Abstract
BACKGROUND
We compared the IQ and academic achievement of adolescents with craniofacial macrosomia (CFM) (“cases) and unaffected children (“controls”). Among cases we analyzed cognitive functioning by facial phenotype.
METHODS
We administered standardized tests of intelligence, reading, spelling, writing and math to 142 cases and 316 controls recruited from 26 cities across the U.S. and Canada. Phenotypic classification was based on integrated data from photographic images, health history, and medical chart reviews. Hearing screens were conducted for all participants.
RESULTS
After adjustment for demographics, cases’ average scores were lower than controls’ on all measures, but the magnitude of differences was small (standardized effect sizes (ES) = −0.04 to −0.2). There was little evidence that hearing status modified case-control group differences (Wald p>0.05 for all measures). Twenty-five percent of controls and 38% of cases were classified as having learning problems (adjusted OR=1.5, 95% CI 0.9–2.4). Comparison of cases with and without learning problems indicated that those with learning problems were more likely to be male, Hispanic and to come from lower income, bilingual families. Analyses by facial phenotype showed that case-control group differences were largest for cases with both microtia and mandibular hypoplasia (ES = −0.25 to −0.5).
CONCLUSIONS
The highest risk of cognitive-academic problems was observed in patients with combined microtia and mandibular hypoplasia. Developmental surveillance of this subgroup is recommended, especially in the context of high socioeconomic risk and bilingual families. Given the early stage of research on CFM and neurodevelopment, replication of these findings is needed.
INTRODUCTION
Craniofacial microsomia (CFM), also known as hemifacial microsomia, refers to a spectrum of congenital malformations primarily involving hypoplasia of facial structures. It occurs in approximately 1 in 3,500 to 5.600 live births1 with higher prevalence among Hispanic and Native American families2 CFM is a highly variable condition that can include facial asymmetry due to maxillary and/or mandibular hypoplasia; malformations of the external ear (microtia) that are usually associated with hearing impairment; preauricular or facial tags; and lateral oral clefts (macrostomia.3 Depending on phenotype, a wide range of surgical interventions may be used to restore craniofacial form and function including surgeries for the mandible, ear, facial nerve, eye and orbit.4
The neurodevelopment of patients with CFM has been rarely examined, although it is potentially significant in terms of etiology, treatment and quality of life.5–9 Most available information is based on medical chart reviews or parent surveys.10,11 One exception is a study in which we evaluated elementary school children with CFM (average age = 7) and demographically similar controls, using standardized tests of verbal and visual-motor skills.12 Children with CFM were two to three times more likely than controls to score in the ‘at-risk’ range on these tests.
The present study followed into adolescence the same cohort of cases and controls (average age = 13). We addressed two primary questions: (1) In comparison with unaffected peers, do adolescents with CFM have continuing problems in two key domains of neurodevelopment, global IQ and academic achievement? (2) Is the cognitive/academic status of adolescents with CFM related to their craniofacial phenotype; i.e., different combinations of anomalies such as microtia with or without other craniofacial malformations? In secondary analyses, we sought to determine whether associations between cases status and neurodevelopmental outcomes differed by hearing status. Two other variables were similarly examined (youth sex and maternal age at birth), as both variables were observed to moderate case-control group differences in the earlier study.12
METHODS
Study Design
The Child and Adolescent Learning and Living Study (CALLS) is Phase 3 of a observational, longitudinal study of prenatal risk factors and neurodevelopment in children with CFM (cases) and children without craniofacial anomalies (controls).13 Participants were initially enrolled between 1996 and 2002 from 26 cities across the U.S. and Canada. Phase 1 of the study focused on demographic and risk factors for CFM.13,14 Families were re-approached and asked to participate in Phase 2 of this research when children were 7 years of age on average, in which we assessed neurodevelopment12 and psychosocial status15. The current Phase 3 occurred between 2011 and 2015 when youth were approximately 13 years of age on average (range = 11 to 17 years). All participating youth were schooled in English since early grade school and all spoke English as their primary language. The study was approved by the institutional review boards of both participating centers (Seattle Children’s Hospital and Boston University).
Cases
Children in the original Phase 1 study were ascertained from craniofacial specialty clinics, eligible if they were < age 48 months; had received a diagnosis of hemifacial microsomia, facial asymmetry, unilateral microtia, OAVS, or Goldenhar syndrome from a craniofacial physician; and did not have a diagnosis of another known syndrome or chromosomal anomaly. Cases without microtia and/or at least 2 CFM-associated malformations were excluded from the current analyses in order to include only cases with the most accepted features of CFM1 and to maximize sample homogeneity.
Controls
Families of control infants were originally recruited during Phase 1 through cases’ pediatricians or from another pediatric practice in close proximity and size. Approximately three controls were recruited for each case and were eligible if they had no known birth defects, had not been adopted, and were within 2 months of the cases’ age at the time of recruitment. For Phase 3, we selected 2 or 3 matching group participants for each case depending on geographic proximity. When there were several potential controls in a given location, we prioritized control group participants who were most similar to the target case in age, sex, and language spoken in the home (English or Spanish).
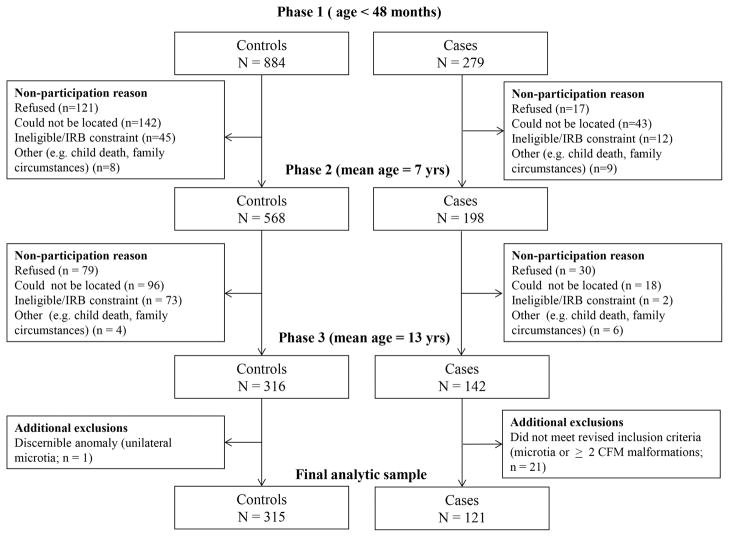
Figure 1 shows the case and control group participation numbers for all three phases of the study and reasons for participant loss.
Figure 1.
Participation and attrition by study phase.
Measures
Intelligence and achievement
Psychometrists supervised by one of the project’s psychologists (BC and MS) traveled to the home communities of participating families and assessed each adolescent individually. We used a two-subtest version of the Wechsler Abbreviated Scale of Intelligence (WASI-2)16–19 to obtain an estimate of global IQ. The subtests were Vocabulary and Matrix Reasoning, which measure verbal and nonverbal cognitive skills, respectively. The Wide Range Achievement Test-IV (WRAT-4)20 was used to assess achievement in spelling, math computation, and sentence comprehension. To assess oral reading, we used the Fluency and Comprehension subtests from the Gray Oral Reading Test – Fourth Edition (GORT-4).21 We created a composite reading score based on the WRAT-420 Sentence Comprehension subtest and the GORT-421 Fluency and Comprehension subtests. Written expression (i.e., ability to formulate ideas in writing) was assessed by the Writing Samples subtest of the Woodcock-Johnson III Tests of Achievement (WJTA-III).22 Age-based standardized scores were used for all measures. All testing was conducted in English.
All testing sessions were video recorded. Inter-examiner reliability was estimated by having one of the psychologist investigators (BC) watch test videos and independently score 20% of all test administrations. Average % agreement between the supervising psychologist and psychometrists was > 95% among all tests given.
Audiometric assessment
Conventional screening audiometry was used to assess hearing status among both cases and controls using a GSI 18™ portable device.23 Trial presentations of tones at 45–55 dB were used to confirm participants’ understanding of the procedure. Both ears were tested at 500, 1000, 2000, and 4000 Hz. A hearing threshold ≤ 40 dB at any of these frequencies was considered a “pass” (40 dB commonly distinguishes “no or mild” from “moderate” or more severe hearing loss).23
Medical history interview and questionnaire
During Phase 1, mothers of children with CFM were interviewed by telephone about demographic, reproductive, and medical factors. At Phase 3, mothers or guardians completed a questionnaire to update the family’s demographic information and medical history (including patients’ medical/surgical histories) and to provide information on any developmental or educational interventions received (e.g., speech, vision, or hearing therapy, physical or occupational therapy, or special education services).
Phenotypic assessment
The details of the methodologic approach for data collection and integration, as well as sub-group identification for this study cohort are described elsewhere.24 Briefly, phenotypic classifications were based on the integration of the following data sources: standardized ratings based on photographs; data from medical questionnaire obtained at the Phase 3 study visit; along with medical chart information taken during Phase 1. The standardized photographic protocol consisted of 16 views of the face25–27 and a classification method described by Birgfeld et al. (2016),26 which used a modified version of the Orbital, Ear, Mandible, Nerve, Soft tissue (OMENS) pictorial rating scale.28–30 Ratings of photos using this method have correlated highly with physical examination for most features26 and we obtained interrater reliability kappa coefficients of 0.7 or greater for each of the OMENS features using physician raters.31 In the current study, one of the investigators, a craniofacial pediatrician, rated all photographs (CH). For each feature on each study participant, data from all 3 sources (i.e. photographic ratings, medical questionnaire, medical chart abstraction) were reviewed in order to establish the phenotype. Three major phenotypic subgroups were identified24: 1) microtia only (in the absence of mandibular hypoplasia, epibulbar dermoids, lateral clefts, preauricular or facial tags, small and/or displaced orbit, nerve palsies; n = 24); 2) microtia and mandibular hypoplasia; n = 46, and 3) other combinations of CFM-associated malformations (two or more were required; n = 51). In the latter subgroup, malformations included preauricular or facial tags (n = 41), mandibular hypoplasia (n = 21), microtia (n = 21), epibulbar dermoids (n=15), nerve palsy (n=9), lateral cleft (n=8), and orbital hypoplasia and displacement (n=3). Further details about all subgroups are provided by Heike et al., 2016.24
Statistical analyses
Linear regression with robust standard errors was used to estimate differences between cases and controls with corresponding 95% confidence intervals (CI). We estimated the magnitude of standardized group differences (standardized effect size; ES) by calculating a modification of Cohen’s d, using the adjusted mean difference and dividing by the root mean square error for the model.32 All analyses were adjusted for youth’s’ age at assessment (in years), youth sex and race/ethnicity (non-Hispanic white, Hispanic and other), family income and the primary caregiver’s highest level of education. We examined differences in adjusted IQ and achievement among the three phenotypic categories using Wald tests; controls served as the referent category.
Logistic regression with robust standard errors was used to compare the proportion of cases and controls with learning problems, after adjustment for all confounds used in the primary linear regressions. A well-established definition of learning problems was used: 33, 34 scores below the 25th percentile on test norms for one or more of the achievement measures given. We also tabulated cases’ demographic and CFM-related characteristics by learning problem status (yes/no).
We examined the effect of attrition bias by repeating the primary analysis using inverse probability weighting (IPW).35 The predicted probability of participation in the adolescent follow-up (vs. non-participation due to refusal or loss of contact) was estimated with characteristics collected at the school-age assessment or earlier including case status, gender, race, language spoken at home (Spanish vs. English) and neurodevelopmental tests and behavior questionnaires.12 We also conducted sensitivity analyses that excluded Spanish-speaking case and control group families.
Secondary analyses
We used censored normal regression36 to examine whether the receipt of interventions designed to improve academic performance may have influenced observed group differences. This approach assumes that the scores of children who received interventions would be at least as low as those observed in the absence of intervention, i.e. that they are “left-censored.”
We conducted stratified analyses of outcomes by case and hearing status to explore the effect of hearing status on IQ and achievement, regardless of phenotype. Controls were considered the referent category. Controls who failed the hearing screen were excluded from these analyses. We used linear regression to evaluate whether IQ and achievement scores differed by youth sex or maternal age at delivery. Evidence for effect modification was evaluated using Wald tests.
RESULTS
The demographic characteristics of the sample are shown in Table 1. Comparisons of participants who did and did not participate in the adolescent follow-up indicate that the latter group was more likely to be non-white or Hispanic, Spanish-speaking, and to have slightly lower neurodevelopmental test scores at the younger age point.
Table 1.
Demographic characteristics of adolescents with and without CFM
| Characteristic1 | Controls N=315 | Cases N=121 | ||
|---|---|---|---|---|
|
| ||||
| N | % | N | % | |
| Age (years) | ||||
| <12 | 64 | 20.4 | 19 | 16.0 |
| 12–14 | 152 | 48.4 | 59 | 49.6 |
| >14 | 98 | 31.2 | 41 | 34.5 |
| Gender | ||||
| Female | 162 | 51.4 | 44 | 36.4 |
| Male | 153 | 48.6 | 77 | 63.6 |
| Race/ethnicity | ||||
| White | 251 | 79.7 | 87 | 71.9 |
| Hispanic | 33 | 10.5 | 28 | 23.1 |
| Black | 19 | 6.0 | 0 | 0.0 |
| Asian/Pacific Islander | 9 | 2.9 | 4 | 3.3 |
| Native American | 3 | 1.0 | 2 | 1.7 |
| Language | ||||
| English | 304 | 96.5 | 106 | 87.6 |
| Spanish | 11 | 3.5 | 15 | 12.4 |
| Grade | ||||
| 5–6 | 101 | 33.7 | 42 | 35.9 |
| 7–8 | 127 | 42.3 | 55 | 47.0 |
| 9–12 | 70 | 23.3 | 20 | 17.1 |
| Family income | ||||
| <$25,000 | 32 | 10.2 | 17 | 14.4 |
| $25,000–34,999 | 23 | 7.3 | 20 | 16.9 |
| $35,000–64,999 | 47 | 15.0 | 19 | 16.1 |
| ≥ $65,000 | 211 | 67.4 | 62 | 52.5 |
| Primary caregiver’s highest level of education | ||||
| <12 years | 11 | 3.5 | 9 | 7.7 |
| High school/GED | 73 | 23.3 | 43 | 36.8 |
| Associate’s degree | 59 | 18.8 | 17 | 14.5 |
| Bachelor’s degree | 104 | 33.2 | 37 | 31.6 |
| Graduate degree | 66 | 21.1 | 11 | 9.4 |
| Site | ||||
| Boston | 221 | 70.2 | 74 | 61.2 |
| Seattle | 94 | 29.8 | 47 | 38.8 |
| Phenotype case group | ||||
| Microtia only | 0 | 0 | 24 | 19.8 |
| Microtia and mandibular hypoplasia | 0 | 0 | 46 | 38.0 |
| Other CFM-associated anomalies | 0 | 0 | 51 | 42.1 |
| No discernible anomaly | 315 | 100.0 | 0 | 0 |
| Receipt of interventions | ||||
| Speech therapy | 47 | 15.8 | 68 | 59.6 |
| Physical therapy | 41 | 13.8 | 40 | 33.9 |
| Occupational therapy | 14 | 4.7 | 35 | 29.7 |
| Special education services | 46 | 15.5 | 38 | 32.2 |
| Any intervention | 96 | 32.5 | 84 | 72.4 |
| Hearing impairment2 | ||||
| No | 298 | 99.3 | 33 | 30.0 |
| Yes | 2 | 0.7 | 77 | 70.0 |
Percentages represent proportion with non-missing values. Missing data: age (1 controls, 2 cases), grade (17 controls, 4 cases), income (2 controls, 3 cases), education (2 controls, 4 cases), interventions (20 controls, 5 cases), hearing impairment status (15 controls, 11 cases)
Defined as a hearing threshold >40 dB at frequencies of 500, 1000, 2000, or 4000 Hz
Case-Control Differences
The adjusted mean scores for cases were lower than controls for all measures of IQ and achievement (Table 2). The magnitude of estimated average differences between cases and controls ranged from −0.1 to −3.7 points, ES = −0.01 to −0.3 (p-values ranged from 0.01 to 0.92). The largest observed deficits were for the reading composite (ES = −0.3) and for the WJTA-III Writing Sample (ES = −0.3).
Table 2.
Comparison of mean test scores for adolescents with and without CFM
| Controls | Cases | Cases vs. Controls | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted1 | |||||||||||||||
|
| ||||||||||||||||
| Test | Measure | N | Mean | SD | N | Mean | SD | Difference | 95% CI | p-value | Difference | 95% CI | p-value | ES2 | ||
| WASI | Vocabulary | 311 | 54.0 | 9.5 | 112 | 50.5 | 11.0 | −3.5 | −5.8 | −1.2 | 0.003 | −1.8 | −3.9 | 0.3 | 0.10 | −0.2 |
| Matrix Reasoning | 311 | 49.9 | 8.5 | 114 | 48.7 | 10.0 | −1.2 | −3.3 | 0.8 | 0.24 | −0.1 | −2.2 | 2.0 | 0.92 | −0.01 | |
| Full Scale IQ | 311 | 103.5 | 13.6 | 113 | 100.0 | 14.9 | −3.4 | −6.6 | −0.3 | 0.03 | −1.2 | −4.2 | 1.8 | 0.44 | −0.1 | |
| WRAT4 | Spelling | 310 | 107.7 | 13.9 | 113 | 104.8 | 16.2 | −2.9 | −6.2 | 0.5 | 0.09 | −1.3 | −4.9 | 2.2 | 0.46 | −0.1 |
| Math Computation | 308 | 108.9 | 15.3 | 113 | 104.7 | 16.1 | −4.2 | −7.6 | −0.8 | 0.02 | −2.3 | −5.7 | 1.2 | 0.20 | −0.2 | |
| Reading Composite | 308 | 103.2 | 14.6 | 110 | 97.2 | 16.7 | −6.0 | −9.5 | −2.5 | 0.001 | −3.7 | −7.0 | −0.3 | 0.04 | −0.3 | |
| WJTA-III | Writing Sample | 298 | 104.4 | 12.1 | 107 | 99.4 | 14.2 | −5.0 | −8.1 | −2.0 | 0.001 | −3.6 | −6.5 | −0.7 | 0.01 | −0.3 |
Standard scores used for all analyses; adjusted for age at assessment (continuous), gender, race/ethnicity (white non-Hispanic, Hispanic, other), income (categorical), primary caregiver’s highest level of education (categorical)
ES = Standardized effect size
Analyses using IPW to account for attrition increased the magnitude of all case deficits (see Table, Supplemental Digital Content 1, which shows the Comparison of mean test scores for adolescents with and without CFM after adjustment for attrition using inverse probability weighting, INSERT LINK.). With IPW, effect sizes for case-control differences ranged from −0.1 to −0.4 (p-values ranged from 0.004 to 0.62) and were greatest for WASI-2 Vocabulary, the Reading Composite and the WJTA-III Writing Sample. Case-control differences were attenuated after the exclusion of 11 controls and 15 cases from Spanish-speaking families, with effect size estimates ranging from −0.04 to −0.3 (p-values ranged from 0.03 to 0.79; see Table, Supplemental Digital Content 2, which shows the Comparison of mean test scores for adolescents with and without CFM after adjustment for attrition using inverse probability weighting, INSERT LINK).
Twenty-five percent (74/301) of controls and 38% (44/115) of cases were classified as having learning problems (adjusted OR=1.5, 95% CI 0.9–2.4). Compared to cases without learning problems, cases with learning problems were more likely to come from families that were Hispanic, bilingual (Spanish and English), and with a family income of <$35,000. Cases with learning problems were also marginally more likely to be male, to fail the hearing screen, and to have microtia alone or microtia plus mandibular hypoplasia (Table 3).
Table 3.
Characteristics of cases, by learning status
| Characteristic1 | No learning problem N=71 | Learning problem N=44 | ||
|---|---|---|---|---|
| N | % | N | % | |
| Sex | ||||
| Female | 29 | 40.8 | 15 | 34.1 |
| Male | 42 | 59.2 | 29 | 65.9 |
| Race/ethnicity | ||||
| Non-Hispanic white | 57 | 80.3 | 25 | 56.8 |
| Hispanic | 11 | 15.5 | 16 | 36.4 |
| Other | 3 | 4.2 | 3 | 6.8 |
| Language | ||||
| English | 66 | 93.0 | 34 | 77.3 |
| Spanish | 5 | 7.0 | 10 | 22.7 |
| Family income | ||||
| < $25,000 | 5 | 7.2 | 12 | 27.9 |
| $25,000–$34,999 | 8 | 11.6 | 12 | 27.9 |
| $35,000–$64,999 | 9 | 13.0 | 9 | 20.9 |
| ≥ $65,000 | 47 | 68.1 | 10 | 23.3 |
| Phenotype | ||||
| Microtia only | 13 | 18.3 | 11 | 25.0 |
| Microtia and mandibular hypoplasia | 24 | 33.8 | 18 | 40.9 |
| Other CFM-associated anomalies | 34 | 47.9 | 15 | 34.1 |
| No discernible anomaly | 0 | 0.0 | 0 | 0.0 |
| Hearing impairment2 | ||||
| No | 24 | 34.8 | 9 | 22.5 |
| Yes | 45 | 65.2 | 31 | 77.5 |
Percentages represent proportion with non-missing values. Missing data: income (3); hearing impairment (6)
Defined as a hearing threshold >40 dB at frequencies of 500, 1000, 2000, or 4000 Hz
Analyses by phenotype
Adjusted case-control differences were variable across phenotype (Table 4). Cases with microtia and mandibular hypoplasia (n=46) had consistently lower IQ and achievement scores relative to controls for all measures, with case deficits ranging from ES = −0.02 to −0.6 (p-values ranged from 0.003 to 0.92). The largest differences were found on the WJTA-III Writing Sample and the Reading Composite (ES ≥ −0.6), and the smallest difference observed on WASI Matrix Reasoning (ES < −.03). Estimates for the 24 cases with microtia only or the 51 cases with other related anomalies were variable and there was no consistent direction in association.
Table 4.
Comparison of mean test scores for adolescents with and without CFM, by phenotype1
| Test | Measure | Controls | Cases | p for group differences | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Microtia only | Microtia and mandibular hypoplasia | Other CFM-associated anomalies | ||||||||||||||||
|
| ||||||||||||||||||
| Difference | 95% CI | p-value | ES2 | Difference | 95% CI | p-value | ES2 | Difference | 95% CI | p-value | ES2 | |||||||
| WASI | Vocabulary | Ref | 0.4 | −3.3 | 4.1 | 0.83 | 0.04 | −3.7 | −6.9 | −0.4 | 0.03 | −0.4 | −0.8 | −3.8 | 2.1 | 0.58 | −0.1 | 0.16 |
| Matrix Reasoning | Ref | 2.1 | −1.6 | 5.8 | 0.27 | 0.2 | −0.2 | −3.3 | 3.0 | 0.92 | −0.02 | −1.0 | −4.1 | 2.0 | 0.52 | −0.1 | 0.60 | |
| Full Scale IQ | Ref | 2.0 | −3.1 | 7.2 | 0.44 | 0.2 | −2.7 | −7.3 | 1.8 | 0.24 | −0.3 | −1.0 | −5.1 | 3.2 | 0.64 | −0.1 | 0.50 | |
| WRAT4 | Spelling | Ref | −3.1 | −10.2 | 4.0 | 0.39 | −0.2 | −4.0 | −8.8 | 0.7 | 0.10 | −0.3 | 2.0 | −3.3 | 7.3 | 0.46 | 0.1 | 0.23 |
| Math Computation | Ref | −1.2 | −7.1 | 4.7 | 0.70 | −0.1 | −4.3 | −9.9 | 1.2 | 0.13 | −0.3 | −0.7 | −5.4 | 3.9 | 0.76 | −0.05 | 0.49 | |
| Reading Composite | Ref | −5.4 | −11.5 | 0.7 | 0.08 | −0.4 | −6.4 | −11.2 | −1.5 | 0.01 | −0.5 | −0.2 | −5.0 | 4.6 | 0.93 | −0.01 | 0.03 | |
| WJTA-III | Writing Sample | Ref | −1.8 | −5.9 | 2.4 | 0.41 | −0.1 | −7.6 | −12.5 | −2.6 | 0.003 | −0.6 | −0.7 | −4.5 | 3.0 | 0.70 | −0.1 | 0.03 |
Standard scores used for all analyses; adjusted for age at assessment (continuous), gender, race/ethnicity (white non-Hispanic, Hispanic, other), income (categorical), primary caregiver’s highest level of education (categorical)
ES = Standardized effect size
Secondary analyses
Seventy-two percent of cases and 33% of controls had received or were currently receiving some form of intervention. Estimates using censored normal regression to account for effects of interim intervention shifted case-control differences by −0.7 to −0.9 SD points (p-values <0.001 for all measures).
Seventy percent of cases (77/110) and 1% of controls (2/300) failed the hearing screen. However, there was little evidence that case deficits differed by hearing status (Table 5). Cases with and without hearing impairment scored more poorly than controls on nearly all measures, but case deficits were not consistently greater in one group over the other and there was no evidence for effect modification (Wald p>0.05 for all measures). There was also little evidence of effect modification by maternal age or youth sex (Wald p>0.05; see Table, Supplemental Digital Content 3, Comparison of mean test scores for adolescents with and without CFM, by maternal age, INSERT LINK and see Table, Supplemental Digital Content 4, Comparison of mean test scores for adolescents with and without CFM, by sex, INSERT LINK). However, on nearly all measures case-control differences were greater among adolescents whose mothers were ≤ 25 years old at the child’s birth than among those whose mothers were > 25. Math Computation was the only exception.
Table 5.
Comparison of mean test scores for adolescents with and without CFM, by hearing impairment
| Test | Measure | Controls | Cases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hearing Impairment1 | |||||||||||||
|
| |||||||||||||
| No | Yes | p for group differences | |||||||||||
| Difference2 | 95% CI | p-value | ES3 | Difference2 | 95% CI | p-value | ES3 | ||||||
| WASI | Vocabulary | Ref | −2.2 | −4.8 | 0.4 | 0.10 | −0.2 | −1.2 | −3.9 | 1.5 | 0.39 | −0.1 | 0.22 |
| Matrix Reasoning | Ref | −1.3 | −4.5 | 2.0 | 0.45 | −0.1 | 0.6 | −2.0 | 3.1 | 0.66 | 0.1 | 0.65 | |
| Full Scale IQ | Ref | −3.1 | −7.2 | 1.1 | 0.15 | −0.2 | −0.1 | −3.8 | 3.6 | 0.96 | −0.01 | 0.35 | |
| WRAT4 | Spelling | Ref | 0.7 | −4.9 | 6.3 | 0.81 | 0.05 | −0.5 | −4.7 | 3.8 | 0.83 | −0.03 | 0.94 |
| Math Computation | Ref | −3.9 | −8.6 | 0.9 | 0.11 | −0.3 | −0.4 | −4.5 | 3.7 | 0.87 | −0.02 | 0.28 | |
| Reading Composite | Ref | −0.7 | −5.1 | 3.7 | 0.75 | −0.1 | −4.1 | −8.2 | 0.0 | 0.05 | −0.3 | 0.15 | |
| WJTA-III | Writing Sample | Ref | −1.1 | −4.7 | 2.6 | 0.57 | −0.1 | −4.0 | −7.6 | −0.4 | 0.03 | −0.3 | 0.09 |
Defined as a hearing threshold >40 dB at frequencies of 500, 1000, 2000, or 4000 Hz. 2 controls with hearing impairment excluded from analysis; 12 controls and 10 cases missing hearing screen; 3 controls and 1 case excluded due to room noise
Standard scores used for all analyses; adjusted for age at assessment (continuous), gender, race/ethnicity (white, Hispanic, other), income (categorical), primary caregiver’s highest level of education (categorical)
ES = Standardized effect size
DISCUSSION
The current study re-examined the neurodevelopmental status of youth with and without CFM who were previously assessed in early elementary school.12 After adjusting for demographic variables associated with neurodevelopment, we found that cases scored lower on average than unaffected controls on standardized measures of IQ and academic achievement. However, these effects were relatively modest and case-control group differences were smaller than those observed in the elementary school assessment. The proportion of youth with learning problems—an important metric for identifying children in need of special education—was higher among cases than controls (38% vs. 25%, respectively), but this effect was also relatively small.
Several factors likely accounted for the roughly equivalent performance of the two groups. Biased attrition affected sample composition due to a disproportionate loss of non-white, Spanish-speaking families and youth who had lower neurodevelopmental test scores during their elementary school assessment. Analyses using inverse probability weighting indicated that differential attrition reduced the magnitude of case-control group differences, particularly in areas in which cases appeared most vulnerable (vocabulary, reading and written expression). Furthermore, over twice as many cases as controls received developmental or educational interventions over the course of their childhood. Censored normal regression analyses indicated that in the absence of intervention, group differences might have been much larger, as much as a full SD. Finally, cases’ averaged scores may have poorly represented the performance of the case group overall, due to the wide range of phenotypic variation in CFM. This idea is supported by our finding that in comparison to the control group, cases with ear and mandibular malformations consistently scored lower than those with only microtia and cases with other combinations of CFM-related malformations. The largest phenotypic subgroup differences were observed on the Reading Composite and Written Expression tests, with mean differences on both measures approximately a half SD, a potentially significant difference from an educational perspective.
Replication of the latter finding is needed before concluding that children with both microtia and mandibular hypoplasia have greater risk for academic problems than children with other phenotypic presentations of CFM. However, the plausibility of this hypothesis is supported by the cumulative number of neurodevelopmental risk factors associated with this combination of facial malformations. These include hearing impairment, speech difficulties, upper airway obstruction possibly leading to sleep disordered breathing.37 multiple surgeries and associated anesthesia exposures,38–42 facial paresis or palsy,43 and the social-psychological effects of anomalous facial appearance.44,45 Although most children with CFM will have some of these risk factors, those with combined microtia and mandibular hypoplasia may experience a greater number of risks and at higher levels of severity, with possibly synergistic effects (e.g., hearing loss and facial paresis may in combination produce greater liability than the sum of their individual effects). Future research is needed to establish the specific neurodevelopmental risks and level of risk associated with each of the major phenotypic categories of CFM and to examine the contribution of these factors to academic outcomes.
In addition to CFM-related risk factors, it is important to anticipate the potential effects of demographic and social-economic variables on neurodevelopment, as these factors have been correlated with academic performance in studies of other pediatric populations.46,47 In our comparisons of cases with and without learning problems, we found that cases with learning problems were nearly 3 times more likely than cases without learning problems to come from families in which Spanish was spoken and from families that earned less than $35,000 annually. These data suggest that social-economic and sociolinguistic factors should be considered in the identification and treatment of learning related problems in children with CFM. Bilingual home environments may be especially important, given the effect of bilingualism on the development of reading skills48,49 and the disproportionate number of Hispanic families in the CFM population.
This study was limited in several respects, including the biased sample attrition described above. In addition, the itinerant nature of our assessments required the use of screening audiometry with a dichotomous outcome (pass/fail), rather than a full hearing test that would have measured degree of hearing loss. Another limitation is related to our phenotypic classification method, which substantially improved upon earlier methods in its use of multiple data sources, but was limited by the available data in this cohort (e.g., incomplete information about extracranial anomalies, lack of intra-oral photos to assess dentition).24
Summary and Conclusions
At this early stage of research on CFM and neurodevelopment, our findings should be viewed primarily as hypothesis-generating, rather than as firm conclusions. Four findings are of particular interest in this regard: First, when disregarding phenotype, children with and without CFM perform similarly on tests of IQ and achievement, though cases perform consistently lower on average. Second, facial phenotype may be an important predictor of academic problems, with heightened risk among cases who have both microtia and mandibular hypoplasia. Third, when patients with CFM do encounter cognitive-academic difficulties, deficits may be primarily related to verbal processing--including vocabulary, reading and writing--with less vulnerability in areas such as math and visual-spatial skills. Finally, cases’ academic performance may be as strongly influenced by family demographic and sociolinguistic characteristics as by CFM-related factors, such as hearing loss. We plan to test these hypotheses in a new, prospective cohort of infants and young children with CFM, currently being recruited.50 In the meantime, we recommend close developmental surveillance of children and youth with CFM, especially those with both ear and jaw malformations, higher social-economic risk, and exposure to bilingual home environments.
Supplementary Material
Supplemental Digital Content 1, see Table, which shows the Comparison of mean test scores for adolescents with and without CFM after adjustment for attrition using inverse probability weighting, INSERT LINK.
Supplemental Digital Content 2, see Table, Comparison of mean test scores for adolescents with and without CFM after exclusion of Spanish-speaking families, INSERT LINK.
Supplemental Digital Content 3, see Table, Comparison of mean test scores for adolescents with and without CFM, by maternal age, INSERT LINK.
Supplemental Digital Content 4, see Table, Comparison of mean test scores for adolescents with and without CFM, by sex, INSERT LINK.
Acknowledgments
Funding Source: All phases of this study were supported by NIH grant R01 DE 11939 from the National Institute of Dental and Craniofacial Research to Dr. Werler.
Footnotes
Financial disclosure statement: The authors have no financial relationships to disclose that pertain to this article.
References
- 1.Poswillo D. The aetiology and pathogenesis of craniofcial deformity. Development. 1988;103:207–212. doi: 10.1242/dev.103.Supplement.207. [DOI] [PubMed] [Google Scholar]
- 2.Harris J, Kallen B, Robert E. The epidemiology of anotia and microtia. Journal of Medical Genetics. 1996;33(10):809–13. doi: 10.1136/jmg.33.10.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heike CL, Luquetti DV, Hing AV. Gene Reviews. Seattle, WA: University of Washington, Seattle; Mar 19, 2009. Craniofacial Microsomia Overview. http://www.ncbi.nlm.gov/books/NBK5199. 1993–2016. Updated Oct 9 2014. [PubMed] [Google Scholar]
- 4.Birgfeld C, Heike C. Craniofacial microsomia. Seminars in Plastic Surgery. 2012 May;26(2):91–104. doi: 10.1055/s-0032-1320067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kjaer I. Human prenatal craniofacial development related to brain development under normal and pathologic conditions. Acta Odontol Scand. 1995;53(3):135–143. doi: 10.3109/00016359509005963. [DOI] [PubMed] [Google Scholar]
- 6.Werler MM, Starr JR, Cloonan YK, Speltz ML. Hemifacial microsomia: From gestation to childhood. J Craniofac Surg. 2009;20(Suppl 1):664–669. doi: 10.1097/SCS.0b013e318193d5d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warschausky S, Kay JB, Buchman S, Halberg A, Berger M. Health-related quality of life in children with craniofacial anomalies. Plast Reconstr Surg. 2002;110(2):409–14. doi: 10.1097/00006534-200208000-00004. discussion 415–6. [DOI] [PubMed] [Google Scholar]
- 8.Edwards TC, Patrick DL, Topolski TD, Aspinall CL, Mouradian WE, Speltz ML. Approaches to craniofacial-specific quality of life assessment in adolescents. Cleft Palate Craniofac J. 2005;42(1):19–24. doi: 10.1597/03-097.2.1. [DOI] [PubMed] [Google Scholar]
- 9.Khetani MA, Collett BR, Speltz ML, Werler MM. Health-related quality of life in children with hemifacial microsomia: Parent and child perspectives. Journal of Developmental and Behavioral Pediatrics. 2013 Nov-Dec;34(9):661–668. doi: 10.1097/DBP.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen MS, Samango-Sprouse CA, Stern HJ, et al. Neurodevelopmental profile of infants and toddlers with oculo-auriculo-vertebral spectrum and the correlation of prognosis with physical findings. Am J Med Genet. 1995;60(6):535–540. doi: 10.1002/ajmg.1320600610. [DOI] [PubMed] [Google Scholar]
- 11.Morrison PJ, Mulholland HC, Craig BG, Nevin NC. Cardiovascular abnormalities in the oculo-auriculo-vertebral spectrum (goldenhar syndrome) Am J Med Genet. 1992;44(4):425–428. doi: 10.1002/ajmg.1320440407. [DOI] [PubMed] [Google Scholar]
- 12.Collett BR, Speltz ML, Cloonan YK, Leroux BG, Kelly JP, Werler MM. Neurodevelopmental outcomes in children with hemifacial microsomia. Arch Pediatr Adolesc Med. 2011;165(2):134–140. doi: 10.1001/archpediatrics.2010.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werler MM, Sheehan JE, Hayes C, Padwa BL, Mitchell AA, Mulliken JB. Demographic and reproductive factors associated wtih hemifacial microsomia. Cleft Palate Craniofac J. 2004;41(5):494–500. doi: 10.1597/03-110.1. [DOI] [PubMed] [Google Scholar]
- 14.Werler MM, Sheehan JE, Hayes C, Mitchell AA, Mulliken JB. Vasoactive exposures, vascular events, and hemifacial microsomia. Birth Defects Res A Clin Mol Teratol. 2004;70:389–395. doi: 10.1002/bdra.20022. [DOI] [PubMed] [Google Scholar]
- 15.Dufton LM, Speltz ML, Kelly JP, Leroux B, Collett BR, Werler MM. Psychosocial outcomes in children with hemifacial microsomia. J Pediatr Psychol. 2011;36(7):794–805. doi: 10.1093/jpepsy/jsq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 17.Axelrod BN. Validity of the wechsler abbreviated scale of intelligence and other very short forms of estimating intellectual functioning. Assessment. 2002 Mar;9(1):17–23. doi: 10.1177/1073191102009001003. [DOI] [PubMed] [Google Scholar]
- 18.Canivez GL, Konold TR, Collins JM, Wilson G. Construct validity of the wechsler abbreviated scale of intelligence and wide range intelligence test: Convergent and structural validity. School Psychology Quarterly. 2009 Dec;24(4):252–265. [Google Scholar]
- 19.Ryan JJ, Carruthers CA, Miller LJ, Souheaver GT, Gontkovsky ST, Zehr MD. Exploratory factor analysis of the wechsler abbreviated scale of intelligence (WASI) in adult standardization and clinical samples. Appl Neuropsychol. 2003 Dec;10(4):252–256. doi: 10.1207/s15324826an1004_8. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson GS, Robertson GJ. WRAT4 Wide Range Achievement Test. Lutz, FL: PAR Psychological Assessment Resources, Inc; 2006. [Google Scholar]
- 21.Wiederholt JL, Bryant B. Gray Oral Reading Tests--Fourth Edition. Austin, TX: Pro-Ed; 2001. [Google Scholar]
- 22.Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson® III Tests of Achievement. Rolling Meadows, IL: Riverside Publishing; 2007. [Google Scholar]
- 23.Audiology Information Series. American Speech-Language-Hearing Association; 2015. Type, degree and configuration of hearing loss. [Google Scholar]
- 24.Heike CL, Wallace E, Speltz ML, Saltzman BS, Werler M, Hing AV, et al. Characterizing facial characteristics in individuals with craniofacial microsomia: a systematic approach for clinical research. Birth Defects Res A Clin Mol Teratol. 2016 doi: 10.1002/bdra.23560. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birgfeld CB, Luquetti DV, Gougoutas AJ, et al. A phenotypic assessment tool for craniofacial microsomia. Plast Reconstr Surg. 2011;127(1):313–320. doi: 10.1097/PRS.0b013e3181f95d15. [DOI] [PubMed] [Google Scholar]
- 26.Birgfeld CB, Heike CL, Saltzman BS, Leroux BG, Evans KN, Luquetti DV. Reliable classification of facial phenotypic variation in craniofacial microsomia: A comparison of physical exam and photographs. Head & Face Medicine. 2016;12(14) doi: 10.1186/s13005-016-0109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heike CL, Stueckle LP, Stuhaug ET, et al. Photographic protocol for image acquisition in craniofacial microsomia. Head Face Med. 2011;7:25. doi: 10.1186/1746-160X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gougoutas AJ, Singh DJ, Low DW, Bartlett SP. Hemifacial microsomia: Clinical features and pictographic representations of the OMENS classification system. Plast Reconstr Surg. 2007;120(7):112e–120e. doi: 10.1097/01.prs.0000287383.35963.5e. [DOI] [PubMed] [Google Scholar]
- 29.Horgan JE, Padwa BL, ALR, Mulliken JB. OMENS-plus: Analysis of craniofacial and extracraniofacial anomalies in hemifacial microsomia. Cleft Palate-Craniofacial Journal. 1995;32(5):405–412. doi: 10.1597/1545-1569_1995_032_0405_opaoca_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 30.Poon CC, Meara JG, Heggie AA. Plastic & reconstructive surgery. 2003;111:1011–1018. doi: 10.1097/01.PRS.0000046245.44567.D6. [DOI] [PubMed] [Google Scholar]
- 31.Birgfeld CB, Saltzman BS, Luquetti DV, Latham K, Starr JR, Heike CL. Comparison of two-dimensional and three-dimensional images for phenotypic assessment of craniofacial microsomia. Cleft Palate Craniofac J. 2013;50(3):305–314. doi: 10.1597/11-173. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. p. 400. [Google Scholar]
- 33.Fletcher JM, Francis DJ, Morris RD, Lyon GR. Evidence-based assessment of learning disabilities in children and adolescents. J Clin Child Adolesc Psychol. 2005;34(3):506–522. doi: 10.1207/s15374424jccp3403_7. [DOI] [PubMed] [Google Scholar]
- 34.Lyon GR, Fletcher JM, Barnes MC. Learning disabilities. In: Mash EJ, Barkely RA, editors. Child Psychopathology. 2. New York: Guilford Press; 2003. pp. 520–586. [Google Scholar]
- 35.Robins JM, Rotnitzky A, Zhao LP. Estimation of regression coefficients when some regressors are not always observed. Journal of the American Statistical Association. 1994;89(427):846–866. [Google Scholar]
- 36.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: Antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24(19):2911–2935. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- 37.Cloonan YK, Kifle Y, Davis S, Speltz ML, Werler MM, Starr JR. Sleep outcomes in children with hemifacial microsomia and controls: A follow-up study. Pediatrics. 2009;124(2):e313–21. doi: 10.1542/peds.2008-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birgfeld C, Heike C. Craniofacial microsomia. Seminars in Plastic Surgery. 2012 May;26(2):91–104. doi: 10.1055/s-0032-1320067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naumann HL, Haberkern CM, Pietila KE, et al. Duration of exposure to cranial vault surgery: Associations with neurodevelopment among children with single-suture craniosynostosis. Pediatric Anesthesia. 2012;22:1053–1061. doi: 10.1111/j.1460-9592.2012.03843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DiMaggio C, Sun LS, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011;113(5):1143–1151. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansen TG, Pedersen JK, Henneberg SW, et al. Academic performance in adolescence after inguinal hernia repair in infancy: A nationwide cohort study. Anesthesiology. 2011;114(5):1076–1085. doi: 10.1097/ALN.0b013e31820e77a0. [DOI] [PubMed] [Google Scholar]
- 42.Flick RP, Katusic SK, Colligan RC, et al. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128(5):e1053–61. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cline JM, Hicks KE, Patel KG. Characterization of facial paresis in hemifacial microsomia. Otolaryngol Head Neck Surg. 2014;150(2):188–193. doi: 10.1177/0194599813512775. [DOI] [PubMed] [Google Scholar]
- 44.Dufton LM, Speltz ML, Kelly JP, Leroux B, Collett BR, Werler MM. Psychosocial outcomes in children with hemifacial microsomia. J Pediatr Psychol. 2011;36(7):794–805. doi: 10.1093/jpepsy/jsq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feragen K, Stock N. A longitudinal study of 340 young people with or without a visible difference: The impact of teasing on self-perceptions of appearance and depressive symptoms. Body image. 2016;16:133–42. doi: 10.1016/j.bodyim.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Armstrong F. Neurodevelopment and chronic illness: Mechanisms of disease and treatment. Mental retardation and developmental disabilities research reviews. 2006;12(3):168–73. doi: 10.1002/mrdd.20114. [DOI] [PubMed] [Google Scholar]
- 47.Evans GW, Li D, Whipple SS. Cumulative risk and child development. Psychological Bulletin. 2013;139(6):1342–1396. doi: 10.1037/a0031808. [DOI] [PubMed] [Google Scholar]
- 48.Davison M, Hammer C, Lawrence F. Associations between preschool language and first grade reading outcomes in bilingual children. Journal of communication disorders. 2011;44(4):444–58. doi: 10.1016/j.jcomdis.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammer CS, Miccio AW. Early language and reading development of bilingual preschoolers from low-income families. Topics in Language Disorders. 2006;26(4):322–337. doi: 10.1097/00011363-200610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luquetti D, Heike C, Saltzman B, Johns A, Drake A, Sarwer D, Leroux B, Kapp-Simon K, Speltz M. Craniofacial microsomia: longitudinal outcomes in children pre-kindergarten (CLOCK). Poster presented at the 72nd Annual American Cleft Palate-Craniofacial Association Conference; Palm Springs, CA. 2015. Apr, [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1, see Table, which shows the Comparison of mean test scores for adolescents with and without CFM after adjustment for attrition using inverse probability weighting, INSERT LINK.
Supplemental Digital Content 2, see Table, Comparison of mean test scores for adolescents with and without CFM after exclusion of Spanish-speaking families, INSERT LINK.
Supplemental Digital Content 3, see Table, Comparison of mean test scores for adolescents with and without CFM, by maternal age, INSERT LINK.
Supplemental Digital Content 4, see Table, Comparison of mean test scores for adolescents with and without CFM, by sex, INSERT LINK.