Abstract
Objectives
The hypothesis of this study was that there are multiple factors that are dominant in causing external apical root resorption (EARR). The objective of this investigation was to better understand the clinical factors that may lead to EARR.
Material and Methods
Maxillary cone-beam computed tomography (CBCT) scans of 18 subjects who were treated with bilateral canine retractions during orthodontics were used to calculate EARR. The subjects were treated using well-calibrated segmental T-loops for delivering a 124 cN retraction force and the moment-to-force ratio suitable for moving the canine under either translation or controlled tipping. The subjects’ age, gender, treatment duration, and genotypes were collected.
Results
Six subjects out of eighteen showed definite EARR, meaning that load was not the only causing factor. All five subjects with the genotype identified had G/G genotype of IL-1β rs11143634, indicating people with this genotype may be at high risk. Longer treatment duration, female, and older age may also contribute to EARR although the findings were not statistically significant.
Conclusion
EARR appears to be related to multiple factors. The orthodontic load and the genotype should be the focuses for future studies.
Introduction
External apical root resorption (EARR) is a side effect that occurs during orthodontic treatments. It is characterized by root shortening or shrinking.1 EARR occurs only in certain patients. It is very important to identify the dominant factors causing EARR so that clinicians may adjust treatment to prevent it. EARR is a multifactorial issue1. Level of orthodontic load sensed by the tooth2–5, treatment type1, 6–14, duration1, 2, 10, 15–27, genotype1, 28–33, and age1, 16, 23, 34–37 are considered as the potential contributing factors. Common consensus is that the elevated stress due to the orthodontic load on a tooth, especially in the periodontal ligament (PDL), causes EARR38; longer treatment also increases the chance of EARR1, 2, 10, 15–27. Al-Qawasmi et al.28 found that the Interleukin IL-1β gene contributes to EARR. Previous studies also indicated that older people are vulnerable1, 39. Most systematic studies were on animals. Clinical data are critical to validate the findings. The major obstacles to validate these clinically had been the ability to control the orthodontic loading and to reliably assess the EARR.
Clinical orthodontic load systems reported in literatures were normally qualitative. Typically, the loads provided by the actuators (like segmental wire or spring) were reported, which were not the load sensed by the tooth. The load on a tooth is difficult to quantify in-vivo. Root length change had been widely reported as evidence of EARR. 2D images were primarily used for measuring root shortening. Errors due to difficulty to align the images taken at different time points could have occurred.40, 41 Studies on premolar extraction cases proved the existence of EARR. 2–4 Limited information is available for understanding clinical effects of orthodontic treatment on EARR. A clinical study with well-controlled orthodontic load and reliable tooth length and volume measurement will help understand the dominant factors to EARR.
The hypothesis of this study was that there are multiple factors that are dominant in causing EARR. The objective of this study was to investigate EARR associated with well-controlled canine retraction treatment. The evaluated factors include treatment strategies, level of orthodontic force, the genotype, the age, the gender, and treatment duration.
Materials and Methods
Eighteen subjects (7 males and 11 females) who needed bilateral maxillary canine retractions were involved in this study. The study was approved by the Institutional Review Board. The inclusion criteria included necessity of extraction of both maxillary 1st premolars and maxillary canine retraction as a part of the orthodontic treatment. The average age of subjects was 19±9 years old. The age ranged from 12 to 47 years old. One of the subjects was 47 years old, one was 35 years old, and the other sixteen subjects were between 12 to 22 years old. The subjects went through the canine retractions. The cone-beam computed tomography (CBCT) scans of the 18 subjects were used for assessing EARRs.
Prior to the study, the right and left 1st premolars were extracted and the upper dental arch was leveled and aligned with 0.019×0.025-inch stainless steel archwire engaged in .022×.028-inch slot brackets. The maxillary second molars were included in the archwire and were co-ligated to the maxillary second premolar and first molar with a .010 stainless steel wire on each side, which served as anchorage. The bilateral first molars were connected with a transpalatal arch for anchorage reinforcement. Segmental T-loops designed for providing 124 cN initial retraction force and the desired moment-to-force ratios (M/Fs) were attached to the corresponding first molar and the canine by clinicians. The M/F, which was based on the location of the center of resistance obtained by using finite element (FE) method, was accomplished by adjusting the gable bends. The controlled tipping (CT) load had relatively lower M/F than translation (TR). The load system delivered was quantified by an orthodontic force tester. Details of the experimental protocol, the specially designed T-loops, and the repeatability testing results were reported previously.42 For each subject, customized segmental T-loops were randomly assigned to the right or left canines to implement either TR or CT force. The treatment period varied depending on the size of initial space, appointment, and inter-subject variations. The average was 4.9 months. The age and treatment duration for each subject were recorded. To maintain the desired force and M/F, the T-loops were replaced with new loops when a canine displaced >1 mm.
All CBCT scans were performed on the same i-CAT Imaging System (Imaging Sciences i-CAT) of the Indiana University School of Dentistry. The voxel size was 0.25 mm and the scan time was 26.9 seconds. The scans of each subject were taken immediately before and after the canine retraction. The same setting was used for all the scans.
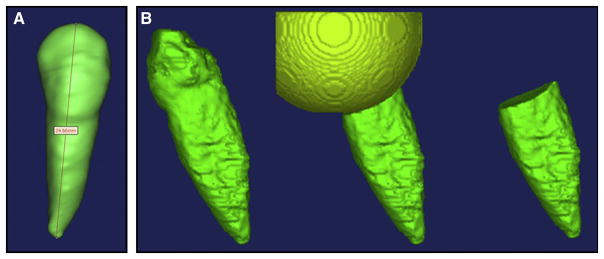
The EARR was quantified by using the CBCT scans obtained immediately before and after canine retraction. The CBCT images were processed with MIMICS 13.0 (Materialise, Belgium). The canine was segmented first, and the tooth length was easily measured by using the 3D length measuring function in MIMICS. The tooth length was defined as the distance between crown tip and root tip. (See in Figure 1a) The length reduction measured from the pre- and post-treatment scans was used to quantify EARR.
Figure 1.
(a) Tooth length measurement by using MIMICS (b) Root volume was measured by the tooth volume minus the crown volume enclosed in the sphere with 10 mm diameter
The metal bracket causes reflection blur at the crown area in the CBCT images, which leads to unreliable contour recognition. The crown portion was removed because the EARR primarily occurs at the apical portion of the root. To make a consistent cut for all teeth, a sphere with 10 mm diameter and centered at the crown tip was created, and then the sphere part including the entire crown was cut from the tooth. The volume of the remaining part of the tooth was considered as the root volume. (See Figure 1b)
If the difference in tooth length between pre- and post-treatment canine CBCTs was > 0.5mm, this was considered EARR. A difference less than 0.5 mm was considered uncertain because of the 0.25 mm voxel size of the CBCT image, thus was not counted as EARR. For volume change, because the average volume of the layer of the root surface with one voxel thickness was 73±11 mm3, only the volume change larger than that were determined as definite volume change.
The maxillary canines and incisors were evaluated for root resorptions. During the canine retraction using the segmental T-loops, only the canines were under orthodontic force, but the incisors were not. Therefore, it was expected that there should be no root resorption on the incisors.
Subjects’ saliva samples were collected using the DNA Collection Kit (OG-100, DNA Genotek, Canada) and genomic DNA from saliva was extracted using Oragnene Purifier (OG-L2P, DNA Genotek, Canada and quantified spectrophotometrically using the default OD260nm absorbance algorithm, then stored at −20°C until use. Automated polymerserase chain reaction (PCR) were performed on PTC-100 Programmable Thermal Controller (MJ Research, Canada) and allelic discrimination were done using the 7300 Sequence Detection System (Applied Biosystem, Foster City, CA), TaqMan® polymerase probes and primers using the method provided by DNA Genotek (TaqMan® SNP Genotyping Assays Protocol). Rs1143643 (Applied Biosystems TaqMan® C_1839949_10), rs1143634 (Applied Biosystems TaqMan® C_9546517_10), rs1143629 (Applied Biosystems TaqMan® C_1839945_1_) for IL-1β, and rs1794065 (Applied Biosystems TaqMan® C_3133518_10), rs315952 (Applied Biosystems TaqMan® C_1151247_10), and rs315951 (Applied Biosystems TaqMan® C_948691_1_) for IL-1RA were genotyped.
Associations of gender and of the distribution of each genotype with EARR were analyzed using Mantel-Haenszel chi-square tests and age and treatment duration were evaluated using two-sample t-tests.
Results
Some of the maxillary canines showed definite EARR while all incisors evaluated had no definite EARR. Table 1 shows the calculated root length and volume changes of the canine on the TR and CT sides as well as the apical tooth displacements43 as functions of subject’s gender, age, treatment duration, and genotype. The incisor root shortenings were all less than 0.5 mm, thus were not included in Table 1. The apical tooth displacements were reported previously.43 The root reduction equal to or greater than 0.5 mm was marked red, which were considered as the definite EARR. The volume changes were less than 75 mm3, the resolution due to the voxel size of the CBCT images, thus were considered as non-definite. The p-values of these factors are shown in Tables 2 and 3.
Table 1.
Results from the eighteen patients, including age, gender, treatment duration, EARR on the CT and TR sides, volume change on the CT and TR sides, patient genotypes, and apical root displacement. The subjects who have definite EARR are highlighted in yellow. The length change exceeding 0.5 mm are highlighted in red. Negative length values indicate root shortening. CT-controlled tipping side; TR-translation side; RR-root resorption, RRV-root volume change
| Age | Gender | Duration (D) | Length Change | Volume Change | Subject Genotype | Apical Movements Intrusion (+) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR_CT | RR_TR | RRV_TR | RRV_CT | IL-1 β rs1143629 |
IL-1 β rs1143634 |
IL-1 β rs1143643 |
IL-1 RA rs315951 |
IL-1 RA rs315952 |
IL-1 RA rs1794065 |
||||||
| (mm) | (mm) | (mm3) | (mm3) | CT(mm) | TR(mm) | ||||||||||
| P01 | 20 | F | 292 | −0.9 | −0.6 | −17 | −51 | 0.73 | 0.35 | ||||||
| P02 | 18 | F | 176 | −0.1 | −0.2 | 26 | 3 | A/A | G/A | C/C | C/C | T/T | A/G | −0.16 | −0.17 |
| P03 | 22 | F | 175 | −0.3 | 0.3 | −6 | −11 | G/G | G/A | C/C | C/C | T/T | G/G | −0.28 | −0.73 |
| P04 | 15 | M | 203 | −0.5 | −0.7 | −23 | −11 | A/G | G/G | C/T | C/C | T/T | A/A | −0.35 | −1.03 |
| P05 | 47 | F | 132 | −0.3 | −0.7 | −15 | −25 | G/G | G/G | C/C | C/C | T/T | A/G | 0.07 | −0.41 |
| P06 | 15 | M | 184 | 0.3 | 0.1 | −1 | 5 | A/A | G/A | C/T | C/C | T/T | G/G | 0.11 | −0.04 |
| P07 | 35 | F | 357 | 0.3 | −0.5 | −3 | 5 | A/G | G/G | C/C | C/G | C/T | G/G | 1.03 | −0.06 |
| P08 | 14 | F | 196 | −0.3 | 0.1 | 10 | 12 | A/G | G/A | C/C | C/G | C/T | G/G | −1.18 | −1.84 |
| P09 | 18 | M | 98 | 0.1 | 0.4 | −24 | −12 | A/G | G/A | C/T | C/G | C/T | A/G | −0.86 | −0.42 |
| P10 | 17 | M | 68 | −0.2 | −0.3 | 23 | 27 | −0.57 | 0.16 | ||||||
| P11 | 16 | F | 120 | −0.3 | −0.2 | −2 | −17 | −0.06 | −0.62 | ||||||
| P12 | 19 | F | 107 | −0.3 | −0.5 | −23 | −1 | A/G | G/G | C/T | C/G | C/T | G/G | −2.38 | −3.53 |
| P13 | 14 | F | 58 | 0.3 | 0.4 | 6 | 5 | A/G | G/G | C/T | C/C | T/T | A/A | 0.00 | 0.35 |
| P14 | 15 | M | 98 | −0.1 | −0.3 | −2 | 9 | −0.76 | −1.00 | ||||||
| P15 | 12 | F | 134 | −2 | −2.1 | −12 | −58 | A/A | G/G | C/C | G/G | C/C | G/G | 1.70 | −2.12 |
| P16 | 15 | M | 62 | −0.2 | 0 | 54 | 55 | A/G | G/A | C/C | C/G | C/T | G/G | 0.03 | 0.30 |
| P17 | 14 | M | 65 | 0.1 | 0 | 15 | 4 | A/A | G/G | C/T | C/C | T/T | G/G | −0.82 | −0.63 |
| P18 | 22 | F | 99 | 0.2 | 0.3 | 12 | 9 | A/G | G/G | C/T | C/C | T/T | A/G | 1.43 | −0.34 |
Table 2.
Genotype distribution and allele frequencies for IL 1β gene cluster SNPs (rs1143629, rs1143634, and rs1143643) and IL-1RA gene cluster SNPs (rs315951, rs315952, and rs1794065) in patients with high and low levels of EARR
|
|
||||||
|---|---|---|---|---|---|---|
| Group | Genotype, n | Allele, n | ||||
|
| ||||||
| AA | AG | GG | A | G | ||
| IL-1β rs1143629 (p=0.460) | High EARR (n=5) | 1 (20.0%) | 3 (60.0%) | 1 (20.0%) | 5 (50.0%) | 5 (50.0%) |
| Low EARR (n=12) | 4 (33.3%) | 7 (58.3%) | 1 (8.3%) | 15 (62.5%) | 9 (37.5%) | |
|
| ||||||
| GG | GA | AA | G | A | ||
|
|
||||||
| IL-1β rs1143634 (p=0.031) | High EARR (n=5) | 5 (100.0%) | 0 (0.0%) | 0 (0.0%) | 10 (100.0%) | 0 (0.0%) |
| Low EARR (n=12) | 5 (41.7%) | 7 (58.3%) | 0 (0.0%) | 17 (70.8%) | 7 (29.2%) | |
|
| ||||||
| CC | CT | TT | C | T | ||
|
|
||||||
| IL-1β rs1143643 (p=0.503) | High EARR (n=5) | 3 (60.0%) | 2 (40.0%) | 0 (0.0%) | 8 (80.0%) | 2 (20.0%) |
| Low EARR (n=12) | 5 (41.7%) | 7 (58.3%) | 0 (0.0%) | 17 (70.8%) | 7 (29.2%) | |
|
| ||||||
| CC | CG | GG | C | G | ||
|
|
||||||
| IL-1RA rs315951 (p=0.160) | High EARR (n=5) | 2 (40.0%) | 2 (40.0%) | 1 (20.0%) | 6 (60.0%) | 4 (40.0%) |
| Low resorption (n=12) | 8 (66.7%) | 4 (33.3%) | 0 (0.0%) | 20 (83.3%) | 4 (16.7%) | |
|
| ||||||
| TT | TC | CC | T | C | ||
|
|
||||||
| IL-1RA rs315952 (p=0.160) | High EARR (n=5) | 2 (40.0%) | 2 (40.0%) | 1 (20.0%) | 6 (60.0%) | 4 (40.0%) |
| Low EARR (n=12) | 8 (66.7%) | 4 (33.3%) | 0 (0.0%) | 20 (83.3%) | 4 (16.7%) | |
|
| ||||||
| AA | AG | GG | A | G | ||
|
|
||||||
| IL-1RA rs1794065 (p=0.631) | High EARR (n=5) | 1 (20.0%) | 1 (20.0%) | 3 (60.0%) | 3 (30.0%) | 7 (70.0%) |
| Low EARR (n=12) | 1 (8.3%) | 3 (12.5%) | 8 (66.7%) | 5 (20.8%) | 19 (79.2%) | |
Table 3.
The p values of the effects of other factors, including, treatment duration, age, and gender. H – Definite EARR, L- Non-definite EARR
| H | L | p-value | |
|---|---|---|---|
| Duration in days, Mean (SD) | 204 (101) | 117 (52) | 0.0255 |
| Age, Mean (SD) | 24.7 (13.5) | 16.7 (2.9) | 0.06 |
| Female, N (%) | 5 (83%) | 6 (50%) | 0.18 |
The genotype distribution and allele frequencies of single nucleotide polymorphisms (SNPs) in subjects with high and low levels of EARR are presented in Table 2. Although the sample size is small, all five subjects who have their genotype tested and with high level of EARR have the GG genotype of IL-1β rs1143634, which is statistically significant (p=0.031). None of the tests for the other SNPs reached statistical significance.
Discussion
This study investigated EARR associated with well-controlled canine retractions. The orthodontic load on each subject was calibrated so that the treatments were similar. The EARR was evaluated using the teeth’s 3D images, which is more accurate. Thus, the results are more accurate and reliable.
The limitation is the small sample size. The sample size needs to be adequate to support meaningful conclusions. It depends on the data’s variation level controlled by the variation of individual control factor. There are multiple factors causing root resorption, such as orthodontic force level, treatment type, and methods of quantification of root resorption. Controlling of these factors have been a major challenge in previous studies. The previous studies used data from various types of clinical cases and evaluated root resorption based on 2D radiographs. The orthodontic force level was not assessed nor controlled, which might vary greatly; the treatment was not consistent; and the 2D evaluation of root resorption might introduce large errors [ref]. These factors introduce large variations, causing the needs to have large samples to raise the statistical power. Two recent review papers called for matching cases/controls in future genetic study of root resorption, which is what we were trying to do. In our study, we have identical clinical case, the well-controlled orthodontic load, and more reliable 3D assessment of root resorption. These will significantly reduce the variations of the control factors and increase the accuracy of quantification of root resorption. These measures will reduce variation and the needs of large sample size. Therefore, the study does provide clues on the dominant factors upon which to focus when studying EARR.
The tipping and translation here represented treatment intentions, which were implemented using different M/F, higher M/F for translation and lower M/F for tipping. They did not represent actual clinical displacement patterns due to the fact that the T-Loop’s M/F was very sensitive to interbracket distance change due to tooth movement so that it changed as the tooth moves. 42
EARR due to canine retraction was investigated in this clinical study. Well-controlled orthodontic loads were applied to the subjects and the EARR was measured from 3D CBCT images, which eliminated the errors due to misalignment that may occur in EARR assessment when 2D radiograph are used.40, 41 In this study, “tooth length” was used instead of “root length” to determine EARR, which will eliminate the effects of different methods to define the root. It is generally accepted that crown length does not change during orthodontic treatment.
The resolution of this method should be two times the CBCT’s voxel size (0.25 mm). Therefore, only root change greater than 0.5 mm was considered conservatively as definite EARR. There were definite EARR on canines in six subjects. Other changes were all negative, meaning root shortening. For the same subjects, the incisors that had no orthodontic load applied, did not show definite EARR. The results indicate:
EARR does occur during canine retraction under a 124 cN retraction force.
The load may trigger EARR because the incisors that have no orthodontic load do not have it.
The orthodontic load is not the only dominant factor causing EARR because it occurs in only few subjects.
Orthodontically induced EARR is believed to be related to multiple factors including orthodontic load, age, treatment duration, and genotype. The effects of these factors on EARR were evaluated. It appears that apical tooth displacements did not show clear patterns in terms of intrusion or extrusion, thus their impacts on EARR were not assessed.
Effect of orthodontic load
Our study suggested that orthodontic load is a contributing factor causing EARR, which agrees with the reports from previous studies. These studies showed that heavy force produces more EARR39, 44. Our results showed that a retraction force at 124 cN level is sufficient to cause EARR. This study also indicated that the load may not be the only dominant factor. All subjects had received the well-controlled orthodontic load on either the TR or CT side. If the load system is the only dominant factor, then all subjects would have consistent clinical outcomes on CT or TR side, which was not observed. In our study, of the 6 out of the 18 subjects showing definite EARR on the TR side, 3 subjects exhibited EARR on the CT side, which indicated that biological factors may also strongly contribute to EARR.
No root showed definite root lengthening, indicating root shortening is dominant during canine retraction. Although it was not statistically significant (p=0.83), teeth on the TR side had more definite root shortenings and higher average root shortening than on the CT side. It might imply that TR may cause more EARR. When designing the treatment strategy, with the same retraction force, TR side had higher M/F than CT side, meaning higher correction moment, which may be one of the causes for EARR.
Theoretically, EARR may be characterized by the root shortening and surface cavities. Both of them result in volume reduction. The volume change was also calculated and shown in Table 1. The measured change due to canine retraction was not severe enough to be considered as definite EARR because of the current CBCT resolution. Thus, the conclusion was not decisive. Images with better resolution will help provide more accurate evaluation, although exposing subjects with more radiation.
Treatment duration
It has been commonly accepted that longer treatment causes EARR.39, 44 Our results support this. The average length of the treatment for the six identified EARR cases was 204 days vs. 117 days for the none definite cases (p=0.0255). However, long treatment time alone may not cause it because several subjects with long treatment did not show definite EARR.
Age
Limited clinical knowledge is available on the age effect on EARR. Jiang et al. reported in an clinical study that older people tend to have more EARR.39 Our study did not show statistically that the age makes significant difference (p=0.06); however, the data did agree with Jiang’s finding. The two elder subjects with ages 47 and 35 had definite EARRs. The other four subjects who had EARR had average age similar to those who did not show definite resorption (16.5 vs. 16.7 years of age). Although the sample size was small so that no conclusion could be made, the data suggested that the older subjects might be more at risk for EARR.
Gender
It is not clear whether gender is a factor of EARR. However, our results show that female patients tended to be more likely to experience EARR, since five of the six patients who have EARR are female. However, the difference did not reach statistical significance (p=0.18).
Genotype
The interleukin-1 (IL-1) family consists of at least three structurally related polypeptides: IL-1α, IL-1β, and IL-1 receptor antagonist (IL-RN)45. IL-1RN inhibits IL-1α or β activity by specifically binding to the IL-1 receptor46 and the overall balance between IL-1 and IL-1RN determines physiological function and may be involved in disease pathogenesis47. Genes of IL-1α, IL-1β and IL-1RN are polymorphic and recently, Al-Qawasmi et al. have found that the IL-1β and its polymorphisms may contribute to EARR28. In our study, the genotype of fourteen out of the eighteen subjects was identified. The results were compared with EARR to see if it occurs only in subjects with a particular genotype. Five of them have definite EARRs. The others had the tooth length difference less than 0.5 mm, thus were not counted due to our established criterion. Eight of them have GG genotype of IL-1β rs1143634. However, all five EARR subjects have this genotype. There were no other genotypes that were common in this subject group. The result indicates that GG genotype of IL-1β rs1143634 may be a necessary indicator of a patient who is vulnerable to root resorption, although it is not sufficient. Other indicators will also need to be identified. Although the sample size is small for us to make a strong conclusion, the results do indicate that subjects with GG genotype of IL-1β rs1143634 may be vulnerable for EARR.
Conclusions
The results of this study support the following conclusions.
Canine retraction under a retraction force of 124 cN may result in EARR.
Teeth with no orthodontic load do not have EARR.
Patients with GG genotype of IL-1β rs1143634 may be at higher risk for EARR.
Highlights.
Canine retraction under a retraction force of 124 cN may result in EARR.
The teeth that have no orthodontic load do not have EARR.
The orthodontic force alone is not sufficient to cause EARR. The high risk factors causing EARRs may be older, female, longer treatment time, high moment-to-force ratio and patients with GG genotype of IL-1β rs1143634.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sameshima GT, Sinclair PM. Predicting and preventing root resorption: Part II. Treatment factors Am J Orthod Dentofacial Orthop. 2001;119(5):511–5. doi: 10.1067/mod.2001.113410. [DOI] [PubMed] [Google Scholar]
- 2.Casa MA, Faltin RM, Faltin K, Sander FG, Arana-Chavez VE. Root resorptions in upper first premolars after application of continuous torque moment. Intra-individual study. J Orofac Orthop. 2001;62(4):285–95. doi: 10.1007/pl00001936. [DOI] [PubMed] [Google Scholar]
- 3.Chan E, Darendeliler MA. Physical properties of root cementum: Part 5. Volumetric analysis of root resorption craters after application of light and heavy orthodontic forces. Am J Orthod Dentofacial Orthop. 2005;127(2):186–95. doi: 10.1016/j.ajodo.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Chan E, Darendeliler MA. Physical properties of root cementum: part 7. Extent of root resorption under areas of compression and tension. Am J Orthod Dentofacial Orthop. 2006;129(4):504–10. doi: 10.1016/j.ajodo.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 5.Weltman B, Vig KW, Fields HW, Shanker S, Kaizar EE. Root resorption associated with orthodontic tooth movement: a systematic review. Am J Orthod Dentofacial Orthop. 2010;137(4):462–76. doi: 10.1016/j.ajodo.2009.06.021. discussion 12A. [DOI] [PubMed] [Google Scholar]
- 6.Blake M, Woodside DG, Pharoah MJ. A radiographic comparison of apical root resorption after orthodontic treatment with the edgewise and Speed appliances. Am J Orthod Dentofacial Orthop. 1995;108(1):76–84. doi: 10.1016/s0889-5406(95)70069-2. [DOI] [PubMed] [Google Scholar]
- 7.Janson GR, De Luca Canto G, Martins DR, Henriques JF, De Freitas MR. A radiographic comparison of apical root resorption after orthodontic treatment with 3 different fixed appliance techniques. Am J Orthod Dentofacial Orthop. 2000;118(3):262–73. doi: 10.1067/mod.2000.99136. [DOI] [PubMed] [Google Scholar]
- 8.Malmgren O, Goldson L, Hill C, et al. Root resorption after orthodontic treatment of traumatized teeth. Am J Orthod. 1982;82(6):487–91. doi: 10.1016/0002-9416(82)90317-7. [DOI] [PubMed] [Google Scholar]
- 9.Goldson L, Henrikson CO. Root resorption during Begg treatment; a longitudinal roentgenologic study. Am J Orthod. 1975;68(1):55–66. doi: 10.1016/0002-9416(75)90159-1. [DOI] [PubMed] [Google Scholar]
- 10.Beck BW, Harris EF. Apical root resorption in orthodontically treated subjects: analysis of edgewise and light wire mechanics. Am J Orthod Dentofacial Orthop. 1994;105(4):350–61. doi: 10.1016/S0889-5406(94)70129-6. [DOI] [PubMed] [Google Scholar]
- 11.Parker RJ, Harris EF. Directions of orthodontic tooth movements associated with external apical root resorption of the maxillary central incisor. Am J Orthod Dentofacial Orthop. 1998;114(6):677–83. doi: 10.1016/s0889-5406(98)70200-8. [DOI] [PubMed] [Google Scholar]
- 12.Pandis N, Nasika M, Polychronopoulou A, Eliades T. External apical root resorption in patients treated with conventional and self-ligating brackets. Am J Orthod Dentofacial Orthop. 2008;134(5):646–51. doi: 10.1016/j.ajodo.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 13.McNab S, Battistutta D, Taverne A, Symons AL. External apical root resorption following orthodontic treatment. Angle Orthod. 2000;70(3):227–32. doi: 10.1043/0003-3219(2000)070<0227:EARRFO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Alexander SA. Levels of root resorption associated with continuous arch and sectional arch mechanics. Am J Orthod Dentofacial Orthop. 1996;110(3):321–4. doi: 10.1016/s0889-5406(96)80017-5. [DOI] [PubMed] [Google Scholar]
- 15.Vlaskalic V, Boyd RL, Baumrind S. Etiology and sequelae of root resorption. Semin Orthod. 1998;4(2):124–31. doi: 10.1016/s1073-8746(98)80009-1. [DOI] [PubMed] [Google Scholar]
- 16.Levander E, Malmgren O. Evaluation of the risk of root resorption during orthodontic treatment: a study of upper incisors. Eur J Orthod. 1988;10(1):30–8. doi: 10.1093/ejo/10.1.30. [DOI] [PubMed] [Google Scholar]
- 17.Odenrick L, Brattstrom V. Nailbiting: frequency and association with root resorption during orthodontic treatment. Br J Orthod. 1985;12(2):78–81. doi: 10.1179/bjo.12.2.78. [DOI] [PubMed] [Google Scholar]
- 18.Sharpe W, Reed B, Subtelny JD, Polson A. Orthodontic relapse, apical root resorption, and crestal alveolar bone levels. Am J Orthod Dentofacial Orthop. 1987;91(3):252–8. doi: 10.1016/0889-5406(87)90455-0. [DOI] [PubMed] [Google Scholar]
- 19.Hendrix I, Carels C, Kuijpers-Jagtman AM, Van THM. A radiographic study of posterior apical root resorption in orthodontic patients. Am J Orthod Dentofacial Orthop. 1994;105(4):345–9. doi: 10.1016/S0889-5406(94)70128-8. [DOI] [PubMed] [Google Scholar]
- 20.Taithongchai R, Sookkorn K, Killiany DM. Facial and dentoalveolar structure and the prediction of apical root shortening. Am J Orthod Dentofacial Orthop. 1996;110(3):296–302. doi: 10.1016/s0889-5406(96)80014-x. [DOI] [PubMed] [Google Scholar]
- 21.Otis LL, Hong JS, Tuncay OC. Bone structure effect on root resorption. Orthod Craniofac Res. 2004;7(3):165–77. doi: 10.1111/j.1601-6343.2004.00282.x. [DOI] [PubMed] [Google Scholar]
- 22.Segal GR, Schiffman PH, Tuncay OC. Meta analysis of the treatment-related factors of external apical root resorption. Orthod Craniofac Res. 2004;7(2):71–8. doi: 10.1111/j.1601-6343.2004.00286.x. [DOI] [PubMed] [Google Scholar]
- 23.DeShields RW. A study of root resorption in treated Class II, Division I malocclusions. Angle Orthod. 1969;39(4):231–45. doi: 10.1043/0003-3219(1969)039<0231:ASORRI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Baumrind S, Korn EL, Boyd RL. Apical root resorption in orthodontically treated adults. Am J Orthod Dentofacial Orthop. 1996;110(3):311–20. doi: 10.1016/s0889-5406(96)80016-3. [DOI] [PubMed] [Google Scholar]
- 25.Jimenez-Pellegrin C, Arana-Chavez VE. Root resorption in human mandibular first premolars after rotation as detected by scanning electron microscopy. Am J Orthod Dentofacial Orthop. 2004;126(2):178–84. doi: 10.1016/j.ajodo.2003.06.020. discussion 84–5. [DOI] [PubMed] [Google Scholar]
- 26.Sameshima GT, Sinclair PM. Characteristics of patients with severe root resorption. Orthod Craniofac Res. 2004;7(2):108–14. doi: 10.1111/j.1601-6343.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- 27.Dermaut LR, De Munck A. Apical root resorption of upper incisors caused by intrusive tooth movement: a radiographic study. Am J Orthod Dentofacial Orthop. 1986;90(4):321–6. doi: 10.1016/0889-5406(86)90088-0. [DOI] [PubMed] [Google Scholar]
- 28.Al-Qawasmi RA, Hartsfield JK, Jr, Everett ET, et al. Genetic predisposition to external apical root resorption. Am J Orthod Dentofacial Orthop. 2003;123(3):242–52. doi: 10.1067/mod.2003.42. [DOI] [PubMed] [Google Scholar]
- 29.Harris EF, Kineret SE, Tolley EA. A heritable component for external apical root resorption in patients treated orthodontically. Am J Orthod Dentofacial Orthop. 1997;111(3):301–9. doi: 10.1016/s0889-5406(97)70189-6. [DOI] [PubMed] [Google Scholar]
- 30.Sameshima GT, Sinclair PM. Predicting and preventing root resorption: Part I. Diagnostic factors. Am J Orthod Dentofacial Orthop. 2001;119(5):505–10. doi: 10.1067/mod.2001.113409. [DOI] [PubMed] [Google Scholar]
- 31.Hartsfield JK, Jr, Everett ET, Al-Qawasmi RA. Genetic Factors in External Apical Root Resorption and Orthodontic Treatment. Crit Rev Oral Biol Med. 2004;15(2):115–22. doi: 10.1177/154411130401500205. [DOI] [PubMed] [Google Scholar]
- 32.Ngan DC, Kharbanda OP, Byloff FK, Darendeliler MA. The genetic contribution to orthodontic root resorption: a retrospective twin study. Aust Orthod J. 2004;20(1):1–9. [PubMed] [Google Scholar]
- 33.Al-Qawasmi RA, Hartsfield JK, Jr, Everett ET, et al. Genetic predisposition to external apical root resorption in orthodontic patients: linkage of chromosome-18 marker. J Dent Res. 2003;82(5):356–60. doi: 10.1177/154405910308200506. [DOI] [PubMed] [Google Scholar]
- 34.Linge BO, Linge L. Apical root resorption in upper anterior teeth. Eur J Orthod. 1983;5(3):173–83. doi: 10.1093/ejo/5.3.173. [DOI] [PubMed] [Google Scholar]
- 35.Linge L, Linge BO. Patient characteristics and treatment variables associated with apical root resorption during orthodontic treatment. Am J Orthod Dentofacial Orthop. 1991;99(1):35–43. doi: 10.1016/S0889-5406(05)81678-6. [DOI] [PubMed] [Google Scholar]
- 36.Mavragani M, Boe OE, Wisth PJ, Selvig KA. Changes in root length during orthodontic treatment: advantages for immature teeth. Eur J Orthod. 2002;24(1):91–7. doi: 10.1093/ejo/24.1.91. [DOI] [PubMed] [Google Scholar]
- 37.Bishara SE, Vonwald L, Jakobsen JR. Changes in root length from early to mid-adulthood: resorption or apposition? Am J Orthod Dentofacial Orthop. 1999;115(5):563–8. doi: 10.1016/s0889-5406(99)70281-7. [DOI] [PubMed] [Google Scholar]
- 38.Viecilli RF, Katona TR, Chen J, Hartsfield JK, Jr, Roberts WE. Orthodontic mechanotransduction and the role of the P2X7 receptor. Am J Orthod Dentofacial Orthop. 2009;135(6):694, e1–16. doi: 10.1016/j.ajodo.2008.10.018. discussion 94–5. [DOI] [PubMed] [Google Scholar]
- 39.Jiang RP, McDonald JP, Fu MK. Root resorption before and after orthodontic treatment: a clinical study of contributory factors. Eur J Orthod. 2010;32(6):693–7. doi: 10.1093/ejo/cjp165. [DOI] [PubMed] [Google Scholar]
- 40.Katona TR. Flaws in root resorption assessment algorithms: role of tooth shape. Am J Orthod Dentofacial Orthop. 2006;130(6):698, e19–27. doi: 10.1016/j.ajodo.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Katona TR. The flaws in tooth root resorption assessment algorithms: the role of source position. Dentomaxillofac Radiol. 2007;36(6):311–6. doi: 10.1259/dmfr/52061649. [DOI] [PubMed] [Google Scholar]
- 42.Xia Z, Chen J, Jiang F, et al. Load system of segmental T-loops for canine retraction. Am J Orthod Dentofacial Orthop. 2013;144(4):548–56. doi: 10.1016/j.ajodo.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li S, Xia Z, Liu SS, Eckert G, Chen J. Three-dimensional canine displacement patterns in response to translation and controlled tipping retraction strategies. Angle Orthod. 2015;85(1):18–25. doi: 10.2319/011314-45.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weltman B, Vig KW, Fields HW, Shanker S, Kaizar EE. Root resorption associated with orthodontic tooth movement: a systematic review. Am J Orthod Dentofacial Orthop. 2010;137(4):462–76. doi: 10.1016/j.ajodo.2009.06.021. discussion 12A. [DOI] [PubMed] [Google Scholar]
- 45.Nicklin MJ, Weith A, Duff GW. A physical map of the region encompassing the human interleukin-1 alpha, interleukin-1 beta, and interleukin-1 receptor antagonist genes. Genomics. 1994;19(2):382–4. doi: 10.1006/geno.1994.1076. [DOI] [PubMed] [Google Scholar]
- 46.Dinarello CA. Cytokines as mediators in the pathogenesis of septic shock. Curr Top Microbiol Immunol. 1996;216:133–65. doi: 10.1007/978-3-642-80186-0_7. [DOI] [PubMed] [Google Scholar]
- 47.Firestein GS, Boyle DL, Yu C, et al. Synovial interleukin-1 receptor antagonist and interleukin-1 balance in rheumatoid arthritis. Arthritis Rheum. 1994;37(5):644–52. doi: 10.1002/art.1780370507. [DOI] [PubMed] [Google Scholar]