Abstract
Cutaneous eruptions are among the most common immune-related adverse events (irAEs) associated with anti-PD-1/PD-L1 therapy, and are often clinically and histologically characterized as lichenoid. Non-lichenoid patterns may also occur and are likely to be encountered by surgical pathologists, given the increasing clinical use of these agents. The purpose of this study is to describe the histopathologic features of non-lichenoid cutaneous irAEs from patients receiving anti-PD-1/PD-L1 therapies for a variety of underlying advanced malignancies. Sixteen patients with 17 biopsied eruptions were included from two academic institutions with extensive experience administering and monitoring responses to immune checkpoint blockade as well as treating the potential side effects. Eruptions occurred a median of 10 days (range 1 day to 11.4 months) after treatment initiation. Nearly half of specimens demonstrated either a psoriasiform/spongiotic or an urticarial-type reaction pattern on histologic review. Patterns consistent with Grover’s disease, bullous pemphigoid, and granulomatous dermatitis were also observed. Nearly two-thirds of patients required systemic corticosteroids for treatment of the cutaneous irAE, and 19% of patients discontinued immunotherapy due to their skin eruptions. 75% of patients showed an objective antitumor response. The diverse array of non-lichenoid cutaneous irAE presented here should reflect and inform the scope of histologic patterns encountered by the practicing surgical pathologist. Such eruptions are seen in patients with a variety of underlying tumor types, many of whom ultimately demonstrate a favorable response to immune checkpoint blockade.
Keywords: PD-1/PD-L1, dermatitis, irAE, immunotherapy, pathology
INTRODUCTION
Therapeutic anti-PD-1/PD-L1 monoclonal antibodies (mAbs) unleash tumor-specific immune cells that can mediate impressive antitumor activity.1,2 This unbridled immune response can result in durable clinic benefit, but in some cases may trigger immune-related adverse events (irAEs) in multiple organ systems. Cutaneous irAEs develop in approximately 15–20% of patients receiving anti-PD-1/PD-L11,2 and have been reported to occur relatively early in the course of treatment.3 Notably, the incidence of cutaneous irAEs seems to vary by tumor type: approximately 40–46% of patients with melanoma4 and 41% of patients with renal cell carcinoma5 will develop cutaneous irAEs associated with anti-PD-1 therapy, as compared to 17% in patients with non-small cell lung cancer (NSCLC)4 and 18% of patients with squamous-cell carcinoma of the head and neck.6
Patients present commonly with nonspecific morbilliform eruptions, but alternate clinical presentations reported with anti-PD-1/PD-L1 include psoriasis,7–11 bullous pemphigoid (BP),12–16 sarcoidosis,17–19 and Stevens Johnson syndrome/toxic epidermal necrolysis.20 For a detailed, comprehensive review of the broad array of presentations published to date, see Curry, et al. 2016.21 Histologic presentations may also vary, with the lichenoid (or interface) pattern being the most commonly recognized.22–29 Immunohistochemical analysis of anti-PD-1-induced lichenoid dermatitis shows similarity to idiopathic lichen planus24 and suggests a T-cell-mediated process,23 which is consistent with the recognized mechanism of action for these agents.
While immune-related lichenoid reactions are frequently seen in patients who are biopsied for cutaneous irAEs, other histologic patterns may be encountered in clinical practice. The purpose of this study was thus to comprehensively describe the clinicopathologic characteristics of the non-lichenoid dermatologic toxicities associated with these agents. Here, we define the spectrum of reaction patterns seen at two major academic institutions that are leading the investigations into usage of these agents in a wide variety of tumor types. We assessed the time to onset of the biopsy-confirmed eruptions, treatment of the eruptions, the association of non-lichenoid cutaneous irAE with irAE in other organ systems, and correlation of the irAE with response to treatment. Many of the observed histologic patterns have been published singularly or in conjunction with cohorts that also included lichenoid eruptions, however, the associated clinical correlates specific to non-lichenoid patterns were either not captured or were not collected and reported in a standardized fashion.
MATERIALS AND METHODS
Institutional Review Board approval for the study was obtained from both Johns Hopkins Hospital (JHH) and Memorial Sloan Kettering Cancer Center (MSKCC). JHH patients who developed a cutaneous eruption following treatment with anti-PD-1/PD-L1 monotherapy or combination therapy with anti-CTLA-4 for any advanced malignancy were eligible for inclusion. Patients were referred by their treating oncologist and/or dermatologist. MSKCC patients had underlying NSCLC and were referred to dermatology for eruptions while receiving anti-PD-1/PD-L1 monotherapy or in combination with anti-CTLA-4. Only patients with biopsy specimens available for review were included. Histologic patterns were assessed by two dermatopathologists (JMT and JDC) who were blinded to treatment outcome. Clinical information was gathered retrospectively from the medical record (Table 1, Supplemental Table 1). Patient response to therapy was classified according to Response Evaluation Criteria in Solid Tumors (RECIST).
Table 1.
Clinical and Histologic Descriptions of Patients with Cutaneous Eruptions While Receiving anti-PD-1/PD-L1
| Pt No. | Age (y), Sex | Oncologic Agent | Cancer Type | Tumor Response | Follow-up (m) | Days to Rash | Clinical Morphologya | Anatomic Distributiona | Histologic Patternb | Treatment of Rash | Treatment Discontinued for Rash | Other irAE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 61, M | anti-PD-1 | HNSCC | PR | 9.97 | 10 | Psoriasiform plaques, onycholysisc,d | Palms, soles, elbow, penis | Psorasiform and spongiotic dermatitis | Clobetasol, desonide, calcipotriene | No (delayed 2 cycles, resumed with less frequent dosing) | Pneumonitis, arthritis |
| 2 | 62, M | anti-PD-1 | MM | CR | 85.3 | 75 | Depigmented patches | Face, neck, extremities | Vitiligo | None | No | Thyroiditis, lymphopenia, LFT elevatione |
| 3 | 42, M | anti-PD-1 | RCC | PD | 30.33 | 34 | Psoriasiform plaquesf | Bilateral lower extremities, trunk | Psoriasiform and spongiotic dermatitis | None | No | LFT elevation |
| 4 | 40, M | anti-PD-1 | CRC (MSI-H) | CR | 35.23 | 341g | Urticarial plaques and tense bullae | Abdomen, extremities | Bullous pemphigoid | Prednisone | No (held due to rash, then achieved CR) | None |
| 5 | 64, M | anti-PD-1 | NSCLC | PR | 68.47 | 5d | Erythematous plaques | Back, neck, bilateral axillae | Urticarial-type reaction pattern* | None | No | Arthritis, colitis |
| 6 | 85, M | anti-PD-1 | NSCLC | PR | 32.67 | 84 | Acneiform | Abdomen, arms, back | Bullous pemphigoid | Prednisone, clobetasol, dapsone | Yes | None |
| 7 | 74, M | anti-PD-1 | NSCLC | CR | 43.17 | 68 | Erythematous | Arms, trunk | Urticarial-type reaction pattern* | Methyl-prednisolone, prednisone, traimcinolone, fluocinonide | No (delayed 1 cycle) | None |
| 8 | 75, M | anti-PD-L1 | Breast | SD | 4.17 | 7 | Psoriasiform and eczematous plaquesc | Scalp, extremities, penis | Psoriasiform and spongiotic dermatitis | Clobetasol, calcipotriene | Yes | None |
| 9 | 65, Mh | anti-PD-L1 | NSCLC | PR | 23.07 | 284 | Bullous dermatitis | Extremities, trunk | Bullous pemphigoid | Clobetasol spray, prednisone | Yes (delayed several cycles, resumed with recurrence of irAE and discontinued) | Arthralgia |
| 10 | 60, M | anti-PD-1 + anti-CTLA-4 | MM | PD | 24.4 | 9 | Erythematous papules | Trunk, distal extremities | Grover’s-like eruption | Prednisone, triamcinolone | No (dose delayed, then resumed) | None |
| 11 | 51, F | anti-PD-1 + anti-CTLA-4 | MM | CR | 28.77 | 3d | Erythematous to hyperpigmented papules | Trunk | Grover’s-like eruption | Prednisone | No (dose delayed, then resumed) | Hypophysitis, arthritis |
| 12 | 82, M | anti-PD-1 + anti-CTLA-4 | MM | PR | 31.7 | 7i | Diffuse urticarial papules coalescing into plaques | Trunk, extremities | Urticarial-type reaction pattern* | Prednisone | No | Thyroiditis, vitiligo |
| 7j | Solitary psoriasiform plaquec | Lower leg | Psorasiform and spongiotic dermatitis | Prednisone | No | |||||||
| 13 | 74, M | anti-PD-1 + anti-CTLA-4 | NSCLC | PR | 2.6 | 47 | EM-like | Trunk, arms, legs | Grover’s-like eruption* | Prednisone, triamcinolone | No | Colitis |
| 14 | 54, F | anti-PD-1 + anti-CTLA-4 | NSCLC | PD | 4 | 6 | Maculopapular | Trunk, legs | Psoriasiform and spongiotic dermatitis | Methyl-prednisolone, fluocinonide | No (delayed 1 cycle) | None |
| 15 | 53, M | anti-PD-1 + anti-CTLA-4 | NSCLC | CR | 27.7 | 1 | Maculopapular | Trunk, proximal arms | Granulomatous dermatatitis | Prednisone, clobetasol foam | No (delayed 1 cycle) | Pneumonitis, adrenal insufficiency, hypothyroidism |
| 16 | 58, F | anti-PD-1 + anti-CTLA-4 | NSCLC | PR | 30.57 | 140 | Erythematous | Arms | Granulomatous dermatatitis | Clobetasol | No | None |
Legend:
At time of biopsy
Histologic pattern determined by consensus (JMT, JDC)
Drug-related exacerbation of possible pre-existing psoriasis
Rash recurred with subsequent treatment infusions
LFT elevations attributed to combination of patient’s underlying Gilbert’s disease, alcohol intake, and anti-PD-1 regimen
Based on limited description, no clinical photo available
Pruritic rash approximately three months prior to appearance of bullae
Patient had an initial biopsy done also demonstrating bullous pemphigoid, 161 days after first infusion and located on back; treated with clobetasol spray, dose delayed but was resumed
Patient initially presented with diffuse macular eruption 7 days after first dose of checkpoint blocking agent, which was controlled with a prednisone taper; patient continued on low-dose prednisone and was taken off protocol approximately one year later, continuing with low-dose steroids for another 9 months; at this point prednisone was tapered further and cutaneous eruption was reported to recur approximately after one month on lower dose, with flaring of longstanding psoriasiform plaque on leg; biopsies were performed 4 days later.
Thought to be drug-related irAE superimposed on chronic psoriasiform eruption
Some cases (often those with the urticarial-type reaction pattern) showed small foci of spongiosis or vacuolar interface reaction
Abbreviations: CTLA-4, cytotoxic T lymphocyte associated antigen 4; CR, complete response; CRC, colorectal adenocarcinoma; HNSCC, head and neck squamous cell carcinoma; irAE, immune-related adverse event; LFT, liver function test; MM, metastatic melanoma; MSI-H, microsatellite unstable; N/A, not applicable; NSCLC, non-small cell lung cancer; PD, progression of disease; PD-1, programmed cell death protein 1; PD-L1, PD1 ligand; PR, partial response; RCC, renal cell carcinoma; SD, stable disease.
RESULTS
From 2010–2016, 18 patients at JHH were biopsied for treatment-associated cutaneous eruptions. One patient had two different skin morphologies that were each biopsied. Among these 19 specimens, 9 (47%) were histopathologically characterized as lichenoid and excluded (Supplemental Figure 1). Seven additional non-lichenoid skin specimens from 2012–2015 were obtained from unique patients treated at MSKCC. Patient characteristics and treatment course for the final cohort of 16 patients with 17 non-lichenoid eruptions are described in Table 1.
The reported time to onset ranged from 1 day to 11.4 months (median 10 days, mean 66 days) after the first anti-PD-1/PD-L1 treatment. A broad spectrum of clinical and histologic presentations were observed (Figures 1–5). Almost half of the patients demonstrated a psoriasiform and spongiotic dermatitis or an urticarial-type reaction on histology. Notably, three of the five patients with eruptions demonstrating psoriasiform features reported a prior history of lesions in keeping with possible psoriasis, but did not carry a formal diagnosis. Focal acantholytic dermatosis, i.e., Grover’s disease-like eruptions, were observed in three patients, all of whom also reported an associated pruritis. The age/sex of the patients, distribution of lesions, and temporal relationship to treatment initiation support that these were drug-related and not incidental, concurrent idiopathic presentations of Grover’s disease.30 Two patients, both with underlying NSCLC treated with combined anti-PD-1 and anti-CTLA-4, demonstrated a granulomatous dermatitis on histology; neither had a prior history of sarcoidosis. Three patients presented with direct immunofluorescence-confirmed BP, two of whom halted anti-PD-1/PD-L1 due to the eruption; the other patient was dose-delayed due to ongoing corticosteroid treatment for his BP, but achieved a complete tumor response during this time and did not restart anti-PD-1 therapy.
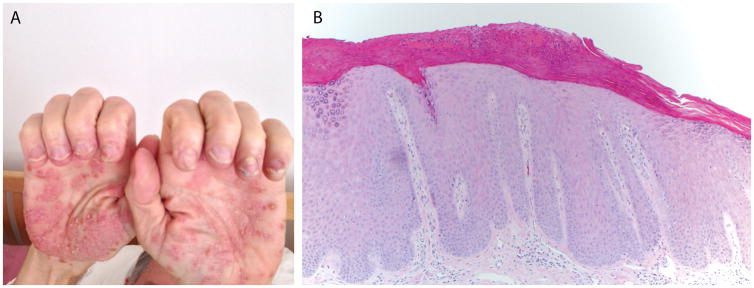
Figure 1.
Patient 1. (A) Well-demarcated scaly plaques with 1–2 mm isolated vesicles on the bilateral palms; (B) Psoriasiform epidermal acanthosis and hypogranulosis with overlying parakeratotic scale containing neutrophils (100× original magnification).
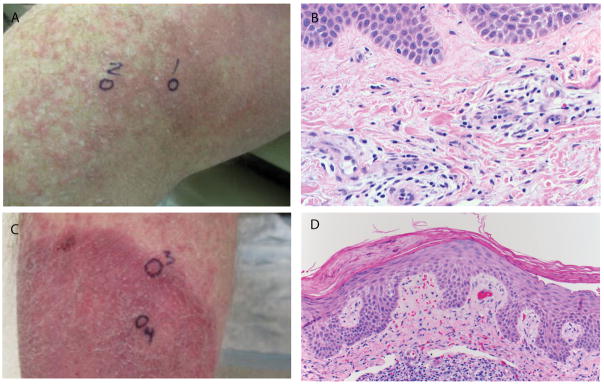
Figure 5.
Patient 12. (A) Urticarial plaques on medial thigh; (B) Superficial perivascular dermatitis including scattered eosinophils and rare neutrophils (400× original magnification); (C) Psoriasiform plaque on lower leg; (D) Psoriasiform and spongiotic dermatitis (200× original magnification). All photomicrographs are of skin biopsy specimens stained with hematoxylin and eosin (H&E).
Three patients required cessation of therapy due to the cutaneous irAE, 10 patients (63%) received systemic corticosteroids to control the eruption, and 9 patients (56%) had irAEs affecting other organ systems (see Table 1). The majority of patients were able to continue treatment and antitumor responses were favorable, with 75% (12/16) of patients demonstrating a partial or complete response and one additional patient demonstrating stable disease for greater than 6 months, with a median follow-up time of 30 months.
DISCUSSION
Immune checkpoint blockade agents are frequently associated with dermatologic irAEs31 demonstrating variable clinical and histologic morphologies (see Table 2 for biopsy confirmed non-lichenoid patterns, and Curry, et al.21 for a comprehensive literature review of studies reporting cutaneous irAE). Previous reports of cutaneous toxicities associated with checkpoint blockers have reported lichenoid patterns in 54–94% of biopsied cases (Supplemental Table 2). We found that 47% of biopsied eruptions encountered during routine service by the general surgical pathologists and dermatopathologists showed a lichenoid histology. Herein, we report the other histologic patterns observed, which accounted for the other half of biopsies received and includes a spectrum of non-lichenoid patterns.
Table 2.
Clinicopathologic studies of non-lichenoid eruptions following treatment with anti-PD-1
| Study | Cutaneous eruption | Tumor typea | Oncologic agenta | Clinical morphologya | Histologic patterna |
|---|---|---|---|---|---|
| Carlos et al.1 | Bullous pemphigoid | Melanoma | anti-PD-1 | Erythematous papules and plaques, few intact and ruptured vesicles and bullae | Subepidermal blisters with eosinophilic infiltration, +DIFb |
| Naidoo et al.2 | Bullous pemphigoid |
|
|
|
|
| Jour et al.3 | Bullous pemphigoid (patients 1–4) Bullous erythema multiforme (patient 5) |
|
|
|
|
| Ohtsuka et al.4 | Psoriasis | Melanoma | anti-PD-1 | Well-demarcated, scaly, erythematous plaques | Parakeratotic hyperkeratosis, irregular elongation of the rete ridges, dermal mononuclear cell infiltration |
| Totonchy et al.5 | Inverse psoriasiform eruption | Melanoma | anti-PD-1 | Vaginal and intergluteal pink-red plaque with erosions and yellow crusting | Psoriasiform epidermal hyperplasia, spongiosis, and predominantly lymphocytic inflammatory infiltrate with scattered eosinophils and dermal edema |
| Matsumura et al.6 | Psoriasis exacerbation | Melanoma | anti-PD-1 | Psoriatic plaques | Mild parakeratotic hyperkeratosis, regular acanthosis, mild telangiectasia in the papillary dermis, mild lymphocytic infiltrate |
| Kato et al.7 | Psoriasis exacerbation | Melanoma | anti-PD-1 | Sharply bordered, scaly, erythematous plaques | Perivascular lymphocytic infiltration, slight elongation of rete ridges, dilated vessels in papillary dermis, thinning of granular layer, neutrophils in cornified layer |
| Chia et al.8 | Psoriasis exacerbation | NSCLC | anti-PD-1 | Erythematous, scaly plaque-like lesions | Minor hyperkeratosis (after 2 months of topical steroids and daily phototherapy) |
| Murata et al.9 | Psoriasis | Melanoma | anti-PD-1 | Erythematous, pruritic and sharp-bordered plaques with silvery scale | Uniform elongation of the rete ridges, thinning of the suprapapillary plate with intermittent parakeratosis, hyperkeratosis, loss of the granular layer, neutrophil aggregation in the stratum corneum |
| Law-Ping-Man et al.10 | Psoriasis | NSCLC | anti-PD-1 | Erythematous and scaly lesions | Epidermal hyperplasia with parakeratotic hyperkeratosis, acanthosis, dilated superficial dermal capillaries, moderate lymphocytic infiltrate in upper dermis |
| Danlos et al.11 | Sarcoid-like granulomatous reaction | Melanoma | anti-PD-1 | Subcutaneous nodule | Dermal granulomatous infiltrate consistent with sarcoidosis |
| Suozzi et al.12 | Cutaneous sarcoidosis | Lung | anti-PD-1 + anti-CTLA-4 |
>50 skin-colored to pink firm papules coalescing into annular plaques, some umbilicated | Epithelioid granulomatous infiltrate |
| Nayar et al.13 | Toxic epidermal necrolysis | Melanoma | anti-PD-1 | Widespread maculopapular skin rash with bullae and areas of skin detachment | Interface dermatitis with lymphocytic infiltrate in the dermal-epidermal junction and apoptotic keratinocytes; complete necrosis of the epidermis with minimal infiltrate |
| Mutgi et al.14 | Pityriasis lichenoides chronica-like drug eruption | Melanoma | anti-PD-1 | Pruritic 3–4 mm red-brown thin papules with centrally adherent micaceous scale | Parakeratotic, spongiotic, and focally acanthotic epidermis with exocytosis of cytologically bland-appearing lymphocytes and rare neutrophils; focal basal layer vacuolar degeneration with interface and perivascular lymphocytic inflammation and extravasated red blood cells |
| Shi et al.15 | N/A | Lung | anti-PD-1 + erlotinib, then anti-PD-1 alone | Papular | Vacuolar interface dermatitis |
| Belum et al.16 | N/A |
|
anti-PD-1 (all patients) |
|
|
| Goldinger et al.17 | N/A | Melanoma | anti-PD-1 | Psoriasiform lesions | Two biopsies over time from the same patient: (1) Focal acanthosis and spongiosis and some apoptotic keratinocytes with lichenoid aspect; (2) lymphocytic infiltration of the adnexa |
Abbreviations:
CTLA-4, cytotoxic T lymphocyte associated antigen 4; DIF, direct immunofluorescence; HNSCC, head and neck squamous cell carcinoma; NSCLC, non-small cell lung carcinoma; PD-1, programmed cell death protein 1; PD-L1, PD1 ligand; SCLC, small cell lung carcinoma.
Legend:
Per patient
Immunofluorescence findings diagnostic for BP (linear deposition of IgG and C3 at the dermal-epidermal junction)
References
Carlos G, Anforth R, Chou S, Clements A, Fernandez-Penas P. A case of bullous pemphigoid in a patient with metastatic melanoma treated with pembrolizumab. Melanoma Res. 2015;25(3):265–268.
Naidoo J, Schindler K, Querfeld C, et al. Autoimmune Bullous Skin Disorders with Immune Checkpoint Inhibitors Targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4(5):383–389.
Jour G, Glitza IC, Ellis RM, et al. Autoimmune dermatologic toxicities from immune checkpoint blockade with anti-PD-1 antibody therapy: a report on bullous skin eruptions. J Cutan Pathol. 2016;43(8):688–696.
Ohtsuka M, Miura T, Mori T, Ishikawa M, Yamamoto T. Occurrence of Psoriasiform Eruption During Nivolumab Therapy for Primary Oral Mucosal Melanoma. JAMA Dermatol. 2015;151(7):797–799.
Totonchy MB, Ezaldein HH, Ko CJ, Choi JN. Inverse Psoriasiform Eruption During Pembrolizumab Therapy for Metastatic Melanoma. JAMA Dermatol. 2016;152(5):590–592.
Matsumura N, Ohtsuka M, Kikuchi N, Yamamoto T. Exacerbation of Psoriasis During Nivolumab Therapy for Metastatic Melanoma. Acta Derm Venereol. 2016;96(2):259–260.
Kato Y, Otsuka A, Miyachi Y, Kabashima K. Exacerbation of psoriasis vulgaris during nivolumab for oral mucosal melanoma. J Eur Acad Dermatol Venereol. 2015.
Chia PL, John T. Severe Psoriasis Flare After Anti-Programmed Death Ligand 1 (PD-L1) Therapy for Metastatic Non-Small Cell Lung Cancer (NSCLC). J Immunother. 2016;39(5):202–204.
Murata S, Kaneko S, Harada Y, Aoi N, Morita E. Case of de novo psoriasis possibly triggered by nivolumab. J Dermatol. 2016.
Law-Ping-Man S, Martin A, Briens E, Tisseau L, Safa G. Psoriasis and psoriatic arthritis induced by nivolumab in a patient with advanced lung cancer. Rheumatology (Oxford). 2016.
Danlos FX, Pages C, Baroudjian B, et al. Nivolumab-Induced Sarcoid-Like Granulomatous Reaction in a Patient With Advanced Melanoma. Chest. 2016;149(5):e133–136.
Suozzi KC, Stahl M, Ko CJ, et al. Immune-related sarcoidosis observed in combination ipilimumab and nivolumab therapy. JAAD Case Rep. 2016;2(3):264–268.
Nayar N, Briscoe K, Fernandez Penas P. Toxic Epidermal Necrolysis-like Reaction With Severe Satellite Cell Necrosis Associated With Nivolumab in a Patient With Ipilimumab Refractory Metastatic Melanoma. J Immunother. 2016;39(3):149–152.
Mutgi KA, Milhem M, Swick BL, Liu V. Pityriasis lichenoides chronica-like drug eruption developing during pembrolizumab treatment for metastatic melanoma. JAAD Case Rep. 2016;2(4):343–345.
Shi VJ, Rodic N, Gettinger S, et al. Clinical and Histologic Features of Lichenoid Mucocutaneous Eruptions Due to Anti-Programmed Cell Death 1 and Anti-Programmed Cell Death Ligand 1 Immunotherapy. JAMA Dermatol. 2016.
Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12–25.
Goldinger SM, Stieger P, Meier B, et al. Cytotoxic Cutaneous Adverse Drug Reactions during Anti-PD-1 Therapy. Clin Cancer Res. 2016;22(16):4023–4029.
For the non-lichenoid eruptions, the median time to onset was 10 days, but a subset of patients experienced a notably delayed onset. Among these were the patients with BP who presented between 2.8 and 11.4 months after starting treatment. This finding of delayed immunobullous eruptions is consistent with previous reports.12,15 Interestingly, our cohort also included patients with vitiligo, granulomatous dermatitis, and an urticarial-type reaction, which also occurred over two months after treatment initiation. Ten of 16 (63%) patients required systemic steroids (again including a number of patients without BP), which is a higher proportion than reported for biopsied lichenoid eruptions.24,25 Over half of patients experienced irAEs in other organ systems, including arthritis/arthralgia in four patients, endocrinopathies in four patients, and two patients each affected by colitis, pneumonitis, and LFT elevations. Collectively, the patterns of delayed onset, the need for oral corticosteroids in the majority of cases, and the systemic autoimmune effects (Table 1) observed in patients with non-lichenoid eruptions suggest a potential mechanism of systemic inflammation stimulated by immune checkpoint blockade that may persist long after the initial activation event and possibly mediating both sustained antitumor effect and both cutaneous and non-cutaneous irAEs.12
The occurrence of cutaneous eruptions from checkpoint agents have been associated with overall survival in patients with melanoma receiving anti-PD-13 and improved antitumor responses in non-small cell lung cancer.32 For lichenoid eruptions in particular, response rates as high as 69% have been reported.25 The 75% objective response rate described here thus confirms this previous finding and extends it to include the non-lichenoid pattern of irAEs. Our findings also indicate that the association between non-lichenoid irAEs and therapeutic clinical benefit includes patients with tumor types beyond melanoma and NSCLC.
Several clinical features favored the anti-PD-1/PD-L1 immunotherapy as the causative agent in these patients. In some cases, the cutaneous eruption was temporally very proximal to anti-PD-1 treatment initiation (patients 1, 5, 8, 10, 12, 14, 15); additionally, the dermatologic toxicity was seen to improve with anti-PD-1/PD-L1 treatment cessation and/or reappeared or worsened after each dose (patients 1, 4, 5, 11). Some patients had atypical eruptions that diverged from the classical clinical presentations. For example, BP is unusual in a 40-year-old patient such as patient 4 (BP typically affects those 70 years of age or older.33 In addition, the blisters on this patient’s skin improved but were persistent up to 4 months after discontinuation of the anti-PD-1 agent, which is consistent with a previous study of immunobullous eruptions associated with anti-PD-1 therapy and in contrast to other types of drug-related BP.12
A definitive understanding of the immune subsets and target antigens underlying irAEs, and the factors that differentiate irAEs in skin as compared to other organ systems, remains to be elucidated. Multiple T cell populations have been implicated in the pathogenesis of cutaneous irAEs, including CD4+ and CD8+ T cells,23,24 Th1/Th17 cells,7 and regulatory T cell depletion.34 By contrast, the development of BP may be due to antibody recognition of a common antigen displayed at both the basement membrane of the skin and on underlying tumor cells.12 The longer time to onset in these patients suggests that B-cell-mediated antibody generation is prolonged compared to a T-cell-mediated response. A distinct mechanism of action is suggested by the appearance of Grover’s disease-like eruptions, i.e., a disorder of keratinocyte proliferation due to paradoxical activation of the MAP kinase pathway.35 The variety of histologic patterns presented in our cohort underscores the potential complexity of the involved immune response, which may comprise both diverse T cell populations and antibody-mediated mechanisms of action as well as alternate signaling pathways.
In conclusion, the diverse clinical and histologic presentations and often delayed onset of irAEs in patients receiving anti-PD-1/PD-L1 checkpoint blockade highlight the importance of clinicopathologic correlation in attributing these varied histologic patterns to the use of checkpoint agents, as many of these non-lichenoid patterns may mimic their idiopathic counterparts. Surgical pathologists will undoubtedly receive an increasing number of biopsies from sites of irAEs as these immunoactive agents become standard of care in an increasing number of tumor types. In describing the diverse array of histologic patterns encountered at two academic institutions, we approach the prevalence of patterns that may be encountered in practice. Further study is warranted to more directly query the immune-mediated mechanisms that underlie diverse irAEs in patients undergoing treatment with immune checkpoint blockade.
Supplementary Material
Supplemental Figure 1. Lichenoid pattern.
Supplemental Table 1. Concurrent medications and peripheral eosinophil counts
Supplemental Table 2. Summary and literature review of biopsy-proven lichenoid dermatitis induced by treatment with anti-PD-1/PD-L1
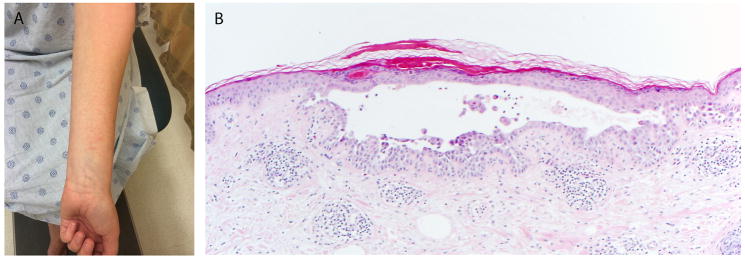
Figure 2.
Patient 10. (A) Multiple erythematous discrete 2–3 mm urticarial papules on distal forearm; (B) Focal acantholytic dyskeratosis, consistent with a Grover’s-like eruption (100×, original magnification).
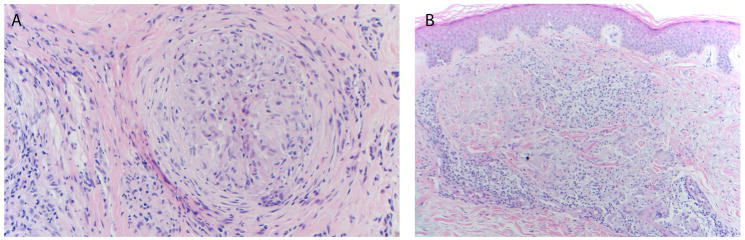
Figure 3.
Two patients (15 and 16) with underlying NSCLC developed granulomatous dermatitis after receiving combination anti-PD-1 and anti-CTLA-4. Histologic patterns were of (A) sarcoidal-type granulomas and (B) a granuloma-annulare pattern (200× and 100× original magnification, respectively).
Figure 4.
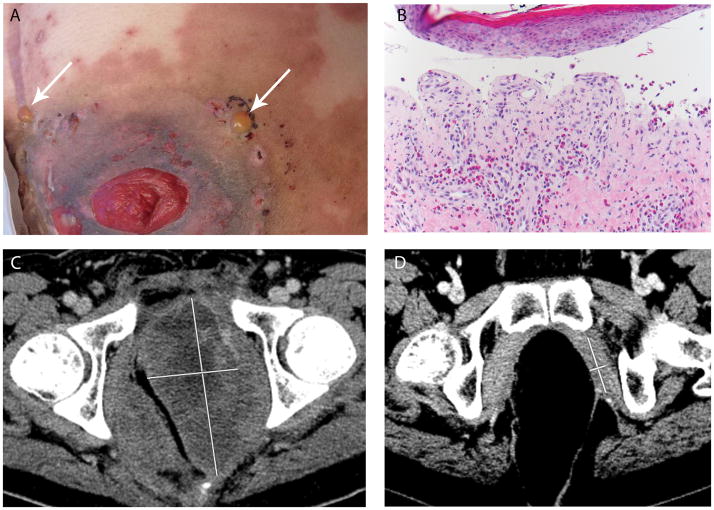
Patient 4. (A) Two tense bullae (white arrows) on urticarial base adjacent to ostomy site on lower abdomen; (B) Subepidermal bullous dermatosis with prominent eosinophils, consistent with bullous pemphigoid (200×, original magnification); (C) Pre-treatment computed tomographic (CT) scan showing 12 × 6 cm left pelvic tumor mass (early tumor regression was observed at the time of first post-treatment CT scan, 2 weeks after initiation of treatment); (D) An additional follow-up CT scan image 16.5 months after treatment initiation and approximately 5 months after bullous pemphigoid eruption, showing marked tumor regression (4.6 × 1.3 cm).
Acknowledgments
Financial support: This work was supported by the Dermatology Foundation (JMT), W.W. Smith Foundation (JMT), NIH R01 CA142779 (JMT), the Bloomberg~Kimmel Institute for Cancer Immunotherapy at Johns Hopkins, and a Memorial Sloan Kettering Core Grant (P30 CA008748) (HR, TJH, MEL, MDH). JMT was also supported by a Stand Up To Cancer—Cancer Research Institute Cancer Immunology Translational Research Grant (SU2C-AACR-DT 1012). Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research.
The authors thank Robert Egbers, MD, Grant Anhalt, MD, Timothy Wang, MD, Mary Sheu, MD, and Helena Pasieka, MD (Johns Hopkins University School of Medicine) for helpful discussions. This work was supported by the Dermatology Foundation (JMT), W.W. Smith Foundation (JMT), NIH R01 CA142779 (JMT), the Bloomberg~Kimmel Institute for Cancer Immunotherapy at Johns Hopkins, and a Memorial Sloan Kettering Core Grant (P30 CA008748) (HR, TJH, MEL, MDH). JMT was also supported by a Stand Up To Cancer—Cancer Research Institute Cancer Immunology Translational Research Grant (SU2C-AACR-DT 1012). Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research.
Footnotes
Conflict of interest disclosures: JMT has received research support from Bristol-Myers Squibb and is a consultant for Bristol-Myers Squibb, Merck, and AstraZeneca. JRB has received funding from Bristol-Myers Squibb and is an advisory board member (uncompensated). DTL has received research support from Merck, Bristol-Myers Squibb, and Aduro Biotech and speaking honoraria from Merck, and is a consultant for Merck. LAD is a founder of PapGene, Inc., Personal Genome Diagnostics (PGDx) and PagerBox and a consultant to Merck, Illumina, PGDx and Cell Design Labs. The first two of these companies, as well as other companies, have licensed technologies from Johns Hopkins University, on which LAD is an inventor. These licenses and relationships are associated with equity or royalty payments to LAD. The terms of these arrangements are being managed by Johns Hopkins University in accordance with its conflict of interest policies. CGD has received research funding from Bristol-Myers Squibb (IIoN grant), has consulted for Merck, Genentech, and MedImmune (AstraZeneca), and has licensed patents to MedImmune (AstraZeneca), Bristol-Myers Squibb, and Potenza Therapeutics. EJL has received research support from Genentech and is a consultant for Bristol-Myers Squibb, EMD Serono, Merck, and Novartis. MDH has received research funding from Bristol-Myers Squibb and Genentech, and has received consulting fees from Merck, Genentech, Bristol-Myers Squibb, AstraZeneca, Neon, and Inovio. For the remaining authors none were declared.
Institutional Review Board approval was obtained from Johns Hopkins Hospital and Memorial Sloan Kettering Cancer Center.
References
- 1.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin Cancer Res. 2016;22(4):886–894. doi: 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eigentler TK, Hassel JC, Berking C, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev. 2016;45:7–18. doi: 10.1016/j.ctrv.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohtsuka M, Miura T, Mori T, Ishikawa M, Yamamoto T. Occurrence of Psoriasiform Eruption During Nivolumab Therapy for Primary Oral Mucosal Melanoma. JAMA Dermatol. 2015;151(7):797–799. doi: 10.1001/jamadermatol.2015.0249. [DOI] [PubMed] [Google Scholar]
- 8.Kato Y, Otsuka A, Miyachi Y, Kabashima K. Exacerbation of psoriasis vulgaris during nivolumab for oral mucosal melanoma. J Eur Acad Dermatol Venereol. 2015 doi: 10.1111/jdv.13336. [DOI] [PubMed] [Google Scholar]
- 9.Law-Ping-Man S, Martin A, Briens E, Tisseau L, Safa G. Psoriasis and psoriatic arthritis induced by nivolumab in a patient with advanced lung cancer. Rheumatology (Oxford) 2016 doi: 10.1093/rheumatology/kew281. [DOI] [PubMed] [Google Scholar]
- 10.Matsumura N, Ohtsuka M, Kikuchi N, Yamamoto T. Exacerbation of Psoriasis During Nivolumab Therapy for Metastatic Melanoma. Acta Derm Venereol. 2016;96(2):259–260. doi: 10.2340/00015555-2212. [DOI] [PubMed] [Google Scholar]
- 11.Murata S, Kaneko S, Harada Y, Aoi N, Morita E. Case of de novo psoriasis possibly triggered by nivolumab. J Dermatol. 2016 doi: 10.1111/1346-8138.13450. [DOI] [PubMed] [Google Scholar]
- 12.Naidoo J, Schindler K, Querfeld C, et al. Autoimmune Bullous Skin Disorders with Immune Checkpoint Inhibitors Targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4(5):383–389. doi: 10.1158/2326-6066.CIR-15-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlos G, Anforth R, Chou S, Clements A, Fernandez-Penas P. A case of bullous pemphigoid in a patient with metastatic melanoma treated with pembrolizumab. Melanoma Res. 2015;25(3):265–268. doi: 10.1097/CMR.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 14.Damsky W, Kole L, Tomayko MM. Development of bullous pemphigoid during nivolumab therapy. JAAD Case Rep. 2016;2(6):442–444. doi: 10.1016/j.jdcr.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang SJ, Carlos G, Chou S, Wakade D, Carlino MS, Fernandez-Penas P. Bullous pemphigoid, an autoantibody-mediated disease, is a novel immune-related adverse event in patients treated with anti-programmed cell death 1 antibodies. Melanoma Res. 2016;26(4):413–416. doi: 10.1097/CMR.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 16.Jour G, Glitza IC, Ellis RM, et al. Autoimmune dermatologic toxicities from immune checkpoint blockade with anti-PD-1 antibody therapy: a report on bullous skin eruptions. J Cutan Pathol. 2016;43(8):688–696. doi: 10.1111/cup.12717. [DOI] [PubMed] [Google Scholar]
- 17.Danlos FX, Pages C, Baroudjian B, et al. Nivolumab-Induced Sarcoid-Like Granulomatous Reaction in a Patient With Advanced Melanoma. Chest. 2016;149(5):e133–136. doi: 10.1016/j.chest.2015.10.082. [DOI] [PubMed] [Google Scholar]
- 18.Reuss JE, Kunk PR, Stowman AM, Gru AA, Slingluff CL, Jr, Gaughan EM. Sarcoidosis in the setting of combination ipilimumab and nivolumab immunotherapy: a case report & review of the literature. J Immunother Cancer. 2016;4:94. doi: 10.1186/s40425-016-0199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suozzi KC, Stahl M, Ko CJ, et al. Immune-related sarcoidosis observed in combination ipilimumab and nivolumab therapy. JAAD Case Rep. 2016;2(3):264–268. doi: 10.1016/j.jdcr.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nayar N, Briscoe K, Fernandez Penas P. Toxic Epidermal Necrolysis-like Reaction With Severe Satellite Cell Necrosis Associated With Nivolumab in a Patient With Ipilimumab Refractory Metastatic Melanoma. J Immunother. 2016;39(3):149–152. doi: 10.1097/CJI.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 21.Curry JL, Tetzlaff MT, Nagarajan P, et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol. 2017;44(2):158–176. doi: 10.1111/cup.12858. [DOI] [PubMed] [Google Scholar]
- 22.Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: A single-institution cohort. J Am Acad Dermatol. 2016;74(3):455–461. e451. doi: 10.1016/j.jaad.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 23.Joseph RW, Cappel M, Goedjen B, et al. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-1 therapy. Cancer Immunol Res. 2015;3(1):18–22. doi: 10.1158/2326-6066.CIR-14-0134. [DOI] [PubMed] [Google Scholar]
- 24.Schaberg KB, Novoa RA, Wakelee HA, et al. Immunohistochemical analysis of lichenoid reactions in patients treated with anti-PD-L1 and anti-PD-1 therapy. J Cutan Pathol. 2016;43(4):339–346. doi: 10.1111/cup.12666. [DOI] [PubMed] [Google Scholar]
- 25.Shi VJ, Rodic N, Gettinger S, et al. Clinical and Histologic Features of Lichenoid Mucocutaneous Eruptions Due to Anti-Programmed Cell Death 1 and Anti-Programmed Cell Death Ligand 1 Immunotherapy. JAMA Dermatol. 2016 doi: 10.1001/jamadermatol.2016.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12–25. doi: 10.1016/j.ejca.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldinger SM, Stieger P, Meier B, et al. Cytotoxic Cutaneous Adverse Drug Reactions during Anti-PD-1 Therapy. Clin Cancer Res. 2016;22(16):4023–4029. doi: 10.1158/1078-0432.CCR-15-2872. [DOI] [PubMed] [Google Scholar]
- 28.Chou S, Hwang SJ, Carlos G, Wakade D, Fernandez-Penas P. Histologic Assessment of Lichenoid Dermatitis Observed in Patients With Advanced Malignancies on Antiprogramed Cell Death-1 (anti-PD-1) Therapy With or Without Ipilimumab. Am J Dermatopathol. 2017;39(1):23–27. doi: 10.1097/DAD.0000000000000587. [DOI] [PubMed] [Google Scholar]
- 29.Tetzlaff MT, Nagarajan P, Chon S, et al. Lichenoid Dermatologic Toxicity From Immune Checkpoint Blockade Therapy: A Detailed Examination of the Clinicopathologic Features. Am J Dermatopathol. 2017;39(2):121–129. doi: 10.1097/DAD.0000000000000688. [DOI] [PubMed] [Google Scholar]
- 30.Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis) Arch Pathol Lab Med. 2009;133(9):1490–1494. doi: 10.5858/133.9.1490. [DOI] [PubMed] [Google Scholar]
- 31.Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasan Ali O, Diem S, Markert E, et al. Characterization of nivolumab-associated skin reactions in patients with metastatic non-small cell lung cancer. Oncoimmunology. 2016;5(11):e1231292. doi: 10.1080/2162402X.2016.1231292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cozzani E, Gasparini G, Burlando M, Drago F, Parodi A. Atypical presentations of bullous pemphigoid: Clinical and immunopathological aspects. Autoimmun Rev. 2015;14(5):438–445. doi: 10.1016/j.autrev.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Blake SJ, Harjunpaa H, et al. Assessing Immune-Related Adverse Events of Efficacious Combination Immunotherapies in Preclinical Models of Cancer. Cancer Res. 2016;76(18):5288–5301. doi: 10.1158/0008-5472.CAN-16-0194. [DOI] [PubMed] [Google Scholar]
- 35.Anforth RM, Blumetti TC, Kefford RF, et al. Cutaneous manifestations of dabrafenib (GSK2118436): a selective inhibitor of mutant BRAF in patients with metastatic melanoma. Br J Dermatol. 2012;167(5):1153–1160. doi: 10.1111/j.1365-2133.2012.11155.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Lichenoid pattern.
Supplemental Table 1. Concurrent medications and peripheral eosinophil counts
Supplemental Table 2. Summary and literature review of biopsy-proven lichenoid dermatitis induced by treatment with anti-PD-1/PD-L1