SYNOPSIS
A survey of the development of dental ceramics has been presented. Such an attempt provides a better understanding of the rationale behind the development and clinical indications of each class of ceramic material. Knowledge of the composition, microstructure, and properties of a material is critical for selecting the right material for specific applications. The key to successful ceramic restorations rests on material selection, manufacturing technique, and restoration design. This also includes the balancing of several factors such as residual stresses, tooth contact conditions, tooth size and shape, elastic modulus of the adhesives and tooth structure, surface state, etc.
Keywords: Dental ceramics, all-ceramic restorations, metal-ceramic restorations, porcelain, glass-ceramics, zirconia, ceramic-polymer interpenetrating network
Introduction
According to the American College of Prosthodontists, 178 million people in the US, which represents 55% of the US population, are missing at least one tooth and this number is expected to grow over the next two decades due to an aging population. Teeth play a critically important role in human life as loss of function reduces one’s ability to eat a balanced diet, with negative consequences for systemic health. Loss of esthetics can also negatively impact social function. Both function and esthetics can be restored with dental crowns and fixed dental prostheses (FDPs). Ceramics have become increasingly popular as restorative materials because of their esthetics, inertness, and biocompatibility. Today, 80.2% of crowns and fixed prostheses produced in the US are all-ceramic restorations, 16.9% are porcelain fused to metal (PFM), and the remaining 2.2% are full cast and 0.7% resin-based composite.1 Demands for more esthetic and metal-free restorations as well as soaring metal prices will likely increase further the number of all-ceramic prostheses.2 However, a major clinical concern is that ceramics are brittle and subject to fracture.3, 4 The financial drivers for developing fracture resistant and esthetic ceramics are high: the European crown and FDP market approached $2 billion in 2007;5 the global crown and FDP market was estimated to be $25 billion in 2010 and over $30 billion in 2015.6 This article provides an overview of the background and the current knowledge base associated with dental ceramics for restoration and metal-veneering including a historical review of the development of ceramic restorations and their limitations. It also includes a summary of the current state of the art of porcelain, glass-ceramics and polycrystalline ceramics. Finally, materials design considerations for dental prostheses are discussed.
The history of dental ceramics
Shortly after the introduction of porcelain into Europe in the early 18th century, Alexis Duchateau, a Parisian apothecary, introduced ceramics to dentistry when he successfully replaced his ivory dentures with porcelain. With the help of a Parisian dentist, Nicholas Dubois de Chemant, Duchateau, working in concert with a new, high-technology porcelain manufacturer in 1774, created a complete set of porcelain dentures. They must have been very well-made as they lasted Duchateau the rest of his life. The development of porcelain dentures was revolutionary in terms of esthetics and oral hygiene, and was recognized as such by and Edward Jenner (developer of the smallpox vaccine) and Faculty of Medicine Paris: “…united the qualities of beauty, solidity and comfort with the exigencies of hygiene.” Because the then-popular ivory-based or wood-based dentures often using cadaver teeth were all porous, they absorb oral fluids and eventually become badly stained and highly unhygienic. Also, these early porcelain dentures were dysfunctional because patients had to remove them in order to eat. In addition, those complete porcelain dentures were only intended for edentulous patients, requiring the removal of the remaining teeth from a patient’s mouth, a very painful procedure prior to the discovery of anesthesia by Horace Wells in the middle of the 19th century.
Porcelain inlays, onlays, and crowns were introduced by Charles Land in 1886,7 which ultimately led to the creation of esthetic and functional ceramic restorations. However, the original dental porcelain contained a high feldspathic glass content and was extremely brittle and weak (σ ~ 60 MPa, σ stands for strength).8, 9 Therefore, despite the esthetic advantage, the early version porcelain restorations were not widely applied in dentistry.10 Dental ceramics have become increasingly popular as restorative materials due to improvements in strength and the increased goodness of fit with development of pressing and CAD/CAM processes. The timeline of the development of dental ceramics from the inception of initial porcelain materials to modern ceramic compositions, along with processing technologies, are shown in Figure 1. The main compositions and pertinent mechanical properties of various dental ceramic materials, representative of major material classes and developments, are shown in Table 1.
Figure 1.
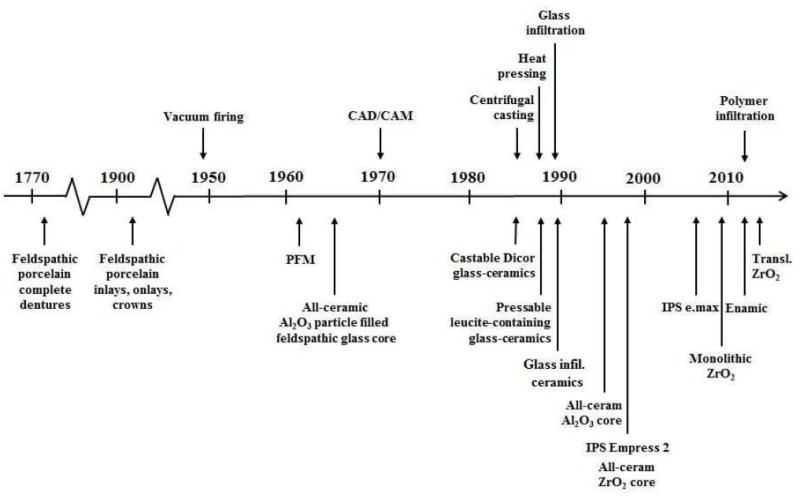
The timeline of the development of dental ceramics and their processing technologies.
Table 1.
Properties of various dental ceramic materials
| Material | Crystalline Phase (vol%) | Modulus E (GPa) | Hardness H (GPa) | Toughness T (MPa·m1/2) | Strength σ (MPa) |
|---|---|---|---|---|---|
| Porcelain | |||||
| Feldspathic ceramic (Vita Mark II) | Albite (<20) | 72 | 6.2 | 1.2 | 122 |
| Veneer for ceramic (LAVA Ceram) | Leucite (6) | 80 | 5.2 | 1.1 | 85 |
| Veneer for metal (d.SIGN) | Leucite/apatite (25) | 68 | 5.9 | 1.1 | 104 |
| Glass-ceramic | |||||
| Mica glass-ceramic (Dicor MGC) | Fluormica (70) | 69 | 6.0 | 1.2 | 229 |
| Leucite glass-ceramic (IPS Empress CAD) | Leucite (35-45) | 65 | 6.2 | 1.3 | 160 |
| Lithium disilicate-ceramic | |||||
| (IPS Empress 2) | Lithium disilicate (65) | 96 | 5.5-6.3 | 2.9-3.2 | 306-420 |
| (IPS e.max CAD) | Lithium disilicate (70) | 95 | 5.8 | 2.3 | 480 |
| (IPS e.max Press) | Lithium disilicate (70) | 95 | 5.8 | 2.8 | 400 |
| Ceramic-glass interpenetrating network | |||||
| Glass-infiltrated spinel | Spinel (68) | 185 | - | 2.5 | 350 |
| Glass-infiltrated alumina | Alumina (68) | 274 | 11.8 | 3.6 | 548 |
| Glass-infiltrated zirconia | Zirconia toughened alumina (67) | 245 | 13.1 | 3.5 | 700 |
| Polycrystalline ceramic | |||||
| Alumina (dense, fine grain) | Alumina (>99) | 372 | 19.6 | 3.1 | 572 |
| Zirconia (LAVA Plus) | 3 mol% Y-TZP (>99) | 210 | 14.0 | 4.0 | 1200 |
| Zirconia (Zpex smile) | Cubic/tetragonal zirconia (>99) | 210 | 13.4 | 2.4 | 485 |
| Ceramic-resin interpenetrating network | |||||
| Resin-infiltrated porcelain (Enamic) | Feldspathic ceramic (75) | 30 | 1.7 | 1.3 | 159 |
| Tooth | |||||
| Dentin | Hydroxyapatite (50) | 18 | 0.6 | 3.1 | 34-98 |
| Enamel | Hydroxyapatite (95) | 94 | 3.2 | 0.8 | 12-42 |
Since the Weinsteins solved the problem of the coefficient of thermal expansion (CTE) mismatch between the porcelain veneer and metal framework in 1962,11, 12 great improvements have been made in PFM systems. Until very recently, it was estimated that 70 – 80 % of fixed prostheses produced in the US were PFM (Private communications with Ivoclar Vivadent, 3M ESPE, Jensen Dental, Marotta Dental Studio, and Glidewell Laboratories). On the other hand, the dental community has long recognized that to realize the full potential of dental prostheses, all-ceramic restorations are necessary. Several strategies have been developed to improve the strength and fit of dental ceramics over the past 50 years. Other improvements in longevity have involved the use of high elastic moduli cores and build-up materials and cements to protect single crowns against bulk fracture.
One well-grounded approach to strengthening porcelain is to add uniformly dispersed filler particles to the glass matrix, a technique referred to as ‘dispersion strengthening’. One of the most successful particle fillers used in dental ceramics is leucite, a crystalline mineral possessing an index of refraction similar to that of feldspathic glasses.13 Commercial dental ceramics containing leucite as a strengthener include IPS Empress (σ ~ 138 MPa) (Ivoclar Vivadent, Schaan, Liechtenstein) and Finesse All-ceramic (σ ~ 125 MPa) (Dentsply International). Particle strengthening can also be achieved by heat-treating the glass to facilitate the precipitation and subsequent growth of crystallites within the glass, a process termed ‘ceraming’. Dental ceramics produced using the ceraming process are called glass-ceramics. Several commercial products such as Dicor (σ ~ 229 MPa) (Dentsply International), IPS Empress II (σ ~ 350 MPa) (Ivoclar Vivadent) and, more recently, IPS e.max Press and IPS e.max CAD (σ ~ 480 MPa) (Ivoclar Vivadent) fall into this category. The leucite-strengthened porcelains and the glass-ceramics are translucent, so single layer (monolithic) restorations can be made from these materials. The drawback is that only moderate strength increases can be achieved via the particle strengthening techniques. Therefore, monolithic ceramic restorations experienced high failure rates range from 4 – 6 % for Dicor molar crowns14, 15 and 3 – 4 % per year for IPS Empress crowns.16, 17
The traditional approach to the fracture problem of monolithic glass-ceramic restorations is to use a layer-structure with esthetics but weak porcelain veneers fused onto strong but opaque ceramic cores. The history of the development of higher-strength ceramic cores involves an increase in crystalline content (from ~40 vol% to 99.9 vol%) accompanied by a reduction in glass content. The first successful strengthened core ceramic was made of feldspathic glass filled with ~40 vol% of alumina particles.18 The alumina fillers increased the flexural strength of the ceramic to ~120 MPa with a trade off in translucency; hence veneering was required. In 1983 Coors Biomedical (Golden, CO) developed Cerestore all-ceramic restorations with a ceramic core containing ~60 wt% of Al2O3, 9 wt% MgO, a barium aluminosilicate glass at 13 wt%, and sufficient silicone (12 wt%) and kaolin clay (4 wt%) to impart sufficient plasticity for transfer molding at 160°C.19 It was reported that the alumina reacted with magnesia to form magnesium aluminate spinel – expanding to become ‘net-shape’. It is highly unlikely that this reaction occurred given the relatively low firing temperature of 1300°C and short firing time. Subsequent analysis showed that the ‘net-shape’ ability occurred due to oxidation of silicone-based releasing gaseous products leading to the crown blowing up like a loaf of bread contained within its mold.20 However, following universal problems with fractured restorations, the manufacturer withdrew the system. A similar product from the same era, the Hi-Ceram restorative system (Vita Zahnfabrik, Bad Säckingen, Germany) with its core material containing around the same amount of alumina as the Cerestore core, also failed to meet the requirements for posterior restorations.21 The Hi-Ceram system was replaced by In-Ceram (Vita Zahnfabrik) in 1990. The In-Ceram restoration had a core that was fabricated by lightly sintering an alumina powder compact and then infiltrating the still porous alumina matrix with a low viscosity glass containing lanthanum which lowered viscosity and increased the index of refraction of the infiltration glass. In contrast to Hi-Ceram, where ~60 vol% alumina particles were added to a glass matrix, In-Ceram alumina was derived from Sadoum’s invention, where glass was added (via high-temperature infiltration) into an alumina scaffold, resulting in an alumina-glass interpenetrating network structure. The final product contained ~70 vol% of alumina and had a flexural strength of ~450 MPa.22 Products along the same line are In-Ceram spinel and In-Ceram zirconia (toughened alumina). The former has a higher translucency but lower strength while the latter has a higher strength but lower translucency, relative to In-Ceram alumina. In 1993, Procera (Nobel Biocare, Göteborg, Sweden) presented a new all-ceramic restoration concept,23 where the fully dense core material contained 99.9 vol% alumina and displayed a flexural strength of 675 MPa. Several years later, even stronger Y-TZP ceramic was introduced to dentistry as a core material with a flexural strength over 1200 MPa.
Despite significant improvements in the performance of dental ceramics, the structural stability of all-ceramic systems remains less reliable than PFM systems where only non-biological complications are considered.24 Clinical studies have revealed that the primary cause of failure for lithium disilicate and alumina restorations are fracture in both veneer and framework, whereas that for zirconia-based restorations is cohesive fracture of the veneering porcelain.25 In an effort to circumvent the problem of veneer chipping and fracture, translucent glass-ceramic materials and, more recently, ‘cubic’ zirconias have been developed for monolithic restoration applications. However, these translucent ceramic materials are considerably weaker than the traditional dental tetragonal zirconia (Y-TZP), and thus cannot be used to replace the strong but more opaque Y-TZP.
The state of the art dental ceramics
Porcelain
Dental ceramics that best mimic the optical properties of natural teeth are predominantly glassy materials, which derive principally from feldspar-quartz-kaolin triaxial porcelain compositions.20, 26 Many technological advances have contributed to the use of porcelain in fixed prosthodontics, such as the development of the vacuum firing technology in 1949; the invention of the high-speed handpiece; the discovery of elastomeric impression materials; and the advent of pressing and CAD/CAM technologies in the 1980s.27 From a materialistic viewpoint, porcelain compositions have evolved from the original hard-paste Meissen porcelain, which contained a higher clay content and thus required a higher firing temperature, to the modern soft-paste porcelains that are comprised of mostly feldspar with no kaolin or quartz and possessed excellent translucency. Unfortunately, dental porcelains with the most desirable esthetics also tend to have the lowest strength and resistance to crack propagation, which severely limit their clinical indications.28-31 One major breakthrough came in 1962, when the Weinsteins, with the help of Koenig, developed a leucite-containing porcelain composition that could be fired directly onto common dental alloys.20 Leucite is a rock-forming mineral that is comprised of potassium alumino-silicate. At room temperature, leucite possesses a tetragonal structure. However, the crystal structure undergoes a tetragonal to cubic phase transformation at 625°C. This phase transformation is accompanied by a volume expansion of 1.2%, resulting in a high CTE (20 – 25 × 10-6/°C).32 Feldspar glass, on the other hand, has a relatively low CTE (~8 × 10-6/°C). Therefore, by varying the proportions of leucite and feldspar glass, porcelain frits with average CTEs matching that (12 – 14 × 10-6/°C) of dental alloys can be produced. A matching CTE between porcelain veneer and metal alloy coping prevents the development of deleterious thermal stresses upon cooling from firing temperatures. In fact, dental manufactures have also discovered that having the porcelain with a slightly lower CTE than the metal (typically differing from less than 1 × 10-6/°C) can place the porcelain in slight compression, thus increasing the fracture resistance of the restoration. The leucite content for tailoring the CTE of porcelain can vary from several wt% when coupled with ceramic frameworks to 17 – 25 wt% when matched with common metal alloys. Leucite is also an effective material for the dispersion strengthening of feldspar glass, since a large amount of leucite (up to 35 – 50 wt%) can be incorporated without significantly compromising its translucency. This is because the reflective index of leucite (n = 1.51) is very close to that of the feldspar glass (n = 1.52 – 1.53). In addition, owing to preferential etching of leucite crystals relative to the glass matrix, the leucite-containing feldspar glasses can be acid etched to create micromechanical features for resin bonding, thus making the restorations more fracture resistant. The microstructures of several commercial leucite-containing feldspathic ceramics used as veneers for ceramics and metals, as well as dispersion strengthened monolithic glass-ceramics are shown in Figure 2.
Figure 2.
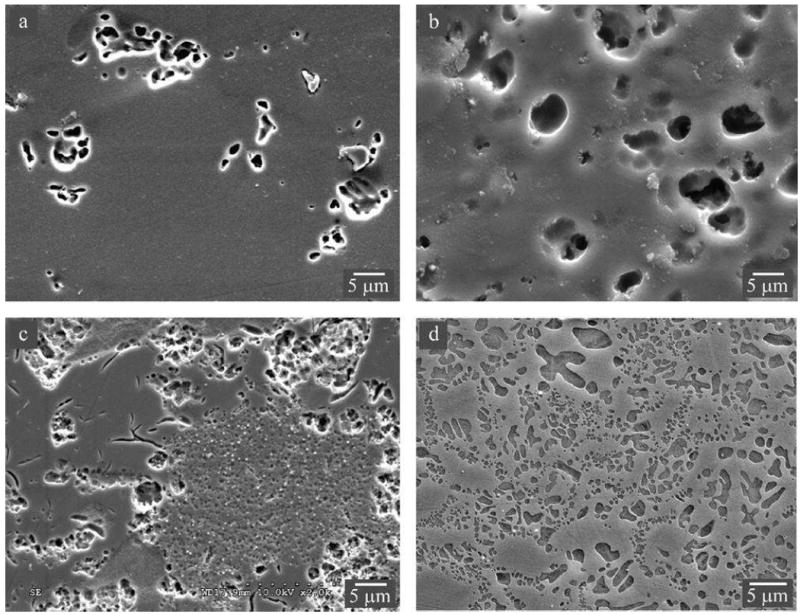
Microstructures of leucite-containing feldspathic ceramics. Images were taken using secondary electrons in a SEM. Feldspathic overlay porcelains for zirconia (A) LAVA Ceram and (B) Vita VM9. Porcelain overlay for metal (C) d.SIGN. A dispersion strengthened glass-ceramic (D) Empress CAD. Acid-etched surface revealing craters once occupied by leucite crystals and microcracks in the glassy matrix. Note: the leucite content increases from porcelain veneers for ceramic to metal to dispersion strengthened glass-ceramic.
Leucite feldspathic porcelain materials remain as some of the most esthetic and widely used dental ceramics. Their clinical indications include inlays, onlays, partial crowns, and crowns, as well as veneers for ceramics and metals. Clinical studies have shown that feldspathic porcelain restorations have excellent long-term success rates when bonded to and supported by primarily enamel structures. For example, the survival rate of inlays and onlays is 92% at 8 years;33 veneers 94% at 12 years34 and crowns 95% at 11 years.35 These findings suggest that this class of materials is ideal for cases where a significant amount of healthy tooth structure and enamel remain.28
The PFM technology has made it possible to fabricate more structurally demanding dental restorations, such as crowns and FDPs. PFM restorations are ideal for cases where minimal-to-no tooth structures remain28 and splinted restorations are required.36 The esthetic qualities of PFM are at their best when a high gold content framework material (e.g. Captek) is used.28 However, the trade-off is that the low-modulus of the high-gold framework provides little support to the porcelain veneer, resulting in a greater tendency for veneer fracture and chipping.37
Glass-ceramics
Glass-ceramics are much stronger and tougher, but also have lower translucency relative to porcelain. The strengthening and toughening of glass-ceramics are achieved by a ‘ceraming’ process, where crystals are precipitated under controlled heat-treatments from homogeneous glass through the nucleation and growth processes. The material Dicor was the first glass-ceramic material used for the fabrication of dental restorations. It consisted of fluormica crystals in the form of individual sheets or plates embedded in a glass matrix. Its microstructure, somewhat analogous to a house-of-cards, provides an interlocking mechanism for strengthening. However, due to its relatively poor mechanical performance in clinical applications, Dicor was withdrawn from the market. Some current leucite reinforced glasses are also produced via the ‘ceraming’ process. However, currently the most widely used and, arguably, the strongest and toughest dental glass-ceramics are made with lithium disilicate reinforcement.
The first dental lithium disilicate ceramic was fabricated from a base glass composition (SiO2-Li2O-Al2O3-K2O-P2O5-ZnO-La2O3) plus some additives for color and fluorescence. A homogeneous base glass ingot, containing a limited amount of lithium meta-silicate, was heated until it reached a viscous state, and then pressed into a mold. Through a judiciously controlled heat-treatment, a glass-ceramic containing ~70 vol% of elongated lithium disilicate crystals could be precipitated from the base glass to produce an interlocked microstructure. The resulting material possessed a flexural strength of 350 MPa and fracture toughness 2.9 MPa·m1/2, which were more than twice that of leucite-based glass-ceramics. The material was commercialized for dental framework use and marketed under the trade name IPS Empress 2. However, this material had high clinical failure rates at 9 – 50% after 24 – 60 months with a higher tendency of framework fracture in the connector area of shortspan posterior FDPs.38-40 These findings indicate insufficient flexural strength of the IPS Empress 2 framework for multi-unit prostheses. Subsequently, a new and improved lithium disilicate glass-ceramic (IPS e.max) with a much higher flexural strength (440 – 480 MPa) was developed. The improvements were made through the refinement of the base glass composition as well as by improving the quality of the initial glass-ingot (with fewer defects and pores). Compared to the base glass for IPS Empress 2, the new glass composition (SiO2-Li2O-Al2O3-K2O-P2O5-ZrO2) contained up to 4 wt% ZrO2 additives, while diminishing the ZnO and La2O3 contents (< 0.1 wt%).
The IPS e.max glass-ceramics came in two forms, Press and CAD (Figure 3), reflecting differences in processing conditions.41, 42 The IPS e.max Press ingots are heat-pressed at 920°C for 20 min. The IPS e.max CAD ingots are first heat treated to form the intermediate lithium meta-silicate glass-ceramics, which are easier to machine to shape. These are then heated to 840°C for 7 min, during which the lithium meta-silicate glass-ceramic is transformed to a chemically more stable and esthetically pleasing lithium disilicate glass-ceramic. Lithium disilicate Press and CAD have a glass matrix containing ~70% elongated, needle-like crystals. In the Press grade the crystallites are ~4 μm long and ~0.6 μm wide and somewhat aligned perpendicular to the external surfaces, whereas in the CAD grade the crystallites are ~1 μm long and ~0.4 μm wide and more randomly oriented. The Press grade exhibits slightly higher toughness because of the greater impedance to crack propagation by the larger grains (i.e. crystals). However, it also has slightly lower strength because these same grains introduce larger starting flaws into the structure (Table 1). Lithium disilicate glass-ceramics are indicated for veneers, anterior crowns, and posterior inlays and onlays. However, when fabricated to monolithic restorations and luted with resin cements, they are also suitable for single-unit, full-coverage crowns for molar teeth. In addition, the large elongated grains in lithium disilicate Press are thought to improve the fracture toughness by crack bridging and deflection. This is especially true in the connector areas of a FDP, where elongated crystals are preferentially oriented parallel to the tensile surface. Such a ‘logs-on-the-river’ structure can effectively improve the fracture resistance of the restoration. Indeed, long-term clinical data support the use of lithium disilicates as single restorations anywhere in the mouth43 and as shortspan FDPs in the anterior region.44
Figure 3.
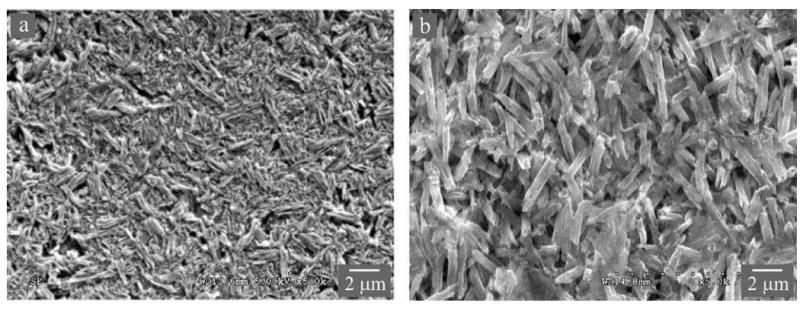
Microstructures of lithium disilicate glass-ceramics (A) CAD and (B) Press. Images were taken on an acid-etched surface using secondary electrons in a SEM, revealing elongated lithium disilicate crystallites. Note in the Press material (B), the preferential orientation of the ‘coarse’ elongated lithium disilicate crystallites.
Polycrystalline ceramics
Recent advances have created stronger and tougher ceramics, predominantly yttria-stabilized tetragonal zirconia polycrystals (Y-TZP) (Figure 4). However, Y-TZP has severe clinical deficiencies owing to its low translucency. The opacity of zirconia becomes a problem especially when placing an anterior crown or shortspan FDPs in the presence of natural teeth. In that case, the reflectance and light scattering do not appear natural. In order to create space for a porcelain veneer thick enough to cover an opaque zirconia core and to match the optical properties of the adjacent natural dentition, a substantial reduction of existing tooth structure is required. In addition, clinical research and practice have revealed that while zirconia frameworks are very fracture resistant, chipping45-52 and delamination53, 54 of the porcelain veneer are frequent problems. In 25 clinical trials on a variety of brands and makes of zirconia-based crowns and FDPs, chips and delaminations were consistently reported at 6–10% in 3–5 years in single crowns and 20–32% in 5–10 years in FDPs.51, 52, 55-75 In contrast, crowns and FDPs with metal frameworks revealed substantially lower fracture rates, ranging from 2.7% to 6% up to 15 years.76-79 One of the primary reasons for the poor clinical performance of PVZ bilayer prostheses is the low thermal conductivity of zirconia core relative to the metal coping, which could result in a large temperature gradient in the porcelain veneer on cooling, and thus residual thermal stresses become locked into the material system.80 While it is evident that the high chipping/fracture rate is due predominantly to these residual stresses, a comprehensive knowledge of the governing material (elastic modulus and CTE), design (veneer/core thickness ratio), and processing (cooling rate) parameters remains largely absent.80-84 Thus, this continues to be an active research area.
Figure 4.

Scanning electron micrograph, showing a typical fine-grained microstructure of high-strength dental zirconias (Y-TZP). Specimen surface was polished and thermally etched.
In an effort to avoid veneer chipping and delamination, monolithic zirconia is often used in full arch restorations, posterior crowns and FDPs.85-88 In all these cases, the opacity of Y-TZP zirconia remains a serious issue, although the white, opaque monolithic Y-TZP restorations may be suitable for bleached teeth.
After a decade of research and development, progress has been made in improving the translucency of Y-TZP by reducing porosity, decreasing grain size and eliminating any alumina added as a sintering aid.89 However, close examinations have revealed that unless they are thin (i.e. < 0.5 mm), so-called commercial translucent Y-TZP restorative materials remain largely opaque.90 Eliminating porosity and impurities alone is not sufficient to significantly improve the translucency of Y-TZP. Tetragonal zirconia is birefringent, meaning that the index of refraction is anisotropic in different crystallographic directions.89, 91 This causes reflection and refraction at grain boundaries, thus reducing light transmittance. Theory predicts that to make a Y-TZP ceramic sufficiently translucent while preserving strength, a sub-100 nm grain size is necessary, so that light may penetrate without substantial scattering.89, 91-93 However, it is technologically challenging to achieve densification without substantial grain growth beyond the critical 100 nm size.
The current approach to this problem is to introduce an optically isotropic cubic zirconia phase into an ordinarily tetragonal material (e.g. DDcubeX2 by Dental Direkt Materials and Zpex Smile by Tosoh Corporation). However, biphasic tetragonal/cubic zirconia is weaker and more brittle compared to its tetragonal counterpart. For instance, the flexural strength and fracture toughness of Zpex Smile (609 MPa and 2.4 MPa m1/2) are only just over one half of that of Y-TZP. They are in fact more like a dental alumina material (Procera alumina, Nobel Biocare),32, 94 and are also subject to low-temperature degradation. In general, increasing yttria content leads to a larger amount of cubic phase and thus greater translucency. The trade-off is that strength and toughness diminish as the cubic content increases. This has led to the development of several translucent dental zirconia materials containing various amounts of cubic phase. For example, the Katana ultra-translucent zirconia material has a flexural strength of 557 MPa, whereas their super-translucent and high-translucent zirconias have flexural strength of 748 and 1125 MPa, respectively. These translucent zirconia pucks also feature multi-layered color with a lighter shade in the occlusal 1/3 thickness and a darker shade at the gingival 1/3, sandwiching two relatively thinner transition layers. However, the mechanical integrity of these multilayered structures has yet to be evaluated.
New classes of materials on the horizon
The current esthetic and high fracture resistant restorative materials are either high crystalline ceramics or heavily particle filled resin composites. The elastic properties of these materials are not compatible with enamel or dentin substrate. Therefore, there is a higher tendency for restoration fracture to occur when a much stiffer ceramic material is used, and underlying tooth fracture to occur when a low modulus resin composite material is utilized.95 In addition, the current advent of great interest in minimally invasive dentistry and chairside one-visit restorations has resulted in the widespread usage of CAD/CAM technology. Ceramic restorative materials are susceptible to machining damage, especially when the restoration or part of the restoration is thin (marginal chipping, for example).96, 97
Recently, a new class of material—ceramic-polymer interpenetrating network (CPIN) material (Vita Enamic)—has been developed. The impetus for developing the CPIN material is to tailor the material properties, such as elastic modulus, strength, toughness, and hardness through judicious control of its composition and microstructure. The Enamic material consists of 86 wt% (75 vol%) of a feldspathic ceramic matrix into which is infiltrated by an organic phase of dimethacrylate resin containing UDMA and TEGDMA.98 The fabrication process of this material involves two steps: first, a porous pre-sintered ceramic network is produced and conditioned by a coupling agent; then, the network structure is infiltrated with the monomers by capillary action.99, 100 The resulting microstructure exhibits a hybrid structure with interpenetrating networks of ceramic and polymer (Figure 5), mimicking the interlocking of prism bands in natural teeth. The flexural strength, elastic modulus, hardness and fracture toughness of the Enamic material have been evaluated by several investigators,99, 100 and revealed similar properties to those of natural tooth structure (Table 1). Compared to ceramic restorative materials, Enamic has reliable millability and edge stability in terms of its ability to be fast milled into thin (< 0.5 mm) restorations with excellent precision.101 A full contour posterior crown takes just a little over 5 minutes to mill, while eliminating the need for post milling firing. The material is also easy to adjust and polish. Thus, it is an ideal material for chairside one-visit restorations.
Figure 5.
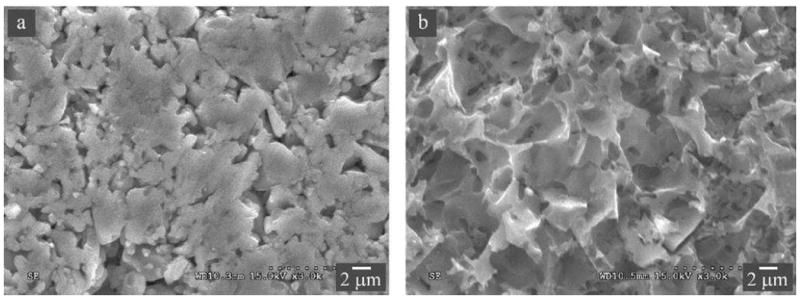
Microstructure of Vita Enamic observed using secondary electrons in a SEM. (A) A polished and then thermally etched surface, revealing a ceramic network structure consisting of ~25 vol% porosity following selective removal of the polymer phase. (B) A polished and then acid etched surface, showing the polymer network after selective removal of the surface ceramic material.
The 3-D interconnected dual network structure of CPIN differs from the resin-based composite (RBC) materials in which only the resin matrix is continuous. The most recent generation of lab fabricated millable RBC blocks, for example LAVA Ultimate from 3M and Cerasmart from GC, are heavily particle filled resins cured at a higher temperature and pressure. The filler particles in LAVA Ultimate are composed of dispersed silica (~20 nm) and zirconia (4 – 11 nm) nanoparticles, as well as silica/zirconia nanoparticle clusters (0.6 – 10 μm) (Figure 6). The rationale behind the usage of nanoclusters is that, compared to the traditional hard micron-sized filler particles, the nanoparticle clusters (analogous to a bunch of grapes) may not be as effective in terms of crack deflection and strengthening, but they are very effective for polish retention. The ‘large’ nanoclusters break down to nanoparticles upon mastication, leading to a smooth wear surface. However, the nanoclusters inevitably consist of defects and voids, which can soak up oral fluids, resulting in the discoloration and degradation of the RBC. Although the filler loading (80 – 90 wt% or 65 – 77 vol%) in the millable RBCs is similar to that of CPIN, their elastic properties and fracture behavior are quite different. In the case of CPIN materials, the interconnectivity of the ceramic phase provides stiffness and hardness that are necessary for the resistance to plastic deformation and wear. The ductile polymer network, on the other hand, is able to effectively distribute stresses in all directions.102 As a result, the 3-D interpenetrating dual network materials possess enhanced resistances to a variety of breakdown phenomena, including contact and flexural damage as well as fatigue crack growth and wear.98, 101-104
Figure 6.
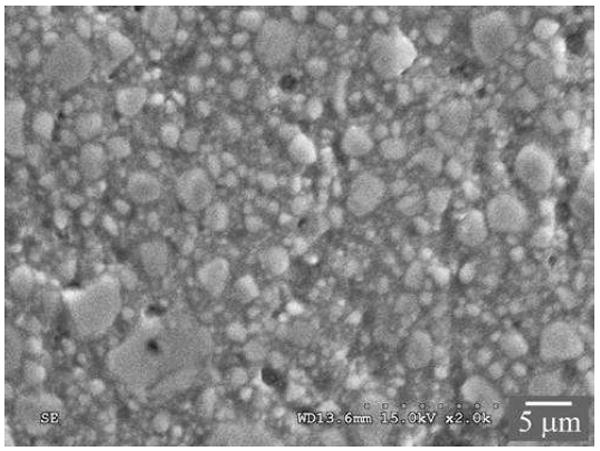
Scanning electron micrograph of a resin-based composite, Lava Ultimate. The material surface was polished down to 1 μm prior to imaging.
The CPIN material also differs from another interpenetrating network material (i.e. In-Ceram alumina) where alumina powders consisting of both coarse and fine particles were slip cast to ~70% density. The cast objects were sintered at 1000 – 1200°C to facilitate the formation of necks between the individual particles, while preventing significant shrinkage of the components. This was achieved by the presence of the coarse grains which prevented contraction and resulted in an interconnected porous structure throughout the object. The porous structure was then infiltrated with a low viscosity lanthanum-containing glass at 950 – 1000°C, during which infiltrating glass completely wetted the alumina scaffold under the influence of capillary forces. The resultant material consisted of a 3-D alumina (~70 vol%) and glass interpenetrating network structure. However, since both alumina and glass are brittle materials, only limited toughening mechanics (i.e. crack deflection) may be achieved and no significant stress distribution can occur.
It seems quite desirable to develop a new restorative material that combines the elastic modulus of RBC, which is much lower than that of dentin and even more so than enamel, with the long lasting esthetics of ceramics. This new CPIN material may offer a unique biomimetic alternative to traditional composites and ceramics. Clinically, Vita Enamic is suitable for single tooth restorations such as inlays, onlays, veneers, and crowns, including implant supported crowns and posterior restorations. There are no credible clinical data available concerning the longevity of Enamic restorations at this time. However, laboratory studies have shown that Enamic exhibits excellent resistance to wear and fatigue damage relative to traditional ceramic restorative materials.98, 101
Materials design considerations
Since the clinical performance, in particular the fracture resistance, of dental restorations is influenced by a host of variables, the restoration design and materials selection involve balancing several factors considered below. In addition, for reader convenience, some of the commonly observed clinical fracture modes are sketched in Figure 7.
Figure 7.
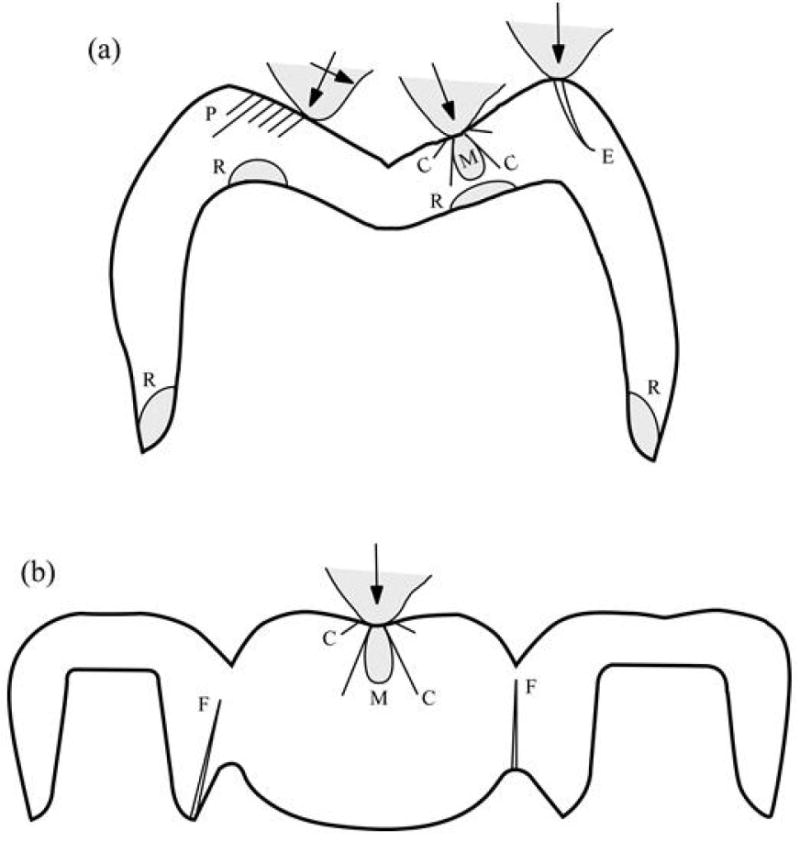
Schematic diagram illustrating various fracture modes in all-ceramic (A) crown and (B) FDP structures: axisymmetric cone (C) and median (M) cracks; partial cone (P) cracks; edge chipping (E) cracks; radial (R) cracks at cementation surfaces; flexure (F) cracks at connectors. Linear-trace cracks (C, P, E, F) extend out of the plane of diagram, shaded (R, M) cracks extend within the plane of diagram.
Modified from Zhang Y, Sailer I, Lawn BR. Fatigue of dental ceramics. Journal of Dentistry 2013; 41(12): 1136; with permission.
Material properties
Fracture in ceramics is governed by toughness and strength, and to a lesser extent by elastic modulus and hardness.105 For crown-like structures, increasing strength simply increases the resistance to crack initiation in these structures, while increasing toughness gives rise to the resistance to crack propagation.106-109 In many clinical trials covering numerous ceramic systems, fracture toughness of the core ceramic tracks well with clinical success. This fact was taken into consideration when designing a new ceramic classification system based upon known clinical indications now in the international standard ISO 6872. In addition, strength may be more relevant to FDP structures, where failure can occur by slow crack growth from a surface flaw, usually on the gingival side of connectors (Figure 7b). A higher modulus reduces layer flexure on a dentin base, actually lowering the failure trends for flexural radial (R) fracture (Figure 7a).3, 110 Increased hardness diminishes the susceptibility to quasi-plastic deformation (contact-induced plastic deformation in brittle materials, which is a precursor of median (M) cracks) and wear at the top surface, and therefore suppresses contact damage (Figure 7a). Interestingly, zirconia has higher toughness and strength than alumina, but lower modulus and hardness. Zirconia is also subject to other forms of long-term degradation, e.g. ‘aging’ from hydrothermal degradation associated with phase transformations.111-114 Porcelains are most vulnerable to damage, while glass–ceramics such as lithium disilicate occupy a middle ground. Accordingly, choice of material is a balancing act, and requires a fundamental materials science understanding.
Microstructure
Ultimately, material properties are determined by the underlying microstructure.115 Current dental ceramic technology borrows heavily from the science of materials fabrication, involving a complexity of starting powder preparation, processing additives and sintering treatments. Veneering ceramics are generally leucite-containing feldspathic porcelains, with the leucite in the form of crystallites to toughen the structure as well as to create a material thermally compatible with the ceramic framework.27, 116, 117 Glass–ceramics are likewise formed by heat-treatment crystallization of glass compositions. The key to superior properties is the choice of constituent starting powders and heat treatments. Lithium disilicates comprise the most recent and most durable of the glass-ceramics.41, 118 Up to 70 vol% needle-like crystallites result in moderately high strengths and toughness by virtue of their crack-containment properties.119 Alumina ceramics have been prepared in a variety of microstructures, but are now supplanted by zirconias. Zirconia properties are governed by many factors, including transformation phases (which confer toughness) and grain size.120 Translucent zirconias are fabricated via refinement of processing routes, beginning with ultra-fine equiaxed powders with yttrium stabilizer, reduction or elimination of light-scattering sintering aids and porosities, and higher sintering temperatures.121 Judicious microstructural control holds the key to future dental materials development.
Residual stresses
Residual stresses can develop in a porcelain veneer from CTE mismatch between the veneer and ceramic framework, and from rapid cooling during processing, especially in frameworks with low thermal diffusivities.80, 83, 84, 122-128 In some layer structures, thermal stresses may be beneficial, e.g. by placing a weak outer porcelain veneer into compression. However, thermal stresses must average out to zero across any layer section, so that compression in one part of a prosthesis must inevitably be counterbalanced by tension elsewhere.110 Moreover, these stresses are never uniform across the section, so any given layer may experience compression at one surface but tension at the other. Monolithic prostheses are not subject to the same concerns, although even there some stresses can arise from rapid cooling during processing, owing to the presence of substantial thermal gradients. Such stresses can have a profound influence on service lifetime.110
Monolithic versus veneered structures
Porcelain-veneered ceramics have superior esthetics, but are more vulnerable to fracture, especially chipping. Veneered crowns and FDPs still constitute mainstream dental practice, but are gradually being supplanted by monolithic prostheses fabricated from more resilient ceramics. Full-contour monoliths are much less susceptible to either occlusal surface or cementation fracture damage. The key to the advance of monoliths is improved esthetics. In modern-day zirconias, this is being achieved by fabricating more translucent microstructures, or by infiltrating glass into outer surfaces to produce graded structures.129-135
Layer thickness
In accord with intuition, thicker layers provide greater protection against fracture, partly because they diminish flexure and membrane stresses at any given occlusal load (a thickness squared relationship) and partly because they increase the distance cracks have to propagate before encountering a weak internal interface (veneered structures) or opposite surface (monoliths). The influence is strongest for radial cracks at the intaglio surface, with greater fatigue life with increased net layer thickness (Figure 7a).106, 107, 136 Interestingly, in veneered structures the critical bite forces to produce flexural radial cracks at the intaglio surface are only mildly sensitive to relative veneer-to-core thickness.137, 138 This allows one to tailor the veneer/core thickness ratio to optimize the residual stress profile while retaining the flexural strength of the veneered restoration.
Tooth contact conditions
Changes in contact geometry primarily affect the ease and extent of occlusal surface damage 139. Sharper, harder contacts in axial loading distribute the load over smaller areas, increasing local stresses and thereby making it easier to initiate cone (C) cracks (Figure 7).139 Such contacts are also likely to promote wear and abrasion damage and to initiate median (M) cracks.140 However, once these cracks grow away from the contact into the far field, they become less influenced by the nature of the contact.106, 107, 109 Radial cracks (especially at the margins) are relatively insensitive to contact conditions. Off-axis contacts can enhance the failure process by initiating partial cones (P) (sliding contacts) or edge chipping (E) (near-edge contacts) (Figure 7a). From a design aspect, it is advisable to avoid sharp cusps near the edges of crowns, to prevent incurring damage in the first place. Sharp cusps are also more prone to quasi-plastic deformation and wear. Contacts with soft materials relative to tooth modulus or hardness, e.g. normal food items, or with blunt objects, may suppress initiation of occlusal surface damage altogether by spreading the load over a greater area.141
Tooth size and shape
The geometry of prosthesis, most notably the dispositions of different cuspal shapes and connector configurations, plays a governing role in fracture resistance. Essentially, the greater the curvature (i.e. the smaller the radius) of a contacting surface, the lower the bite force to initiate cracks associated with layer flexure.142 Also, the smaller the crown height, the lower the force to drive longitudinal cracks around a side wall.143 Clearly, these geometrical factors will be governed by the spatial restrictions imposed by opposing and adjacent dentition.
Substrate modulus
The modulus of tooth dentin is about one fifth that of enamel and an even smaller fraction than that of most ceramics used in crowns and FDPs.144 A compliant substrate is an additional source of enhanced flexure,145-148 hence of radial fracture.149-152 The modulus of cements or adhesives used to bond the dental prostheses to the underlying tooth structure is a factor of two to five times lower still, further degrading the load-bearing capacity,145, 148, 153, 154 and even thin cement layers (e.g. < 0.1 mm) can substantially enhance crown flexure. The use of high-modulus build-up materials and dental cements would appear to be a useful strategy for minimizing flexural fractures.145
Surface state
It is evident that some precautions need to be taken in the preparation of prosthesis surfaces to stop cracks forming in the first place. Surface treatments can lead to the introduction of flaws that diminish strength. Aggressive sandblasting procedures with hard, coarse abrasive particles under high air pressure used to provide greater adhesion at the cementation surfaces of crowns fall into this category.155-157 Likewise, the use of coarse diamond burs to grind down crown cusps in order to adjust the occlusal surface enhances the prospect of crack initiation. On the other hand, while compromising the load-bearing capacity of a restoration, prematurely initiated cracks from such damage may arrest within the structure, with relatively little consequent effect on the final fracture condition.142
Summary
Ceramic restorations are developed for esthetics, biocompatibility, and chemical durability. The composition, microstructure, and properties of ceramic materials determine the clinical indications of various classes of dental ceramics. Other factors that influence material selection include restoration designs (monolithic or layered structure), layer thickness, residual stresses, tooth contact conditions, tooth size and shape, elastic modulus of the adhesives and substrate (enamel or dentin), and surface state. Successful application of ceramic restorations ultimately depends upon material selection, manufacturing technique, and restoration design.
KEY POINTS.
A facile understanding of the development, composition, microstructure, properties, and indications of various classes of ceramic dental materials.
Knowledge of the rationale behind the choice and usage of dental ceramics to maximize esthetics and durability.
To appreciate that successful ceramic restorations depend on the balancing of multiple factors.
Footnotes
DISCLOSURE
The Authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yu Zhang, Department of Biomaterials and Biomimetics, 433 First Avenue, Room 810, Department of Biomaterials and Biomimetics, New York University College of Dentistry, New York, NY 10010, USA.
J Robert Kelly, Department of Biomedical Engineering, UCONN Health, University of Connecticut, Farmington, CT 06030-1615, USA.
References
- 1.Christensen GJ. Is the rush to all-ceramic crowns justified? J Am Dent Assoc. 2014;145(2):192–194. doi: 10.14219/jada.2013.19. [DOI] [PubMed] [Google Scholar]
- 2.Chan C. US Markets for Crowns and Bridges 2011. Millennium Research Group, Inc.; Dec, 2010. p. 142025. [Google Scholar]
- 3.Lawn BR, Deng Y, Thompson VP. Use of contact testing in the characterization and design of all-ceramic crownlike layer structures: a review. J Prosthet Dent. 2001;86(5):495–510. doi: 10.1067/mpr.2001.119581. [DOI] [PubMed] [Google Scholar]
- 4.Griggs JA. Recent advances in materials for all-ceramic restorations. Dent Clin North Am. 2007;51(3):713–727, viii. doi: 10.1016/j.cden.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Markets for Crowns & Bridges 2008. Toronto, Ontario, Canada: Millennium Research Group; Dec, 2007. [2007]. [Google Scholar]
- 6.Palmer R. Dentistry without borders. dlpmagazinecom; 2010. [Google Scholar]
- 7.Land CH. Porcelain dental art: No. II.*. Dent Cosmos. 1903;45(8):615–620. [Google Scholar]
- 8.McLean JW. The Science and Art of Dental Ceramics. Chicago: Quintessence Publishing Co Inc.; 1979. [Google Scholar]
- 9.Binns D. The Chemical and Physical Properties of Dental Porcelain. Chicago: Quintessence Publishing Co Inc.; 1983. [Google Scholar]
- 10.van Noort R. Introduction to dental materials. 2. London: Mosby Ltd.; 2002. pp. 231–246. [Google Scholar]
- 11.Weinstein M, Katz S, Weinstein AB. 3 052 982. US patent. 1962
- 12.Weinstein M, Weinstein AB. 3 052 983. US patent. 1962
- 13.Denry IL. Recent advances in ceramics for dentistry. Crit Rev Oral Biol Med. 1996;7(2):134–143. doi: 10.1177/10454411960070020201. [DOI] [PubMed] [Google Scholar]
- 14.Malament KA, Socransky SS. Survival of Dicor glass-ceramic dental restorations over 14 years: part I. Survival of Dicor complete coverage restorations and effect of internal surface acid etching, tooth position, gender and age. J Prosthet Dent. 1999;81:23–32. doi: 10.1016/s0022-3913(99)70231-3. [DOI] [PubMed] [Google Scholar]
- 15.Sjogren G, Lantto R, Tillberg A. Clinical evaluation of all-ceramic crowns (Dicor) in general practice. J Prosthet Dent. 1999;81:277–284. doi: 10.1016/s0022-3913(99)70269-6. [DOI] [PubMed] [Google Scholar]
- 16.Fradeani M, Aquilano A. Clinical experience with Empress crowns. Int J Prosthodont. 1997;10(3):241–247. [PubMed] [Google Scholar]
- 17.Sjogren G, Lantto R, Granberg A, et al. Clinical examination of leucite-reinforced glass-ceramic crowns (Empress) in general practice: a retrospective study. Int J Prosthodont. 1999;12:122–128. [PubMed] [Google Scholar]
- 18.McLean JW, Hughs TH. The reinforcement of dental porcelain with ceramic oxides. Br Dent J. 1965;119:251–267. [PubMed] [Google Scholar]
- 19.Sozio RB, Riley EJ. The shrink-free ceramic crown. J Prosthet Dent. 1983;69:1982–1985. doi: 10.1016/0022-3913(83)90497-3. [DOI] [PubMed] [Google Scholar]
- 20.Kelly JR. Ceramics in restorative and prosthetic dentistry. Annu Rev Mater Sci. 1997;27:443–468. [Google Scholar]
- 21.Bieniek KW, Marx R. Die mechanische belastbarkeit neuer vollkeramischer kronen- und bruckenmaterialen. Schweitz Monatsschr Zahnmed. 1994;104:284–289. [PubMed] [Google Scholar]
- 22.Probster L, Diehl J. Slip-casting alumina ceramics for crown and bridge restorations. Quintessence Int. 1992;23(1):25–31. [PubMed] [Google Scholar]
- 23.Anderson M, Oden A. A new all-ceramic crown. A dense-sintered, high-purity alumina coping with porcelain. Acta Odontol Scand. 1993;51:59–64. doi: 10.3109/00016359309041149. [DOI] [PubMed] [Google Scholar]
- 24.Goodacre CJ, Bernal G, Rungcharassaeng K, et al. Clinical complications in fixed prosthodontics. J Prosthet Dent. 2003;90(1):31–41. doi: 10.1016/s0022-3913(03)00214-2. [DOI] [PubMed] [Google Scholar]
- 25.Conrad HJ, Seong WJ, Pesun IJ. Current ceramic materials and systems with clinical recommendations: a systematic review. J Prosthet Dent. 2007;98(5):389–404. doi: 10.1016/S0022-3913(07)60124-3. [DOI] [PubMed] [Google Scholar]
- 26.Kelly JR. Dental ceramics: current thinking and trends. Dent Clin N Am. 2004;48(2):513–530. doi: 10.1016/j.cden.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Kelly JR, Benetti P. Ceramic materials in dentistry: historical evolution and current practice. Aust Dent J. 2011;56(Suppl 1):84–96. doi: 10.1111/j.1834-7819.2010.01299.x. [DOI] [PubMed] [Google Scholar]
- 28.McLaren EA, Figueira J. Updating classifications of ceramic dental materials: a guide to material selection. Compend Contin Educ Dent. 2015;36(6):400–405. quiz 406, 416. [PubMed] [Google Scholar]
- 29.Peterson IM, Pajares A, Lawn BR, et al. Mechanical characterization of dental ceramics by hertzian contacts. J Dent Res. 1998;77(4):589–602. doi: 10.1177/00220345980770041201. [DOI] [PubMed] [Google Scholar]
- 30.Peterson IM, Wuttiphan S, Lawn BR, et al. Role of microstructure on contact damage and strength degradation of micaceous glass-ceramics. Dent Mater. 1998;14(1):80–89. doi: 10.1016/s0109-5641(98)00013-x. [DOI] [PubMed] [Google Scholar]
- 31.Scherrer SS, Kelly JR, Quinn GD, et al. Fracture toughness (KIc) of a dental porcelain determined by fractographic analysis. Dent Mater. 1999;15(5):342–348. doi: 10.1016/s0109-5641(99)00055-x. [DOI] [PubMed] [Google Scholar]
- 32.Denry IL, Holloway JA. Ceramics for dental applications: a review. Mater. 2010;3:351–368. [Google Scholar]
- 33.Kramer N, Frankenberger R. Clinical performance of bonded leucite-reinforced glass ceramic inlays and onlays after eight years. Dent Mater. 2005;21(3):262–271. doi: 10.1016/j.dental.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Fradeani M, Redemagni M, Corrado M. Porcelain laminate veneers: 6- to 12-year clinical evaluation--a retrospective study. Int J Periodontics Rest Dent. 2005;25(1):9–17. [PubMed] [Google Scholar]
- 35.Fradeani M, Redemagni M. An 11-year clinical evaluation of leucite-reinforced glass-ceramic crowns: a retrospective study. Quintessence Int. 2002;33(7):503–510. [PubMed] [Google Scholar]
- 36.Malament KA. Reflections on modem dental ceramics. Dent Today. 2015;34(11):10–12. [PubMed] [Google Scholar]
- 37.Kim B, Zhang Y, Pines M, et al. Fracture of porcelain-veneered structures in fatigue. J Dent Res. 2007;86(2):142–146. doi: 10.1177/154405910708600207. [DOI] [PubMed] [Google Scholar]
- 38.Esquivel-Upshaw JF, Anusavice KJ, Young H, et al. Clinical performance of a lithia disilicate-based core ceramic for three-unit posterior FPDs. Int J Prosthodont. 2004;17(4):469–475. [PubMed] [Google Scholar]
- 39.Marquardt P, Strub JR. Survival rates of IPS empress 2 all-ceramic crowns and fixed partial dentures: results of a 5-year prospective clinical study. Quintessence Int. 2006;37(4):253–259. [PubMed] [Google Scholar]
- 40.Taskonak B, Sertgoz A. Two-year clinical evaluation of lithia-disilicate-based all-ceramic crowns and fixed partial dentures. Dent Mater. 2006;22(11):1008–1013. doi: 10.1016/j.dental.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 41.Holand W, Schweiger M, Watzke R, et al. Ceramics as biomaterials for dental restoration. Expert Rev Med Devices. 2008;5(6):729–745. doi: 10.1586/17434440.5.6.729. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Lee JJ, Srikanth R, et al. Edge chipping and flexural resistance of monolithic ceramics. Dent Mater. 2013;29(12):1201–1208. doi: 10.1016/j.dental.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gehrt M, Wolfart S, Rafai N, et al. Clinical results of lithium-disilicate crowns after up to 9 years of service. Clin Oral Investig. 2013;17(1):275–284. doi: 10.1007/s00784-012-0700-x. [DOI] [PubMed] [Google Scholar]
- 44.Kern M, Sasse M, Wolfart S. Ten-year outcome of three-unit fixed dental prostheses made from monolithic lithium disilicate ceramic. J Am Dent Assoc. 2012;143(3):234–240. doi: 10.14219/jada.archive.2012.0147. [DOI] [PubMed] [Google Scholar]
- 45.Al-Amleh B, Lyons K, Swain M. Clinical trials in zirconia: a systematic review. J Oral Rehabil. 2010;37(8):641–652. doi: 10.1111/j.1365-2842.2010.02094.x. [DOI] [PubMed] [Google Scholar]
- 46.Christensen GJ. Porcelain-fused-to-metal versus zirconia-based ceramic restorations, 2009. J Am Dent Assoc. 2009;140(8):1036–1039. doi: 10.14219/jada.archive.2009.0316. [DOI] [PubMed] [Google Scholar]
- 47.Denry I, Kelly JR. State of the art of zirconia for dental applications. Dent Mater. 2008;24(3):299–307. doi: 10.1016/j.dental.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Sailer I, Feher A, Filser F, et al. Five-year clinical results of zirconia frameworks for posterior fixed partial dentures. Int J Prosthodont. 2007;20(4):383–388. [PubMed] [Google Scholar]
- 49.Sailer I, Pjetursson BE, Zwahlen M, et al. A systematic review of the survival and complication rates of all-ceramic and metal-ceramic reconstructions after an observation period of at least 3 years. Part II: Fixed dental prostheses. Clin Oral Implan Res. 2007;18(Suppl 3):86–96. doi: 10.1111/j.1600-0501.2007.01468.x. [DOI] [PubMed] [Google Scholar]
- 50.Larsson C, Vult Von Steyern P. Implant-supported full-arch zirconia-based mandibular fixed dental prostheses. Eight-year results from a clinical pilot study. Acta Odontol Scand. 2012;71(5):1118–1122. doi: 10.3109/00016357.2012.749518. [DOI] [PubMed] [Google Scholar]
- 51.Ortorp A, Kihl ML, Carlsson GE. A 5-year retrospective study of survival of zirconia single crowns fitted in a private clinical setting. J Dent. 2012;40(6):527–530. doi: 10.1016/j.jdent.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 52.Schmitter M, Mussotter K, Rammelsberg P, et al. Clinical performance of long-span zirconia frameworks for fixed dental prostheses: 5-year results. J Oral Rehabil. 2012;39(7):552–557. doi: 10.1111/j.1365-2842.2012.02311.x. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y, Liu G, Wang Y, et al. Failure modes and fracture origins of porcelain veneers on bilayer dental crowns. Int J Prosthodont. 2014;27(2):147–150. doi: 10.11607/ijp.3608. [DOI] [PubMed] [Google Scholar]
- 54.Pang Z, Chughtai A, Sailer I, et al. A fractographic study of clinically retrieved zirconia-ceramic and metal-ceramic fixed dental prostheses. Dent Mater. 2015;31(10):1198–1206. doi: 10.1016/j.dental.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sax C, Hammerle CH, Sailer I. 10-year clinical outcomes of fixed dental prostheses with zirconia frameworks. Int J Comput Dent. 2011;14(3):183–202. [PubMed] [Google Scholar]
- 56.Larsson C, Vult Von Steyern P. Implant-supported full-arch zirconia-based mandibular fixed dental prostheses. Eight-year results from a clinical pilot study. Acta Odontol Scand. 2013;71(5):1118–1122. doi: 10.3109/00016357.2012.749518. [DOI] [PubMed] [Google Scholar]
- 57.Larsson C, Vult von Steyern P, Nilner K. A prospective study of implant-supported full-arch yttria-stabilized tetragonal zirconia polycrystal mandibular fixed dental prostheses: three-year results. Int J Prosthodont. 2010;23(4):364–369. [PubMed] [Google Scholar]
- 58.Tsumita M, Kokubo Y, Ohkubo C, et al. Clinical evaluation of posterior all-ceramic FPDs (Cercon): a prospective clinical pilot study. J Prosthodont Res. 2010;54(2):102–105. doi: 10.1016/j.jpor.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 59.Schmitter M, Mussotter K, Rammelsberg P, et al. Clinical performance of extended zirconia frameworks for fixed dental prostheses: two-year results. J Oral Rehabil. 2009;36(8):610–615. doi: 10.1111/j.1365-2842.2009.01969.x. [DOI] [PubMed] [Google Scholar]
- 60.Bornemann G, Rinke S, Huels A. Prospective clinical trial with conventionally luted zirconia-based fixed partial dentures - 18-month results. J Dent Res. 2003;82:B117–B117. [Google Scholar]
- 61.Raigrodski AJ, Yu A, Chiche GJ, et al. Clinical efficacy of veneered zirconium dioxide-based posterior partial fixed dental prostheses: five-year results. J Prosthet Dent. 2012;108(4):214–222. doi: 10.1016/S0022-3913(12)60165-6. [DOI] [PubMed] [Google Scholar]
- 62.Salido MP, Martinez-Rus F, del Rio F, et al. Prospective clinical study of zirconia-based posterior four-unit fixed dental prostheses: four-year follow-up. Int J Prosthodont. 2012;25(4):403–409. [PubMed] [Google Scholar]
- 63.Pelaez J, Cogolludo PG, Serrano B, et al. A four-year prospective clinical evaluation of zirconia and metal-ceramic posterior fixed dental prostheses. Int J Prosthodont. 2012;25(5):451–458. [PubMed] [Google Scholar]
- 64.Schmitt J, Holst S, Wichmann M, et al. Zirconia posterior fixed partial dentures: a prospective clinical 3-year follow-up. Int J Prosthodont. 2009;22(6):597–603. [PubMed] [Google Scholar]
- 65.Raigrodski AJ, Chiche GJ, Potiket N, et al. The efficacy of posterior three-unit zirconium-oxide-based ceramic fixed partial dental prostheses: a prospective clinical pilot study. J Prosthet Dent. 2006;96(4):237–244. doi: 10.1016/j.prosdent.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 66.Pospiech P, Rountree P, Nothdurft F. Clinical evaluation of zirconia-based all-ceramic posterior bridges: two-year results. J Dent Res. 2003;82:114. [Google Scholar]
- 67.Crisp RJ, Cowan AJ, Lamb J, et al. A clinical evaluation of all-ceramic bridges placed in UK general dental practices: first-year results. Br Dent J. 2008;205(9):477–482. doi: 10.1038/sj.bdj.2008.937. [DOI] [PubMed] [Google Scholar]
- 68.Ohlmann B, Rammelsberg P, Schmitter M, et al. All-ceramic inlay-retained fixed partial dentures: preliminary results from a clinical study. J Dent. 2008;36(9):692–696. doi: 10.1016/j.jdent.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 69.Sorrentino R, De Simone G, Tete S, et al. Five-year prospective clinical study of posterior three-unit zirconia-based fixed dental prostheses. Clin Oral Investig. 2012;16(3):977–985. doi: 10.1007/s00784-011-0575-2. [DOI] [PubMed] [Google Scholar]
- 70.Ortorp A, Kihl ML, Carlsson GE. A 3-year retrospective and clinical follow-up study of zirconia single crowns performed in a private practice. J Dent. 2009;37(9):731–736. doi: 10.1016/j.jdent.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 71.Tinschert J, Schulze KA, Natt G, et al. Clinical behavior of zirconia-based fixed partial dentures made of DC-Zirkon: 3-year results. Int J Prosthodont. 2008;21(3):217–222. [PubMed] [Google Scholar]
- 72.Vult von Steyern P, Carlson P, Nilner K. All-ceramic fixed partial dentures designed according to the DC-Zirkon technique. A 2-year clinical study. J Oral Rehabil. 2005;32(3):180–187. doi: 10.1111/j.1365-2842.2004.01437.x. [DOI] [PubMed] [Google Scholar]
- 73.Molin MK, Karlsson SL. Five-year clinical prospective evaluation of zirconia-based Denzir 3-unit FPDs. Int J Prosthodont. 2008;21(3):223–227. [PubMed] [Google Scholar]
- 74.Larsson C, Vult von Steyern P, Sunzel B, et al. All-ceramic two- to five-unit implant-supported reconstructions. A randomized, prospective clinical trial. Swed Dent J. 2006;30(2):45–53. [PubMed] [Google Scholar]
- 75.Edelhoff D, Florian B, Florian W, et al. HIP zirconia fixed partial dentures--clinical results after 3 years of clinical service. Quintessence Int. 2008;39(6):459–471. [PubMed] [Google Scholar]
- 76.Valderhaug J. A 15-year clinical evaluation of fixed prosthodontics. Acta Odontol Scand. 1991;49(1):35–40. doi: 10.3109/00016359109041138. [DOI] [PubMed] [Google Scholar]
- 77.Walton TR. A 10-year longitudinal study of fixed prosthodontics: clinical characteristics and outcome of single-unit metal-ceramic crowns. Int J Prosthodont. 1999;12(6):519–526. [PubMed] [Google Scholar]
- 78.Walton TR. An up to 15-year longitudinal study of 515 metal-ceramic FPDs: Part 1. Outcome. Int J Prosthodont. 2002;15(5):439–445. [PubMed] [Google Scholar]
- 79.Walton TR. An up to 15-year longitudinal study of 515 metal-ceramic FPDs: Part 2. Modes of failure and influence of various clinical characteristics. Int J Prosthodont. 2003;16(2):177–182. [PubMed] [Google Scholar]
- 80.Swain MV. Unstable cracking (chipping) of veneering porcelain on all-ceramic dental crowns and fixed partial dentures. Acta Biomater. 2009;5(5):1668–1677. doi: 10.1016/j.actbio.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 81.Al-Amleh B, Neil Waddell J, Lyons K, et al. Influence of veneering porcelain thickness and cooling rate on residual stresses in zirconia molar crowns. Dent Mater. 2014;30(3):271–280. doi: 10.1016/j.dental.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 82.Baldassarri M, Stappert CF, Wolff MS, et al. Residual stresses in porcelain-veneered zirconia prostheses. Dent Mater. 2012;28(8):873–879. doi: 10.1016/j.dental.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Belli R, Monteiro S, Jr, Baratieri LN, et al. A photoelastic assessment of residual stresses in zirconia-veneer crowns. J Dent Res. 2012;91(3):316–320. doi: 10.1177/0022034511435100. [DOI] [PubMed] [Google Scholar]
- 84.Mainjot AK, Schajer GS, Vanheusden AJ, et al. Residual stress measurement in veneering ceramic by hole-drilling. Dent Mater. 2011;27(5):439–444. doi: 10.1016/j.dental.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 85.Beuer F, Stimmelmayr M, Gueth JF, et al. In vitro performance of full-contour zirconia single crowns. Dent Mater. 2012;28(4):449–456. doi: 10.1016/j.dental.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 86.Christensen R. Focus on: Monolithic crowns. Dent Today. 2013;32(3):22. [PubMed] [Google Scholar]
- 87.Rinke S, Fischer C. Range of indications for translucent zirconia modifications: clinical and technical aspects. Quintessence Int. 2013;44(8):557–566. doi: 10.3290/j.qi.a29937. [DOI] [PubMed] [Google Scholar]
- 88.Stober T, Bermejo JL, Rammelsberg P, et al. Enamel wear caused by monolithic zirconia crowns after 6 months of clinical use. J Oral Rehabil. 2014;41(4):314–322. doi: 10.1111/joor.12139. [DOI] [PubMed] [Google Scholar]
- 89.Zhang HB, Li ZP, Kim BN, et al. Effect of alumina dopant on transparency of tetragonal zirconia. J Nanomater. 2012:269065. Article ID 269064. [Google Scholar]
- 90.Tong H, Tanaka CB, Kaizer MR, et al. Characterization of three commercial Y-TZP ceramics produced for their high-translucency, high-strength and high-surface area. Ceram Int. 2016;42(1, Part B):1077–1085. doi: 10.1016/j.ceramint.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klimke J, Trunec M, Krell A. Transparent tetragonal yttria-stabilized zirconia ceramics: influence of scattering caused by birefringence. J Am Ceram Soc. 2011;94(6):1850–1858. [Google Scholar]
- 92.Zhang Y. Making yttria-stabilized tetragonal zirconia translucent. Dent Mater. 2014;30(10):1195–1203. doi: 10.1016/j.dental.2014.08.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anselmi-Tamburini U, Woolman JN, Munir ZA. Transparent nanometric cubic and tetragonal zirconia obtained by high-pressure pulsed electric current sintering. Adv Funct Mater. 2007;17(16):3267–3273. [Google Scholar]
- 94.Zeng K, Oden A, Rowcliffe D. Evaluation of mechanical properties of dental ceramic core materials in combination with porcelains. Int J Prosthodont. 1998;11(2):183–189. [PubMed] [Google Scholar]
- 95.Zhang Y, Mai Z, Barani A, et al. Fracture-resistant monolithic dental crowns. Dent Mater. 2016;32(3):442–449. doi: 10.1016/j.dental.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Giannetopoulos S, van Noort R, Tsitrou E. Evaluation of the marginal integrity of ceramic copings with different marginal angles using two different CAD/CAM systems. J Dent. 2010;38(12):980–986. doi: 10.1016/j.jdent.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 97.Tsitrou EA, Northeast SE, van Noort R. Brittleness index of machinable dental materials and its relation to the marginal chipping factor. J Dent. 2007;35(12):897–902. doi: 10.1016/j.jdent.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 98.El Zhawi H, Kaizer MR, Chughtai A, et al. Polymer infiltrated ceramic network structures for resistance to fatigue fracture and wear. Dent Mater. 2016;32(11):1352–1361. doi: 10.1016/j.dental.2016.08.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Coldea A, Swain MV, Thiel N. Mechanical properties of polymer-infiltrated-ceramic-network materials. Dent Mater. 2013;29(4):419–426. doi: 10.1016/j.dental.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 100.Della Bona A, Corazza PH, Zhang Y. Characterization of a polymer-infiltrated ceramic-network material. Dent Mater. 2014;30(5):564–569. doi: 10.1016/j.dental.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Swain MV, Coldea A, Bilkhair A, et al. Interpenetrating network ceramic-resin composite dental restorative materials. Dent Mater. 2016;32(1):34–42. doi: 10.1016/j.dental.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 102.Feng XQ, Mai YW, Qin QH. A micromechanical model for interpenetrating multiphase composites. Comput Mater Sci. 2003;28(3-4):486–493. [Google Scholar]
- 103.Coldea A, Fischer J, Swain MV, et al. Damage tolerance of indirect restorative materials (including PICN) after simulated bur adjustments. Dent Mater. 2015;31(6):684–694. doi: 10.1016/j.dental.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 104.Coldea A, Swain MV, Thiel N. Hertzian contact response and damage tolerance of dental ceramics. J Mech Behav Biomed Mater. 2014;34:124–133. doi: 10.1016/j.jmbbm.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 105.Lawn BR. Fracture of Brittle Solids. Second ed. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- 106.Bhowmick S, Zhang Y, Lawn BR. Competing fracture modes in brittle materials subject to concentrated cyclic loading in liquid environments: Bilayer structures. J Mater Res. 2005;20(10):2792–2800. [Google Scholar]
- 107.Hermann I, Bhowmick S, Zhang Y, et al. Competing fracture modes in brittle materials subject to concentrated cyclic loading in liquid environments: Trilayer structures. J Mater Res. 2006;21(2):512–521. [Google Scholar]
- 108.Lawn BR, Bhowmick S, Bush MB, et al. Failure modes in ceramic-based layer structures: a basis for materials design of dental crowns. J Am Ceram Soc. 2007;90(6):1671–1683. [Google Scholar]
- 109.Zhang Y, Bhowmick S, Lawn BR. Competing fracture modes in brittle materials subject to concentrated cyclic loading in liquid environments: monoliths. J Mater Res. 2005;20(8):2021–2029. [Google Scholar]
- 110.Hermann I, Bhowmick S, Lawn BR. Role of core support material in veneer failure of brittle layer structures. J Biomed Mater Res B Appl Biomater. 2007;82(1):115–121. doi: 10.1002/jbm.b.30712. [DOI] [PubMed] [Google Scholar]
- 111.Chevalier J. What future for zirconia as a biomaterial? Biomaterials. 2006;27:534–543. doi: 10.1016/j.biomaterials.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 112.Chevalier J, Cales B, Drouin JM. Low-temperature aging of Y-TZP ceramics. J Am Ceram Soc. 1999;82(8):2150–2154. [Google Scholar]
- 113.Chevalier J, Olagnon C, Fantozzi G. Crack propagation and fatigue in zirconia-based composites. Compos Part A Appl Sci and Manuf. 1999;30(4):525–530. [Google Scholar]
- 114.Kim JW, Covel NS, Guess PC, et al. Concerns of hydrothermal degradation in CAD/CAM zirconia. J Dent Res. 2010;89(1):91–95. doi: 10.1177/0022034509354193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Giordano R, McLaren EA. Ceramics overview: classification by microstructure and processing methods. Compend Contin Educ Dent. 2010;31(9):682–684, 686, 688 passim. quiz 698, 700. [PubMed] [Google Scholar]
- 116.Denry IL, Mackert JR, Jr, Holloway JA, et al. Effect of cubic leucite stabilization on the flexural strength of feldspathic dental porcelain. J Dent Res. 1996;75(12):1928–1935. doi: 10.1177/00220345960750120301. [DOI] [PubMed] [Google Scholar]
- 117.Mackert JR, Jr, Evans AL. Effect of cooling rate on leucite volume fraction in dental porcelains. J Dent Res. 1991;70(2):137–139. doi: 10.1177/00220345910700020801. [DOI] [PubMed] [Google Scholar]
- 118.Culp L, McLaren EA. Lithium disilicate: the restorative material of multiple options. Compend Contin Educ Dent. 2010;31(9):716–720, 722. 724–715. [PubMed] [Google Scholar]
- 119.Chai H, Lee JJ, Lawn BR. On the chipping and splitting of teeth. J Mech Behav Biomed Mater. 2011;4(3):315–321. doi: 10.1016/j.jmbbm.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 120.Hannink RHJ, Kelly PM, Muddle BC. Transformation toughening in zirconia-containing ceramics. J Am Ceram Soc. 2000;83(3):461–487. [Google Scholar]
- 121.Stawarczyk B, Ozcan M, Hallmann L, et al. The effect of zirconia sintering temperature on flexural strength, grain size, and contrast ratio. Clin Oral Investig. 2013;17(1):269–274. doi: 10.1007/s00784-012-0692-6. [DOI] [PubMed] [Google Scholar]
- 122.Baldassarri M, Zhang Y, Thompson VP, et al. Reliability and failure modes of implant-supported zirconium-oxide fixed dental prostheses related to veneering techniques. J Dent. 2011;39(7):489–498. doi: 10.1016/j.jdent.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Benetti P, Kelly JR, Della Bona A. Analysis of thermal distributions in veneered zirconia and metal restorations during firing. Dent Mater. 2013;29(11):1166–1172. doi: 10.1016/j.dental.2013.08.212. [DOI] [PubMed] [Google Scholar]
- 124.Mainjot AK, Schajer GS, Vanheusden AJ, et al. Influence of cooling rate on residual stress profile in veneering ceramic: measurement by hole-drilling. Dent Mater. 2011;27(9):906–914. doi: 10.1016/j.dental.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 125.Mainjot AK, Schajer GS, Vanheusden AJ, et al. Influence of zirconia framework thickness on residual stress profile in veneering ceramic: Measurement by hole-drilling. Dent Mater. 2012;28(4):378–384. doi: 10.1016/j.dental.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 126.Mainjot AK, Schajer GS, Vanheusden AJ, et al. Influence of veneer thickness on residual stress profile in veneering ceramic: measurement by hole-drilling. Dent Mater. 2012;28(2):160–167. doi: 10.1016/j.dental.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 127.Meira JB, Reis BR, Tanaka CB, et al. Residual stresses in Y-TZP crowns due to changes in the thermal contraction coefficient of veneers. Dent Mater. 2013;29(5):594–601. doi: 10.1016/j.dental.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 128.Tholey MJ, Swain MV, Thiel N. Thermal gradients and residual stresses in veneered Y-TZP frameworks. Dent Mater. 2011;27(11):1102–1110. doi: 10.1016/j.dental.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 129.Ren L, Janal MN, Zhang Y. Sliding contact fatigue of graded zirconia with external esthetic glass. J Dent Res. 2011;90(9):1116–1121. doi: 10.1177/0022034511412075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang Y. Overview: Damage resistance of graded ceramic restorative materials. J Eur Ceram Soc. 2012;32(11):2623–2632. doi: 10.1016/j.jeurceramsoc.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang Y, Chai H, Lawn BR. Graded structures for all-ceramic restorations. J Dent Res. 2010;89(4):417–421. doi: 10.1177/0022034510363245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang Y, Kim JW. Graded structures for damage resistant and aesthetic all-ceramic restorations. Dent Mater. 2009;25(6):781–790. doi: 10.1016/j.dental.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang Y, Kim JW. Graded zirconia glass for resistance to veneer fracture. J Dent Res. 2010;89(10):1057–1062. doi: 10.1177/0022034510375289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang Y, Ma L. Optimization of ceramic strength using elastic gradients. Acta Mater. 2009;57:2721–2729. doi: 10.1016/j.actamat.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang Y, Sun MJ, Zhang DZ. Designing functionally graded materials with superior load-bearing properties. Acta Biomater. 2012;8(3):1101–1108. doi: 10.1016/j.actbio.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bhowmick S, Melendez-Martinez JJ, Zhang Y, et al. Design maps for failure of all-ceramic layer structures in concentrated cyclic loading. Acta Mater. 2007;55(7):2479–2488. doi: 10.1016/j.actamat.2006.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Deng Y, Miranda P, Pajares A, et al. Fracture of ceramic/ceramic/polymer trilayers for biomechanical applications. J Biomed Mater Res A. 2003;67(3):828–833. doi: 10.1002/jbm.a.10161. [DOI] [PubMed] [Google Scholar]
- 138.Dibner AC, Kelly JR. Fatigue strength of bilayered ceramics under cyclic loading as a function of core veneer thickness ratios. J Prosthet Dent. 2016;115(3):335–340. doi: 10.1016/j.prosdent.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 139.Bhowmick S, Melendez-Martinez JJ, Hermann I, et al. Role of indenter material and size in veneer failure of brittle layer structures. J Biomed Mater Res B Appl Biomater. 2007;82(1):253–259. doi: 10.1002/jbm.b.30728. [DOI] [PubMed] [Google Scholar]
- 140.Ren L, Zhang Y. Sliding contact fracture of dental ceramics: principles and validation. Acta Biomater. 2014;10(7):3243–3253. doi: 10.1016/j.actbio.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Qasim T, Ford C, Bush MB, et al. Margin failures in brittle dome structures: relevance to failure of dental crowns. J Biomed Mater Res B Appl Biomater. 2007;80(1):78–85. doi: 10.1002/jbm.b.30571. [DOI] [PubMed] [Google Scholar]
- 142.Qasim T, Bush MB, Hu X, et al. Contact damage in brittle coating layers: influence of surface curvature. J Biomed Mater Res B Appl Biomater. 2005;73(1):179–185. doi: 10.1002/jbm.b.30188. [DOI] [PubMed] [Google Scholar]
- 143.Barani A, Keown AJ, Bush MB, et al. Role of tooth elongation in promoting fracture resistance. J Mech Behav Biomed Mater. 2012;8:37–46. doi: 10.1016/j.jmbbm.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 144.Xu HH, Smith DT, Jahanmir S, et al. Indentation damage and mechanical properties of human enamel and dentin. J Dent Res. 1998;77(3):472–480. doi: 10.1177/00220345980770030601. [DOI] [PubMed] [Google Scholar]
- 145.Zhang Y, Kim JW, Bhowmick S, et al. Competition of fracture mechanisms in monolithic dental ceramics: flat model systems. J Biomed Mater Res B Appl Biomater. 2009;88(2):402–411. doi: 10.1002/jbm.b.31100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Scherrer SS, de Rijk WG, Belser UC, et al. Effect of cement film thickness on the fracture resistance of a machinable glass-ceramic. Dent Mater. 1994;10(3):172–177. doi: 10.1016/0109-5641(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 147.Scherrer SS, de Rijk WG. The fracture resistance of all-ceramic crowns on supporting structures with different elastic moduli. Int J Prosthodont. 1993;6(5):462–467. [PubMed] [Google Scholar]
- 148.Ma L, Guess PC, Zhang Y. Load-bearing properties of minimal-invasive monolithic lithium disilicate and zirconia occlusal onlays: finite element and theoretical analyses. Dent Mater. 2013;29(7):742–751. doi: 10.1016/j.dental.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang Y, Lawn B. Long-term strength of ceramics for biomedical applications. J Biomed Mater Res B Appl Biomater. 2004;69(2):166–172. doi: 10.1002/jbm.b.20039. [DOI] [PubMed] [Google Scholar]
- 150.Lee KS, Jung Y-G, Peterson IM, et al. Model for cyclic fatigue of quasiplastic ceramics in contact with spheres. J Am Ceram Soc. 2000;83(9):2255–2262. [Google Scholar]
- 151.Lawn BR, Pajares A, Zhang Y, et al. Materials design in the performance of all-ceramic crowns. Biomaterials. 2004;25(14):2885–2892. doi: 10.1016/j.biomaterials.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 152.Kim JW, Bhowmick S, Chai H, et al. Role of substrate material in failure of crown-like layer structures. J Biomed Mater Res B Appl Biomater. 2007;81(2):305–311. doi: 10.1002/jbm.b.30666. [DOI] [PubMed] [Google Scholar]
- 153.Chai H, Lawn BR. Role of adhesive interlayer in transverse fracture of brittle layer structures. J Mater Res. 2000;15(4):1017–1024. [Google Scholar]
- 154.Kim JH, Miranda P, Kim DK, et al. Effect of an adhesive interlayer on the fracture of a brittle coating on a supporting substrate. J Mater Res. 2003;18(1):222–227. [Google Scholar]
- 155.Zhang Y, Lawn BR, Malament KA, et al. Damage accumulation and fatigue life of particle-abraded ceramics. Int J Prosthodont. 2006;19(5):442–448. [PubMed] [Google Scholar]
- 156.Zhang Y, Lawn BR, Rekow ED, et al. Effect of sandblasting on the long-term performance of dental ceramics. J Biomed Mater Res B Appl Biomater. 2004;71(2):381–386. doi: 10.1002/jbm.b.30097. [DOI] [PubMed] [Google Scholar]
- 157.Guess PC, Zhang Y, Kim JW, et al. Damage and reliability of Y-TZP after cementation surface treatment. J Dent Res. 2010;89(6):592–596. doi: 10.1177/0022034510363253. [DOI] [PMC free article] [PubMed] [Google Scholar]


