Neutrophils have a short circulating life-span (~18.5 hours) and die as they age [1]. This type of cell death is called “spontaneous” death, and it is critical to maintaining homeostasis. Apoptosis has long been considered the primary mechanism of cell death for aging neutrophils, but inhibition of caspases, which are critical mediators of apoptosis, delay but do not completely abolish cell death, suggesting that other mechanisms of neutrophil spontaneous death exist [2, 3]. There is currently no direct evidence implicating other types of cell death in neutrophil spontaneous death, and the precise molecular mechanisms of this type of cell death remain unknown. Here we discuss our recent observation that neutrophil spontaneous death is heterogeneous and includes other types of cell death in addition to apoptosis.
Does neutrophil spontaneous death equal apoptosis?
Apoptosis is known to be a major cause of neutrophil spontaneous death, since aging neutrophils show typical apoptotic morphology including cell body shrinkage, DNA fragmentation, chromatin condensation, and cytoplasmic vacuolation [4–6]. The apoptotic characteristics of aging neutrophils are often investigated by flow cytometry with anti-annexin V antibodies, which bind to phosphatidylserine on apoptotic cells [7] and together with the membrane-impermeable dye propidium iodide (PI) form the gold standard evaluation of neutrophil death. We regularly use this protocol to analyze neutrophil spontaneous death but, when capturing time-lapse video of spontaneous death with the same staining reagents, we noticed that the time-lapse analysis did not correlate with our flow cytometry results.
In our FACS analyses, about 30–50% of human neutrophils were annexin V/PI double-negative (healthy), 40–60% annexin V single-positive (early apoptosis), 5–10% annexin V/PI double-positive (late apoptosis), and only a small fraction PI single-positive (necrosis) at 24 hours [2]. However, by microscopy, we could see remarkably heterogeneous neutrophil spontaneous death (Fig. 1A and Movie 1). The results were unexpected: there were many PI single-positive cells (10% in total), which are usually infrequent by flow cytometry (Fig. 1B). Approximately 33.7% of neutrophils were visibly annexin V/PI double-positive at 24 hours, significantly higher than the proportion generally seen by flow cytometory. Intriguingly, most neutrophils with enlarged and swollen morphology (herein described as “puffed” cells) appeared to be the predominant population of dead cells, consisting of about 35% of total at 24 hours.
Figure 1. Heterogeneity of neutrophil spontaneous death.
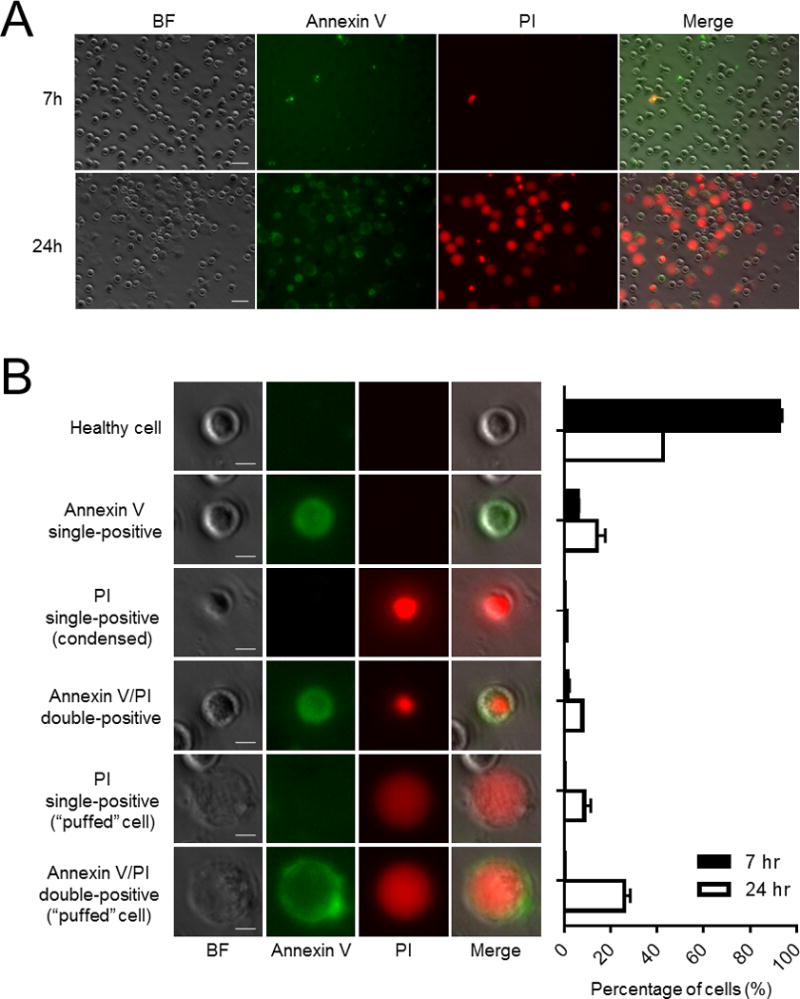
Freshly isolated human neutrophils were cultured in 1 ml RPMI 1640 supplemented with CaCl2 (2 mM), HEPES (20 mM), 20% heat inactivated low endotoxin FBS and 1% Penicillin/Streptomycin at density of 5 × 105 cells/ml at 37°C in high humidity. A non-treated 24-well plate was used for cell culturing. Cells were stained with Annexin V-FITC (BD Pharmigen) (10 μl) and propidium iodide (PI) (AnaSpec.Inc) (200 ng/ml). (A) Neutrophil morphology at 7 hr and 24 hr. Bars indicate 20 μm. (B) Classification of cell types based on Annexin V/PI staining and morphology. Bar graphs show percentage of each cell types at 7 hr and 24 hr. Bars indicate 5 μm.
We observed two types of “puffed” cells, namely PI single-positive cells and annexin V/PI double-positive cells. No annexin V single-positive “puffed” cells were seen on time-lapse microscopy, and all “puffed” cells were PI positive, suggesting that the membrane integrity was disrupted in these “puffed” cells. The size of “puffed” cells was significantly larger (15–20 um) than average neutrophil size (around 8–10 um). Based on this morphology, the “puffed” cells were clearly distinct from apoptotic neutrophils.
We next considered whether these were necrotic neutrophils. Apoptotic neutrophils are engulfed by macrophages and cleared by efferocytosis in vivo [4, 8]. If apoptotic neutrophils are not sufficiently eliminated, neutrophils undergo secondary necrosis that can produce unwanted inflammation and tissue damage. Unlike apoptotic neutrophils, significant numbers of the “puffed” cells became PI single-positive (annexin V negative, thus bypassing apoptotic pathways). They are therefore highly unlikely to be generated by secondary necrosis. Consistently, while necrosis can occur when microorganisms stimulate neutrophils, it is not considered a major cause of neutrophil spontaneous death [9].
Some activated neutrophils undergo lytic cell death, known as NETosis. NETosis is stepwise process of signal transduction, chromatin decondensation, membrane rupture, and release of chromatin together with granule proteins [10]. Although it is possible that the “puffed” cells may contain decondensed chromatin, they did not release DNA fibers into the extracellular space. Moreover, neutrophils need to be activated by specific stimuli to induce NETosis, whereas the “puffed” cells were spontaneously generated during cell culture. Thus, the “puffed” cells were not the products of NETosis-mediated cell death. Taken together, the “puffed” cells are undefined dead neutrophils that appear to undergo cell death via an unknown and distinct mechanism. Further studies are needed to understand how these “puffed” cells are generated.
“Puffed” cells are easily destroyed by physical stimuli
There has traditionally been a consensus that neutrophil spontaneous death primarily occurs via apoptosis. Nevertheless, we did observe high numbers of PI single-positive and annexin V/PI double-positive “puffed” cells under the microscope. Why have we hitherto missed these numerous “puffed” cells?
We found that physical stimuli such as pipetting efficiently fragmented the “puffed” cells, probably due to their fragile membrane structures. Ten up and down pipetting actions, usually a necessary part of flow cytometry protocols, almost completely eliminated both PI single-positive and annexin V/PI double-positive “puffed” cells (Fig. 2A). This changed the composition of the cell population at 24 hours and allowed the annexin V single-positive cells to dominate, consistent with typical flow cytometry results for aging neutrophils. Thus, we may well have ignored the dead “puffed” cells when analyzing neutrophil death by flow cytometry and in doing so misread the true ratio of dead to healthy cells. Some protocols fix neutrophils prior to flow cytometry, which could stabilize and protect the “puffed” cells from physical damage. To test this possibility, we fixed the neutrophils in 4% paraformaldehyde prior to pipetting them up and down ten times and checking the remaining cells by light microscopy. As shown in Fig. 2B, most of the “puffed” cells fragmented and disappeared by pipetting, indicating that fixation did not prevent damage to these cells. These results demonstrate that there is a progressive increase in fragile “puffed” cells and apoptotic cells during the course of neutrophil aging, generating a heterogeneous aging population. This should be taken into account when working on neutrophils in vitro.
Figure 2. “Puffed” neutrophils are easily destroyed by physical stimuli.
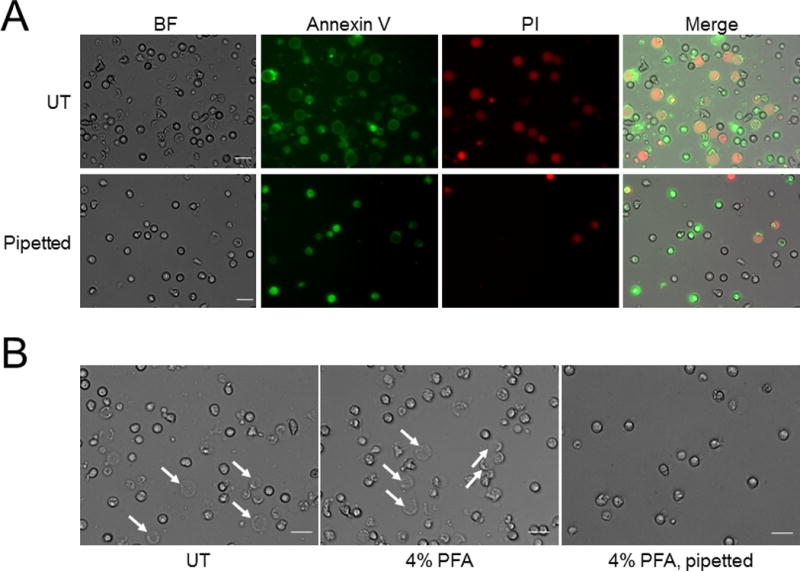
Freshly isolated human neutrophils were cultured as described in Figure 1. (A) After staining with Annexin V-FITC and PI, cells were gently pipetted up and down using P1000 tip 10 times. Bars indicate 20 μm. (B) After culturing, cells were fixed with 4% Paraformaldehyde (PFA) for 30 min at room temperature, then gently pipetted up and down using P1000 tip 10 times. Arrows indicate “puffed” cells. Bars indicate 20 μm.
In summary, here we unexpectedly found a population of “puffed” cells as neutrophils aged. Neutrophil spontaneous death consists not of only apoptosis but rather a heterogeneous collection of different types of cell death. “Puffed” neutrophils are easily fragmented by physical stimuli (e.g., pipetting) and therefore may have hitherto been neglected when analyzing neutrophil populations by flow cytometry or other methods that require pippeting. It will be interesting to examine the physiological roles of these “puffed” cells and whether they are involved in inflammatory diseases.
Supplementary Material
Supplemental Movie 1. Time-lapse images of human neutrophils stained with Annexin V and PI. Freshly isolated human neutrophils were cultured as described in Figure 1. Images were taken every 20 min for 17 hr.
Acknowledgments
The authors thank Leslie Silberstein, John Manis, and Li Chai for helpful discussions. Yan Teng is sponsored by China Scholarship Council (201606240106). H. Luo is supported by National Institutes of Health grants (R01AI103142, R01HL092020, and P01 HL095489) and a grant from FAMRI (CIA 123008).
Footnotes
Author Contributions
Y. Teng and H. Kambara designed and carried out experiments, analyzed data and prepared manuscript. H. Luo designed experiments, analyzed data, and wrote paper.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Lahoz-Beneytez J, Elemans M, Zhang Y, Ahmed R, Salam A, Block M, et al. Human neutrophil kinetics: modeling of stable isotope labeling data supports short blood neutrophil half-lives. Blood. 2016;127:3431–3438. doi: 10.1182/blood-2016-03-700336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loison F, Zhu H, Karatepe K, Kasorn A, Liu P, Ye K, et al. Proteinase 3-dependent caspase-3 cleavage modulates neutrophil death and inflammation. J Clin Invest. 2014;124:4445–4458. doi: 10.1172/JCI76246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo HR, Loison F. Constitutive neutrophil apoptosis: mechanisms and regulation. Am J Hematol. 2008;83:288–295. doi: 10.1002/ajh.21078. [DOI] [PubMed] [Google Scholar]
- 4.Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payne CM, Glasser L, Tischler ME, Wyckoff D, Cromey D, Fiederlein R, et al. Programmed cell death of the normal human neutrophil: an in vitro model of senescence. Microsc Res Tech. 1994;28:327–344. doi: 10.1002/jemt.1070280408. [DOI] [PubMed] [Google Scholar]
- 6.Guejes L, Zurgil N, Deutsch M, Gilburd B, Shoenfeld Y. The influence of different cultivating conditions on polymorphonuclear leukocyte apoptotic processes in vitro, I: the morphological characteristics of PMN spontaneous apoptosis. Ultrastruct Pathol. 2003;27:23–32. doi: 10.1080/01913120309947. [DOI] [PubMed] [Google Scholar]
- 7.Homburg CH, de Haas M, von dem Borne AE, Verhoeven AJ, Reutelingsperger CP, Roos D. Human neutrophils lose their surface Fc gamma RIII and acquire Annexin V binding sites during apoptosis in vitro. Blood. 1995;85:532–540. [PubMed] [Google Scholar]
- 8.Greenlee-Wacker MC. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol Rev. 2016;273:357–370. doi: 10.1111/imr.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iba T, Hashiguchi N, Nagaoka I, Tabe Y, Murai M. Neutrophil cell death in response to infection and its relation to coagulation. J Intensive Care. 2013;1:13. doi: 10.1186/2052-0492-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol. 2012;198:773–783. doi: 10.1083/jcb.201203170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Movie 1. Time-lapse images of human neutrophils stained with Annexin V and PI. Freshly isolated human neutrophils were cultured as described in Figure 1. Images were taken every 20 min for 17 hr.


