Abstract
Substance P (SP) is an undecapeptide present in the central nervous system, and the peripheral nervous system. SP released from the peripheral nerves exerts its biological and immunological activity via high affinity neurokinin 1 receptor (NK1R). SP is also produced by immune cells, and acts as an autocrine or paracrine fashion to regulate the function of immune cells. In addition to its proinflammatory role, SP and its metabolites in combination with insulin-like growth factor-1 (IGF-1), are shown to promote the corneal epithelial wound healing. Recently, we showed an altered ocular surface homeostasis in unmanipulated NK1R-/- mice, suggesting the role of SP-NK1R signaling in ocular surface homeostasis under steady state. The current review summarizes the immunobiology of SP, and its effect on immune cells and immunity to microbial infection. In addition, the effect of SP in inflammation, wound healing and corneal epithelial homeostasis in the eye is discussed.
Keywords: Substance P, neurokinin receptor, inflammation and wound healing
Introduction
Substance P (SP) is an eleven-amino acid long neuropeptide, which is produced by neuronal and non-neuronal cells, including the immune cells. SP is known to exert its biological activity through G-Protein coupled neurokinin receptors named neurokinin 1 receptor (NK1R), neurokinin 2 receptor (NK2R), and neurokinin 3 receptor (NK3R). Among the three, NK1R, has the highest affinity for SP. SP-NK1R interaction is widely reported to regulate immune cell's function and the immunity to microbial infection. In addition, SP is also reported to promote ocular inflammation, wound healing, and the tissue homeostasis. The focus of this review is threefold: (1) To provide an overview of the biology of SP, (2) To describe the effect of SP on immune cells and the immunity to microbial infection, and (3) To describe the role of SP in ocular inflammation, wound healing and tissue homeostasis.
Discovery, Chemical composition and the biosynthesis of SP
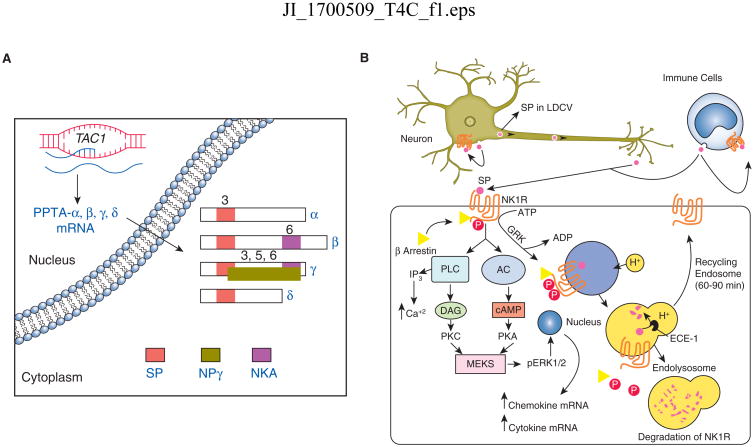
In 1931, von Euler and Gaddum discovered that the powdered extracts of the equine (horse) brain and intestine had hypotensive and spasmogenic activity (1). Since the activity of this factor resided in the water soluble powdered extracts of the brain and intestinal tissue, it was initially named as “preparation P” and the later “Substance P”. In the early seventies, Chang and Leeman determined the amino acid composition of SP (2). SP is comprised of eleven amino acids (Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH2) and has a net positive charge under physiological pH (2). SP is a member to the tachykinin family of neuropeptides, and is encoded by Tachykinin 1 (TAC1) gene (3). In humans, the major mammalian TAC genes are TAC1, TAC3 and TAC4. The human TAC1 gene consists of seven exons and encodes for SP, neurokinin A (NKA), neuropeptide K, and neuropeptide-γ (NPγ) (4, 5). TAC1 encodes for preprotachykinin A mRNA (PPTA), whereas TAC3 and TAC4 encode for preprotachykinin B (PPTB) and preprotachykinin C (PPTC) mRNA, respectively (6, 7). PPTA mRNA undergoes alternative splicing to give rise to αPPTA, βPPTA, γPPTA, and δPPTA mRNA transcripts (Figure 1A). Out of the four isoforms of PPTA mRNA, α- and δ-PPTA gives rise to SP peptide only, whereas β-PPTA yields to SP and NKA peptide, and the γ-PPTA transcript encodes for SP, NKA, and NPγ neuropeptide (8).
Figure 1.
(A) Schematic of human TAC1 gene mRNA splice variants. The neuropeptide products are indicated by colored boxes on mRNA structures and the number denotes the coding exon for each tachykinin derived from the various isoforms of preprotachykinin A (PPTA). (B) Schematic representation of Substance P (SP) production by a neuronal cell and an immune cell type. The outcome of SP binding to a target cell expressing high affinity SP receptor, NK1R, is depicted as NK1R associated signaling events along with an internalization of SP-NK1R complex, and the recycling of free NK1R to the cell surface after degradation of SP in endosomal complex. LDCV (large dense-core vesicles), GRK (G-protein coupled receptor kinases), ECE-1 (endothelin-converting enzyme-1), PLC (phospholipase C), AC (adenylate cyclase), DAG (diacylglycerol), IP3 (inositol triphosphate), PKC (protein kinase C), MEKS (mitogen activated protein kinase), pERK1/2 (phosphorylated extracellular signal related kinases).
SP is present in a large amount in the central nervous system (CNS) and the peripheral nervous system (PNS) (9). In neurons, SP is expressed in the soma. Once synthesized, SP is transported in large dense-core vesicles (LDCVs), and the peptide is released through the exocytosis of LDCVs either at axonal terminals or at the neuronal soma (Figure 1B) (10). Once exocytosed, SP either binds to membrane-bound SP receptor expressed on same cell or the neighboring cells. Unbound SP can be degraded by a cell-surface metalloendopeptidase named neprilysin (NEP), and thereby suggests a shorter half-life of SP in tissues (11, 12). However, SP can prolong its half-life by interacting with high molecular weight factors such as fibronectin (13). In support, SP is reported to be more stable in blood plasma (14). Thus, SP may form a complex with other molecules to prolong its half-life in tissue or in the blood.
Neurokinin receptors
Biological activity of SP is mediated through neurokinin receptors (NKRs). Neurokinin receptors belong to the class I (rhodopsin-like) family of G-protein coupled receptors (15, 16). There are three different types of NKRs: NK1R, NK2R, and NK3R. The affinity of these NKRs in binding to SP is in the order of NK1R>NK2R>NK3R (17). In humans, two naturally-occurring isoforms of NK1R are found in neuronal and immune cell types (18-21). They are named as full-length NK1R or NK1R and truncated NK1R (NK1R-T). NK1R is made up of 407 amino acid residues and has one C-terminal intracellular domain, whereas NK1R-T is 311 amino acids long, but lacks the 90-amino acid long C-terminal intracellular domain (22).
NK1R signaling involves phosphorylation of the carboxyl terminal domain of NK1R by G-protein coupled receptor kinases (GRKs), resulting in the interaction of NK1R with β-arrestin adapter proteins. β-arrestin plays a central role in desensitization of cells to SP signaling (8, 23). The desensitization process involves β-arrestin associated internalization of SP-NK1R complex in an endosome (Figure 1B). The endosomal acidification dissociates SP from NK1R complex followed by degradation of SP by endothelin-converting enzyme-1 (ECE-1), a membrane metalloendopeptidase (8). The degradation of SP frees NK1R to recycle back to the cell surface (Figure 1B). Recycling of NK1R is also regulated by the concentration of SP. After stimulation with low concentration of SP (<1nM), NK1R is minimally phosphorylated and gets internalized into endosomes, but rapidly dissociate from β-arrestin to recycle back to the cell surface (24). However, high concentration of SP (> 10 nM) causes extensive NK1R phosphorylation, its internalization into endosomes and prolonged association with β-arrestin (23). After prolonged stimulation with SP, the NK1R gets ubiquitinated and traffics to lysosomes, where it gets degraded (Figure 1B) (25).
SP binding to NK1R also activates phospholipase C (PLC), which causes the formation of inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 increases the level of cytosolic Ca2+, whereas DAG activates protein kinase C (PKC). NK1R signaling can also activate adenylyl cyclase, which causes the generation of cyclic adenosine monophosphate (cAMP), and the latter activates protein kinase A (PKA) (Figure 1B). In NK1R transfected rat kidney epithelial cells (KNRK), these signaling events cause an activation of extracellular signal-related kinases 1/2 (ERK1/2), a member of mitogen activated protein kinases (MAPKs) (26). The nuclear translocation of ERK1/2 is necessary for the proliferation and anti-apoptotic effect of SP (26). NK1R signaling may also cause an activation of p38MAPK and NF-κB transcription factor (27, 28). In macrophages, SP induced NF-κB activation requires ERK1/2 and p38 MAPK activation, which results into the expression of MIP-2 and MCP-1 chemokine (29).
In contrast to NK1R, the signaling property of NK1R-T is less well studied. NK1R-T does not interact with β-arrestin resulting in the impairment of SP induced desensitization and endocytosis of SP-NK1R complex (26). The NK1R-T is found on human monocytes, macrophages, colonic epithelial cells of the colitis patients, and in the peripheral tissues, including heart, lung, prostate and spleen (19, 30). Furthermore, NK1R-T has 10-fold lower affinity to SP than full-length NK1R (20, 31). The intracellular signaling pathways for NK1R and NK1R-T are different, for example, SP stimulation of NK1R-T did not activate NF-κB and showed reduced mRNA expression of IL-8 (32, 33). NK1R-T stimulation in HEK 293 cells cause ERK1/2 phosphorylation, but the effects are seen after 15 minutes of incubation with SP (20, 32). In contrast, full-length NK1R stimulation results in a rapid activation of ERK (with in 1-2 minutes). NK1R-T stimulation increases CCL5- induced intracellular calcium level in monocytes/macrophages (19). These results demonstrate a cross-talk between NK1R-T and CCR5, while interacting with their respective ligands. However, more detailed studies are needed to address the factors involved in up regulating NK1RT in chronic inflamed tissues, and whether an increased level of NK1RT has beneficial or detrimental effects in resolving the inflammation and promoting the tissue repair.
Substance P and NK1R expression in immune cell types
Nerves containing SP are reported to innervate primary (thymus and bone-marrow) and secondary lymphoid organs (spleen, lymph nodes, tonsils, and lymphoid aggregates in the gut) (34-36). This suggests that SP may act as a mediator of cross talk between the nervous and immune system. In addition to their production from neurons, SP and its receptor NK1R are well documented to express in different immune cell types. The expression of SP and its effect on immune cells are summarized in Table I. The brief description of the role of SP in regulating the function of different immune cell types is shown below.
Table I. Expression and effects of SP in immune cell types.
| Immune cell types | SP Expression is | NK1R mediated effect is | SP or NK1R agonist signaling on immune cells | References |
|---|---|---|---|---|
| Dendritic cells | shown in human and mouse | shown in human and mouse | Potentiate immunostimulatory function and survival of DCs, IL-12 production and generation of type I immunity. | (75-79) |
| Monocytes/Macrophages | shown in human and mouse | shown in human and mouse | NK1R-T stimulation in monocytes augments CCR5 signaling and promotes M-tropic HIV infection. NK1R signaling enhances IL-12 production in macrophages. | (67-70) |
| Eosinophils | shown in human and mouse | not shown | SP effects are NK2R mediated. Inhibit spontaneous apoptosis of cells in culture, exerts chemotactic effect and causes the release of O2-. | (51, 52) |
| Neutrophils | Shown in human and mouse | shown in human and mouse | Causes superoxide production, enhances phagocytosis, increases expression of chemokine and chemokine receptor. | (55,57,58, 62) |
| Mast cells | not shown | shown in human and mouse | Causes mast cell degranulation, TNF-α and VEGF secretion. | (40,41,45, 46) |
| Natural Killer Cells | shown in human and mouse | shown in human and mouse | Increases IFN-γ production, either inhibits or enhances NK cell cytotoxicity under defined set of condition. | (81,83-86) |
| T lymphocytes | shown in human and mouse | shown in human and mouse | Enhances IL-2 and IFN-γ production, promotes T cell proliferation, generation of memory Th17 from non-committed memory CD4 T cells. | (89,90,92, 94) |
Mast cells
Mast cells are shown in close proximity to SP-positive nerves in many tissues, including lung, intestine, dura matter, diaphragm, and skin suggesting the involvement of mast cells in neurogenic inflammation (37-39). Earlier studies showed that SP can induce the histamine release by mast cell degranulation, and this SP mediated activity was considered to be the outcome of direct G-protein mediated activation rather than a neurokinin receptor-mediated process (40, 41). However, later studies in both rat and human cultured mast cells showed the expression of high affinity SP receptor NK1R (42, 43), while the human intestinal mast cells (mucosal type) do not respond to substance P, as they do not express any of the three neurokinin receptors under homeostatic condition (44). Together, these studies suggest SP exerts its function in NK1R independent and dependent fashion on mast cells. In addition, studies show a bidirectional signal between mast cells and SP expressing nerves. SP released from sensory neurons acts on mast cells to produce TNF-α and vascular endothelial growth factor (VEGF) (45, 46). On the other hand, mast cells can release the tryptase enzyme, which activates protease-activated receptor-2 (PAR-2) on neurons, and mediates the release of SP to induce neurogenic inflammation (47).
Eosinophils
SP is shown to present in human eosinophils (48). Similarly, immunostaining of SP is noted in eosinophils derived from liver granulomas of mice infected with murine schistosomiasis (48-50). Eosinophil activation by SP causes their degranulation and release of 02- (51). This effect of SP is mediated via its N-terminal and was thought to be a receptor independent event. However, more recently, eosinophils isolated from atopic dermatitis patients were shown to express the higher levels of NK2R, but not NK1R, at mRNA and protein levels (52). SP stimulation of eosinophils showed an increased influx of Ca2+ and inhibited their spontaneous apoptosis in cultures (52). SP was also shown to have a chemotactic effect on eosinophils derived from atopic dermatitis patients (52), suggesting that eosinophils may propagate the itch cycle in atopic conditions via SP.
Neutrophils
Neutrophils expressing SP are found in granulomas and inflamed bronchoalveolar lavage (53, 54). SP stimulation of human neutrophils causes superoxide production (55). This biological activity of SP is NK1R dependent and involves an increased level of intracellular Ca2+ (56). SP also enhances the phagocytic activity of neutrophils, and regulates the influx of neutrophils in inflamed tissues (57). The influx of neutrophils in inflamed tissue is NK1R mediated. A reduced influx of neutrophils and MPO activity was reported in the pancreas and lung tissue of NK1R deficient mice in an acute pancreatitis mouse model (58). Similarly, IL-1β induced neutrophil accumulation in a murine air-pouch model was impaired in NK1R-/- mice (59). SP administration in the skin has shown to cause the neutrophil accumulation in the skin tissue of mice, and the effect is dependent on skin resident mast cells (60, 61). However, in vitro SP stimulation of primary mouse neutrophils, purified from the peripheral blood by density gradient centrifugation, showed an increased expression of CCL3, CXCL2 chemokines and CCR1, CXCR2 chemokine receptors (62). These effects were NK1R dependent. Together, these studies suggest a direct and indirect effect of SP in regulating neutrophil function and influx in inflamed tissues.
Monocytes/macrophages
Monocytes/macrophages in both human and rodents are reported to express SP and its receptor NK1R (63-65). In human mononuclear phagocytes, NK1R antagonist treatment down-regulates SP mRNA expression (66). While determining, which form of NK1R is expressed, primary human monocytes and undifferentiated human monocyte cell line THP-1 are reported to express only NK1R-T (19, 67). The NK1R-T expressing THP-1 cells, when stimulated with SP, did not trigger Ca2+ response, but SP stimulation augmented CCL5-induced intracellular levels of Ca2+ in undifferentiated THP-1 cells as well as in primary human monocytes (67). These studies suggest a critical interaction between NK1R-T and CCR5 in augmenting CCL5 induced effects in monocytes. In fact, the NK1R antagonists have been shown to inhibit HIV infection of macrophages, suggesting the importance of SP via NK1R-T in enhancing M-tropic HIV infection (68, 69). In mice, SP induces p35 and p40 mRNA in cultured macrophages via NK1R, and LPS stimulation further augments the secretion of bioactive IL-12p70 (70). Moreover, IL-12 induces SP (PPTA) expression in splenic macrophages, suggesting IL-12 and SP regulate each other's expression in murine macrophages (71). In addition to IL-12, IL-23 has also been reported to regulate SP levels in mouse macrophages, which is subject to inhibition by TGF-β cytokine (72). SP exerts its action on mouse macrophages through high affinity NK1R, and it causes NF-κB activation without increasing the intracellular Ca2+ levels (29, 73). NK1R expression in mouse peritoneal macrophages are up regulated by Th1 (IFN-γ) and Th2 (IL-4) cytokines (74). Together, these reports provide convincing evidence for the expression and function of SP in both human and rodent monocytes/macrophages.
Dendritic cells
Dendritic cells (DCs) play an important role for the generation of an adaptive T cell response to nonself-antigens. SP and its receptors NK1R and NK2R are present in both mouse and human dendritic cells (75, 76). In mice, bone marrow derived dendritic cells (BMDCs) were shown to express the gamma-transcript of PPTA gene, and produce SP at protein levels as determined by immunostaining (75). BMDCs expressing SP potentiate allogeneic T cell proliferation through NK1R (75). In addition, BMDCs stimulated with NK1R agonist produce IL-12, and when injected in vivo induces type I immunity (77). Similarly, in vivo administration of NK1R agonist has been shown to potentiate the immunostimulatory functions of skin-resident Langerhans cells to induce an antigen specific Type-I immunity in a mouse model (78). NK1R signaling of BMDCs has also been shown to promote the survival of DCs (79), suggesting a possible in vivo role of SP in maintaining tissue-resident DC populations under homeostatic condition, especially in tissues highly innervated with sensory nerves such as the skin and cornea.
Natural Killer cells
Natural killer (NK) cells are granular lymphocytes, which are primarily involved in controlling viral load in inflamed tissues by targeting virus-infected cells. Human NK cells are reported to express functional NK1R, and a dose-dependent effect of SP on NK cell migration was seen in vitro with or without IL-2 stimulation (80). SP-NK1R interaction has also been shown to promote IFN-γ production from murine NK cells in a bacterial infection model (81). On the other hand, the role of SP in inhibiting the cytotoxicity of NK cells is well described in studies related with HIV-seropositive subjects. In patients with HIV infection, higher amounts of SP are seen in human plasma, and it correlates with lower number and depressed function of NK cells (82). The ex-vivo experiments carried out on peripheral-blood mononuclear cells isolated from HIV-seropositive subjects showed that substance P antagonist CP-96345 increases the cytolytic activity of NK cells (83). A detailed study further showed that human NK cells express both full length NK1R and NK1R-T, and a preincubation with SP inhibits the NK cell's contact-dependent cytotoxicity. The inhibitory effect of SP was evident in NK cell line (YTS) as well as in NK cells purified from the human blood of healthy volunteers (84). In this study, preincubation with SP did not affect NF-kB activation, but inhibited the prolonged increase in Ca2+ level in NK cell after its interaction with the target cell. SP preincubation also inhibited activation-receptor induced phosphorylation of ERK in NK cells. However, in the absence of preincubation and the use of different concentrations, SP has been shown to stimulate the cytotoxicity of NK cells (85, 86). Thus, under defined set of conditions, SP can inhibit the cytotoxicity of NK cells.
T lymphocytes
Resting T cells do not express SP and its receptor, but activated human T lymphocytes are shown to express PPTA gene and produce SP neuropeptide (87). Similarly, in rodents, activated T cells express NK1R, and SP released from activated T cells may act in an autocrine fashion to regulate T cell proliferation (75). It is reported that IL-12 augments, whereas IL-10 blocks the SP production from murine T cells (72). IL-12 also induces SP receptor NK1R in murine T cells (88). SP has also been shown to enhance IL-2 expression in activated human T cells and promote T cell proliferation (89, 90). In human T lymphocytes, SP is reported to enhance the expression of macrophage inflammatory protein-1β (MIP-1β), suggesting that SP action on T cell may promote the chemotaxis of CCR5 (MIP-1β receptor) expressing immune cells (91). Recently, in the presence of monocytes, SP-NK1R interaction has been shown to promote the generation of human memory Th17 cells from non-Th17 committed memory CD4 T cells (92). In murine schistosomiasis parasitic infection, granuloma eosinophils produce SP and CD4 T cells isolated from the granulomas are shown to express NK1R, suggesting that SP-NK1R interaction in granulomas may involve CD4 T cells (93). In fact, SP is reported to enhance schistosome egg antigen induced IFN-γ production in NK1R expressing T cells from schistosome infected mice (94). Together, these studies show the functional role of SP on activated, but not naive T cells.
Role of Substance P in regulating immune response to microbial infection
Multiple studies have documented the role of substance P in viral, bacterial, and parasitic infection (95-97). The known roles of SP in regulating the immunity to viral, bacterial, and parasitic infections are summarized below.
SP and its receptor in viral infections
Among viral infection, role of SP is well reported in murine gamma herpes virus 68 (HV-68) infection. HV-68 is a gamma-2 herpes virus, which infects the epithelial cells and establishes the latency in immune cell types such as B cells, macrophages and dendritic cells (98, 99). HV-68 infection of C57BL/6 (B6) mice is shown to cause an increased mRNA expression of PPTA and substance P receptor in spleen and mesenteric lymph node of infected mice (100). In the same study, NK-1R deficient mice exhibited reduced CTL response and an increased level of latent virus. Similarly, mice genetically deficient for PPTA gene, after HV-68 infection, were shown to have an approximately 100-fold higher viral load in the lungs in comparison to control B6 mice (101). This was associated with an increased lung pathology, and an increased level of latent virus in the spleens of PPTA deficient mice. Together, these studies suggest that, in the absence of SP or its receptor, the host immunity to HV-68 infection is significantly compromised.
The role of SP has also been investigated in detail in HIV infection. Increased level of SP is reported in the blood plasma of HIV infected men and women (82, 102). In addition, HIV infection of cultured macrophages increases the expression of SP in the immune cell (103). On the other hand, in vitro addition of SP has been shown to enhance the replication of HIV in blood-isolated mononuclear phagocytes (104, 105). This effect of SP is mediated via NK1R-T. The latter enhances HIV entry into immune cells via CCR5, as the use of non-peptide SP antagonist (CP-96,345) inhibits HIV infection of macrophages and down-regulates CCR5 (68). In comparison to CP-96,345, NK1R antagonist aprepitant is more potent ex vivo to suppress the HIV infection of myeloid derived macrophages (69). Aprepitant treatment has also been shown to reduce viral load in SIV-infected macaques, and reduce the pro-inflammatory cytokines in HIV positive individuals, suggesting that NK1R antagonist might be use as an adjunct therapy in HIV infection (106).
SP is also reported to play an important role during infection with paramyxoviruses. Sendai virus is a single-stranded RNA virus of the Paramyxoviridae family. Sendai virus infection of Guinea pig increases the expression of SP in the cell body of the afferent neurons in nodose ganglia (107). The inducible expression of SP suggests its involvement in virus-induced airway inflammation. In fact, NK1R antagonist treatment of Guinea pigs, infected with Sendai virus, was shown to limit virus-induced bronchoconstriction (108). Similarly, in a rat model of respiratory syncytial virus (RSV) infection, an increased expression of SP receptor was noted following lung infection with RSV (109). Challenge of RSV infected rats with capsaicin resulted in airway inflammation, and NK1R antagonist treatment abrogated the effect of capsaicin (110).
Similar to HIV, measles virus (MV) spreading from one cell to another is partly dependent on SP receptor NK1R. The fusion protein of MV shares the carboxyl terminal sequence of SP and pharmacological inhibition of the NK1R is shown to reduce the spreading of MV (111, 112). Neurkinin-1 receptor signaling is also reported to regulate innate immune defense to genital herpes virus infection (113). NK1R deficient mice exhibited an enhanced level of HSV-2 in the genital tract, which was associated with an impaired NK cell cytotoxicity in the vaginal tissue. This study suggested that lack of NK1R signaling compromises innate immunity to HSV-2 infection. NK1R signaling is also reported to regulate corneal HSV-1 infection induced inflammation, as described under ocular inflammation section.
SP and its receptor in Bacterial infections
The best studied role of SP in augmenting the immunity to bacterial infection was shown in a mouse model of Salmonella infection. The successful clearance of Salmonella infection requires IL-12 induced production of IFN-γ, and the latter enhances macrophage and dendritic cell activation to eliminate the Salmonella infection (114). Oral infection with salmonella causes a rapid and dramatic up regulation of SP and NK1R mRNA in mucosal tissues (115, 116). Mice pretreated with spantide II, NK1R antagonist, prior to oral challenge with Salmonella became highly susceptible to bacterial infection, as spantide II treatment reduced IL-12p40 mRNA expression and impeded the bacterial clearance (116). In a murine model of pneumococcal meningitis, therapeutic targeting of NK1R was shown to limit neuroinflammation (117). Another proinflammatory role of SP was described in two clinically relevant bacterial CNS pathogens- Neisseria meningitides and Borrelia burgdorferi. Following exposure to either of these two pathogens, SP was shown to augment proinflammatory cytokine production in the isolated cultures of CNS resident microglia and astrocyte cell types (118). Moreover, NK1R deficient mice demonstrated a decreased level of inflammatory cytokines after CNS infection of these pathogens (118).
SP and its receptor in parasitic infections
The role of SP in parasitic infection is also well documented. It is reported that SP is involved in regulating the size of granuloma formation after infection with larval cysts of the cestode taenia solium. Mice deficient in substance P precursor or NK1R showed much smaller granulomas, and the lesser level of IL-6, TNF-α and IL-1β protein in comparison to wild-type infected mice (119). Mice can get infected with schistosomes, which colonize human intestine and thus, mice are considered as a good model to study schistosomiasis. Schistosome eggs in liver induced chronic granulomatous inflammation, and SP expression in granulomas was shown to control the levels of inflammatory cytokines (120). NK1R knockout mice in response to schistosome infection develop smaller granulomas and show reduced levels of IFN-γ and IgG2a (120, 121). SP involvement has also been shown in a murine model of Trypanosoma brucei infection, as the infected mice treated with SP antagonist RP67,580 developed reduced neuroinflammation (122). However, Trypanosoma infection of NK1R knockout mice showed more severe neuroinflammation than wild-type mice (123). Interestingly, treatment with NK2 and NK3 antagonist reduced neuroinflammation in the knockout mice, suggesting that in the absence of NK1R signaling SP can still exert its function through weaker affinity receptors NK2R and NK3R. In fact, NK1R has been shown to act as a negative feedback for basal and activity induced release of SP from neurons, and an increased level of SP is noted in the skin of NK1R-/- mice (124).
Role of Substance P in ocular inflammation
Multiple reports have shown the role of SP in ocular inflammation (125). Human samples obtained from the patients with Pterygium showed the expression of SP and NK1R in Pterygium fibroblasts. Moreover, the cell culture studies show that SP via NK1R induces the migration of pterygium fibroblast and microvascular endothelial cells, suggesting that SP may contribute to the pathogenesis of Pterygia through its profibrogenic and angiogenic action (126). In allergic conjunctivitis patients, a higher level of SP was found in the tear fluid (127). The treatment of conjunctivitis in patients with ocular allergy and in animal model is reported to reduce the SP level in tears (128-130). Subconjunctival injections of SP are also shown to cause conjunctivitis and increase the permeability of conjunctival blood vessels (129). Recently, SP-NK1R interaction is reported to cause the loss of Anterior chamber associated immune deviation (ACAID) after retinal laser burns (RLB) in a rodent model (131). The study showed that RLB in one eye resulted in an increase in NK1R expression in retina of both eyes, and the local use of NK1R antagonists prevented the bilateral loss of ACAID. SP has also been shown to cause the rejection of second corneal allograft transplant (132). Using a mouse model of penetrating keratoplasty, it was shown that severing the corneal nerves during surgery released SP in both eyes, which disabled CD4+CD25+regulatory T cells (Treg) that were required for allograft survival (132). Although the underlying mechanism by which SP abolished the immunosuppressive activity of Treg is not clear, the study showed the in vivo ability of SP to regulate Treg function in an animal model.
SP has also been shown to stimulate the corneal neovascularization through NK1R (133). The possible underlying mechanism could be the direct action of SP on vascular endothelial cells, as functional NK1R is known to express on human umbilical vascular endothelial cells (HUVECs), and the growth promoting effects of SP on vascular endothelial cells are reported in serum free culture condition (134, 135). SP may also indirectly promote hemangiogenesis in tissues by recruiting granulocytes with angiogenic potential from the blood circulation (136). Under these conditions, SP can perform its chemotactic activity or cause mast cell degranulation to recruit the granulocytes in inflamed tissue (137). In addition to the development of new blood vessels, SP via NK1R is known to promote the vasodilation of existing blood vessels resulting in plasma extravasation (138, 139). Recently, SP has been shown to regulate lymphangiogenesis in an animal model of diet-induced obesity (140). The study showed that NK1R antagonist treatment reduced abnormal lymphangiogenesis in visceral adipose tissue (VAT) of allergen sensitized obese mice. SP is also known to effectively modulate the contractility of lymphatic vessels via its direct effect on lymphatic muscle cells (141). The direct effect of SP is mediated via NK1R and NK3R molecules, which are expressed on lymphatic muscle cells and are involved in the activation of p38MAPK and ERK1/2 molecules (142). The development of hemangiogenesis and lymphangiogenesis in avascular cornea is likely to affect the visual acuity by causing edema and the influx of immune cells in corneal tissue (143). Recently, in two different models of corneal angiogenesis (alkali burn and sutures), topical application of NK1R antagonist Lanepitant is shown to effectively reduce both hemangiogenesis and lymphangiogenesis in the inflamed cornea (144). Moreover, treatment with NK1R antagonist Fosaprepitant is shown to inhibit corneal (hem and lymph) angiogenesis after this has been established in an animal model.
SP has also been reported to regulate virus and bacteria induced inflammation in the cornea. In a mouse model of corneal herpes simplex virus-1 infection, SP has been shown to regulate the severity of herpes stromal keratitis (HSK) induced in response to corneal herpes simplex virus-1 infection (145). In this study, intense SP staining was noted in the posterior stroma of the eyes with severe HSK, whereas the eyes with mild HSK showed SP staining on the apical surface of the corneal epithelium. The intense SP staining noted in the corneal stroma could be the outcome of SP expression by stromal keratocytes/fibrobalsts, diffusion of SP from aqueous humor, SP secreted from the sympathetic nerves. A recent study has shown hyper innervation of the corneal stroma of HSK developing eyes with sympathetic nerves that are originating from superior cervical ganglion (SCG) (146). The cultured explants of SCG are reported to increase the level of SP in response to stimulation with IL-1β cytokine (147). In HSV-1 infected corneas, NK1R expression was seen in both CD45- and CD45+ cells, suggesting that SP is likely exerting its action on both immune and non-immune cells. Moreover, subconjunctival injection of NK1R antagonist spantide I, during the clinical phase of HSK, was shown to reduce the corneal opacity and angiogenesis (145). In addition to HSK, SP is also reported to regulate the severity of bacterial keratitis induced in response to corneal Pseudomonas aeruginosa infection in resistant BALB/c and susceptible C57BL/6 (B6) mouse model (81, 148). In these studies, SP was shown to adversely affect the disease by elevating the levels of pro-inflammatory cytokines and the growth factors in infected corneas (149). The use of SP antagonist spantide I was shown to reduce type I and enhanced type II cytokine in the infected cornea of B6 mice, leading to an improved disease outcome (150). SP was also reported to delay the apoptosis of polymorphonuclear cells (PMN) and blocking SP interaction with NK1R promotes an earlier apoptosis and improves disease outcome in susceptible B6 mice (151). Together, these studies show the involvement of SP in promoting infection induced corneal inflammation.
Role of Substance P in corneal wound healing
In addition to its role in microbial inflammation, SP is reported to play an important role in corneal wound healing. Substance P is present in corneal nerves, normal tears, and is also expressed in the corneal epithelium and stromal keratocytes (152-156). Although normal cornea has resident immune cell population such as macrophages and dendritic cells, they are not reported to express SP in the corneal tissue. Among SP receptors, NK1R is expressed in corneal epithelial and keratocytes (155, 156). SP via NK1R increases the half-life of IL-8 transcripts in human corneal epithelial cells resulting in the enhancement of IL-8 synthesis (154). Similarly, the primary human keratocytes expressing full-length form of NK1R upon SP stimulation secrete increased level of chemotactic IL-8 protein, which contributes significantly to SP-enhanced keratocytes migration (155). Together, these events suggest the SP-NK1R interaction promotes corneal wound healing. In fact, SP is reported to promote diabetic corneal epithelial wound healing via NK1R (157). In an alkali-burn model of mouse and rabbit eyes, SP was shown to mobilize CD29+ stromal cells from the bone marrow into the circulation, and subsequently to the injured tissue to accelerate the process of wound healing (158). The process of corneal epithelial wound healing involves migration of corneal epithelial cells over the denuded surface followed by their proliferation to increase the normal thickness of the epithelium (159). Unlike in a diabetic mouse model, topical application of SP treatment alone is not effective to promote injury induced corneal epithelial wound healing in rabbit model (160). However, SP is known to act synergistically with insulin growth factor-1 (IGF-1) to promote corneal epithelial cell migration and their attachment to extracellular matrix proteins in ex-vivo cultures (161). The combined effect of SP/IGF-1 was concentration dependent of each factor and was mediated by NK-1R, but not by NK2R and NK3R (162). The signaling events associated with the combined treatment involve protein kinase C activation, p38 MAPK activation, and upregulation of α5 integrin (163-165). The latter is expressed on actively migrating corneal epithelial cells. In Rabbits, the administration of eye drops containing both SP and IGF-1 was shown to promote corneal epithelial wound healing (166). SP/IGF-1 treatment was also reported to improve the barrier function and enhance epithelial wound healing in animal models of neurotrophic keratopathy; a persistent corneal epithelial defect condition that results from insults to the trigeminal nerve (167, 168).
In addition to full-length SP, the four-amino acid sequence (FGLM-amide) derived from the carboxyl terminal end of SP along with IGF-1 effectively stimulates the corneal epithelial cell migration in a rabbit model of corneal wound healing (169). SP-derived peptide and IGF-1 have also been shown to promote corneal epithelial wound healing in diabetic rats (170). Similarly, a tetrapeptide (SSSR) derived from the C-domain of IGF-1 acts synergistically with FGLM-amide to promote corneal epithelial wound healing (171). Shortening of SP and IGF-1 retained their synergistic effect, but prevented their undesirable side effects, such as SP induced miosis (172), and IGF-1 induced angiogenesis (173). These encouraging observations resulted in the clinical trials to treat persistent corneal epithelial defects with topical application of SP or SP-derived peptide along with IGF-1 molecule. Eye drops containing FGLM-amide and IGF-1 were shown to treat a patient with neurotrophic keratopathy (174). The treatment resulted in reduced corneal opacity and the closure of the corneal epithelial defect. Similarly, eye drops containing FGLM-amide and SSSR peptides were shown to induce a rapid resurfacing of the corneal epithelium in individuals with neurotrophic keratopathy (175). Together, these studies show the beneficial effect of SP in association with IGF-1 to induce corneal epithelial wound healing under experimental and clinical condition.
Role of Substance P in corneal epithelial homeostasis
The cornea is highly innervated with sensory nerves that produce SP and cGRP neuropeptides (176). The neuronal soma of sensory nerves innervating the corneal tissue is localized in trigeminal ganglia (TG). Electrical stimulation of the trigeminal ganglia is reported to cause an increase of tear secretion, which is dependent on the release of SP from the sensory nerve endings (177). This study suggested the role of SP in reflex tear production. Recently, we showed that mice genetically deficient for functional NK1R had reduced level of basal tears in comparison to wild type B6 mice as measured with phenol red thread test and developed dry eye disease associated clinical features (178). SP and its metabolites are reported to present in normal human tears without inducing any overt inflammation, suggesting SP may have a physiological role in maintaining the corneal epithelium homeostasis (153, 179). In fact, SP has recently been shown to inhibit hyperosmotic stress induced apoptosis of the corneal epithelial cells in ex-vivo cultures (180). SP-NK1R interaction is also reported to regulate the expression of E-cadherin and ZO-1 tight junction proteins in the corneal epithelial cells and thereby demonstrate its protective role in preserving the corneal epithelial barrier function (181-183). Our results showed an excessive exfoliation of the apical corneal epithelial cells in unmanipulated NK1R-/- mice, possibly because of the dysregulation in the expression or localization of adhesion/tight junction proteins in corneal epithelial cells (178). Together, these studies suggest an important physiological role of SP-NK1R interaction in maintenance of the corneal epithelium homeostasis under steady-state condition.
Conclusions
The existing literature strongly supports the role of SP in promoting an inflammatory condition. The current experimental strategy to control SP induced inflammatory events is heavily dependent upon blocking its high affinity receptor NK1R via peptide or non-peptide NK1R antagonists. However, this strategy does not take into account the interaction of available SP in inflamed tissue with lesser affinity SP receptor NK2R/NK3R, and its outcome on an ongoing tissue inflammation. Future experimental and clinical studies should look into strategies to further increase the efficacy of the treatments targeting SP induced inflammation. At the same time, one should not overlook the beneficial effects of SP, especially on wound healing or in homeostasis of the corneal epithelium.
Acknowledgments
Funding Sources: This research was supported by National Eye Institute Grant EY022417 awarded to Dr. Suvas, Research to Prevent Blindness (RPB) to the Department of Ophthalmology, and Core Grant for Vision Research EY004068 awarded to Dr. Hazlett.
References
- 1.VE US, Gaddum JH. An unidentified depressor substance in certain tissue extracts. J Physiol. 1931;72:74–87. doi: 10.1113/jphysiol.1931.sp002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang MM, Leeman SE, Niall HD. Amino-acid sequence of substance P. Nat New Biol. 1971;232:86–87. doi: 10.1038/newbio232086a0. [DOI] [PubMed] [Google Scholar]
- 3.Pennefather JN, Lecci A, Candenas ML, Patak E, Pinto FM, Maggi CA. Tachykinins and tachykinin receptors: a growing family. Life Sci. 2004;74:1445–1463. doi: 10.1016/j.lfs.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 4.Krause JE, Chirgwin JM, Carter MS, Xu ZS, Hershey AD. Three rat preprotachykinin mRNAs encode the neuropeptides substance P and neurokinin A. Proc Natl Acad Sci U S A. 1987;84:881–885. doi: 10.1073/pnas.84.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nawa H, Hirose T, Takashima H, Inayama S, Nakanishi S. Nucleotide sequences of cloned cDNAs for two types of bovine brain substance P precursor. Nature. 1983;306:32–36. doi: 10.1038/306032a0. [DOI] [PubMed] [Google Scholar]
- 6.Bonner TI, Affolter HU, Young AC, Young WS., 3rd A cDNA encoding the precursor of the rat neuropeptide, neurokinin B. Brain Res. 1987;388:243–249. doi: 10.1016/0169-328x(87)90031-3. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Lu L, Furlonger C, Wu GE, Paige CJ. Hemokinin is a hematopoietic-specific tachykinin that regulates B lymphopoiesis. Nat Immunol. 2000;1:392–397. doi: 10.1038/80826. [DOI] [PubMed] [Google Scholar]
- 8.Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev. 2014;94:265–301. doi: 10.1152/physrev.00031.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hokfelt T, Vincent S, Dalsgaard CJ, Skirboll L, Johansson O, Schultzberg M, Lundberg JM, Rosell S, Pernow B, Jancso G. Distribution of substance P in brain and periphery and its possible role as a co-transmitter. Ciba Found Symp. 1982:84–106. doi: 10.1002/9780470720738.ch6. [DOI] [PubMed] [Google Scholar]
- 10.De Camilli P, Jahn R. Pathways to regulated exocytosis in neurons. Annu Rev Physiol. 1990;52:625–645. doi: 10.1146/annurev.ph.52.030190.003205. [DOI] [PubMed] [Google Scholar]
- 11.Erdos EG, Skidgel RA. Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J. 1989;3:145–151. [PubMed] [Google Scholar]
- 12.Skidgel RA, Engelbrecht S, Johnson AR, Erdos EG. Hydrolysis of substance p and neurotensin by converting enzyme and neutral endopeptidase. Peptides. 1984;5:769–776. doi: 10.1016/0196-9781(84)90020-2. [DOI] [PubMed] [Google Scholar]
- 13.Rameshwar P, Oh HS, Yook C, Gascon P, Chang VT. Substance p-fibronectin-cytokine interactions in myeloproliferative disorders with bone marrow fibrosis. Acta Haematol. 2003;109:1–10. doi: 10.1159/000067268. [DOI] [PubMed] [Google Scholar]
- 14.Corbally N, Powell D, Tipton KF. The binding of endogenous and exogenous substance-P in human plasma. Biochem Pharmacol. 1990;39:1161–1166. doi: 10.1016/0006-2952(90)90257-l. [DOI] [PubMed] [Google Scholar]
- 15.Maggi CA, Patacchini R, Rovero P, Giachetti A. Tachykinin receptors and tachykinin receptor antagonists. J Auton Pharmacol. 1993;13:23–93. doi: 10.1111/j.1474-8673.1993.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 16.Gerard NP, Bao L, Xiao-Ping H, Gerard C. Molecular aspects of the tachykinin receptors. Regul Pept. 1993;43:21–35. doi: 10.1016/0167-0115(93)90404-v. [DOI] [PubMed] [Google Scholar]
- 17.Mantyh PW. Neurobiology of substance P and the NK1 receptor. J Clin Psychiatry. 2002;63(11):6–10. [PubMed] [Google Scholar]
- 18.Lai JP, Cnaan A, Zhao H, Douglas SD. Detection of full-length and truncated neurokinin-1 receptor mRNA expression in human brain regions. J Neurosci Methods. 2008;168:127–133. doi: 10.1016/j.jneumeth.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai JP, Ho WZ, Kilpatrick LE, Wang X, Tuluc F, Korchak HM, Douglas SD. Full-length and truncated neurokinin-1 receptor expression and function during monocyte/macrophage differentiation. Proc Natl Acad Sci U S A. 2006;103:7771–7776. doi: 10.1073/pnas.0602563103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fong TM, Anderson SA, Yu H, Huang RR, Strader CD. Differential activation of intracellular effector by two isoforms of human neurokinin-1 receptor. Mol Pharmacol. 1992;41:24–30. [PubMed] [Google Scholar]
- 21.Baker SJ, Morris JL, Gibbins IL. Cloning of a C-terminally truncated NK-1 receptor from guinea-pig nervous system. Brain Res Mol Brain Res. 2003;111:136–147. doi: 10.1016/s0169-328x(03)00002-0. [DOI] [PubMed] [Google Scholar]
- 22.Douglas SD, Leeman SE. Neurokinin-1 receptor: functional significance in the immune system in reference to selected infections and inflammation. Ann N Y Acad Sci. 2011;1217:83–95. doi: 10.1111/j.1749-6632.2010.05826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McConalogue K, Dery O, Lovett M, Wong H, Walsh JH, Grady EF, Bunnett NW. Substance P-induced trafficking of beta-arrestins. The role of beta-arrestins in endocytosis of the neurokinin-1 receptor. J Biol Chem. 1999;274:16257–16268. doi: 10.1074/jbc.274.23.16257. [DOI] [PubMed] [Google Scholar]
- 24.Roosterman D, Cottrell GS, Schmidlin F, Steinhoff M, Bunnett NW. Recycling and resensitization of the neurokinin 1 receptor. Influence of agonist concentration and Rab GTPases. J Biol Chem. 2004;279:30670–30679. doi: 10.1074/jbc.M402479200. [DOI] [PubMed] [Google Scholar]
- 25.Cottrell GS, Padilla B, Pikios S, Roosterman D, Steinhoff M, Gehringer D, Grady EF, Bunnett NW. Ubiquitin-dependent down-regulation of the neurokinin-1 receptor. J Biol Chem. 2006;281:27773–27783. doi: 10.1074/jbc.M603369200. [DOI] [PubMed] [Google Scholar]
- 26.DeFea KA, Vaughn ZD, O'Bryan EM, Nishijima D, Dery O, Bunnett NW. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta -arrestin-dependent scaffolding complex. Proc Natl Acad Sci U S A. 2000;97:11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiebich BL, Schleicher S, Butcher RD, Craig A, Lieb K. The neuropeptide substance P activates p38 mitogen-activated protein kinase resulting in IL-6 expression independently from NF-kappa B. J Immunol. 2000;165:5606–5611. doi: 10.4049/jimmunol.165.10.5606. [DOI] [PubMed] [Google Scholar]
- 28.Lieb K, Fiebich BL, Berger M, Bauer J, Schulze-Osthoff K. The neuropeptide substance P activates transcription factor NF-kappa B and kappa B-dependent gene expression in human astrocytoma cells. J Immunol. 1997;159:4952–4958. [PubMed] [Google Scholar]
- 29.Sun J, Ramnath RD, Zhi L, Tamizhselvi R, Bhatia M. Substance P enhances NF-kappaB transactivation and chemokine response in murine macrophages via ERK1/2 and p38 MAPK signaling pathways. Am J Physiol Cell Physiol. 2008;294:C1586–1596. doi: 10.1152/ajpcell.00129.2008. [DOI] [PubMed] [Google Scholar]
- 30.Gillespie E, Leeman SE, Watts LA, Coukos JA, O'Brien MJ, Cerda SR, Farraye FA, Stucchi AF, Becker JM. Truncated neurokinin-1 receptor is increased in colonic epithelial cells from patients with colitis-associated cancer. Proc Natl Acad Sci U S A. 2011;108:17420–17425. doi: 10.1073/pnas.1114275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Leeman SE, Slack BE, Hauser G, Saltsman WS, Krause JE, Blusztajn JK, Boyd ND. A substance P (neurokinin-1) receptor mutant carboxyl-terminally truncated to resemble a naturally occurring receptor isoform displays enhanced responsiveness and resistance to desensitization. Proc Natl Acad Sci U S A. 1997;94:9475–9480. doi: 10.1073/pnas.94.17.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai JP, Lai S, Tuluc F, Tansky MF, Kilpatrick LE, Leeman SE, Douglas SD. Differences in the length of the carboxyl terminus mediate functional properties of neurokinin-1 receptor. Proc Natl Acad Sci U S A. 2008;105:12605–12610. doi: 10.1073/pnas.0806632105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorton D, Bellinger DL, Felten SY, Felten DL. Substance P innervation of the rat thymus. Peptides. 1990;11:1269–1275. doi: 10.1016/0196-9781(90)90162-x. [DOI] [PubMed] [Google Scholar]
- 35.Lorton D, Bellinger DL, Felten SY, Felten DL. Substance P innervation of spleen in rats: nerve fibers associate with lymphocytes and macrophages in specific compartments of the spleen. Brain Behav Immun. 1991;5:29–40. doi: 10.1016/0889-1591(91)90005-u. [DOI] [PubMed] [Google Scholar]
- 36.Hukkanen M, Konttinen YT, Rees RG, Gibson SJ, Santavirta S, Polak JM. Innervation of bone from healthy and arthritic rats by substance P and calcitonin gene related peptide containing sensory fibers. J Rheumatol. 1992;19:1252–1259. [PubMed] [Google Scholar]
- 37.Skofitsch G, Savitt JM, Jacobowitz DM. Suggestive evidence for a functional unit between mast cells and substance P fibers in the rat diaphragm and mesentery. Histochemistry. 1985;82:5–8. doi: 10.1007/BF00502084. [DOI] [PubMed] [Google Scholar]
- 38.Stead RH, Tomioka M, Quinonez G, Simon GT, Felten SY, Bienenstock J. Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proc Natl Acad Sci U S A. 1987;84:2975–2979. doi: 10.1073/pnas.84.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dimitriadou V, Rouleau A, Trung Tuong MD, Newlands MD, Miller HR, Luffau G, Schwartz JC, Garbarg M. Functional relationships between sensory nerve fibers and mast cells of dura mater in normal and inflammatory conditions. Neuroscience. 1997;77:829–839. doi: 10.1016/s0306-4522(96)00488-5. [DOI] [PubMed] [Google Scholar]
- 40.Repke H, Bienert M. Structural requirements for mast cell triggering by substance P-like peptides. Agents Actions. 1988;23:207–210. doi: 10.1007/BF02142542. [DOI] [PubMed] [Google Scholar]
- 41.Columbo M, Horowitz EM, Kagey-Sobotka A, Lichtenstein LM. Substance P activates the release of histamine from human skin mast cells through a pertussis toxin-sensitive and protein kinase C-dependent mechanism. Clin Immunol Immunopathol. 1996;81:68–73. doi: 10.1006/clin.1996.0159. [DOI] [PubMed] [Google Scholar]
- 42.Okada T, Hirayama Y, Kishi S, Miyayasu K, Hiroi J, Fujii T. Functional neurokinin NK-1 receptor expression in rat peritoneal mast cells. Inflamm Res. 1999;48:274–279. doi: 10.1007/s000110050459. [DOI] [PubMed] [Google Scholar]
- 43.Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123:398–410. doi: 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bischoff SC, Schwengberg S, Lorentz A, Manns MP, Bektas H, Sann H, Levi-Schaffer F, Shanahan F, Schemann M. Substance P and other neuropeptides do not induce mediator release in isolated human intestinal mast cells. Neurogastroenterol Motil. 2004;16:185–193. doi: 10.1111/j.1365-2982.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- 45.Ansel JC, Brown JR, Payan DG, Brown MA. Substance P selectively activates TNF-alpha gene expression in murine mast cells. J Immunol. 1993;150:4478–4485. [PubMed] [Google Scholar]
- 46.Theoharides TC, Zhang B, Kempuraj D, Tagen M, Vasiadi M, Angelidou A, Alysandratos KD, Kalogeromitros D, Asadi S, Stavrianeas N, Peterson E, Leeman S, Conti P. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci U S A. 2010;107:4448–4453. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vergnolle N, Wallace JL, Bunnett NW, Hollenberg MD. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol Sci. 2001;22:146–152. doi: 10.1016/s0165-6147(00)01634-5. [DOI] [PubMed] [Google Scholar]
- 48.Aliakbari J, Sreedharan SP, Turck CW, Goetzl EJ. Selective localization of vasoactive intestinal peptide and substance P in human eosinophils. Biochem Biophys Res Commun. 1987;148:1440–1445. doi: 10.1016/s0006-291x(87)80293-0. [DOI] [PubMed] [Google Scholar]
- 49.Weinstock JV, Blum A, Walder J, Walder R. Eosinophils from granulomas in murine schistosomiasis mansoni produce substance P. J Immunol. 1988;141:961–966. [PubMed] [Google Scholar]
- 50.Metwali A, Blum AM, Ferraris L, Klein JS, Fiocchi C, Weinstock JV. Eosinophils within the healthy or inflamed human intestine produce substance P and vasoactive intestinal peptide. J Neuroimmunol. 1994;52:69–78. doi: 10.1016/0165-5728(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 51.Kroegel C, Giembycz MA, Barnes PJ. Characterization of eosinophil cell activation by peptides. Differential effects of substance P, melittin, and FMET-Leu-Phe. J Immunol. 1990;145:2581–2587. [PubMed] [Google Scholar]
- 52.Raap M, Rudrich U, Stander S, Gehring M, Kapp A, Raap U. Substance P activates human eosinophils. Exp Dermatol. 2015;24:557–559. doi: 10.1111/exd.12717. [DOI] [PubMed] [Google Scholar]
- 53.Tuncer LI, Alacam T, Oral B. Substance P expression is elevated in inflamed human periradicular tissue. J Endod. 2004;30:329–332. doi: 10.1097/00004770-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Killingsworth CR, Shore SA, Alessandrini F, Dey RD, Paulauskis JD. Rat alveolar macrophages express preprotachykinin gene-I mRNA-encoding tachykinins. Am J Physiol. 1997;273:L1073–1081. doi: 10.1152/ajplung.1997.273.5.L1073. [DOI] [PubMed] [Google Scholar]
- 55.Serra MC, Bazzoni F, Della Bianca V, Greskowiak M, Rossi F. Activation of human neutrophils by substance P. Effect on oxidative metabolism, exocytosis, cytosolic Ca2+ concentration and inositol phosphate formation. J Immunol. 1988;141:2118–2124. [PubMed] [Google Scholar]
- 56.Tanabe T, Otani H, Bao L, Mikami Y, Yasukura T, Ninomiya T, Ogawa R, Inagaki C. Intracellular signaling pathway of substance P-induced superoxide production in human neutrophils. Eur J Pharmacol. 1996;299:187–195. doi: 10.1016/0014-2999(95)00816-0. [DOI] [PubMed] [Google Scholar]
- 57.Bar-Shavit Z, Goldman R, Stabinsky Y, Gottlieb P, Fridkin M, Teichberg VI, Blumberg S. Enhancement of phagocytosis - a newly found activity of substance P residing in its N-terminal tetrapeptide sequence. Biochem Biophys Res Commun. 1980;94:1445–1451. doi: 10.1016/0006-291x(80)90581-1. [DOI] [PubMed] [Google Scholar]
- 58.Bhatia M, Saluja AK, Hofbauer B, Frossard JL, Lee HS, Castagliuolo I, Wang CC, Gerard N, Pothoulakis C, Steer ML. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc Natl Acad Sci U S A. 1998;95:4760–4765. doi: 10.1073/pnas.95.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahluwalia A, De Felipe C, O'Brien J, Hunt SP, Perretti M. Impaired IL-1beta-induced neutrophil accumulation in tachykinin NK1 receptor knockout mice. Br J Pharmacol. 1998;124:1013–1015. doi: 10.1038/sj.bjp.0701978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yano H, Wershil BK, Arizono N, Galli SJ. Substance P-induced augmentation of cutaneous vascular permeability and granulocyte infiltration in mice is mast cell dependent. J Clin Invest. 1989;84:1276–1286. doi: 10.1172/JCI114295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsuda H, Kawakita K, Kiso Y, Nakano T, Kitamura Y. Substance P induces granulocyte infiltration through degranulation of mast cells. J Immunol. 1989;142:927–931. [PubMed] [Google Scholar]
- 62.Sun J, Ramnath RD, Bhatia M. Neuropeptide substance P upregulates chemokine and chemokine receptor expression in primary mouse neutrophils. Am J Physiol Cell Physiol. 2007;293:C696–704. doi: 10.1152/ajpcell.00060.2007. [DOI] [PubMed] [Google Scholar]
- 63.Ho WZ, Lai JP, Zhu XH, Uvaydova M, Douglas SD. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J Immunol. 1997;159:5654–5660. [PubMed] [Google Scholar]
- 64.Bost KL, Breeding SA, Pascual DW. Modulation of the mRNAs encoding substance P and its receptor in rat macrophages by LPS. Reg Immunol. 1992;4:105–112. [PubMed] [Google Scholar]
- 65.Germonpre PR, Bullock GR, Lambrecht BN, Van De Velde V, Luyten WH, Joos GF, Pauwels RA. Presence of substance P and neurokinin 1 receptors in human sputum macrophages and U-937 cells. Eur Respir J. 1999;14:776–782. doi: 10.1034/j.1399-3003.1999.14d08.x. [DOI] [PubMed] [Google Scholar]
- 66.Lai JP, Ho WZ, Yang JH, Wang X, Song L, Douglas SD. A non-peptide substance P antagonist down-regulates SP mRNA expression in human mononuclear phagocytes. J Neuroimmunol. 2002;128:101–108. doi: 10.1016/s0165-5728(02)00164-9. [DOI] [PubMed] [Google Scholar]
- 67.Chernova I, Lai JP, Li H, Schwartz L, Tuluc F, Korchak HM, Douglas SD, Kilpatrick LE. Substance P (SP) enhances CCL5-induced chemotaxis and intracellular signaling in human monocytes, which express the truncated neurokinin-1 receptor (NK1R) J Leukoc Biol. 2009;85:154–164. doi: 10.1189/jlb.0408260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lai JP, Ho WZ, Zhan GX, Yi Y, Collman RG, Douglas SD. Substance P antagonist (CP-96,345) inhibits HIV-1 replication in human mononuclear phagocytes. Proc Natl Acad Sci U S A. 2001;98:3970–3975. doi: 10.1073/pnas.071052298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X, Douglas SD, Lai JP, Tuluc F, Tebas P, Ho WZ. Neurokinin-1 receptor antagonist (aprepitant) inhibits drug-resistant HIV-1 infection of macrophages in vitro. J Neuroimmune Pharmacol. 2007;2:42–48. doi: 10.1007/s11481-006-9059-6. [DOI] [PubMed] [Google Scholar]
- 70.Kincy-Cain T, Bost KL. Substance P-induced IL-12 production by murine macrophages. J Immunol. 1997;158:2334–2339. [PubMed] [Google Scholar]
- 71.Arsenescu R, Blum AM, Metwali A, Elliott DE, Weinstock JV. IL-12 induction of mRNA encoding substance P in murine macrophages from the spleen and sites of inflammation. J Immunol. 2005;174:3906–3911. doi: 10.4049/jimmunol.174.7.3906. [DOI] [PubMed] [Google Scholar]
- 72.Blum A, Setiawan T, Hang L, Stoyanoff K, Weinstock JV. Interleukin-12 (IL-12) and IL-23 induction of substance p synthesis in murine T cells and macrophages is subject to IL-10 and transforming growth factor beta regulation. Infect Immun. 2008;76:3651–3656. doi: 10.1128/IAI.00358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marriott I, Mason MJ, Elhofy A, Bost KL. Substance P activates NF-kappaB independent of elevations in intracellular calcium in murine macrophages and dendritic cells. J Neuroimmunol. 2000;102:163–171. doi: 10.1016/s0165-5728(99)00182-4. [DOI] [PubMed] [Google Scholar]
- 74.Marriott I, Bost KL. IL-4 and IFN-gamma up-regulate substance P receptor expression in murine peritoneal macrophages. J Immunol. 2000;165:182–191. doi: 10.4049/jimmunol.165.1.182. [DOI] [PubMed] [Google Scholar]
- 75.Lambrecht BN, Germonpre PR, Everaert EG, Carro-Muino I, De Veerman M, de Felipe C, Hunt SP, Thielemans K, Joos GF, Pauwels RA. Endogenously produced substance P contributes to lymphocyte proliferation induced by dendritic cells and direct TCR ligation. Eur J Immunol. 1999;29:3815–3825. doi: 10.1002/(SICI)1521-4141(199912)29:12<3815::AID-IMMU3815>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 76.Marriott I, Bost KL. Expression of authentic substance P receptors in murine and human dendritic cells. J Neuroimmunol. 2001;114:131–141. doi: 10.1016/s0165-5728(00)00466-5. [DOI] [PubMed] [Google Scholar]
- 77.Janelsins BM, Sumpter TL, Tkacheva OA, Rojas-Canales DM, Erdos G, Mathers AR, Shufesky WJ, Storkus WJ, Falo LD, Jr, Morelli AE, Larregina AT. Neurokinin-1 receptor agonists bias therapeutic dendritic cells to induce type 1 immunity by licensing host dendritic cells to produce IL-12. Blood. 2013;121:2923–2933. doi: 10.1182/blood-2012-07-446054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mathers AR, Tckacheva OA, Janelsins BM, Shufesky WJ, Morelli AE, Larregina AT. In vivo signaling through the neurokinin 1 receptor favors transgene expression by Langerhans cells and promotes the generation of Th1- and Tc1-biased immune responses. J Immunol. 2007;178:7006–7017. doi: 10.4049/jimmunol.178.11.7006. [DOI] [PubMed] [Google Scholar]
- 79.Janelsins BM, Mathers AR, Tkacheva OA, Erdos G, Shufesky WJ, Morelli AE, Larregina AT. Proinflammatory tachykinins that signal through the neurokinin 1 receptor promote survival of dendritic cells and potent cellular immunity. Blood. 2009;113:3017–3026. doi: 10.1182/blood-2008-06-163121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feistritzer C, Clausen J, Sturn DH, Djanani A, Gunsilius E, Wiedermann CJ, Kahler CM. Natural killer cell functions mediated by the neuropeptide substance P. Regul Pept. 2003;116:119–126. doi: 10.1016/s0167-0115(03)00193-9. [DOI] [PubMed] [Google Scholar]
- 81.Lighvani S, Huang X, Trivedi PP, Swanborg RH, Hazlett LD. Substance P regulates natural killer cell interferon-gamma production and resistance to Pseudomonas aeruginosa infection. Eur J Immunol. 2005;35:1567–1575. doi: 10.1002/eji.200425902. [DOI] [PubMed] [Google Scholar]
- 82.Douglas SD, Ho WZ, Gettes DR, Cnaan A, Zhao H, Leserman J, Petitto JM, Golden RN, Evans DL. Elevated substance P levels in HIV-infected men. AIDS. 2001;15:2043–2045. doi: 10.1097/00002030-200110190-00019. [DOI] [PubMed] [Google Scholar]
- 83.Evans DL, Lynch KG, Benton T, Dube B, Gettes DR, Tustin NB, Lai JP, Metzger D, Douglas SD. Selective serotonin reuptake inhibitor and substance P antagonist enhancement of natural killer cell innate immunity in human immunodeficiency virus/acquired immunodeficiency syndrome. Biol Psychiatry. 2008;63:899–905. doi: 10.1016/j.biopsych.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Monaco-Shawver L, Schwartz L, Tuluc F, Guo CJ, Lai JP, Gunnam SM, Kilpatrick LE, Banerjee PP, Douglas SD, Orange JS. Substance P inhibits natural killer cell cytotoxicity through the neurokinin-1 receptor. J Leukoc Biol. 2011;89:113–125. doi: 10.1189/jlb.0410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lang K, Drell TL, Niggemann B, Zanker KS, Entschladen F. Neurotransmitters regulate the migration and cytotoxicity in natural killer cells. Immunol Lett. 2003;90:165–172. doi: 10.1016/j.imlet.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 86.Flageole H, Senterman M, Trudel JL. Substance P increases in vitro lymphokine-activated-killer (LAK) cell cytotoxicity against fresh colorectal cancer cells. J Surg Res. 1992;53:445–449. doi: 10.1016/0022-4804(92)90088-h. [DOI] [PubMed] [Google Scholar]
- 87.Lai JP, Douglas SD, Ho WZ. Human lymphocytes express substance P and its receptor. J Neuroimmunol. 1998;86:80–86. doi: 10.1016/s0165-5728(98)00025-3. [DOI] [PubMed] [Google Scholar]
- 88.Blum AM, Metwali A, Crawford C, Li J, Qadir K, Elliott DE, Weinstock JV. Interleukin 12 and antigen independently induce substance P receptor expression in T cells in murine schistosomiasis mansoni. FASEB J. 2001;15:950–957. doi: 10.1096/fj.00-0379. [DOI] [PubMed] [Google Scholar]
- 89.Calvo CF, Chavanel G, Senik A. Substance P enhances IL-2 expression in activated human T cells. J Immunol. 1992;148:3498–3504. [PubMed] [Google Scholar]
- 90.Payan DG, Brewster DR, Goetzl EJ. Specific stimulation of human T lymphocytes by substance P. J Immunol. 1983;131:1613–1615. [PubMed] [Google Scholar]
- 91.Guo CJ, Lai JP, Luo HM, Douglas SD, Ho WZ. Substance P up-regulates macrophage inflammatory protein-1beta expression in human T lymphocytes. J Neuroimmunol. 2002;131:160–167. doi: 10.1016/s0165-5728(02)00277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cunin P, Caillon A, Corvaisier M, Garo E, Scotet M, Blanchard S, Delneste Y, Jeannin P. The tachykinins substance P and hemokinin-1 favor the generation of human memory Th17 cells by inducing IL-1beta, IL-23, and TNF-like 1A expression by monocytes. J Immunol. 2011;186:4175–4182. doi: 10.4049/jimmunol.1002535. [DOI] [PubMed] [Google Scholar]
- 93.Cook GA, Elliott D, Metwali A, Blum AM, Sandor M, Lynch R, Weinstock JV. Molecular evidence that granuloma T lymphocytes in murine schistosomiasis mansoni express an authentic substance P (NK-1) receptor. J Immunol. 1994;152:1830–1835. [PubMed] [Google Scholar]
- 94.Blum AM, Metwali A, Elliott DE, Weinstock JV. T cell substance P receptor governs antigen-elicited IFN-gamma production. Am J Physiol Gastrointest Liver Physiol. 2003;284:G197–204. doi: 10.1152/ajpgi.00271.2002. [DOI] [PubMed] [Google Scholar]
- 95.Bost KL. Tachykinin-modulated anti-viral responses. Front Biosci. 2004;9:1994–1998. doi: 10.2741/1376. [DOI] [PubMed] [Google Scholar]
- 96.Pascual DW. The role of tachykinins on bacterial infections. Front Biosci. 2004;9:3209–3217. doi: 10.2741/1473. [DOI] [PubMed] [Google Scholar]
- 97.Weinstock JV. The role of substance P, hemokinin and their receptor in governing mucosal inflammation and granulomatous responses. Front Biosci. 2004;9:1936–1943. doi: 10.2741/1375. [DOI] [PubMed] [Google Scholar]
- 98.Sunil-Chandra NP, Efstathiou S, Nash AA. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J Gen Virol. 1992;73(Pt 12):3275–3279. doi: 10.1099/0022-1317-73-12-3275. [DOI] [PubMed] [Google Scholar]
- 99.Flano E, Husain SM, Sample JT, Woodland DL, Blackman MA. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J Immunol. 2000;165:1074–1081. doi: 10.4049/jimmunol.165.2.1074. [DOI] [PubMed] [Google Scholar]
- 100.Elsawa SF, Taylor W, Petty CC, Marriott I, Weinstock JV, Bost KL. Reduced CTL response and increased viral burden in substance P receptor-deficient mice infected with murine gamma-herpesvirus 68. J Immunol. 2003;170:2605–2612. doi: 10.4049/jimmunol.170.5.2605. [DOI] [PubMed] [Google Scholar]
- 101.Payne CM, Heggie CJ, Brownstein DG, Stewart JP, Quinn JP. Role of tachykinins in the host response to murine gammaherpesvirus infection. J Virol. 2001;75:10467–10471. doi: 10.1128/JVI.75.21.10467-10471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Douglas SD, Cnaan A, Lynch KG, Benton T, Zhao H, Gettes DR, Evans DL. Elevated substance P levels in HIV-infected women in comparison to HIV-negative women. AIDS Res Hum Retroviruses. 2008;24:375–378. doi: 10.1089/aid.2007.0207. [DOI] [PubMed] [Google Scholar]
- 103.Ho WZ, Lai JP, Li Y, Douglas SD. HIV enhances substance P expression in human immune cells. FASEB J. 2002;16:616–618. doi: 10.1096/fj.01-0655fje. [DOI] [PubMed] [Google Scholar]
- 104.Ho WZ, Douglas SD. Substance P and neurokinin-1 receptor modulation of HIV. J Neuroimmunol. 2004;157:48–55. doi: 10.1016/j.jneuroim.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 105.Ho WZ, Cnaan A, Li YH, Zhao H, Lee HR, Song L, Douglas SD. Substance P modulates human immunodeficiency virus replication in human peripheral blood monocyte-derived macrophages. AIDS Res Hum Retroviruses. 1996;12:195–198. doi: 10.1089/aid.1996.12.195. [DOI] [PubMed] [Google Scholar]
- 106.Barrett JS, Spitsin S, Moorthy G, Barrett K, Baker K, Lackner A, Tulic F, Winters A, Evans DL, Douglas SD. Pharmacologic rationale for the NK1R antagonist, aprepitant as adjunctive therapy in HIV. J Transl Med. 2016;14:148. doi: 10.1186/s12967-016-0904-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carr MJ, Hunter DD, Jacoby DB, Undem BJ. Expression of tachykinins in nonnociceptive vagal afferent neurons during respiratory viral infection in guinea pigs. Am J Respir Crit Care Med. 2002;165:1071–1075. doi: 10.1164/ajrccm.165.8.2108065. [DOI] [PubMed] [Google Scholar]
- 108.Jacoby DB, Yost BL, Elwood T, Fryer AD. Effects of neurokinin receptor antagonists in virus-infected airways. Am J Physiol Lung Cell Mol Physiol. 2000;279:L59–65. doi: 10.1152/ajplung.2000.279.1.L59. [DOI] [PubMed] [Google Scholar]
- 109.Piedimonte G, Rodriguez MM, King KA, McLean S, Jiang X. Respiratory syncytial virus upregulates expression of the substance P receptor in rat lungs. Am J Physiol. 1999;277:L831–840. doi: 10.1152/ajplung.1999.277.4.L831. [DOI] [PubMed] [Google Scholar]
- 110.Auais A, Adkins B, Napchan G, Piedimonte G. Immunomodulatory effects of sensory nerves during respiratory syncytial virus infection in rats. Am J Physiol Lung Cell Mol Physiol. 2003;285:L105–113. doi: 10.1152/ajplung.00004.2003. [DOI] [PubMed] [Google Scholar]
- 111.Harrowe G, Mitsuhashi M, Payan DG. Measles virus-substance P receptor interactions. Possible novel mechanism of viral fusion. J Clin Invest. 1990;85:1324–1327. doi: 10.1172/JCI114571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Makhortova NR, Askovich P, Patterson CE, Gechman LA, Gerard NP, Rall GF. Neurokinin-1 enables measles virus trans-synaptic spread in neurons. Virology. 2007;362:235–244. doi: 10.1016/j.virol.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Svensson A, Kaim J, Mallard C, Olsson A, Brodin E, Hokfelt T, Eriksson K. Neurokinin 1 receptor signaling affects the local innate immune defense against genital herpes virus infection. J Immunol. 2005;175:6802–6811. doi: 10.4049/jimmunol.175.10.6802. [DOI] [PubMed] [Google Scholar]
- 114.Kincy-Cain T, Clements JD, Bost KL. Endogenous and exogenous interleukin-12 augment the protective immune response in mice orally challenged with Salmonella dublin. Infect Immun. 1996;64:1437–1440. doi: 10.1128/iai.64.4.1437-1440.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bost KL. Inducible preprotachykinin mRNA expression in mucosal lymphoid organs following oral immunization with Salmonella. J Neuroimmunol. 1995;62:59–67. doi: 10.1016/0165-5728(95)00103-9. [DOI] [PubMed] [Google Scholar]
- 116.Kincy-Cain T, Bost KL. Increased susceptibility of mice to Salmonella infection following in vivo treatment with the substance P antagonist, spantide II. J Immunol. 1996;157:255–264. [PubMed] [Google Scholar]
- 117.Chauhan VS, Kluttz JM, Bost KL, Marriott I. Prophylactic and therapeutic targeting of the neurokinin-1 receptor limits neuroinflammation in a murine model of pneumococcal meningitis. J Immunol. 2011;186:7255–7263. doi: 10.4049/jimmunol.1100721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chauhan VS, Sterka DG, Jr, Gray DL, Bost KL, Marriott I. Neurogenic exacerbation of microglial and astrocyte responses to Neisseria meningitidis and Borrelia burgdorferi. J Immunol. 2008;180:8241–8249. doi: 10.4049/jimmunol.180.12.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Garza A, Tweardy DJ, Weinstock J, Viswanathan B, Robinson P. Substance P signaling contributes to granuloma formation in Taenia crassiceps infection, a murine model of cysticercosis. J Biomed Biotechnol. 2010;2010:597086. doi: 10.1155/2010/597086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Blum AM, Metwali A, Cook G, Mathew RC, Elliott D, Weinstock JV. Substance P modulates antigen-induced, IFN-gamma production in murine Schistosomiasis mansoni. J Immunol. 1993;151:225–233. [PubMed] [Google Scholar]
- 121.Blum AM, Metwali A, Kim-Miller M, Li J, Qadir K, Elliott DE, Lu B, Fabry Z, Gerard N, Weinstock JV. The substance P receptor is necessary for a normal granulomatous response in murine schistosomiasis mansoni. J Immunol. 1999;162:6080–6085. [PubMed] [Google Scholar]
- 122.Kennedy PG, Rodgers J, Jennings FW, Murray M, Leeman SE, Burke JM. A substance P antagonist, RP-67,580, ameliorates a mouse meningoencephalitic response to Trypanosoma brucei brucei. Proc Natl Acad Sci U S A. 1997;94:4167–4170. doi: 10.1073/pnas.94.8.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kennedy PG, Rodgers J, Bradley B, Hunt SP, Gettinby G, Leeman SE, de Felipe C, Murray M. Clinical and neuroinflammatory responses to meningoencephalitis in substance P receptor knockout mice. Brain. 2003;126:1683–1690. doi: 10.1093/brain/awg160. [DOI] [PubMed] [Google Scholar]
- 124.Lever IJ, Grant AD, Pezet S, Gerard NP, Brain SD, Malcangio M. Basal and activity-induced release of substance P from primary afferent fibres in NK1 receptor knockout mice: evidence for negative feedback. Neuropharmacology. 2003;45:1101–1110. doi: 10.1016/s0028-3908(03)00298-3. [DOI] [PubMed] [Google Scholar]
- 125.Bignami F, Rama P, Ferrari G. Substance P and its Inhibition in Ocular Inflammation. Curr Drug Targets. 2016;17:1265–1274. doi: 10.2174/1389450116666151019100216. [DOI] [PubMed] [Google Scholar]
- 126.Chui J, Di Girolamo N, Coroneo MT, Wakefield D. The role of substance P in the pathogenesis of pterygia. Invest Ophthalmol Vis Sci. 2007;48:4482–4489. doi: 10.1167/iovs.07-0123. [DOI] [PubMed] [Google Scholar]
- 127.Fujishima H, Takeyama M, Takeuchi T, Saito I, Tsubota K. Elevated levels of substance P in tears of patients with allergic conjunctivitis and vernal keratoconjunctivitis. Clin Exp Allergy. 1997;27:372–378. [PubMed] [Google Scholar]
- 128.Tamura T. Olopatadine ophthalmic solution suppresses substance P release in the conjunctivitis models. Asia Pac Allergy. 2012;2:115–121. doi: 10.5415/apallergy.2012.2.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yamaji M, Takada M, Fujiwara R, Ohishi H, Izushi K, Sugimoto Y, Kamei C. Role of substance P in experimental allergic conjunctivitis in guinea pigs. Methods Find Exp Clin Pharmacol. 1997;19:637–643. [PubMed] [Google Scholar]
- 130.Callebaut I, Vandewalle E, Hox V, Bobic S, Jorissen M, Stalmans I, De Vries A, Scadding G, Hellings PW. Nasal corticosteroid treatment reduces substance P levels in tear fluid in allergic rhinoconjunctivitis. Ann Allergy Asthma Immunol. 2012;109:141–146. doi: 10.1016/j.anai.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 131.Lucas K, Karamichos D, Mathew R, Zieske JD, Stein-Streilein J. Retinal laser burn-induced neuropathy leads to substance P-dependent loss of ocular immune privilege. J Immunol. 2012;189:1237–1242. doi: 10.4049/jimmunol.1103264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Paunicka KJ, Mellon J, Robertson D, Petroll M, Brown JR, Niederkorn JY. Severing corneal nerves in one eye induces sympathetic loss of immune privilege and promotes rejection of future corneal allografts placed in either eye. Am J Transplant. 2015;15:1490–1501. doi: 10.1111/ajt.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ziche M, Morbidelli L, Pacini M, Geppetti P, Alessandri G, Maggi CA. Substance P stimulates neovascularization in vivo and proliferation of cultured endothelial cells. Microvasc Res. 1990;40:264–278. doi: 10.1016/0026-2862(90)90024-l. [DOI] [PubMed] [Google Scholar]
- 134.Greeno EW, Mantyh P, Vercellotti GM, Moldow CF. Functional neurokinin 1 receptors for substance P are expressed by human vascular endothelium. J Exp Med. 1993;177:1269–1276. doi: 10.1084/jem.177.5.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Villablanca AC, Murphy CJ, Reid TW. Growth-promoting effects of substance P on endothelial cells in vitro. Synergism with calcitonin gene-related peptide, insulin, and plasma factors. Circ Res. 1994;75:1113–1120. doi: 10.1161/01.res.75.6.1113. [DOI] [PubMed] [Google Scholar]
- 136.Kohara H, Tajima S, Yamamoto M, Tabata Y. Angiogenesis induced by controlled release of neuropeptide substance P. Biomaterials. 2010;31:8617–8625. doi: 10.1016/j.biomaterials.2010.07.079. [DOI] [PubMed] [Google Scholar]
- 137.Iwamoto I, Tomoe S, Tomioka H, Yoshida S. Substance P-induced granulocyte infiltration in mouse skin: the mast cell-dependent granulocyte infiltration by the N-terminal peptide is enhanced by the activation of vascular endothelial cells by the C-terminal peptide. Clin Exp Immunol. 1992;87:203–207. doi: 10.1111/j.1365-2249.1992.tb02975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lembeck F, Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979;310:175–183. doi: 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- 139.Eglezos A, Giuliani S, Viti G, Maggi CA. Direct evidence that capsaicin-induced plasma protein extravasation is mediated through tachykinin NK1 receptors. Eur J Pharmacol. 1991;209:277–279. doi: 10.1016/0014-2999(91)90183-q. [DOI] [PubMed] [Google Scholar]
- 140.Ramalho R, Almeida J, Fernandes R, Costa R, Pirraco A, Guardao L, Delgado L, Moreira A, Soares R. Neurokinin-1 receptor, a new modulator of lymphangiogenesis in obese-asthma phenotype. Life Sci. 2013;93:169–177. doi: 10.1016/j.lfs.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 141.Davis MJ, Lane MM, Davis AM, Durtschi D, Zawieja DC, Muthuchamy M, Gashev AA. Modulation of lymphatic muscle contractility by the neuropeptide substance P. Am J Physiol Heart Circ Physiol. 2008;295:H587–597. doi: 10.1152/ajpheart.01029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chakraborty S, Nepiyushchikh Z, Davis MJ, Zawieja DC, Muthuchamy M. Substance P activates both contractile and inflammatory pathways in lymphatics through the neurokinin receptors NK1R and NK3R. Microcirculation. 2011;18:24–35. doi: 10.1111/j.1549-8719.2010.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bachmann B, Taylor RS, Cursiefen C. The association between corneal neovascularization and visual acuity: a systematic review. Acta Ophthalmol. 2013;91:12–19. doi: 10.1111/j.1755-3768.2011.02312.x. [DOI] [PubMed] [Google Scholar]
- 144.Bignami F, Giacomini C, Lorusso A, Aramini A, Rama P, Ferrari G. NK1 receptor antagonists as a new treatment for corneal neovascularization. Invest Ophthalmol Vis Sci. 2014;55:6783–6794. doi: 10.1167/iovs.14-14553. [DOI] [PubMed] [Google Scholar]
- 145.Twardy BS, Channappanavar R, Suvas S. Substance P in the corneal stroma regulates the severity of herpetic stromal keratitis lesions. Invest Ophthalmol Vis Sci. 2011;52:8604–8613. doi: 10.1167/iovs.11-8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yun H, Lathrop KL, Hendricks RL. A Central Role for Sympathetic Nerves in Herpes Stromal Keratitis in Mice. Invest Ophthalmol Vis Sci. 2016;57:1749–1756. doi: 10.1167/iovs.16-19183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Freidin M, Kessler JA. Cytokine regulation of substance P expression in sympathetic neurons. Proc Natl Acad Sci U S A. 1991;88:3200–3203. doi: 10.1073/pnas.88.8.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McClellan SA, Zhang Y, Barrett RP, Hazlett LD. Substance P promotes susceptibility to Pseudomonas aeruginosa keratitis in resistant mice: anti-inflammatory mediators downregulated. Invest Ophthalmol Vis Sci. 2008;49:1502–1511. doi: 10.1167/iovs.07-1369. [DOI] [PubMed] [Google Scholar]
- 149.Foldenauer ME, McClellan SA, Barrett RP, Zhang Y, Hazlett LD. Substance P affects growth factors in Pseudomonas aeruginosa-infected mouse cornea. Cornea. 2012;31:1176–1188. doi: 10.1097/ICO.0b013e31824d6ffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hazlett LD, McClellan SA, Barrett RP, Liu J, Zhang Y, Lighvani S. Spantide I decreases type I cytokines, enhances IL-10, and reduces corneal perforation in susceptible mice after Pseudomonas aeruginosa infection. Invest Ophthalmol Vis Sci. 2007;48:797–807. doi: 10.1167/iovs.06-0882. [DOI] [PubMed] [Google Scholar]
- 151.Zhou Z, Barrett RP, McClellan SA, Zhang Y, Szliter EA, van Rooijen N, Hazlett LD. Substance P delays apoptosis, enhancing keratitis after Pseudomonas aeruginosa infection. Invest Ophthalmol Vis Sci. 2008;49:4458–4467. doi: 10.1167/iovs.08-1906. [DOI] [PubMed] [Google Scholar]
- 152.Jones MA, Marfurt CF. Peptidergic innervation of the rat cornea. Exp Eye Res. 1998;66:421–435. doi: 10.1006/exer.1997.0446. [DOI] [PubMed] [Google Scholar]
- 153.Yamada M, Ogata M, Kawai M, Mashima Y, Nishida T. Substance P and its metabolites in normal human tears. Invest Ophthalmol Vis Sci. 2002;43:2622–2625. [PubMed] [Google Scholar]
- 154.Tran MT, Lausch RN, Oakes JE. Substance P differentially stimulates IL-8 synthesis in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:3871–3877. [PubMed] [Google Scholar]
- 155.Sloniecka M, Le Roux S, Zhou Q, Danielson P. Substance P Enhances Keratocyte Migration and Neutrophil Recruitment through Interleukin-8. Mol Pharmacol. 2016;89:215–225. doi: 10.1124/mol.115.101014. [DOI] [PubMed] [Google Scholar]
- 156.Watanabe M, Nakayasu K, Iwatsu M, Kanai A. Endogenous substance P in corneal epithelial cells and keratocytes. Jpn J Ophthalmol. 2002;46:616–620. doi: 10.1016/s0021-5155(02)00617-2. [DOI] [PubMed] [Google Scholar]
- 157.Yang L, Di G, Qi X, Qu M, Wang Y, Duan H, Danielson P, Xie L, Zhou Q. Substance P promotes diabetic corneal epithelial wound healing through molecular mechanisms mediated via the neurokinin-1 receptor. Diabetes. 2014;63:4262–4274. doi: 10.2337/db14-0163. [DOI] [PubMed] [Google Scholar]
- 158.Hong HS, Lee J, Lee E, Kwon YS, Lee E, Ahn W, Jiang MH, Kim JC, Son Y. A new role of substance P as an injury-inducible messenger for mobilization of CD29(+) stromal-like cells. Nat Med. 2009;15:425–435. doi: 10.1038/nm.1909. [DOI] [PubMed] [Google Scholar]
- 159.Nishida T. Neurotrophic mediators and corneal wound healing. Ocul Surf. 2005;3:194–202. doi: 10.1016/s1542-0124(12)70206-9. [DOI] [PubMed] [Google Scholar]
- 160.Kingsley RE, Marfurt CF. Topical substance P and corneal epithelial wound closure in the rabbit. Invest Ophthalmol Vis Sci. 1997;38:388–395. [PubMed] [Google Scholar]
- 161.Nishida T, Nakamura M, Ofuji K, Reid TW, Mannis MJ, Murphy CJ. Synergistic effects of substance P with insulin-like growth factor-1 on epithelial migration of the cornea. J Cell Physiol. 1996;169:159–166. doi: 10.1002/(SICI)1097-4652(199610)169:1<159::AID-JCP16>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 162.Nakamura M, Ofuji K, Chikama T, Nishida T. The NK1 receptor and its participation in the synergistic enhancement of corneal epithelial migration by substance P and insulin-like growth factor-1. Br J Pharmacol. 1997;120:547–552. doi: 10.1038/sj.bjp.0700923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ofuji K, Nakamura M, Nishida T. Signaling regulation for synergistic effects of substance P and insulin-like growth factor-1 or epidermal growth factor on corneal epithelial migration. Jpn J Ophthalmol. 2000;44:1–8. doi: 10.1016/s0021-5155(99)00168-9. [DOI] [PubMed] [Google Scholar]
- 164.Nakamura M, Chikama T, Nishida T. Up-regulation of integrin alpha 5 expression by combination of substance P and insulin-like growth factor-1 in rabbit corneal epithelial cells. Biochem Biophys Res Commun. 1998;246:777–782. doi: 10.1006/bbrc.1998.8704. [DOI] [PubMed] [Google Scholar]
- 165.Nakamura M, Chikama T, Nishida T. Participation of p38 MAP kinase, but not p44/42 MAP kinase, in stimulation of corneal epithelial migration by substance P and IGF-1. Curr Eye Res. 2005;30:825–834. doi: 10.1080/02713680591006129. [DOI] [PubMed] [Google Scholar]
- 166.Nakamura M, Ofuji K, Chikama T, Nishida T. Combined effects of substance P and insulin-like growth factor-1 on corneal epithelial wound closure of rabbit in vivo. Curr Eye Res. 1997;16:275–278. doi: 10.1076/ceyr.16.3.275.15409. [DOI] [PubMed] [Google Scholar]
- 167.Nagano T, Nakamura M, Nakata K, Yamaguchi T, Takase K, Okahara A, Ikuse T, Nishida T. Effects of substance P and IGF-1 in corneal epithelial barrier function and wound healing in a rat model of neurotrophic keratopathy. Invest Ophthalmol Vis Sci. 2003;44:3810–3815. doi: 10.1167/iovs.03-0189. [DOI] [PubMed] [Google Scholar]
- 168.Nakamura M, Kawahara M, Nakata K, Nishida T. Restoration of corneal epithelial barrier function and wound healing by substance P and IGF-1 in rats with capsaicin-induced neurotrophic keratopathy. Invest Ophthalmol Vis Sci. 2003;44:2937–2940. doi: 10.1167/iovs.02-0868. [DOI] [PubMed] [Google Scholar]
- 169.Nakamura M, Chikama T, Nishida T. Synergistic effect with Phe-Gly-Leu-Met-NH2 of the C-terminal of substance P and insulin-like growth factor-1 on epithelial wound healing of rabbit cornea. Br J Pharmacol. 1999;127:489–497. doi: 10.1038/sj.bjp.0702550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nakamura M, Kawahara M, Morishige N, Chikama T, Nakata K, Nishida T. Promotion of corneal epithelial wound healing in diabetic rats by the combination of a substance P-derived peptide (FGLM-NH2) and insulin-like growth factor-1. Diabetologia. 2003;46:839–842. doi: 10.1007/s00125-003-1105-9. [DOI] [PubMed] [Google Scholar]
- 171.Yamada N, Yanai R, Kawamoto K, Nagano T, Nakamura M, Inui M, Nishida T. Promotion of corneal epithelial wound healing by a tetrapeptide (SSSR) derived from IGF-1. Invest Ophthalmol Vis Sci. 2006;47:3286–3292. doi: 10.1167/iovs.05-1205. [DOI] [PubMed] [Google Scholar]
- 172.Soloway MR, Stjernschantz J, Sears M. The miotic effect of substance P on the isolated rabbit iris. Invest Ophthalmol Vis Sci. 1981;20:47–52. [PubMed] [Google Scholar]
- 173.Shigematsu S, Yamauchi K, Nakajima K, Iijima S, Aizawa T, Hashizume K. IGF-1 regulates migration and angiogenesis of human endothelial cells. Endocr J. 1999;46(1):S59–62. doi: 10.1507/endocrj.46.suppl_s59. [DOI] [PubMed] [Google Scholar]
- 174.Chikama T, Fukuda K, Morishige N, Nishida T. Treatment of neurotrophic keratopathy with substance-P-derived peptide (FGLM) and insulin-like growth factor I. Lancet. 1998;351:1783–1784. doi: 10.1016/s0140-6736(98)24024-4. [DOI] [PubMed] [Google Scholar]
- 175.Yamada N, Matsuda R, Morishige N, Yanai R, Chikama TI, Nishida T, Ishimitsu T, Kamiya A. Open clinical study of eye-drops containing tetrapeptides derived from substance P and insulin-like growth factor-1 for treatment of persistent corneal epithelial defects associated with neurotrophic keratopathy. Br J Ophthalmol. 2008;92:896–900. doi: 10.1136/bjo.2007.130013. [DOI] [PubMed] [Google Scholar]
- 176.Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 177.Kovacs I, Ludany A, Koszegi T, Feher J, Kovacs B, Szolcsanyi J, Pinter E. Substance P released from sensory nerve endings influences tear secretion and goblet cell function in the rat. Neuropeptides. 2005;39:395–402. doi: 10.1016/j.npep.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 178.Gaddipati S, Rao P, Jerome AD, Burugula BB, Gerard NP, Suvas S. Loss of Neurokinin-1 Receptor Alters Ocular Surface Homeostasis and Promotes an Early Development of Herpes Stromal Keratitis. J Immunol. 2016;197 doi: 10.4049/jimmunol.1600836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yamada M, Ogata M, Kawai M, Mashima Y, Nishida T. Substance P in human tears. Cornea. 2003;22:S48–54. doi: 10.1097/00003226-200310001-00007. [DOI] [PubMed] [Google Scholar]
- 180.Yang L, Sui W, Li Y, Qi X, Wang Y, Zhou Q, Gao H. Substance P Inhibits Hyperosmotic Stress-Induced Apoptosis in Corneal Epithelial Cells through the Mechanism of Akt Activation and Reactive Oxygen Species Scavenging via the Neurokinin-1 Receptor. PLoS One. 2016;11:e0149865. doi: 10.1371/journal.pone.0149865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ko JA, Murata S, Nishida T. Up-regulation of the tight-junction protein ZO-1 by substance P and IGF-1 in A431 cells. Cell Biochem Funct. 2009;27:388–394. doi: 10.1002/cbf.1587. [DOI] [PubMed] [Google Scholar]
- 182.Ko JA, Yanai R, Nishida T. Up-regulation of ZO-1 expression and barrier function in cultured human corneal epithelial cells by substance P. FEBS Lett. 2009;583:2148–2153. doi: 10.1016/j.febslet.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 183.Araki-Sasaki K, Aizawa S, Hiramoto M, Nakamura M, Iwase O, Nakata K, Sasaki Y, Mano T, Handa H, Tano Y. Substance P-induced cadherin expression and its signal transduction in a cloned human corneal epithelial cell line. J Cell Physiol. 2000;182:189–195. doi: 10.1002/(SICI)1097-4652(200002)182:2<189::AID-JCP7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]