Abstract
Background
The discovery of signaling networks that drive oncogenic processes has led to the development of targeted anticancer agents. The burden of pigmentary adverse events from these drugs is unknown.
Objective
To conduct a systematic review and meta-analysis of published clinical trials, and determine the incidence and risk of developing targeted therapy-induced pigmentary changes.
Methods
A comprehensive search was conducted to identify studies reporting targeted therapy-induced pigmentary changes. The incidence and relative risk were calculated. Case reports and series were reviewed to understand clinical characteristics.
Results
8,052 patients from 36 clinical trials were included. The calculated overall incidences of targeted cancer therapy-induced all-grade pigmentary changes in the skin and hair were 17.7% (95% CI, 11.9–25.4) and 21.5% (95% CI, 14.9–30.1), respectively. The relative risk of all-grade pigmentary changes of skin and hair were 93.7 (95% CI: 5.86–1497.164) and 20.1 (95% CI: 8.35–48.248). Across 54 case reports/series (n=75 patients), EGFR and Bcr-abl inhibitors were the most common offending agents.
Limitations
Potential underreporting and variability in oncologists reporting these events.
Conclusion
There is a significant risk of developing pigmentary changes during treatment with targeted anticancer therapies. Appropriate counseling and management are critical to minimize psychosocial impairment and deterioration in quality of life.
Keywords: Cabozantinib, Imatinib, Ipilimumab, Nivolumab, Pazopanib, Pembrolizumab, Sorafenib, Sunitinib, Pigmentary, Hypopigmentation, Hyperpigmentation, Depigmentation, Repigmentation, Dyspigmentation, Vitiligo
INTRODUCTION
The discovery of intracellular signaling networks that drive oncogenic processes when aberrantly activated has led to the development of molecularly targeted agents for the treatment of various cancers [1, 2]. Their targeted action spares normal cells, thus improving efficacy and health-related quality of life (HRQoL). While systemic adverse events (AEs) characteristic of conventional cytotoxic agents (e.g. myelosuppression, nausea, vomiting) [3] are typically not encountered, dermatologic AEs (affecting the skin, hair, nails, mucosae) are common because some of the signaling pathways inhibited are also essential for cutaneous homeostasis [4]. Skin eruptions (rashes), xerosis, pruritus, photosensitivity, pigmentary changes, fissures, hand-foot skin reaction, and hair/nail changes are some of the most commonly encountered targeted therapy-induced dermatologic AEs [5]. Although not life-threatening, they can negatively impact patients’ HRQoL, and impair psychosocial functioning and activities of daily living (ADL) [6, 7]. Furthermore, they often result in dose reductions, interruptions, or even discontinuation of therapy, which may lead to suboptimal management of the cancer itself and result in poorer outcomes [8].
Whereas the incidence and risk of some of the targeted therapy-induced dermatologic AEs have been previously estimated [9, 10], that of dermatologic pigmentary AEs (dpAEs) is not known. The latter are of particular concern because of their persistence, resistance to therapy, and negative impact on psychosocial well-being and HRQoL. Therefore, we conducted a systematic review and meta-analysis of the literature to determine the incidence and risk of targeted therapy-induced dermatologic pigmentary AEs.
METHODS
Data source
We searched all targeted anticancer agents (n=64, Appendix I) approved by the Food and Drug Administration (www.FDA.gov) in January 2017. A PubMed search was conducted using the generic name of targeted agents (e.g. “afatinib”) as the keyword. The search was limited to phase II and phase III randomized and non-randomized clinical trials (RCT, NRCT) published in English (January 1998 through January 2017). We also reviewed abstracts and virtual meeting presentations (January 2004 through January 2017) posted on the American Society of Clinical Oncology (ASCO) website to further identify relevant clinical trials. In addition, an independent search on the Web of Science database was also conducted to ensure that no other studies were missed. We reviewed each publication and retrieved data only from complete and/or the most recent reports if duplicate publications were identified. Extracted information included patient characteristics, study design, treatment regimen, study results, and safety data.
Study selection
The U.S. Food and Drug Administration (USFDA) approves targeted therapies at a specific dose in the treatment of cancer. Therefore, we excluded clinical trials employing drugs at unapproved doses (e.g. phase I studies) in order to determine the incidence and risk of dpAEs at the dosing level meaningful for clinicians. We also excluded trials that combined targeted agents with other chemotherapeutic agents and/or treatment modalities. The dpAEs in the studies were reported as: “hyperpigmentation,” “hypopigmentation,” “depigmentation,” “repigmentation,” “dyspigmentation,” “discoloration,” “color change,” and “vitiligo” of either the skin/ hair/ nails. Studies that met the following criteria were selected for final analysis: (1) prospective phase II and III clinical trials in patients with cancer; (2) assignment of participants to treatment with the targeted agent at the approved dose; and (3) availability of data regarding the incidence of pigmentary changes.
Clinical end points
The clinical endpoints were extracted from the safety profile in each trial. The dpAEs for skin were recorded according to the National Cancer Institute’s Common Toxicity Criteria (CTCv2.0), or the Common Terminology Criteria for AEs (CTCAE v3.0 and v4.0). The grading of dpAEs in the skin in version 2.0 is described as follows: grade 0, none; grade 1, localized; grade 2, generalized. In version 3.0, the description was updated to hyperpigmentation and hypopigmentation, as follows: grade 1, slight or localized; grade 2, marked or generalized. Version 4.0 further stratifies hyperpigmentation and hypopigmentation by body surface area (BSA) involvement as follows: grade 1, covering <10% BSA—no psychosocial impact; grade 2, covering >10% BSA—associated psychosocial impact. However, none of the studies in our meta-analysis utilized CTCAE v4.0. Lastly, given that pigmentary changes are not considered life threatening, there is no high-grade designation for these AEs.
Statistical analysis
All statistical analysis was performed using Comprehensive Meta-Analysis program (v2.0, Biostat, Englewood, NJ). The number of patients with pigmentary AEs in treatment and control groups (as applicable) was identified from the selected clinical trials. The incidence and 95% confidence intervals (CIs) were calculated for each trial. For studies with a control arm, the relative risk (RR) of pigmentary AEs was also calculated.
For meta-analysis, both the fixed-effects (weighted with inverse variance) and the random-effects model were given consideration for meta-analysis. The Cochran Q statistic was calculated for each meta-analysis to determine the heterogeneity of the included trials. For P value of Cochran Q statistic less than 0.1, the assumption of homogeneity was deemed invalid, and the random-effects model was employed after exploring the cause of heterogeneity. Barring this phenomenon, both the fixed-effects and random-effects models were reported. A two-tailed P value of less than 0.05 was established as statistically significant.
Systematic review of published case reports and case series
We also reviewed case reports and series to understand the clinical characteristics of dpAEs, as they are not reported in clinical trial publications. For this portion of the study, the following PubMed search strategy was used (last performed in January 2017): generic drug name AND (albinism OR bronz* OR dark* OR darkening OR depigmentation OR discoloration OR dyschromia OR excessive pigmentation OR hyperpigmentation OR hypopigmentation OR light* OR lightening OR melanosis OR poliosis OR repigmentation OR vitiligo OR whit*). The results were narrowed down to case reports and case series published in English. In addition, a manual search of the bibliography from retrieved reports was also performed. One of the authors (JD) reviewed all the identified manuscripts and extracted the following data onto an excel spreadsheet: age, gender, race, underlying cancer, clinical findings, types of dyspigmentation including sites of involvement, pathology findings (if available), drug (including dosing), dose alterations, outcomes, number of cases, first author, year of publication.
RESULTS
Search results
Our literature search yielded a total of 7,604 potentially relevant studies, of which 36 clinical trials that involved targeted anticancer therapies met the inclusion criteria, and were included in the final analysis (Fig 1). The latter included phase II [11–34] (n=24) and phase III [35–46] (n=12) trials—all investigating solid organ malignancies. In all, 8,052 patients (controls, n=3,648; drug, n=4,404) were analyzed across trials employing cabozantinib, imatinib, ipilimumab, nivolumab, pazopanib, pembrolizumab, sorafenib, or sunitinib, which represented 8 major drugs of the 64 (12.5%) included in the search. The data was analyzed separately for dpAEs of the skin and hair.
Figure 1.
Selection process for studies included in meta-analysis.
Incidence of all-grade pigmentary changes in skin
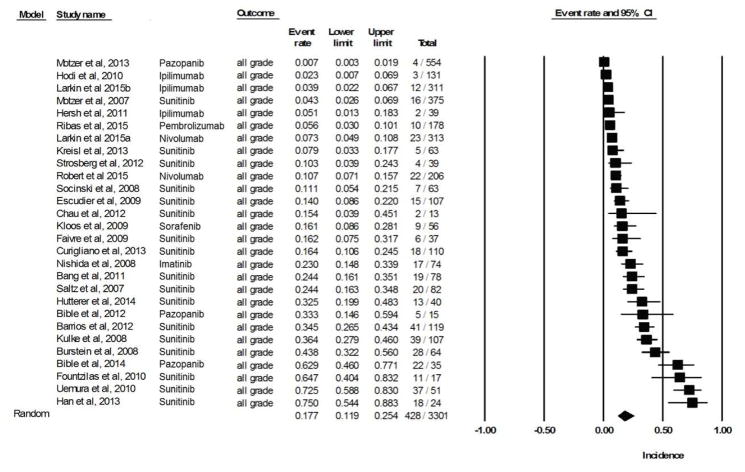
Data for all-grade dpAEs of skin was available for 6,538 patients (across 28 clinical trials) treated with a targeted agent. The calculated overall incidence across all studies was 17.7% (95% CI, 11.9–25.4) according to the random-effects model (heterogeneity test: Q = 416.4, I2 = 93.5, P <0.001) (Fig 2A). The lowest incidence, 0.7%, was noted in the pazopanib arm (n=554) of a randomized, open-label, phase III trial involving metastatic renal-cell carcinoma patients [39]. The highest incidence, 75%, was noted in a phase II study of sunitinib (n=24) in patients with relapsed or refractory small cell lung cancer [17]. The drug-wise summary incidences of all-grade pigmentary changes are provided in Table 1.
Figure 2.
Figure 2A. Incidence of all-grade targeted therapy-induced pigmentary changes in skin.
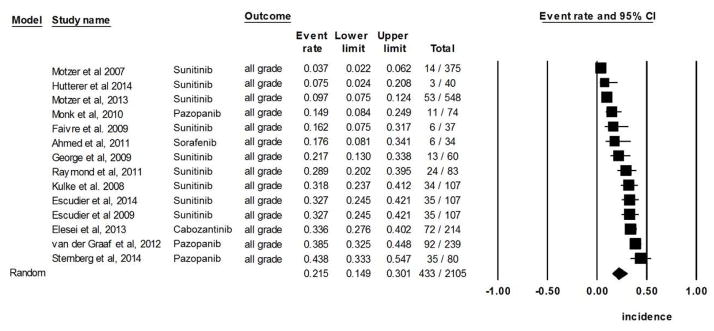
Figure 2B. Incidence of all-grade targeted therapy-induced pigmentary changes in hair.
Table 1.
Incidence of all-grade pigmentary changes with approved targeted agents in monotherapy.
| Drug | Primary molecular targets | Incidence of all-grade pigmentary changes (95% CI) | |
|---|---|---|---|
| Skin | Hair | ||
| Cabozantinib39 | VEGF-R1/-R2/-R3, Flt-3, MET, RET, KIT, AXL, TRKB, TEK, TIE-2 | Not yet reported | 33.6% (27.6%–40.2%) |
| Imatinib11 | BCR-ABL, PDGFR-α/β, KIT | 23.0% (14.8%–33.9%) | Not yet reported |
| Ipilimumab12,35–36 | CTLA-4 | 3.6% (2.3%–5.8%) | Not yet reported |
| Nivolumab37–38 | PD-1 | 8.8% (6.1%–12.7%) | Not yet reported |
| Pazopanib13–14,32,39,43–44 | VEGF-R1/-R2/-R3, PDGFR-α/β, KIT, RAF | 15.6% (0.7%–83.4%) | 31.7% (18.9%–48.0%) |
| Pembrolizumab40 | PD-1 | 5.6% (3.0%–10.1%) | Not yet reported |
| Sorafenib15,33 | VEGF-R1/-R2/-R3, PDGFR-β, KIT, RET, RAF (CRAF & BRAF) | 16.1% (8.6%–28.1%) | 17.6% (8.1%–34.1%) |
| Sunitinib16–31,34,41,45–46 | VEGF-R1/-R2/-R3, PDGFR, KIT, RET, CSF-1R, Flt-3 | 25.5% (17.0%–36.4%) | 17.9% (10.5%–28.7%) |
Incidence of all-grade pigmentary changes in hair
In all, we identified 14 clinical trials (involving 3,319 evaluable patients) that reported hair color changes as a result of treatment with a targeted agent. The calculated overall incidence was 21.5% (95% CI, 14.9–30.1) according to the random-effects model (heterogeneity test: Q=191.3, I2=93.2, P<0.001) (Fig 2B). The lowest incidence, 3.7%, was noted in the sunitinib arm (n=375) of a randomized, double-blinded, phase III trial involving metastatic renal-cell carcinoma patients [41]; in an open-label extension study to evaluate the safety and efficacy of pazopanib in patients with advanced renal-cell carcinoma (n=80), the incidence of hair color changes was highest at 43.8% [43].
Relative risk of all-grade pigmentary changes in skin
In order to estimate the relative risk (RR) of these changes in patients receiving targeted therapies as compared to placebo, a pooled meta-analysis was performed by using RCTs as the control arm. All-grade skin pigmentary changes were noted in 428/3301 patients receiving targeted therapies [11–31, 35–41], as opposed to none (0/360) among patients who received best supportive care (BSC) alone [41]. The calculated overall RR for all-grade changes was 93.7 (95% CI: 5.86–1497.164; P <0.001), according to the random-effects model. The calculated high-grade RR was 2.371 (95% CI: 0.134–42.003; P=0.556).
Relative risk of all-grade pigmentary changes in hair
A meta-analysis of RR for all-grade hair color changes associated with targeted agents versus controls was performed on 3 RCTs [42,44,46]. All-grade hair color changes were noted in 187/536 patients receiving targeted therapies, as compared to 5/314 patients who received BSC alone. The calculated RR was 20.1 (95% CI: 8.35–48.248; P <0.001), according to the fixed-effects model. The calculated high-grade RR was 2.134 (95% CI: 0.224–20.355; P=0.510).
Case reports and Case series
Our search strategy yielded 54 publications (2002–2017) reporting on targeted anticancer therapy-induced dpAEs involving the skin, hair, nails, and mucosae: 45 were case reports (n=45 patients) and 9 were case series (n=30 patients), with single-case reporting representing the majority (45/54 patients, 83%). Given the case-level nature of the reports, we conducted a pooled analysis, and provided a summary of our findings (Table 2)—the raw data pertaining to all cases, their description, and references are provided in Appendix II. The mean patient age was 49.8 years (range: 8 years to 83 years), with a slight preponderance of females (41/75, 54.7%). The time to onset ranged from “immediately” to up to 10 years after initiation of treatment, and most reports (n=30/54, 55.6%) pertained to imatinib (43/75 patients, 57.3%). Accordingly, nearly half of the cases (n=33/75, 44.0%) had been treated for chronic myeloid leukemia (CML). Importantly, only 6/75 cases (8.0%) experienced dose alterations due to dpAEs.
Table 2.
Published case reports/series of pigmentary changes during treatment with targeted anticancer agents (n=54).
| # | Dermatologic AE | Primary mechanism of action | Targeted agent | Number of cases |
|---|---|---|---|---|
| 1. Skin change | ||||
| Hyperpigmentation | EGFR inhibitor | Gefitinib1 | 2 | |
| Bcr-abl inhibitor | Imatinib2–8 | 15 | ||
| Repigmentation | Bcr-abl inhibitor | Imatinib9 | 1 | |
| JAK inhibitor | Ruxolitinib10 | 1 | ||
| Hypopigmentation | EGFR inhibitor | Iefitinib11 | 1 | |
| Bcr-abl inhibitor(s) | Imatinib5,12–19 | 14 | ||
| Dasatinib20–22 | 3 | |||
| Immunomodulator | IL-223 | 1 | ||
| VEGFR inhibitor(s) | Pazopanib24 | 1 | ||
| Sunitinib25–26 | 2 | |||
| BRAF inhibitor | Vemurafenib27 | 1 | ||
| PD-1 inhibitor | Pembrolizumab28 | 1 | ||
| Other dyschromias (blue/gray, yellow) | Bcr-abl inhibitor | Imatinib29–31 | 3 | |
| VEGFR inhibitor | Sorafenib32 | 1 | ||
| EGFR/ VEGFR inhibitor | Vandetanib33–34 | 3 | ||
| 2. Hair change | ||||
| Repigmentation | EGFR inhibitor | Erlotinib35 | 1 | |
| Hypopigmentation | EGFR inhibitor | Cetuximab36 | 1 | |
| Bcr-abl inhibitor(s) | Imatinib37 | 1 | ||
| Dasatinib20–21,38–39 | 4 | |||
| VEGFR inhibitor(s) | Pazopanib24,40 | 2 | ||
| Regorafenib41 | 1 | |||
| Sunitinib25,42 | 2 | |||
| PD-1 inhibitor | Pembrolizumab28 | 1 | ||
| 3. Nail change | ||||
| Hyperpigmentation | EGFR inhibitor | Gefitinib43 | 1 | |
| Bcr-abl inhibitor | Imatinib44–46 | 3 | ||
| Other dyschromias (yellow) | mTOR inhibitor | Temsirolimus47 | 1 | |
| 4. Mucosal change of hard palate | ||||
| Other dyschromias | Bcr-abl inhibitor | Imatinib5,29–31,44,46,48–54 | 17 | |
The skin appeared to be the most commonly affected site, followed by involvement of the mucosa, hair, and nails. While generalized skin involvement did occur (n=10), localized affliction of the face (n=36), trunk (n=10), hands/feet (n=8) and legs (n=8), and arms (n=7) was also seen; no specific patterns were identifiable. The outcome of skin dpAEs was noted in 11/50 (22.0%) cases; resolution in 7/50, 14.0%, and persistence in 4/50, 8.0% cases. The information pertaining to reversibility was not described in the rest. The Bcr-abl inhibitor, imatinib, was responsible for the majority of skin-related dpAEs in this pooled analysis of cases. In cases where hair was affected, scalp hair involvement predominated (10/13, 76.9%), although virtually all hair-bearing areas appear susceptible; inhibitors of the Bcr-abl and VEGFR were the most common culprits. Nail dpAEs included hyperpigmentation and yellow discoloration in a total of 5 cases. Mucosal dpAEs were exclusively seen with imatinib (n=17) and described as a blue-gray to brown discoloration.
DISCUSSION
In this study, we determined the incidence and risk of targeted anticancer therapy-induced dpAEs from the safety data of published clinical trials and attempted to analyze the clinical characteristics by reviewing pertinent case reports/series. We found that the overall incidence of dpAEs in patients exposed to targeted anticancer therapies is high—skin, 17.7% and hair, 21.5%. The targeted agents imatinib, cabozantinib, nivolumab, pazopanib, pembrolizumab, sorafenib, and sunitinib appeared to be the most common culprits.
The pathophysiology of targeted anticancer therapy-induced dpAEs appears to be multifactorial, and remains poorly understood [47]. Pigmentary changes associated with imatinib, a tyrosine kinase inhibitor, are well documented in the literature, with the most commonly described clinical phenotype being reversible, dose-related hypopigmentation [48, 49]. In vitro studies demonstrate that imatinib may decrease skin pigmentation by inhibiting tyrosinase activity, likely through blockade of the c-KIT pathway and PDGF inhibition [50]. Interestingly, paradoxical cases of imatinib-associated hyperpigmentation have also been described [51–53], although the mechanisms underlying these differential reactions remain unclear.
Similarly, dpAEs associated with multikinase (MKI) inhibitors are likely due to inhibition of c-KIT, a known regulator of melanogenesis. c-KIT is uniquely expressed in melanocytes and plays a critical role in melanocyte development, differentiation, and maintenance [54]. Mutations in c-KIT are associated with hypopigmentation syndromes such as piebaldism and vitiligo [55, 56]. The non-selective MKIs, cabozantinib, pazopanib, sorafenib, and sunitinib are probably associated with c-KIT inhibition, though perhaps not through a direct effect on the KIT receptor, as with imatinib.
In the case of ipilimumab, however, the pigmentary changes appear to be a direct result of CTLA-4 inhibition and consequent immune system activation, [57] including against the melanocytes [58]. Surprisingly, clinical depigmentation may serve as a surrogate marker for responsiveness to anticancer treatment, with the appearance of vitiligo-like melanoma-associated hypopigmentation portending a favorable response to therapy [59]. Finally, vitiligo-like lesions that occur during treatment with selective PD-1 inhibitors, such as pembrolizumab and nivolumab, have been reported in up to 25% of patients and may be associated with a clinical benefit [60]. A recent study suggests a unique clinical phenotype and pathophysiological pathway that implicates a CD8 T-cell immune response distinct from spontaneously occurring vitiligo [61].
Current management strategies focus on pre-emptive approaches and patient education rather than symptom management, because termination of drug exposure typically leads to resolution of the dpAEs. Patients should also be advised to use appropriate UV protection, as individuals who experience hypopigmentation may be at an increased risk for photosensitivity disorders. Our meta-analysis has several limitations. First, dpAEs are asymptomatic and patients are less likely to notice and/or report them. Second, the assessment and reporting of dpAEs may be variable across healthcare providers and institutions, which could have impacted safety reporting in clinical trials. Therefore, these inconsistencies may have resulted in the underreporting, and consequently, an underestimation of the incidence of targeted anticancer therapy-induced dpAEs.
The study of AEs, especially dermatologic, is yet to keep up with the pace at which newer targeted anticancer drugs are being approved. Herein, we have shown that dpAEs are being encountered by a significant number of cancer patients. This phenomenon is of particular importance because these events bear the potential to negatively impact patients’ quality of life and psychosocial well-being, in addition to being long-lasting and challenging to treat. Moreover, the use of these drugs is widening, suggesting that these AEs could be increasingly encountered. Therefore, there is an urgent need to educate patients and healthcare providers and develop effective management strategies. Further investigation into the pathophysiology and management of dpAEs is warranted to ensure optimal therapy and improve patients’ quality of life. By understanding the pathogenesis and clinical manifestations of these AEs, dermatologists play a critical role in guiding oncologic therapy by minimizing unwarranted dose reduction and dose stoppage.
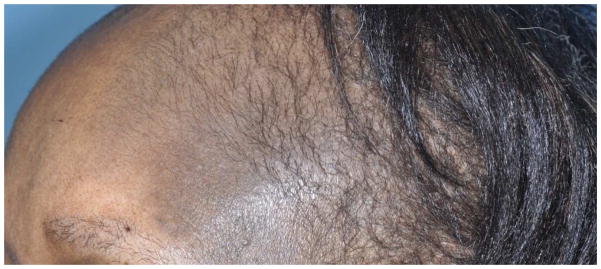
Figure 3.
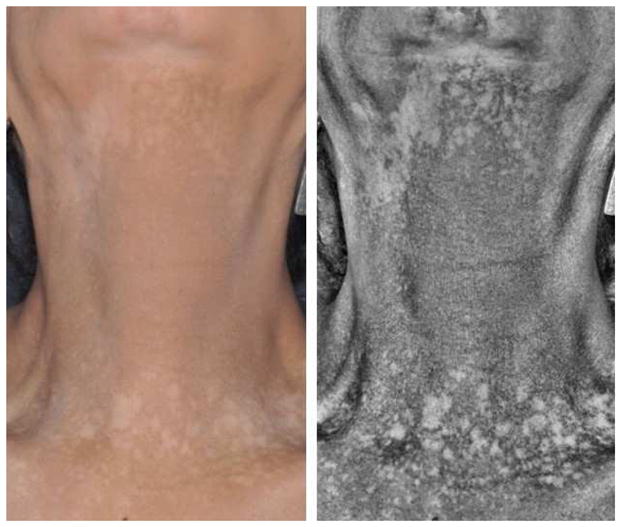
Figure 3A. Gray-colored imatinib-induced hyperpigmentation predominantly on the face of a 65-year-old female with gastrointestinal stromal tumor.
Figure 3B. Well-defined asymptomatic depigmented macules (enhanced under Wood’s light) predominantly on the face and neck in a 65-year-old female receiving MK-3475 (pembrolizumab) for melanoma.
Acknowledgments
Funding Support: This study was supported in part by the NIH/NCI Cancer Center Support Grant P20 CA008748. M.E.L. and V.R.B. are supported by the RJR Oncodermatology Fund. Funding/Sponsors were not involved in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
ABBREVIATIONS
- ADL
activities of daily living
- AE
adverse event
- ASCO
American Society of Clinical Oncology
- Bcr-abl
breakpoint cluster region-abelson
- BRAF
B-rapidly accelerated fibrosarcoma proto-oncogene serine–threonine-protein kinase
- BSA
body surface area
- BSC
best supportive care
- CI
confidence interval
- CML
chronic myeloid leukemia
- CSF
colony-stimulating factor
- CTC
Common toxicity criteria
- CTCAE
Common Terminology Criteria for Adverse Events
- CTLA-4
cytotoxic T-lymphocyte antigen-4
- dpAE
dermatologic pigmentary adverse events
- EGFR/ EGFRI
epidermal growth factor receptor/ EGFR inhibitor
- Flt
fms-like tyrosine kinase
- HRQoL
health-related quality of life
- Kit
KIT protein
- mAb
monoclonal antibody
- MEK
MAPK/ERK (Extracellular signal-Regulated Kinase) Kinase
- MET
mesenchymal-epithelial transition factor
- MKIs
multikinase inhibitor(s)
- MSKCC
Memorial Sloan Kettering Cancer Center
- mTOR
mammalian target of rapamycin
- NRCT
non-randomized controlled trial
- PDGF/ PDGFR
platelet derived growth factor/ PDGF receptor
- Ras
rat sarcoma
- Ret
rearranged during transfection
- RR
relative risk
- RCT
randomized controlled trial
- TEK
Tyrosine kinase, endothelial
- TIE
Tyrosine kinase with immunoglobulin-like and EGF-like domains
- TKI
tyrosine kinase inhibitor
- TRKB
tropomyosin receptor kinase B
- USFDA
United States Food and Drug Administration
- VEGF/ VEGFR
vascular endothelial growth factor/ VEGF receptor
APPENDIX
Appendix I. List of all targeted agents searched to identify studies reporting dermatologic pigmentary adverse events (n=64)
Ado-trastuzumab emtansine (Kadcyla)
Afatinib dimaleate (Gilotrif)
Alectinib (Alecensa)
Alemtuzumab (Campath)
Atezolizumab (Tecentriq)
Axitinib (Inlyta)
Belinostat (Beleodaq)
Bevacizumab (Avastin)
Blinatumomab (Blincyto)
Bortezomib (Velcade)
Bosutinib (Bosulif)
Brentuximab vedotin (Adcetris)
Cabozantinib (Cometriq)
Carfilzomib (Kyprolis)
Ceritinib (Zykadia)
Cetuximab (Erbitux)
Cobimetinib (Cotellic)
Crizotinib (Xalkori)
Dabrafenib (Tafinlar)
Daratumumab (Darzalex)
Dasatinib (Sprycel)
Dinutuximab (Unituxin)
Elotuzumab (Empliciti)
Erlotinib hydrochloride (Tarceva)
Everolimus (Afinitor)
Gefitinib (Iressa)
Ibrutinib (Imbruvica)
Idelalisib (Zydelig)
Imatinib mesylate (Gleevec)
Ipilimumab (Yervoy)
Ixazomib (Ninlaro)
Lapatinib ditosylate (Tykerb)
Lenvatinib (Lenvima)
Necitumumab (Portrazza)
Nilotinib (Tasigna)
Nivolumab (Opdivo)
Obinutuzumab (Gazyva)
Ofatumumab (Arzerra)
Olaparib (Lynparza)
Olaratumab (Lartruvo)
Osimertinib (Tagrisso)
Palbociclib (Ibrance)
Panitumumab (Vectibix)
Panobinostat (Farydak)
Pazopanib hydrochloride (Votrient)
Pembrolizumab (Keytruda)
Pertuzumab (Perjeta)
Ponatinib (Iclusig)
Ramucirumab (Cyramza)
Regorafenib (Stivarga)
Rituximab (Rituxan)
Romidepsin (Istodax)
Ruxolitinib (Jakafi)
Sorafenib tosylate (Nexavar)
Sonidegib (Odomzo)
Sunitinib malate (Sutent)
Temsirolimus (Torisel)
Trametinib (Mekinist)
Trastuzumab (Herceptin)
Vandetanib (Caprelsa)
Vemurafenib (Zelboraf)
Vismodegib (Erivedge)
Vorinostat (Zolinza)
Ziv-aflibercept (Zaltrap)
Appendix II. Published case reports/series of pigmentary changes during treatment with targeted anticancer agents (n=54)
Reference
- 1.Chang GC, Yang TY, Chen KC, Yin MC, Wang RC, Lin YC. Paronychia and skin hyperpigmentation induced by Gefitinib in advanced non-small-cell lung cancer. J Clin Oncol. 2004;22:4646–7. doi: 10.1200/JCO.2004.02.168. [DOI] [PubMed] [Google Scholar]
- 2.Alexandrescu DT, Dasanu CA, Farzanmehr H, Kauffman CL. Persistent cutaneous hyperpigmentation after tyrosine kinase inhibition with Imatinib for GIST. Derm Online J. 2008;14(7):7. [PubMed] [Google Scholar]
- 3.Prasad N, Deshmukh C, Biswas G, Bakshi A, Sastry PS, Parikh PM. Dermatological toxicity of Imatinib mesylate. J Assoc Physicians India. 2005;53:298. [PubMed] [Google Scholar]
- 4.Hamza I, Gaies E, Kastalli E, Daghfous R, El Aidli S. Facial hyperpigmentation during Imatinib therapy for gastrointestinal stromal tumor. Therapie. 2014;69(3):245–7. doi: 10.2515/therapie/2014018. [DOI] [PubMed] [Google Scholar]
- 5.Singh N, Bakshi S. Imatinib-induced dental hyperpigmentation in childhood chronic myeloid leukemia. J Pediatr Hematol Oncol. 2007;29(3):208. doi: 10.1097/MPH.0b013e318033a76c. [DOI] [PubMed] [Google Scholar]
- 6.Ghunawat S1, Sarkar R1, Garg VK1. Imatinib induced melasma-like pigmentation: Report of five cases and review of literature. Indian J Dermatol Venereol Leprol. 2016 Jul-Aug;82(4):409–412. doi: 10.4103/0378-6323.182387. [DOI] [PubMed] [Google Scholar]
- 7.Valizadeh N. Imatinib Induced Facial Skin Hyperpigmentation in a Case of Chronic Myelogenous Leukemia. Shiraz E-Medical Journal. 2011 Jul;12(3) [Google Scholar]
- 8.Ghunawat S, Sarkar R, Garg VK. Imatinib induced melasma-like pigmentation: Report of five cases and review of literature. Indian J Dermatol Venereol Leprol. 2016 Jul-Aug;82(4):409–412. doi: 10.4103/0378-6323.182387. [DOI] [PubMed] [Google Scholar]
- 9.Han H, Yu YY, Wang YH. Imatinib mesylate—induced repigmentation of vitiligo lesions in a patient with recurrent gastrointestinal stromal tumors. J Am Acad Dermatol. 2008;59:S80–83. doi: 10.1016/j.jaad.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 10.Harris JE, Rashighi M, Nguyen N, Jabbari A, Ulerio G, Clynes R, Christiano AM, Mackay-Wiggan J. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA) J Am Acad Dermatol. 2016 Feb;74(2):370–1. doi: 10.1016/j.jaad.2015.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jalalat SZ, Cohen PR. Gefitinib-associated vitiligo: report in a man with parotid squamous cell carcinoma and review of drug-induced hypopigmentation. Derm Online J. 2013;19(10):4. [PubMed] [Google Scholar]
- 12.Tsao AS, Kantarjian H, Cortes J, O’Brien S, Talpaz M. Imatinib mesylate causes hypopigmentation in the skin. Cancer. 2003;98(11):2483–7. doi: 10.1002/cncr.11812. [DOI] [PubMed] [Google Scholar]
- 13.Raanani P, Goldman JM, Ben-Bassat I. Depigmentation in a chronic myeloid leukemia patient treated with STI-471. J Clin Oncol. 2002;20:869–70. doi: 10.1200/JCO.2002.20.3.869. [DOI] [PubMed] [Google Scholar]
- 14.Hasan S, DInh K, Lombardo F, Dawkins F, Kark J. Hypopigmentation in an African patient treated with Imatinib mesylate: a case report. J Natl Med Assoc. 2003;95(8):722–4. [PMC free article] [PubMed] [Google Scholar]
- 15.Grossman WJ, Wilson DB. Hypopigmentation from Imatinib mesylate (Gleevac) J Pediatr Hematol Oncol. 2004;26:214. doi: 10.1097/00043426-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Brazzelli V, Roveda E, Prestinarl F, Barbagallo T, Bellani E, Trevisan V, et al. Vitiligo-like lesions and diffuse lightening of the skin in a pediatric patient treated with Imatinib mesylate: a noninvasive colormetric assessment. Pediatr Dermatol. 2006;23(2):175–8. doi: 10.1111/j.1525-1470.2006.00208.x. [DOI] [PubMed] [Google Scholar]
- 17.Legros L, Cassuto JP, Ortonne JP. Imatinib mesilate (Glivec): a systemic depigmenting agent for extensive vitiligo? Br J Dermatol. 2005;153:691–2. doi: 10.1111/j.1365-2133.2005.06813.x. [DOI] [PubMed] [Google Scholar]
- 18.McPartlin S, Leach M. Loss of skin pigment caused by imatinib therapy. Br J Haematol. 2005;129:448. doi: 10.1111/j.1365-2141.2005.05431.x. [DOI] [PubMed] [Google Scholar]
- 19.Cerchione C, Fabbricini R, Pane F, Luciano L. Vitiligo-like lesions in an adult patient treated with Imatinib mesylate. Leuk Res. 2009;33(8):e104–5. doi: 10.1016/j.leukres.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Brazzelli V, Grasso V, Barbaccia V, Giambattista M, Rivetti N, Zecca M, et al. Hair depigmentation and vitiligo-like lesions in a leukaemic paediatric patient during chemotherapy with Dasatinib. Acta Derm Venereol. 2012;92:193–220. doi: 10.2340/00015555-1289. [DOI] [PubMed] [Google Scholar]
- 21.Fujimi A, Ibata S, Kanisawa Y, Shibata T, Sakamoto H, Yamada S, et al. Reversible skin and hair depigmentation during chemotherapy with Dasatinib for chronic myeloid leukemia. J Dermatol. 2016;43(1):106–7. doi: 10.1111/1346-8138.13150. [DOI] [PubMed] [Google Scholar]
- 22.Boudadi K, Chugh R. Diffuse Hypopigmentation Followed by Hyperpigmentation in an African American Woman with Hemangiopericytoma Treated with Dasatinib. J Clin Diagn Res. 2014 Nov;8(11):QD01–QD02. doi: 10.7860/JCDR/2014/8055.5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gathings R, Lewallen R, Yosipovitch G. Immunotherapy-induced leukoderma from treatment of melanoma with IL-2: a case report and a review of the literature. Acta Derm Venereol. 2015;95:197–200. doi: 10.2340/00015555-1897. [DOI] [PubMed] [Google Scholar]
- 24.Sideras K, Menefee ME, Burton JK, Erlichman C, Bible KC. Profound hair and skin hypopigmentation in an African American woman treated with the multi-targeted tyrosine kinase inhibitor Pazopanib. J Clin Oncol. 2010;28:e312–3. doi: 10.1200/JCO.2009.26.4432. [DOI] [PubMed] [Google Scholar]
- 25.Hartmann JT, Kanz L. Sunitinib and periodic hair depigmentation due to temporary c-KIT inhibition. Arch Dermatol. 2008;144:1525–6. doi: 10.1001/archderm.144.11.1525. [DOI] [PubMed] [Google Scholar]
- 26.Al Enazi MM, Kadry R, Mitwali H. Skin depigmentation induced by Sunitinib treatment of renal cell carcinoma. J Am Acad Dermatol. 2009;61:905–6. doi: 10.1016/j.jaad.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 27.Alonso-Castro L, Rios-Buceta L, Vano-Galvan S, Moreno C, Soria-Rivas A, Jaen P. Vitiligo in 2 patients receiving Vemurafenib for metastatic melanoma. J Am Acad Dermatol. 2013;69(1):e28–9. doi: 10.1016/j.jaad.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Wolner ZJ, Marghoob AA, Pulitzer MP, Postow MA, Marchetti MA. A case report of disappearing pigmented skin lesions associated with pembrolizumab treatment for metastatic melanoma. Br J Dermatol. 2017 Jan 28; doi: 10.1111/bjd.15354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kagimoto Y, Mizuashi M, Kikuchi K, Aiba S. Lichenoid drug eruption with hyperpigmentation caused by Imatinib mesylate. Int J Dermatol. 2014;53:e161–2. doi: 10.1111/ijd.12174. [DOI] [PubMed] [Google Scholar]
- 30.Resende RG, Teixeira RGL, Vasconcelos FO, Silva MES, Abreu MHG, Gomez RS. Imatinib-associated hyperpigmentation of the palate in post-HSCT patient. J Craniomaxillofac Surg. 2012;40:e140–3. doi: 10.1016/j.jcms.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Song HS, Kang HY. Imatinib mesylate-induced hyperpigmentation of the nose and palate. Ann Dermatol. 2014 Aug;26(4):532–3. doi: 10.5021/ad.2014.26.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dasanu CA, Dutcher J, Alexandrescu DT. Yellow skin discoloration associated with Sorafenib use for treatment of metastatic renal cell carcinoma. South Med J. 2007;100(3):328–30. doi: 10.1097/SMJ.0b013e31802f01a9. [DOI] [PubMed] [Google Scholar]
- 33.Kong HH, Fine HA, Stern JB, Chanco Turner ML. Cutaneous pigmentation after photosensitivity induced by Vandetanib therapy. Arch Dermatol. 2009;145:923–5. doi: 10.1001/archdermatol.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brooks S, Linehan WM, Srinivasan R, Kong HH. Successful laser treatment of Vandetanib-associated cutaneous pigmentation. Arch Dermatol. 2011;147:364–5. doi: 10.1001/archdermatol.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng YP, Chen HJ, Chiu HC. Erlotinib-induced hair repigmentation. Int J Dermatol. 2014;53:e55–7. doi: 10.1111/j.1365-4632.2011.05422.x. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez NA, Ascaso FJ. Trichomegaly and poliosis of the eyelashes during Cetuximab treatment of metastatic colorectal cancer. J Clin Oncol. 2011;29:e532–3. doi: 10.1200/JCO.2011.34.6858. [DOI] [PubMed] [Google Scholar]
- 37.Mariani S, Abruzzese E, Basciani S, Fiore D, Persichetti A, Watanabe M, et al. Reversible hair depigmentation in a patient treated with Imatinib. Leuk Res. 2011;35(6):e64–6. doi: 10.1016/j.leukres.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 38.Sun A, Akin RS, Cobos E, Smith J. Hair depigmentation during chemotherapy with Dasatinib, a dual Bcr-Abl/Src family tyrosine kinase inhibitor. J Drugs Dermatol. 2009;8(4):395–8. [PubMed] [Google Scholar]
- 39.Samimi S, Chu E, Seykora J, Loren A, Vittorio C, Rook A, Rosenbach M, Kim EJ. Dasatinib-induced leukotrichia in a patient with chronic myelogenous leukemia. JAMA Dermatol. 2013 May;149(5):637–9. doi: 10.1001/jamadermatol.2013.75. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi E, Koyama T, Kobayashi K, Setsu N, Kawashima M, Kawai A. Reversible hair depigmentation in a Japanese female treated with Pazopanib. J Dermatol. 2014;41(11):1021–2. doi: 10.1111/1346-8138.12654. [DOI] [PubMed] [Google Scholar]
- 41.Sibaud V, Munsch C, Lamant L. Eruptive nevi and hair depigmentation related to Regorafenib. Eur J Dermatol. 2015;25(1):85–6. doi: 10.1684/ejd.2014.2462. [DOI] [PubMed] [Google Scholar]
- 42.Brzezniak C, Szabo E. Sunitinib-associated hair depigmentation. N Engl J Med. 2014;370:e27. doi: 10.1056/NEJMicm1309906. [DOI] [PubMed] [Google Scholar]
- 43.Huan TC, Ho CL. Blue-black discoloration of the nails associated with Gefitinib. Acta Clinica Belgica. 2011;66:72. doi: 10.2143/ACB.66.1.2062522. [DOI] [PubMed] [Google Scholar]
- 44.Steele JC, Triantafyllou A, Rajlawat BP, Field EA. Oral mucosal hyperpigmentation and horizontal melanonychia caused by imatinib. Clin Exp Dermatol. 2012;37:432–447. doi: 10.1111/j.1365-2230.2011.04196.x. [DOI] [PubMed] [Google Scholar]
- 45.Prabhash K, Biswas G, Prasad N, Karant N, Sastry PSRK, Parikh PM. Imatinib-induced nail hyperpigmentation in chronic myeloid leukemia. 2006;72:63–4. doi: 10.4103/0378-6323.19727. [DOI] [PubMed] [Google Scholar]
- 46.Mcpherson T, Sherman V, Turner R. Imatinib-associated hyperpigmentation, a side effect that should be recognized. J Eur Acad Dermatol Venereol. 2009;23(1):82–3. doi: 10.1111/j.1468-3083.2008.02706.x. [DOI] [PubMed] [Google Scholar]
- 47.Peuvrel L, Quereux G, Brocard A, Saint-Jean M, Dreno B. Onychopathy induced by Temsirolimus, a mammalian target of rapamycin inhibitor. Dermatology. 2012;224:204–8. doi: 10.1159/000338893. [DOI] [PubMed] [Google Scholar]
- 48.Roeker LE, Wolanskyj AP. Imatinib-associated melanosis of the palate. Am J Hematol. 2014;89:564. doi: 10.1002/ajh.23589. [DOI] [PubMed] [Google Scholar]
- 49.Wong M, Sade S, Gilbert M, Klieb HBE. Oral melanosis after tyrosine kinase inhibition with Imatinib for chronic myelogenous leukemia: report of a case and review of the literature. Derm Online J. 2011;17(6):4. [PubMed] [Google Scholar]
- 50.Li CC, Malik SM, Blaeser BF, Dehni WJ, Kabani SP, Boyle N, et al. Mucosal pigmentation caused by Imatinib: report of three cases. Head Neck Pathol. 2012;6:290–5. doi: 10.1007/s12105-011-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewis DM. Diffuse pigmentation of the palate. J Okla Dent Assoc. 2009;100(8):24–5. [PubMed] [Google Scholar]
- 52.Khoo TL, Catalano A, Supple S, Chong L, Yeoh SC, Yeung S, et al. Hyperpigmentation of the hard palate associated with Imatinib therapy for chronic myeloid leukemia with a genetic variation in the proto-oncogene c-KIT. Leuk Lymphoma. 2013;54(1):186–8. doi: 10.3109/10428194.2012.702904. [DOI] [PubMed] [Google Scholar]
- 53.Mattsson U, Halbritter S, Serikoff EM, Christerson L, Warfvinge G. Oral pigmentation in the hard palate associated with Imatinib mesylate therapy: a report of three cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(5):e12–6. doi: 10.1016/j.tripleo.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 54.Yu YH, Shere Y, Vigneswaran N. Oral and maxillofacial pathology case of the month. Palatal melanosis associated with Imatinib mesylate therapy. Tex Dent J. 2012;129(8):764–5. [PubMed] [Google Scholar]
Footnotes
No prior presentations have been performed regarding this research.
COI Disclosure Statement: JD and VRB have nothing to disclose. SW has a speaking arrangement with Novartis, Bayer-Onyx, Pfizer, and Mediavation. VS has a speaking, consultant or advisory role with Roche, GlaxoSmithKline, Pierre Fabre, Merck, Bristol-Myers Squibb, Bayer and Boehringer Ingelheim. MEL has a speaking, consultant or advisory role with Abbvie, Quintiles, Boehringer Ingelheim, AstraZeneca pharmaceuticals, Legacy Healthcare, Foamix, Adgero Bio Pharmaceuticals, Janssen R&D, Novartis, and Novocure. MEL receives research grants from Berg and Bristol-Myers Squibb.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Soreide K, Berg M, Skudal BS, Nedreboe BS. Advances in the understanding and treatment of colorectal cancer. Discov Med. 2011;12:393–404. [PubMed] [Google Scholar]
- 2.Ricciardi S, Tomao S, de Marinis F. Toxicity of targeted therapy in non-small-cell lung cancer management. Clin Lung Cancer. 2009;10:28–35. doi: 10.3816/CLC.2009.n.004. [DOI] [PubMed] [Google Scholar]
- 3.Vokes EE, Chu E. Anti-EGFR therapies: clinical experience in colorectal, lung, and head and neck cancers. Oncology (Williston Park) 2006;20:15–25. [PubMed] [Google Scholar]
- 4.Jost M, Kari C, Rodeck U. The EGF receptor, an essential regulator of multiple epidermal functions. Eur J Dermatol. 2000;10:505–10. [PubMed] [Google Scholar]
- 5.Balagula Y, Lacouture ME, Cotliar JA. Dermatologic toxicities of targeted anticancer therapies. J Support Oncol. 2010;8:149–161. [PubMed] [Google Scholar]
- 6.Wagner LI, Berg SR, Gandhi M, Hlubocky FJ, Webster K, Aneja M, et al. The development of a functional assessment of cancer therapy (FACT) questionnaire to assess dermatologic symptoms associated with epidermal growth factor receptor inhibitors (FACT-EGFRI-18) Support Care Cancer. 2013;21:1033–41. doi: 10.1007/s00520-012-1623-4. [DOI] [PubMed] [Google Scholar]
- 7.Rosen AC, Case EC, Dusza SW, Balagula Y, Gordon J, West DP, Lacouture ME. Impact of dermatologic adverse events on quality of life in 283 cancer patients: A questionnaire study in a dermatology referral clinic. Am J Clin Dermatol. 2013;14:327–33. doi: 10.1007/s40257-013-0021-0. [DOI] [PubMed] [Google Scholar]
- 8.Boone SL, Rademaker A, Liu D, Pfeiffer C, Mauro DJ, Lacouture ME. Impact and management of skin toxicity associated with anti-epidermal growth factor receptor therapy: survey results. Oncology. 2007;72:152–9. doi: 10.1159/000112795. [DOI] [PubMed] [Google Scholar]
- 9.Ensslin CJ, Rosen AC, Wu S, Lacouture ME. Pruritus in patients treated with targeted cancer therapies: systematic review and meta-analysis. J Am Acad Dermatol. 2013;69(5):708–20. doi: 10.1016/j.jaad.2013.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valentine J, Belum VR, Duran J, Ciccolini K, Schindler K, Wu S, Lacouture ME. Incidence and risk of xerosis with targeted anticancer therapies. J Am Acad Dermatol. 2015;72:656–67. doi: 10.1016/j.jaad.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Nishida T, Shirao K, Sawaki A, Koseki M, Okamura T, Ohtsu A, et al. Efficacy and safety profile of Imatinib mesylate (ST1571) in Japanese patients with advanced gastrointestinal stromal tumors: a phase II study (STI571B1202) Int J Clin Oncol. 2008;13:244–51. doi: 10.1007/s10147-007-0746-y. [DOI] [PubMed] [Google Scholar]
- 12.Hersh EM, O’Day SJ, Powderly J, Khan KD, Pavlick AC, Cranmer LD, et al. A phase II multicenter study of Ipilimumab with or without dacarbazine in chemotherapy-naïve patients with advanced melanoma. Invest New Drugs. 2011;29:489–98. doi: 10.1007/s10637-009-9376-8. [DOI] [PubMed] [Google Scholar]
- 13.Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, et al. A multicenter phase II trial of Pazopanib in metastatic and progress medullary thyroid carcinoma: MC057H. J Clin Endocrinol Metab. 2014;99(5):1687–93. doi: 10.1210/jc.2013-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bible KC, Suman VJ, Menefee ME, Smallridge RC, Molina JR, Maples WJ, et al. A multiinstitutional phase II trial of Pazopanib monotherapy in advanced anaplastic thyroid cancer. J Clin Endocrinol Metab. 2012;97:3179–84. doi: 10.1210/jc.2012-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R, et al. Phase II trial of Sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675–84. doi: 10.1200/JCO.2008.18.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curigliano G, Pivot X, Cortes J, Elias A, Cesari, Khosravan R, et al. Randomized phase II study of sunitinib versus standard of care for patients with previously treated advanced triple-negative breast cancer. The Breast. 2013;22:650–6. doi: 10.1016/j.breast.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 17.Han JY, Kim HY, Lim KY, Han JH, Lee YJ, Kwak MH, et al. A phase II study of Sunitinib in patients with relapsed or refractory small cell lung cancer. Lung Cancer. 2013;79:137–42. doi: 10.1016/j.lungcan.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Strosberg JR, Weber JM, Choi J, Campos TL, Valone TL, Han G, et al. A phase II clinical trial of Sunitinib following hepatic transarterial embolization for metastatic neuroendocrine tumors. Ann Oncol. 2012;23(9):2335–41. doi: 10.1093/annonc/mdr614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreisl TN, Smith P, Sul J, Salgado C, Iwamoto FM, Shih JH, Fine HA. Continuous daily Sunitinib for recurrent glioblastoma. J Neurooncol. 2013;111:41–8. doi: 10.1007/s11060-012-0988-z. [DOI] [PubMed] [Google Scholar]
- 20.Chau NG, Hotte SJ, Chen EX, Chin SF, Turner S, Wang L, Siu LL. A phase II study of Sunitinib in recurrent and/or metastatic adenoid cystic carcinoma (ACC) of the salivary glands: current progress and challenges in evaluating molecularly targeted agents in ACC. Ann Oncol. 2012;23:1562–70. doi: 10.1093/annonc/mdr522. [DOI] [PubMed] [Google Scholar]
- 21.Barrios CH, Hernandez-Barajas D, Brown MP, Lee SH, Fein L, Liu JH, et al. Phase II trial of continuous once-daily dosing of Sunitinib as first-line treatment in patients with metastatic renal cell carcinoma. Cancer. 2012;118:1252–9. doi: 10.1002/cncr.26440. [DOI] [PubMed] [Google Scholar]
- 22.Bang YJ, Kang YK, Kang WK, Boku N, Chung HC, Chen JS, et al. Phase II study of Sunitinib as second-line treatment for advanced gastric cancer. Invest New Drugs. 2011;29:1449–58. doi: 10.1007/s10637-010-9438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uemura H, Shinohara N, Yuasa T, Tomita Y, Fujimoto H, Niwakawa M, et al. A phase II study of Sunitinib in Japanese patients with metastatic renal cell carcinoma: insights into treatment, efficacy, and safety. Jpn J Clin Oncol. 2010;40(3):194–202. doi: 10.1093/jjco/hyp146. [DOI] [PubMed] [Google Scholar]
- 24.Escudier B, Roigas J, Gillessen S, Harmenberg U, Srinivas S, Mulder SF, et al. Phase II study of Sunitinib administered in a continuous once-daily dosing regimen in patients with cytokine-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27:4068–75. doi: 10.1200/JCO.2008.20.5476. [DOI] [PubMed] [Google Scholar]
- 25.Faivre S, Raymond E, Boucher E, Douillard J, Lim HY, Kim JS, et al. Safety and efficacy of Suntinib in patients with advanced hepatocellular carcinoma: an open-label, multicentre, phase II study. Lancet Oncol. 2009;10:794–800. doi: 10.1016/S1470-2045(09)70171-8. [DOI] [PubMed] [Google Scholar]
- 26.Kulke MH, Lenz HJ, Meropol NJ, Posey J, Ryan DP, Picus J, et al. Activity of Sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol. 2008;26:3403–10. doi: 10.1200/JCO.2007.15.9020. [DOI] [PubMed] [Google Scholar]
- 27.Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, et al. Phase II study of Sunitinib malate, on oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26:1810–16. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 28.Socinski MA, Novella S, Brahmer JR, Rosell R, Sanchez JM, Belami CP, et al. Multicenter, phase II trial of Sunitinib in previously treated, advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:650–56. doi: 10.1200/JCO.2007.13.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saltz LB, Rosen LS, Marshall JL, Belt RJ, Hurwitz HI, Eckhardt SG, et al. Phase II trial of Sunitinib in patients with metastatic colorectal cancer after failure of standard therapy. J Clin Oncol. 2007;25:4793–4799. doi: 10.1200/JCO.2007.12.8637. [DOI] [PubMed] [Google Scholar]
- 30.Hutterer M, Nowosielski M, Haybaeck J, Embacher S, Stockhammer F, Gotwald T, et al. A single-arm phase II Austrian/German multicenter trial on continuous daily Sunitinib in primary glioblastoma at first recurrence (SURGE 01–07) Neuro-Oncol. 2014;16(1):92–102. doi: 10.1093/neuonc/not161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fountzilas G, Fragkoulidi A, Kalogera-Fountzila A, Nikolaidou M, Bobos M, Calderaro J, et al. A phase II study of Sunitinib in patients with recurrent and/or metastatic non-nasopharyngeal head and neck cancer. Cancer Chemother Pharmacol. 2010;65:649–60. doi: 10.1007/s00280-009-1070-1. [DOI] [PubMed] [Google Scholar]
- 32.Monk BJ, Mas Lopez L, Zarba JJ, Oaknin A, Tarpin C, Termrungruanglert W, et al. Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. J Clin Oncol. 2010 Aug 1;28(22):3562–9. doi: 10.1200/JCO.2009.26.9571. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed M, Barbachano Y, Riddell A, Hickey J, Newbold KL, Viros A. Analysis of the efficacy and toxicity of sorafenib in thyroid cancer: a phase II study in a UK based population. Eur J Endocrinol. 2011 Aug;165(2):315–22. doi: 10.1530/EJE-11-0129. [DOI] [PubMed] [Google Scholar]
- 34.George S, Merriam P, Maki RG, Van den Abbeele AD, Yap JT, Akhurst T, et al. Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J Clin Oncol. 2009;27(19):3154–60. doi: 10.1200/JCO.2008.20.9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with Ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 37.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 39.Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus Sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:711–31. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 40.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–18. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus Interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 42.Elisei R, Schlumberger MJ, Müller SP, Schöffski P, Brose MS, Shah MH, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013 Oct 10;31(29):3639–46. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sternberg CN, Davis ID, Deen KC, Sigal E, Hawkins RE. An open-label extension study to evaluate safety and efficacy of pazopanib in patients with advanced renal cell carcinoma. Oncology. 2014;87(6):342–50. doi: 10.1159/000366227. [DOI] [PubMed] [Google Scholar]
- 44.van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012 May 19;379(9829):1879–86. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 45.Escudier B, Porta C, Bono P, Powles T, Eisen T, Sternberg CN, et al. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES Study. J Clin Oncol. 2014;32(14):1412–8. doi: 10.1200/JCO.2013.50.8267. [DOI] [PubMed] [Google Scholar]
- 46.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501–13. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 47.Robert C, Sibaud V, Mateus C, Cherpelis BS. Advances in the management of cutaneous toxicities of targeted therapies. Semin Oncol. 2012;39:227–240. doi: 10.1053/j.seminoncol.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 48.Tsao AS, Kantarjian H, Cortes J, O’Brien S, Talpaz M. Imatinib mesylate causes hypopigmentation in the skin. Cancer. 2003;98:2483–7. doi: 10.1002/cncr.11812. [DOI] [PubMed] [Google Scholar]
- 49.Cario-Andre M, Ardilouze L, Pain C, Gauthier Y, Mahon FX, Taieb A. Imatinib mesilate inihibits melanogenesis in vitro. Br J Dermatol. 2006;155:493–4. doi: 10.1111/j.1365-2133.2006.07359.x. [DOI] [PubMed] [Google Scholar]
- 50.Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000 Oct;295(1):139–45. [PubMed] [Google Scholar]
- 51.Heidary N, Naik H, Burgin S. Chemotherapeutic agents and the skin: an update. J Am Acad Dermatol. 2008;58:545–570. doi: 10.1016/j.jaad.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Basso FG, Boer CC, Correa ME, et al. Skin and oral lesions associated to imatinib mesylate therapy. Support Care Cancer. 2009;17:465–8. doi: 10.1007/s00520-008-0536-8. [DOI] [PubMed] [Google Scholar]
- 53.McPherson T, Sherman V, Turner R. Imatinib-associated hyperpigmentation, a side effect that should be recognized. J Eur Acad Dermatol Venereol. 2009;23:82–3. doi: 10.1111/j.1468-3083.2008.02706.x. [DOI] [PubMed] [Google Scholar]
- 54.Picardo M, Cardinali G. The genetic determination of skin pigmentation: KITLG and the KITLG/c-Kit pathway as key players in the onset of human familial pigmentary diseases. J Invest Dermatol. 2011;131:1182–85. doi: 10.1038/jid.2011.67. [DOI] [PubMed] [Google Scholar]
- 55.Spritz RA. The molecular basis of human piebaldism. Pigment Cell Res. 1992;5:3403. doi: 10.1111/j.1600-0749.1992.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 56.Grimes PE. New insights and new therapies in vitiligo. JAMA. 2005;293:730–5. doi: 10.1001/jama.293.6.730. [DOI] [PubMed] [Google Scholar]
- 57.Tarhini A. Immune-mediated adverse events associated with ipilimumab ctla-4 blockade therapy: the underlying mechanisms and clinical management. Scientifica (Cairo) 2013;2013:857519. doi: 10.1155/2013/857519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 59.Pavlick AC, Ott PA, Kannan K, et al. Hair depigmentation as an indicator of a durable response to CTLA-4 therapy. J Clin Oncol. 2010;28 article 15s, abstract no. 8571. [Google Scholar]
- 60.Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, et al. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol. 2016;152(1):45–51. doi: 10.1001/jamadermatol.2015.2707. [DOI] [PubMed] [Google Scholar]
- 61.Larsabal M, Marti A, Jacquemin C, Rambert J, Thiolat D, Dousset L, et al. Vitiligo-like lesions occurring in patients receiving anti-programmed cell death-1 therapies are clinically and biologically distinct from vitiligo. J Am Acad Dermatol. 2017 Jan 13; doi: 10.1016/j.jaad.2016.10.044. epublication ahead of print. [DOI] [PubMed] [Google Scholar]