Abstract
Objectives
Patients with bipolar disorder spend the most time in the depressed phase and that phase is associated with the most morbidity and mortality. Treatment of bipolar depression lacks a test to determine who will respond to treatment. White matter disruptions have been found in bipolar disorder. Previous reports suggest that white matter disruptions may be associated with resistance to antidepressant medication, but this has never been studied in a prospective study using an FDA-approved medication.
Methods
18 subjects with bipolar disorder who were in a major depressive episode and off all medications were recruited. Magnetic resonance imaging was acquired using a 64 direction diffusion tensor imaging sequence on a 3T scanner. Subjects were treated with eight weeks of open-label lurasidone. Montgomerey Asberg Depression Rating Scale (MADRS) was completed weekly. Tract-Based Spatial Statistics were utilized to perform a regression analysis of fractional anisotropy (FA) data with treatment outcome as assessed by percent change in MADRS as a regressor while controlling for age and sex, using a threshold of p (threshold-free cluster enhancement-corrected) <0.05.
Results
FA was positively correlated with antidepressant treatment response in multiple regions of the mean FA skeleton bilaterally, including tracts in the frontal and parietal lobes.
Conclusions
Greater disruptions in the white matter tracts in bipolar disorder were associated with poorer antidepressant response to lurasidone. The disruptions may potentially indicate treatment with a different antidepressant medication class. These results are limited by the open-label study design, sample size and lack of healthy control group.
Keywords: Bipolar Disorder, antidepressive agents, diffusion tensor imaging, lurasidone hydrochloride, white matter, magnetic resonance imaging
Introduction
Bipolar disorder remains a significant public health problem. The World Health Organization ranks it in the top 10 causes of disability worldwide (1). The depressed phase of the disorder accounts for the most disability and risk for suicide (2). Only three medications are FDA approved for the treatment of bipolar depression: lurasidone, quetiapine, and a combination pill of olanzapine and fluoxetine. Moreover, treatment is a trial and error process, as no tests exist to select the best treatment option for individual patients. The medications often take weeks to take effect, so the treatment course is often drawn out. The pathophysiology of the disorder remains largely unknown (3) making it harder to develop new, more effective treatments.
Previous studies have identified disruptions in white matter tracts on magnetic resonance imaging (MRI) scans using diffusion tensor imaging (DTI) or magnetic transfer ratio sequences in bipolar patients when compared to healthy volunteers (4–6). Greater rates of white matter hyperintensities (WMH’s) on T2 weighted FLAIR images, also indicative of disruptions in white matter tracts, have been reported in bipolar disorder (7). The clinical significance of these white matter disruptions has not been fully elucidated.
Previous retrospective studies found that higher rates of WMH’s in bipolar disorder were associated with poor clinical outcomes, indicating that white matter disruptions may characterize a particular subtype of bipolar depression that is more treatment resistant (8, 9). These studies were naturalistic in design, however, so the subjects had had different courses of treatment. Other studies have reported an association between white matter disruptions and treatment outcome in geriatric major depressive disorder (10, 11), providing further evidence that white matter disruptions may be important to clinical response to medications.
One previous prospective study was performed to identify whether DTI signal was associated with response to an acute antidepressant treatment course (12). That study used an experimental treatment, however, that included sleep deprivation and light therapy. A significant association was found between more DTI disruptions and poorer response to the treatment. To our knowledge, there are no prospective studies of white matter disruptions on MRI and treatment response to an FDA approved medication for bipolar depression. Here we studied whether white matter integrity was associated with the antidepressant response to lurasidone, an atypical antipsychotic recently approved for the treatment of depression in bipolar disorder.
Methods
Subject recruitment
20 patients with bipolar disorder (I, II or NOS) who were currently in a major depressive episode, and scored ≥16 on the Quick Inventory of Depressive Symptomatology-Self Rated version (QIDS-SR) were recruited (13). Subjects were excluded if they had current psychosis, significant suicide risk, recent substance abuse (within the last 2 months) or substance dependence (within the last 6 months), contraindications to MRI imaging such as known metal in the body or claustrophobia, previous lack of response to lurasidone, onset of mood disorder after age 40 years or taking medications that precluded a trial of lurasidone. One subject withdrew from the study before starting medication, and one was excluded from the analysis because of having remitted from their depression before starting medication. Data presented here are from the remaining 18 subjects. Patients were medication free for eight weeks before MRI.
MRI acquisition
Pretreatment brain MRI scans were obtained with a 3T Signa HDx scanner with a 32 channel head coil was used for acquisition. 64-direction diffusion tensor imaging sequence was used with a single shot sequence (TR=8500 ms, TE=85 ms, flip angle 90°, voxel size 1.88 mm X 1.88 mm X 2.5 mm, 60 axial slices, acquisition matrix 96 X 96).
Clinical treatment
After MRI, patients received open-label 8 week treatment trial of lurasidone at standard doses. Patients started at 20 mg PO daily and was raised by an additional 20 mg if the subject did not report intolerable side effects and did not score less than 3 on the Clinical Global Impression - Improvement scale (CGI-I). The dose of lurasidone was increased in this manner at weeks 2 and 4, and then weekly for the last four weeks to a maximum of 120 mg/day. Treatment response was measured with the Montgomery-Asberg Depression Rating Scale (MADRS) each week (14). A curve was fitted for each subject’s MADRS values over the eight-week course using a previously published mathematical model that uses information on the slope and curvature of the treatment response (15). The extrapolated values from those curves at the baseline timepoint and at the last MADRS measurement were used to quantify response (Supplemental Figure 1).
MRI data analysis and statistics
Voxelwise statistical analysis of FA data was carried out using TBSS (Tract-Based Spatial Statistics) (16), part of FSL (17). First, DTI images were motion corrected using FSL's eddy correct module, then they were brain-extracted using BET (18), then FA images were created by fitting a tensor model to the raw diffusion data using FDT. All subjects' FA data were then aligned into a common space using the nonlinear registration tool FNIRT (19), which uses a b-spline representation of the registration warp field (20). Next, a mean FA image was created and thinned to create a mean FA skeleton which represents the centers of all tracts common to the group. Each subject's aligned FA data was then projected onto this skeleton and the resulting data was fed into voxelwise cross-subject statistics. Statistics were done with FSL's randomise tool (21), which was used to perform a regression analysis on the FA data using percent change in MADRS as a regressor while controlling for age and sex, using a threshold of p (cluster corrected) <0.05. Corrections for multiple comparisons were performed with threshold-free cluster enhancement [TFCE] (17). The Institutional Review Board of the New York State Psychiatric Institute approved the study and all participants gave written informed consent. All research procedures were in accordance with the Helsinki Declaration of 1975.
Results
Demographic data
18 subjects (11 men and 7 women), mean age 33.4 ± 9.8 years. The majority (61%) met DSM IV criteria for Bipolar II disorder, two (11%) for Bipolar I Disorder, and five (28%) for Bipolar Disorder, Not Otherwise Specified. Three (17%) were Hispanic, seven (39%) were of a minority race and 14 (78%) were either single or divorced. Most were employed full-time (61%) and had completed a mean of 15.4±1.7 years of education. Mean age of onset of depressive illness was 16.6 ± 9.5 years. Lifetime co-morbid anxiety disorders included Social Phobia 6 (33%), and Panic Disorder and PTSD 3 each (17%). Although current substance use disorder was an exclusion criterion, lifetime co-morbid substance use disorders did occur, but were uncommon [two with past Alcohol Dependence (11%) and one each with Stimulant Dependence and Marijuana Abuse/Dependence (11%)].
Clinical response
Mean dosage of lurasidone at Week 8 was 43.3 (SD 23.3) mg/day. The mean MADRS score was 24.3 (SD 6.4) at baseline and 8.0 (SD 8.2) after treatment. Fifty percent (9/18) subjects remitted on treatment defined as >50% decrease in MADRS score and final MADRS score <10. The course of treatment response is depicted in Figure 1.
Figure 1.
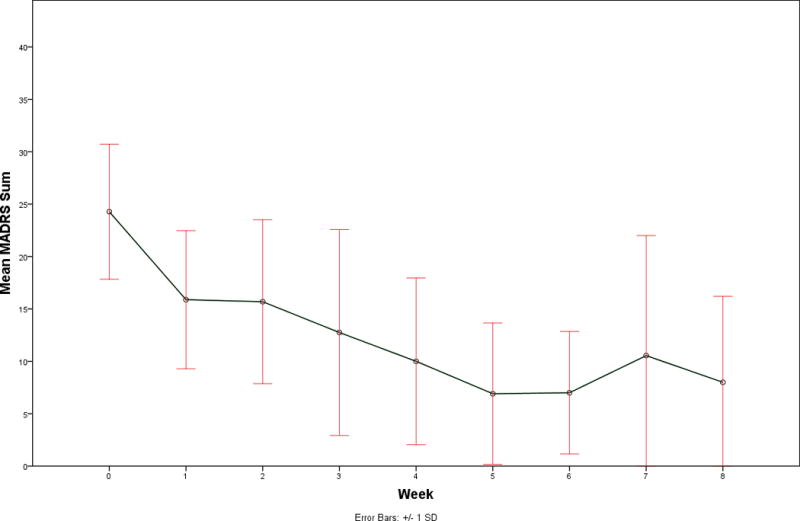
Clinical antidepressant response to the 8-week open label lurasidone treatment for bipolar depression as measured by weekly Montgomery Asberg Depression Rating Scales. Mean values are plotted and error bars represent standard deviations.
Imaging Data
TBSS analysis of white matter tracts demonstrated significant positive correlations between better antidepressant response to lurasidone and greater fractional anisotropy (FA) in multiple regions of the skeleton with age and sex were included as covariates (Figure 2). The significant white matter tract FA correlations with outcome were widespread throughout the frontal and parietal lobes, but did not include the cerebellum. No negative correlations were found. The mean FA in the significant regions was assessed in post hoc analyses to evaluate associations with other demographic measures. The mean FA did not correlate with the duration of illness (r=0.15, p=0.50) or baseline MADRS value (r=−0.14, p=0.57) with age and sex as covariates. Mean FA of subjects with a history of substance use disorder (t=1.12, p=0.28) or anxiety disorders (t=0.56, p=0.58) did not differ from those without such a history with age and sex as covariates.
Figure 2.
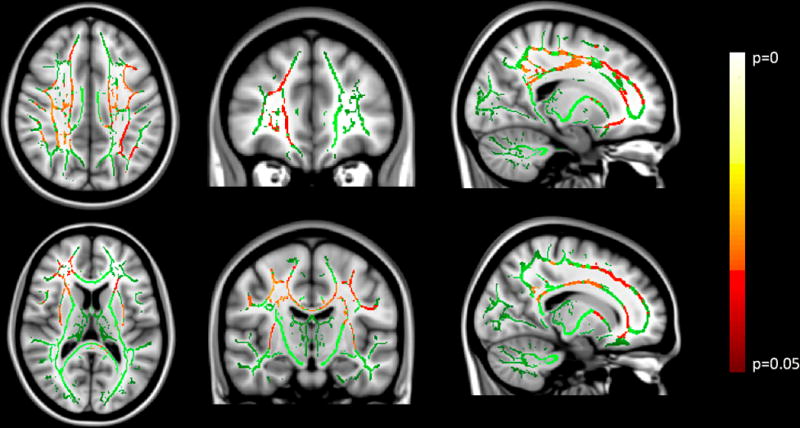
Results from the tract based spatial statistics (TBSS) analysis overlayed on a T1 structural MRI image. Two representative images of each view are provided. Green color demarcates the TBSS skeleton of major white matter tracts. The red-yellow color scheme indicates regions where DTI data significantly correlated with percent change of MADRS score, the clinical outcome measure of antidepressant treatment response to lurasidone in bipolar depression (n=18) with age and sex as covariates.
Discussion
These data suggest that greater white matter integrity is associated with better treatment response to an acute course of an FDA approved medication for bipolar depression, lurasidone. The association is widespread throughout the frontal and parietal cortices, but did not involve the cerebellum.
White matter pathology has been reported previously in bipolar disorder when compared to healthy volunteers using a number of MRI imaging modalities (4, 5, 7). The cause of these abnormalities is largely unknown. White matter disruptions can be vascular in etiology, and are associated with cardiovascular risk factors in the population in general. They also increase in prevalence with age (22). Increased rates of white matter hyperintensities in bipolar disorder have been found to be present in the adolescent bipolar population without significant cardiovascular pathology, indicating that the white matter disruptions may not be wholly due to these risk factors (23). Disruptions of white matter on MRI is not a bipolar disorder specific finding, as other psychiatric conditions including schizophrenia and major depressive disorder have demonstrated these disruptions (7). Post mortem brain studies have not reported gross pathological findings in the white matter such as scars or plaques, as found in neurodegenerative disorders. However, molecular studies have suggested greater inflammation and mitochondrial oxidative damage in post mortem brain tissue of bipolar disorder than in healthy volunteers (24–27). Taken together, it is likely that the findings reflect a neuropathologic process of unknown mechanism affecting white matter tracts in bipolar disorder.
Disruptions in white matter tracts have been associated with greater neurocognitive deficits in bipolar disorder (28, 29). Patients with MDD with a history of suicide attempts have also found to have higher rates of WMH’s than those without attempts, indicating that the white matter disruptions may play a role in suicidal behavior (30, 31). The latter finding complements our data to suggest that subjects with white matter disruptions comprise a more severe clinical group.
A previous meta-analysis of white matter disruptions in bipolar disorder found that the posterior cingulate cortex was the most reliably disrupted when compared to healthy volunteers (4). Here, we found white matter disruptions in tracts near the posterior cingulate to be associated with poor clinical treatment response to lurasidone. The FA of the white matter tracts near the posterior cingulate were found to be correlated with treatment response to lurasidone in this study. A number of previous DTI studies focused on the disruptions of white matter in the frontal lobe in bipolar disorder and found differences from healthy volunteers, consistent with our results (32, 33). Frontal lobe white matter deficits may explain a frontal-limbic deficit in bipolar disorder that have been delineated through task-based functional MRI studies (34). Frontal lobe deficits are also consistent with a number of symptoms of bipolar disorder, including distractibility, involvement in activities with high potential for painful consequences, and less cognitive regulation of emotion (35). Our results were not limited to the frontal region, however, so it is not possible to determine from our study whether the frontal lobe disruptions are essential to the disruption in antidepressant response.
Lurasidone’s antipsychotic mechanism is thought to be through either its serotonin 2A receptor or dopamine D2 receptor antagonism. However, its antidepressant mechanism remains largely unknown. One previous report found that clozapine, also an atypical antipsychotic, increased white matter FA in the brain of subjects with schizophrenia (36). Another pilot study found that increased myelin integrity was associated with treatment response to antipsychotic medications in schizophrenia (37). However, not all studies have replicated this finding (38). Therefore, more work is needed to determine if atypical antipsychotics, and lurasidone in particular, can reverse the white matter disruptions in bipolar disorder. Our data suggest that white matter disruptions impede response to treatment. The results are consistent with lurasidone acting through either a monoaminergic mechanism or a reparative mechanism that targets white matter tracts.
Our study has several limitations. There was no healthy volunteer group to compare to the bipolar subjects. Therefore, there is no way to test whether the white matter disruptions are in fact a pathophysiological effect as has been reported in other studies. There was no placebo treatment or active comparator arm to the study, and the treatment was not masked to participants or raters. Therefore, there is no way to know if the association with treatment outcome is related to lurasidone specifically or clinical improvement in general. There were no post-treatment scans, so there is no way to determine if lurasidone changes the white matter integrity. The results are also limited by the sample size. A larger study with these control groups and post treatment scans could address these limitations. Future studies may also focus on alternate treatments that could target the white matter pathology of the disorder. For example, lithium has demonstrated neuroprotective properties, and previous pilot studies have reported better response to lithium in patients with more white matter hyperintensities.
Supplementary Material
Examples of clinical curves used to quantify antidepressant treatment response to lurasidone for two individual subjects. Curves were fit to the time course of MADRS scores using previously published mathematical modeling. The extrapolated depression severity at the beginning timepoint of treatment and at the last observation of treatment were used to quantify antidepressant response (arrows point to those values in A). Percent change, the primary outcome measure used in the analysis was calculated for each subject as a ratio of the extrapolated value at the baseline timepoint (left arrow) to the extrapolated value at the final observation (right arrow). This approach incorporates all of the clinical data instead of the standard approach of relying on just two depression severity measurements.
Acknowledgments
We would like to thank Dr. Francesca Zanderigo with the manuscript, Dr. R Todd Ogden for his consultation on statistical considerations and Vinushini Arunagiri for her efforts in coordinating the project. We would like to thank all of the research participants for their contributions.
Disclosures
This work was funded by an Independent Medical Education Grant from Sunovian Pharmaceuticals. ML was also supported by a grant from the National Institutes of Health (K23MH104688). Dr. Mann receives royalties from the Research Foundation for Mental hygiene for the commercial use of the Columbia Suicide Severity Rating Scale (C-SSRS). Sunovion Pharmaceuticals did not write or edit this manuscript, and did not design or supervise the conduct of the study.
References
- 1.The World Health Report 2002:Reducing Risks, Promoting Healthy Life. Geneva, Switzerland: World Health Organization; 2002. 2002. [DOI] [PubMed] [Google Scholar]
- 2.Baldessarini RJ, Vieta E, Calabrese JR, Tohen M, Bowden CL. Bipolar depression: overview and commentary. Harv Rev Psychiatry. 2010;18(3):143–57. doi: 10.3109/10673221003747955. [DOI] [PubMed] [Google Scholar]
- 3.Grande I, Berk M, Birmaher B, Vieta E. Bipolar disorder. Lancet. 2016;387(10027):1561–72. doi: 10.1016/S0140-6736(15)00241-X. [DOI] [PubMed] [Google Scholar]
- 4.Nortje G, Stein DJ, Radua J, Mataix-Cols D, Horn N. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. J Affect Disord. 2013;150(2):192–200. doi: 10.1016/j.jad.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 5.Vederine FE, Wessa M, Leboyer M, Houenou J. A meta-analysis of whole-brain diffusion tensor imaging studies in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(8):1820–6. doi: 10.1016/j.pnpbp.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Bruno SD, Barker GJ, Cercignani M, Symms M, Ron MA. A study of bipolar disorder using magnetization transfer imaging and voxel-based morphometry. Brain : a journal of neurology. 2004;127(Pt 11):2433–40. doi: 10.1093/brain/awh274. [DOI] [PubMed] [Google Scholar]
- 7.Beyer JL, Young R, Kuchibhatla M, Krishnan KR. Hyperintense MRI lesions in bipolar disorder: A meta-analysis and review. Int Rev Psychiatry. 2009;21(4):394–409. doi: 10.1080/09540260902962198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore PB, Shepherd DJ, Eccleston D, Macmillan IC, Goswami U, McAllister VL, et al. Cerebral white matter lesions in bipolar affective disorder: relationship to outcome. Br J Psychiatry. 2001;178:172–6. doi: 10.1192/bjp.178.2.172. [DOI] [PubMed] [Google Scholar]
- 9.Dupont RM, Jernigan TL, Butters N, Delis D, Hesselink JR, Heindel W, et al. Subcortical abnormalities detected in bipolar affective disorder using magnetic resonance imaging. Clinical and neuropsychological significance. Arch Gen Psychiatry. 1990;47(1):55–9. doi: 10.1001/archpsyc.1990.01810130057008. [DOI] [PubMed] [Google Scholar]
- 10.Sneed JR, Culang-Reinlieb ME, Brickman AM, Gunning-Dixon FM, Johnert L, Garcon E, et al. MRI signal hyperintensities and failure to remit following antidepressant treatment. J Affect Disord. 2011;135(1–3):315–20. doi: 10.1016/j.jad.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 11.Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Latoussakis V, Kanellopoulos D, Klimstra S, et al. Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry. 2008;165(2):238–44. doi: 10.1176/appi.ajp.2007.07050744. [DOI] [PubMed] [Google Scholar]
- 12.Bollettini I, Poletti S, Locatelli C, Vai B, Smeraldi E, Colombo C, et al. Disruption of white matter integrity marks poor antidepressant response in bipolar disorder. J Affect Disord. 2015;174:233–40. doi: 10.1016/j.jad.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 14.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 15.Tarpey T, Petkova E, Ogden RT. Profiling Placebo Responders by Self-Consistent Partitioning of Functional Data. Journal of the American Statistical Association. 2003;98(464):850–8. [Google Scholar]
- 16.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 17.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 18.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersson JLR JM, Smith S. FMRIB tehnical report TR07JA2. 2007. Non-linear registration, aka Spatial normalization. [Google Scholar]
- 20.Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18(8):712–21. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- 21.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–97. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longstreth WT, Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27(8):1274–82. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 23.Serafini G, Pompili M, Borgwardt S, Houenou J, Geoffroy PA, Jardri R, et al. Brain changes in early-onset bipolar and unipolar depressive disorders: a systematic review in children and adolescents. Eur Child Adolesc Psychiatry. 2014;23(11):1023–41. doi: 10.1007/s00787-014-0614-z. [DOI] [PubMed] [Google Scholar]
- 24.Kaminsky Z, Tochigi M, Jia P, Pal M, Mill J, Kwan A, et al. A multi-tissue analysis identifies HLA complex group 9 gene methylation differences in bipolar disorder. Mol Psychiatry. 2012;17(7):728–40. doi: 10.1038/mp.2011.64. [DOI] [PubMed] [Google Scholar]
- 25.de Baumont A, Maschietto M, Lima L, Carraro DM, Olivieri EH, Fiorini A, et al. Innate immune response is differentially dysregulated between bipolar disease and schizophrenia. Schizophrenia research. 2015;161(2–3):215–21. doi: 10.1016/j.schres.2014.10.055. [DOI] [PubMed] [Google Scholar]
- 26.Gigante AD, Andreazza AC, Lafer B, Yatham LN, Beasley CL, Young LT. Decreased mRNA expression of uncoupling protein 2, a mitochondrial proton transporter, in post-mortem prefrontal cortex from patients with bipolar disorder and schizophrenia. Neurosci Lett. 2011;505(1):47–51. doi: 10.1016/j.neulet.2011.09.064. [DOI] [PubMed] [Google Scholar]
- 27.Andreazza AC, Wang JF, Salmasi F, Shao L, Young LT. Specific subcellular changes in oxidative stress in prefrontal cortex from patients with bipolar disorder. J Neurochem. 2013;127(4):552–61. doi: 10.1111/jnc.12316. [DOI] [PubMed] [Google Scholar]
- 28.Liu JX, Chen YS, Hsieh JC, Su TP, Yeh TC, Chen LF. Differences in white matter abnormalities between bipolar I and II disorders. J Affect Disord. 2010;127(1–3):309–15. doi: 10.1016/j.jad.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 29.Kieseppa T, Mantyla R, Tuulio-Henriksson A, Luoma K, Mantere O, Ketokivi M, et al. White matter hyperintensities and cognitive performance in adult patients with bipolar I, bipolar II, and major depressive disorders. Eur Psychiatry. 2014;29(4):226–32. doi: 10.1016/j.eurpsy.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Grangeon MC, Seixas C, Quarantini LC, Miranda-Scippa A, Pompili M, Steffens DC, et al. White matter hyperintensities and their association with suicidality in major affective disorders: a meta-analysis of magnetic resonance imaging studies. CNS Spectr. 2010;15(6):375–81. doi: 10.1017/s1092852900029242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pompili M, Innamorati M, Mann JJ, Oquendo MA, Lester D, Del Casale A, et al. Periventricular white matter hyperintensities as predictors of suicide attempts in bipolar disorders and unipolar depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1501–7. doi: 10.1016/j.pnpbp.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Adler CM, Holland SK, Schmithorst V, Wilke M, Weiss KL, Pan H, et al. Abnormal frontal white matter tracts in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord. 2004;6(3):197–203. doi: 10.1111/j.1399-5618.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- 33.Haznedar MM, Roversi F, Pallanti S, Baldini-Rossi N, Schnur DB, Licalzi EM, et al. Fronto-thalamo-striatal gray and white matter volumes and anisotropy of their connections in bipolar spectrum illnesses. Biol Psychiatry. 2005;57(7):733–42. doi: 10.1016/j.biopsych.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Townsend J, Altshuler LL. Emotion processing and regulation in bipolar disorder: a review. Bipolar Disord. 2012;14(4):326–39. doi: 10.1111/j.1399-5618.2012.01021.x. [DOI] [PubMed] [Google Scholar]
- 35.The Diagnostic and Statistical Manual of Mental Disorders (5th ed; DSM-5) Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 36.Ozcelik-Eroglu E, Ertugrul A, Oguz KK, Has AC, Karahan S, Yazici MK. Effect of clozapine on white matter integrity in patients with schizophrenia: a diffusion tensor imaging study. Psychiatry Res. 2014;223(3):226–35. doi: 10.1016/j.pscychresns.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Garver DL, Holcomb JA, Christensen JD. Compromised myelin integrity during psychosis with repair during remission in drug-responding schizophrenia. Int J Neuropsychopharmacol. 2008;11(1):49–61. doi: 10.1017/S1461145707007730. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q, Cheung C, Deng W, Li M, Huang C, Ma X, et al. White-matter microstructure in previously drug-naive patients with schizophrenia after 6 weeks of treatment. Psychol Med. 2013;43(11):2301–9. doi: 10.1017/S0033291713000238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Examples of clinical curves used to quantify antidepressant treatment response to lurasidone for two individual subjects. Curves were fit to the time course of MADRS scores using previously published mathematical modeling. The extrapolated depression severity at the beginning timepoint of treatment and at the last observation of treatment were used to quantify antidepressant response (arrows point to those values in A). Percent change, the primary outcome measure used in the analysis was calculated for each subject as a ratio of the extrapolated value at the baseline timepoint (left arrow) to the extrapolated value at the final observation (right arrow). This approach incorporates all of the clinical data instead of the standard approach of relying on just two depression severity measurements.


