Abstract
Prior mouse genetic research has set the stage for a deep understanding of appetite regulation. This goal is now being realized through the use of recent technological advances, such as the ability to map connectivity between neurons, manipulate neural activity in real time, and measure neural activity during behavior. Indeed, major progress has been made with regards to meal-related gut control of appetite, arcuate nucleus-based hypothalamic circuits linking energy state to the motivational drive, hunger, and finally limbic and cognitive processes that bring about hunger-mediated increases in reward value and perception of food. Unexpected findings are also being made, for example, the rapid regulation of homeostatic neurons by cues that predict future food consumption. The aim of this review is to 1) to cover the major underpinnings of appetite regulation, 2) to describe recent advances resulting from the new technologies, and 3) to synthesize these findings into an updated view of appetite regulation.
Introduction
Eating provides nutrients that are critical for survival. But eating too little or too much, or at inopportune times, has adverse consequences. For these reasons, the desire to eat – appetite – is heavily regulated. This regulation is mediated by short-term feedback from the gut to ensure that amounts of food ingested can be accommodated, by longer-term feedback from adipose tissue to ensure that energy stores are adequate, and by cues from the environment to a) promote eating when food is obtainable, to b) balance the drive to eat with other competing survival drives (including drives to avoid predators, mate and drink) and to c) complement the above-mentioned “error detection”-based feedback regulation with “error prevention” feed-forward anticipatory regulation.
Because appetite is dysfunctional in conditions such as anorexia nervosa and obesity, it is important to understand the processes that control it: specifically the neural circuits that regulate appetite and the synaptic mechanisms that operate within these circuits. Efforts to achieve this goal, however, have been hampered by the brain’s complexity – many different neurons controlling many different functions, intermixed and entangled in ways that have defied targeted investigation. Fortunately, with new neuroscience technologies, which can be applied in neuron-specific ways through the use of viral vectors and mice expressing DNA recombinases, it is now possible to manipulate and measure the activity of specified neurons in behaving animals, and to selectively map their afferent and efferent connections (Lerner et al., 2016; Nassi et al., 2015; Sjulson et al., 2016). Such approaches are particularly effective when studying appetite because researchers have access to a vast array of neuron-selective, recombinase-expressing mice – a byproduct of the remarkable diversity in gene expression by neurons in and around the hypothalamus (Campbell et al., 2017; Romanov et al., 2016). In addition to allowing for dissection of hypothalamic circuitry, these genetically-accessible nodes also serve as valuable entry points for studying extrahypothalamic contributions to appetite control. With these tools in hand, one can now establish the function of a given neuron and determine the larger wiring diagram within which it is embedded. The goal of this review is to discuss neural regulation of appetite, with special emphasis on recent advances.
Section 1: Clarifications
1.1 Hunger versus Appetite, Anorexia versus Satiety and Satiation
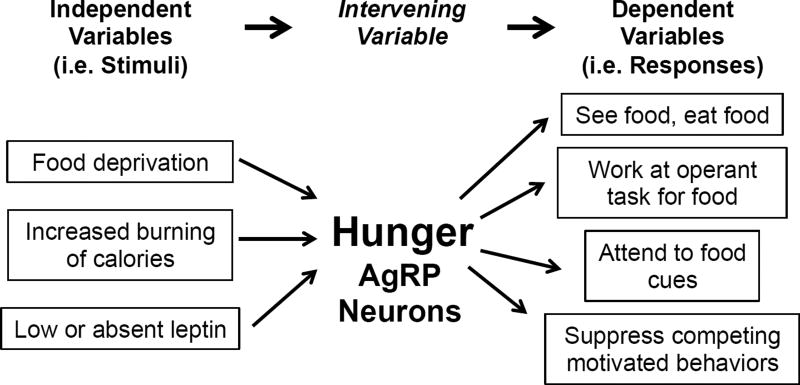
The desire to eat can have many causes ranging from prior caloric deficiency, to the sight and smell of palatable food, and to contexts related to time-of-day, social events, and risk/danger (Begg and Woods, 2013). Because of this, it can be difficult to determine why eating is initiated at any given moment. Consequently, terms used to describe the desire to eat, particularly ones ascribing an underlying cause, can at times be problematic. A case in point is the term hunger. To many, it means the desire (or drive) to eat in response to prior caloric deprivation. However, hunger as such cannot be measured – at least at present. Instead, hunger is operationally quantified by assessing amounts of food eaten or effort exerted to obtain food, or in humans, by also asking if they are hungry. Such assessments are agnostic to cause. Since many things can cause the desire to eat, it is difficult to know if and how much “hunger” is present. The term “appetite”, on the other hand, is more consistent with the aforementioned operational assessments as this term does not imply any specific underlying cause. As such, hunger can be viewed as the intervening variable (Figure 1) or drive linking caloric deficiency with appetite (Berridge, 2004; Miller, 1971). But in the end, if we can’t measure it and can’t visualize it, does the term hunger have utility? Is it a real entity? The answer would seem to be yes. Evidence for this comes from studies of agouti-related peptide (AgRP)-expressing neurons in the hypothalamic arcuate nucleus. AgRP neurons become active when animals are calorically deficient and are less active when animals are calorically replete. When AgRP neurons are opto- or chemo-genetically forced into an activated state in otherwise replete animals, intense eating occurs (Aponte et al., 2011; Krashes et al., 2011). In this sense, AgRP neurons and the wiring diagram within which they operate can be viewed as the physical embodiment of the intervening variable, hunger. Consistent with this, as alluded to in Figure 1, activation of AgRP neurons induces many of the behavioral effects associated with hunger (Aponte et al., 2011; Burnett et al., 2016; Krashes et al., 2011; Livneh et al., 2017).
Figure 1. Hunger and AgRP neurons as the “intervening variable”.
The intervening variable concept of motivational drive simplifies the linking of different stimuli (independent variables) with many different responses (dependent variables) (as proposed by Neal Miller (Miller, 1971) and modified by (Berridge, 2004)).
An equally complex term is satiety. Most commonly it is used to mean the opposite of hunger – decreased desire to eat in response to resolution of caloric deficiency (i.e. restoration of fuel storage deficits). As the antonym for hunger, this term also suffers from the issues outlined above. Staying with the AgRP neuron “metaphor”, natural or experimental manipulations that decrease eating by restoring lower levels of AgRP neuron activity, and/or by reversing effects of AgRP neuron activation on downstream circuitry, are often viewed as causing satiety (Atasoy et al., 2012; Fenselau et al., 2017; Garfield et al., 2015). The term satiation also means decreased desire to eat, but in contrast to satiety, it refers to the termination of meals, brought about by meal-related short-term feedback signals from the gut which precede assimilation of ingested calories (Cummings and Overduin, 2007). But confusion occurs here too, as meal-related feedback signals are often referred to as satiety factors. Finally, anorexia also means decreased desire to eat, but unlike satiety or satiation, it is used by some to imply pathophysiologic causes such as illness, fear or pain – things unrelated to energy balance or normal gastrointestinal feedback. Clearly the various terms can be ambiguous and confusing. However, their existence is testimony to the many different ways in which appetite can be affected.
1.2 Homeostatic versus Hedonic Appetite – Eating to Live or Living to Eat
Clearly both prior caloric deficit and proximity to tasty foods, such as desserts, stimulate appetite. The striking differences in these two causes of appetite have at times led to the view that they are entirely separate entities: homeostatic versus hedonic eating. While there is no doubt that aspects of homeostatic and hedonic eating are different, there are also aspects that are interrelated and shared (Bessesen, 2011; Ferrario et al., 2016; Fulton, 2010; Lockie and Andrews, 2013; Morton et al., 2014; Padilla et al., 2017; Perello and Dickson, 2015; Williams, 2014). This becomes clear when one considers the reward value of food – often measured by assessing how hard animals will work for food. When calorically replete, rodents will perform little work to obtain standard chow. However, when calorically deficient, these same animals will perform intense work for food. Thus, caloric deficiency greatly increases the reward value and/or incentive salience of food and related cues (Fulton, 2010). Similarly, peripheral homeostatic feedback signals from fat and the gut, such as leptin and ghrelin, also affect the rewarding aspects of food (Domingos et al., 2011; Fulton, 2010; Lockie and Andrews, 2013; Williams, 2014). Indeed, receptors for these and related hormones have been found on neurons classically viewed as controlling reward. But it isn’t even necessary to invoke direct effects of homeostatic hormones on reward pathways because experimental activation of AgRP neurons, the quintessential “homeostatic” hunger neuron, also greatly increases the rewarding aspects of food - indeed to the same high level as that seen in fasted animals (Krashes et al., 2011). Thus, it’s clear that these two forms of appetite regulation should not be viewed separately, but instead as having different afferent signals but working through converging, common downstream circuitry to ultimately increase appetite. In essence, we eat to live by living to eat.
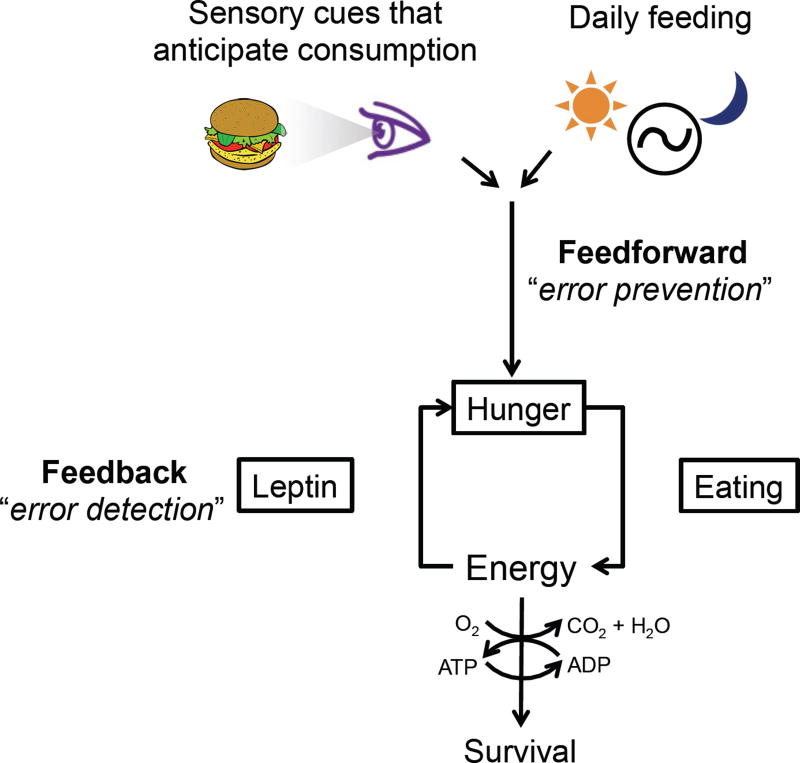
Another term requiring clarification is homeostasis (Figure 2). Traditionally, it implies regulation in response to feedback from perturbed physiologic variables (i.e. fat stores, blood osmolarity, body temperature, etc.). Issues emerge, however, when seemingly homeostatic actions take place in the absence of such perturbations. For example, daily home-cage feeding on standard chow is often considered an example of “homeostatic” feeding. Yet this type of feeding, and indeed initiation of most meals, occurs in the apparent absence of current metabolic deficits (Rogers and Brunstrom, 2016; Strubbe and Woods, 2004; Woods and Ramsay, 2007). Consumption of standard chow in ad libitum fed mice varies with circadian rhythms (Strubbe and Woods, 2004) and likely serves to prevent future caloric deficit (Rogers and Brunstrom, 2016). Similarly, the increased willingness to consume calorically-dense and easily ingestible foods may have emerged (via prior experience and/or evolution) as a way to decrease the risk of future energy deficit while minimizing time spent foraging and exposure to predators. This anticipation of future as well as present needs is a common feature of adaptive regulation of many physiologic variables (Somjen, 1992; Woods and Ramsay, 2007), and in the cardiovascular field has been termed feedforward homeostatic control (Dampney, 2016). In this light, the recently discovered food/water cue-based and circadian control of AgRP hunger neurons, subfornical (SFO) and organum vasculosum of the lamina terminalis (OVLT) thirst neurons, and water-excretion controlling vasopressin neurons, to be discussed later in greater detail, could be viewed as serving anticipatory, feedforward “homeostatic” functions (Betley et al., 2015; Chen et al., 2015; Gizowski et al., 2016; Mandelblat-Cerf et al., 2017; Mandelblat-Cerf et al., 2015; Zimmerman et al., 2016).
Figure 2. Homeostasis and the role of feedback and feedforward control.
Feedback is reactive in that it detects perturbations, i.e. “errors”, and regulates hunger to restore energy balance. Feedforward regulation anticipates future needs and regulates hunger to prevent future disturbances in energy balance.
Section 2: Feedback from the Gut
2.1 Satiation and Termination of Meals
Many factors promote meal initiation including food availability, cues associated with food, circadian time and time elapsed since the previous meal (Petrovich, 2013; Strubbe and Woods, 2004). Meal termination, in contrast, is controlled predominantly by the gut. There are a number of excellent reviews on satiation and meal termination (Chambers et al., 2013; Cummings and Overduin, 2007; Grill and Hayes, 2012; Moran and Ladenheim, 2016; Steinert et al., 2017).
Information related to ingested food is transmitted from the stomach and small intestine to the hindbrain via the afferent arm of the vagus nerve (Berthoud and Neuhuber, 2000; Brookes et al., 2013) (Figure 3). Like most sensory nerves, it is composed of pseudounipolar neurons, the cell bodies for which are located primarily in the nodose ganglia. Their “sensory” axons target visceral organs where they are activated directly by luminal stretch or in a paracrine fashion by factors secreted in response to nutrients by enteroendocrine cells located in the gut epithelium (Brookes et al., 2013; Dockray, 2013; Gribble and Reimann, 2016). Their “CNS-directed” axons travel centrally to the dorsal hindbrain where they synapse on neurons in the caudal end of the nucleus of the solitary tract (NTS).
Figure 3. Satiation and the role of the afferent vagus nerve and NTS.
Vagal afferents detect stretch of the gut wall and nutrient-induced release of paracrine signals such as CCK and serotonin (5HT) by gut enteroendocrine cells. These afferents activate neurons in the NTS to bring about satiation.
Potential satiation factors released by enteroendocrine cells include cholecystokinin (CCK), glucagon-like peptide-1 (GLP-1), peptide YY (PYY) and serotonin (5-HT). CCK is particularly noteworthy as it has been extensively studied and is universally accepted as an important satiation factor. In response to nutrients, it is released by cells in the upper small intestine where it activates vagal afferents by binding to CCK1 receptors. Blocking these actions of CCK delays meal termination, increasing meal size, while physiologic administration of CCK does the opposite (Moran and Ladenheim, 2016). Strikingly, manipulation of CCK’s actions does not affect total daily food intake as changes in meal size are offset by compensatory changes in meal frequency. This selective action on meal termination is a hallmark of satiation factors. Highlighting this point are the contrasting effects of the adiposity-related satiety factor, leptin. It regulates both meal size and total daily food intake. Leptin decreases total daily food intake by increasing satiety – primarily by its actions on hypothalamic neurons. It reduces meal size, on the other hand, by enhancing the efficacy of satiation signaling (Moran and Ladenheim, 2016; Morton et al., 2014). Thus, when adipose stores and leptin are low, meal sizes are increased. The circuit mechanisms by which long-term adiposity signals regulate short-term gut satiation signaling are an area of active investigation. Likely mechanisms include regulation of hindbrain satiation circuits by descending projections from hypothalamic satiety circuits, and/or direct actions of satiety hormones on these same hindbrain satiation circuits or on the vagal afferents themselves (de Lartigue et al., 2014; Grill and Hayes, 2012; Li et al., 2011; Morton et al., 2014; Peters et al., 2006).
The role of gut-released GLP-1 and PYY in satiation per se is uncertain and continues to be the subject of investigation (Chambers et al., 2013; Manning and Batterham, 2014; Steinert et al., 2017). Serotonin, as discussed below, is an important coupling factor that links nutrient activation of enterochromaffin cells, a subset of enteroendocrine cells, to stimulation of vagal afferents. Amylin, unlike the above-mentioned satiation factors, is co-released with insulin by pancreatic β-cells. Amylin increases within minutes of eating, as does insulin, and it causes satiation by engaging neurons in the area postrema, a circumventricular organ located just dorsal and medial to the NTS (Lutz, 2013).
2.2 “Labeled Line” Transmission by the Afferent Vagus
The afferent vagus nerve relays multimodal sensory information from the gut, but also the lungs and cardiovascular system, with each modality serving very different regulatory functions (Berthoud and Neuhuber, 2000). Similarly, gut information regarding stretch versus nutrient content serve related but not identical functions (Dockray, 2013). Given this diversity of information transmitted by the sensory vagus nerve and its ~2,300 constituent neurons, a key question has been: what are the coding mechanisms used for transmitting these discrete signals? Afferents from different organs, or from different segments of the GI tract, send viscerotopic projections to subdomains of the NTS (Berthoud and Neuhuber, 2000; Travagli and Anselmi, 2016), suggesting the presence of “labeled lines” for organ-specific information. But beyond that, it has been difficult to establish coding mechanisms for different sensory modalities from within a given organ and/or specific region of the gastrointestinal (GI) tract. The absence of tools for the selective targeting of neuron subtypes has made it difficult to address this question.
Recent genetic studies have made important advances in this area. Specifically, distinct vagal afferent subtypes were found to differentially express the following G-protein coupled receptors (GPCRs): P2ry1, Npy2r, Gpr65 and Glp1r (Chang et al., 2015; Williams et al., 2016). Selective assessment of these subtypes was then achieved by generating corresponding Cre knockin mice (Chang et al., 2015; Williams et al., 2016). P2ry1- and Npy2r-expressing afferents were found to play key roles in different aspects of respiratory physiology (Chang et al., 2015), while Gpr65- and Glp1r-expressing afferents were found to play key roles in gastrointestinal physiology. Of note, Gpr65- and Glp1r-expressing afferents respectively innervate different aspects of the gut (intestinal villi of the proximal small intestine versus enteric ganglia between muscle layers of the stomach and small intestine), send projections to different domains of the NTS (commissural zone versus medial subnucleus), detect different sensory stimuli (nutrient-evoked release of serotonin by enterochromaffin cells versus stretch of the stomach and intestine), and evoke different reflex responses when optogenetically stimulated. Thus, Gpr65-expressing afferents sense nutrients in the very proximal small intestine and then, via a vago-vagal reflex (i.e. Gpr65 afferents → NTS → vagus motor nerve), inhibit stomach contractions (Figure 4). This reflex prevents excessive transit of stomach contents into the small intestine. The Glp1r-expressing afferents, in contrast, sense stomach and intestinal stretch and then, via a vago-vagal reflex, may provide positive feedback to ongoing contractions – although additional experiments are required to establish the effect on stomach contractions (Figure 4). Of interest, the mechanosensing Glp1r neurons express receptors for and are activated by CCK, the previously mentioned “gold-standard” satiation factor. This is of interest since stomach stretch has previously been shown to inhibit feeding (Phillips and Powley, 1996), and CCK is thought to work, at least in part, by increasing sensitivity of stretch detection (Blackshaw and Grundy, 1990; Moran and Ladenheim, 2016; Schwartz et al., 1991). Interestingly, these same mechanosensing afferents may also be regulated by leptin (de Lartigue et al., 2014; Li et al., 2011; Peters et al., 2006). Surprisingly, neither type of gut afferent is responsive to the enteroendocrine factor GLP-1 as assessed by Ca2+ imaging, including the subset marked by its receptor, Glp1r. It is speculated that GLP-1 agonists might instead affect Glp1r-expressing neurons by modulating their function, perhaps by binding to receptors on terminals in the NTS, regulating synaptic release of glutamate. As the above-mentioned studies were performed on anesthetized animals, the predicted role of these afferents in mediating satiation remains to be determined, and will clearly be a focus for future investigations. In total, these studies clearly demonstrate labeled line transmission of different sensory modalities by the vagus nerve. In addition, the above-mentioned Cre-driver mice, and other Cre- and Flp-driver mice that exist or will be generated, in conjunction with intersectional approaches to facilitate “capture” of increasingly selective subsets of vagal afferents, will provide unprecedented access to these “labeled lines”, greatly facilitating research into their function.
Figure 4. “Labeled line” transmission by Glp1r- and Gpr65-expressing vagal afferents (Williams et al., 2016).
(A) Vagal afferents marked by expression of Glpr1 detect stretch and, via a vago-vagal reflex, appear to produce stomach contractions. As stomach stretch has been shown to inhibit feeding (Phillips and Powley, 1996), these neurons likely engage NTS neurons that bring about satiation.
(B) Vagal afferents marked by expression of Gpr65 detect nutrient-induced release of 5HT, and via a vago-vagal reflex, inhibit stomach contractions. This serves to prevent excessively rapid transit of nutrients from the stomach into the small intestine. The effect of Gpr65-expressing afferents on behavior is presently unknown.
2.3 Relay of Visceral Information to NTS, LPBN and Beyond
Vagal afferents transmit multimodal visceral information to the caudal NTS, where they excite NTS neurons by releasing glutamate (Travagli and Anselmi, 2016). NTS neurons receive additional information on satiation from amylin-responsive neurons in the area postrema, and on adipose stores via direct actions of leptin and via descending projections from the hypothalamus (Grill and Hayes, 2012; Lutz, 2013). NTS neurons integrate and then route this information to nearby parasympathetic preganglionic neurons in the dorsal motor nucleus of the vagus (DMV) to evoke vago-vagal reflexes, to other hindbrain sites to control sympathetic outflow, and to more rostral sites to control behavior, autonomic outflow and neuroendocrine responses (Ghosal et al., 2017; Grill and Hayes, 2012; Rinaman, 2010; Trapp and Cork, 2015; Travagli and Anselmi, 2016). NTS neurons release many transmitters including glutamate and GABA, and the neuromodulators norepinephrine, GLP-1, and CCK (Figure 5) (D'Agostino et al., 2016; Rinaman, 2010; Roman et al., 2016; Trapp and Cork, 2015; Travagli and Anselmi, 2016). The noradrenergic NTSNE neurons belong to the A2 cell group and are marked by the enzymes tyrosine hydroxylase (TH) and dopamine β-hydroxylase (DBH). Some overlap exists between NTSGLP1 and NTSCCK neurons, but both are distinct from NTSNE neurons (Garfield et al., 2012; Zheng et al., 2015). Importantly, most if not all NTSNE, NTSGLP1 and NTSCCK neurons co-release glutamate (Roman et al., 2016; Stornetta et al., 2002; Trapp and Cork, 2015; Zheng et al., 2015).
Figure 5. Satiation: NTS → LPBN and beyond.
NTS neurons activate LPBNCGRP neurons and this promotes meal termination via projections to the central amygdala (CeA) (Campos et al., 2016; Carter et al., 2013; Roman et al., 2016). NTSCCK neurons also project to the PVH where they promote satiety (D'Agostino et al., 2016). Similarly, NTSGLP1 neurons can promote satiety (Gaykema et al., 2017), perhaps via their projections to the PVH and ARC.
Information from the NTS reaches behavioral and autonomic control sites by direct projection, and by relay through the lateral parabrachial nucleus (LPBN) (Figure 5) (Grill and Hayes, 2012; Herbert et al., 1990; Saper and Loewy, 1980). For example, NTSNE, NTSGLP1 and NTSCCK neurons target the LPBN, and also many other sites within the medulla, hypothalamus, central amygdala (CeA) and bed nucleus of the stria terminalis (BNST) (D'Agostino et al., 2016; Rinaman, 2010; Roman et al., 2016; Trapp and Cork, 2015). Of note, chemogenetic activation of NTSCCK neurons (D'Agostino et al., 2016) and NTSGLP1 neurons (Gaykema et al., 2017) was recently shown to cause satiety. The satiety-inducing effects of NTSCCK neurons are likely mediated via projections to the PVH where they appear to engage melanocortin-4 receptor (MC4R)-expressing neurons (D'Agostino et al., 2016). The effects of NTSGLP1 neurons could similarly be mediated by neurons in the PVH (Trapp and Cork, 2015), and/or possibly also the arcuate nucleus (Secher et al., 2014), as chemogenetic activation of NTSGLP1 neurons induces c-Fos in both of these sites (Gaykema et al., 2017).
Recently an important appetite-suppressing circuit, centered on CGRP-expressing neurons in the LPBN, was identified. These neurons receive visceral information and relay it to higher structures, ultimately affecting appetite (Figure 5) (Campos et al., 2016; Carter et al., 2013; Roman et al., 2016). When opto- or chemogenetically activated, LPBNCGRP neurons decrease appetite. They do this via excitatory projections to the CeA, where they likely engage PKC-δ-expressing CeA neurons, previously reported to affect appetite (Cai et al., 2014). Importantly, NTSNE and NTSCCK neurons, activated by meal ingestion, directly excite LPBNCGRP neurons (Kreisler et al., 2014; Roman et al., 2016). Equally important, silencing LPBNCGRP neuron output delays meal termination and increases meal size (Campos et al., 2016), but does not affect daily food intake due to compensatory decreases in meal frequency. As mentioned earlier, changes in meal size that are not accompanied by changes in daily food intake are characteristic of alterations in satiation. Surprisingly, these same LPBNCGRP neurons mediate visceral malaise-induced anorexia (Carter et al., 2013), and activation of LPBNCGRP neurons is necessary and sufficient to induce conditioned taste aversion (Carter et al., 2015). As physiologic satiation is generally not viewed as being aversive, and since inability to induce conditioned taste aversion has been a key criterion for bona fide satiation factors (Chambers et al., 2013; Smith, 1998), these results suggest one of two possibilities: that there are functionally distinct subsets of LPBNCGRP neurons that mediate satiation versus aversion, and/or that aversion may be the result of particularly high level LPBNCGRP neuron activation, which might occur when eating excessively large meals (Saper, 2016). Regardless, the observation of increased meal size in mice with silenced LPBNCGRP neurons clearly demonstrates that this pathway contributes to satiation.
2.4 The Curious Case of the Stomach Hormone Ghrelin
Many aspects of ghrelin biology have recently been comprehensively reviewed (Muller et al., 2015; Steinert et al., 2017). A few key points will be made here. Ghrelin is produced by enteroendocrine cells in the stomach, but in many ways it is unlike other gut hormones. First, the secretory cells that release ghrelin do not have access to, nor are they regulated by, luminal contents or stomach stretch. Instead, ghrelin secretion appears to be controlled by autonomic input (Muller et al., 2015; Steinert et al., 2017). Second, and very remarkably, ghrelin is the only systemic circulating factor known to stimulate appetite. Its levels are highest before a meal, fall with eating, and increase with subsequent fasting. This has led to the suggestion that it promotes meal initiation. Ghrelin’s receptor, the growth hormone secretagogue receptor (GHSR), is expressed by many neurons in the brain and, indeed, appetite is increased following injection of ghrelin into many of these sites. AgRP neurons, which are directly activated by ghrelin, are one important target for appetite stimulation (Chen et al., 2004; Luquet et al., 2007; Wang et al., 2014). Surprisingly, deletion of ghrelin’s gene or the gene encoding its receptor, or genetic ablation of ghrelin-secreting endocrine cells, does not affect feeding. Thus, either ghrelin is not normally involved in feeding or aspects of these studies may have obscured detection of effects. For example, redundant processes may have compensated for deficient ghrelin signaling or, alternatively, the methods employed to assess appetite in these animals may not have been poised to identify ghrelin‘s true function (Walker et al., 2012). On the other hand, disruption of ghrelin function is able to cause profound hypoglycemia in starved mice (Goldstein et al., 2011; McFarlane et al., 2014; Wang et al., 2014). This has raised the possibility that ghrelin’s primary function may be to resist hypoglycemia.
Section 3: Hypothalamic Regulation of Hunger/Satiety
In contrast to short-term feedback from the gut which is relayed by vagal afferents to the NTS, longer-term feedback from energy stores is transmitted by hormones and primarily impacts neurons in the hypothalamus (Morton et al., 2014). This section focuses on the hypothalamic circuits transducing long-term feedback signals into hunger/satiety.
3.1 Leptin, the Long-Term Feedback Signal from Adipose Tissue
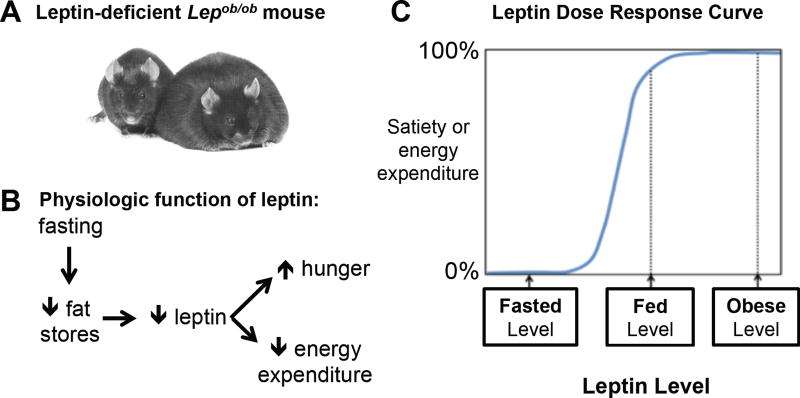
In the summer of 1949, scientists at Jackson Labs noted the appearance of “some very plump young mice”, and they called the new mutant allele obese (ob) (Figure 6) (Ingalls et al., 1950). Forty-four years later, obesity in these mice was found to be due to deficiency of the novel adipocyte hormone, leptin (Zhang et al., 1994). Shortly thereafter, deficiency of leptin’s receptor (LEPR) was found to cause obesity in another mutant mouse (Chen et al., 1996; Chua et al., 1996; Lee et al., 1996). LEPR is an IL6-type class I cytokine receptor that signals via the JAK2 / STAT3 pathway (Flak and Myers, 2016). Decades of research have determined that leptin circulates in proportion to fat stores (Considine et al., 1996), that its ability to regulate energy balance is mediated largely by receptors on hypothalamic neurons (Flak and Myers, 2016), and that humans with deficiency of leptin or its receptor are massively obese – hence leptin’s function is highly conserved (Farooqi and O'Rahilly, 2014). Finally, leptin’s primary role is to signal whether fat stores are adequate, or when low, inadequate (Figure 6) (Ahima et al., 1996). Specifically, falling leptin levels bring about key adaptive responses, including hunger and decreased sympathetically-mediated energy expenditure to conserve limited fuel stores (Ahima et al., 1996; Rosenbaum and Leibel, 2014). Conversely, when fat stores are adequate, increases in leptin such as those that occur with obesity or exogenous leptin treatment are largely ineffective in resisting weight gain (Ravussin et al., 2014; Rosenbaum and Leibel, 2014). Thus, leptin’s role in maintaining energy balance is asymmetric – it’s very strong in restoring fat stores when they are low, and very weak in resisting obesity (Figure 6). Leptin- and LEPR-deficient mice develop obesity because their brains operate as if fat stores are low. Consequently, they have all the adaptations of starvation including intense hunger and low energy expenditure – a bad combination when unlimited food is available. As will be discussed below, the principal targets of leptin are energy balance-regulating neurons in the arcuate nucleus.
Figure 6. The adipocyte hormone, leptin.
Low levels of leptin signal to the brain that fat stores are inadequate (Ahima et al., 1996; Rosenbaum and Leibel, 2014).
(A) A control sibling (left) sitting next to a homozygous leptin-deficient, very obese mouse (right). Image courtesy of R.L. Leibel (2008) Int. J. Obes. 32, S98, 2008.
(B) Decreased food intake reduces fat stores and lowers blood leptin levels. This is sensed by the brain, which then brings about adaptive changes in appetite and energy expenditure – both aimed at restoring fat stores.
(C) A schematized dose response curve for blood leptin levels and their effects on satiety and energy expenditure. The effective range for leptin is between the low levels seen with fasting and the normal levels seen in the ad libitum fed, non-obese state. Levels above this, as occur with obesity or with exogenous leptin treatment, produce little additional effects – i.e. the dose response curve at higher levels of leptin is relatively flat.
3.2 POMC and AgRP Neurons in the Arcuate, MC4Rs in the PVH
Many years ago it was noted that POMC-expressing neurons reside in the arcuate nucleus (Dube et al., 1978) and that central injection of αMSH, a processed peptide of pro-opiomelanocortin (POMC), decreases food intake (Poggioli et al., 1986). Originally, these findings were of unknown significance, but they soon came to the forefront as two lines of investigation converged – one involving αMSH receptors and the other an obese, hyperphagic mutant mouse with striking yellow hair (Ay/a mice). Molecular cloning originally identified five melanocortin (MC) receptors, all GPCRs (Mountjoy et al., 1992), three of which are relevant to this story. MC1R is expressed by skin melanocytes, MC3R and MC4R by neurons in the brain, and each is activated by αMSH. Obese yellow Ay/a mice were found to have a genomic deletion, placing Agouti, a pigment-regulating gene, under the transcriptional control of Raly, a ubiquitously expressed ribonucleoprotein (Bultman et al., 1992; Michaud et al., 1993). Agouti is normally expressed only in the skin and in a temporally and spatially discrete pattern. Agouti was subsequently found to block MC1R and MC4R suggesting mechanisms for yellow fur and obesity in Ay/a mice (Lu et al., 1994). Also, semi-selective agonists and antagonists of MC3R/MC4R were found to decrease and increase feeding, respectively (Fan et al., 1997). The dominant role for MC4R was ultimately proven when its deficiency in both mice and humans was linked to hyperphagia and obesity (Huszar et al., 1997; Vaisse et al., 1998; Yeo et al., 1998). αMSH’s key role was similarly proven when POMC deficiency in both mice and humans was also found to cause massive obesity (Krude et al., 1998; Yaswen et al., 1999). Thus, as with leptin and its receptor, the roles played by αMSH and MC4R are highly conserved.
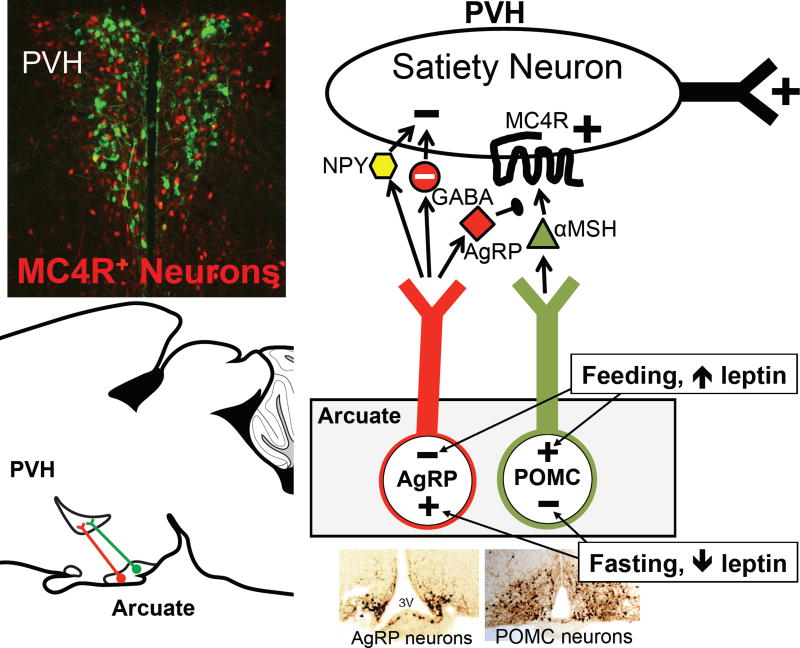
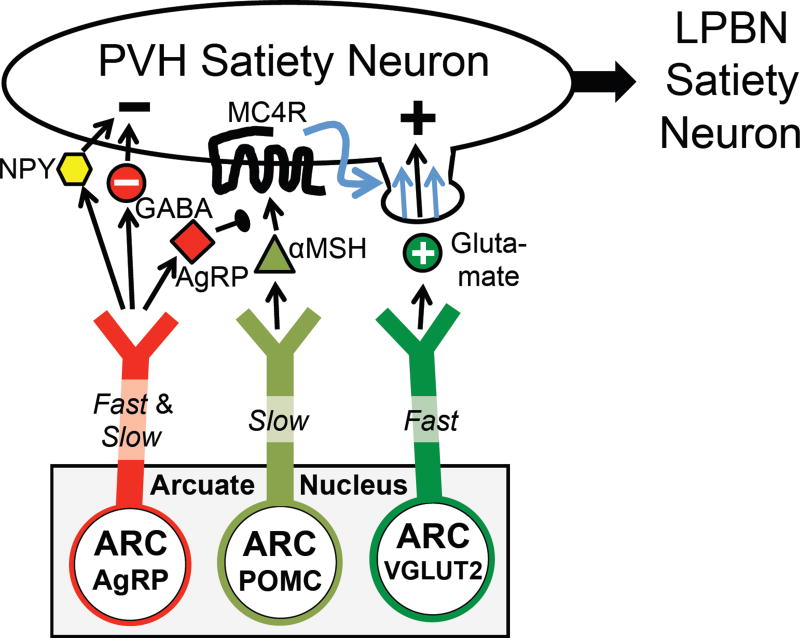
In a dramatic twist, which we now know to be of great importance, a homologue of agouti was discovered – agouti-related peptide (AgRP) (Graham et al., 1997; Ollmann et al., 1997; Shutter et al., 1997). AgRP is expressed only by neurons in the arcuate nucleus, and these neurons are distinct from ARCPOMC neurons (Figure 7). AgRP antagonizes MC3R and MC4R, and causes marked hyperphagia and obesity when it is transgenically overexpressed or centrally injected (Graham et al., 1997; Ollmann et al., 1997; Rossi et al., 1998). In addition to the MC3/4R antagonist, ARCAgRP neurons also release two inhibitory transmitters, GABA and NPY (Cowley et al., 2001; Hahn et al., 1998). The latter is of interest as NPY potently stimulates feeding when injected centrally (Allen et al., 1985; Levine and Morley, 1984; Stanley and Leibowitz, 1984). The importance of ARCAgRP neurons became clear when their genetic ablation was found to profoundly reduce feeding (Gropp et al., 2005; Luquet et al., 2005). Finally, as befits two neurons “destined” to regulate hunger/satiety, both fasting and leptin deficiency (which mimics fasting) coordinately activate ARCAgRP neurons and inhibit ARCPOMC neurons (Figure 7) (Hahn et al., 1998; Mizuno et al., 1998; Ollmann et al., 1997; Schwartz et al., 1997; Shutter et al., 1997). In total, the above-mentioned studies established the importance of ARCAgRP neurons, ARCPOMC neurons, and MC4Rs.
Figure 7. The ARC to PVH satiety circuit.
See text for details.
Upper left insert – PVH neurons. MC4R–expressing neurons (red) were identified by crossing Mc4r-2a–Cre mice with lox-tdTomato reporter mice. The section was counterstained with an antibody against oxytocin (green). Note that oxytocin neurons do not express MC4Rs and that Mc4r–expressing neurons are a minor yet functionally important subset of all PVH neurons.
A key question involves the downstream site(s) mediating melanocortin regulation of energy balance. The paraventricular hypothalamic nucleus (PVH) is a region of particular interest. PVH-directed lesions (Gold et al., 1977; Leibowitz et al., 1981) and haploinsufficiency of SIM1, a transcription factor required for PVH development, cause obesity (Holder et al., 2000; Michaud et al., 2001). Also, PVH neurons express abundant MC4Rs (Kishi et al., 2003; Liu et al., 2003; Mountjoy et al., 1994), they receive strong input from ARCAgRP and ARCPOMC neurons (Bagnol et al., 1999; Cowley et al., 1999), and injection of MC3/4R ligands into the PVH affects feeding (Cowley et al., 1999; Giraudo et al., 1998; Kask and Schioth, 2000; Skibicka and Grill, 2009). However, MC4Rs are expressed broadly in the brain, ARCAgRP and ARCPOMC neurons project to additional sites, and injection of MC3/4R agonists into sites other than the PVH also affects feeding (Skibicka and Grill, 2009). Thus, the specific role of MC4Rs on PVH neurons was unclear. To address this, Mc4rlox/lox and Mc4rloxTB/loxTB mice were generated to, respectively, delete and test necessity or re-express and test sufficiency of MC4Rs (Balthasar et al., 2005; Shah et al., 2014). MC4R expression was then manipulated in either genetically defined neurons or in discrete anatomical loci by crossing Mc4rlox/lox and Mc4rloxTB/loxTB mice with Sim1-Cre BAC transgenic mice, as well as a panel of neuropeptide/neurotransmitter-specific Cre knockin mice, or by stereotaxically injecting them with adeno-associated virus expressing Cre (AAV-Cre) (Balthasar et al., 2005; Shah et al., 2014). These studies found that MC4Rs on PVH neurons are both necessary and sufficient for regulating hunger/satiety (Figure 7), and that these PVHMC4R satiety neurons are glutamatergic and do not express the following PVH neuropeptides: oxytocin, corticotropin releasing hormone (CRH), vasopressin or dynorphin. While αMSH/MC4R signaling also regulates energy expenditure in addition to hunger, this action is not mediated by MC4R-expressing neurons in the PVH (Balthasar et al., 2005; Garfield et al., 2015). Instead, regulation of energy expenditure is brought about by MC4Rs on cholinergic preganglionic sympathetic neurons in the intermediolateral nucleus of the spinal cord (IML) (Berglund et al., 2014; Rossi et al., 2011), and possibly also by neurons in the anterior hypothalamus and hindbrain (Monge-Roffarello et al., 2014; Skibicka and Grill, 2009). Thus, MC4R-regulated neural circuits controlling hunger/satiety versus energy expenditure diverge. Hunger/satiety is regulated by the PVHMC4R neurons.
3.3 Opto- and Chemogenetics Reveal Structure / Function of ARC→PVH Circuitry
Acute manipulation of ARCAgRP neurons demonstrates their remarkable capacity to drive hunger. Specifically, opto- and chemogenetic activation rapidly (within minutes) increases feeding, indeed to the same high level as that seen in fasted mice (Aponte et al., 2011; Krashes et al., 2011). Importantly, ARCAgRP neuron stimulation also increases reward value of food as assessed by instrumental breakpoint assays, again to the same high level seen with fasting (Atasoy et al., 2012; Krashes et al., 2011). Conversely, opto- and chemogenetic activation of ARCPOMC neurons inhibits feeding, but in contrast to ARCAgRP neurons, it takes many hours for this to occur and the observed effects are small (Aponte et al., 2011; Fenselau et al., 2017; Zhan et al., 2013). Consistent with the PVH being an important target, optogenetic stimulation of ARCAgRP terminals in the PVH increases feeding (Atasoy et al., 2012) – but so too does stimulation of ARCAgRP terminals in the bed nucleus of the stria terminalis (BNST), lateral hypothalamus (LH), paraventricular thalamus (PVT) and medial amygdala (Betley et al., 2013; Padilla et al., 2016). Finally, as assessed by ChR2-assisted circuit mapping (CRACM), ARCAgRP neurons make GABAergic inhibitory synaptic connections with a subset of PVH neurons (Atasoy et al., 2012; Krashes et al., 2014). While ARCPOMC neurons also project to the PVH, similar CRACM studies are not possible because their PVH projections are ineffective in releasing fast-acting transmitters (Atasoy et al., 2014; Fenselau et al., 2017), a necessary precondition for CRACM, and the postsynaptic actions of light/ChR2-evoked αMSH release are difficult to detect. In summary, ARCAgRP neurons induce hunger by inhibiting “satiety” neurons in the PVH (Figure 7); ARCPOMC neurons, via αMSH/MC4R signaling, presumably activate these neurons. Indeed, αMSH has been shown to increase firing of PVH neurons in ex vivo brain slices (Ghamari-Langroudi et al., 2015; Liu et al., 2003).
The PVH is complex, containing many subsets of neurons subserving very different neuroendocrine, autonomic and behavioral functions (Sutton et al., 2016). To determine which subset is targeted by ARCAgRP neurons, CRACM was used to assess connectivity to genetically identified PVH neurons, including neurons marked by a Mc4r2a-Cre knockin allele (Garfield et al., 2015). Because Mc4r mRNA is expressed at low levels and since there are no MC4R antibodies suitable for immunohistochemistry, it has previously been difficult to visualize MC4R expression with cellular resolution. Consequently, the percentage of PVH neurons expressing MC4R has been unknown. Remarkably, as revealed by Mc4r2a-Cre mice, Cre-dependent reporter alleles and viruses, only a fraction of PVH neurons express MC4R (Garfield et al., 2015) – thus MC4R marks a distinct subset (or subsets) of PVH neurons. Consistent with this, while ARCAgRP neurons make GABAergic connections with only a modest percentage of unidentified or SIM1-expressing PVH neurons (Atasoy et al., 2012; Krashes et al., 2014), they connect to the majority of PVHMC4R neurons (83%), to few MC4R-negative PVH neurons (20%) and to few or no CRH- or oxytocin-expressing PVH neurons as visualized using Crhires-Cre and Oxtires-Cre knockin mice (Garfield et al., 2015). Thus, ARCAgRP neurons, and presumably αMSH-releasing ARCPOMC neurons, preferentially target PVHMC4R neurons (Figure 7).
As previously mentioned, opto- and chemogenetic stimulation of ARCAgRP neurons, but not ARCPOMC neurons, rapidly affects feeding within minutes. This rapid control is mediated by GABA and NPY (Atasoy et al., 2012; Krashes et al., 2013). Released AgRP also increases feeding but only after 4 hours of ARCAgRP neuron stimulation (Krashes et al., 2013). The ability of ARCPOMC neurons to inhibit feeding, which is slow in onset (hours), is mediated exclusively by αMSH as it is blocked in agouti-overexpressing Ay obese mice (Aponte et al., 2011). A common feature of these two slow acting factors, AgRP and αMSH, is that they both engage MC4Rs. Given the above findings and the inability of ARCPOMC → PVH projections to release fast-acting transmitters (Atasoy et al., 2014; Fenselau et al., 2017), it is likely that the transmitter profiles of these two neurons accounts for their temporally distinct actions (fast and slow for ARCAgRP neurons and only slow for ARCPOMC neurons).
This then raises two questions. First, is there any fast-acting satiety neuron in the ARC → PVH circuit? And second, why is the effect of ARCPOMC neuron stimulation so delayed and weak (suggesting that it is not sufficient), when the effects of αMSH and MC4R deficiency, on the other hand, are so large (establishing their clear necessity)? This striking contrast suggests that αMSH/MC4R signaling may interact with and hence require another component – perhaps a rapidly acting satiety neuron. Given that fast actions of ARCAgRP neurons are mediated by inhibitory transmitters, if such a fast-acting satiety neuron exists it would presumably release the excitatory transmitter, glutamate. This possibility is of interest as it was recently discovered that PVH neurons, by virtue of their expression of the voltage-gated sodium channel Nav1.7, sum excitatory synaptic inputs over greatly extended timescales, thus magnifying effects of excitatory inputs on neuronal firing (Branco et al., 2016). Importantly, deletion of Nav1.7 in PVH neurons diminishes this summation, decreases firing and causes massive obesity. Thus, glutamatergic input from some unknown source likely plays an important role in activating PVH satiety neurons and in causing satiety.
Related to the above discussion, it was recently shown that a novel group of glutamatergic neurons in the arcuate nucleus, marked by expression of VGLUT2 and oxytocin receptor, rapidly decreases / increases feeding when stimulated / inhibited (Fenselau et al., 2017). These excitatory neurons inhibit feeding via the PVH where they preferentially target and synaptically converge with inhibitory ARCAgRP neurons on downstream PVHMC4R neurons (Figure 8). Indeed, individual PVH neurons receive dual synaptic input from ARCAgRP and ARCGlutamatergic neurons. ARCPOMC neurons presumably also target the same PVHMC4R neurons (Atasoy et al., 2014; Bagnol et al., 1999; Cowley et al., 1999). This is relevant because excitatory transmission across the ARCGlutamatergic → PVHMC4R synapse is strongly, postsynaptically potentiated by αMSH (Fenselau et al., 2017). Thus, αMSH/MC4R signaling appears to cause satiety by two mechanisms: by directly activating PVHMC4R neurons (Ghamari-Langroudi et al., 2015; Liu et al., 2003) and by inducing synaptic plasticity – specifically, by upregulating transmission across the excitatory ARCGlutamatergic → PVHMC4R synapse (Figure 8) (Fenselau et al., 2017). Given that ARCPOMC neuron stimulation takes hours to affect feeding, synaptic plasticity may be the dominant mechanism. Direct activation, if it occurred, should in principle rapidly activate PVHMC4R neurons as it does when aMSH is added ex vivo to brain slices. Of interest, if synaptic plasticity is the primary means by which aMSH activates PVHMC4R neurons and causes satiety, then it would explain why αMSH/MC4R signaling is so necessary on the one hand, and why αMSH release by ARCPOMC neurons is generally so insufficient on the other hand. Stated another way, for endogenously released αMSH to be effective, it requires its “substrate” – namely, ongoing transmission across the ARCGlutamatergic → PVHMC4R synapse. Finally, these novel ARCGlutamatergic neurons may regulate satiety in humans, as well as in mice, since they, unlike most other arcuate neurons, are enriched for mRNA transcripts of genes linked with BMI in human GWAS studies (Campbell et al., 2017).
Figure 8. The complete ARC→ PVH circuit includes ARCVGLUT2 satiety neurons.
Slow (AgRP) and fast (NPY and GABA) mediators of hunger are released by one set of neurons, ARCAgRP neruons. Slow (αMSH) and fast (glutamate) mediators of satiety, on the other hand, are released by two parallel-projecting neurons, ARCPOMC and ARCVGLUT2 neurons (Fenselau et al., 2017). αMSH/MC4R signaling in PVHMC4Rneurons causes satiety by two mechanisms: by directly activating the MC4R–PVH neurons and via synaptic plasticity - upregulating excitatory transmission across the ARCVGLUT2 → PVHMC4R synapse (as indicated by the blue line) (Fenselau et al., 2017). PVHMC4R neurons project to the lateral parabrachial nucleus (LPBN) where they promote satiety (Garfield et al., 2015).
3.4 Structure and Function of the PVHMC4R → LPBN Circuit
Consistent with mediating the appetite-regulating actions of ARCAgRP, ARCPOMC and ARCGlutamatergic neurons, chemogenetic stimulation / inhibition of PVHMC4R neurons decreases / increases feeding, respectively (Garfield et al., 2015). And as was true for ARCAgRP neurons, hunger induced by inhibiting PVHMC4R neurons increases the reward value of food. Energy expenditure, on the other hand, is not affected by PVHMC4R neuron stimulation (Garfield et al., 2015) – which further demonstrates the divergence of MC4R-regulated pathways controlling satiety versus energy expenditure (Balthasar et al., 2005). Additional neurons capable of promoting satiety may reside in the PVH, as hyperphagia seen with PVHMC4R neuron inhibition, while marked, may not be as great as that seen following inhibition of most PVH neurons using Sim1-Cre mice (Atasoy et al., 2012; Garfield et al., 2015; Stachniak et al., 2014). Sim1, unlike MC4R, is expressed by most PVH neurons.
PVHMC4R neurons project heavily to a number of sites including the central region of the lateral parabrachial nucleus (LPBN), the dorsal vagal complex (NTS/DMV), and the median eminence. The LPBN-projecting neurons are a discrete subset. As revealed by terminal-specific TVA-only rabies mapping (Betley et al., 2013), they do not send collaterals to the other above-mentioned sites (Garfield et al., 2015). The LPBN-projecting PVHMC4R neurons are responsible for causing satiety as optogenetic stimulation of ChR2-expressing PVHMC4R terminals in the LPBN, and not in other sites, reduces feeding (Garfield et al., 2015). As per CRACM studies, they form excitatory synapses on neurons in the central LPBN, which are themselves likely to be glutamatergic (Garfield et al., 2015; Shah et al., 2014). Importantly, they do not connect with any of the CGRP-expressing neurons in the external LPBN. As previously mentioned, LPBNCGRP neurons receive interoceptive information from the viscera, promote satiation and illness-induced anorexia, and are aversive when activated. Consistent with a role in mediating satiety (which presumably is not aversive), activation of the PVHMC4R → LPBN projections is rewarding as assessed using an in vivo optogenetic stimulation real-time place-preference assay (Garfield et al., 2015). Notably, this functional aspect of the circuit is state-dependent in that activation is rewarding when mice are hungry, but not when they are sated. Given that PVHMC4R neurons are glutamatergic, the LPBN must harbor satiety neurons that are activated by the PVHMC4R satiety neurons. Consistent with this, chemogenetic inhibition of glutamatergic neurons in and around the central LPBN markedly increases feeding (Garfield et al., 2015). Thus, ARCAgRP, ARCPOMC and ARCGlutamatergic neurons converge on PVHMC4R neurons which then, via excitatory projections, affect hunger/satiety by regulating LPBN satiety neurons.
3.5 Hormonal and Neuronal Regulation of the Arcuate Hunger/Satiety System
Hunger-promoting ARCAgRP neurons are inhibited by the fed state and activated by the fasted state (Hahn et al., 1998), while satiety-promoting ARCPOMC and ARCGlutamatergic neurons, which are synaptically downstream of inhibitory ARCAgRP neurons, respond in an opposite fashion (Atasoy et al., 2012; Fenselau et al., 2017; Mizuno et al., 1998). The precise means by which the fed / fasted state accomplishes this regulation is not entirely understood but clearly involves leptin, likely also ghrelin, and possibly insulin and other hormones and metabolites (Flak and Myers, 2016; Morton et al., 2014; Muller et al., 2015). The ghrelin receptor (GHSR) is expressed by ARCAgRP neurons (Zigman et al., 2006) while the receptor for leptin (LEPR) is expressed by subsets of both ARCPOMC and ARCAgRP neurons (Flak and Myers, 2016; Williams et al., 2010). Ghrelin excites ARCAgRP neurons (Cowley et al., 2003), and this mediates the hyperphagic effects of ghrelin administration (Chen et al., 2004; Luquet et al., 2007; Wang et al., 2014). However, leptin regulation of ARCPOMC and ARCAgRP neurons, as well as energy balance, is more complex. It is mediated, in part, by direct actions; leptin activates and inhibits subsets of ARCPOMC and ARCAgRP neurons, respectively (Cowley et al., 2001; Elias et al., 1999; Takahashi and Cone, 2005; van den Top et al., 2004). However, these direct actions account for only a fraction of leptin’s ability to regulate energy balance, as genetic deletion of LEPRs from ARCPOMC and ARCAgRP neurons produces smaller than expected effects on food intake and body weight (Balthasar et al., 2004; van de Wall et al., 2008). Recall that mice lacking LEPRs everywhere are massively obese. Deletion of LEPRs from GABAergic, Nxk2.1-expressing or NOS1-expressing neurons, on the other hand, causes marked hyperphagia and obesity (Leshan et al., 2012; Ring and Zeltser, 2010; Vong et al., 2011). Putting these findings together, the energy balance-regulating effects of leptin are mediated predominantly by GABAergic neurons in the hypothalamus. It is speculated that local LEPR-expressing GABAergic neurons in the arcuate provide “indirect” regulation of ARCPOMC neurons, and probably also ARCAgRP neurons, and that this indirect pathway is an important means by which leptin regulates energy balance (Vong et al., 2011). Indeed, as per CRACM studies, LEPR-expressing arcuate neurons provide strong GABAergic input to both ARCPOMC and ARCAgRP neurons (Garfield et al., 2016). A fraction of this local input to ARCPOMC neurons comes from GABAergic LEPR-expressing ARCAgRP neurons (Atasoy et al., 2012; Cowley et al., 2001). In contrast, all local GABAergic input to ARCAgR neurons comes from a novel source as ARCAgRP neurons do not receive synaptic input from either ARCAgRP or ARCPOMC neurons (Atasoy et al., 2012). One or more of the recently discovered arcuate LEPR-expressing GABAergic neurons, for example the subsets marked by expression of Trh/Cxcl12 or Tbx19, are strong candidates to mediate indirect leptin regulation of ARCPOMC and ARCAgRP neurons (Campbell et al., 2017).
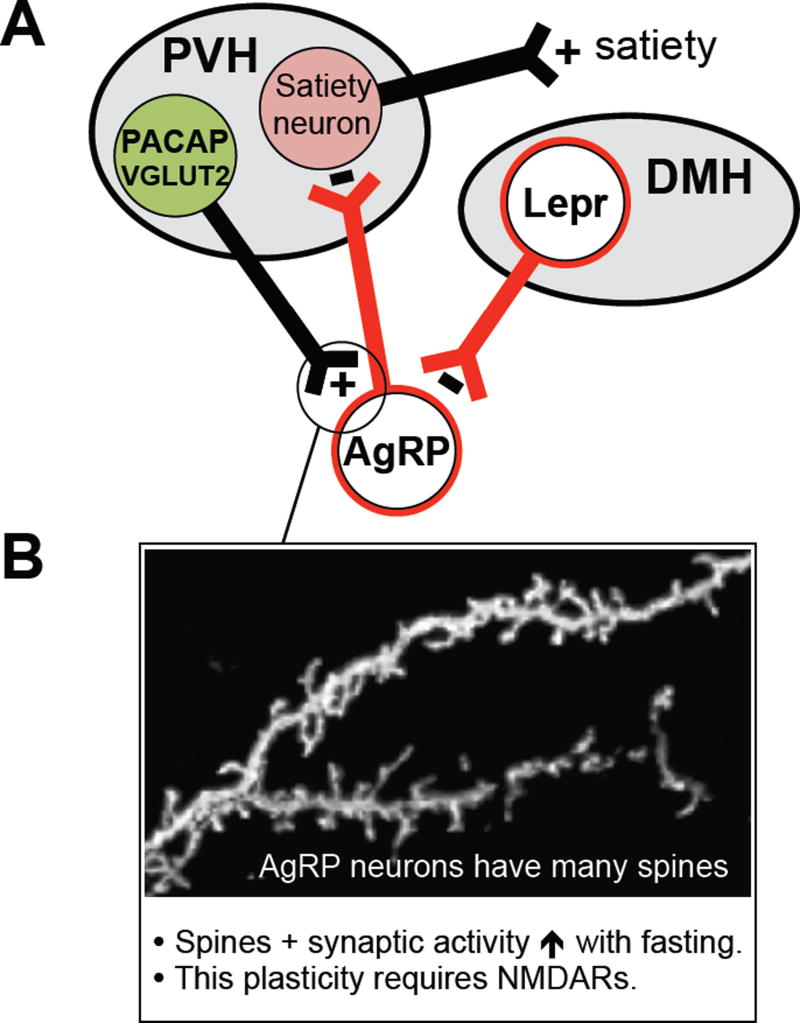
In addition to the local afferents mentioned above, monosynaptic rabies mapping and CRACM studies have established that ARCAgRP neurons also receive strong glutamatergic and GABAergic input from beyond the arcuate (Garfield et al., 2016; Krashes et al., 2014; Wang et al., 2015). Of note, a subset of glutamatergic neurons in the PVH provides very strong excitatory drive to ARCAgRP neurons (Figure 9) (Krashes et al., 2014). Chemogenetic stimulation / inhibition of these excitatory PVH afferents increases / decreases feeding, and the increase in feeding is blocked if ARCAgRP neurons are simultaneously inhibited. Thus, in addition to satiety neurons that lie downstream of and are inhibited by ARCAgRP neurons (i.e. PVHMC4R neurons), the PVH also contains hunger-promoting, excitatory neurons that target ARCAgRP neurons. This reciprocal PVH excitatory → ARCAgRP inhibitory → PVH satiety circuit (Figure 9) highlights complexity of hypothalamic connections and the need for cell-specific techniques to elucidate circuit-based mechanisms. Excitatory afferent control of ARCAgRP neurons is likely meaningful, as synaptic plasticity of excitatory synapses on ARCAgRP neurons is an important control point (Figure 9). Fasting, ghrelin and low leptin levels increase excitatory synapses, dendritic spines and excitatory synaptic activity in ARCAgRP neurons (Liu et al., 2012; Pinto et al., 2004; Yang et al., 2011). Furthermore, this fasting-induced plasticity, which requires NMDA receptors on ARCAgRP neurons, contributes importantly to fasting-induced activation of ARCAgRP neurons (Kong et al., 2016; Liu et al., 2012). Of interest, this fasting-induced plasticity is mediated by a signal transduction pathway that involves AMP-activated protein kinase and p21-activated kinase (Kong et al., 2016).
Figure 9. Long range afferent regulation of AgRP neurons.
(A) Strong excitatory afferents come from the PVH and drive hunger via a reciprocal PVH → ARC — | PVH → satiety circuit (Krashes et al., 2014). Strong inhibition comes from LEPR-expressing neurons in the DMH, and these afferents promote rapid food cue-induced regulation of AgRP neurons (Garfield et al., 2016). The excitatory afferents may also promote rapid, food cue-induced regulation – although this has yet to be tested.
(B) AgRP neurons have many dendritic spines and their excitatory synapses are very plastic. Fasting markedly increases spine number and synaptic activity, and these fasting-induced plasticity responses, which require NMDARs on AgRP neurons, contribute to fasting activation of AgRP neurons (Liu et al., 2012). Photo of dendritic spines courtesy of Dong Kong.
ARCAgRP neurons also receive strong long-range inhibitory drive, including from LEPR-expressing neurons in the dorsomedial nucleus of the hypothalamus (DMH) (Figure 9), which are also marked by dynorphin (Garfield et al., 2016). Remarkably, optogenetic activation of these inhibitory inputs rapidly decreases feeding – even after it has already commenced. Chemogenetic inhibition of these afferents in sated mice, on the other hand, is without apparent effect on feeding – suggesting that these afferents are not a source of tonic, satiety-related feedback inhibition of ARCAgRP neurons. Instead, as revealed by GCaMP6 fiber photometry recordings of neuronal calcium activity in vivo, these DMHLEPR GABAergic afferents are rapidly activated by environmental cues that anticipate ingestion of food (Garfield et al., 2016). As will be discussed later, they likely play an important role in mediating cue-related anticipatory feedforward inhibition of ARCAgRP neurons (Betley et al., 2015; Chen et al., 2015; Mandelblat-Cerf et al., 2015). Finally, it was recently shown that cholinergic neurons in the forebrain suppress appetite (Herman et al., 2016). Strikingly, impairment of their function causes marked hyperphagia and obesity. The ability of these cholinergic neurons to control appetite appears to be mediated, at least in part, by projections to the arcuate, where released acetylcholine (Ach) may engage AChRs on ARCPOMC neurons and/or possibly ARCGlutamatergic neurons (Herman et al., 2016; Mineur et al., 2011). The function of such forebrain cholinergic regulation is not presently understood. Thus, in addition to being targeted by circulating hormones, ARC neurons are also strongly controlled by neuronal afferents. As will be discussed below, this latter neuronal regulation must mediate rapid, cue-induced feedforward control of ARC neurons.
3.6. Feed-forward control of hypothalamic activity: external cues regarding mealtimes and food availability regulate feeding to prevent future homeostatic perturbations
The findings described in the previous sections often use homeostatic challenges such as 24-hr acute food restriction, which drive large changes in plasma levels of leptin and ghrelin. In contrast, many common forms of feeding occur in the absence of major deviations in energy balance, such as ‘spontaneous feeding’ (e.g., initiation of feeding during ad libitum access to food; (Watts, 2012)). In this section, we consider the implications of recent studies showing that the activity of hypothalamic cell types whose roles have been considered ‘reactive’ and ‘homeostatic’ is profoundly modulated both prior to and immediately following ingestion of food or water.
AgRP neurons gradually increase their activity across hours (i) throughout the light cycle in ad libitum fed mice (Mandelblat-Cerf et al., 2015), (ii) in food-entrained mice prior to scheduled feeding (Tan et al., 2014), and (iii) during acute fasting (Betley et al., 2015; Mandelblat-Cerf et al., 2015). These findings are consistent with a longstanding model of AgRP neurons as sensors of slow signals of energy balance that, in turn, coordinate counter-regulatory actions to gradually restore energy homeostasis. Recently, this ‘feedback-dominateD' model of AgRP function has been called into question in light of surprising findings involving extremely rapid changes in AgRP neuron activity. In food-restricted mice, the sudden appearance of food or learned external sensory cues predicting upcoming food consumption causes a rapid (~seconds) drop in the activity of most AgRP neurons (Betley et al., 2015; Chen et al., 2015; Mandelblat-Cerf et al., 2015), beginning prior to food ingestion. This response to food-predicting cues scaled with the caloric content of the offered food in food-restricted mice (Chen et al., 2015), and was largely absent in fed mice. One ‘feedforwarD' driver of these rapid drops is a population of DMHLEPR GABAergic neurons that synapse onto AgRP neurons. These neurons were found to rapidly increase their activity following presentation of food, beginning prior to food consumption (Garfield et al., 2016) and with response magnitudes proportional to the caloric content of the associated food. In contrast to the rapid drop in AgRP neurons, POMC neurons, which are known to be suppressed by AgRP input (Atasoy et al., 2012; Cowley et al., 2001), showed rapid increases in activity upon presentation of a food cue within seconds (Chen et al., 2015; Mandelblat-Cerf et al., 2015). These rapid increases are likely driven, in part, by food cue-evoked reduction in activity of inhibitory AgRP inputs to POMC neurons. In addition, long-range inputs such as those from cholinergic neurons in the diagonal band of Broca (Herman et al., 2016) may also contribute to cue-evoked increases in POMC neuron activity, as other basal forebrain cholinergic neurons were shown to be rapidly activated by detection of unexpected food cues (Parikh et al., 2007).
What might be the function of this rapid, feedforward regulation of AgRP neurons? Many hypotheses have been put forward (Betley et al., 2015; Chen and Knight, 2016; Chen et al., 2015; Mandelblat-Cerf et al., 2015; Seeley and Berridge, 2015; Sternson and Eiselt, 2017). Below, we review evidence that the brain does not typically drive foraging in response to hormonal ‘feedback’ signals of acute energy deficit. Rather, we suggest that ‘feedforwarD' signals such as sensory food cues cause a decrease in AgRP neuron activity proportional to the anticipated amount of food to be consumed, contributing to a rapid decrease in residual hunger drive which helps prevent overconsumption.
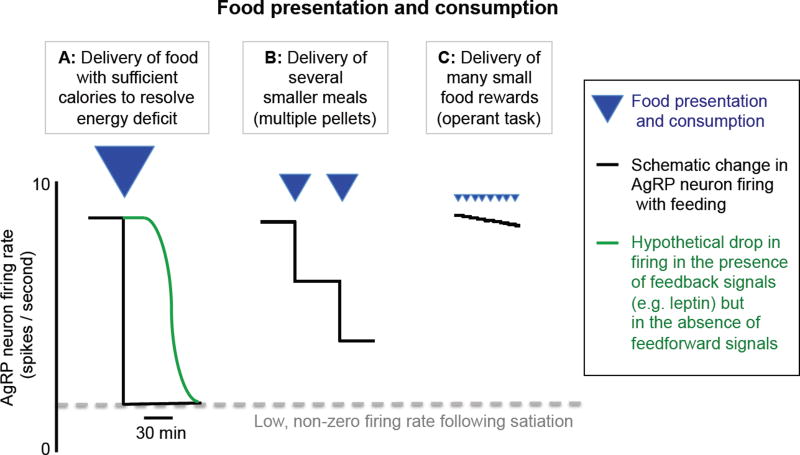
This concept is illustrated in Figure 10: suppose that a food-restricted mouse is presented with and consumes a large quantity of food (Figure 10A). This results in changes in leptin, ghrelin, and other hormones that ultimately lead to a new, lower level of AgRP neuron spiking activity. If digestion and hormone changes were instantaneous, they could immediately reset AgRP neuron firing to this new level, thus properly adjusting subsequent hunger drive and short-term decisions regarding food consumption. However, these digestive processes and feedback signals are too slow (minutes to hours) to achieve this goal. Recent findings show a far more rapid food-induced drop in of AgRP activity within seconds which then remains at a relatively stable level (Betley et al., 2015; Chen et al., 2015; Mandelblat-Cerf et al., 2015). We and others suggest that this new plateau level of firing matches the level driven (at much later times, long after meal termination) by slower post-ingestive hormonal feedback signals (Figure 10A, blue line). In this way, the magnitude of the rapid drop in AgRP neuron activity following cues predicting upcoming food consumption may reflect the expected reduction in energy deficit once this immediately available food is consumed, digested, and absorbed. Consistent with this view, when food consumption instead consists of several small meals, each anticipated to be less than that required to restore energy homeostasis (Figure 10B,C), AgRP firing undergoes successive drops to lower firing levels (Betley et al., 2015; Chen et al., 2015; Livneh et al., 2017; Sternson and Eiselt, 2017). Consumption of even smaller quantities of food, each replenishing only a tiny fraction of the total energy deficit (as is the case during many operant behavioral tasks) results in AgRP activity remaining elevated (Figure 10C), with only slight drops following food detection (Livneh et al., 2017). In all cases, the residual AgRP firing immediately following consumption would likely drive subsequent foraging, albeit with reduced vigor (Aponte et al., 2011). In contrast, if the quantity of newly available food exceeds homeostatic deficits, AgRP neuron firing would quickly drop to a low but non-zero level (Mandelblat-Cerf et al., 2015) to prevent ‘overshoots’ in calorie intake (Carpenter, 2004) due to overconsumption and additional foraging. This notion may help explain the somewhat paradoxical finding that direct or indirect artificial suppression of AgRP activity (Garfield et al., 2016; Krashes et al., 2011), including soon after meal onset, can actually suppress feeding. Artificial suppression likely drives AgRP neuron firing to a level below that attained following anticipatory ingestive and pre-ingestive drops in firing, thereby preventing not only overconsumption but any immediate food consumption.
Figure 10. Rapid drops in AgRP neuron activity during food presentation.
Presentation of a quantity of food sufficient to replenish energy deficit causes a large drop in AgRP neuron activity within seconds (A, black line), albeit not to quiescent levels. If ‘feedforwarD' multisensory signals predicting the consequences of consumption of this food on energy deficit were not available, the drop in firing would only occur over tens of minutes or more (A, blue line), due to systemic feedback (e.g. from increased leptin, etc.). When mice are presented with smaller quantities of food that will only partially restore energy balance, the rapid drop in AgRP activity is proportionately smaller (B). In the limit of very small food rewards (C), AgRP firing should remain elevated and roughly constant across many food presentations.
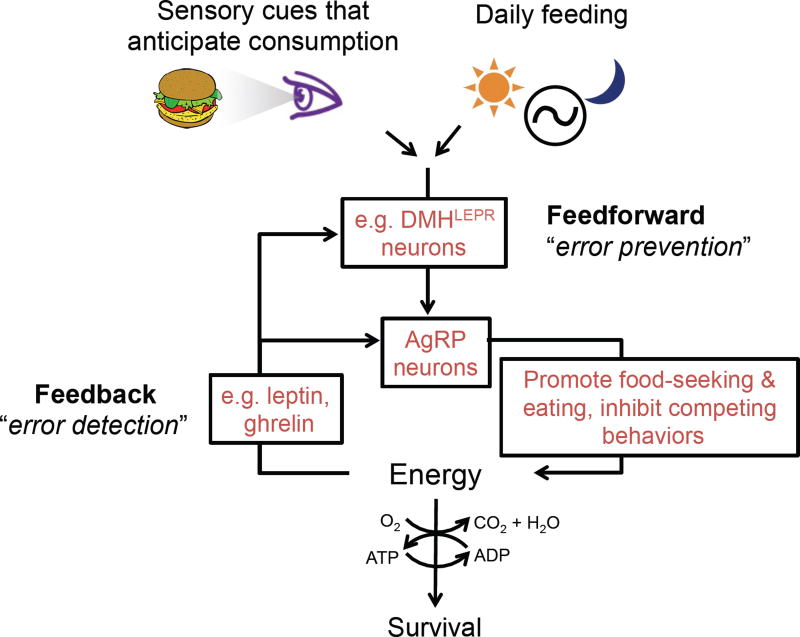
Feedback signals may not only act on AgRP neurons, but also by adjusting the gain of feedforward inputs to AgRP neurons (Figure 11), such as DMHLEPR GABAergic neurons (Garfield et al., 2016). Indeed, leptin was found to depolarize DMHLEPR GABAergic neurons, potentially elevating tonic inhibition of AgRP neurons while reducing the capacity for food cues to drive additional phasic inhibition of these neurons (Garfield et al., 2016). Similarly, leptin and ghrelin may adjust the gain on excitatory feedforward inputs to AgRP neurons via effects on synaptic plasticity (Liu et al., 2012; Yang et al., 2011).
Figure 11. A more detailed model of feedback and feedforward control.
Recent studies implicate AgRP neurons as a key ‘intervening variable’ between estimation of current and future caloric needs and orchestration of diverse food seeking behaviors (compare to Figure 2). Estimates of current energy deficit and upcoming energy balance following consumption are controlled by feedback signals to AgRP neurons (e.g. leptin, ghrelin), as well as feedforward signals (e.g. DMHLepR neuron inputs to AgRP neurons) and direct feedback modulation of these feedforward inputs.
A complementary role for rapid changes in AgRP neuron activity was proposed by Sternson and colleagues (Betley et al., 2015; Sternson and Eiselt, 2017). These authors found that AgRP neuron stimulation was mildly aversive, and suggest that the drop in AgRP activity may provide rapid relief from this aversive state and act as a teaching signal to reinforce learning of cues predicting food (but see (Chen et al., 2016) for an alternative interpretation). Thus, in addition to guiding decisions regarding immediate food-seeking and consumption, this rapid estimate of upcoming food consumption may also increase the value ascribed to novel food-associated cues, allowing earlier predictions of the availability of that food. This, in turn, may facilitate pre-ingestive drops in AgRP neuron activity during subsequent presentation of these same food cues.
The framework of feedforward and feedback signals may help clarify the regulation of AgRP neuron activity across many types of feeding, including spontaneous and daily scheduled feeding (potentially driven by feedforward input from predictive circadian oscillators), feeding following prolonged food restriction (driven by feedback signals indicating negative energy balance), and feeding driven by the sudden appearance of a highly palatable, calorically-dense food source that could help defend against future energy deficit. In this framework, AgRP neurons act less as high-fidelity primary interoceptive sensors of food hormones, and more as high-level command neurons that coordinate diverse aspects of the hunger drive to maintain relatively constant current and future energy balance.
The ubiquitous nature of rapid, bidirectional changes in “homeostatic” neuron activity
The surprisingly fast timescale on which learned pre-ingestive and ingestion-related cues can influence hypothalamic AgRP neurons may be the rule and not the exception. Indeed, the presence of both feedback signals and feedforward predictive signals is a hallmark of adaptive control systems, which have previously been suggested to be important in the control of feeding circuits (Carpenter, 2004; Somjen, 1992; Woods and Ramsay, 2007) and in the control of other aspects of body homeostasis (e.g. in the cardiovascular system (Dampney, 2016; Korner, 1995)). One useful comparison is between AgRP neurons and other hypothalamic neurons involved in regulation of blood osmolarity, including Nos1+ neurons in the subfornical organ (SFONos1) that drive thirst, as well as supraoptic nucleus neuroendocrine neurons that project to the posterior pituitary and release vasopressin (SONVP neurons) to inhibit water excretion by the kidney (Bourque, 2008). As with AgRP neurons, these SFONos1 and SONVP neurons receive direct systemic feedback (in this case, feedback regarding blood osmolarity via osmosensors (Prager-Khoutorsky and Bourque, 2015)). Early studies showed that plasma vasopressin levels dropped in thirsty animals and humans within minutes of drinking onset, even prior to systemic osmotic feedback, thus potentially preventing undershoots in plasma osmolarity upon absorption of water (reviewed in (Stricker and Hoffmann, 2007)).
Recently, we performed electrophysiology and calcium recordings in identified SONVP neurons, and observed a drop in activity within seconds of presentation of a learned water cue, and a further drop immediately following drinking onset (Mandelblat-Cerf et al., 2017). Importantly, as with AgRP neurons, the drop in SONVP neuron activity stabilized at this lower level of tonic activity, prior to systemic feedback, and showed no additional change in level in the minutes following return of plasma osmolality to baseline levels (Mandelblat-Cerf et al., 2017). Assessing the role of these changes in SONVP neuron activity is comparatively simple, as these pituitary-projecting neurons regulate body physiology and are unlikely to be co-opted, for example, for reinforcement learning, as they send very few axon collaterals to other brain regions. Similar to SONVP neurons, SFONos1 excitatory neurons (which project to the supraoptic nucleus and paraventricular hypothalamus (Zimmerman et al., 2016) where they likely contact SONVP neurons) also display rapid post-ingestive (but not pre-ingestive) drops in activity following cues signaling water availability (Zimmerman et al., 2016). These data illustrate that SONVP and SFONos1 neurons can exhibit rapid modulation of activity by learned and innate visual, olfactory, thermosensory, gustatory and other cues in a manner that appears to pre-emptively adjust levels of activity to those predicted to occur when post-ingestive systemic feedback signals reach steady-state levels.
Feedforward control is also likely to dominate at longer timescales. In particular, circadian rhythms are likely to anticipate hunger as well as thirst, by pre-emptively modulating appropriate hypothalamic populations (Gizowski et al., 2016; Mandelblat-Cerf et al., 2015; Tan et al., 2014). Together, these data support longstanding hypotheses suggesting that feedforward control may be a ubiquitous feature of “homeostatic” processes (Carpenter, 2004; Dampney, 2016; Somjen, 1992; Woods and Ramsay, 2007). Notably, the recent measurements of activity in defined hypothalamic populations refine these early hypotheses by demonstrating that feedforward signals do not work in parallel and/or apart from homeostatic neurons, but instead work through homeostatic neurons. Carpenter (Carpenter, 2004) goes so far as to suggest that “the whole of the brain may be regarded as a way of helping the hypothalamus to do a better job, by making better predictions of what is going to happen next, and what is likely to follow from one course of action rather than another.”
Section 4: Behavioral Mechanisms and Role of Higher Cognitive Structures in Hunger
Section 4.1: Gating of ‘cognitive’ areas underlying sensory attention, perception, and action by caloric deficiency and the ARC: case studies involving selective cortical responses to food cues
The concept that internal need states (e.g. caloric restriction) likely interact with external cues (e.g. signals of food availability) at multiple levels of neural processing has long been recognized as essential to understanding the genesis of specific motivated behaviors (see (Bindra, 1974; Toates, 1986); for detailed models, see (Carpenter, 2004)). As reviewed below, sensory processing of food cues in the environment may already be selectively attenuated in states of satiety vs. hunger, thereby contributing to the reduction in food-seeking behaviors.
While a wide range of sensory cues and contexts have been used to study the relationship between hunger state and reactions to the sensory environment, we focus here on studies of neural responses to visual food cues in animals and humans. Such studies have many advantages, including the ability to assess neural responses to the identical sensory stimulus in both hungry and sated conditions. As with food advertisements in modern societies, the visual cues predicting food in these studies are often learned: the sight of the Mars Bar wrapper is only attention-grabbing and appealing because one has learned that there is delicious chocolate inside. Parallel studies in humans and animal models illustrate broader efforts to understand how energy deficit can influence ‘cognitive’ cortical and limbic neural circuits to selectively bias attention, sensory perception, memory recall, and actions towards food cues.
Human neuroimaging studies have helped address the question of where in the brain selective processing of food cues might emerge, by capitalizing on the broad coverage of cortical and limbic brain areas and the ease of recordings across slowly changing motivational states afforded, for example, by functional magnetic resonance imaging. These studies reveal that, in hungry human subjects, visual food-associated cues drive larger neural responses than non-food cues in temporal lobe association cortical areas including parahippocampal gyrus and insular cortex (InsCtx), as well as in the basolateral amygdala (BLA) (Cornier et al., 2009; Frank et al., 2013; Huerta et al., 2014; LaBar et al., 2001; Siep et al., 2009). Interestingly, such food-cue-response biases do not exist in early visual cortex, suggesting that they may emerge in higher cortical areas that represent objects (e.g. candy wrappers) rather than low-level sensory features (e.g. luminance). Strikingly, in healthy human subjects, food-cue-response biases are abolished when subjects are imaged a second time following meal consumption (Cornier et al., 2009; Frank et al., 2013; Huerta et al., 2014; LaBar et al., 2001; Siep et al., 2009). These findings are of significant clinical relevance given the obesogenic effects of marketing advertisements involving branding of learned cues associated with high-calorie foods. Further, individuals with certain eating disorders, obesity, or elevated propensity for weight gain show InsCtx food-cue responses that are less attenuated by meal consumption than responses in control subjects (Aotani et al., 2012; Cornier et al., 2009; Jastreboff et al., 2014; Santel et al., 2006; Stoeckel et al., 2008).
Studies in humans have also provided initial clues as to how hunger may selectively enhance cortical and limbic sensitivity to food cues. For example, gastric banding and gastric bypass both help reverse the aberrant neural responses to food cues observed in obese subjects, potentially due to the associated changes in gut-released hormone levels (Bruce et al., 2012; Ochner et al., 2012). As well, circulating levels of the gut hormone ghrelin correlate with food-cue responses in a network of reward-related brain areas including BLA and InsCtx (Kroemer et al., 2013; Sun et al., 2014). Moreover, systemic injection of ghrelin increases subjective hunger ratings as well as visual food-cue response magnitude and food cue neural response biases in human BLA and InsCtx (Malik et al., 2008). As discussed above, AgRP neurons are a key neural target for actions of ghrelin (Betley et al., 2015; Chen et al., 2015; Nakazato et al., 2001). Thus, studies on ghrelin’s effects on food-cue responses in human cortex provide indirect evidence that AgRP neuron activity may contribute to hunger-dependent cortical processing of food cues.
We recently tested this hypothesis directly, by establishing a chronic cellular-resolution imaging platform in behaving mice for evaluating hunger-dependent processing of learned food cues (i) in the same individual cortical neurons across slowly changing states of natural hunger and satiety, and (ii) during selective chemogenetic activation of AgRP neurons (Livneh et al., 2017). Food-restricted mice were trained to lick following presentation of a visual food cue in order to obtain a highly palatable food reward, and to avoid licking to other cues to avoid delivery of quinine, an aversive tastant (Burgess et al., 2016; Livneh et al., 2017). Selective behavioral responses to the food cue were absent following satiation (Burgess et al., 2016; Livneh et al., 2017). Strikingly, this selective responding to the food cue could be fully restored by chemogenetic activation of AgRP neurons (Livneh et al., 2017). As in bulk imaging studies in humans, food cue neuronal response biases in mice were not present in early visual cortex but emerged in temporal association cortex, and were abolished following satiation (Burgess et al., 2016). Further, food cue biases were even stronger in lateral amygdala neurons, which project back to association cortex but not to early sensory cortex (Burgess et al., 2016), suggesting a possible mechanism driving hunger-dependent biases in association cortical responses and in selective attention.
In humans, the most consistent and profound modulation of food cue responses by hunger and satiety occurs in InsCtx (see above). Studies in rodents found that InsCtx neurons respond to learned cues in hungry rodents (Gardner and Fontanini, 2014; Kusumoto-Yoshida et al., 2015; Samuelsen et al., 2012) and that InsCtx is required for behavioral responses involving retrieval of the incentive value of foods and food-associated cues (Balleine and Dickinson, 2000; Kusumoto-Yoshida et al., 2015). Previous recordings of InsCtx sensory responses at cellular resolution, while highly informative, were limited to relatively brief electrophysiologic recordings (rodents: e.g. (de Araujo et al., 2006; Fontanini and Katz, 2009; Kusumoto-Yoshida et al., 2015); non-human primates: (Rolls, 2007; Yaxley et al., 1988)), or to imaging during acute recordings in anesthetized mice (Chen et al., 2011). To assess effects of hunger on food cue responses in InsCtx across slowly changing states of hunger and satiety, we developed a method for imaging activity in the same InsCtx neurons across hours and days in behaving mice (Livneh et al., 2017). We found that, in hungry mice, InsCtx neurons showed a strong response bias towards learned food cues. Food-cue responses were abolished by satiation but was restored, in sated mice, by selective activation of AgRP neurons. Genetic circuit mapping techniques revealed a major pathway from AgRP neurons to InsCtx via the paraventricular thalamus (PVT) and BLA (Livneh et al., 2017). In particular, AgRP neurons preferentially synapse onto and inhibit the subset of PVT neurons that project to the BLA. PVT has been implicated in gating of motivationally salient sensory cues (Do-Monte et al., 2015; Haight and Flagel, 2014; James and Dayas, 2013). Further, stimulation of inhibitory AgRP axon terminals in PVT (Betley et al., 2013), as well as pharmacologic inhibition of all PVT neurons (Stratford and Wirtshafter, 2013) and selective chemogenetic inhibition of PVT projections to BLA (Livneh et al., 2017), all drive increased homecage food intake.
The dialogue between BLA and InsCtx (and nearby orbitofrontal cortex) is believed to be critical for encoding and retrieval of the value of rewards and reward-predicting cues (Clark et al., 2012; Grossman et al., 2008; Johnson et al., 2009; Lasseter et al., 2011; Parkes and Balleine, 2013; Samuelsen et al., 2012). Indeed, silencing of specific BLA neurons that project to InsCtx selectively abolished learned visual food cue responses but not taste responses in InsCtx (Livneh et al., 2017), further supporting a specific role for the AgRP→PVT→BLA→InsCtx pathway in biasing sensory processing towards learned food cues (Figure 12). Given the integration of interoceptive, gustatory, visual, and other stimuli in InsCtx and BLA ((Cechetto and Saper, 1987; Chen et al., 2011); for reviews, see (Craig, 2003; Naqvi et al., 2014)), we suggest that these regions are essential for estimating the integrated interoceptive value of ingesting a food associated with a learned or innate predictive sensory cue (Livneh et al., 2017). As such, these two areas are well suited to recruit motor circuitry to drive approach or avoidance of available foods. For example, InsCtx and BLA projections can promote reward seeking via projections to the ventral striatum (Parkes et al., 2015; Reynolds and Zahm, 2005), and InsCtx projections may be particularly relevant when food seeking and ingestion carry both positive and negative consequences (Seif et al., 2013).
Figure 12. A neural pathway illustrating hunger-dependent processing of food-cue sensory processing.
Many studies in humans and rodents implicate the basolateral amygdala (BLA) and insular cortex (InsCtx) in assessing the valence and salience of learned food cues, and addressing the question of expected interoceptive value: how will eating this food make me feel, now and later? If the answer is net positive, then motor circuits are recruited by BLA and InsCtx. This answer is dependent on hunger state (and therefore on tonic input from AgRP neurons, in part via paraventricular thalamus, PVT), and on the salience of learned food cue inputs to BLA (from thalamus and association cortex). The estimation of interoceptive consequences of food consumption in InsCtx is likely shaped by previous experience involving visceral and gustatory input. LP: lateral posterior nucleus of the thalamus. POR: postrhinal cortex. VPL: ventral posterolateral thalamus. VPM: ventral posteromedial thalamus. PBN: parabrachial nucleus. NTS: nucleus of the solitary tract. NAc: nucleus accumbens (Livneh et al., 2017).
The context-dependent decision to engage in food-seeking and feeding vs. other behaviors is likely regulated by the many non-collateralizing sets of AgRP projections. These projections target diverse downstream brain areas (Betley et al., 2013), where some drive feeding while others play roles in suppressing defensive, territorial and other behaviors (Betley et al., 2013; Burnett et al., 2016; Dietrich et al., 2015; Jikomes et al., 2016; Padilla et al., 2017; Padilla et al., 2016). The above findings highlight key features of the hunger circuit: first, AgRP neurons serve as an intervening variable between need states and the driving or suppression of diverse behaviors required to effectively promote food-seeking and feeding in different environmental contexts. As illustrated in Figure 1, the presence of this intervening variable simplifies the linking of needs with effector systems, as opposed to schemes in which many competing need states directly act on the many effector pathways, an inefficient strategy that could promote antagonistic or mutually-exclusive behaviors. Finally, these data support the notion that each AgRP projection may target a brain region involved in one or many broad classes of behaviors, while higher-order projections from each AgRP target region may regulate increasingly specific components of each behavior in increasingly specific contexts. For example, InsCtx silencing impairs feeding, but only when the food is contingent on operant responding to a learned cue (Livneh et al., 2017).
4.2. Context-dependent behavioral effects of AgRP stimulation
The previous section discussed how AgRP neuron stimulation in sated mice not only restores homecage feeding but can also restore engagement in an operant visual discrimination task as well as associated neural responses to food cues in InsCtx. An important question emerges: Does AgRP stimulation itself carry positive or negative valence, and how does this relate to an animal’s motivation to work for food? As mentioned above, Betley and colleagues found that AgRP neuron stimulation is inherently mildly aversive, in that mice will avoid a spatial location that was previously paired with optogenetic stimulation of AgRP neurons (Betley et al., 2015). Further, inhibition of AgRP neurons in food-restricted mice during consumption of food with a specific flavor led to a conditioned flavor preference (Betley et al., 2015), consistent with the aversive nature of states of elevated AgRP neuron activity in the absence of food. Garfield and colleagues subsequently investigated PVHMC4R neurons, which receive strong inhibitory input from AgRP neurons and are thus likely to be suppressed during food-restriction in the absence of food. Stimulation of PVHMC4R neurons drove real-time place preference, but only in fasted mice (Garfield et al., 2015). Thus, the mild negative valence associated with hunger likely results, in part, from AgRP activation and consequent inhibition of positive valence-promoting PVHMC4R neurons.
At the same time, AgRP neuron stimulation may also act by increasing the rewarding nature of food consumption, such that animals work more to obtain a small amount of food. Indeed, breakpoint analyses in progressive ratio feeding experiments show that fasted mice will nose poke many more times for a single food pellet than fed mice, and that chemogenetic stimulation of AgRP neurons in fed mice restores this behavior to levels observed in fasted mice (Krashes et al., 2011), with qualitatively similar findings obtained by inhibiting downstream PVHMC4R neurons (Garfield et al., 2015). Further, while sated mice avoid places associated with AgRP stimulation in the absence of food (Betley et al., 2015), sated mice will actually press a lever for AgRP stimulation when conditioning occurs in the presence of food (Chen et al., 2016). Based on these and other experiments, these authors conclude that artificial stimulation or self-stimulation of AgRP neurons produces a long lasting potentiation in the rewarding properties of food (Chen et al., 2016). The above findings all seem compatible with one another and with aforementioned theories of motivation (Bindra, 1974; Toates, 1986) that argue that food-seeking behavior requires not only a need state but also food availability, including food that is accessible upon performance of a learned task. In the absence of such food availability, AgRP stimulation is net aversive (Betley et al., 2015). If efforts to find food are futile, AgRP stimulation may then drive other repetitive behaviors (Dietrich et al., 2015). Thus, artificial stimulation of AgRP neurons will result in different behaviors depending on the availability of food and food-associated cues, as well as other competing need states and defensive states (Jikomes et al., 2016; Padilla et al., 2016).
Section 5: Future Directions and Important Questions
Recent technical advancements have made it possible to delineate wiring diagrams and assess neural activity in a cell-specific manor. Such approaches are greatly accelerating progress with regards to understanding the neural control of appetite. These efforts, however, have also raised new questions – some of which were previously “unknown unknowns” – for example, the role of rapid, feed-forward regulation of classically-viewed homeostatic hunger neurons. Below, we list important outstanding issues that seem ripe for future investigation.
-
“Labeled lines” in the afferent vagus and their CNS circuits regulating satiation (Section 2).
A major future goal will be to discover additional “labeled lines” of information flow in the afferent vagus, identify or generate Cre/Flp-driver mice that will provide selective access to each, and then to link each labeled line with DMV motor neurons mediating vago-vagal reflexes and with CNS circuits controlling different aspects of behavior.
-
The role of ghrelin (Section 2.4).
Many aspects of ghrelin biology and gain-of-function ghrelin injection studies strongly support an important role for ghrelin in controlling appetite. Loss-of-function studies, on the other hand, have produced confusing negative results. Future studies will need to reconcile these results and clearly define ghrelin’s true role in regulating appetite – which seems likely.
-
ARCAgRP projections to the PVH versus other sites – what’s the purpose / logic with regards to regulation of hunger/satiety (Section 3.3)?
ARCAgRP projections to the PVH play an important role in regulating hunger / satiety. However, optogenetic terminal stimulation studies have also shown that ARCAgRP projections to other sites, the LH, BNST and PVT, can also cause hunger. It is striking that such different downstream sites, with such different connectivity, can drive behavior which at first pass seems similar. Future efforts are required to determine how similar the evoked behaviors are, and to uncover the purpose and wiring diagram “logic” that underlies such diverse downstream effector targets.
What is the role of the lateral hypothalamus (LH) in regulation of appetite? While not covered in this review, the LH receives projections from ARCAgRP and ARCPOMC neurons, and manipulation of neuronal activity in the LH produces effects on consumption. It is unclear, however, if LH neurons specifically control hunger/satiety as do ARCAgRP, ARCPOMC and PVHMC4R neurons, or alternatively, if LH neurons control reward processing and thus consequently, indirectly affect appetite as well as many other drive-related behaviors – one example being thirst. Prevailing evidence would seem to favor the latter. See (Sternson and Eiselt, 2017) for excellent coverage of the contributions of LH to the regulation of feeding-related behaviors.
-
ARC → PVH → LPBN → ? → ? → hunger/satiety (Section 3.4)?
A major target of the ARC → PVH appetite circuit are satiety neurons in the LPBN. Going forward it will be important to identify these LPBN satiety neurons, and their projection sites by which they decrease appetite. Given prior assessments of LPBN projections, these neurons are likely to engage interesting structures, one example being the amygdala, which is involved in valence-encoding.
-
Rapid feed-forward regulation of “homoestatic” ARCAgRP hunger neurons (Section 3.6).
This unexpected discovery in 2015 has prompted a number of hypotheses regarding it function. Establishing its true function(s) is important and will require some method to selectively abolish this rapid regulation. One approach could involve selective inhibition of afferents that mediate this rapid regulation, followed by an assessment of consequences.
-
High-level processing of sensory cues brings about rapid, feed-forward regulation of ARCAgRP hunger neurons (Section 3.6).
This premise, that high-level processing is involved, is based upon the findings that sensory cue-mediated rapid regulation occurs only when animals anticipate, based upon their state or their desire for a palatable food, that consumption will occur. Also, the degree of regulation is strikingly proportional to amount of anticipated consumption. Given that highly processed information reaches and regulates ARCAgRP neurons, which are themselves potent drivers of appetite, it raises the question as to whether classically-viewed abnormalities of cognitive processing, such as anorexia nervosa, or possibly even obesity per se, could involve dysregulation of rapid, feed-forward regulation and that this could contribute to these disorders.
Which other regulators of appetite work upstream of ARCAgRP neurons (Section 3.6)? Given that cues predicting consumption of food unexpectedly regulate ARCAgRP hunger neurons, it begs the question as to what other regulators of appetite work via ARCAgRP neurons, or instead work downstream of or in parallel to ARCAgRP neurons. A few examples worthy of future investigation include circadian regulation of appetite, lights on/lights off regulation of appetite and dehydration-induced anorexia.
-
Maintaining “drive” specificity in higher circuits (Section 4)?
Hunger and thirst, and likely all need-based motivational drives, increase reward value and perception of goals and cues related to goals (i.e. food or water). It is likely that increased reward value and perception induced by each of the different drive states involves similar higher structures and similar processing within these structures. With this premise in mind, a key question becomes, how is specificity for the goal (food versus water) maintained as these higher structures receive and process polysynaptic input from drive-specific neurons (i.e. ARCAgRP neurons for hunger and SFO neurons for thirst)? This, perhaps, may be one of the biggest questions in our field.
Acknowledgments
We thank members of the Lowell and Andermann labs for many stimulating discussions and for providing feedback on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- Allen LG, Kalra PS, Crowley WR, Kalra SP. Comparison of the effects of neuropeptide Y and adrenergic transmitters on LH release and food intake in male rats. Life Sci. 1985;37:617–623. doi: 10.1016/0024-3205(85)90428-x. [DOI] [PubMed] [Google Scholar]
- Aotani D, Ebihara K, Sawamoto N, Kusakabe T, Aizawa-Abe M, Kataoka S, Sakai T, Iogawa H, Ebihara C, Fujikura J, et al. Functional magnetic resonance imaging analysis of food-related brain activity in patients with lipodystrophy undergoing leptin replacement therapy. J Clin Endocrinol Metab. 2012;97:3663–3671. doi: 10.1210/jc.2012-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Li WP, Su HH, Sertel SM, Scheffer LK, Simpson JH, Fetter RD, Sternson SM. A genetically specified connectomics approach applied to long-range feeding regulatory circuits. Nat Neurosci. 2014;17:1830–1839. doi: 10.1038/nn.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, Akil H, Barsh GS, Watson SJ. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J Neurosci. 1999;19:RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. The effect of lesions of the insular cortex on instrumental conditioning: evidence for a role in incentive memory. J Neurosci. 2000;20:8954–8964. doi: 10.1523/JNEUROSCI.20-23-08954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Begg DP, Woods SC. The endocrinology of food intake. Nat Rev Endocrinol. 2013;9:584–597. doi: 10.1038/nrendo.2013.136. [DOI] [PubMed] [Google Scholar]
- Berglund ED, Liu T, Kong X, Sohn JW, Vong L, Deng Z, Lee CE, Lee S, Williams KW, Olson DP, et al. Melanocortin 4 receptors in autonomic neurons regulate thermogenesis and glycemia. Nat Neurosci. 2014;17:911–913. doi: 10.1038/nn.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- Bessesen DH. Regulation of body weight: what is the regulated parameter? Physiol Behav. 2011;104:599–607. doi: 10.1016/j.physbeh.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Xu S, Cao ZF, Gong R, Magnus CJ, Yu Y, Sternson SM. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521:180–185. doi: 10.1038/nature14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindra D. A motivational view of learning, performance, and behavior modification. Psychol Rev. 1974;81:199–213. doi: 10.1037/h0036330. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Effects of cholecystokinin (CCK-8) on two classes of gastroduodenal vagal afferent fibre. J Auton Nerv Syst. 1990;31:191–201. doi: 10.1016/0165-1838(90)90185-l. [DOI] [PubMed] [Google Scholar]
- Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9:519–531. doi: 10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- Branco T, Tozer A, Magnus CJ, Sugino K, Tanaka S, Lee AK, Wood JN, Sternson SM. Near-Perfect Synaptic Integration by Nav1.7 in Hypothalamic Neurons Regulates Body Weight. Cell. 2016;165:1749–1761. doi: 10.1016/j.cell.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJ, Spencer NJ, Costa M, Zagorodnyuk VP. Extrinsic primary afferent signalling in the gut. Nat Rev Gastroenterol Hepatol. 2013;10:286–296. doi: 10.1038/nrgastro.2013.29. [DOI] [PubMed] [Google Scholar]
- Bruce JM, Hancock L, Bruce A, Lepping RJ, Martin L, Lundgren JD, Malley S, Holsen LM, Savage CR. Changes in brain activation to food pictures after adjustable gastric banding. Surg Obes Relat Dis. 2012;8:602–608. doi: 10.1016/j.soard.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Bultman SJ, Michaud EJ, Woychik RP. Molecular characterization of the mouse agouti locus. Cell. 1992;71:1195–1204. doi: 10.1016/s0092-8674(05)80067-4. [DOI] [PubMed] [Google Scholar]
- Burgess CR, Ramesh RN, Sugden AU, Levandowski KM, Minnig MA, Fenselau H, Lowell BB, Andermann ML. Hunger-Dependent Enhancement of Food Cue Responses in Mouse Postrhinal Cortex and Lateral Amygdala. Neuron. 2016;91:1154–1169. doi: 10.1016/j.neuron.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett CJ, Li C, Webber E, Tsaousidou E, Xue SY, Bruning JC, Krashes MJ. Hunger-Driven Motivational State Competition. Neuron. 2016;92:187–201. doi: 10.1016/j.neuron.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Haubensak W, Anthony TE, Anderson DJ. Central amygdala PKC-delta(+) neurons mediate the influence of multiple anorexigenic signals. Nat Neurosci. 2014;17:1240–1248. doi: 10.1038/nn.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JN, Macosko EZ, Fenselau H, Pers TH, Lyubetskaya A, Tenen D, Goldman M, Versegen AMJ, Resch JM, McCarroll SA, et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci. 2017;20:484–496. doi: 10.1038/nn.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos CA, Bowen AJ, Schwartz MW, Palmiter RD. Parabrachial CGRP Neurons Control Meal Termination. Cell Metab. 2016;23:811–820. doi: 10.1016/j.cmet.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RH. Homeostasis: a plea for a unified approach. Adv Physiol Educ. 2004;28:180–187. doi: 10.1152/advan.00012.2004. [DOI] [PubMed] [Google Scholar]
- Carter ME, Han S, Palmiter RD. Parabrachial calcitonin gene-related peptide neurons mediate conditioned taste aversion. J Neurosci. 2015;35:4582–4586. doi: 10.1523/JNEUROSCI.3729-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503:111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Sandoval DA, Seeley RJ. Integration of satiety signals by the central nervous system. Curr Biol. 2013;23:R379–388. doi: 10.1016/j.cub.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD. Vagal Sensory Neuron Subtypes that Differentially Control Breathing. Cell. 2015;161:622–633. doi: 10.1016/j.cell.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, Shen Z, Marsh DJ, Feighner SD, Guan XM, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- Chen X, Gabitto M, Peng Y, Ryba NJ, Zuker CS. A gustotopic map of taste qualities in the mammalian brain. Science. 2011;333:1262–1266. doi: 10.1126/science.1204076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Knight ZA. Making sense of the sensory regulation of hunger neurons. Bioessays. 2016;38:316–324. doi: 10.1002/bies.201500167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lin YC, Kuo TW, Knight ZA. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell. 2015;160:829–841. doi: 10.1016/j.cell.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lin YC, Zimmerman CA, Essner RA, Knight ZA. Hunger neurons drive feeding through a sustained, positive reinforcement signal. Elife. 2016:5. doi: 10.7554/eLife.18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua SC, Jr, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, Leibel RL. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- Clark JJ, Hollon NG, Phillips PE. Pavlovian valuation systems in learning and decision making. Curr Opin Neurobiol. 2012;22:1054–1061. doi: 10.1016/j.conb.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Rojas DC, Tregellas JR. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS One. 2009;4:e6310. doi: 10.1371/journal.pone.0006310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino G, Lyons DJ, Cristiano C, Burke LK, Madara JC, Campbell JN, Garcia AP, Land BB, Lowell BB, Dileone RJ, et al. Appetite controlled by a cholecystokinin nucleus of the solitary tract to hypothalamus neurocircuit. Elife. 2016:5. doi: 10.7554/eLife.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dampney RA. Central neural control of the cardiovascular system: current perspectives. Adv Physiol Educ. 2016;40:283–296. doi: 10.1152/advan.00027.2016. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Gutierrez R, Oliveira-Maia AJ, Pereira A, Jr, Nicolelis MA, Simon SA. Neural ensemble coding of satiety states. Neuron. 2006;51:483–494. doi: 10.1016/j.neuron.2006.07.009. [DOI] [PubMed] [Google Scholar]
- de Lartigue G, Ronveaux CC, Raybould HE. Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity. Mol Metab. 2014;3:595–607. doi: 10.1016/j.molmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MO, Zimmer MR, Bober J, Horvath TL. Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell. 2015;160:1222–1232. doi: 10.1016/j.cell.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do-Monte FH, Quinones-Laracuente K, Quirk GJ. A temporal shift in the circuits mediating retrieval of fear memory. Nature. 2015;519:460–463. doi: 10.1038/nature14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray GJ. Enteroendocrine cell signalling via the vagus nerve. Curr Opin Pharmacol. 2013;13:954–958. doi: 10.1016/j.coph.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Domingos AI, Vaynshteyn J, Voss HU, Ren X, Gradinaru V, Zang F, Deisseroth K, de Araujo IE, Friedman J. Leptin regulates the reward value of nutrient. Nat Neurosci. 2011;14:1562–1568. doi: 10.1038/nn.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube D, Lissitzky JC, Leclerc R, Pelletier G. Localization of alpha-melanocyte-stimulating hormone in rat brain and pituitary. Endocrinology. 1978;102:1283–1291. doi: 10.1210/endo-102-4-1283. [DOI] [PubMed] [Google Scholar]
- Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, O'Rahilly S. 20 years of leptin: human disorders of leptin action. J Endocrinol. 2014;223:T63–70. doi: 10.1530/JOE-14-0480. [DOI] [PubMed] [Google Scholar]
- Fenselau H, Campbell JN, Verstegen AM, Madara JC, Xu J, Shah BP, Resch JM, Yang Z, Mandelblat-Cerf Y, Livneh Y, et al. A rapidly acting glutamatergic ARC-->PVH satiety circuit postsynaptically regulated by alpha-MSH. Nat Neurosci. 2017;20:42–51. doi: 10.1038/nn.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Labouebe G, Liu S, Nieh EH, Routh VH, Xu S, O'Connor EC. Homeostasis Meets Motivation in the Battle to Control Food Intake. J Neurosci. 2016;36:11469–11481. doi: 10.1523/JNEUROSCI.2338-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak JN, Myers MG., Jr Minireview: CNS Mechanisms of Leptin Action. Mol Endocrinol. 2016;30:3–12. doi: 10.1210/me.2015-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanini A, Katz DB. Behavioral modulation of gustatory cortical activity. Ann N Y Acad Sci. 2009;1170:403–406. doi: 10.1111/j.1749-6632.2009.03922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Kullmann S, Veit R. Food related processes in the insular cortex. Front Hum Neurosci. 2013;7:499. doi: 10.3389/fnhum.2013.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton S. Appetite and reward. Front Neuroendocrinol. 2010;31:85–103. doi: 10.1016/j.yfrne.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Gardner MP, Fontanini A. Encoding and tracking of outcome-specific expectancy in the gustatory cortex of alert rats. J Neurosci. 2014;34:13000–13017. doi: 10.1523/JNEUROSCI.1820-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, Campbell JN, Gavrilova O, Lee CE, Olson DP, et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat Neurosci. 2015;18:863–871. doi: 10.1038/nn.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield AS, Patterson C, Skora S, Gribble FM, Reimann F, Evans ML, Myers MG, Jr, Heisler LK. Neurochemical characterization of body weight-regulating leptin receptor neurons in the nucleus of the solitary tract. Endocrinology. 2012;153:4600–4607. doi: 10.1210/en.2012-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield AS, Shah BP, Burgess CR, Li MM, Li C, Steger JS, Madara JC, Campbell JN, Kroeger D, Scammell TE, et al. Dynamic GABAergic afferent modulation of AgRP neurons. Nat Neurosci. 2016;19:1628–1635. doi: 10.1038/nn.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaykema RP, Newmyer BA, Ottolini M, Raje V, Warthen DM, Lambeth PS, Niccum M, Yao T, Huang Y, Schulman IG, et al. Activation of murine pre-proglucagon-producing neurons reduces food intake and body weight. J Clin Invest. 2017;127:1031–1045. doi: 10.1172/JCI81335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Digby GJ, Sebag JA, Millhauser GL, Palomino R, Matthews R, Gillyard T, Panaro BL, Tough IR, Cox HM, et al. G-protein-independent coupling of MC4R to Kir7.1 in hypothalamic neurons. Nature. 2015;520:94–98. doi: 10.1038/nature14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S, Packard AE, Mahbod P, McKlveen JM, Seeley RJ, Myers B, Ulrich-Lai Y, Smith EP, D'Alessio DA, Herman JP. Disruption of Glucagon-Like Peptide 1 Signaling in Sim1 Neurons Reduces Physiological and Behavioral Reactivity to Acute and Chronic Stress. J Neurosci. 2017;37:184–193. doi: 10.1523/JNEUROSCI.1104-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudo SQ, Billington CJ, Levine AS. Feeding effects of hypothalamic injection of melanocortin 4 receptor ligands. Brain Res. 1998;809:302–306. doi: 10.1016/s0006-8993(98)00837-3. [DOI] [PubMed] [Google Scholar]
- Gizowski C, Zaelzer C, Bourque CW. Clock-driven vasopressin neurotransmission mediates anticipatory thirst prior to sleep. Nature. 2016;537:685–688. doi: 10.1038/nature19756. [DOI] [PubMed] [Google Scholar]
- Gold RM, Jones AP, Sawchenko PE. Paraventricular area: critical focus of a longitudinal neurocircuitry mediating food intake. Physiol Behav. 1977;18:1111–1119. doi: 10.1016/0031-9384(77)90019-1. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Zhao TJ, Li RL, Sherbet DP, Liang G, Brown MS. Surviving starvation: essential role of the ghrelin-growth hormone axis. Cold Spring Harb Symp Quant Biol. 2011;76:121–127. doi: 10.1101/sqb.2011.76.010447. [DOI] [PubMed] [Google Scholar]
- Graham M, Shutter JR, Sarmiento U, Sarosi I, Stark KL. Overexpression of Agrt leads to obesity in transgenic mice. Nat Genet. 1997;17:273–274. doi: 10.1038/ng1197-273. [DOI] [PubMed] [Google Scholar]
- Gribble FM, Reimann F. Enteroendocrine Cells: Chemosensors in the Intestinal Epithelium. Annu Rev Physiol. 2016;78:277–299. doi: 10.1146/annurev-physiol-021115-105439. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012;16:296–309. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- Grossman SE, Fontanini A, Wieskopf JS, Katz DB. Learning-related plasticity of temporal coding in simultaneously recorded amygdala-cortical ensembles. J Neurosci. 2008;28:2864–2873. doi: 10.1523/JNEUROSCI.4063-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- Haight JL, Flagel SB. A potential role for the paraventricular nucleus of the thalamus in mediating individual variation in Pavlovian conditioned responses. Front Behav Neurosci. 2014;8:79. doi: 10.3389/fnbeh.2014.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Herman AM, Ortiz-Guzman J, Kochukov M, Herman I, Quast KB, Patel JM, Tepe B, Carlson JC, Ung K, Selever J, et al. A cholinergic basal forebrain feeding circuit modulates appetite suppression. Nature. 2016;538:253–256. doi: 10.1038/nature19789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder JL, Jr, Butte NF, Zinn AR. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum Mol Genet. 2000;9:101–108. doi: 10.1093/hmg/9.1.101. [DOI] [PubMed] [Google Scholar]
- Huerta CI, Sarkar PR, Duong TQ, Laird AR, Fox PT. Neural bases of food perception: coordinate-based meta-analyses of neuroimaging studies in multiple modalities. Obesity (Silver Spring) 2014;22:1439–1446. doi: 10.1002/oby.20659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41:317–318. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- James MH, Dayas CV. What about me…? The PVT: a role for the paraventricular thalamus (PVT) in drug-seeking behavior. Front Behav Neurosci. 2013;7:18. doi: 10.3389/fnbeh.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff AM, Lacadie C, Seo D, Kubat J, Van Name MA, Giannini C, Savoye M, Constable RT, Sherwin RS, Caprio S, et al. Leptin is associated with exaggerated brain reward and emotion responses to food images in adolescent obesity. Diabetes Care. 2014;37:3061–3068. doi: 10.2337/dc14-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jikomes N, Ramesh RN, Mandelblat-Cerf Y, Andermann ML. Preemptive Stimulation of AgRP Neurons in Fed Mice Enables Conditioned Food Seeking under Threat. Curr Biol. 2016;26:2500–2507. doi: 10.1016/j.cub.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW, Gallagher M, Holland PC. The basolateral amygdala is critical to the expression of pavlovian and instrumental outcome-specific reinforcer devaluation effects. J Neurosci. 2009;29:696–704. doi: 10.1523/JNEUROSCI.3758-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kask A, Schioth HB. Tonic inhibition of food intake during inactive phase is reversed by the injection of the melanocortin receptor antagonist into the paraventricular nucleus of the hypothalamus and central amygdala of the rat. Brain Res. 2000;887:460–464. doi: 10.1016/s0006-8993(00)03034-1. [DOI] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- Kong D, Dagon Y, Campbell JN, Guo Y, Yang Z, Yi X, Aryal P, Wellenstein K, Kahn BB, Sabatini BL, et al. A Postsynaptic AMPK-->p21-Activated Kinase Pathway Drives Fasting-Induced Synaptic Plasticity in AgRP Neurons. Neuron. 2016;91:25–33. doi: 10.1016/j.neuron.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner PI. Circulatory control and the supercontrollers. J Hypertens. 1995;13:1508–1521. [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Koda S, Lowell BB. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab. 2013;18:588–595. doi: 10.1016/j.cmet.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238–242. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisler AD, Davis EA, Rinaman L. Differential activation of chemically identified neurons in the caudal nucleus of the solitary tract in non-entrained rats after intake of satiating vs. non-satiating meals. Physiol Behav. 2014;136:47–54. doi: 10.1016/j.physbeh.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer NB, Krebs L, Kobiella A, Grimm O, Pilhatsch M, Bidlingmaier M, Zimmermann US, Smolka MN. Fasting levels of ghrelin covary with the brain response to food pictures. Addict Biol. 2013;18:855–862. doi: 10.1111/j.1369-1600.2012.00489.x. [DOI] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- Kusumoto-Yoshida I, Liu H, Chen BT, Fontanini A, Bonci A. Central role for the insular cortex in mediating conditioned responses to anticipatory cues. Proc Natl Acad Sci U S A. 2015;112:1190–1195. doi: 10.1073/pnas.1416573112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115:493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- Lasseter HC, Wells AM, Xie X, Fuchs RA. Interaction of the basolateral amygdala and orbitofrontal cortex is critical for drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2011;36:711–720. doi: 10.1038/npp.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Hammer NJ, Chang K. Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol Behav. 1981;27:1031–1040. doi: 10.1016/0031-9384(81)90366-8. [DOI] [PubMed] [Google Scholar]
- Lerner TN, Ye L, Deisseroth K. Communication in Neural Circuits: Tools, Opportunities, and Challenges. Cell. 2016;164:1136–1150. doi: 10.1016/j.cell.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshan RL, Greenwald-Yarnell M, Patterson CM, Gonzalez IE, Myers MG., Jr Leptin action through hypothalamic nitric oxide synthase-1-expressing neurons controls energy balance. Nat Med. 2012;18:820–823. doi: 10.1038/nm.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AS, Morley JE. Neuropeptide Y: a potent inducer of consummatory behavior in rats. Peptides. 1984;5:1025–1029. doi: 10.1016/0196-9781(84)90165-7. [DOI] [PubMed] [Google Scholar]
- Li Y, Wu X, Zhou S, Owyang C. Low-affinity CCK-A receptors are coexpressed with leptin receptors in rat nodose ganglia: implications for leptin as a regulator of short-term satiety. Am J Physiol Gastrointest Liver Physiol. 2011;300:G217–227. doi: 10.1152/ajpgi.00356.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci. 2003;23:7143–7154. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, Ding JB, Yang Z, Sabatini BL, Lowell BB. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73:511–522. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh Y, Ramesh RN, Burgess CR, Levandowski KM, Madara JC, Fenselau H, Goldey G, Diaz V, Jikomes N, Resch JM, et al. Homeostatic circuits selectively gate food cue responses in insular cortex. Nature. 2017 doi: 10.1038/nature22375. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockie SH, Andrews ZB. The hormonal signature of energy deficit: Increasing the value of food reward. Mol Metab. 2013;2:329–336. doi: 10.1016/j.molmet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T, Luther M, Chen W, Woychik RP, Wilkison WO, et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. 1994;371:799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Luquet S, Phillips CT, Palmiter RD. NPY/AgRP neurons are not essential for feeding responses to glucoprivation. Peptides. 2007;28:214–225. doi: 10.1016/j.peptides.2006.08.036. [DOI] [PubMed] [Google Scholar]
- Lutz TA. The interaction of amylin with other hormones in the control of eating. Diabetes Obes Metab. 2013;15:99–111. doi: 10.1111/j.1463-1326.2012.01670.x. [DOI] [PubMed] [Google Scholar]
- Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Mandelblat-Cerf Y, Kim A, Burgess CR, Subramanian S, Tannous BA, Lowell BB, Andermann ML. Bidirectional Anticipation of Future Osmotic Challenges by Vasopressin Neurons. Neuron. 2017;93:57–65. doi: 10.1016/j.neuron.2016.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelblat-Cerf Y, Ramesh RN, Burgess CR, Patella P, Yang Z, Lowell BB, Andermann ML. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. Elife. 2015:4. doi: 10.7554/eLife.07122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning S, Batterham RL. The role of gut hormone peptide YY in energy and glucose homeostasis: twelve years on. Annu Rev Physiol. 2014;76:585–608. doi: 10.1146/annurev-physiol-021113-170404. [DOI] [PubMed] [Google Scholar]
- McFarlane MR, Brown MS, Goldstein JL, Zhao TJ. Induced ablation of ghrelin cells in adult mice does not decrease food intake, body weight, or response to high-fat diet. Cell Metab. 2014;20:54–60. doi: 10.1016/j.cmet.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud EJ, Bultman SJ, Stubbs LJ, Woychik RP. The embryonic lethality of homozygous lethal yellow mice (Ay/Ay) is associated with the disruption of a novel RNA-binding protein. Genes Dev. 1993;7:1203–1213. doi: 10.1101/gad.7.7a.1203. [DOI] [PubMed] [Google Scholar]
- Michaud JL, Boucher F, Melnyk A, Gauthier F, Goshu E, Levy E, Mitchell GA, Himms-Hagen J, Fan CM. Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum Mol Genet. 2001;10:1465–1473. doi: 10.1093/hmg/10.14.1465. [DOI] [PubMed] [Google Scholar]
- Miller NE. Neal E. Miller: selected papers. Chicago: Aldine, Atherton; 1971. [Google Scholar]
- Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, Gundisch D, Diano S, De Biasi M, Horvath TL, Gao XB, et al. Nicotine decreases food intake through activation of POMC neurons. Science. 2011;332:1330–1332. doi: 10.1126/science.1201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno TM, Kleopoulos SP, Bergen HT, Roberts JL, Priest CA, Mobbs CV. Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting and [corrected] in ob/ob and db/db mice, but is stimulated by leptin. Diabetes. 1998;47:294–297. doi: 10.2337/diab.47.2.294. [DOI] [PubMed] [Google Scholar]
- Monge-Roffarello B, Labbe SM, Lenglos C, Caron A, Lanfray D, Samson P, Richard D. The medial preoptic nucleus as a site of the thermogenic and metabolic actions of melanotan II in male rats. Am J Physiol Regul Integr Comp Physiol. 2014;307:R158–166. doi: 10.1152/ajpregu.00059.2014. [DOI] [PubMed] [Google Scholar]
- Moran TH, Ladenheim EE. Physiologic and Neural Controls of Eating. Gastroenterol Clin North Am. 2016;45:581–599. doi: 10.1016/j.gtc.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci. 2014;15:367–378. doi: 10.1038/nrn3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- Muller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F, et al. Ghrelin. Mol Metab. 2015;4:437–460. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Gaznick N, Tranel D, Bechara A. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci. 2014;1316:53–70. doi: 10.1111/nyas.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassi JJ, Cepko CL, Born RT, Beier KT. Neuroanatomy goes viral! Front Neuroanat. 2015;9:80. doi: 10.3389/fnana.2015.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochner CN, Laferrere B, Afifi L, Atalayer D, Geliebter A, Teixeira J. Neural responsivity to food cues in fasted and fed states pre and post gastric bypass surgery. Neurosci Res. 2012;74:138–143. doi: 10.1016/j.neures.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Padilla SL, Qiu J, Nestor CC, Zhang C, Smith AW, Whiddon BB, Ronnekleiv OK, Kelly MJ, Palmiter RD. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc Natl Acad Sci U S A. 2017;114:2413–2418. doi: 10.1073/pnas.1621065114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla SL, Qiu J, Soden ME, Sanz E, Nestor CC, Barker FD, Quintana A, Zweifel LS, Ronnekleiv OK, Kelly MJ, et al. Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat Neurosci. 2016;19:734–741. doi: 10.1038/nn.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes SL, Balleine BW. Incentive memory: evidence the basolateral amygdala encodes and the insular cortex retrieves outcome values to guide choice between goal-directed actions. J Neurosci. 2013;33:8753–8763. doi: 10.1523/JNEUROSCI.5071-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes SL, Bradfield LA, Balleine BW. Interaction of insular cortex and ventral striatum mediates the effect of incentive memory on choice between goal-directed actions. J Neurosci. 2015;35:6464–6471. doi: 10.1523/JNEUROSCI.4153-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perello M, Dickson SL. Ghrelin signalling on food reward: a salient link between the gut and the mesolimbic system. J Neuroendocrinol. 2015;27:424–434. doi: 10.1111/jne.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JH, Ritter RC, Simasko SM. Leptin and CCK selectively activate vagal afferent neurons innervating the stomach and duodenum. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1544–1549. doi: 10.1152/ajpregu.00811.2005. [DOI] [PubMed] [Google Scholar]
- Petrovich GD. Forebrain networks and the control of feeding by environmental learned cues. Physiol Behav. 2013;121:10–18. doi: 10.1016/j.physbeh.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Gastric volume rather than nutrient content inhibits food intake. Am J Physiol. 1996;271:R766–769. doi: 10.1152/ajpregu.1996.271.3.R766. [DOI] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- Poggioli R, Vergoni AV, Bertolini A. ACTH-(1–24) and alpha-MSH antagonize feeding behavior stimulated by kappa opiate agonists. Peptides. 1986;7:843–848. doi: 10.1016/0196-9781(86)90104-x. [DOI] [PubMed] [Google Scholar]
- Prager-Khoutorsky M, Bourque CW. Mechanical basis of osmosensory transduction in magnocellular neurosecretory neurones of the rat supraoptic nucleus. J Neuroendocrinol. 2015;27:507–515. doi: 10.1111/jne.12270. [DOI] [PubMed] [Google Scholar]
- Ravussin Y, Leibel RL, Ferrante AW., Jr A missing link in body weight homeostasis: the catabolic signal of the overfed state. Cell Metab. 2014;20:565–572. doi: 10.1016/j.cmet.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Zahm DS. Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci. 2005;25:11757–11767. doi: 10.1523/JNEUROSCI.3432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010;1350:18–34. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring LE, Zeltser LM. Disruption of hypothalamic leptin signaling in mice leads to early-onset obesity, but physiological adaptations in mature animals stabilize adiposity levels. J Clin Invest. 2010;120:2931–2941. doi: 10.1172/JCI41985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers PJ, Brunstrom JM. Appetite and energy balancing. Physiol Behav. 2016;164:465–471. doi: 10.1016/j.physbeh.2016.03.038. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Sensory processing in the brain related to the control of food intake. Proc Nutr Soc. 2007;66:96–112. doi: 10.1017/S0029665107005332. [DOI] [PubMed] [Google Scholar]
- Roman CW, Derkach VA, Palmiter RD. Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat Commun. 2016;7:11905. doi: 10.1038/ncomms11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Zeisel A, Bakker J, Girach F, Hellysaz A, Tomer R, Alpar A, Mulder J, Clotman F, Keimpema E, et al. Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nat Neurosci. 2016 doi: 10.1038/nn.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M, Leibel RL. 20 years of leptin: role of leptin in energy homeostasis in humans. J Endocrinol. 2014;223:T83–96. doi: 10.1530/JOE-14-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, et al. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139:4428–4431. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- Samuelsen CL, Gardner MP, Fontanini A. Effects of cue-triggered expectation on cortical processing of taste. Neuron. 2012;74:410–422. doi: 10.1016/j.neuron.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santel S, Baving L, Krauel K, Munte TF, Rotte M. Hunger and satiety in anorexia nervosa: fMRI during cognitive processing of food pictures. Brain Res. 2006;1114:138–148. doi: 10.1016/j.brainres.2006.07.045. [DOI] [PubMed] [Google Scholar]
- Saper CB. The House Alarm. Cell Metab. 2016;23:754–755. doi: 10.1016/j.cmet.2016.04.021. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, McHugh PR, Moran TH. Integration of vagal afferent responses to gastric loads and cholecystokinin in rats. Am J Physiol. 1991;261:R64–69. doi: 10.1152/ajpregu.1991.261.1.R64. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- Secher A, Jelsing J, Baquero AF, Hecksher-Sorensen J, Cowley MA, Dalboge LS, Hansen G, Grove KL, Pyke C, Raun K, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124:4473–4488. doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley RJ, Berridge KC. The hunger games. Cell. 2015;160:805–806. doi: 10.1016/j.cell.2015.02.028. [DOI] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, et al. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci. 2013;16:1094–1100. doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah BP, Vong L, Olson DP, Koda S, Krashes MJ, Ye C, Yang Z, Fuller PM, Elmquist JK, Lowell BB. MC4R–expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proc Natl Acad Sci U S A. 2014;111:13193–13198. doi: 10.1073/pnas.1407843111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutter JR, Graham M, Kinsey AC, Scully S, Luthy R, Stark KL. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997;11:593–602. doi: 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 2009;198:149–158. doi: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Sjulson L, Cassataro D, DasGupta S, Miesenbock G. Cell-Specific Targeting of Genetically Encoded Tools for Neuroscience. Annu Rev Genet. 2016;50:571–594. doi: 10.1146/annurev-genet-120215-035011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibicka KP, Grill HJ. Hypothalamic and hindbrain melanocortin receptors contribute to the feeding, thermogenic, and cardiovascular action of melanocortins. Endocrinology. 2009;150:5351–5361. doi: 10.1210/en.2009-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GP. Satiation : from gut to brain. New York: Oxford University Press; 1998. [Google Scholar]
- Somjen GG. The Missing Error Signal—Regulation Beyond Negative Feedback. Physiology. 1992;7:184–185. [Google Scholar]
- Stachniak TJ, Ghosh A, Sternson SM. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus-->midbrain pathway for feeding behavior. Neuron. 2014;82:797–808. doi: 10.1016/j.neuron.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley BG, Leibowitz SF. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984;35:2635–2642. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- Steinert RE, Feinle-Bisset C, Asarian L, Horowitz M, Beglinger C, Geary N. Ghrelin, CCK, GLP-1, and PYY(3–36): Secretory Controls and Physiological Roles in Eating and Glycemia in Health, Obesity, and After RYGB. Physiol Rev. 2017;97:411–463. doi: 10.1152/physrev.00031.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM, Eiselt AK. Three Pillars for the Neural Control of Appetite. Annu Rev Physiol. 2017;79:401–423. doi: 10.1146/annurev-physiol-021115-104948. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Sevigny CP, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. J Comp Neurol. 2002;444:191–206. doi: 10.1002/cne.10141. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D. Injections of muscimol into the paraventricular thalamic nucleus, but not mediodorsal thalamic nuclei, induce feeding in rats. Brain Res. 2013;1490:128–133. doi: 10.1016/j.brainres.2012.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker EM, Hoffmann ML. Presystemic signals in the control of thirst, salt appetite, and vasopressin secretion. Physiol Behav. 2007;91:404–412. doi: 10.1016/j.physbeh.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Strubbe JH, Woods SC. The timing of meals. Psychol Rev. 2004;111:128–141. doi: 10.1037/0033-295X.111.1.128. [DOI] [PubMed] [Google Scholar]
- Sun X, Veldhuizen MG, Wray AE, de Araujo IE, Sherwin RS, Sinha R, Small DM. The neural signature of satiation is associated with ghrelin response and triglyceride metabolism. Physiol Behav. 2014;136:63–73. doi: 10.1016/j.physbeh.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton AK, Myers MG, Jr, Olson DP. The Role of PVH Circuits in Leptin Action and Energy Balance. Annu Rev Physiol. 78:207–221. doi: 10.1146/annurev-physiol-021115-105347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi KA, Cone RD. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology. 2005;146:1043–1047. doi: 10.1210/en.2004-1397. [DOI] [PubMed] [Google Scholar]
- Tan K, Knight ZA, Friedman JM. Ablation of AgRP neurons impairs adaption to restricted feeding. Mol Metab. 2014;3:694–704. doi: 10.1016/j.molmet.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toates FM. Motivational systems. Cambridge Cambridgeshire; New York: Cambridge University Press; 1986. [Google Scholar]
- Trapp S, Cork SC. PPG neurons of the lower brain stem and their role in brain GLP-1 receptor activation. Am J Physiol Regul Integr Comp Physiol. 2015;309:R795–804. doi: 10.1152/ajpregu.00333.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli RA, Anselmi L. Vagal neurocircuitry and its influence on gastric motility. Nat Rev Gastroenterol Hepatol. 2016;13:389–401. doi: 10.1038/nrgastro.2016.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7:493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- Vong L, Ye C, Yang Z, Choi B, Chua S, Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, Ibia IE, Zigman JM. Disruption of cue-potentiated feeding in mice with blocked ghrelin signaling. Physiol Behav. 2012;108:34–43. doi: 10.1016/j.physbeh.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, He X, Zhao Z, Feng Q, Lin R, Sun Y, Ding T, Xu F, Luo M, Zhan C. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front Neuroanat. 2015;9:40. doi: 10.3389/fnana.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liu C, Uchida A, Chuang JC, Walker A, Liu T, Osborne-Lawrence S, Mason BL, Mosher C, Berglund ED, et al. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol Metab. 2014;3:64–72. doi: 10.1016/j.molmet.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG. Chapter 14 - Neuroendocrine Regulation of Food Intake A2 - Fink, George. In: Pfaff DW, Levine JE, editors. Handbook of Neuroendocrinology. San Diego: Academic Press; 2012. pp. 331–354. [Google Scholar]
- Williams DL. Neural integration of satiation and food reward: role of GLP-1 and orexin pathways. Physiol Behav. 2014;136:194–199. doi: 10.1016/j.physbeh.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell. 2016;166:209–221. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010;30:2472–2479. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC, Ramsay DS. Homeostasis: beyond Curt Richter. Appetite. 2007;49:388–398. doi: 10.1016/j.appet.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- Yaxley S, Rolls ET, Sienkiewicz ZJ. The responsiveness of neurons in the insular gustatory cortex of the macaque monkey is independent of hunger. Physiol Behav. 1988;42:223–229. doi: 10.1016/0031-9384(88)90074-1. [DOI] [PubMed] [Google Scholar]
- Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O'Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20:111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, Wu P, Luo M. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci. 2013;33:3624–3632. doi: 10.1523/JNEUROSCI.2742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zheng H, Stornetta RL, Agassandian K, Rinaman L. Glutamatergic phenotype of glucagon-like peptide 1 neurons in the caudal nucleus of the solitary tract in rats. Brain Struct Funct. 2015;220:3011–3022. doi: 10.1007/s00429-014-0841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman CA, Lin YC, Leib DE, Guo L, Huey EL, Daly GE, Chen Y, Knight ZA. Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature. 2016;537:680–684. doi: 10.1038/nature18950. [DOI] [PMC free article] [PubMed] [Google Scholar]