Abstract
Lipoxins are an important class of lipid mediators that induce the resolution of inflammation and arise from transcellular exchange of arachidonic acid (AA)-derived lipoxygenase products. Human epithelial 15-lipoxygenase-2 (h15-LOX-2), the major lipoxygenase in macrophages, has exhibited strict regiospecificity, catalyzing only the hydroperoxidation of carbon 15 of AA. To determine the catalytic potential of h15-LOX-2 in transcellular synthesis events, we reacted it with the three lipoxygenase-derived monohydroperoxy-eicosatetraenoic acids (HPETE) in humans: 5-HPETE, 12-HPETE, and 15-HPETE. Only 5-HPETE was a substrate for h15-LOX-2, and the steady-state catalytic efficiency (kcat/Km) of this reaction was 31% of the kcat/Km of AA. The only major product of h15-LOX-2’s reaction with 5-HPETE was the proposed lipoxin intermediate, 5,15-dihydroperoxy-eicosatetraenoic acid (5,15-diHPETE). However, h15-LOX-2 did not react further with 5,15-diHPETE to produce lipoxins. This result is consistent with the specificity of h15-LOX-2 despite the increased reactivity of 5,15-diHPETE. Density functional theory calculations determined that the radical, after abstracting the C10 hydrogen atom from 5,15-diHPETE, had an energy 5.4 kJ/mol lower than that of the same radical generated from AA, demonstrating the facility of 5,15-diHPETE to form lipoxins. Interestingly, h15-LOX-2 does react with 5S,6R-diHETE, forming LipoxinA4, indicating the gemdiol does not prohibit h15-LOX-2 reactivity. Taken together, these results demonstrate the strict regiospecificity of h15-LOX-2 that circumscribes its role in transcellular synthesis.
Graphical abstract
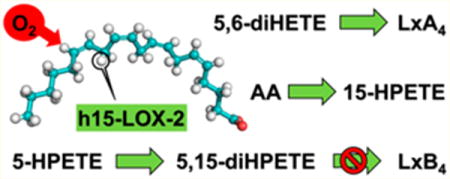
The acute inflammatory response is essential for host defense from pathogens or injury and is typically stimulated by lipid autacoids that recruit leukocytes to the affected area. The recruited leukocytes then release more lipid autacoids and other signaling molecules to either amplify the inflammatory response or resolve it.1 The regulation of this response and its resolution are crucial for homeostasis, as uncontrolled or chronic inflammation can result in a number of diseases, including atherosclerosis,2–4 diabetes,5,6 periodontal disease,7 autoimmune disorders,8 and cancer.9–13
Although many factors contribute to the overall inflammatory response and resolution, an important class of regulatory molecules, the eicosanoids, has demonstrated the ability to recruit neutrophils and macrophages, induce thrombus formation, regulate vasodilation/constriction, and even contribute to apoptosis.9,14–16 These eicosanoids are derived from arachidonic acid (AA) in leukocytes and other cells by cyclooxygenases or lipoxygenases. In humans, there are six known lipoxygenases (LOX): leukocyte 5-LOX (h5-LOX), platelet 12-LOX (h12-LOX), 12R-LOX, epidermal LOX-3, reticulocyte 15-LOX-1 (h15-LOX-1 or 12/15-LOX), and epithelial 15-LOX-2 (h15-LOX-2). Each LOX is classified by the carbon position of AA that is predominately oxygenated and the tissue in which each LOX was originally found.14 Lipoxygenases are non-heme iron-containing dioxygenases that synthesize eicosanoids by sequential hydroperoxidation of polyunsaturated fatty acids (PUFAs), either individually or in concert transcellularly.17–22 The lipoxygenase products that are of particular interest in this study are the lipoxins, which mediate inflammation catabasis via vasodilation/constriction, suppression of leukotriene-mediated inflammation, M2 macrophage recruitment, and effects on cytokine signaling.8,23,24 These lipoxins (lipoxygenase interaction products) are trihydroxylated eicosatetraenoic acids that result from the transcellular exchange of lipoxygenase products.21 Several transcellular exchange routes have been demonstrated to produce lipoxins in vivo and in vitro (Scheme 1).18,21,22,25–31 The first route starts with 15-hydroperoxy-5Z,8Z,10Z,13E-eicosatetraenoic acid (15-HPETE), produced by either of the h15-LOXs in reticulocytes, macrophages, endothelial cells, etc.17,21,26,32–34 This 15-HPETE can then be converted into 5S,15S-dihydroperoxy-6E,8Z,10Z,13E-eicosatetraenoic acid (5,15-diHPETE) by h5-LOX in neutrophils or other cells. The resulting 5,15-diHPETE can be further epoxidated by h5LOX to form 5S-trans-5,6-oxido-15S-hydroperoxy-7E,9E,11Z,14Z-eicosatetraenoic acid (15S-hydroperoxy-LTA4). 15S-hydroperoxy-LTA4 can be hydrolyzed by soluble epoxide hydrolase and reduced by glutathione peroxidase (GP) to form 5S,6R,15S-trihydroxy-7E,9E,11Z,13E-eicosatetraenoic acid (LipoxinA4 (LxA4)).21,26,34–36 Due to the instability of the 5,6-epoxide on 15S-hydroperoxy-LTA4, this intermediate can also be hydrolyzed nonenzymatically at carbon 6 and reduced by GP to form 5S,6S,15S-trihydroxy-7E,9E,11Z,13E-eicosate-traenoic acid or at carbon 14 to yield 5S,14R(or S),15S-trihydroxy-6E,8E,10E,12E-eicosatetraenoic acid (LxB4, all trans isomers).26,30 A similar lipoxin pathway involving a 5,15-diHPETE intermediate starts instead with 5-hydroperoxy-6E,8Z,11Z,14Z-eicosatetraenoic acid (5-HPETE) from h5-LOX.21,26 This 5-HPETE can then react with h15-LOX-1 to produce 5,15-diHPETE. This 5,15-diHPETE can then be epoxidated by h12-LOX or h15-LOX-1 to form 5S-hyroperoxy-15S-trans-14,15-oxido-6E,8Z,10E,12E-eicosatetraenoic acid. This product can be hydrolyzed by soluble epoxide hydrolase and reduced by GP to form 5S,14R,15S-trihydroxy-6E,8Z,10E,12E-eicosatetraenoic acid (LipoxinB4(LxB4)).21,26,31,37,38 Also, as with 15S-hydroperoxy-LTA4, the 14,15-epoxide is not stable and can be hydrolyzed non-enzymatically to form 5S,14S,15S-trihydroxy-6E,8Z,10E,12E-eicosatetraenoic acid or 5S,6R(or S),15S-trihydroxy-7E,9E,11E,13E-eicosatetraenoic acid (LxA4, all trans isomers).31,37–39 Yet another route can be initiated by h5-LOX’s major product, 5S-trans-5,6-oxido-7E,9E,11Z,14Z-eicosatetraenoic acid (LTA4), which can then be converted into LxA4 via hydroperoxidation by either h15-LOX-1 in reticulocytes or h12-LOX in platelets; hydrolysis of the epoxide nonenzymatically or by epoxide hydrolase and reduction by GP are needed to convert the resulting 15S-hydroperoxy-LTA4 into the final product LxA4.18,22,27,28 Additionally, LTA4 compounds that are not hydrolyzed to their main product (5S,12R-dihydroxy-6Z,8E,10E,14Z-eicosatetraenoic acid (LTB4)) can also be converted to 5S,6R-dihydroxy-7E,9E,11Z,14Z-eicosatetraenoic acid (5,6-diHETE) by soluble epoxide hydrolase or non-enzymatic hydrolysis to either 5S,6R-diHETE or 5S,6S-diHETE.18,29,40,41 5S,6R-diHETE can then react with h12-LOX to generate LxA4, while 5S,6S-diHETE will generate 6S-LxA4.18 Finally, lipoxins can be generated by the 6R-oxygenase and 14R-oxygenase activities of h5-LOX and h12-LOX, which are not illustrated here.31,40 Thus, as their name implies, lipoxins arise from the many possible exchanges of lipoxygenase AA products.
Scheme 1. Biosynthetic Routes to Lipoxinsa.
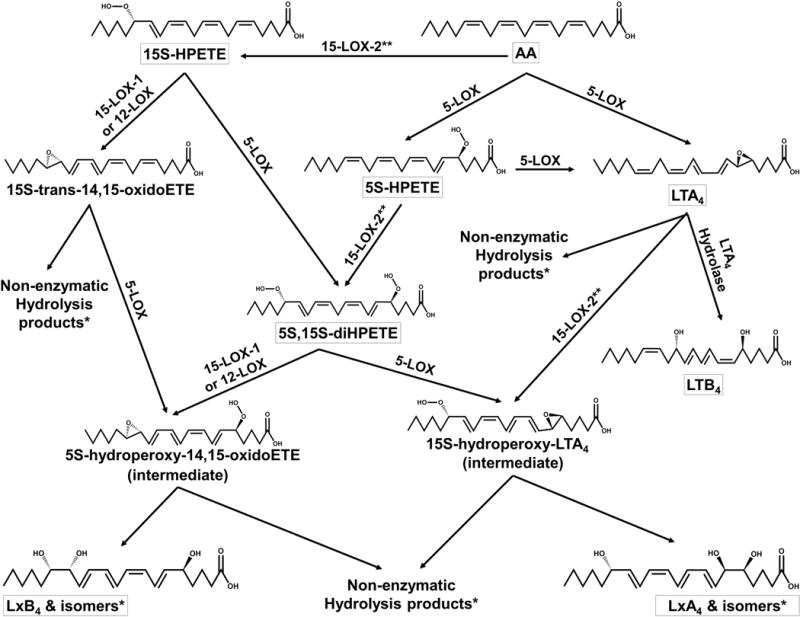
aLipoxinA4, LipoxinB4, and their respective isomers can arise from arachidonic acid (AA) through the pathways laid out in this scheme. The names of major products are boxed, such as LTA4 and LxB4. Enzymes are listed above the arrows of the reactions performed. The arrows with no enzyme listed are hydrolysis reactions that either are catalyzed by soluble epoxide hydrolase or are nonenzymatic, as indicated by the products. The numerous products observed that arise from nonenzymatic hydrolysis, indicated by a single asterisk, are listed in Figure S2. Reactions that h15-LOX-2 can perform are indicated by two asterisks; however, these reactions can also be performed by h15-LOX-1. Please note that this scheme has been simplified. For example, the required reductions of the hydroperoxy moieties to hydroxyl moieties are typically performed by glutathione peroxidase. These reactions, along with the 14R-oxygenase and 6R-oxygenase activities of h12-LOX and h5-LOX, have been excluded for clarity.
In this study, we aim to elucidate the potential of h15-LOX-2 to synthesize lipoxins. h15-LOX-2 was originally discovered as a distinct LOX isoform in epithelial cells and exhibited product regiospecificity that set it apart from h15-LOX-1.42 Principally, h15-LOX-2 converts AA into 15-HPETE exclusively, producing none of the 12-HPETE product seen in the h15-LOX-1 reaction with AA (Scheme 2). On the basis of its original expression patterns, h15-LOX-2 was not considered as a key player in atherosclerosis and other vascular diseases, but recent studies have revealed that h15-LOX-2 is the major lipoxygenase expressed in macrophages, is found in high abundance in atherosclerotic plaques, and is induced by hypoxia and other inflammation factors.2,4,43 Additionally, using macrophages from cystic fibrosis patients, the Urbach lab established a correlation between the mRNA levels of h15-LOX-2 and the ratio of two key opposing lipid mediators: LxA4 and LTB4.32 Given this fresh perspective, the role of h15-LOX-2 in transcellular synthesis may be antagonistic to the leukotriene-mediated inflammatory pathways of h5-LOX, but its direct biosynthetic potential has not been evaluated.
Scheme 2. Positional Specificity of 15-Lipoxygenasesa.
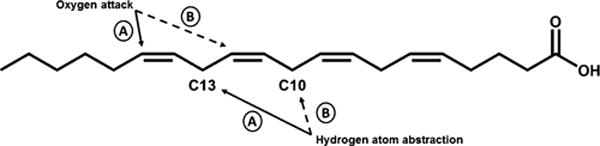
a(A) h15-LOX-2 has demonstrated the ability to only abstract the hydrogen atom at C13 and allow oxygen attack at C15 (solid arrows) of arachidonic acid. (B) h15-LOX-1 can abstract a hydrogen atom from C13 and oxygenate at C15 as well but also abstracts the C10 hydrogen atom and facilitates oxygen attack at the C12 position (dashed arrows).
Previous work has demonstrated the abilities of porcine 5-LOX, porcine 15-LOX-1, and h12-LOX to turn over AA secondary metabolites (e.g., mono- and dihydroperoxy-eicosatetraenoic acids) into lipoxins.17,22,31,34 Additionally, in studies by Green20 and Zhao et al.,44 human keratinocytes containing 15-lipoxygenase activity were shown to turn over 5-HETE into 5,15-diHETE. However, no study to date has demonstrated the potential of h15-LOX-2 to synthesize lipoxins or determined its kinetics with AA secondary metabolites. In this work, we investigated both the kinetics and the in vitro product profile of h15-LOX-2 with a variety of oxylipins and defined the catalytic bounds of h15-LOX-2.
EXPERIMENTAL PROCEDURES
Chemicals
The lipid mass spectrometry standards for 5,15-diHETE, 5,6-diHETE, LxA4, and LxB4 were purchased from Cayman Chemical. AA was purchased from Nu Chek Prep, Inc., and used to synthesize 5-HPETE, 5-HETE, 12-HPETE, 15-HPETE, 13S-hydroxy-9Z,11E-octadecadienoic acid (13-HODE), 13S-hydroperoxy-9Z,11E-octadecadienoic acid (13-HPODE), and 5S,5,15-diHPETE as follows. Syntheses of 5-HPETE, 5-HETE, 12-HPETE, 15-HPETE, 13-HODE, and 13-HPODE were performed as previously described.45–47 5,15-diHPETE was synthesized from 15-HPETE as follows. Twenty micromolar 15-HPETE was reacted in 1 L of 50 mM HEPES (pH 7.5), 50 mM NaCl, and 100 μM EDTA (buffer A) with 200 μM ATP and 1 g of h5-LOX ammonium sulfate precipitate, prepared as previously described.45 This reaction was monitored at 254 nm to completion, quenched with 0.5% (v/v) glacial acetic acid, extracted with 1 L of DCM, and evaporated to dryness. 5,15-diHPETE was then purified via high-performance liquid chromatography (HPLC) on a Higgins Haisil semipreparative (5 μm, 250 mm × 10 mm) C18 column with an isocratic elution of 50/50 acetonitrile/water. Purity was assessed via liquid chromatography–mass spectrometry to be greater than 90%.
Expression and Purification of h15-LOX-2
Over-expression and purification of wild-type h15-LOX-2 was performed as previously described.48 The purity of the enzyme was assessed by SDS gel electrophoresis to be greater than 85%. Metal content was assessed on a Finnigan inductively coupled plasma-mass spectrometer (ICP-MS) via comparison with an iron standard solution. Cobalt-EDTA was used as an internal standard.
Analysis of h15-LOX-2 Products from 5-HETE, 5-HPETE, 12-HPETE, 15-HPETE, 5,6-diHETE, and 5,15-diHPETE
h15-LOX-2 (0.6 pmol) was reacted in 6 mL of 25 mM HEPES (pH 7.5) at ambient temperature with 10 μM oxylipin (5-HETE, 5-HPETE, 12-HPETE, 15-HPETE, 5,15-diHPETE, or 5,6-diHETE) for 1 h, quenched with 0.5% glacial acetic acid, extracted with 6 mL of DCM, reduced with trimethylphosphite, and evaporated under a stream of N2 to dryness. The reaction mixtures were then reconstituted in 30 μL of methanol, further diluted with 60 μL of 0.1% formic acid in water, and analyzed via LC-MS/MS. Control reactions without h15-LOX-2 were run to ensure that the products formed were not a result of oxylipin degradation. Additional reactions were performed for 1 and 10 min to determine relative turnover rates of the secondary substrates. Chromatographic separation was performed on a Dionex UltiMate 3000 UHPLC with a C18 column (Phenomenex Kinetex, 1.7 μm, 150 mm × 2.1 mm). The autosampler was held at 4 °C, and the injection volume was 20 μL. Mobile phase A consisted of water with 0.1% (v/v) formic acid, and mobile phase B consisted of acetonitrile with 0.1% formic acid. The flow rate was 0.350 mL/min. The initial condition (40% mobile phase B) was ramped up to 45% over 19 min. Mobile phase B was then ramped up to 75% over an additional 19 min and returned to 40% to equilibrate for 10 min. The chromatography system was coupled to a Velos Pro linear ion trap (Thermo Scientific) for mass analysis. Analytes were ionized via heated electrospray ionization with −4.0 kV spray voltage and 35, 10, and 0 arbitrary units for sheath, auxiliary, and sweep gas, respectively. The radiofrequency amplitude of the S-Lens was 52.5%, and the probe and capillary temperatures were 45 and 350 °C, respectively. All analyses were performed in negative ionization mode at the normal resolution setting. MS2 was performed at 35% normalized collision energy in a targeted manner with a mass list containing the following m/z ratios ±0.1: 319.2, 335.2, 351.2, and 367.2. The UV detectors used were Thermo PDA Plus and Dionex Ultimate-3000 DAD. The DAD is slightly blue-shifted and not as sensitive as the Thermo PDA Plus, while the PDA Plus can lose small spectral features by its spectrum averaging. Both detectors were used for identification in all cases, but the most informative spectrum for each is included in the figures. We identified the products by matching retention times, UV spectra, and fragmentation patterns to those of known standards (Figure S1).
Steady-State Kinetics of h15-LOX-2 with 5-HETE and 5-HPETE
Reactions were performed at ambient temperature in a quartz cuvette containing 2 mL of buffer A with substrate (AA, 5-HPETE, or 5-HETE). AA concentrations were varied from 0 to 29 μM; 5-HPETE concentrations were varied from 0 to 35 μM, and 5-HETE concentrations were varied from 0 to 32 μM. Higher concentrations were avoided to prevent the formation of micelles. We determined the concentration of AA by measuring the amount of 15-HPETE produced from complete reaction with soybean lipoxygenase-1 (sLO-1). The concentrations of 5-HETE and 5-HPETE were determined from the absorbance at 234 nm. Reactions were initiated by the addition of 0.2 pmol of h15-LOX-2 and monitored on a Hewlitt-Packard 8453 UV–vis spectrophotometer. Product formation was determined by the change in absorbance at 234 nm for 15-HPETE (ε234nm = 25000 M−1 cm−1) and 254 nm for 5,15-diHPETE. The absorbance maximum for 5,15-diHPETE (247 nm) was not used due to the overlap from the decaying 5-HPETE absorbance at 234 nm. The molar extinction coefficient for 5,15-diHPETE at 254 nm was derived semiempirically from the literature value (ε247nm = 33500 M−1 cm−1 in methanol)49 and standard dilutions to be 24300 ± 30 M−1 cm−1 in methanol and 21900 ± 700 M−1 cm−1 in buffer A. KaleidaGraph (Synergy) was used to fit initial rates (at less than 20% turnover) as well as the second order derivatives (kcat/Km) to the Michaelis–Menten equation for the calculation of kinetic parameters.
Computational Methods
Density functional theory (DFT) calculations were performed using the Gaussian 09 software package50 with a combination of the Becke exchange functional (B)51 and the Lee–Yang–Parr correlation functional (LYP).52,53 The 6-311G(d,p) basis set was employed to describe the system.54 No symmetry constraints were applied during the geometry optimization, and the obtained minimum was confirmed by frequency calculation (no imaginary frequencies). Low-energy conformers were obtained through manual adjustment of dihedral angles with subsequent reoptimization. Higher energy conformers (typically 4–5 kcal/mol higher in energy) are not discussed.
RESULTS AND DISCUSSION
Product Profiles of h15-LOX-2 with AA and Monohydro(pero)xy-eicosatetraenoic Acids
In previous studies, h15-LOX-2 has demonstrated complete regiospecificity in contrast to h15-LOX-1.42,55,56 In particular, h15-LOX-1 can produce both 15-HPETE and 12-HPETE from AA by hydrogen abstraction at the C13 and C10 positions, respectively55,56 (Scheme 2). On the other hand, h15-LOX-2 has been shown to produce only 15-HPETE from AA, selectively abstracting only the C13 hydrogen atom.42 In our experiments, we observed this selectivity as well. Reactions of h15-LOX-2 with AA produced only 15-HPETE (data not shown), and no further reaction was seen with 15-HPETE (Figure S3A). Despite the availability of a C13 hydrogen atom to abstract and a C15 to oxygenate, h15-LOX-2 did not react with 12-HPETE (Figure S3B). This is not surprising given that the C13 hydrogen atoms are no longer bisallylic due to the location of the C12 hydroperoxy moiety. Additionally, this hydroxyl may also block the proper insertion of 12-HPETE into the active site. Despite h15-LOX-2’s lack of reactivity with 12-HPETE and 15-HPETE, we tested these HPETEs to ensure that no reactions were available via nontraditional binding modes. However, when h15-LOX-2 was reacted with 5-HPETE, it produced 5,15-diHETE (Figure 1). 5,15-diHETE has been observed in previous reactions with keratinocytes containing 15-lipoxygenase activity20,44 and was suggested to be an intermediate in lipoxin biosynthesis.17,20
Figure 1.
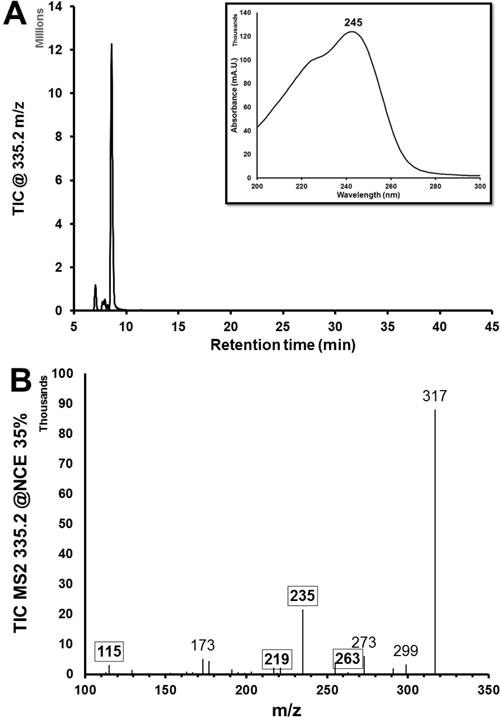
h15-LOX-2 converts 5-HPETE to 5,15-diHPETE. (A) Total ion count (TIC) chromatogram of h15-LOX-2’s reaction with 5-HPETE, which displays ions with a parent m/z of 335.2. The large peak has a retention time of 8.9 min and a λmax value of 245 nm, as seen in the UV–vis spectra of the peak (inset), which matches the 5,15-diHETE standard. (B) Mass spectrum of the peak at 8.9 min. The diagnostic peaks for 5,15-diHETE are bolded and boxed.
Steady-State Kinetics of 5-HPETE and Formation of the Lipoxin Intermediate 5,15-diHPETE
In addition to demonstrating h15-LOX-2’s ability to convert 5-H(P)ETE to 5,15-diH(P)ETE, we determined the catalytic efficiency of this turnover. The steady-state kinetic parameters of h15-LOX-2 were obtained for AA, 5-HETE, and 5-HPETE (Table 1). The absolute kcat (catalytic rate) and kcat/Km (catalytic efficiency) for h15-LOX-2 with AA (normalized to metal content) were 0.64 ± 0.02 s−1 and 0.16 ± 0.02 μM−1 s−1, respectively, which corroborates previously reported values.47 Kinetic kcat and kcat/Km parameters of the secondary metabolites are reported relative to AA. As reported in Table 1, h15-LOX-2 displayed kcat values for 5-HETE and 5-HPETE greater than that for AA, but the Km (Michaelis constant) values for these secondary metabolites were much higher than that for AA. The net result was a lower catalytic efficiency (kcat/Km) relative to AA for 5-HETE and 5-HPETE (25% and 31%, respectively). These relative kcat/Km values are large, considering that the relative kcat/Km value of h5-LOX with 5-HPETE is 2% of its catalytic efficiency with AA.45 These results can be explained by the fact that h15-LOX-2 is abstracting its “native” hydrogen atom from 5-HETE (C13), while h5-LOX abstracts the less-preferred hydrogen (C10 versus C7 for AA). Nonetheless, the fact that the catalytic efficiency of h15-LOX-2 with 5-HETE is large indicates the minor effect the hydroxyl of C5 has on catalysis.
Table 1.
Steady-State Parameters for h15-LOX-2 Hydroperoxidation of 5-HETE and 5-HPETE
| relative kcata | Km (μM) | relative kcat/Kma | |
|---|---|---|---|
| AA | 1.00 ± 0.04 | 4.0 ± 0.6 | 1.0 ± 0.1 |
| 5-HETE | 2.1 ± 0.2 | 33 ± 5 | 0.25 ± 0.01 |
| 5-HPETE | 1.5 ± 0.1 | 19 ± 2 | 0.31 ± 0.02 |
All kcat and kcat/Km values are relative to the kcat and kcat/Km values of AA, which are 0.64 ± 0.02 s−1 and 0.16 ± 0.02 μM−1 s−1, respectively.
Energetics of C10 Abstraction from 5,15-diHPETE
Considering the potential of 5,15-diHPETE as a lipoxin intermediate, we modeled its reactivity through homolytic CH bond cleavage by employing DFT methods. We calculated the energy required for C10 hydrogen abstraction and the resulting radical stabilization. DFT calculations were performed on model compounds (Figure 2, m1–m3) that correspond to the conjugated systems of AA (m1), 5(or 15)-HPETE (m2), and 5,15-diHPETE (m3). The simplest case, model m1, contains four alkene moieties that are separated with methylene spacers and terminated with methyl groups. The two conceivable hydrogen abstraction pathways, denoted A (C10 abstraction) and B (C7 or C13 abstraction), both yield radical systems that can delocalize over five carbon atoms and are uphill by 67.2 kcal/mol, independent of position. In model m2, the abstraction pathway A leads to a radical that can delocalize over seven carbon centers, and the intermediate is calculated to have an energy 3.0 kcal/mol (64.2 kcal/mol) lower than that of the intermediate generated through pathway B. The latter is practically identical in stability to the radical intermediates computed for model m1 (67.1 kcal/mol). Finally, model m3 yielded a fully conjugated radical (nine carbon centers) upon hydrogen atom abstraction, and the bond dissociation energy was lowered by an additional 2.5 kcal/mol (61.8 kcal/mol). As noted in Computational Methods, these energies correspond to the lowest energy conformers in each case, and the conformations to which these molecules would be constrained in the active site may differ. Even so, the ability of the C10 radical to extend over nine carbon centers instead of seven or five lends to the ease of hydrogen atom abstraction and subsequent epoxidation in which the 5,15-diHETE intermediate could be converted into lipoxins.
Figure 2.
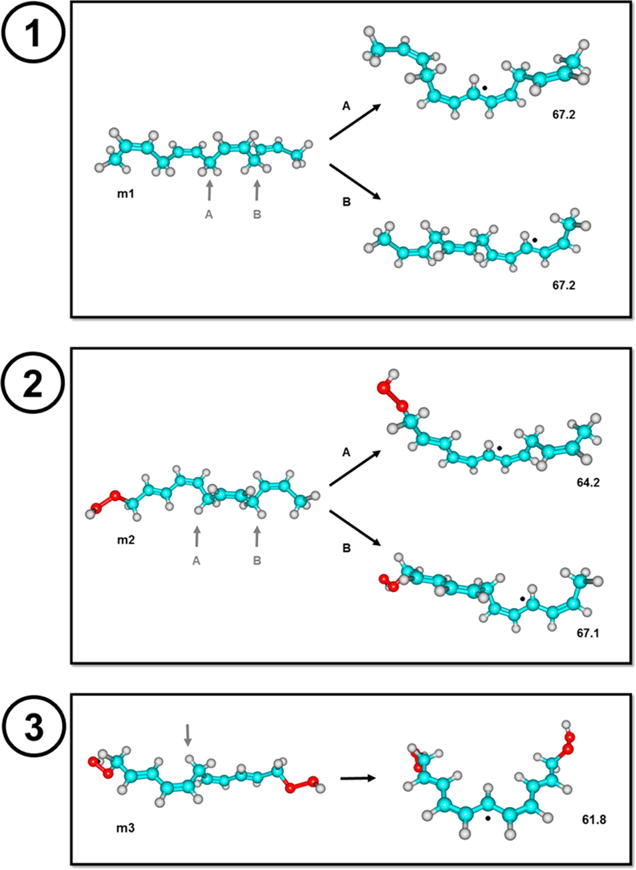
Structures of in silico models and dehydrogenation mechanisms. (1) Model m1 represents arachidonic acid’s conjugated system and the two hydrogen atom abstractions A and B that result in two radical structures with similar energies. (2) Model m2 represents 5-HPETE and 15-HPETE’s conjugated system and the two hydrogen atom abstractions A and B that result in two radical structures with different energies. (3) Model m3 represents 5,15-diHPETE and the resulting low energy radical structure from a C10 hydrogen atom abstraction.
Product Profiles of h15-LOX-2 with Dihydro(pero)xy-eicosatetraenoic acids
In view of this lowered energy for C10 hydrogen atom abstraction, we reasoned that h15-LOX-2 may be capable of lipoxin formation from 5,15-diHPETE. Thus, we reacted h15-LOX-2 with 5,15-diHPETE to determine if this lowered bond energy allows for an exception to h15-LOX-2’s stringent regiospecificity; however, h15-LOX-2 continued to demonstrate strict C13 hydrogen atom abstraction. h15-LOX-2 did not react with 5,15-diHPETE (Figure 3) to form either of the lipoxins. Although a small peak with the same retention time and parent mass as LxA4 was observed in the h15-LOX-2 reaction, the MS2 spectra did not match those of LxA4. There was, however, a trace amount of an unknown product produced by h15-LOX-2 in both its reaction with 5-HPETE and 5,15-diHPETE, which is consistent with a previous report on 15-lipoxygenase activity.20 This unidentified product was determined not to be LxB4 or LxA4 due to its longer retention time and UV–vis absorbance spectrum (Figure S4). As a positive control, h15-LOX-1 was reacted with 5,15-diHPETE and was able to form LxB4.17,26 With the consideration that h15-LOX-2 has an allosteric site for 13-HODE and is readily activated by 13-HPODE,57,58 h15-LOX-2 was reacted with 5,15-diHPETE in the presence of 10 μM 13-HPODE. However, even with excess 13-HPODE, h15-LOX-2 was unable to generate lipoxins from this 5,15-diHPETE intermediate (data not shown). In other words, h15-LOX-2 continues to demonstrate regiospecificity for the C13 hydrogen abstraction and C15 oxygen attack even with the lowered abstraction energy for 5,15-diHPETE.
Figure 3.
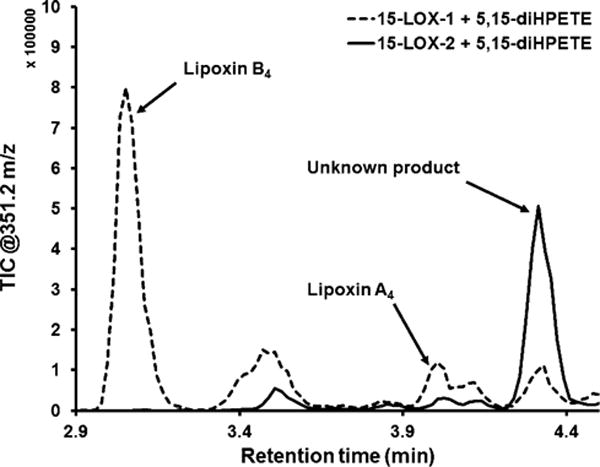
h15-LOX-2 cannot synthesize lipoxins from the 5,15-diHPETE intermediate. A TIC chromatogram of the reactions of h15-LOX-2 (solid line) and h15-LOX-1 (dashed line) with 5,15-diHPETE, displaying ions with a parent m/z of 351.2. In the h15-LOX-1 reactions, LipoxinB4 and -A4 peaks were confirmed with retention times, UV–vis spectra, and MS spectra as compared to the standards. The tiny peak at the retention time of LxA4 in the h15-LOX-2 reaction was not LxA4, as determined by its MS2 spectra. Thus, no lipoxin peaks were seen for h15-LOX-2.
This regiospecificity does not, however, preclude h15-LOX-2 from the formation of lipoxins in light of the h5-LOX products available (Scheme 1).18,22 Given cellular conditions, the second major product of human and porcine h5-LOX is LTA4,59–62 which is unstable in water and easily hydrolyzed into various 5,6-diHETE epimers nonenzymatically or by epoxide hydrolase.18,29 This is a minor pathway in the cell, however, with the main hydrolysis product being LTB4 formed by LTA4 hydrolase.63,64 We demonstrated that h15-LOX-2 reacts with 5-HPETE, and considering that the locations of the hydroperoxy and epoxy moieties were far from the methyl end of 5,6-diHETE and LTA4, respectively, we suspected that h15-LOX-2 could generate LxA4 from 5,6-diHETE and/or LTA4. Unfortunately, we could not test whether h15-LOX-2 could generate LxA4 from LTA4 because h15-LOX-2’s in vitro catalytic efficiency is slower than LTA4’s in vitro hydrolysis rate in pH 7.4 buffer (∼2.2 μM/s).65 Currently, no in vitro technique exists that can stabilize LTA4 and trap 15-hydroxy-LTA4, which makes the existence of 15-hydroxy-LTA4 in lipoxin biosynthesis speculative. However, we were able to demonstrate that h15-LOX-2 does hydroperoxidate C15 of 5S,6R-diHETE to generate LxA4 (Figure 4), and the relative rate of this reaction was 99.5% of h15-LOX-2’s rate with 5-HPETE. Thus, the addition of a hydroxyl at C6 had little effect on the rate. With respect to LTA4 reactivity, we speculate that because h15-LOX-2 generates LxA4 from 5S,6R-diHETE, it most likely could convert LTA4 to LxA4 as 5,6-diHETE is bulkier and more polar than a 5,6-epoxide.
Figure 4.
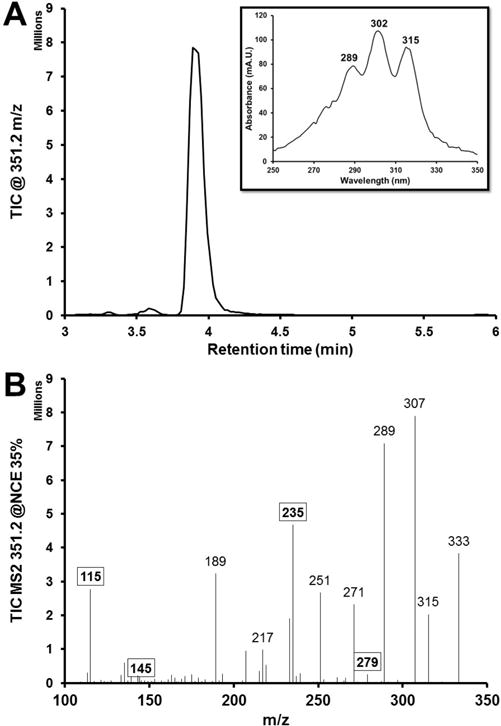
h15-LOX-2 converts 5S,6R-diHETE to LipoxinA4. (A) A TIC chromatogram of h15-LOX-2’s reaction with 5S,6R-diHETE, displaying ions with a parent m/z of 351.2. The large peak has a retention time of 3.9 min and a λmax value of 302 nm, as seen in the UV–vis spectrum of the peak (inset), which matches the LipoxinA4 standard. (B) Mass spectrum of the peak at 3.9 min. The diagnostic peaks for LipoxinA4 are bolded and boxed.
Implications of the Findings: h15-LOX-2’s Role in Transcellular Synthesis
With its expression in macrophages and endothelial and epithelial tissues, h15-LOX-2 has the potential to play a role in many inflammatory events involving transcellular syntheses. However, as observed in this study and previous studies, h15-LOX-2 has strict regiospecificity for C15 hydroperoxidation, which constrains its role in these transcellular syntheses. In particular, we found that h15-LOX-2 will not react with its own AA product (15-HPETE) or h12-LOX’s AA product (12-HPETE). Additionally, h15-LOX-2 cannot generate LxB4 from 5-HPETE or 5,15-diHPETE due to its inability to abstract a C10 hydrogen atom. However, h15-LOX-2 does generate 15-HPETE from AA, which can then be exchanged with h5-LOX to form lipoxins.26,30,31,39 In addition, this study demonstrates that h15-LOX-2 can convert h5-LOX-derived pro-inflammatory mediators (5-HETE and 5,6-diHETE) into anti-inflammatory precursors (i.e., LxA4). This antagonism between h15-LOX-2 and h5-LOX may be an important switch between pro- and anti-inflammatory mediators in vivo, as demonstrated in studies of prostate cancer cells10–12 and cystic fibrosis macrophages.32 For example, 5-HETE has been implicated in the proliferation of prostate cancer,13,66,67 and an increase in h15-LOX-2 expression results in decreased proliferation of prostate cancer cells.10–12 Perhaps h15-LOX-2’s efficient reaction with 5-HETE lends to the reduction of the cellular level of this compound and promotes antitumorigenic effects. In a similar vein, a more specific correlation was seen in macrophages of cystic fibrosis patients in which the ratio of LxA4 to LTB4 correlated directly with h15-LOX-2 mRNA levels.32 This correlation lends credence to the “class-switching” ability of h15-LOX-2 in that LTA4 can be converted to the potent inflammatory LTB4 via LTA4 hydrolase or to the potent anti-inflammatory LxA4 via h15-LOX-2. In addition, h15-LOX-2 could convert 5,6-diHETE, which has demonstrated some inflammatory effects,68,69 into LxA4. This directly demonstrates h15-LOX-2’s ability to efficiently switch h5-LOX products into their anti-inflammatory counterparts. Altogether, the role that h15-LOX-2 plays in the complex network of pro-inflammatory and pro-resolving lipid mediators has yet to be fully understood, but we have demonstrated, at the enzymatic level, h15-LOX-2’s ability to efficiently “class-switch” h5-LOX pro-inflammatory mediators into anti-inflammatory intermediates and delineated its potential to participate in transcellular syntheses.
Supplementary Material
Acknowledgments
Funding
This work was supported by NIH Grants NS081180 and GM56062.
ABBREVIATIONS
- LOX
lipoxygenase
- h15-LOX-2
human epithelial 15-lipoxygenase-2
- h15-LOX-1
human reticulocyte 15-lipoxygenase-1
- sLO-1
soybean lipoxygenase-1
- 5-LOX
leukocyte 5-lipoxygenase
- 12-LOX
human platelet 12-lipoxygenase
- GP
glutathione peroxidase
- AA
arachidonic acid
- HETE
hydoxy-eicosatetraenoic acid
- HPETE
hydroperoxy-eicosatetraenoic acid
- diHETEs
dihydroxy-eicosatetraenoic acids
- 5-HETE
5-hydroxy- 6E,8Z,11Z,14Z-eicosatetraenoic acid
- 5-HPETE
5-hydroperoxy-6E,8Z,11Z,14Z-eicosatetraenoic acid
- 12-HPETE
12-hydroperoxy-5Z,8Z,10E,14Z-eicosatetraenoic acid
- 15-HPETE
15-hydroperoxy-5Z,8Z,10Z,13E-eicosatetraenoic acid
- 5,15-HETE
5S,15S-dihydroxy-6E,8Z,10Z,13E-eicosatetraenoic acid
- 5,15-diHPETE
5,15-dihydroperoxy-6E,8Z,10Z,13E-eicosatetraenoic acid
- 5,6-diHETE
5S,6R-dihydroxy-7E,9E,11Z,14Z-eicosatetraenoic acid
- LTA4
5S-trans-5,6-oxido-7E,9E,11Z,14Z-eicosatetraenoic acid
- LTB4
5S,12R-dihydroxy-6Z,8E,10E,14Z-eicosatetraenoic acid
- LipoxinA4 (LxA4)
5S,6R,15S-trihydroxy-7E,9E,11Z,13E-eicosatetraenoic acid
- LipoxinB4 (LxB4)
5S,14R,15S-trihydroxy-6E,8Z,10E,12E-eicosatetraenoic acid
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.biochem.5b01339.
Notes
The authors declare no competing financial interest.
References
- 1.Kuehl FA, Egan RW. Prostglandins, arachidonic acid and inflammation. Science. 1980;210:978–984. doi: 10.1126/science.6254151. [DOI] [PubMed] [Google Scholar]
- 2.Hulten LM, Olson FJ, Aberg H, Carlsson J, Karlstrom L, Boren J, Fagerberg B, Wiklund O. 15-Lipoxygenase-2 is expressed in macrophages in human carotid plaques and regulated by hypoxia-inducible factor-1α. Eur J Clin Invest. 2010;40:11–17. doi: 10.1111/j.1365-2362.2009.02223.x. [DOI] [PubMed] [Google Scholar]
- 3.Gertow K, Nobili E, Folkersen L, Newman JW, Pedersen TL, Ekstrand J, Swedenborg J, Kühn H, Wheelock CE, Hansson GK, Hedin U, Haeggström JZ, Gabrielsen A. 12- and 15-lipoxygenases in human carotid atherosclerotic lesions: Associations with cerebrovascular symptoms. Atherosclerosis. 2011;215:411–416. doi: 10.1016/j.atherosclerosis.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Rydberg EK. Hypoxia Increases LDL Oxidation and Expression of 15-Lipoxygenase-2 in Human Macrophages. Arterioscler, Thromb, Vasc Biol. 2004;24:2040–2045. doi: 10.1161/01.ATV.0000144951.08072.0b. [DOI] [PubMed] [Google Scholar]
- 5.Weaver JR, Holman TR, Imai Y, Jadhav A, Kenyon V, Maloney DJ, Nadler JL, Rai G, Simeonov A, Taylor-Fishwick DA. Integration of pro-inflammatory cytokines, 12-lipoxygenase and NOX-1 in pancreatic islet beta cell dysfunction. Mol Cell Endocrinol. 2012;358:88–95. doi: 10.1016/j.mce.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Cole BK, Lieb DC, Dobrian AD, Nadler JL. 12- and 15-lipoxygenases in adipose tissue inflammation. Prostaglandins Other Lipid Mediators. 2013;104–105:84–92. doi: 10.1016/j.prostaglandins.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 2003;82:82–90. doi: 10.1177/154405910308200202. [DOI] [PubMed] [Google Scholar]
- 8.Mangino MJ, Brounts L, Harms B, Heise C. Lipoxin biosynthesis in inflammatory bowel disease. Prostaglandins Other Lipid Mediators. 2006;79:84–92. doi: 10.1016/j.prostaglandins.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Greene ER, Huang S, Serhan CN, Panigrahy D. Regulation of inflammation in cancer by eicosanoids. Prostaglandins Other Lipid Mediators. 2011;96:27–36. doi: 10.1016/j.prostaglandins.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suraneni M, Moore J, Zhang D, Badeaux M, Macaluso M, DiGiovanni J, Kusewitt D, Tang DG. Tumor-suppressive functions of 15-Lipoxygenase-2 and RB1CC1 in prostate cancer. Cell Cycle. 2014;13:1798–1810. doi: 10.4161/cc.28757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang S. Evidence That Arachidonate 15-Lipoxygenase 2 Is a Negative Cell Cycle Regulator in Normal Prostate Epithelial Cells. J Biol Chem. 2002;277:16189–16201. doi: 10.1074/jbc.M111936200. [DOI] [PubMed] [Google Scholar]
- 12.Tang Y, Wang MT, Chen Y, Yang D, Che M, Honn KV, Akers GD, Johnson SR, Nie D. Downregulation of vascular endothelial growth factor and induction of tumor dormancy by 15-lipoxygenase-2 in prostate cancer. Int J Cancer. 2009;124:1545–1551. doi: 10.1002/ijc.24118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shureiqi I, Lippman SM. Lipoxygenase modulation to reverse carcinogenesis. Cancer Res. 2001;61:6307–6312. [PubMed] [Google Scholar]
- 14.Kuhn H, Banthiya S, van Leyen K. Mammalian lipoxygenases and their biological relevance. Biochim Biophys Acta, Mol Cell Biol Lipids. 2015;1851:308–330. doi: 10.1016/j.bbalip.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vijil C, Hermansson C, Jeppsson A, Bergström G, Hultén LM. Arachidonate 15-Lipoxygenase Enzyme Products Increase Platelet Aggregation and Thrombin Generation. PLoS One. 2014;9:88546. doi: 10.1371/journal.pone.0088546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lefer AM, Stahl GL, Lefer DJ, Brezinski ME, Nicolaou KC, Veale CA, Abe Y, Smith JB. Lipoxins A4 and B4: comparison of icosanoids having bronchoconstrictor and vasodilator actions but lacking platelet aggregatory activity. Proc Natl Acad Sci U S A. 1988;85:8340–8344. doi: 10.1073/pnas.85.21.8340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhn H, Wiesner R, Alder L, Fitzsimmons BJ, Rokach J, Brash AR. Formation of lipoxin B by the pure reticulocyte lipoxygenase via sequential oxygenation of the substrate. Eur J Biochem. 1987;169:593–601. doi: 10.1111/j.1432-1033.1987.tb13650.x. [DOI] [PubMed] [Google Scholar]
- 18.Tornhamre S, Gigou A, Edenius C, Lellouche JP, Lindgren JA. Conversion of 5,6-dihydroxyeicosatetraenoic-acids: A novel pathway for lipoxin formation by human platelets. FEBS Lett. 1992;304:78–82. doi: 10.1016/0014-5793(92)80593-6. [DOI] [PubMed] [Google Scholar]
- 19.Wecksler AT, Kenyon V, Deschamps JD, Holman TR. Substrate Specificity Changes for Human Reticulocyte and Epithelial 15-Lipoxygenases Reveal Allosteric Product Regulation†. Biochemistry. 2008;47:7364–7375. doi: 10.1021/bi800550n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green FA. Transformations of 5-HETE by activated keratinocyte 15-lipoxygenase and the activation mechanism. Lipids. 1990;25:618–623. doi: 10.1007/BF02536012. [DOI] [PubMed] [Google Scholar]
- 21.Serhan CN, Hamberg M, Samuelsson B. Trihydroxytetraenes: A novel series of compounds fromed from arachidonic acid in human leukocytes. Biochem Biophys Res Commun. 1984;118:943–949. doi: 10.1016/0006-291x(84)91486-4. [DOI] [PubMed] [Google Scholar]
- 22.Tornhamre S, Elmqvist A, Lindgren JA. 15-lipoxygenation of leukotriene A4: Studies of 12- and 15-lipoxygenase efficiency to catalyze lipoxin formation. Biochim Biophys Acta, Mol Cell Biol Lipids. 2000;1484:298–306. doi: 10.1016/s1388-1981(00)00017-2. [DOI] [PubMed] [Google Scholar]
- 23.Chiang N, Arita M, Serhan CN. Anti-inflammatory circuitry: Lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins, Leukotrienes Essent Fatty Acids. 2005;73:163–177. doi: 10.1016/j.plefa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Buckley CD, Gilroy DW, Serhan CN. Proresolving Lipid Mediators and Mechanisms in the Resolution of Acute Inflammation. Immunity. 2014;40:315–327. doi: 10.1016/j.immuni.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonnans C, Gras D, Chavis C, Mainprice B, Vachier I, Godard P, Chanez P. Synthesis and anti-inflammatory effect of lipoxins in human airway epithelial cells. Biomed Pharmacother. 2007;61:261–267. doi: 10.1016/j.biopha.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Serhan CN, Hamberg M, Samuelsson B, Morris J, Wishka DG. On the stereochemistry and biosynthesis of lipoxin B. Proc Natl Acad Sci U S A. 1986;83:1983–1987. doi: 10.1073/pnas.83.7.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiore S, Serhan CN. Formation of lipoxins and leukotrienes during receptor-mediated interactions of human platelets and recombinant human granulocyte/macrophage colony-stimulating factor-primed neutrophils. J Exp Med. 1990;172:1451–1457. doi: 10.1084/jem.172.5.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romano M, Chen XS, Takahashi Y, Yamamoto S, Funk CD, Serhan CN. Lipoxin synthase activity of human platelet 12-lipoxygenase. Biochem J. 1993;296:127–133. doi: 10.1042/bj2960127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haeggström JZ, Meijer J, Radmark O. Leukotriene A4: enzymatic conversion into 5,6-dihydroxy-7,9,11,14-eicosatetraenoic acid by mouse liver cytosolic epoxide hydrolase. J Biol Chem. 1986;261:6332–6337. [PubMed] [Google Scholar]
- 30.Ueda N, Yamamoto S, Fitzsimmons BJ, Rokach J. Lipoxin synthesis by arachidonate 5-lipoxygenase purified from porcine leukocytes. Biochem Biophys Res Commun. 1987;144:996–1002. doi: 10.1016/s0006-291x(87)80062-1. [DOI] [PubMed] [Google Scholar]
- 31.Ueda N, Yokoyama C, Yamamoto S, Fitzsimmons BJ, Rokach J, Oates JA, Brash AR. Lipoxin synthesis by arachidonate 12-lipoxygenase purified from porcine leukocytes. Biochem Biophys Res Commun. 1987;149:1063–1069. doi: 10.1016/0006-291x(87)90516-x. [DOI] [PubMed] [Google Scholar]
- 32.Ringholz FC, Buchanan PJ, Clarke DT, Millar RG, McDermott M, Linnane B, Harvey BJ, McNally P, Urbach V. Reduced 15-lipoxygenase 2 and lipoxin A4/leukotriene B4 ratio in children with cystic fibrosis. Eur Respir J. 2014;44:394–404. doi: 10.1183/09031936.00106013. [DOI] [PubMed] [Google Scholar]
- 33.Levy BD, Romano M, Chapman HA, Reilly JJ, Drazen J, Serhan CN. Human alveolar macrophages have 15-lipoxygenase and generate 15 (S)-hydroxy-5, 8, 11-cis-13-trans-eicosatetraenoic acid and lipoxins. J Clin Invest. 1993;92:1572–1579. doi: 10.1172/JCI116738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ueda N, Kaneko S, Yoshimoto T, Yamamoto S. Purification of arachidonate 5-lipoxygenase from porcine leukocytes and its reactivity with hydroperoxyeicosatetraenoic acids. J Biol Chem. 1986;261:7982–7988. [PubMed] [Google Scholar]
- 35.Chavis C, Vachier I, Chanez P, Bousquet J. 5(S),15(S)-Dihydroxyeicosatetraenoic acid and lipoxin generation in human polymorphonuclear cells: dual specificity of 5-lipoxygenase towards endogenous and exogenous precursors. J Exp Med. 1996;183:1633–1643. doi: 10.1084/jem.183.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bryant RW, Simon TC, Bailey MJ. Role of glutathione peroxidase and hexose monophosphate shunt in the platelet lipoxygenase pathway. J Biol Chem. 1982;257:14937–14943. [PubMed] [Google Scholar]
- 37.Maas RL, Brash AR. Evidence for a lipoxygenase mechanism in the biosynthesis of epoxide and dihydroxy leukotrienes from 15 (S)-hydroperoxyicosatetraenoic acid by human platelets and porcine leukocytes. Proc Natl Acad Sci U S A. 1983;80:2884–2888. doi: 10.1073/pnas.80.10.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lundberg U, Radmark O, Malmsten C, Samuelsson B. Transformation of 15-hydroperoxy-5,9,11,13-eicosatetraenoic acid into novel leukotrienes. FEBS Lett. 1981;126:127–132. doi: 10.1016/0014-5793(81)81050-2. [DOI] [PubMed] [Google Scholar]
- 39.Serhan CN, Nicolaou KC, Webber SE, Veale CA, Dahlen SE, Puustinen TJ, Samuelsson B. LipoxinA4 stereochemistry and biosynthesis. J Biol Chem. 1986;261:16340–16345. [PubMed] [Google Scholar]
- 40.Ueda N, Yamamoto S. The 6R-oxygenase activity of arachidonate 5-lipoxygenase purified from porcine leukocytes. J Biol Chem. 1988;263:1937–1941. [PubMed] [Google Scholar]
- 41.Borgeat P, Samuelsson B. Metabolism of arachidonic acid in polymorphonuclear leukocytes. Structural analysis of novel hydroxylated compounds. J Biol Chem. 1979;254:7865–7869. [PubMed] [Google Scholar]
- 42.Brash AR, Boeglin WE, Chang MS. Discovery of a second 15S-lipoxygenase in humans. Proc Natl Acad Sci U S A. 1997;94:6148–6152. doi: 10.1073/pnas.94.12.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wuest SJA, Crucet M, Gemperle C, Loretz C, Hersberger M. Expression and regulation of 12/15-lipoxygenases in human primary macrophages. Atherosclerosis. 2012;225:121–127. doi: 10.1016/j.atherosclerosis.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 44.Zhao H, Richards-Smith B, Baer AN, Green FA. Lipoxygenase mRNA in cultured human epidermal and oral keratinocytes. J Lipid Res. 1995;36:2444–2449. [PubMed] [Google Scholar]
- 45.Smyrniotis CJ, Barbour SR, Xia Z, Hixon MS, Holman TR. ATP Allosterically Activates the Human 5-Lipoxygenase Molecular Mechanism of Arachidonic Acid and 5(S)-Hydroperoxy-6(E),8(Z),11(Z),14(Z)-eicosatetraenoic Acid. Biochemistry. 2014;53:4407–4419. doi: 10.1021/bi401621d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis ER, Johansen E, Holman TR. Large Competitive Kinetic Isotope Effects in Human 15-Lipoxygenase Catalysis Measured by a Novel HPLC Method. J Am Chem Soc. 1999;121:1395–1396. [Google Scholar]
- 47.Wecksler AT, Kenyon V, Garcia NK, Deschamps JD, van der Donk WA, Holman TR. Kinetic and Structural Investigations of the Allosteric Site in Human Epithelial 15-Lipoxygenase-2. Biochemistry. 2009;48:8721–8730. doi: 10.1021/bi9009242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jameson JB, Kenyon V, Holman TR. A high-throughput mass spectrometric assay for discovery of human lipoxygenase inhibitors and allosteric effectors. Anal Biochem. 2015;476:45–50. doi: 10.1016/j.ab.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Os CPA, Rijke-Schilder GPM, Van Halbeek H, Verhagen J, Vliegenthart JFG. Double dioxygenation of arachidonic acid by soybean lipoxygenase-1: Kinetics and regio-stereo specificities of the reaction steps. Biochim Biophys Acta, Lipids Lipid Metab. 1981;663:177–193. doi: 10.1016/0005-2760(81)90204-6. [DOI] [PubMed] [Google Scholar]
- 50.Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, Peterson G, Nakatsuji H, Caricato M, Li X, Hratchian H, Izmaylov A, Bloino J, Zheng G, Sonnenberg J, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr, Peralta J, Ogliaro F, Bearpark M, Heyd J, Brothers E, Kudin K, Starovervov V, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant J, Iyengar S, Tomasi J, Cossi M, Rega N, Millam J, Klene M, Knox J, Cross J, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann R, Yazyev O, Austin A, Cammi R, Pomelli C, Ochterski J, Martin R, Morokuma K, Zakrzewski V, Voth G, Salvador P, Dannenberg J, Dapprich S, Daniels A, Farkas O, Foresman J, Otriz J, Cioslowski J, Fox D. Gaussian 09. Gaussian, Inc.; Wallingford, CT: 2009. [Google Scholar]
- 51.Becke AD. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A: At, Mol, Opt Phys. 1988;38:3098–3100. doi: 10.1103/physreva.38.3098. [DOI] [PubMed] [Google Scholar]
- 52.Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B: Condens Matter Mater Phys. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 53.Miehlich B, Savin A, Stoll H, Preuss H. Results obtained with the correlation energy density functionals of Becke and Lee, Yang and Parr. Chem Phys Lett. 1989;157:200–206. [Google Scholar]
- 54.Krishnan R, Binkley JS, Seeger R, Pople JA. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys. 1980;72:650–654. [Google Scholar]
- 55.Bryant RW, Bailey MJ, Schewe T, Rapoport SM. Positional specificity of a reticulocyte lipoxygenase. Conversion of arachidonic acid to 15-S-hydroperoxy-eicosatetraenoic acid. J Biol Chem. 1982;257:6050–6055. [PubMed] [Google Scholar]
- 56.Kuhn H, Barnett J, Grunberger D, Baecker P, Chow J, Nguyen B, Bursztyn-Pettegrew H, Chan H, Sigal E. Overexpression, purification, and characterization of human recombinant 15-lipoxygenase. Biochim Biophys Acta, Lipids Lipid Metab. 1993;1169:80–89. doi: 10.1016/0005-2760(93)90085-n. [DOI] [PubMed] [Google Scholar]
- 57.Joshi N, Hoobler EK, Perry S, Diaz G, Fox B, Holman TR. Kinetic and Structural Investigations into the Allosteric and pH Effect on the Substrate Specificity of Human Epithelial 15-Lipoxygenase-2. Biochemistry. 2013;52:8026–8035. doi: 10.1021/bi4010649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoobler EK, Holz C, Holman TR. Pseudoperoxidase investigations of hydroperoxides and inhibitors with human lipoxygenases. Bioorg Med Chem. 2013;21:3894–3899. doi: 10.1016/j.bmc.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abramovitz M, Wong E, Cox ME, Richardson CD, Li C, Vickers PJ. 5-lipoxygenase-activating protein stimulates the utilization of arachidonic acid by 5-lipoxygenase. Eur J Biochem. 1993;215:105–111. doi: 10.1111/j.1432-1033.1993.tb18012.x. [DOI] [PubMed] [Google Scholar]
- 60.Rakonjac M, Fischer L, Provost P, Werz O, Steinhilber D, Samuelsson B, Radmark O. Coactosin-like protein supports 5-lipoxygenase enzyme activity and up-regulates leukotriene A4 production. Proc Natl Acad Sci U S A. 2006;103:13150–13155. doi: 10.1073/pnas.0605150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gerstmeier J, Weinigel C, Barz D, Werz O, Garscha U. An experimental cell-based model for studying the cell biology and molecular pharmacology of 5-lipoxygenase-activating protein in leukotriene biosynthesis. Biochim Biophys Acta, Gen Subj. 2014;1840:2961–2969. doi: 10.1016/j.bbagen.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 62.Shimizu T, Radmark O, Samuelsson B. Enzyme with dual lipoxygenase activities catalyzes leukotriene A4 synthesis from arachidonic acid. Proc Natl Acad Sci U S A. 1984;81:689–693. doi: 10.1073/pnas.81.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Radmark O, Malmsten C, Samuelsson B. Leukotriene A: stereochemistry and enzymatic conversion to leukotriene B. Biochem Biophys Res Commun. 1980;92:954–961. doi: 10.1016/0006-291x(80)90795-0. [DOI] [PubMed] [Google Scholar]
- 64.Radmark O, Shimizu T, Jornvall H, Samuelsson B. Leukotriene A4 hydrolase in human leukocytes: purification and properties. J Biol Chem. 1984;259:12339–12345. [PubMed] [Google Scholar]
- 65.Fitzpatrick FA, Morton DR, Wynalda MA. Albumin stabilizes leukotriene A4. J Biol Chem. 1982;257:4680–4683. [PubMed] [Google Scholar]
- 66.Ghosh J, Myers CE. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc Natl Acad Sci U S A. 1998;95:13182–13187. doi: 10.1073/pnas.95.22.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gupta S, Srivastava M, Ahmad N, Sakamoto K, Bostwick DG, Mukhtar H. Lipoxygenase-5 is overexpressed in prostate adenocarcinoma. Cancer. 2001;91:737–743. doi: 10.1002/1097-0142(20010215)91:4<737::aid-cncr1059>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 68.Cristol JP, Sirois P. Comparative activity of leukotriene D4, 5,6-dihydroxy-eicostetraenoic acid, and lipoxin A on guinea pig lung parenchyma and ileum smooth muscle. Res Commun Chem Pathol Pharmacol. 1988;59:423–426. [PubMed] [Google Scholar]
- 69.Muller A, Rechencq E, Kugel C, Lellouche JP, Beaucourt JP, Niel G, Girard JP, Rossi JC, Bonne C. Comparative biological activities of the four synthetic (5,6)-dihete isomers. Prostaglandins. 1989;38:635–644. doi: 10.1016/0090-6980(89)90046-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


