. Significance was accepted at P ≤ 0.05. Means with different letters are significantly different from each other at the same time point. n = 20. C-GLU, carbohydrate, glucose drink; C-WB, carbohydrate, white bread; F-GLU, fat, glucose drink; F-WB, fat, white bread; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; NEFA, nonesterified fatty acid; P-GLU, protein, glucose drink; P-WB, protein, white bread; TAG, triacylglycerol.
DISCUSSION
Average meal or dietary GI and GL values have been estimated for eating occasions or total diet with the use of dietary questionnaire–derived data. The values are estimated by summing the GI contributions of each carbohydrate-containing food, which are determined by multiplying the GI value of each individual food by the percentage of available carbohydrates of the food relative to the total available carbohydrates in the eating occasion or total diet (16, 22, 45). The estimate does not adjust for the macronutrient composition of the background diet or prior meal of study participants. These variables may alter the GI value of individual foods that are used in the calculation. Our study was designed to address this issue by investigating the effect of breakfasts that varied in macronutrient compositions on the glycemic response and determinations of GI and GL values of a subsequent standard test food (white bread containing 50 g available carbohydrate) that was consumed 4 h thereafter.
Consumption of a high-protein breakfast attenuated the subsequent rise in the postprandial glucose response and resulted in glucose AUCi values that were lower than those after consumption of the high-carbohydrate and high-fat breakfasts, thereby resulting in concomitant lower calculated GI and GL values for white bread. Consistent with these findings, the effect of a prior protein preload or snack blunting the glycemic response to a subsequent carbohydrate-rich food or meal has been reported for a wide range of protein types and amounts (18–90 g) (38, 39, 47, 48). The mechanism underlying what has been referred to as the second meal effect of protein has been attributed to the sustained release of gut-derived signals including glucagon-like peptide-1 (GLP-1), cholecystokinin, and gastric inhibitory polypeptides (39, 47, 49–52). These gut hormones have been shown to circulate in plasma for hours after ingestion of a protein preload (39, 47, 49–52). The presence of protein and elevated gut hormones collectively contribute to the slowing of gastric emptying rates, which result in a reduced glycemic response to the subsequent food or meal consumption (39, 53, 54). Our data show that consumption of the high-protein breakfast before the white-bread challenge resulted in a slower rise in blood glucose concentrations between 15 and 30 min than was shown with consumption of high-carbohydrate and high-fat breakfasts. In addition, glucose concentrations with the high-protein breakfast peaked at 90 min, which was later than with the high-carbohydrate and high-fat breakfasts, which peaked at 60 min. These observations, taken together, suggest that the slowing of gastric emptying rates by consumption of a high-protein breakfast may have contributed to the reduced glycemic response to the subsequent white-bread challenge.
The effect of protein on second meal glucose suppression has also been attributed to an increase in the insulin response, which may be mediated via direct pancreatic stimulation of insulin secretion by certain amino acids such as leucine, valine, and lysine (39, 47, 49, 51, 55). In contrast, our data indicated that the high-protein breakfast resulted in a lower mean insulin AUCi value for white bread than was shown with the high-fat breakfast and a similar value compared with that with the high-carbohydrate breakfast in healthy individuals. These data suggest that the lower glucose response that is induced by a high-protein breakfast may result in less stimulation of insulin release. Similar results have been reported in response to a high-protein snack that was consumed before a high-carbohydrate breakfast in patients with type 2 diabetes (38). In this case, the rise in the blood glucose concentration was blunted without a concomitant change in insulin concentrations (38). In a separate study, consumption of a high-protein breakfast has been reported to induce greater insulin responses to a subsequent lunch than does consumption of a high-carbohydrate breakfast, whereas the postlunch glucose responses were similar between the 2 breakfasts (40). Collectively, these findings suggest that the data that support the hypothesis that insulin mediates the protein effect on second meal glucose suppression are inconsistent.
Previous studies that have explored the second meal effect have focused on the impact of high- compared with low-GI or -GL carbohydrate-containing foods or meals on glycemic responses to the subsequent food or meal. Some studies have suggested that low-GI or -GL meals that are consumed 4 h or the night before a test meal result in lower blood glucose concentrations or glucose AUCi values than do high-GI or GL meals (34–36, 41–43, 56, 57). This effect has been attributed to the prolonged glucose absorption and slower gastric emptying that are related to the effect of short-chain fatty acids and GLP-1 that are stimulated from the colonic fermentation of indigestible carbohydrate (34, 41–43, 57–60). However, in our study, although the calculated meal GI values of the high-carbohydrate, high-protein, and high-fat breakfasts (57.0, 56.8, and 53.3, respectively) were similar, the high-protein breakfast resulted in significantly lower glycemic response to the white-bread challenge than the other 2 breakfasts did. Our results are consistent with previous findings that breakfast or dinner meals with different GI or GL values result in similar postprandial glucose responses after a standard subsequent test meal in healthy adults (56, 59, 61) and adults with type 2 diabetes (37), thereby suggesting that the second meal effect may not be affected by GI or GL values of the prior food or meal. Taken together, these results suggest that the second meal effect may be altered by the macronutrient composition, but not the GI value, of the prior meal. Hence, the use of individual GI values to calculate the average GI value of a meal or snack may overestimate the actual effect when high-protein foods are ingested earlier.
Compared with the high-protein breakfast, the high-fat breakfast resulted in higher glucose and insulin AUCi in response to the white-bread challenge. This observation was unexpected because fat has been previously reported to induce the secretion of gastric inhibitory polypeptides and GLP-1 ≥5 h postconsumption (52). Fat has also been reported to attenuate the gastric emptying rate ≥3 h after ingestion, similar to the effect of protein (52, 62). Our results are not consistent with prior work suggesting that adding fat into carbohydrate-rich breakfasts attenuates postlunch glucose responses (62–65). Further exploration of mechanisms is needed to explain the reason for these discrepancies. The high-fat breakfast resulted in transient higher serum NEFA concentrations between 60 and 90 min after the glucose drink than were shown with the high-carbohydrate and -protein breakfasts, and this observation was similar to previous findings (65–67). All breakfasts had similar effects on serum postprandial HDL-cholesterol, LDL-cholesterol, and triacylglycerol concentrations during our 2-h study period to the white-bread challenge.
There are several strengths of this study. A complete breakfast, rather than a macronutrient preload or snack, was used before the determination of GI values to approximate common eating occasions. The foods that were included in the breakfasts were frequently eaten, the macronutrient compositions of the meals were within the range of recommended daily intakes, and meal GI values of breakfasts were similar. Moreover, this study was tightly controlled in other aspects including subject characteristics, the study environment, physical activity intensity, and times of tests and measurements. The limitations of the current study are that potential mechanistic measurements were not assessed concurrently with glucose responses, and fasting serum glucose concentrations were not measured before the test breakfasts.
In conclusion, consuming a high-protein meal relative to a high-carbohydrate or high-fat meal results in a lower glycemic response and GI and GL values for a subsequent white-bread challenge. These findings suggest that the macronutrient composition of the prior meal, which is referred to as the second meal effect, together with other methodologic and physiologic factors that are documented in previous studies (18–20, 68, 69) may collectively cause variability in the determination of GI values of individual foods. The use of previously published individual GI values (31) to calculate the average GI and GL values of a meal or dietary intake from dietary questionnaire–derived data on the basis of a previously published formula (22) may overestimate the actual effect when high-protein foods are ingested before the test food. Future studies are needed to determine whether the background food macronutrient composition influences average meal or dietary GI and GL values that are calculated from dietary questionnaire–derived data, which may influence the interpretation of the associations between these values and chronic disease risk.
Acknowledgments
We thank the study coordinators Janey Ronxhi and Jean Galluccio and the Metabolic Research Unit and Nutrition Evaluation Laboratory for assistance.
The authors’ responsibilities were as follows—HM: performed the data analysis and interpretation, and wrote the initial draft of the manuscript; AHL, NRM, and LMA: designed and conducted the research; AHL: had primary responsibility for the final content of the manuscript; and all authors: contributed to the critical review of the manuscript, and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: AUCi, incremental AUC; GI, glycemic index; GL, glycemic load; GLP-1, glucagon-like peptide-1; II, insulin index; MRU, Metabolic Research Unit; NEFA, nonesterified fatty acid.
REFERENCES
- 1.Liu S, Willett WC, Stampfer MJ, Hu FB, Franz M, Sampson L, Hennekens CH, Manson JE. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr 2000;71:1455–61. [DOI] [PubMed] [Google Scholar]
- 2.Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC. Glycemic index, glycemic load, and chronic disease risk–a meta-analysis of observational studies. Am J Clin Nutr 2008;87:627–37. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins DJ, Kendall CW, Vuksan V, Faulkner D, Augustin LS, Mitchell S, Ireland C, Srichaikul K, Mirrahimi A, Chiavaroli L, et al. Effect of lowering the glycemic load with canola oil on glycemic control and cardiovascular risk factors: a randomized controlled trial. Diabetes Care 2014;37:1806–14. [DOI] [PubMed] [Google Scholar]
- 4.Salmerón J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, Stampfer MJ, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 1997;20:545–50. [DOI] [PubMed] [Google Scholar]
- 5.Salmerón J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 1997;277:472–7. [DOI] [PubMed] [Google Scholar]
- 6.Schulze MB, Liu S, Rimm EB, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr 2004;80:348–56. [DOI] [PubMed] [Google Scholar]
- 7.Zhang C, Liu S, Solomon CG, Hu FB. Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care 2006;29:2223–30. [DOI] [PubMed] [Google Scholar]
- 8.Bhupathiraju SN, Tobias DK, Malik VS, Pan A, Hruby A, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. Am J Clin Nutr 2014;100:218–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer KA, Kushi LH, Jacobs DR Jr, Slavin J, Sellers TA, Folsom AR. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr 2000;71:921–30. [DOI] [PubMed] [Google Scholar]
- 10.Stevens J, Ahn K, Juhaeri, Houston D, Steffan L, Couper D. Dietary fiber intake and glycemic index and incidence of diabetes in African-American and white adults: the ARIC study. Diabetes Care 2002;25:1715–21. [DOI] [PubMed] [Google Scholar]
- 11.van Woudenbergh GJ, Kuijsten A, Sijbrands EJ, Hofman A, Witteman JC, Feskens EJ. Glycemic index and glycemic load and their association with C-reactive protein and incident type 2 diabetes. J Nutr Metab 2011;2011:623076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sluijs I, Beulens JW, van der Schouw YT, van der A DL, Buckland G, Kuijsten A, Schulze MB, Amiano P, Ardanaz E, Balkau B, et al. Dietary glycemic index, glycemic load, and digestible carbohydrate intake are not associated with risk of type 2 diabetes in eight European countries. J Nutr 2013;143:93–9. [DOI] [PubMed] [Google Scholar]
- 13.van Dam RM, Visscher AW, Feskens EJ, Verhoef P, Kromhout D. Dietary glycemic index in relation to metabolic risk factors and incidence of coronary heart disease: the Zutphen Elderly Study. Eur J Clin Nutr 2000;54:726–31. [DOI] [PubMed] [Google Scholar]
- 14.Levitan EB, Mittleman MA, Hakansson N, Wolk A. Dietary glycemic index, dietary glycemic load, and cardiovascular disease in middle-aged and older Swedish men. Am J Clin Nutr 2007;85:1521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vega-López S, Ausman LM, Griffith JL, Lichtenstein AH. Interindividual variability and intra-individual reproducibility of glycemic index values for commercial white bread. Diabetes Care 2007;30:1412–7. [DOI] [PubMed] [Google Scholar]
- 16.Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G, Wolever TM. Glycaemic index methodology. Nutr Res Rev 2005;18:145–71. [DOI] [PubMed] [Google Scholar]
- 17.Meng H, Matthan NR, Ausman LM, Lichtenstein AH. Effect of macronutrients and fiber on postprandial glycemic responses and meal glycemic index and glycemic load value determinations. Am J Clin Nutr 2017;105:842–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthan NR, Ausman LM, Meng H, Tighiouart H, Lichtenstein AH. Estimating the reliability of glycemic index values and potential sources of methodological and biological variability. Am J Clin Nutr 2016;104:1004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams SM, Venn BJ, Perry T, Brown R, Wallace A, Mann JI, Green TJ. Another approach to estimating the reliability of glycaemic index. Br J Nutr 2008;100:364–72. [DOI] [PubMed] [Google Scholar]
- 20.Vrolix R, Mensink RP. Variability of the glycemic response to single food products in healthy subjects. Contemp Clin Trials 2010;31:5–11. [DOI] [PubMed] [Google Scholar]
- 21.Wolever TM, Yang M, Zeng XY, Atkinson F, Brand-Miller JC. Food glycemic index, as given in glycemic index tables, is a significant determinant of glycemic responses elicited by composite breakfast meals. Am J Clin Nutr 2006;83:1306–12. [DOI] [PubMed] [Google Scholar]
- 22.Wolever TM, Jenkins DJ. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr 1986;43:167–72. [DOI] [PubMed] [Google Scholar]
- 23.Brand-Miller JC, Thomas M, Swan V, Ahmad ZI, Petocz P, Colagiuri S. Physiological validation of the concept of glycemic load in lean young adults. J Nutr 2003;133:2728–32. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins DJ, Wolever TM, Jenkins AL, Lee R, Wong GS, Josse R. Glycemic response to wheat products: reduced response to pasta but no effect of fiber. Diabetes Care 1983;6:155–9. [DOI] [PubMed] [Google Scholar]
- 25.Monro J. Redefining the glycemic index for dietary management of postprandial glycemia. J Nutr 2003;133:4256–8. [DOI] [PubMed] [Google Scholar]
- 26.Flint A, Moller BK, Raben A, Pedersen D, Tetens I, Holst JJ, Astrup A. The use of glycaemic index tables to predict glycaemic index of composite breakfast meals. Br J Nutr 2004;91:979–89. [DOI] [PubMed] [Google Scholar]
- 27.Henry CJ, Lightowler HJ, Newens KJ, Pata N. The influence of adding fats of varying saturation on the glycaemic response of white bread. Int J Food Sci Nutr 2008;59:61–9. [DOI] [PubMed] [Google Scholar]
- 28.Marangoni F, Poli A. The glycemic index of bread and biscuits is markedly reduced by the addition of a proprietary fiber mixture to the ingredients. Nutr Metab Cardiovasc Dis 2008;18:602–5. [DOI] [PubMed] [Google Scholar]
- 29.Dodd H, Williams S, Brown R, Venn B. Calculating meal glycemic index by using measured and published food values compared with directly measured meal glycemic index. Am J Clin Nutr 2011;94:992–6. [DOI] [PubMed] [Google Scholar]
- 30.Sun L, Ranawana DV, Leow MK, Henry CJ. Effect of chicken, fat and vegetable on glycaemia and insulinaemia to a white rice-based meal in healthy adults. Eur J Nutr 2014;53:1719–26. [DOI] [PubMed] [Google Scholar]
- 31.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 2002;76:5–56. [DOI] [PubMed] [Google Scholar]
- 32.Aziz A. The glycemic index: methodological aspects related to the interpretation of health effects and to regulatory labeling. J AOAC Int 2009;92:879–87. [PubMed] [Google Scholar]
- 33.Wolever TMS, Bolognesi C. Time of day influences relative glycaemic effect of foods. Nutr Res 1996;16:381–4. [Google Scholar]
- 34.Granfeldt Y, Wu X, Bjorck I. Determination of glycaemic index; some methodological aspects related to the analysis of carbohydrate load and characteristics of the previous evening meal. Eur J Clin Nutr 2006;60:104–12. [DOI] [PubMed] [Google Scholar]
- 35.Liljeberg HG, Akerberg AK, Bjorck IM. Effect of the glycemic index and content of indigestible carbohydrates of cereal-based breakfast meals on glucose tolerance at lunch in healthy subjects. Am J Clin Nutr 1999;69:647–55. [DOI] [PubMed] [Google Scholar]
- 36.Liljeberg H, Bjorck I. Effects of a low-glycaemic index spaghetti meal on glucose tolerance and lipaemia at a subsequent meal in healthy subjects. Eur J Clin Nutr 2000;54:24–8. [DOI] [PubMed] [Google Scholar]
- 37.Clark CA, Gardiner J, McBurney MI, Anderson S, Weatherspoon LJ, Henry DN, Hord NG. Effects of breakfast meal composition on second meal metabolic responses in adults with type 2 diabetes mellitus. Eur J Clin Nutr 2006;60:1122–9. [DOI] [PubMed] [Google Scholar]
- 38.Chen MJ, Jovanovic A, Taylor R. Utilizing the second-meal effect in type 2 diabetes: practical use of a soya-yogurt snack. Diabetes Care 2010;33:2552–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma J, Stevens JE, Cukier K, Maddox AF, Wishart JM, Jones KL, Clifton PM, Horowitz M, Rayner CK. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 2009;32:1600–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park YM, Heden TD, Liu Y, Nyhoff LM, Thyfault JP, Leidy HJ, Kanaley JA. A high-protein breakfast induces greater insulin and glucose-dependent insulinotropic peptide responses to a subsequent lunch meal in individuals with type 2 diabetes. J Nutr 2015;145:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenkins DJ, Wolever TM, Taylor RH, Griffiths C, Krzeminska K, Lawrie JA, Bennett CM, Goff DV, Sarson DL, Bloom SR. Slow release dietary carbohydrate improves second meal tolerance. Am J Clin Nutr 1982;35:1339–46. [DOI] [PubMed] [Google Scholar]
- 42.Wolever TM, Jenkins DJ, Ocana AM, Rao VA, Collier GR. Second-meal effect: low-glycemic-index foods eaten at dinner improve subsequent breakfast glycemic response. Am J Clin Nutr 1988;48:1041–7. [DOI] [PubMed] [Google Scholar]
- 43.Nilsson AC, Ostman EM, Granfeldt Y, Bjorck IM. Effect of cereal test breakfasts differing in glycemic index and content of indigestible carbohydrates on daylong glucose tolerance in healthy subjects. Am J Clin Nutr 2008;87:645–54. [DOI] [PubMed] [Google Scholar]
- 44.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr 1981;34:362–6. [DOI] [PubMed] [Google Scholar]
- 45.Food and Agriculture Organization. Carbohydrates in human nutrition: a summary of the Joint FAO/WHO Expert Consultation, 1997. Rome (Italy): Food and Agriculture Organization (United Nations); 1997. [Google Scholar]
- 46.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc 2001;33:1126–41. [DOI] [PubMed] [Google Scholar]
- 47.Jakubowicz D, Froy O, Ahren B, Boaz M, Landau Z, Bar-Dayan Y, Ganz T, Barnea M, Wainstein J. Incretin, insulinotropic and glucose-lowering effects of whey protein pre-load in type 2 diabetes: a randomised clinical trial. Diabetologia 2014;57:1807–11. [DOI] [PubMed] [Google Scholar]
- 48.Silva Ton WT, das Graças de Almeida C, de Morais Cardoso L, Marvila Girondoli Y, Feliciano Pereira P, Viana Gomes Schitini JK, Galvão Cândido F, Marques Arbex P, de Cássia Gonçalves Alfenas R. Effect of different protein types on second meal postprandial glycaemia in normal weight and normoglycemic subjects. Nutr Hosp 2014;29:553–8. [DOI] [PubMed] [Google Scholar]
- 49.Rayner CK, Ma J, Jones KL, Horowitz M. Comment on: Chen et al. Utilizing the second-meal effect in type 2 diabetes: practical use of a soya-yogurt snack. Diabetes Care 2010;33:2552-2554. Diabetes Care 2011;34:e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knop FK. Comment on: Chen et al. Utilizing the second-meal effect in type 2 diabetes: practical use of a soya-yogurt snack. Diabetes Care 2010;33:2552-2554. Diabetes Care 2011;34:e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jakubowicz D, Froy O. Biochemical and metabolic mechanisms by which dietary whey protein may combat obesity and Type 2 diabetes. J Nutr Biochem 2013;24:1–5. [DOI] [PubMed] [Google Scholar]
- 52.Carr RD, Larsen MO, Winzell MS, Jelic K, Lindgren O, Deacon CF, Ahren B. Incretin and islet hormonal responses to fat and protein ingestion in healthy men. Am J Physiol Endocrinol Metab 2008;295:E779–84. [DOI] [PubMed] [Google Scholar]
- 53.Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care 2001;24:371–81. [DOI] [PubMed] [Google Scholar]
- 54.Karamanlis A, Chaikomin R, Doran S, Bellon M, Bartholomeusz FD, Wishart JM, Jones KL, Horowitz M, Rayner CK. Effects of protein on glycemic and incretin responses and gastric emptying after oral glucose in healthy subjects. Am J Clin Nutr 2007;86:1364–8. [DOI] [PubMed] [Google Scholar]
- 55.Salehi A, Gunnerud U, Muhammed SJ, Ostman E, Holst JJ, Björck I, Rorsman P. The insulinogenic effect of whey protein is partially mediated by a direct effect of amino acids and GIP on beta-cells. Nutr Metab (Lond) 2012;9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nilsson A, Ostman E, Preston T, Bjorck I. Effects of GI vs content of cereal fibre of the evening meal on glucose tolerance at a subsequent standardized breakfast. Eur J Clin Nutr 2008;62:712–20. [DOI] [PubMed] [Google Scholar]
- 57.Stevenson E, Williams C, Nute M, Humphrey L, Witard O. Influence of the glycaemic index of an evening meal on substrate oxidation following breakfast and during exercise the next day in healthy women. Eur J Clin Nutr 2008;62:608–16. [DOI] [PubMed] [Google Scholar]
- 58.Brighenti F, Benini L, Del Rio D, Casiraghi C, Pellegrini N, Scazzina F, Jenkins DJ, Vantini I. Colonic fermentation of indigestible carbohydrates contributes to the second-meal effect. Am J Clin Nutr 2006;83:817–22. [DOI] [PubMed] [Google Scholar]
- 59.Nilsson A, Granfeldt Y, Ostman E, Preston T, Bjorck I. Effects of GI and content of indigestible carbohydrates of cereal-based evening meals on glucose tolerance at a subsequent standardised breakfast. Eur J Clin Nutr 2006;60:1092–9. [DOI] [PubMed] [Google Scholar]
- 60.Drucker DJ. The biology of incretin hormones. Cell Metab 2006;3:153–65. [DOI] [PubMed] [Google Scholar]
- 61.Gabrial SG, Shakib MR, Gabrial GN. Effect of pseudocereal-based breakfast meals on the first and second meal glucose tolerance in healthy and diabetic subjects. Open Access Maced J Med Sci 2016;4:565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gentilcore D, Chaikomin R, Jones KL, Russo A, Feinle-Bisset C, Wishart JM, Rayner CK, Horowitz M. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J Clin Endocrinol Metab 2006;91:2062–7. [DOI] [PubMed] [Google Scholar]
- 63.Reis CE, Ribeiro DN, Costa NM, Bressan J, Alfenas RC, Mattes RD. Acute and second-meal effects of peanuts on glycaemic response and appetite in obese women with high type 2 diabetes risk: a randomised cross-over clinical trial. Br J Nutr 2013;109:2015–23. [DOI] [PubMed] [Google Scholar]
- 64.Mori AM, Considine RV, Mattes RD. Acute and second-meal effects of almond form in impaired glucose tolerant adults: a randomized crossover trial. Nutr Metab (Lond) 2011;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ercan N, Gannon MC, Nuttall FQ. Effect of added fat on the plasma glucose and insulin response to ingested potato given in various combinations as two meals in normal individuals. Diabetes Care 1994;17:1453–9. [DOI] [PubMed] [Google Scholar]
- 66.Robertson MD, Henderson RA, Vist GE, Rumsey RD. Extended effects of evening meal carbohydrate-to-fat ratio on fasting and postprandial substrate metabolism. Am J Clin Nutr 2002;75:505–10. [DOI] [PubMed] [Google Scholar]
- 67.Frape DL, Williams NR, Rajput-Williams J, Maitland BW, Scriven AJ, Palmer CR, Fletcher RJ. Effect of breakfast fat content on glucose tolerance and risk factors of atherosclerosis and thrombosis. Br J Nutr 1998;80:323–31. [DOI] [PubMed] [Google Scholar]
- 68.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, et al. Personalized nutrition by prediction of glycemic responses. Cell 2015;163:1079–94. [DOI] [PubMed] [Google Scholar]
- 69.Korem T, Zeevi D, Zmora N, Weissbrod O, Bar N, Lotan-Pompan M, Avnit-Sagi T, Kosower N, Malka G, Rein M, et al. Bread affects clinical parameters and induces gut microbiome-associated personal glycemic responses. Cell Metab 2017;25:1243–53.e5. [DOI] [PubMed] [Google Scholar]