Abstract
Anaphylaxis is a severe, systemic hypersensitivity reaction that is rapid in onset and characterized by life-threatening airway, breathing, and/or circulatory problems, and that is usually associated with skin and mucosal changes. Because it can be triggered in some people by minute amounts of antigen (e.g. certain foods or single insect stings), anaphylaxis can be considered the most aberrant example of an imbalance between the cost and benefit of an immune response. This review will describe current understanding of the immunopathogenesis and pathophysiology of anaphylaxis, focusing on the roles of IgE and IgG antibodies, immune effector cells, and mediators thought to contribute to examples of the disorder. Evidence from studies of anaphylaxis in humans will be discussed, as well as insights gained from analyses of animal models, including mice genetically deficient in the antibodies, antibody receptors, effector cells, or mediators implicated in anaphylaxis, and mice which have been “humanized” for some of these elements. We also will review possible host factors which may influence the occurrence or severity of anaphylaxis. Finally, we will speculate about anaphylaxis from an evolutionary perspective, and argue that, in the context of severe envenomation by arthropods or reptiles, anaphylaxis may even provide a survival advantage.
Keywords: Anaphylaxis, basophils, cysteinyl leukotrienes, epinephrine, food allergy, histamine, IgE, mast cells, platelet activating factor, urticaria
Introduction
The recent “International Consensus on (ICON) Anaphylaxis” described anaphylaxis as “a serious, generalized or systemic, allergic or hypersensitivity reaction that can be life-threatening or fatal”.1 This definition is intentionally “generic”, in that it doesn't mention any of the specific immune elements that might be involved in particular instances of the disorder, as these may vary depending on individual circumstances. In this review, we will describe the key immune elements, such as antibody isotypes, effector cells, and biological mediators, which can contribute to development and pathophysiological manifestations of anaphylaxis. We in particular will note the extent of evidence implicating these immune components in anaphylaxis in humans versus that induced in mouse models of the disorder, focusing especially on forms of anaphylaxis induced by the reactions of allergens with antigen-specific antibodies. We will not extensively review forms of anaphylaxis induced by the antibody-independent activation of effector cells such as mast cells and basophils, topics which have been reviewed elsewhere.2, 3
Clinical Anaphylaxis
The clinical definition, classification, nomenclature, and treatment of anaphylaxis have been points of controversy, varying among different medical subspecialties and in different countries, and it became clear that an important goal for the field would be to achieve a true international consensus on these important points.4 Subsequently, multinational, multidisciplinary symposia were convened to agree on the definition of anaphylaxis, the clinical criteria for its diagnosis, and its management5. Participants agreed on a description of anaphylaxis as “a serious allergic reaction that is rapid in onset and may cause death”, as well as on three sets of clinical criteria to diagnose anaphylaxis.5 These criteria were re-affirmed in the recent “International Consensus on (ICON) Anaphylaxis”1 and are more extensively reviewed elsewhere in this issue (Castells et al.6). A minority of patients exhibit biphasic allergic reactions, in which signs and symptoms of anaphylaxis recur hours after the early phase of the reaction has waned, and in some patients late phase reactions occur without initial hypotension or airway obstruction.7, 8 In addition to the biphasic reactions observed in some patients with anaphylaxis induced by a variety of causes, patients who have IgE reactive with the oligosaccharide galactose-alpha-1,3-galactose (“alpha-gal”), which is present in mammalian meat and in some therapeutic antibodies, can exhibit anaphylaxis after a delay of several hours during which no signs or symptoms are apparent.9
Although there is broad consensus on many aspects of the treatment of anaphylaxis10-12 (see also Castells et al.6), such recommendations are based largely on observational studies, extrapolation from retrospective case reviews, and a few clinical trials.10, 11 Injectable epinephrine is universally agreed upon as the first line therapy for anaphylaxis,10-12 and may counteract many pathophysiological changes in anaphylaxis by acting through: alpha-1 adrenergic receptors to induce vasoconstriction, which prevents or diminishes tissue/airway edema, hypotension and distributive shock; beta-1 adrenergic receptors to increase heart rate and cardiac contractility; and beta-2 adrenergic receptors to dilate airways.11 In addition, epinephrine's action on beta-2 adrenergic receptors may potentially block further release of mediators (histamine and eicosanoids) by mast cells and perhaps other effector cells.13, 14
Other therapies should be considered second line – and not a substitute for epinephrine. Guidelines generally agree that patients should be placed in a supine position and given crystalloid to maintain perfusion, and oxygen.10, 12 H1 and H2 antihistamines may be helpful in treating cutaneous and upper respiratory signs and symptoms, and corticosteroids may help to prevent biphasic reactions, but neither prevent nor treat airway obstruction or circulatory collapse and therefore can't be considered as alternatives to epinephrine.10-12 Development of novel therapies for anaphylaxis is likely to be guided mainly by limited data from humans and by observations made using animal models.
Immunological mechanisms of anaphylaxis
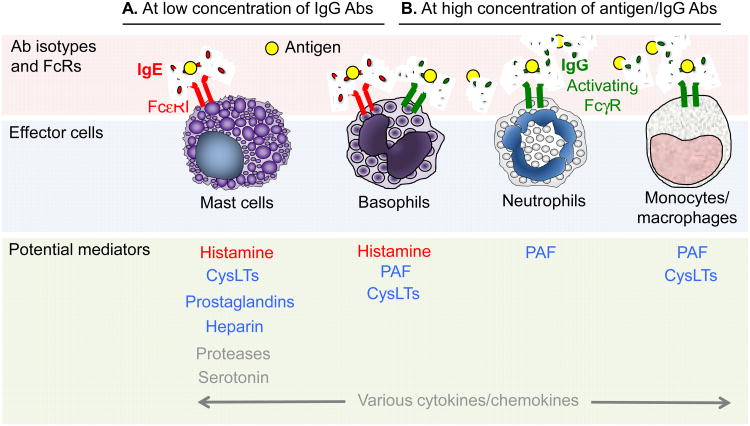
Only limited data on immunological mechanisms of anaphylaxis from human subjects are available due to the life-threatening nature of anaphylaxis and obvious ethical concerns. Human studies of anaphylaxis have included inducing anaphylaxis in volunteers (most often through hymenoptera sting challenge) and collecting samples from patients presenting for emergency management of anaphylaxis. Data obtained in such studies, as well as key findings obtained using mouse models of anaphylaxis, are summarized below, in Figure 1 and in Table 1. The major pathophysiological changes observed during anaphylaxis, and some of the mediators that are thought to contribute to them, are shown in Figure 2.
Figure 1. Multiple potential pathways in antibody-mediated anaphylaxis.
A Antigen-specific IgE antibodies and FcεRI-bearing effector cells (e.g. mast cells, basophils) play a dominant role in anaphylaxis induced (sometimes by very small amounts of antigen) when concentrations of IgG antibodies are low. B. Mouse models of anaphylaxis suggest that IgG antibodies and FcγR-bearing effector cells (e.g. basophils, macrophages, neutrophils, as well as mast cells) can be important effectors of anaphylaxis induced by large amounts of antigen in the presence of high concentrations of IgG antibodies. Some examples of anaphylaxis likely involve both pathways (A and B). Note that co-engagement of ITAM-containing activating FcγRs or FcεRI with the ITIM-bearing FcγRIIB (on mast cells [in mice, but perhaps not in humans] or basophils [in humans and mice]) can act to diminish effector cell activation. In red: Strong evidence for the importance of these mediators in human anaphylaxis induced by antigen; in blue: These elements can participate in models of anaphylaxis in mice but their importance in human anaphylaxis is not yet clear; in grey: Elements with the potential to influence anaphylaxis, but their importance in human or mouse anaphylaxis not yet clear (e.g., human mast cells are thought to make little or no serotonin).
Table 1. Roles (or potential roles) of various antibodies, effector cells and mediators in anaphylaxis in humans and mice.
| Effector mechanisms | Humans | Mice | |
|---|---|---|---|
| Antibody isotypes | IgE | - Elevated IgE levels in individuals with allergic diseases16, 19 - Purified IgE can transfer skin reactivity from a sensitized human subject to a naive host17, 21-23 - The anti-IgE Ab omalizumab can decrease the risks of anaphylaxis30-35 |
- PCA and PSA induced by transfer of antigen-specific IgE into naïve mice and challenge with the antigen24, 25 - IgE-mediated PCA and PSA is abrogated in mice lacking the high affinity IgE receptor FcεRI25 - ASA partially reduced in IgE-deficient or Fceε1-/- mice in some models, but not in others53, 89, 93, 103, 192, 193 |
| IgG | - No definitive evidence to date - Cases of anaphylaxis reported following treatment with therapeutic mAbs without detectable levels of anti-drug IgE58, 194-196 |
- IgG1, IgG2a and IgG2b (but not IgG3) can induce PSA50-60 - IgG-PSA is reduced in FcγRIII-/- mice51, 52 - IgG1- and IgG2b- (but not IgG2a-) PSA is enhanced in FcγRIIB-/- mice52 - Mice deficient in FcεRIα exhibit enhanced systemic anaphylaxis upon challenge with 2.4G2 anti-FcγRII/III Abs20 - Mice deficient for IgG1 or FcγRIII are largely protected in several ASA models89, 102, 103 - ‘Humanized’ mice expressing human FcγRI or FcγRIIA can develop IgG-mediated anaphylaxis150, 151, 153 |
|
| Complement | Anaphylatoxins | - Injection of low doses of C3a, C4a or C5a in the skin of healthy volunteers induces immediate wheal and flare reactions66-69 - Blood levels of C3a, C4a and C5a correlate with the severity of anaphylaxis in humans65 |
- Reduced peanut-induced anaphylaxis in C4-/- mice197 - Reduced IgE-PCA in mice in which mast cells lack C3aR or C5aR198 - Anaphylaxis induced by direct activation of complement by peanut extract in one model177 - C3-/- mice can fully develop IgG-PSA model199 - ASA is not affected in C2-, C5- and C5aR-deficient mice, or after depletion of complement using cobra venom factor192, 200 |
| Effector cells | Mast cells | - Elevated levels of tryptase have been detected during acute anaphylaxis in humans65, 79-82 - High occurrence of anaphylaxis in patients with mastocytosis85-87 |
- IgE-PCA and PSA markedly reduced in various strains of mast cell-deficient mice26-28, 58, 88 - ASA reduced in mast cell-deficient mice in some studies, but not in others51, 54, 89-93, 103, 192, 201 |
| Basophils | - No definitive evidence to date - “basophil activation tests” used to diagnose or confirm allergen sensitization96-99 |
- Controversial: some reports indicate a contribution of basophils to IgG-PSA52, 54, 56 or ASA53, 89, 91, while others found no significant role for basophils52, 92, 103, 199, 202 | |
| Neutrophils | - MPO levels are increased in patients with anaphylaxis as compared to healthy donors106 | - Antibody-mediated neutrophil depletion reduces IgG-PSA and ASA in some52, 53, 56 but not all91, 103 models | |
| Monocytes/ macrophages | - Not yet determined | - Depletion of monocytes/macrophages using clodronate liposomes can reduce IgG-PSA and ASA52, 89, 92, 102, 103 | |
| Platelets | - No definitive evidence to date - Anaphylaxis in humans is associated with platelet activation108 |
- No definitive evidence to date - Depletion of platelets with anti-platelet antibodies (daily for 3 days) or neuraminidase does not reduce ASA102 |
|
| Mediators | Histamine | - Aerosol administration of histamine induces bronchoconstriction in healthy volunteers114, 115 - Intravenous administration of histamine in volunteers can reproduce many of the symptoms of anaphylaxis116, 117 - H1 antihistamines are commonly used as adjunctive therapy for acute anaphylaxis and anaphylactoid reactions119 |
- Histamine injection induces anaphylaxis203, 204 - H1 antihistamine reduces IgE-PSA203 - IgG-PSA and ASA are reduced in mice pre-treated with H1 antihistamine in some models52, 103, 205, but not in others53, 102 - Mice deficient for the histidine decarboxylase (HDC)gene are protected from IgE-PSA203 - H1R- and H2R-deficient mice are partially protected from IgE-PSA204 |
| Cysteinyl leukotrienes (CysLTs) | - Levels of some CysLTs are increased during the onset of anaphylaxis131-133 - Intradermal injection of leukotriene B4 (LTB4), LTC4 and LTD4 induces a wheal and flare reaction in healthy volunteers134 - Aerosol administration of LTC4 and LTD4 in healthy subjects induces bronchoconstriction114, 115, 129 |
- Reduced IgE-PSA in mice deficient for LTC4S135 - Mice deficient for CysLT1R also have significantly reduced IgE-PCA136 |
|
| PAF | - Injection of PAF in the skin of healthy volunteers induces wheal and flare reactions123-125 - Circulating PAF levels increase and circulating PAF-AH activity decreases in proportion to the severity of anaphylaxis65, 82, 128 |
- PAF is released during IgG-PSA and ASA53, 91 - Injection of PAF induces anaphylaxis206 - Reduced ASA in mice deficient for the PAF receptor (PAFR)207 - PAFR antagonists can partially reduce anaphylaxis in IgG-PSA and ASA models52, 53, 57, 58, 91, 102, 103 |
|
| Others | - Anaphylaxis induces increases in levels of many mediators which could potentially contribute (positively or negatively) to the clinical signs and symptoms. This includes various cytokines and chemokines, prostaglandins, tryptase, bradykinin, serotonin, etc. | - Mast cell-derived prostaglandin D2 (PGD2) can limit IgE-PCA and IgE-PSA185 | |
Figure 2. Pathophysiological changes in anaphylaxis and mediators that have been implicated in these processes.
Note: As mentioned in the text, first line treatment of anaphylaxis consists of the rapid administration of epinephrine (see Castells et al.6). Although there is evidence that the mediators shown in the figure, particularly histamine and cysteinyl leukotrienes, contribute to some of the various signs and symptoms of anaphylaxis, and anti-histamines are routinely administered to patients with anaphylaxis, pharmacological targeting of such mediators represents second line treatment and should not be considered as an alternative to epinephrine. In red: Strong evidence for the importance of that mediator, in humans, in the development of some of the signs and symptoms listed in the adjacent box; in blue: these elements can be important in mouse models of anaphylaxis but their importance in human anaphylaxis is not yet clear (studies in human subjects suggest that cysteinyl leukotrienes may contribute importantly to the bronchoconstriction and enhanced vascular permeability associated with anaphylaxis [see text]); in grey: elements with the potential to influence anaphylaxis, but their importance in human or mouse anaphylaxis not yet clear. Note that some mediators (underlined) are likely to contribute to the development of late consequences of anaphylaxis.
Effector molecules and receptors
IgE-dependent anaphylaxis
IgE antibodies undeniably can play an important role in conferring immunological specificity to effector cell activation in anaphylaxis and other allergic diseases.15-18 IgE is by far the isotype found at the lowest concentrations in the circulation (50-200 ng/ml total circulating IgE in healthy individuals vs. ∼10 mg/ml for IgG);15 however, IgE can be found at much higher levels in individuals with allergic diseases.16, 19 IgE binds to the high affinity receptor, FcεRI, on the surface of blood basophils and tissue resident mast cells,20 and (in humans to a greater extent than in mice) other cell types, including neutrophils, eosinophils, monocytes and dendritic cells, and platelets.20 Upon exposure to a bi- or multi-valent allergen, crosslinking of FcεRI-bound IgE induces activation of mast cells and basophils, and the immediate release of preformed mediators such as histamine and various proteases, as well as de novo synthesis of many inflammatory mediators such as certain leukotrienes, prostaglandins, and cytokines.16, 20 The importance of that reaction was demonstrated 50 years ago, when different groups realized that purified IgE was capable of transferring skin reactivity from a sensitized human subjects to naive hosts.17, 21-23 Similarly, transfer of antigen-specific IgE into naïve mice sensitizes the animals to develop anaphylaxis upon subsequent exposure to that allergen.24, 25 Such IgE-mediated anaphylaxis is abrogated in mice lacking the high affinity IgE receptor FcεRI25, as well as in mast cell-deficient mice,26-28 highlighting the importance of IgE-mediated mast cell activation in such models of anaphylaxis.
Ever since the discovery that IgE can transfer allergen reactivity, the development of antigen-specific IgE antibodies has been regarded as a key risk factor for the development of allergy and/or anaphylaxis upon subsequent antigen exposure. Indeed, quantification of specific IgE levels are used as part of the diagnostic evaluation of those thought to have allergic diseases, and is used to identify potential triggers of anaphylaxis in patients with a history of anaphylaxis.29 Several trials have concluded that the use of the anti-IgE therapeutic antibody omalizumab as adjunctive treatment during food or venom immunotherapies can decrease the risks of severe allergic reactions, including anaphylaxis, and in some but not all trials also has been reported to improve the rapidity and efficacy of immunotherapy in achieving desensitization.30-34 In addition, limited clinical data also suggest that omalizumab may prevent spontaneous episodes of anaphylaxis in patients with systemic mastocytosis, a disease characterized by marked increases in mast cell numbers and activity35 (also see the review by Akin et al.36 in this issue of JACI).
Clearly, however, IgE levels alone do not explain an individual's susceptibility to anaphylaxis. Some patients can experience near fatal anaphylaxis despite having low or undetectable levels of circulating allergen-specific IgE.37 Conversely, allergen-specific IgE can be detected in the plasma of many subjects who do not develop clinical symptoms when exposed to that allergen.38 This is particularly true for hymenoptera venom, where the vast majority (∼80%) of people with IgE antibodies specific for hymenoptera venoms have no history of systemic reactions to such venoms.39-42 Therefore, the presence of antigen-specific IgE antibodies, taken in isolation, does not indicate that the person necessarily will exhibit any, let alone severe, clinical reactivity to the recognized antigens.43-49
IgE-independent anaphylaxis
The fact that some patients experience anaphylaxis despite having undetectable levels of circulating allergen-specific IgE37 suggests the existence of IgE-independent pathways of anaphylaxis. However, it should be noted that a lack of detection of free IgE does not mean that such patients don't have enough FcεRI-bound IgE to experience IgE-mediated anaphylaxis. More definitive evidence for IgE-independent anaphylaxis has been obtained using mouse models (Table 1).
Role of IgG and FcγRs
Besides IgE, we now know that mouse IgG also can induce passive systemic anaphylaxis (PSA) reactions, with physiological manifestations similar to those seen in IgE-dependent PSA (mainly hypothermia, vasodilatation and cardiopulmonary changes).50-60 Whether IgG antibodies also mediate anaphylaxis in humans still remains to be proven, and is the topic of a recent review.2 As demonstrated in mice, IgG-mediated anaphylaxis typically requires a much larger dose of antigen than does IgE-mediated anaphylaxis,61 and systemic anaphylaxis also requires systemic absorption of ingested antigen.62 Such conditions could be encountered in the case of anaphylaxis occurring in response to infusion of large quantities of a drug or a therapeutic monoclonal antibody (mAb)2 (Table 1).
Role of complement
Activation of the complement cascade occurs in response to many stimuli, and leads to generation of small polypeptides: C3a, C4a and C5a, also named anaphylatoxins, which are potent inflammatory mediators.63 Multiple lines of evidence suggest that anaphylatoxins might be involved in anaphylaxis. Depletion of complement levels and production of C3a and C5a is observed in human anaphylaxis.64, 65 Anaphylatoxins can activate various myeloid cells, including mast cells and basophils.63 Injection of low doses of C3a, C4a or C5a into the skin of healthy volunteers induces immediate wheal and flare reactions.66-69 In addition, one study showed that blood levels of C3a, C4a and C5a correlated with the severity of anaphylaxis in humans.65 Several transgenic mouse models have been used to study the importance of the complement pathway in anaphylaxis. Data obtained using these transgenic models are reviewed in Table 1, and suggest that, in mice, the effect of complement components on anaphylaxis may be in most cases largely redundant with that of other mediators and may depend on the specific model used.
Potential effector cells of anaphylaxis
Mast cells
Mast cells are viewed as key players in IgE-dependent allergies and anaphylaxis.16, 70 Mast cells ordinarily express large numbers of the high affinity IgE receptor, FcεRI. During IgE-dependent immune responses, the antigen-dependent cross-linking of antigen-specific IgE bound to FcεRI induces the aggregation of FcεRI, promoting the activation of downstream signaling events that lead to the secretion of several biologically active products thought to be implicated in allergic reactions, such as histamine and various cysteinyl leukotrienes (Cys-LTs).16, 71-73 The molecular mechanisms of such IgE-dependent stimulation of mast cells have been extensively reviewed.16, 71, 73-75 There is compelling evidence of activation of mast cells during acute anaphylaxis. Although histamine detection can be used to diagnose anaphylaxis (see Histamine, below), detection of histamine in clinical blood specimens is difficult due to its extremely short half-life, and histamine isn't a mast cell-specific product, since it can also be released by other cells, including basophils76 and neutrophils.77, 78 Tryptase is much more stable than histamine, and is considered to be a largely mast cell-derived product.79 Mature β-tryptase is stored in mast cell granules and released upon activation, such as in anaphylaxis, whereas α- and β- protryptases are secreted constitutively by mast cells and therefore increased blood levels may indicate increased mast cell burden rather than anaphylaxis.79 Elevated levels of tryptase have been detected during acute anaphylaxis in humans.65, 79-82 However, the roles of tryptase or other mast cell-derived proteases in anaphylaxis remain unknown. Moreover, in some patients with anaphylaxis, such as children with food allergen-induced anaphylaxis, elevated blood levels of tryptase have not been detected.83 Additional evidence for a role of mast cells in anaphylaxis comes from the observation that patients suffering from mastocytosis, a disease characterized by the presence of high numbers of mast cells in various organs,84 have a high occurrence of anaphylaxis.85 In children with mastocytosis, increased serum tryptase levels, used as an indicator of mast cell burden, is a risk factor for anaphylaxis and for the severity of anaphylaxis episodes.86, 87
Studies using various strains of mast cell-deficient mice also confirmed the key role of mast cells in IgE-mediated anaphylaxis.26-28, 58, 88 Several reports now demonstrate that mast cell-deficient mice also have reduced peanut-induced anaphylaxis in active systemic anaphylaxis (ASA) models.89-93 However, the role of mast cells in ASA models using other antigens/allergens is more controversial (summarized in Table 1). Therefore, it is likely that mast cells play either dominant or largely redundant roles in anaphylaxis, and that the mast cells' role can be enhanced - or masked - depending on the exact model, adjuvant and allergen used.
Basophils
Human basophils also express high levels of the high affinity IgE receptor FcεRI,94 and express the activating IgG receptor FcγRIIA and the inhibitory IgG receptor FcγRIIB.95 Several lines of evidence suggest that basophils participate in anaphylaxis.76 For example, IgE-dependent activation of human basophils is associated with elevations in the levels of certain basophil cell surface markers, such as CD203c or CD63, and this forms the basis of “basophil activation tests” which can be used to diagnose or confirm allergen sensitization, and to monitor the effects of efforts to treat these conditions with immunotherapy.96-99 However, it is difficult to ascertain how important a contribution basophils make to the pathology of anaphylaxis in humans, given the concomitant mast cell activation that occurs in this setting. Even in mice, the role of basophils in anaphylaxis is unsettled (Table 1).
Monocytes/macrophages
Monocytes and macrophages express high levels of activating FcγRs,100 and can also respond to anaphylatoxins.101 Studies in mice have shown that depletion of monocytes/macrophages using clodronate liposomes can reduce anaphylaxis in both IgG-mediated passive models and active models52, 89, 92, 102, 103 (Table 1). These data suggest that monocytes/macrophages might play an important role in anaphylaxis. However, to the best of our knowledge, the extent to which monocytes/macrophages can contribute to anaphylaxis in humans has not yet been determined.
Neutrophils
The potential functions of neutrophils in anaphylaxis have been recently reviewed in detail.104 Human and mouse neutrophils express several activating FcγRs,104 can produce histamine,77, 78 and can release platelet-activating factor (PAF; please see below for details on the role of PAF in anaphylaxis) in response to stimulation with immune complexes in vitro.53 Moreover, human neutrophils reportedly can express FcεRI, particularly in some patients with asthma.105 The major enzyme stored in neutrophils is myeloperoxidase (MPO). A recent report shows that circulating MPO levels are increased in patients with anaphylaxis as compared to healthy donors.106 Consistent with this, elevated MPO activity can also be detected as soon as two minutes after antigen challenge in an active mouse model of anaphylaxis.53 However, it should be noted that these results do not provide definitive proof of neutrophil activation in anaphylaxis, since MPO could also be potentially released by other cell populations, including macrophages.107 Reduced expression of the activating IgG receptors FcγRIII and FcγRIV on mouse neutrophils occurs after IgG-mediated PSA, which suggest more definitely that neutrophils could be directly activated by IgG immune complexes during anaphylaxis.52, 55 Antibody-mediated neutrophil depletion can reduce anaphylaxis in IgG-mediated PSA52, 53, 56 and mast cell-independent ASA models.53, 103 However, neutrophil-depleting antibodies had no effect in a mast cell-dependent ASA model induced without artificial adjuvants.103 This suggests that neutrophils may be particularly prominent in ASA models induced with adjuvants and that such models may not require any non-redundant contributions of mast cells (Table 1).
Platelets
Anaphylaxis in humans is associated with platelet activation,108 presumably in response to PAF and/or other mechanisms, and activated platelets can release mediators, such as platelet factor 4 (PF4) and serotonin,108 which might contribute to the pathophysiology of anaphylaxis. Moreover, human (but not mouse) platelets can express FcεRI, FcεRII and FcγRIIA,95, 109, 110 and platelets can be activated ex vivo following incubation with serum from allergic patients and subsequent exposure to the relevant allergen.111 Two recent reports have shown that, during basophil activation tests performed in blood specimens ex vivo, basophils (a potential source of PAF) can form associations with platelets,112, 113 identifying this interaction as one which should be investigated further in the context of anaphylaxis.
Potential mediators of anaphylaxis
Histamine
Histamine has long been considered to be an important mediator of anaphylaxis. Woodrow and colleagues showed that aerosol administration of histamine induces bronchoconstriction in healthy volunteers, although the effect of histamine was much less potent than that of leukotrienes (see Leukotrienes, below).114, 115 Intravenous administration of histamine in volunteers can reproduce many of the signs and symptoms of anaphylaxis, including cutaneous flushing, headache, airway obstruction and transient hemodynamic changes, mainly represented by systemic hypotension, tachycardia, and increased left ventricular performance.116, 117 There are four known histamine receptors, named H1-4.118 Studies using receptor antagonists suggest that some of the systemic effects of histamine, including airway obstruction and tachycardia, are mainly mediated through H1R, while some others, including cutaneous flushing and headaches, seem to be mediated through both H1 and H2 receptors.116 H1 antihistamines are commonly used as adjunctive treatment for acute anaphylaxis and anaphylactoid reactions.119 The contribution of histamine to anaphylaxis has also been confirmed using mouse models (summarized in Table 1). Mast cells and basophils likely represent the main sources of histamine in anaphylaxis. In agreement with that, histamine release is abrogated in mast cell-deficient mice in a model of IgE-mediated PSA,27 and increases in plasma histamine levels are also abrogated, in two models of ASA, in mice deficient for both mast cells and basophils.91, 103
Platelet-Activating Factor (PAF)
PAF is a potent phospholipid-derived mediator implicated in platelet aggregation and thought to play important roles in a variety of immune and inflammatory responses. The biology of PAF and its potential role in anaphylaxis have been recently reviewed in detail.120 PAF can be released by a variety of human cells, including purified lung mast cells and blood basophils after ex vivo stimulation with anti-IgE antibodies,121 and by purified neutrophils after incubation in vitro with heat-aggregated human IgG.122 Many of the cell populations which produce PAF can also respond to PAF, including platelets, mast cells, neutrophils and macrophages (reviewed in120). Injection of PAF in the skin of healthy volunteers induces wheal and flare reactions.123-125 Since these reactions could be blocked by H1-antihistamines, it was first proposed that PAF induced wheals via secondary histamine release by dermal mast cells.124, 125 However, unlike human lung mast cells and peripheral blood-derived mast cells, skin mast cells do not degranulate in response to PAF stimulation ex vivo.126 In addition, Krause and collaborators showed that intradermal injection of PAF, unlike that of histamine and codeine, did not cause a statistically significant rise in dermal histamine levels in healthy volunteers.127 A limited number of reports have assessed concentrations of PAF or PAF-acetylhydrolase (PAF-AH) - an enzyme responsible for the rapid degradation of PAF - after anaphylaxis in humans. In these reports, circulating PAF levels were increased and circulating PAF-AH activity was inversely correlated with the severity of anaphylaxis.65, 82, 128
The contribution of PAF to anaphylaxis has been studied in more detail using pharmacologic and genetic approaches in mouse models (reviewed in Table 1). In most models, combined inhibition of histamine and PAF almost entirely blocked anaphylaxis, suggesting additive or synergistic effects of histamine and PAF. The main cellular source of PAF in these reports likely depends on the exact anaphylaxis model used. Using an adjuvant-free active anaphylaxis model, we recently reported that the PAFR antagonist CV-6209 can reduce anaphylaxis in wild-type mice, but has no effect on the residual anaphylaxis observed in monocyte/macrophage-depleted mice, suggesting that monocytes/macrophages represent the major source of PAF in this model.103
Cysteinyl leukotrienes (CysLTs)
A third class of potential mediators of anaphylaxis was originally termed ‘slow-reacting substance of anaphylaxis’ (SRS-A), and consists of three bioactive cysteinyl leukotrienes (CysLTs): leukotriene B4 (LTB4), LTC4 and LTD4 (reviewed in129). CysLTs are synthesized from arachidonic acid by a variety of cells, including mast cells, basophils and macrophages.130 CysLTs and their metabolites can be measured by mass spectrometry, and several reports show that levels of some of these products, namely LTE4, 2,3-dinor-9α,11β-PGF2, and 9α,11β-PGF2, are increased during the onset of anaphylaxis.131-133 While these reports indicate that CysLTs and their metabolites might be good biomarkers of anaphylaxis, they do not prove that these compounds make an important contribution to the clinical manifestations of anaphylaxis. However, multiple observations suggest that CysLTs can promote acute allergic reactions. When injected intradermally in healthy volunteers, each of the three CysLTs elicited a wheal and flare reaction.134 In addition, aerosol administration of LTC4 and LTD4 in healthy subjects induced bronchoconstriction with 1,000-fold more potency than histamine114, 115, 129 (Table 1).
More definitive evidence for a role of CysLTs in anaphylaxis comes from studies in mice. Mice deficient for LTC4S (a protein responsible for biosynthesis of LTC4) or for the Cys-LT receptor CysLT1R have markedly reduced IgE-mediated passive cutaneous anaphylaxis (PCA).135, 136
Other potential mediators
Anaphylaxis induces changes in levels of many other mediators which could potentially contribute (positively or negatively) to the clinical signs and symptoms (Table 1). This includes tryptase,64, 80, 137-139 prostaglandins132, 137 and cytokines/chemokines.65, 138 Depletion of the bradykinin precursor, high molecular weight kininogen, has been observed in anaphylaxis, likely through activation of the plasma contact system and kallakrein.64, 140, 141 Anaphylaxis patients may also experience depletion of clotting factors, including Factors V and VIII, and in extreme cases develop diffuse intravascular coagulation.64, 142 While most patients promptly treated for anaphylaxis recover without obvious sequelae, some develop recurrent signs and symptoms which require continued treatment with epinephrine and for which corticosteroids are administered.10, 143 Such sequelae are thought to reflect the “late” consequences of some of the mediators released by effectors of anaphylaxis, such as cysteinyl leukotrienes, cytokines and chemokines, or by structural cells activated in this setting.143 Finally, mast cells can release adenosine upon IgE-dependent activation, and adenosine can have complex effects, mediated via various adenosine receptors with distinct functions, which have the potential to influence the pathophysiology of anaphylaxis.144 However, more work is needed to define the importance of most of these mediators in anaphylaxis, particularly in humans.
Insights from humanized models of anaphylaxis
Several ‘humanized’ mouse models of anaphylaxis have been developed to investigate the functions of human antibodies, Fc receptors and effector cells in anaphylaxis. Transgenic mice expressing human FcεRI instead of the mouse protein (hFcεRITg mice) were generated, and the expression profile of the hFcεRI transgene is very similar to that found in humans.145-148 hFcεRITg mice can develop systemic anaphylaxis in response to intravenous sensitization with mouse or human IgE (mouse IgE can bind to human FcεRI, while human IgE can't bind to the mouse receptor) followed by systemic antigen challenge,145, 148 cutaneous anaphylaxis when they are sensitized intra-dermally with serum from peanut-allergic patients and then intravenously challenged with peanut extract.149 hFcγRITg and hFcγRIIATg mice have also been generated, and the expression of hFcγRI or hFcγRIIA in such transgenic mice recapitulates that found in humans.150, 151 Each of these transgenic models can develop IgG-mediated anaphylaxis though a mechanism involving monocytes/macrophages and neutrophils.122, 152 More recently, Gillis and collaborators developed a novel mouse strain in which the human low-affinity IgG receptor locus, comprising both activating (hFcγRIIA, hFcγRIIIA, and hFcγRIIIB) and inhibitory (hFcγRIIB) hFcγR genes, has been knocked-in into the equivalent mouse locus.153 These knock-in mice are susceptible to PSA induced by injection of heat-aggregated human intravenous immunoglobulin (IVIg). The contribution of hFcγRIIA to anaphylaxis is predominant in these mice, as revealed in experiments using an anti-FcγRIIA blocking antibody.153 Antibody-mediated depletion of neutrophils, and to a lesser extent basophils, also ameliorated signs of anaphylaxis. Finally, such anaphylaxis also could be partially inhibited using either a PAF receptor antagonist or a histamine receptor 1 antagonist.153
Recently, three groups independently attempted to generate ‘humanized’ models of anaphylaxis using different strains of highly immunodeficient NOD-scid gamma (NSG) mice engrafted with human stem cells.154-156 Bryce and colleagues used NSG mice expressing human SCF, IL3 and GM-CSF transgenes (NSG-SGM3 mice), and engrafted them with human thymus, liver, and hematopoietic stem cells. Such engraftment resulted in the development of large numbers of ‘human’ mast cells in NSG-SGM3 mice in the peritoneal cavity and peripheral tissues.156 The authors could induce both PCA and PSA reactions upon sensitization with a chimeric IgE containing the human constant region, and challenge with the relevant antigen.156 Burton and colleagues used NSG mice carrying a human SCF transgene and engrafted with human hematopoietic stem cells.154 The authors demonstrated that such engrafted mice also develop large numbers of ‘human’ mast cells, produce human IgE (hIgE) in response to gavage with peanut extract, and develop anaphylaxis upon subsequent oral challenge with peanut.154 Importantly, anaphylaxis in this model could be blocked in mice treated with the anti-hIgE antibody omalizumab (which does not recognize mouse IgE).154 Pagovich et al. also developed a ‘humanized’ model of peanut anaphylaxis in NSG mice engrafted with blood mononuclear cells from patients with peanut allergy with a clinical history of anaphylaxis.155 These mice produced human IgE and IgG antibodies in response to intraperitoneal sensitizations with peanut, and developed anaphylaxis upon subsequent oral challenges with peanut.155 Again, anaphylaxis was reduced in mice treated with omalizumab, as well as in mice which had received an adeno-associated virus (AAV) coding for omalizumab.155
Altogether, results from such humanized models of anaphylaxis suggest that both hIgE and hIgG have the potential to induce anaphylaxis through their respective Fc receptors, and also suggest that peanut anaphylaxis is highly dependent on IgE.
Genetic diversity/host factors influencing anaphylaxis
Genetic modifiers may influence mast cell activation and the development of anaphylaxis, as demonstrated in differences observed between the 129/Sv and C57BL/6 strains of mice.157 129/Sv mice demonstrated higher levels of plasma histamine than did C57BL/6 mice following anaphylaxis induced by anti-IgE. Although higher numbers of mast cells and serum IgE levels in the 129/Sv mice could potentially explain these differences, the authors also demonstrated that bone marrow-derived cultured mast cells from 129/Sv mice degranulated more robustly than those from C57BL/6 while synthesizing similar quantities of cytokines.157 However, the specific genetic modifiers responsible for these observed differences between the two strains of mice remain unknown.
Ethnic differences in rates of food allergy and anaphylaxis suggest that genetic modifiers also may exist in human populations.158, 159 Reasons for these ethnic disparities remain unclear, but may reflect true genetic differences, environmental factors, including socioeconomic status, or a combination of factors. Nevertheless, a handful of genetic polymorphisms have been described that may influence development of anaphylaxis. Genetic polymorphisms in IL-4Rα, IL-10, and IL-13 have been linked to the development of anaphylaxis to drugs and latex160-162 but theoretically may influence allergen sensitization more than (or in addition to) effector mechanisms during anaphylaxis.
Polymorphisms affecting metabolism of mediators of anaphylaxis also may influence anaphylaxis severity. As mentioned above, PAF-AH activity levels inversely correlated with severity of anaphylaxis.65, 82, 128 A loss of function mutation in PAF-AH, V279F has been linked with asthma, but not yet with anaphylaxis.163 Individuals with variants in angiotensinogen, i.e. the MM genotype associated with decreased levels of angiotensinogen, were reported to have increased rates of hymenoptera venom allergy and more severe reactions during venom immunotherapy.164 Similarly, amongst patients with tree nut and peanut allergies, lower serum ACE levels were associated with more severe pharyngeal edema, presumably through decreased bradykinin metabolism.165
A few mutations have been described that may influence development and severity of anaphylaxis. An activating mutation in c-KIT, D816V, promotes mast cell proliferation in clonal mast cell disorders including mastocytosis166, 167 (also see Akins et al36 in this issue of JACI). D816V mutations are also found in some patients with recurrent anaphylaxis who do not have increased mast cell numbers on pathology and therefore do not meet criteria for mastocytosis;168 while this suggests that that their mast cells are hyperresponsive, this has not yet been substantiated. In autosomal dominant hyper-IgE syndrome caused by loss-of-function mutations in STAT3, patients have increased levels of total and allergen specific IgEs, but clinically lower rates of anaphylaxis.169 This clinical observation may be explained, at least in part, by decreased mast cell degranulation169 and/or by inhibition of enhanced vascular permeability through increased resilience of adherens junctions in patients and cells with STAT3 loss of function mutations.170
The role of sex hormones in anaphylaxis is unclear. Anaphylaxis occurs more commonly in women than men.171, 172 Moreover, in a model of PSA, female mice exhibited a greater drop in body temperature than did male mice, and this sex difference could be abrogated by ovariectomy or administration of estrogen antagonist to female mice.173 However, analysis of patients in an anaphylaxis registry revealed an increased severity of anaphylaxis in male versus female patients of 13-56 years of age, but no sex differences in anaphylaxis severity for prepubescent individuals or those older than 56 years old.174
Recovery from anaphylaxis
Many of those who have experienced anaphylaxis and were not treated have survived the episode, particularly those with less severe presentations. What is the basis of such recovery? Variations in metabolism of mediators, including PAF and bradykinin, may influence manifestations of anaphylaxis65, 82, 128, 165 and theoretically the ability to recover from these manifestations. In animal models of anaphylaxis and in humans undergoing insect sting challenge, levels of substances with endogenous vasopressor activity, including epinephrine, norepinephrine and angiotensin II, are increased within minutes following development of anaphylaxis,175, 176 likely to compensate for the vasodilation and fluid extravasation occurring during anaphylaxis. Observations that beta-adrenergic blockade can exacerbate systemic anaphylaxis in mouse and rat models177, 178 and in people with severe anaphylaxis due to multiple causes,179-182 particularly when combined with angiotensin converting enzyme (ACE) inhibitors,183 support a role for endogenous vasopressors in limiting the severity of pathophysiological changes in anaphylaxis. Mast cell degranulation releases chymase, which can convert angiotensin I to angiotensin II,184 and may thereby directly contribute to increased angiotensin II levels observed following anaphylaxis. In a recent paper, Nakamura and colleagues showed that mice in which mast cells cannot produce prostaglandin D2 (PGD2) have enhanced manifestations of IgE-mediated anaphylaxis. Therefore, it appears that mast cells also can secrete anti-anaphylactic mediators which might help to limit anaphylactic responses.185 Finally, it is possible that genetically-determined or other differences in mast cell activation or mediator release profiles also might contribute to differences in the manifestations of, or recovery from, anaphylaxis.
Can anaphylaxis be beneficial?
Using mouse models, we recently reported that the development of a type 2 immune response to honeybee venom (BV) could increase the survival of mice challenged with whole BV186. Also, others have shown in mice that a type 2 immune response to BV phospholipase A2 (bvPLA2, which is considered to be the major BV allergen in humans) could diminish the drop in body temperature induced by challenge with a “near-lethal” dose of bvPLA2.187 Importantly, these effects were dependent on IgE,186 and on the high affinity IgE receptor, FcεRI.186, 187 In a follow-up study, we also provided evidence that IgE, FcεRI and mast cells can enhance the survival of mice injected with Russell's viper venom.188 One of the mechanisms by which innate activation of mouse mast cells can enhance the survival of naïve mice upon their first exposure to various arthropod189 or reptile188-190 venoms is the proteolytic reduction of the toxicity of venom components by mast cell-derived carboxypeptidase 3A190, 191 or mouse mast cell protease 4 (chymase).189 Given that snake (or arthropod) envenomation in the field can result in systemic distribution of the venom, one could argue that systemic IgE-dependent mast cell activation in this setting could both produce the clinical picture of anaphylaxis and also result in the systemic release of mediators (i.e. mast cell proteases) that can degrade toxic components of the venom. In such settings, anaphylaxis could be beneficial, if it prevents death by envenomation -- and the unfortunate individual also survives the anaphylaxis. Although we don't know whether human IgE also can enhance resistance to venoms (and we imagine that we would have some trouble enlisting volunteers for such a study), it is tempting to speculate that anaphylaxis induced by small amounts of venom (e.g. a single or wasp bee sting) represents only the most extreme and maladaptive end of a spectrum of acquired IgE-mediated immune responses to venom that includes, at the other end of the spectrum, appropriately regulated immune responses that can enhance resistance to such venoms.
Concluding remarks
Anaphylaxis represents one of the most urgent of medical emergencies, where rapid diagnosis and prompt and appropriate treatment can mean the difference between life and death. While there has been steady progress in our understanding of the antibodies, effector cells and mediators that can contribute to the development and manifestations of anaphylaxis, especially in the context of mouse models of the disorder, the basic clinical management of anaphylaxis has changed little in decades (see Castells et al.6 in this issue of JACI) and Table 2. In a report published in 2005, Sampson et al.5 identified as major research needs both the development of “universally accepted diagnostic criteria” and the importance of identifying “reliable laboratory biomarkers to confirm the clinical impression”. As noted in our Introduction, the first need largely has been addressed by international, interdisciplinary efforts to forge consensus. But the second need remains essentially unfulfilled. It is our hope that further progress in understanding the immunopathogenesis and pathophysiology of anaphylaxis in all of its various forms will help to guide efforts to devise more effective strategies for preventing this disorder and also to provide more effective options for rapidly diagnosing and effectively treating anaphylaxis when it occurs.
Table 2. Key concepts and therapeutic implications.
|
Acknowledgments
Declaration of funding sources: L.L.R. acknowledges support from the European Commission (Marie Skłodowska-Curie Individual Fellowship H2020-MSCA-IF-2014 656086) and the Institut National de la Santé et de la Recherche Médicale (INSERM); S.J.G. acknowledges support from National Institutes of Health grants U19 AI104209, NS 080062, and R01 AR067145 and the Department of Pathology, Stanford University School of Medicine.
Abbreviations
- ASA
Active systemic anaphylaxis
- CysLT
Cysteinyl leukotriene
- DT
Diphtheria toxin
- DTR
Diphtheria toxin receptor
- HDC
Histidine decarboxylase
- Ig
Immunoglobulin
- LT
Leukotriene
- mAb
Monoclonal antibody
- MCPT
Mast cell protease
- MPO
Myeloperoxidase
- OVA
Ovalbumin
- PAF
Platelet-activating factor
- PAF-AH
Platelet-activating factor acetylhydrolase
- PAFR
Platelet-activating factor receptor
- PCA
Passive cutaneous anaphylaxis
- PGD2
Prostaglandin D2
- PSA
Passive systemic anaphylaxis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simons FE, Ardusso LR, Bilo MB, Cardona V, Ebisawa M, El-Gamal YM, et al. International consensus on (ICON) anaphylaxis. World Allergy Organ J. 2014;7:9. doi: 10.1186/1939-4551-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkelman FD, Khodoun MV, Strait R. Human IgE-independent systemic anaphylaxis. J Allergy Clin Immunol. 2016;137:1674–80. doi: 10.1016/j.jaci.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subramanian H, Gupta K, Ali H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J Allergy Clin Immunol. 2016;138:700–10. doi: 10.1016/j.jaci.2016.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bock GG, Goode J, editors. Final Discussion, in Anaphylaxis: Novartis Foundation Symposium. 2004. p. 257. [Google Scholar]
- 5.Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson NF, Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–7. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 6.Castells MC. The diagnosis and management of anaphylaxis. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Golden DB. Patterns of anaphylaxis: acute and late phase features of allergic reactions. Novartis Found Symp. 2004;257:101–10. discussion 110-5, 157-60, 276-85. [PubMed] [Google Scholar]
- 8.Lee S, Sadosty AT, Campbell RL. Update on biphasic anaphylaxis. Curr Opin Allergy Clin Immunol. 2016;16:346–51. doi: 10.1097/ACI.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 9.Commins SP, Jerath MR, Cox K, Erickson LD, Platts-Mills T. Delayed anaphylaxis to alpha-gal, an oligosaccharide in mammalian meat. Allergol Int. 2016;65:16–20. doi: 10.1016/j.alit.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lieberman P, Nicklas RA, Randolph C, Oppenheimer J, Bernstein D, Bernstein J, et al. Anaphylaxis--a practice parameter update 2015. Ann Allergy Asthma Immunol. 2015;115:341–84. doi: 10.1016/j.anai.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Simons FE, Ebisawa M, Sanchez-Borges M, Thong BY, Worm M, Tanno LK, et al. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ J. 2015;8:32. doi: 10.1186/s40413-015-0080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muraro A, Roberts G, Worm M, Bilo MB, Brockow K, Fernandez Rivas M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:1026–45. doi: 10.1111/all.12437. [DOI] [PubMed] [Google Scholar]
- 13.Broder I, Baumal R. Studies of reversed anaphylaxis in the perfused guinea-pig lung. Immunology. 1972;22:651–61. [PMC free article] [PubMed] [Google Scholar]
- 14.Peachell P. Regulation of mast cells by beta-agonists. Clin Rev Allergy Immunol. 2006;31:131–42. doi: 10.1385/CRIAI:31:2:131. [DOI] [PubMed] [Google Scholar]
- 15.Dullaers M, De Bruyne R, Ramadani F, Gould HJ, Gevaert P, Lambrecht BN. The who, where, and when of IgE in allergic airway disease. J Allergy Clin Immunol. 2012;129:635–45. doi: 10.1016/j.jaci.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 16.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oettgen HC. Fifty years later: Emerging functions of IgE antibodies in host defense, immune regulation, and allergic diseases. J Allergy Clin Immunol. 2016;137:1631–45. doi: 10.1016/j.jaci.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–17. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 19.Platts-Mills TA, Schuyler AJ, Erwin EA, Commins SP, Woodfolk JA. IgE in the diagnosis and treatment of allergic disease. J Allergy Clin Immunol. 2016;137:1662–70. doi: 10.1016/j.jaci.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraft S, Kinet JP. New developments in FcepsilonRI regulation, function and inhibition. Nat Rev Immunol. 2007;7:365–78. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 21.Stanworth DR, Humphrey JH, Bennich H, Johansson SG. Specific inhibition of the Prausnitz-Kustner reaction by an atypical human myeloma protein. Lancet. 1967;2:330–2. doi: 10.1016/s0140-6736(67)90171-7. [DOI] [PubMed] [Google Scholar]
- 22.Ishizaka K, Ishizaka T, Richter M. Effect of reduction and alkylation on allergen-combining properties of reaginic antibody. J Allergy. 1966;37:135–44. doi: 10.1016/0021-8707(66)90088-8. [DOI] [PubMed] [Google Scholar]
- 23.Ribatti D. The discovery of immunoglobulin E. Immunol Lett. 2016;171:1–4. doi: 10.1016/j.imlet.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Wershil BK, Mekori YA, Murakami T, Galli SJ. 125I-fibrin deposition in IgE-dependent immediate hypersensitivity reactions in mouse skin. Demonstration of the role of mast cells using genetically mast cell-deficient mice locally reconstituted with cultured mast cells. J Immunol. 1987;139:2605–14. [PubMed] [Google Scholar]
- 25.Dombrowicz D, Flamand V, Brigman KK, Koller BH, Kinet JP. Abolition of anaphylaxis by targeted disruption of the high affinity immunoglobulin E receptor alpha chain gene. Cell. 1993;75:969–76. doi: 10.1016/0092-8674(93)90540-7. [DOI] [PubMed] [Google Scholar]
- 26.Feyerabend TB, Weiser A, Tietz A, Stassen M, Harris N, Kopf M, et al. Cre-mediated cell ablation contests mast cell contribution in models of antibody- and T cell-mediated autoimmunity. Immunity. 2011;35:832–44. doi: 10.1016/j.immuni.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Oka T, Kalesnikoff J, Starkl P, Tsai M, Galli SJ. Evidence questioning cromolyn's effectiveness and selectivity as a ‘mast cell stabilizer’ in mice. Lab Invest. 2012;92:1472–82. doi: 10.1038/labinvest.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lilla JN, Chen CC, Mukai K, BenBarak MJ, Franco CB, Kalesnikoff J, et al. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood. 2011;118:6930–8. doi: 10.1182/blood-2011-03-343962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamilton RG, MacGlashan DW, Jr, Saini SS. IgE antibody-specific activity in human allergic disease. Immunol Res. 2010;47:273–84. doi: 10.1007/s12026-009-8160-3. [DOI] [PubMed] [Google Scholar]
- 30.Nadeau KC, Kohli A, Iyengar S, DeKruyff RH, Umetsu DT. Oral immunotherapy and anti-IgE antibody-adjunctive treatment for food allergy. Immunol Allergy Clin North Am. 2012;32:111–33. doi: 10.1016/j.iac.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 31.MacGinnitie AJ, Rachid R, Gragg H, Little SV, Lakin P, Cianferoni A, et al. Omalizumab facilitates rapid oral desensitization for peanut allergy. J Allergy Clin Immunol. 2017;139:873–81 e8. doi: 10.1016/j.jaci.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood RA, Kim JS, Lindblad R, Nadeau K, Henning AK, Dawson P, et al. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow's milk allergy. J Allergy Clin Immunol. 2016;137:1103–10 e1-11. doi: 10.1016/j.jaci.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boni E, Incorvaia C, Mauro M. Dose-dependence of protection from systemic reactions to venom immunotherapy by omalizumab. Clin Mol Allergy. 2016;14:14. doi: 10.1186/s12948-016-0051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ricciardi L. Omalizumab: A useful tool for inducing tolerance to bee venom immunotherapy. Int J Immunopathol Pharmacol. 2016;29:726–8. doi: 10.1177/0394632016670920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter MC, Robyn JA, Bressler PB, Walker JC, Shapiro GG, Metcalfe DD. Omalizumab for the treatment of unprovoked anaphylaxis in patients with systemic mastocytosis. J Allergy Clin Immunol. 2007;119:1550–1. doi: 10.1016/j.jaci.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 36.Akin C. Mast Cell Activation Syndrome. J Allergy Clin Immunol. 2017 [Google Scholar]
- 37.Simons FE, Frew AJ, Ansotegui IJ, Bochner BS, Golden DB, Finkelman FD, et al. Risk assessment in anaphylaxis: current and future approaches. J Allergy Clin Immunol. 2007;120:S2–24. doi: 10.1016/j.jaci.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125:S116–25. doi: 10.1016/j.jaci.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 39.Bilo BM, Bonifazi F. Epidemiology of insect-venom anaphylaxis. Curr Opin Allergy Clin Immunol. 2008;8:330–7. doi: 10.1097/ACI.0b013e32830638c5. [DOI] [PubMed] [Google Scholar]
- 40.Sturm GJ, Heinemann A, Schuster C, Wiednig M, Groselj-Strele A, Sturm EM, et al. Influence of total IgE levels on the severity of sting reactions in Hymenoptera venom allergy. Allergy. 2007;62:884–9. doi: 10.1111/j.1398-9995.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 41.Haftenberger M, Laussmann D, Ellert U, Kalcklosch M, Langen U, Schlaud M, et al. [Prevalence of sensitisation to aeraoallergens and food allergens: results of the German Health Interview and Examination Survey for Adults (DEGS1)] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:687–97. doi: 10.1007/s00103-012-1658-1. [DOI] [PubMed] [Google Scholar]
- 42.Langen U, Schmitz R, Steppuhn H. [Prevalence of allergic diseases in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1)] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:698–706. doi: 10.1007/s00103-012-1652-7. [DOI] [PubMed] [Google Scholar]
- 43.Bousquet J, Anto JM, Bachert C, Bousquet PJ, Colombo P, Crameri R, et al. Factors responsible for differences between asymptomatic subjects and patients presenting an IgE sensitization to allergens. A GA2LEN project. Allergy. 2006;61:671–80. doi: 10.1111/j.1398-9995.2006.01048.x. [DOI] [PubMed] [Google Scholar]
- 44.Golden DB, Marsh DG, Kagey-Sobotka A, Freidhoff L, Szklo M, Valentine MD, et al. Epidemiology of insect venom sensitivity. JAMA. 1989;262:240–4. [PubMed] [Google Scholar]
- 45.Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125:191–7 e1-13. doi: 10.1016/j.jaci.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Schafer T, Przybilla B. IgE antibodies to Hymenoptera venoms in the serum are common in the general population and are related to indications of atopy. Allergy. 1996;51:372–7. [PubMed] [Google Scholar]
- 47.Sturm GJ, Kranzelbinder B, Schuster C, Sturm EM, Bokanovic D, Vollmann J, et al. Sensitization to Hymenoptera venoms is common, but systemic sting reactions are rare. J Allergy Clin Immunol. 2014;133:1635–43 e1. doi: 10.1016/j.jaci.2013.10.046. [DOI] [PubMed] [Google Scholar]
- 48.Hamilton RG. Allergic sensitization is a key risk factor for but not synonymous with allergic disease. J Allergy Clin Immunol. 2014;134:360–1. doi: 10.1016/j.jaci.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 49.Warrington R. Lack of Correlation between Severity of Clinical Symptoms, Skin Test Reactivity, and Radioallergosorbent Test Results in Venom-Allergic Patients. Allergy Asthma Clin Immunol. 2006;2:62–7. doi: 10.1186/1710-1492-2-2-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harada M, Nagata M, Takeuchi M, Ohara T, Makino S, Watanabe A. Age-dependent difference in susceptibility to IgE antibody- and IgG1 antibody-mediated passive anaphylactic shock in the mouse. Immunol Invest. 1991;20:515–23. doi: 10.3109/08820139109082632. [DOI] [PubMed] [Google Scholar]
- 51.Miyajima I, Dombrowicz D, Martin TR, Ravetch JV, Kinet JP, Galli SJ. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gammaRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis. J Clin Invest. 1997;99:901–14. doi: 10.1172/JCI119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beutier H, Gillis CM, Iannascoli B, Godon O, England P, Sibilano R, et al. IgG subclasses determine pathways of anaphylaxis in mice. J Allergy Clin Immunol. 2017;139:269–80 e7. doi: 10.1016/j.jaci.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jonsson F, Mancardi DA, Kita Y, Karasuyama H, Iannascoli B, Van Rooijen N, et al. Mouse and human neutrophils induce anaphylaxis. J Clin Invest. 2011;121:1484–96. doi: 10.1172/JCI45232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsujimura Y, Obata K, Mukai K, Shindou H, Yoshida M, Nishikado H, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28:581–9. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 55.Khodoun MV, Strait R, Armstrong L, Yanase N, Finkelman FD. Identification of markers that distinguish IgE- from IgG-mediated anaphylaxis. Proc Natl Acad Sci U S A. 2011;108:12413–8. doi: 10.1073/pnas.1105695108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khodoun MV, Kucuk ZY, Strait RT, Krishnamurthy D, Janek K, Clay CD, et al. Rapid desensitization of mice with anti-FcgammaRIIb/FcgammaRIII mAb safely prevents IgG-mediated anaphylaxis. J Allergy Clin Immunol. 2013;132:1375–87. doi: 10.1016/j.jaci.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Finkelman FD, Rothenberg ME, Brandt EB, Morris SC, Strait RT. Molecular mechanisms of anaphylaxis: lessons from studies with murine models. J Allergy Clin Immunol. 2005;115:449–57. doi: 10.1016/j.jaci.2004.12.1125. quiz 58. [DOI] [PubMed] [Google Scholar]
- 58.Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007;120:506–15. doi: 10.1016/j.jaci.2007.07.033. quiz 16-7. [DOI] [PubMed] [Google Scholar]
- 59.Hirayama N, Hirano T, Kohler G, Kurata A, Okumura K, Ovary Z. Biological activities of antitrinitrophenyl and antidinitrophenyl mouse monoclonal antibodies. Proc Natl Acad Sci U S A. 1982;79:613–5. doi: 10.1073/pnas.79.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grey HM, Hirst JW, Cohn M. A new mouse immunoglobulin: IgG3. J Exp Med. 1971;133:289–304. doi: 10.1084/jem.133.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. J Clin Invest. 2006;116:833–41. doi: 10.1172/JCI25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strait RT, Mahler A, Hogan S, Khodoun M, Shibuya A, Finkelman FD. Ingested allergens must be absorbed systemically to induce systemic anaphylaxis. J Allergy Clin Immunol. 2011;127:982–9 e1. doi: 10.1016/j.jaci.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46:2753–66. doi: 10.1016/j.molimm.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith PL, Kagey-Sobotka A, Bleecker ER, Traystman R, Kaplan AP, Gralnick H, et al. Physiologic manifestations of human anaphylaxis. J Clin Invest. 1980;66:1072–80. doi: 10.1172/JCI109936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown SG, Stone SF, Fatovich DM, Burrows SA, Holdgate A, Celenza A, et al. Anaphylaxis: clinical patterns, mediator release, and severity. J Allergy Clin Immunol. 2013;132:1141–9 e5. doi: 10.1016/j.jaci.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 66.Lepow IH, Willms-Kretschmer K, Patrick RA, Rosen FS. Gross and ultrastructural observations on lesions produced by intradermal injection of human C3a in man. Am J Pathol. 1970;61:13–23. [PMC free article] [PubMed] [Google Scholar]
- 67.Wuepper KD, Bokisch VA, Muller-Eberhard HJ, Stoughton RB. Cutaneous responses to human C 3 anaphylatoxin in man. Clin Exp Immunol. 1972;11:13–20. [PMC free article] [PubMed] [Google Scholar]
- 68.Yancey KB, Hammer CH, Harvath L, Renfer L, Frank MM, Lawley TJ. Studies of human C5a as a mediator of inflammation in normal human skin. J Clin Invest. 1985;75:486–95. doi: 10.1172/JCI111724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gorski JP, Hugli TE, Muller-Eberhard HJ. C4a: the third anaphylatoxin of the human complement system. Proc Natl Acad Sci U S A. 1979;76:5299–302. doi: 10.1073/pnas.76.10.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Voehringer D. Protective and pathological roles of mast cells and basophils. Nat Rev Immunol. 2013;13:362–75. doi: 10.1038/nri3427. [DOI] [PubMed] [Google Scholar]
- 71.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–23. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abramson J, Pecht I. Regulation of the mast cell response to the type 1 Fc epsilon receptor. Immunol Rev. 2007;217:231–54. doi: 10.1111/j.1600-065X.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 73.Reber LL, Frossard N. Targeting mast cells in inflammatory diseases. Pharmacol Ther. 2014;142:416–35. doi: 10.1016/j.pharmthera.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 74.Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999;402:B24–30. doi: 10.1038/35037021. [DOI] [PubMed] [Google Scholar]
- 75.Rivera J, Fierro NA, Olivera A, Suzuki R. New insights on mast cell activation via the high affinity receptor for IgE. Adv Immunol. 2008;98:85–120. doi: 10.1016/S0065-2776(08)00403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schroeder JT. Basophils: emerging roles in the pathogenesis of allergic disease. Immunol Rev. 2011;242:144–60. doi: 10.1111/j.1600-065X.2011.01023.x. [DOI] [PubMed] [Google Scholar]
- 77.Alcaniz L, Vega A, Chacon P, El Bekay R, Ventura I, Aroca R, et al. Histamine production by human neutrophils. FASEB J. 2013;27:2902–10. doi: 10.1096/fj.12-223867. [DOI] [PubMed] [Google Scholar]
- 78.Xu X, Zhang D, Zhang H, Wolters PJ, Killeen NP, Sullivan BM, et al. Neutrophil histamine contributes to inflammation in mycoplasma pneumonia. J Exp Med. 2006;203:2907–17. doi: 10.1084/jem.20061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwartz LB. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;26:451–63. doi: 10.1016/j.iac.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 80.Schwartz LB, Metcalfe DD, Miller JS, Earl H, Sullivan T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987;316:1622–6. doi: 10.1056/NEJM198706253162603. [DOI] [PubMed] [Google Scholar]
- 81.Stone SF, Cotterell C, Isbister GK, Holdgate A, Brown SG Emergency Department Anaphylaxis Investigators. Elevated serum cytokines during human anaphylaxis: Identification of potential mediators of acute allergic reactions. J Allergy Clin Immunol. 2009;124:786–92 e4. doi: 10.1016/j.jaci.2009.07.055. [DOI] [PubMed] [Google Scholar]
- 82.Vadas P, Perelman B, Liss G. Platelet-activating factor, histamine, and tryptase levels in human anaphylaxis. J Allergy Clin Immunol. 2013;131:144–9. doi: 10.1016/j.jaci.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 83.De Schryver S, Halbrich M, Clarke A, La Vieille S, Eisman H, Alizadehfar R, et al. Tryptase levels in children presenting with anaphylaxis: Temporal trends and associated factors. J Allergy Clin Immunol. 2016;137:1138–42. doi: 10.1016/j.jaci.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 84.Valent P, Akin C, Hartmann K, Nilsson G, Reiter A, Hermine O, et al. Advances in the Classification and Treatment of Mastocytosis: Current Status and Outlook toward the Future. Cancer Res. 2017 doi: 10.1158/0008-5472.CAN-16-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schuch A, Brockow K. Mastocytosis and Anaphylaxis. Immunol Allergy Clin North Am. 2017;37:153–64. doi: 10.1016/j.iac.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 86.Brockow K, Jofer C, Behrendt H, Ring J. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients. Allergy. 2008;63:226–32. doi: 10.1111/j.1398-9995.2007.01569.x. [DOI] [PubMed] [Google Scholar]
- 87.Alvarez-Twose I, Vano-Galvan S, Sanchez-Munoz L, Morgado JM, Matito A, Torrelo A, et al. Increased serum baseline tryptase levels and extensive skin involvement are predictors for the severity of mast cell activation episodes in children with mastocytosis. Allergy. 2012;67:813–21. doi: 10.1111/j.1398-9995.2012.02812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sawaguchi M, Tanaka S, Nakatani Y, Harada Y, Mukai K, Matsunaga Y, et al. Role of mast cells and basophils in IgE responses and in allergic airway hyperresponsiveness. J Immunol. 2012;188:1809–18. doi: 10.4049/jimmunol.1101746. [DOI] [PubMed] [Google Scholar]
- 89.Arias K, Chu DK, Flader K, Botelho F, Walker T, Arias N, et al. Distinct immune effector pathways contribute to the full expression of peanut-induced anaphylactic reactions in mice. J Allergy Clin Immunol. 2011;127:1552–61 e1. doi: 10.1016/j.jaci.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 90.Burton OT, Noval Rivas M, Zhou JS, Logsdon SL, Darling AR, Koleoglou KJ, et al. Immunoglobulin E signal inhibition during allergen ingestion leads to reversal of established food allergy and induction of regulatory T cells. Immunity. 2014;41:141–51. doi: 10.1016/j.immuni.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reber LL, Marichal T, Mukai K, Kita Y, Tokuoka SM, Roers A, et al. Selective ablation of mast cells or basophils reduces peanut-induced anaphylaxis in mice. J Allergy Clin Immunol. 2013;132:881–8 e1-11. doi: 10.1016/j.jaci.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smit JJ, Willemsen K, Hassing I, Fiechter D, Storm G, van Bloois L, et al. Contribution of classic and alternative effector pathways in peanut-induced anaphylactic responses. PLoS One. 2011;6:e28917. doi: 10.1371/journal.pone.0028917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun J, Arias K, Alvarez D, Fattouh R, Walker T, Goncharova S, et al. Impact of CD40 ligand, B cells, and mast cells in peanut-induced anaphylactic responses. J Immunol. 2007;179:6696–703. doi: 10.4049/jimmunol.179.10.6696. [DOI] [PubMed] [Google Scholar]
- 94.Karasuyama H, Mukai K, Obata K, Tsujimura Y, Wada T. Nonredundant roles of basophils in immunity. Annu Rev Immunol. 2011;29:45–69. doi: 10.1146/annurev-immunol-031210-101257. [DOI] [PubMed] [Google Scholar]
- 95.Bruhns P, Jonsson F. Mouse and human FcR effector functions. Immunol Rev. 2015;268:25–51. doi: 10.1111/imr.12350. [DOI] [PubMed] [Google Scholar]
- 96.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124:292–300. e1–97. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Giavina-Bianchi P, Galvao VR, Picard M, Caiado J, Castells MC. Basophil Activation Test Is a Relevant Biomarker of the Outcome of Rapid Desensitization in Platinum Compounds-Allergy. J Allergy Clin Immunol Pract. 2016 doi: 10.1016/j.jaip.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 98.Kim SY, Kim JH, Jang YS, Choi JH, Park S, Hwang YI, et al. The Basophil Activation Test Is Safe and Useful for Confirming Drug-Induced Anaphylaxis. Allergy Asthma Immunol Res. 2016;8:541–4. doi: 10.4168/aair.2016.8.6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Santos AF, Du Toit G, Douiri A, Radulovic S, Stephens A, Turcanu V, et al. Distinct parameters of the basophil activation test reflect the severity and threshold of allergic reactions to peanut. J Allergy Clin Immunol. 2015;135:179–86. doi: 10.1016/j.jaci.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcgamma receptors in dendritic cells and macrophages. Nat Rev Immunol. 2014;14:94–108. doi: 10.1038/nri3582. [DOI] [PubMed] [Google Scholar]
- 101.Bohlson SS, O'Conner SD, Hulsebus HJ, Ho MM, Fraser DA. Complement, c1q, and c1q-related molecules regulate macrophage polarization. Front Immunol. 2014;5:402. doi: 10.3389/fimmu.2014.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Strait RT, Morris SC, Yang M, Qu XW, Finkelman FD. Pathways of anaphylaxis in the mouse. J Allergy Clin Immunol. 2002;109:658–68. doi: 10.1067/mai.2002.123302. [DOI] [PubMed] [Google Scholar]
- 103.Balbino B, Sibilano R, Starkl P, Marichal T, Gaudenzio N, Karasuyama H, et al. Pathways of immediate hypothermia and leukocyte infiltration in an adjuvant-free mouse model of anaphylaxis. J Allergy Clin Immunol. 2017;139:584–96 e10. doi: 10.1016/j.jaci.2016.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jonsson F, Mancardi DA, Albanesi M, Bruhns P. Neutrophils in local and systemic antibody-dependent inflammatory and anaphylactic reactions. J Leukoc Biol. 2013;94:643–56. doi: 10.1189/jlb.1212623. [DOI] [PubMed] [Google Scholar]
- 105.Gounni AS, Lamkhioued B, Koussih L, Ra C, Renzi PM, Hamid Q. Human neutrophils express the high-affinity receptor for immunoglobulin E (Fc epsilon RI): role in asthma. FASEB J. 2001;15:940–9. doi: 10.1096/fj.00-0378com. [DOI] [PubMed] [Google Scholar]
- 106.Francis A, Bosio E, Stone SF, Fatovich DM, Arendts G, Nagree Y, et al. Neutrophil activation during acute human anaphylaxis: analysis of MPO and sCD62L. Clin Exp Allergy. 2016 doi: 10.1111/cea.12868. [DOI] [PubMed] [Google Scholar]
- 107.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 108.Kasperska-Zajac A, Rogala B. Platelet function in anaphylaxis. J Investig Allergol Clin Immunol. 2006;16:1–4. [PubMed] [Google Scholar]
- 109.Joseph M, Gounni AS, Kusnierz JP, Vorng H, Sarfati M, Kinet JP, et al. Expression and functions of the high-affinity IgE receptor on human platelets and megakaryocyte precursors. Eur J Immunol. 1997;27:2212–8. doi: 10.1002/eji.1830270914. [DOI] [PubMed] [Google Scholar]
- 110.Hasegawa S, Pawankar R, Suzuki K, Nakahata T, Furukawa S, Okumura K, et al. Functional expression of the high affinity receptor for IgE (FcepsilonRI) in human platelets and its' intracellular expression in human megakaryocytes. Blood. 1999;93:2543–51. [PubMed] [Google Scholar]
- 111.Capron A, Joseph M, Ameisen JC, Capron M, Pancre V, Auriault C. Platelets as effectors in immune and hypersensitivity reactions. Int Arch Allergy Appl Immunol. 1987;82:307–12. doi: 10.1159/000234214. [DOI] [PubMed] [Google Scholar]
- 112.Mukai K, Gaudenzio N, Gupta S, Vivanco N, Bendall SC, Maecker HT, et al. Assessing basophil activation by using flow cytometry and mass cytometry in blood stored 24 hours before analysis. J Allergy Clin Immunol. 2017;139:889–99 e11. doi: 10.1016/j.jaci.2016.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tordesillas L, Rahman AH, Hartmann BM, Sampson HA, Berin MC. Mass cytometry profiling the response of basophils and the complete peripheral blood compartment to peanut. J Allergy Clin Immunol. 2016;138:1741–4 e9. doi: 10.1016/j.jaci.2016.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weiss JW, Drazen JM, Coles N, McFadden ER, Jr, Weller PF, Corey EJ, et al. Bronchoconstrictor effects of leukotriene C in humans. Science. 1982;216:196–8. doi: 10.1126/science.7063880. [DOI] [PubMed] [Google Scholar]
- 115.Weiss JW, Drazen JM, McFadden ER, Jr, Weller P, Corey EJ, Lewis RA, et al. Airway constriction in normal humans produced by inhalation of leukotriene D. Potency, time course, and effect of aspirin therapy. JAMA. 1983;249:2814–7. [PubMed] [Google Scholar]
- 116.Kaliner M, Sigler R, Summers R, Shelhamer JH. Effects of infused histamine: analysis of the effects of H-1 and H-2 histamine receptor antagonists on cardiovascular and pulmonary responses. J Allergy Clin Immunol. 1981;68:365–71. doi: 10.1016/0091-6749(81)90134-2. [DOI] [PubMed] [Google Scholar]
- 117.Vigorito C, Russo P, Picotti GB, Chiariello M, Poto S, Marone G. Cardiovascular effects of histamine infusion in man. J Cardiovasc Pharmacol. 1983;5:531–7. doi: 10.1097/00005344-198307000-00004. [DOI] [PubMed] [Google Scholar]
- 118.MacGlashan D., Jr Histamine: A mediator of inflammation. J Allergy Clin Immunol. 2003;112:S53–9. doi: 10.1016/s0091-6749(03)01877-3. [DOI] [PubMed] [Google Scholar]
- 119.Sheikh A, Ten Broek V, Brown SG, Simons FE. H1-antihistamines for the treatment of anaphylaxis: Cochrane systematic review. Allergy. 2007;62:830–7. doi: 10.1111/j.1398-9995.2007.01435.x. [DOI] [PubMed] [Google Scholar]
- 120.Gill P, Jindal NL, Jagdis A, Vadas P. Platelets in the immune response: Revisiting platelet-activating factor in anaphylaxis. J Allergy Clin Immunol. 2015;135:1424–32. doi: 10.1016/j.jaci.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 121.Triggiani M, Schleimer RP, Warner JA, Chilton FH. Differential synthesis of 1-acyl-2-acetyl-sn-glycero-3-phosphocholine and platelet-activating factor by human inflammatory cells. J Immunol. 1991;147:660–6. [PubMed] [Google Scholar]
- 122.Jonsson F, Mancardi DA, Zhao W, Kita Y, Iannascoli B, Khun H, et al. Human FcgammaRIIA induces anaphylactic and allergic reactions. Blood. 2012;119:2533–44. doi: 10.1182/blood-2011-07-367334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Archer CB, Page CP, Paul W, Morley J, MacDonald DM. Inflammatory characteristics of platelet activating factor (PAF-acether) in human skin. Br J Dermatol. 1984;110:45–50. doi: 10.1111/j.1365-2133.1984.tb07310.x. [DOI] [PubMed] [Google Scholar]
- 124.Lai CK, Ollier S, Lau CK, Holgate ST. Effect of azelastine and ketotifen on the bronchial and skin responses to platelet-activating factor in humans. Clin Exp Allergy. 1991;21:489–96. doi: 10.1111/j.1365-2222.1991.tb01690.x. [DOI] [PubMed] [Google Scholar]
- 125.Juhlin L, Pihl-Lundin I. Effects of antihistamines on cutaneous reactions and influx of eosinophils after local injection of PAF, kallikrein, compound 48/80 and histamine in patients with chronic urticaria and healthy subjects. Acta Derm Venereol. 1992;72:197–200. [PubMed] [Google Scholar]
- 126.Kajiwara N, Sasaki T, Bradding P, Cruse G, Sagara H, Ohmori K, et al. Activation of human mast cells through the platelet-activating factor receptor. J Allergy Clin Immunol. 2010;125:1137–45 e6. doi: 10.1016/j.jaci.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 127.Krause K, Gimenez-Arnau A, Martinez-Escala E, Farre-Albadalejo M, Abajian M, Church MK, et al. Platelet-activating factor (PAF) induces wheal and flare skin reactions independent of mast cell degranulation. Allergy. 2013;68:256–8. doi: 10.1111/all.12083. [DOI] [PubMed] [Google Scholar]
- 128.Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T, et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358:28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- 129.Austen KF. The cysteinyl leukotrienes: where do they come from? What are they? Where are they going? Nat Immunol. 2008;9:113–5. doi: 10.1038/ni0208-113. [DOI] [PubMed] [Google Scholar]
- 130.Peters-Golden M, Gleason MM, Togias A. Cysteinyl leukotrienes: multi-functional mediators in allergic rhinitis. Clin Exp Allergy. 2006;36:689–703. doi: 10.1111/j.1365-2222.2006.02498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Higashi N, Mita H, Ono E, Fukutomi Y, Yamaguchi H, Kajiwara K, et al. Profile of eicosanoid generation in aspirin-intolerant asthma and anaphylaxis assessed by new biomarkers. J Allergy Clin Immunol. 2010;125:1084–91 e6. doi: 10.1016/j.jaci.2009.12.977. [DOI] [PubMed] [Google Scholar]
- 132.Ono E, Taniguchi M, Mita H, Fukutomi Y, Higashi N, Miyazaki E, et al. Increased production of cysteinyl leukotrienes and prostaglandin D2 during human anaphylaxis. Clin Exp Allergy. 2009;39:72–80. doi: 10.1111/j.1365-2222.2008.03104.x. [DOI] [PubMed] [Google Scholar]
- 133.Denzlinger C, Haberl C, Wilmanns W. Cysteinyl leukotriene production in anaphylactic reactions. Int Arch Allergy Immunol. 1995;108:158–64. doi: 10.1159/000237133. [DOI] [PubMed] [Google Scholar]
- 134.Soter NA, Lewis RA, Corey EJ, Austen KF. Local effects of synthetic leukotrienes (LTC4, LTD4, LTE4, and LTB4) in human skin. J Invest Dermatol. 1983;80:115–9. doi: 10.1111/1523-1747.ep12531738. [DOI] [PubMed] [Google Scholar]
- 135.Kanaoka Y, Maekawa A, Penrose JF, Austen KF, Lam BK. Attenuated zymosan-induced peritoneal vascular permeability and IgE-dependent passive cutaneous anaphylaxis in mice lacking leukotriene C4 synthase. J Biol Chem. 2001;276:22608–13. doi: 10.1074/jbc.M103562200. [DOI] [PubMed] [Google Scholar]
- 136.Maekawa A, Austen KF, Kanaoka Y. Targeted gene disruption reveals the role of cysteinyl leukotriene 1 receptor in the enhanced vascular permeability of mice undergoing acute inflammatory responses. J Biol Chem. 2002;277:20820–4. doi: 10.1074/jbc.M203163200. [DOI] [PubMed] [Google Scholar]
- 137.Lessof MH, Youlten LJ, Heavey D, Kemeny DM, Dollery C. Local mediator release in bee venom anaphylaxis. Clin Exp Allergy. 1989;19:231–2. doi: 10.1111/j.1365-2222.1989.tb02370.x. [DOI] [PubMed] [Google Scholar]
- 138.Korosec P, Turner PJ, Silar M, Kopac P, Kosnik M, Gibbs BF, et al. Basophils, high-affinity IgE receptors, and CCL2 in human anaphylaxis. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2016.12.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.van der Linden PW, Hack CE, Poortman J, Vivie-Kipp YC, Struyvenberg A, van der Zwan JK. Insect-sting challenge in 138 patients: relation between clinical severity of anaphylaxis and mast cell activation. J Allergy Clin Immunol. 1992;90:110–8. doi: 10.1016/s0091-6749(06)80017-5. [DOI] [PubMed] [Google Scholar]
- 140.van der Linden PW, Hack CE, Eerenberg AJ, Struyvenberg A, van der Zwan JK. Activation of the contact system in insect-sting anaphylaxis: association with the development of angioedema and shock. Blood. 1993;82:1732–9. [PubMed] [Google Scholar]
- 141.Sala-Cunill A, Bjorkqvist J, Senter R, Guilarte M, Cardona V, Labrador M, et al. Plasma contact system activation drives anaphylaxis in severe mast cell-mediated allergic reactions. J Allergy Clin Immunol. 2015;135:1031–43 e6. doi: 10.1016/j.jaci.2014.07.057. [DOI] [PubMed] [Google Scholar]
- 142.Pumphrey RS. Fatal anaphylaxis in the UK, 1992-2001. Novartis Found Symp. 2004;257:116–28. discussion 28-32, 57-60, 276-85. [PubMed] [Google Scholar]
- 143.Lieberman P. Biphasic anaphylactic reactions. Ann Allergy Asthma Immunol. 2005;95:217–26. doi: 10.1016/S1081-1206(10)61217-3. quiz 26, 58. [DOI] [PubMed] [Google Scholar]
- 144.Kalesnikoff J, Galli SJ. Anaphylaxis: Mechanisms of mast cell activation. In: Ring J, editor. Anaphylaxis Chem Immunol Allergy. Vol. 95. Basel: Karger; 2010. pp. 45–66. [DOI] [PubMed] [Google Scholar]
- 145.Dombrowicz D, Brini AT, Flamand V, Hicks E, Snouwaert JN, Kinet JP, et al. Anaphylaxis mediated through a humanized high affinity IgE receptor. J Immunol. 1996;157:1645–51. [PubMed] [Google Scholar]
- 146.Dombrowicz D, Lin S, Flamand V, Brini AT, Koller BH, Kinet JP. Allergy-associated FcRbeta is a molecular amplifier of IgE- and IgG-mediated in vivo responses. Immunity. 1998;8:517–29. doi: 10.1016/s1074-7613(00)80556-7. [DOI] [PubMed] [Google Scholar]
- 147.Mancardi DA, Iannascoli B, Hoos S, England P, Daeron M, Bruhns P. FcgammaRIV is a mouse IgE receptor that resembles macrophage FcepsilonRI in humans and promotes IgE-induced lung inflammation. J Clin Invest. 2008;118:3738–50. doi: 10.1172/JCI36452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fung-Leung WP, De Sousa-Hitzler J, Ishaque A, Zhou L, Pang J, Ngo K, et al. Transgenic mice expressing the human high-affinity immunoglobulin (Ig) E receptor alpha chain respond to human IgE in mast cell degranulation and in allergic reactions. J Exp Med. 1996;183:49–56. doi: 10.1084/jem.183.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu Y, Sun Y, Chang LJ, Li N, Li H, Yu Y, et al. Blockade of peanut allergy with a novel Ara h 2-Fcgamma fusion protein in mice. J Allergy Clin Immunol. 2013;131:213–21 e1-5. doi: 10.1016/j.jaci.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Heijnen IA, van Vugt MJ, Fanger NA, Graziano RF, de Wit TP, Hofhuis FM, et al. Antigen targeting to myeloid-specific human Fc gamma RI/CD64 triggers enhanced antibody responses in transgenic mice. J Clin Invest. 1996;97:331–8. doi: 10.1172/JCI118420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McKenzie SE, Taylor SM, Malladi P, Yuhan H, Cassel DL, Chien P, et al. The role of the human Fc receptor Fc gamma RIIA in the immune clearance of platelets: a transgenic mouse model. J Immunol. 1999;162:4311–8. [PubMed] [Google Scholar]
- 152.Mancardi DA, Albanesi M, Jonsson F, Iannascoli B, Van Rooijen N, Kang X, et al. The high-affinity human IgG receptor FcgammaRI (CD64) promotes IgG-mediated inflammation, anaphylaxis, and antitumor immunotherapy. Blood. 2013;121:1563–73. doi: 10.1182/blood-2012-07-442541. [DOI] [PubMed] [Google Scholar]
- 153.Gillis CM, Jonsson F, Mancardi DA, Tu N, Beutier H, Van Rooijen N, et al. Mechanisms of anaphylaxis in human low-affinity IgG receptor locus knock-in mice. J Allergy Clin Immunol. 2017;139:1253–1265 e14. doi: 10.1016/j.jaci.2016.06.058. [DOI] [PubMed] [Google Scholar]
- 154.Burton OT, Stranks AJ, Tamayo JM, Koleoglou KJ, Schwartz LB, Oettgen HC. A humanized mouse model of anaphylactic peanut allergy. J Allergy Clin Immunol. 2017;139:314–22 e9. doi: 10.1016/j.jaci.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pagovich OE, Wang B, Chiuchiolo MJ, Kaminsky SM, Sondhi D, Jose CL, et al. Anti-hIgE gene therapy of peanut-induced anaphylaxis in a humanized murine model of peanut allergy. J Allergy Clin Immunol. 2016;138:1652–62 e7. doi: 10.1016/j.jaci.2016.03.053. [DOI] [PubMed] [Google Scholar]
- 156.Bryce PJ, Falahati R, Kenney LL, Leung J, Bebbington C, Tomasevic N, et al. Humanized mouse model of mast cell-mediated passive cutaneous anaphylaxis and passive systemic anaphylaxis. J Allergy Clin Immunol. 2016;138:769–79. doi: 10.1016/j.jaci.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yamashita Y, Charles N, Furumoto Y, Odom S, Yamashita T, Gilfillan AM, et al. Cutting edge: genetic variation influences Fc epsilonRI-induced mast cell activation and allergic responses. J Immunol. 2007;179:740–3. doi: 10.4049/jimmunol.179.2.740. [DOI] [PubMed] [Google Scholar]
- 158.Mahdavinia M, Fox SR, Smith BM, James C, Palmisano EL, Mohammed A, et al. Racial Differences in Food Allergy Phenotype and Health Care Utilization among US Children. J Allergy Clin Immunol Pract. 2017;5:352–7 e1. doi: 10.1016/j.jaip.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Buka RJ, Crossman RJ, Melchior CL, Huissoon AP, Hackett S, Dorrian S, et al. Anaphylaxis and ethnicity: higher incidence in British South Asians. Allergy. 2015;70:1580–7. doi: 10.1111/all.12702. [DOI] [PubMed] [Google Scholar]
- 160.Apter AJ, Schelleman H, Walker A, Addya K, Rebbeck T. Clinical and genetic risk factors of self-reported penicillin allergy. J Allergy Clin Immunol. 2008;122:152–8. doi: 10.1016/j.jaci.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 161.Guglielmi L, Fontaine C, Gougat C, Avinens O, Eliaou JF, Guglielmi P, et al. IL-10 promoter and IL4-Ralpha gene SNPs are associated with immediate beta-lactam allergy in atopic women. Allergy. 2006;61:921–7. doi: 10.1111/j.1398-9995.2006.01067.x. [DOI] [PubMed] [Google Scholar]
- 162.Brown RH, Hamilton RG, Mintz M, Jedlicka AE, Scott AL, Kleeberger SR. Genetic predisposition to latex allergy: role of interleukin 13 and interleukin 18. Anesthesiology. 2005;102:496–502. doi: 10.1097/00000542-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 163.Karasawa K, Harada A, Satoh N, Inoue K, Setaka M. Plasma platelet activating factor-acetylhydrolase (PAF-AH) Prog Lipid Res. 2003;42:93–114. doi: 10.1016/s0163-7827(02)00049-8. [DOI] [PubMed] [Google Scholar]
- 164.Niedoszytko M, Ratajska M, Chelminska M, Makowiecki M, Malek E, Sieminska A, et al. The angiotensinogen AGT p.M235T gene polymorphism may be responsible for the development of severe anaphylactic reactions to insect venom allergens. Int Arch Allergy Immunol. 2010;153:166–72. doi: 10.1159/000312634. [DOI] [PubMed] [Google Scholar]
- 165.Summers CW, Pumphrey RS, Woods CN, McDowell G, Pemberton PW, Arkwright PD. Factors predicting anaphylaxis to peanuts and tree nuts in patients referred to a specialist center. J Allergy Clin Immunol. 2008;121:632–8 e2. doi: 10.1016/j.jaci.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 166.Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y, et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci U S A. 1995;92:10560–4. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gulen T, Ljung C, Nilsson G, Akin C. Risk Factor Analysis of Anaphylactic Reactions in Patients With Systemic Mastocytosis. J Allergy Clin Immunol Pract. 2017 doi: 10.1016/j.jaip.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 168.Akin C, Scott LM, Kocabas CN, Kushnir-Sukhov N, Brittain E, Noel P, et al. Demonstration of an aberrant mast-cell population with clonal markers in a subset of patients with “idiopathic” anaphylaxis. Blood. 2007;110:2331–3. doi: 10.1182/blood-2006-06-028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Siegel AM, Stone KD, Cruse G, Lawrence MG, Olivera A, Jung MY, et al. Diminished allergic disease in patients with STAT3 mutations reveals a role for STAT3 signaling in mast cell degranulation. J Allergy Clin Immunol. 2013;132:1388–96. doi: 10.1016/j.jaci.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hox V, O'Connell MP, Lyons JJ, Sackstein P, Dimaggio T, Jones N, et al. Diminution of signal transducer and activator of transcription 3 signaling inhibits vascular permeability and anaphylaxis. J Allergy Clin Immunol. 2016;138:187–99. doi: 10.1016/j.jaci.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Webb LM, Lieberman P. Anaphylaxis: a review of 601 cases. Ann Allergy Asthma Immunol. 2006;97:39–43. doi: 10.1016/S1081-1206(10)61367-1. [DOI] [PubMed] [Google Scholar]
- 172.Worm M, Edenharter G, Ruëff F, Scherer K, Pföhler C, Mahler V, et al. Symptom profile and risk factors of anaphylaxis in Central Europe. Allergy. 2012;67:691–8. doi: 10.1111/j.1398-9995.2012.02795.x. [DOI] [PubMed] [Google Scholar]
- 173.Hox V, Desai A, Bandara G, Gilfillan AM, Metcalfe DD, Olivera A. Estrogen increases the severity of anaphylaxis in female mice through enhanced endothelial nitric oxide synthase expression and nitric oxide production. J Allergy Clin Immunol. 2015;135:729–36 e5. doi: 10.1016/j.jaci.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Francuzik W, Nassiri M, Babina M, Worm M. Impact of sex on anaphylaxis severity—data from the Anaphylaxis Registry. J Allergy Clin Immunol. 2015;136:1425–6. doi: 10.1016/j.jaci.2015.06.052. [DOI] [PubMed] [Google Scholar]
- 175.Piper PJ, Collier HO. Release of catecholamines in the guinea-pig by substances involved in anaphylaxis. Nature. 1967;213:838–40. doi: 10.1038/213838a0. [DOI] [PubMed] [Google Scholar]
- 176.van der Linden PW, Struyvenberg A, Kraaijenhagen RJ, Hack CE, van der Zwan JK. Anaphylactic shock after insect-sting challenge in 138 persons with a previous insect-sting reaction. Ann Intern Med. 1993;118:161–8. doi: 10.7326/0003-4819-118-3-199302010-00001. [DOI] [PubMed] [Google Scholar]
- 177.Khodoun M, Strait R, Orekov T, Hogan S, Karasuyama H, Herbert DR, et al. Peanuts can contribute to anaphylactic shock by activating complement. J Allergy Clin Immunol. 2009;123:342–51. doi: 10.1016/j.jaci.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang W, Shibamoto T, Kurata Y, Kohno H. Effects of β-adrenoceptor antagonists on anaphylactic hypotension in conscious rats. Eur J Pharmacol. 2011;650:303–8. doi: 10.1016/j.ejphar.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 179.Awai LE, Mekori YA. Insect sting anaphylaxis and beta-adrenergic blockade: a relative contraindication. Ann Allergy. 1984;53:48–9. [PubMed] [Google Scholar]
- 180.Jacobs RL, Rake GW, Jr, Fournier DC, Chilton RJ, Culver WG, Beckmann CH. Potentiated anaphylaxis in patients with drug-induced beta-adrenergic blockade. J Allergy Clin Immunol. 1981;68:125–7. doi: 10.1016/0091-6749(81)90170-6. [DOI] [PubMed] [Google Scholar]
- 181.Lang DM. Anaphylactoid and anaphylactic reactions Hazards of beta-blockers. Drug Safety. 1995;12:299–304. doi: 10.2165/00002018-199512050-00002. [DOI] [PubMed] [Google Scholar]
- 182.Lee S, Hess EP, Nestler DM, Bellamkonda Athmaram VR, Bellolio MR, Decker WW, et al. Antihypertensive medication use is associated with increased organ system involvement and hospitalization in emergency department patients with anaphylaxis. J Clin Allergy Immunol. 2013;131:1103–8. doi: 10.1016/j.jaci.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 183.Nassiri M, Babina M, Dölle S, Edenharter G, Ruëff F, Worm M. Ramipril and metoprolol intake aggravate human and murine anaphylaxis: evidence for direct mast cell priming. J Allergy Clin Immunol. 2015;135:491–9. doi: 10.1016/j.jaci.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 184.Li M, Liu K, Michalicek J, Angus JA, Hunt JE, Dell'Italia LJ, et al. Involvement of chymase-mediated angiotensin II generation in blood pressure regulation. J Clin Invest. 2004;114:112–20. doi: 10.1172/JCI20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nakamura TYF, Yamada R, Fujii W, Hamabata T, Lee MY, et al. Mast cell–derived prostaglandin D2 attenuates anaphylactic reactions in mice. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 186.Marichal T, Starkl P, Reber LL, Kalesnikoff J, Oettgen HC, Tsai M, et al. A beneficial role for immunoglobulin E in host defense against honeybee venom. Immunity. 2013;39:963–75. 188. doi: 10.1016/j.immuni.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Palm NW, Rosenstein RK, Yu S, Schenten DD, Florsheim E, Medzhitov R. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity. 2013;39:976–85. doi: 10.1016/j.immuni.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Starkl P, Marichal T, Gaudenzio N, Reber LL, Sibilano R, Tsai M, et al. IgE antibodies, FcepsilonRIalpha, and IgE-mediated local anaphylaxis can limit snake venom toxicity. J Allergy Clin Immunol. 2016;137:246–57 e11. doi: 10.1016/j.jaci.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Akahoshi M, Song CH, Piliponsky AM, Metz M, Guzzetta A, Abrink M, et al. Mast cell chymase reduces the toxicity of Gila monster venom, scorpion venom, and vasoactive intestinal polypeptide in mice. J Clin Invest. 2011;121:4180–91. doi: 10.1172/JCI46139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Metz M, Piliponsky AM, Chen CC, Lammel V, Abrink M, Pejler G, et al. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313:526–30. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- 191.Schneider LA, Schlenner SM, Feyerabend TB, Wunderlin M, Rodewald HR. Molecular mechanism of mast cell mediated innate defense against endothelin and snake venom sarafotoxin. J Exp Med. 2007;204:2629–39. doi: 10.1084/jem.20071262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Oettgen HC, Martin TR, Wynshaw-Boris A, Deng C, Drazen JM, Leder P. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–70. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 193.Dombrowicz D, Flamand V, Miyajima I, Ravetch JV, Galli SJ, Kinet JP. Absence of Fc epsilonRI alpha chain results in upregulation of Fc gammaRIII-dependent mast cell degranulation and anaphylaxis. Evidence of competition between Fc epsilonRI and Fc gammaRIII for limiting amounts of FcR beta and gamma chains. J Clin Invest. 1997;99:915–25. doi: 10.1172/JCI119256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cheifetz A, Smedley M, Martin S, Reiter M, Leone G, Mayer L, et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol. 2003;98:1315–24. doi: 10.1111/j.1572-0241.2003.07457.x. [DOI] [PubMed] [Google Scholar]
- 195.Baker DL, Nakamura GR, Lowman HB, Fischer SK. Evaluation of IgE Antibodies to Omalizumab (Xolair(R)) and Their Potential Correlation to Anaphylaxis. AAPS J. 2016;18:115–23. doi: 10.1208/s12248-015-9821-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Price KS, Hamilton RG. Anaphylactoid reactions in two patients after omalizumab administration after successful long-term therapy. Allergy Asthma Proc. 2007;28:313–9. doi: 10.2500/aap.2007.28.3003. [DOI] [PubMed] [Google Scholar]
- 197.Kodama T, Sekine H, Takahashi M, Iwaki D, Machida T, Kanno K, et al. Role of complement in a murine model of peanut-induced anaphylaxis. Immunobiology. 2013;218:844–50. doi: 10.1016/j.imbio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 198.Schafer B, Piliponsky AM, Oka T, Song CH, Gerard NP, Gerard C, et al. Mast cell anaphylatoxin receptor expression can enhance IgE-dependent skin inflammation in mice. J Allergy Clin Immunol. 2013;131:541–8 e1-9. doi: 10.1016/j.jaci.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jiao D, Liu Y, Lu X, Liu B, Pan Q, Liu Y, et al. Macrophages are the dominant effector cells responsible for IgG-mediated passive systemic anaphylaxis challenged by natural protein antigen in BALB/c and C57BL/6 mice. Cell Immunol. 2014;289:97–105. doi: 10.1016/j.cellimm.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 200.Windbichler M, Echtenacher B, Takahashi K, Ezekowitz RA, Schwaeble WJ, Jenseniuis JC, et al. Investigations on the involvement of the lectin pathway of complement activation in anaphylaxis. Int Arch Allergy Immunol. 2006;141:11–23. doi: 10.1159/000094177. [DOI] [PubMed] [Google Scholar]
- 201.Takeishi T, Martin TR, Katona IM, Finkelman FD, Galli SJ. Differences in the expression of the cardiopulmonary alterations associated with anti-immunoglobulin E-induced or active anaphylaxis in mast cell-deficient and normal mice. Mast cells are not required for the cardiopulmonary changes associated with certain fatal anaphylactic responses. J Clin Invest. 1991;88:598–608. doi: 10.1172/JCI115344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–74. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 203.Makabe-Kobayashi Y, Hori Y, Adachi T, Ishigaki-Suzuki S, Kikuchi Y, Kagaya Y, et al. The control effect of histamine on body temperature and respiratory function in IgE-dependent systemic anaphylaxis. J Allergy Clin Immunol. 2002;110:298–303. doi: 10.1067/mai.2002.125977. [DOI] [PubMed] [Google Scholar]
- 204.Wechsler JB, Schroeder HA, Byrne AJ, Chien KB, Bryce PJ. Anaphylactic responses to histamine in mice utilize both histamine receptors 1 and 2. Allergy. 2013;68:1338–40. doi: 10.1111/all.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Arias K, Baig M, Colangelo M, Chu D, Walker T, Goncharova S, et al. Concurrent blockade of platelet-activating factor and histamine prevents life-threatening peanut-induced anaphylactic reactions. J Allergy Clin Immunol. 2009;124:307–14. 14 e1–2. doi: 10.1016/j.jaci.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 206.Kelefiotis D, Vakirtzi-Lemonias C. In vivo responses of mouse blood cells to platelet-activating factor (PAF): role of the mediators of anaphylaxis. Agents Actions. 1993;40:150–6. doi: 10.1007/BF01984054. [DOI] [PubMed] [Google Scholar]
- 207.Ishii S, Kuwaki T, Nagase T, Maki K, Tashiro F, Sunaga S, et al. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med. 1998;187:1779–88. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]