Summary
The community of microorganisms in the mammalian gastrointestinal tract, referred to as the gut microbiota, influences host physiology and immunity. The last decade of microbiome research has provided significant advancements for the field and highlighted the importance of gut microbes to states of both health and disease. Novel molecular techniques have unraveled the tremendous diversity of intestinal symbionts that potentially influence the host, many proof-of-concept studies have demonstrated causative roles of gut microbial communities in various pathologies, and microbiome-based approaches are beginning to be implemented in the clinic for diagnostic purposes or for personalized treatments. However, several challenges for the field remain: purely descriptive reports outnumbering mechanistic studies and slow translation of experimental results obtained in animal models into the clinics. Moreover, there is a dearth of knowledge regarding how gut microbes, including novel species that have yet to be identified, impact host immune responses. The sheer complexity of the gut microbial ecosystem makes it difficult, in part, to fully understand the microbiota-host networks that regulate immunity. In the present manuscript, we review key findings on the interactions between gut microbiota members and the immune system. Because culturing microbes allows performing functional studies, we have emphasized the impact of specific taxa or communities thereof. We also highlight underlying molecular mechanisms and discuss opportunities to implement minimal microbiome-based strategies.
Keywords: gut microbiota, immune system, anaerobic cultivation, minimal microbiomes, Altered Schaedler Flora, lipids
1 INTRODUCTION
The mammalian gastrointestinal tract is home to a dense microbial population, a community collectively referred to as the gut microbiota. These organisms live a mutualistic lifestyle with their host and provide it with numerous benefits—they supply micronutrients, increase food digestibility and nutrient absorption, influence immune maturation, offer colonization resistance against pathogens and secrete metabolites that aid in nutrition and whole body physiology (1, 2). Despite serving a valuable role in host biology, the gut microbiota has also been implicated in the pathogenesis of several immune-mediated human diseases, including allergy, asthma, multiple sclerosis, inflammatory bowel diseases (3–6). Consequently, much interest exists in understanding the mechanisms by which members of this complex community shape host physiology and immunity.
Studies in germfree mice highlight the importance of the gut microbiota to proper immunological development—these animals have reduced numbers of immune cells present in their intestinal lamina propria and smaller Peyer’s patches compared to conventionally reared mice (7–10). Systemically, they exhibit a diminished capacity for antibody production, fewer plasma cells, smaller mesenteric lymph nodes, and reduced numbers of germinal centers versus their conventional counterparts (11). Immune maturation can be induced in germfree mice by colonization with a complex gut microbiota; however, some notable deficits and distinctions remain into adulthood depending upon the age of mice at the time of microbial exposure (12–16). Indeed, early life represents a critical window for proper immune development (17, 18). Infants are born sterile and establishment of the gut microbiota begins shortly after birth (19–21). During this time, the mammalian host is rapidly exposed to a myriad of different microorganisms (22, 23). The gut microbiota is quite unstable throughout the first months of life, is strongly influenced by breast-feeding, diversifies substantially with the introduction of solid food, and eventually stabilizes after the first two years of life (24, 25). Such temporal variability and succession dynamics add yet another level of complexity to dissecting the interplay between the immune system and gut microbes and are beyond the scope of this review.
Instead, we will focus on the dynamic interactions between cultured members of the microbiota and host immunity. Indeed, depletion of gut microbes in conventional mice via antibiotic treatment significantly impacts the immune system—it reduces spleen size, decreases immune cell numbers and alters expression of genes regulating inflammatory responses and production of antimicrobial factors such that these animals more closely resemble germfree mice than those with a conventional microbiota (26, 27). Cultivation of gut microbes represents an important technique for studying the relationship between this complex ecosystem and its host. Molecular-based methods represent another approach that has been reviewed many times elsewhere (28, 29) and will not be touched upon here. Because molecular techniques allow high-throughput analysis of entire systems, they are very useful for obtaining comprehensive and integrated views of complex interactions not permitted by more reductionist approaches. In contrast, the major advantage of cultivation approaches is the ability to characterize microorganisms in great detail, perform downstream functional studies and identify specific microbial bioactive compounds and their effects on target cells (30). Contratry to frequent introductory statements in the literature that only minor fractions of the mammalian gut microbiota can be cultured, cultivation techniques can indeed capture a substantial amount of the bacterial diversity present in the mammalian gut: a comparison of thousands of high-throughput amplicon datasets with a catalogue of reference sequences from isolates revealed that 35% to 65% of microbial species in the human and mouse gut have representative strains in culture (31). Nonetheless, there are still numerous unknown taxa to be discovered. These taxa represent a considerable pool of novel functions, some of which may have a profound impact upon the immune system.
This review compiles knowledge about the regulation of immune responses by specific members of the gut microbiota, with primary focus on studies using cultured microbes as single strains or minimal consortia to investigate specific interactions at local (gut mucosal immune system) and distant body sites. Even though culture approaches are simplistic, they provide precise knowledge that is eventually useful for proper interpretation of results generated with more comprehensive systems-oriented approaches. We acknowledge that microorganisms other than bacteria can regulate immune responses. However, the bulk of information provided here concerns bacteria as they represent the dominant members of gut ecosystems and are the subject of most reports in the literature. Furthermore, there is exhaustive literature on probiotics and their impact on the immune system. However, because most probiotic studies are based on the use of generic strains and molecular mechanisms are rarely investigated (32, 33), we deliberately excluded probiotic studies from the present review. Finally, sophisticated in vitro systems and functional metagenomic approaches allow assessment of the impact of microbes on the immune system (34, 35). However, because these approaches are still in their infancy and findings eventually require validation in more complex models, this review focuses primarily on data obtained in vivo.
2 SINGLE STRAINS, SPECIFIC EFFECTS
Germfree mice have been used extensively to study the impact of single microorganisms on immune responses. Born and raised in the absence of living body microorganisms, these mice allow targeted manipulations of the gut microbiota and determination of the impact of specific microbial strains, including characterization of underlying molecular mechanisms (36). Here, we highlight only a select set of experimental studies aimed at revealing the specific interactions between single bacterial species and the host (37), some of which utilized germfree mice while others employed mouse models with a complex microbiota (more examples are described in Table 1). For additional reading, we highly recommend Faith et al., 2014 (38), Tan et al., 2016 (39) and Geva-Zatorsky et al., 2017 (40), all of whom have reported extensively on strain specific effects in experimental model systems. The spectrum of host-microbe interactions is presented here by Phylum merely to facilitate discussion; we are mindful that phylogenetic clustering may not provide a consistent phenotype for all members that accurately predicts host responses. We highlight taxa belonging to the most abundant phyla in the murine microbiota (with Firmicutes and Bacteroidetes representing c.90% of commonly detected sequence diversity) as well as members of some other commonly detected but lowly abundant phyla such as Proteobacteria, Fusobacteria, Tenericuites, Deferribacteres, Verrucomicrobia and Actinobacteria (41). Finally, considering the width of strain level diversity (42) and rapid exchange of genetic information (43) that exists within gut microbial ecosystems, the results discussed herein should not be considered as generalizable phenomenon applicable to all members of a particular species, but rather as examples of the complexity and uniqueness of their intricate relationships with the host.
Table 1.
Impact of select microbial strains on the immune system in gnotobiotic mouse models
| Strain | Reference | Impact on phenotype |
|---|---|---|
| Acinetobacter lwoffi F78 | Geva-Zatorsky et al. 2017 (40) | Repressed colonic IL-22 production |
| Akkermansia muciniphila ATCC BAA-835 | Derrien et al. 2011 (193) | Modulated colonic expression of host genes involved in antigen presentation, B and T cell receptor and leukocyte trafficking, IL-4 signalling and complement and coagulation cascades |
| Bacteroides caccae ATCC 43185 | Faith et al. 2014 (38) | Increased number of CD4+ Foxp3+ Treg cells in the colonic lamina propria |
| Bacteroides intestinalis (no strain designation) | Faith et al. 2014 (38) | Increased number of CD4+ Foxp3+ Treg cells in the colonic lamina propria |
| Bacteroides thetaiotaomicron ATCC 29148 | Faith et al. 2014 (38); Geva-Zatorsky et al. 2017 (40); Hoffmann et al. 2016 (38, 40, 194) | Increased number of CD4+ Foxp3+ Treg cells in the colonic lamina propria |
| Bacteroides thetaiotaomicron ATCC 29741 | Geva-Zatorsky et al. 2017 (40) | Increased number of CD4+ Foxp3+ Treg cells in the colonic lamina propria |
| Bacteroides vulgatus mpk | Muller et al. 2008 (38, 40, 195) | Induced DC production of IL-6 in the absence of other pro-inflammatory cytokines |
| Bacteroides vulgatus (no strain designation) | Faith et al. 2014 (38) | Induced expansion of colonic Treg cells |
| Bacteroides vulgatus ATCC 8482 | Geva-Zatorsky et al. 2017 (40) | Induced expansion of plasmacytoid dendritic cells |
| Candida albicans B311 (type A) | Jones-Carson et al. 1997 (196) | Induced development of memory T cells in a B cell-deficient mouse |
| Candida albicans SC5314 | Faith et al. 2014 (38) | Induced production of IFN-g, IL-17 and IL-22 |
| Clostridium sordellii AO32 | Geva-Zatorsky et al. 2017 (40) | Repressed colonic IL22 production |
| Parabacteroides distasonis (no strain designation) | Faith et al. 2014 (38) | Increased number of CD4+ Foxp3+ Treg cells in the colonic lamina propria |
| Ruminococcus gnavus ATCC29149 | Geva-Zatorsky et al. 2017 (40) Hoffmann et al. 2016 (40, 194) |
Up-regulated expression of host genes related to antimicrobial responses as well as amide, lipid and tryptophan metabolism in the colon |
2.1 FIRMICUTES
Clostridium spp
Given the phylogenetic diversity of the Clostridium genus across its many clusters (44), only a few major examples of species-specific interactions with the host are described here, the majority of which promote immune regulation. Conventional mice colonized with C. butyricum MIYAIRI 588 expanded the numbers of both IL-10 secreting macrophages (F4/80+ CD11b+ CD11cint) and inducible CD4+ T regulatory (Treg) cells, the latter of which occurred via increased secretion of TGF-β1 by lamina propria dendritic cells (DC). Moreover, these enhanced regulatory responses were sufficient to prevent experimental colitis in mice via a TLR2-dependent manner (45, 46). When C. ramosum AO31, C. sordelli AO32 or C. histolyticum AO25 were individually mono-associated with mice, each similarly expanded both Foxp3−Rorγt+CD4+ T cells and Foxp3+Rorγt+Helios−CD4+ T cells in the colon and Foxp3+Rorγt+Helios−CD4+ T in the small intestine compared to germfree mice (40). In contrast, C. perfringens ATCC 13124 did not promote expansion of either cell type, demonstrating that there are clear species-specific effects on immune modulation by members of the Clostridium genus (40).
Faecalibacterium prausnitzii
Another Firmicutes member often studied for its immunomodulatory properties is Faecalibacterium prausnitzii. The abundance of this taxa is routinely reduced in the gut microbiome of patients with inflammatory bowel diseases (IBD), particularly during flares (47–49). This decrease is presumably a consequence of an inflammation-driven increase in tissue oxygen tension that creates an environment unfavorable for F. prausnitzii (50, 51). However, the lack of F. prausnitzii may actively contribute to the disease process as this organism possesses anti-inflammatory properties and helps maintain epithelial cell integrity. F. prausnitzii A2-165 induces IL-10 production in human and murine dendritic cells and increases intestinal expression of mouse Claudin-4, which together may explain its protective role in colitis models (52–55). F. prausnitzii A2-165 may mediate these anti-inflammatory effects via secretion of effector proteins that inhibit NF-kB signaling in intestinal epithelial cells (56). Alternatively, F. prausnitzii may confer anti-inflammatory effects via the production of butyrate, which could alter the functions of macrophages and peripheral Treg cells via epigenetic modifications (57, 58). Additional studies are needed to define if these pathways are indeed regulated by F. prausnitzii in vivo to limit host inflammation.
Segmented Filamentous Bacteria (SFB – Candidatus savagella) (59)
SFB are Gram-positive clostridia-related bacteria best characterized as colonizers of the murine small intestine, with similar microorganisms found in humans, non-human primates, cats, dogs, and other animals (60–62). Through intimate contact with small intestinal epithelial cells (IEC), SFB induces the secretion of serum amyloid protein A (SAA) 1/2 (63, 64). In turn, SAA acts on CD11c+ dendritic cells to trigger production of IL-1β and IL-23—the latter of which stimulates group 3 innate lymphoid cells to release IL-22, which then synergizes with IL-1β to maximize IEC secretion of SAA (64). Additionally, IEC also release reactive oxygen species that, when combined with the actions of dendritic cells and CX3CR1+ macrophages, induce expansion of Th17 cells in the small intestine (64–68). Importantly, host-adaptation influences the capacity of SFB isolates to adhere to the epithelium and subsequently induce Th17 cells, as demonstrated by a comparison between mouse and rat isolates with reciprocal colonization of each strain in both hosts (64). Locally, SFB induction of the IL-17 axis is protective against Citrobacter rodentium colitis in mice (65). However, colonization of severe combined immune deficiency (SCID) mice with SFB precipitates the onset of severe colitis following adoptive transfer of CD45highCD4+ T cells (69). SFB can also induce Th17 cells that migrate to distant body sites, such as the joints, to mediate arthritis (70). Collectively, this intricate cross-talk between SFB and the IL-17 axis clearly shapes immunity and may contextually precipitate disease in susceptible hosts. However, whether the same SFB-mediated immunological signaling described in mice is preserved in humans and other host species remains unknown.
2.2 BACTEROIDETES
Bacteroides fragilis
Although multiple members of the Bacteroides genus have been documented to impact host immune responses, our discussion here will focus specifically on the immunomodulatory properties of B. fragilis, which, depending on the strain, can induce either immunoregulation or inflammation. Colonization of germfree mice with B. fragilis NCTC 9343 induces a marked expansion of Tregs in the intestinal mucosa via TLR2 signaling—a response dependent on the presence of polysaccharide-A (PSA), a zwitterionic capsular polysaccharide. This response is sufficient to protect mice from both TNBS- and Helicobacter hepaticus-mediated colitis, in part, via an IL-10-dependent mechanism (40, 71–73). Dendritic cells, including plasmacytoid dendritic cells, have been shown to sense outer membrane vesicle (OMV)-associated PSA via Gadd45-α and TLR2 (74), which promotes CD4+ T cells to secrete IL-10 and suppresses Th17 cell responses (75); OMV signaling via ATG16L1 and NOD2 is also likely important (76) as ATG16L1-deficient dendritic cells are not able to induce Treg responses. B. fragilis NCTC 9343 can also mediate protection against oxazolone-induced colitis in a PSA-independent mechanism by providing a source of bacterial sphingolipids that limits expansion of colonic iNKT cells (77). In addition to regulating mucosal immunity, B. fragilis PSA can also impact systemic immune responses, including protection of experimental autoimmune encephalomyelitis (EAE) in mouse models through the action of TLR2 by inducing expansion of a specific subset of Treg cells (i.e., CD39+Foxp3+CD4+ T cells) (78–80).
In contrast to these examples of immune regulation, a subset of B. fragilis strains called enterotoxigenic B. fragilis (ETBF), including strain 86-5443-2-2, can induce pro-inflammatory responses, severe colitis and colonic tumorigenesis in mice; such responses are accompanied by Stat3 activation and a potent Th17 response that plays a causal role in disease (81). Interestingly, non-toxigenic strains of B. fragilis may mitigate the pathogenic effects of toxigenic strains by competitive exclusion mediated via the Type VI secretion system (82) and/or by reducing toxin expression (83). Together, these reports highlight the profoundly different interactions that can occur between the immune system and B. fragilis depending on which strain(s) are present in the host.
2.3 PROTEOBACTERIA
Escherichia coli
Diversity of the species E. coli represents a spectrum of host relationships that can range from mutualism to opportunistic and specialized pathogenesis (84). Here, we will discuss only the specific examples by which select strains of commensal E. coli are known to modulate host immunity. Studies in germfree guinea pigs, chickens and piglets were the first to describe that replicating E. coli could stimulate both mucosal and systemic immune responses (85–87). Since then, most studies seeking to define specific interactions between the host immune system and E. coli have focused on isolates such as the probiotic strain E. coli Nissle 1917, which provides protection against Salmonella (88) and pathogenic E. coli O157:H7 infections in mice via competition over nutritional resources (89). Nissle 1917 may further promote immune regulation by promoting the expansion of plasmacytoid DC, Foxp3−Rorγt+CD4+ T cells and Foxp3+Rorγt+Helios−CD4+ T cells in the colon and contraction of CD11b+CD11c−F4/80+ monocytes in the small intestine as compared to germfree mice (40).
In contrast to the immunoregulatory and protective effects of E. coli Nissle, other human commensal E. coli are hypothesized to contribute to the development of intestinal inflammation in a subset of Crohn’s disease (CD) patients (90); these opportunists are known collectively as Adherent and Invasive E. coli (AIEC). AIEC strains are generally defined by their ability to adhere to and invade epithelial cells, replicate inside macrophages and induce TNF production from macrophages in vitro (91). Although detailed in vivo studies defining the immunological response induced by these strains are still needed, one recent study elegantly demonstrated that the IgA-coated AIEC strain A2 isolated from CD patients with peripheral spondyloarthritis (SpA), an extra-intestinal manifestation often found in patients with active IBD, induced both mucosal and systemic Th17 responses in germfree mice as compared to non-AIEC E. coli (92). Induction of such Th17 immunity required the E. coli to harbor the virulence-associated metabolic enzyme propanediol dehydratase, and colonization of genetically susceptible mouse models of colitis and arthritis led to more severe inflammation in both instances (92). Altogether, these findings highlight how complex and intricate the effect of various E. coli lineages can be in the host, ranging from responses that mediate protective immunity to immunopathology.
Klebsiella spp. and Proteus mirabilis
Other members of the family Enterobacteriacea, including K. pneumoniae and P. mirabilis have been documented to also promote inflammatory responses and play a causative role in colitis. Using the TRUC (Tbet−/− Rag2−/−) mouse model, Garrett and colleagues showed that K. pneumoniae and P. mirabilis (strain name not provided) act in concert with the resident microbiota to elicit transmissible disease dependent on TNF signaling (93, 94). Disease was improved following the transfer of CD4+CD25+ regulatory T cells (93, 94), indicating that these bacteria negatively impact immunoregulation. More recent studies with two additional Klebsiella species describe the expansion of Th17 CD4+ T cells in both the small intestine and colon of mono-associated mice (39, 40), thereby suggesting a potential mechanism by which Klebsiella may contribute to inflammation. Colonization with P. mirabilis HI4320 can also elicit intestinal inflammation following intestinal epithelial injury; such disease is mediated by a bacterial hemolysin that activates NLRP3 signaling in Ly6Chigh monocytes (95). These monocytes require the chemokine receptor CCR2 to secrete IL-1β and contribute to P. mirabilis-mediated disease progression (95).
Helicobacter hepaticus
Enterohepatic Helicobacter species such as H. hepaticus and H. bilis are well-known for playing a causative role in the development of liver tumors as well as intestinal inflammation and carcinogenesis in immunodeficient mice (96–98). The immunological mechanisms by which these organisms contribute to disease are best described for H. hepaticus and will therefore be highlighted here. Although H. hepaticus (NCI-Frederick isolate 1A) induces Th1 responses specific to its flagellar hook protein (FlgE) during colitis in IL-10−/− mice (99), additional studies using 129/SvEv Rag2−/− mice demonstrated that IL-23, rather than IL-12, secreted by CD11c+ monocytes was essential for developing intestinal inflammation (100–102). IL-23 induced by H. hepaticus 1A can further exacerbate disease by activating ILC-3 cells, a sub-population of innate lymphoid cells (103). IL-1β and MyD88 signaling also play critical roles in disease (104, 105). In immunocompetent mice, however, the relationship between H. hepaticus and the host immune system appears very different. Colonization of wild-type mice with H. hepaticus 1A promotes the development of Treg cells (106). Chow and Mazmanian proposed that H. hepaticus actively induces a tolerogenic response from the host through the action of a Type VI secretion system and perhaps via transcriptional suppression of genes such as TLR4, NF-κB and IL-17R in IECs. This directed effort to modulate the function of IECs may prevent the development of antigen-specific CD4+ T cell responses and intestinal inflammation (107, 108). Although further investigation is still required, it seems that in this instance the pathobiont alters host responses such that the host-microbiota balance tips from tolerance to inflammatory.
2.4 FUSOBACTERIA
Fusobacterium spp
Following characterization of the microbiota associated with colorectal cancer, a potential pathogenic role for Fusobacterium species emerged (109). Using the ApcMin/+ mouse model for tumorigenesis, F. nucleatum (strain EAVG_002; 7/1) was shown to not only potentiates tumor growth but also elicit a pro-inflammatory response similar to that of fusobacteria-associated human carcinomas, including infiltration of myeloid cells (109). Additional studies revealed that F. nucleatum uses its Fap2 protein to bind to a carbohydrate moiety present on tumor cells (110) and render them resistant to NK and T cells cytotoxic activities via direct interaction with the cell surface receptor TIGIT (111). Interestingly, mono-association of wild-type mice with F. nucleatum F0419 induced either little or no expansion of Th17 cells in the colon and small intestine, respectively, (39, 40), indicating that this organism may only significantly modulate host immune responses during disease. In contrast, F. varium robustly stimulates the mucosal immune system when it is mono-associated with wild-type germfree mice. F. varium AO16 expanded the numbers of CD103+CD11b+ dendritic cells, Foxp3−Rorγt+CD4+ T cells, Foxp3+CD4+ T cells and Foxp3+Rorγt+Helios−CD4+ T cells in the colon; however, it did not induce any changes in the populations of immune cells present in the small intestine (40). F. varium also influenced host gene expression patterns in a site-specific manner, including up-regulation of genes involved in triglyceride and arachidonic acid metabolism in the colon and down-regulation of genes in the small intestine involving bile acid and retinol metabolism and those belonging to the cytochrome p450 and Reg3 antimicrobial families (40). Kasper and colleagues hypothesized that such a distinct transcriptional signature could be acting to permit F. varium colonization of the mouse intestine (40). Importantly, these findings highlight the distinct influence of F. varium and F. nucleatum on host phenotypes, including immune responses.
2.5 ACTINOBACTERIA
Bifidobacterium adolescentis
Similar to SFB, mono-colonization of germfree mice with B. adolescentis L2-32 markedly promoted the expansion of Th17 cells in the small intestine without provoking intestinal inflammation (39). However, B. adolescentis L2-32 induced host gene expression patterns distinct from those of SFB, including genes in MHC II-dependent antigen presentation and muscle-related pathways, the latter of which suggests that non-hematopoietic cells may play a role in communicating with the host immune system during B. adolescentis colonization. Of particular interest was the finding that B. adolescentis L2-32, like SFB, exacerbated disease in a murine model of arthritis (39). These results demonstrate how the behavior of a gut symbiont can vary from commensal/mutualistic to pathobiont depending on the strain and/or its relationship with the host.
3 A COMMUNITY IS MORE THAN THE SUM OF ITS PARTS
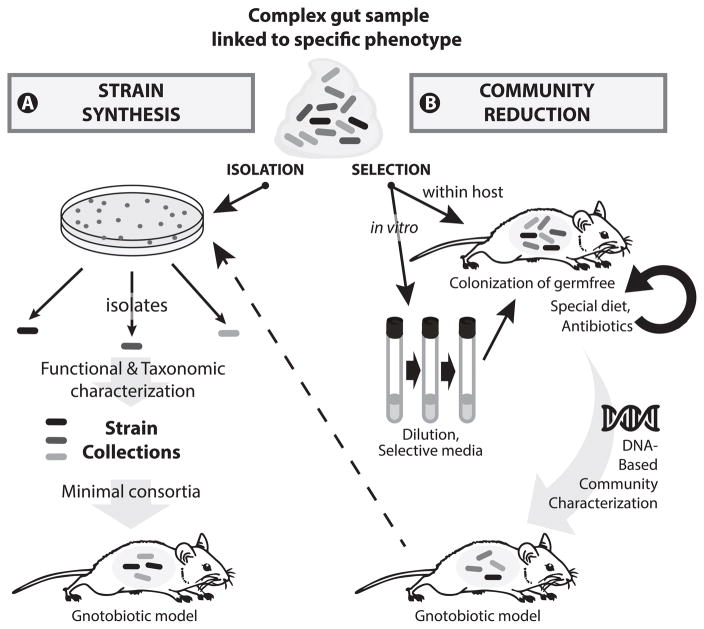
Many studies described above as well as other reports in the literature used germfree animals to test the effects of colonization by single bacterial species on host responses. Although these systems are very powerful for dissecting individual microbe-host interactions and for demonstrating causality, they are somewhat artificial because the complete absence of endogenous microorganisms most likely influences the nature and amplitude of these interactions. Hence, there is a growing interest in developing defined microbial communities of reduced complexity, referred to as minimal microbiomes (10). Figure 1 illustrates concepts for establishing these model communities based on two main approaches detailed in the paragraphs below. It is of course important to eventually test the health-modulating properties of strains under native conditions in animal colonized with endogenous complex communities (112). However, minimal microbiomes allow detailed mechanistic investigation under controlled conditions.
FIGURE 1.
From complex gut microbial community to defined experimental systems. Prerequisite for the workflows presented here is an animal model (a mouse in the present example) with a specific phenotype (e.g. chronic disease with a clear measurable endpoint) or a physiological parameter of interest (e.g. maturation of specific immune cell populations). The phenotype/parameter of interest can be associated with defined gut microbiota signatures and must be absent in germfree animals. Starting from a gut sample collected from an animal with the specific feature of interest, two routes of action can be followed: (A) The classical route is based on cultivation and isolation of strains (e.g. microbial colonies on nutrient agar) with the aim of identifying single components of the complex ecosystem of origin that can drive the phenotype. Each isolate is characterized taxonomically and functionally and deposited in public culture collections for downstream access. Based on expert knowledge or assisted by computational methods, single strains are combined together and used to colonize germfree animals with the goal of mimicking the disease phenotype or physiological parameter of interest. (B) A second, less explored route is the selection of microbial communities with the aim of obtaining the most reduced complexity still associated with the phenotype of interest. This can be achieved via iterative association of germfree animals (circled black arrow) in the presence of selective forces (e.g. special diet or antibiotics), and/or in vitro enrichment of specific microbes (e.g. via selective media or simply by serial dilution) prior to colonization of germfree animals. The advantage of performing an in vitro step prior to association with mice is bulk selection of microorganisms that can indeed grow under laboratory conditions, which can substantially facilitate downstream identification of single members of the final minimal microbiome. The progress of community reduction can be monitored throughout the workflow using targeted (16S rRNA amplicon) or shotgun metagenomic sequencing. Of note, workflow B can be followed by A (dashed arrow) for detailed characterization of single community members. The new gnotobiotic models created by either approach can ultimately be used for detailed experimental testing of microbe-microbe and microbe-host interactions.
3.1 HYPOTHESIS-DRIVEN ASSEMBLAGE VIA STRAIN SYNTHESIS
Hypothesis-driven assemblage consists of combining known microorganisms previously cultured and characterized. The hallmark of this approach is to work with single microorganisms that must be isolated from a complex starting environment.
Cultivation of individual species can be a substantial challenge for certain microorganisms that are highly sensitive to changes in physicochemical properties (e.g. redox potential or nutrient availability). Hence, strain selection hypotheses as generated by high-throughput sequencing are often hampered by the uncultured nature of candidate taxa (113). So far, the assemblage of minimal bacterial consortia has been primarily driven by expert knowledge of the phylogenetic and functional diversity of the native complex ecosystem under investigation and of phylogenetically-related single microorganisms that can be cultured. The Altered Schaedler Flora (ASF) is such a consortium that consists of 8 bacterial strains originally isolated from the intestine of mice (114). Since its establishment in the 1970s and 1980s, it has been used often as a reference defined microbiota and has therefore generated the highest number of publications describing the effects of a minimal bacterial consortium on the immune system. The ASF community has been reviewed recently in detail (115) and we have listed key studies pertaining to ASF-induced immune responses in Table 2. Although major immune parameters are normalized in ASF mice such that they closely resemble their conventionally-raised counterparts, the limited number of strains in the ASF community and the primary rationale for their selection (to standardize mouse models prior to introduction into barrier production and limit colonization by enteric pathogens) explain why not all microbial and host-derived functions are present in ASF-bearing mice, including Th17 cell maturation, numbers of IgA+ plasmablasts, and the expression of RegIII-γ (115). The SIHUMI microbiota represents another consortium of 8 strains (originating this time from the human gut), which harbors metabolic functions not described in ASF mice, such as mucin or bilirubin conversion (116, 117). SIHUMI has been utilized in mouse models of colitis, where inflammatory scores were mirrored by the production of IFN-γ, IL12-p40 and IL17 by colonic tissue explants and by mesenteric lymph node cells stimulated with lysates from members of the SIHUMI community (118); however, additional SIHUMI-induced effects on the immune system have otherwise not been described.
Table 2.
Impact of defined minimal bacterial consortia on immune parameters in vivo
| Minimal consortium (host species) | Reference | Impact on host phenotype |
|---|---|---|
| ASF (mouse) | Jergens et al. 2007 (120) | Induction of systemic IgG |
| ASF (mouse) | Ivanov et al. 2008 (197) | Lack of Th17 cells and increased proportions of Foxp3+ cells in the small intestinal lamina propria; reduced proportion of IgA+ cells compared with SPF mice |
| ASF (mouse) | Hapfelmeier et al. 2010 (198) | Induction of IgA in duodenum |
| ASF (mouse) | Feng et al. 2010 (199) | Homeostatic and spontaneous proliferation of CBir1 TCR transgenic T cells in RAG-knockout mice |
| ASF (mouse) | Geuking et al. 2011 (200) | Activation and de novo generation of colonic Treg cells |
| Bristol microbiota (pig) | Laycock et al. 2012 (133) | Increase in serum levels of immunoglobulins |
| ASF (mouse) | Mosconi et al. 2013 (201) | Induction of Thymic stromal lymphopoietin (TSLP) mRNA expression in colonic lamina propria; Induction of IL-17 and IFN-γ in TSLP-knockout mice but not C57BL/6 wildtypes |
| ASF (mouse) | Natividad et al. 2013 (202) | RegIII-γ expression in ileum and colon lower than that induced by a complex microbiota |
| 17-member clostridia cocktail (mouse) | Atarashi et al. 2013 (136) | Treg cell maturation |
| SIHUMI (mouse) | Eun et al. 2014 (118) | Increased IL-17, IL12-p40, and IFN-γ protein secretion by colonic tissue explants and MLN from IL10-knockout mice |
ASF, Altered Schaedler Flora; MLN, mesenteric lymph node; SIHUMI, simplified human intestinal microbiota; SPF, specific pathogen-free
The low complexity of ASF and SIHUMI makes them versatile systems appropriate for testing the impact of colonization by additional strains. However, there are very few corresponding reports in the literature (119–122), and none on the role of specific bacteria in the regulation of immune responses. This may be due in part to the limited public availability of minimal consortia and gut bacterial strains in general, which prevents experimental implementation. The latter shortage has been at least ameliorated by recent projects focused on the cultivation of mammalian gut bacteria and the discovery of novel taxa (123–125), including our own work that cataloged bacterial strains from the mouse intestine (126). This resource has already been used successfully for the generation of a modular minimal consortium, referred to as Oligo-MM (oligo-mouse microbiota), which conferred colonization resistance against the enteric pathogen Salmonella enterica serovar Typhimurium (127). The Oligo-MM model opens new avenues for detailed mechanistic studies in gnotobiotes under standardized and close-to-native conditions, including effects on immune responses.
Another recent study pushed the current limits of minimal microbiome-based approaches by colonizing mice with a mixture of 92 cultured bacterial strains (128). The paper focused on describing metabolic functions of the microbiota—their impact on the immune system was not described. Nevertheless, the approach is elegant and the work offers new perspectives for the field. Although the authors reported a core of colonizing strains that formed stable, dominant communities across each animal, not all 92 strains established in the mice. Variations were detected for a few taxa and may increase when population densities are lower than those detectable by sequencing. Moreover, bacterial spatial distribution in the gut was not investigated, nor was colonization of different mouse groups with the defined complex mixture. The latter experimental aspect has a high chance to result in varying microbiota composition; one could take advantage of this inter-individual variability by combining it with metabolome characterization, antigen profiling, and detailed immunophenotyping to unravel microbe-host interactions in a highly controlled manner.
Finally, one additional line of promising future research is the use of models other than mice that are better suited for translational medical research, especially pigs (129, 130). Decades ago, studies in germfree pigs/piglets highlighted the impact of gut microbiota on the immune system (131, 132). To the best of our knowledge, there have been very few attempts to implement minimal microbiomes in pig models (133). Logistics associated with the maintenance of gnotobiotic pigs and limited availability of microbial strains from the pig intestine are sound explanations for this paucity of data. However, the impact of demonstrating the causal effects of specific gut microbiota members on immune regulation in animal models with better clinical relevance would match the effort required for such work.
3.2 FUNCTIONAL ENRICHMENT VIA COMMUNITY REDUCTION
Instead of following a culture-based, reductionist approach as described in the previous section, the functional enrichment workflow relies on serial reduction of starting community’s complexity to obtain the most simplified consortium associated with the given phenotype of interest. This approach can include a combination of in vitro and in vivo enrichment strategies, as pictured in Figure 1.
By focusing on target functions of the native ecosystem (e.g. the ability to induce a certain disease phenotype), the major advantage of this second approach to generate minimal microbiomes is that it bypasses the major risk of not recapitulating the desired phenotype when working with single isolates or a combination thereof. However, the workload associated with multiple germfree experiments necessary for functional enrichment can rapidly become a bottleneck, and the final consortium of reduced diversity may still contain unknown taxa that complicate interpretation of results.
To the best of our knowledge, there are very few examples of functional enrichment approaches that generated defined communities. Although not related to immune responses, a recent seminal study illustrates well the principle of in vivo community reduction (134). The authors showed that providing a diet low in fermentable polysaccharides to mice previously colonized with a human fecal microbiota reduced the relative abundance of many taxa (as detected by sequencing), illustrating a loss of dominant microbiota members. Although this effect was reversed when a high polysaccharide diet was re-introduced to the same mice, constant feeding of the low polysaccharide diet across four generations resulted in irreversible, long-term loss of taxa.
Another example of effector strain enrichment, this time in the context of inflammation, is the in vitro enrichment of lactobacilli from the murine gut for treatment of Citrobacter rodentium colitis. These lactobacilli reduced disease severity, improved gut barrier function and decreased mRNA expression of IFN-γ and TNF in the distal colon (135). Of note, treatment of C. rodentium infected mice with MRS alone (the medium used for in vitro enrichment) also tended to provide similar benefits and was associated with increased abundance of endogenous lactobacilli.
Perhaps the best known example of a successful community reduction approach in the context of immune modulation is the induction of Treg cells by a mixture of butyrate-producing clostridia (136, 137). The authors followed an elegant procedure with several steps of culture enrichments (e.g. using chloroform) prior to inoculation into germfree recipient mice; eventually they cultivated single strains and characterized a 17-member clostridium cocktail (138). Unfortunately, the authors did not provide full taxonomic classification of the strains that, although published, still do not have standing in the nomenclature and are not publically available. Moreover, effects of metabolic functions other than butyrate production (e.g. bile acid dehydroxylation by the Clostridium scindens strain) may be worth investigating.
The minimal microbial consortia described here clearly impact host physiology—some are even documented to influence immune responses. Although the exact mechanisms underlying these host-microbe interactions have yet to be described, they likely involve microbial metabolites capable of modulating cellular microenvironments. In the next section, we give examples of microbiota-derived metabolites than can influence immune responses, with a special focus on lipids.
4 LIPIDS AND THEIR IMPACT ON IMMUNE RESPONSES
Lipids represent a very complex family of dietary, host, and microbial compounds. Their direct roles in regulating immunity has been reviewed elsewhere (139, 140); however, the impact of gut microorganisms on lipid-based modulation of the immune system is less known. The immunoregulatory activities of short-chain fatty acids (SCFA) produced by gut bacteria via the fermentation of carbohydrates and proteins has been already studied and reviewed extensively (141, 142) and will be described only briefly in the next section. The gut microbiota can also metabolize dietary lipids, cholesterol, and host-derived cholesterol metabolites such as steroids and bile acids (143–145). Hence, gut bacteria have the potential to alter the bioavailability and bioactivities of these compounds and thereby alter corresponding signaling networks. When investigating the effects of gut microbial metabolites, it is important to keep in mind that the primary function of the digestive system is efficient absorption of dietary components and that many absorptive processes, including those for lipids, take place in proximal parts of the small intestine, whereas the densest microbial communities are found in the colon. That being said, the amount of metabolites that reach distal parts of the intestine may still be substantial depending on environmental (e.g. diet) and host genetic factors (e.g. transporter efficacy, de novo synthesis capacities). Combined with the fact that lipid-dependent signaling pathways are sensitive, it is sound to assume that the gut microbiota influences lipid-driven regulation of immune responses. The implementation of minimal microbiome-based approaches for studying these interactions will open new avenues of research and applications and facilitate testing the causative role of gut microbes in health regulation and identifying direct versus indirect effects.
4.1 SHORT-CHAIN FATTY ACIDS (SCFA)
SCFA are metabolic by-products of gut microbiota fermentation of dietary substrates, including fibers. The most abundant of these well-studied bacterial metabolites include acetate, propionate, and butyrate, the latter of which serves as an important fuel source for IECs (146). Additionally, SCFA regulate the functions of a wide variety of both innate and adaptive immune cells primarily via the G protein-coupled receptors (GPCRs) GPR43 and GPR41. SCFA can also directly impact the actions of immune cells via GPR-independent mechanisms, including inhibition of histone deacetylase (HDAC) activity, which regulates gene transcription (142). Both mechanisms can be observed in SCFA regulation of innate immune cells, including macrophages. Butyrate in particular limits intestinal macrophage production of the pro-inflammatory mediators nitric oxide, IL-6, and IL-12 (57) and regulates intestinal macrophage polarization via histone acetylation (147). Butyrate also signals via Gpr109a on colonic macrophages to limit inflammation and maintain intestinal homeostasis (148). SCFAs can also induce tolerogenic responses in murine mucosal dendritic cells (149) and impart anti-inflammatory effects onto human monocyte-derived dendritic cells (150). SCFAs also impact the functions of polymorphonuclear cells (PMN, e.g., neutrophils), another type of innate immune cell. SCFA promote neutrophil activation and migration via upregulation of adhesion molecules and increased chemokine production (151, 152). However, they can also dampen neutrophil effector functions such as phagocytosis, bacterial killing and cytokine production (153).
Many reports in the literature also describe the profound impact of SCFA on the adaptive immune system, particularly T cell phenotypes. Butyrate induces differentiation of colonic and peripheral Treg cells in mice, potentially via histone modification of the Foxp3 locus (58, 137). Additionally, acetate and propionate regulate colonic Treg cell numbers and functions via GPR43 signaling in mice (154). More recently, optimal development of memory CD8+ T cells has been shown to require acetate (155). Humoral immune responses are also influenced by SCFA. Recently, Kim and colleagues showed that SCFA regulate metabolism and gene expression in B cells, which enhances antibody production (156). Additional studies revealed that acetate influenced vitamin A metabolism via GPR43 signaling in dendritic cells, which ultimately promoted intestinal B cell production of IgA (157). Additional studies are needed to determine if SCFA also impact humoral immunity via HDAC activity.
Considering that SCFA are absorbed by the gut and reach systemic circulation via the portal vein, these immunomodulatory metabolites can impact both local and systemic responses and may be beneficial for treating immune-mediated diseases. The anti-inflammatory properties of SCFA treatments have been observed in mouse models of colitis (137, 158) as well as in human patients, who experienced some improvements in disease following colonic irrigation with SCFA (159). Probiotic treatment with butyrate-producing bacterial strains such as Clostridium butyricum represents another potential therapy and showed promising results when administered to ulcerative colitis patients (160). Studies in mice suggest that C. butyricum limits colitis by triggering IL-10 production from intestinal macrophages (45). More recently, C. butyricum was shown to attenuate steatohepatitis in mice partially through butyrate-induced immunomodulation in the liver that included promoting Treg and limiting Th1 and Th17 cell development (161). Additional efforts to identify strains of gut bacteria that can provide a source of SCFA for treatment of inflammatory diseases are clearly warranted.
Such therapeutic approaches may also one day benefit patients with systemic immune-mediated diseases. Mice fed a high-fiber diet experienced increased circulating levels of SCFA and were protected against allergic airway disease (162). Treatment with propionate seeded the lungs with dendritic cells impaired in their ability to induce T-helper type-2 cell effector functions. In another pre-clinical study, acetate and propionate protected mice against development of experimental autoimmune encephalomyelitis, which was associated with an increase in Treg cells and a decrease in Th1 cells; however, providing SCFA in the drinking water or feeding a high-fiber diet exacerbated antibody-induced arthritis (163). Such dichotomous findings emphasize caution as these novel treatments are explored further.
4.2 OTHER LIPIDS
Lipid-binding receptors are known to influence inflammatory responses (140). Because the nature of these responses depends on lipid type and amount, the metabolic functions of gut bacteria such as fatty acid isomerase (164), lipid storage (165, 166), breakdown of triglycerides by lipase activity (145), or conversion of cholesterol (143, 167) are likely to influence immune reactions. For instance, cholesterol has the potential to induce intestinal inflammation and chemical inhibition of cholesterol absorption was shown to diminish systemic inflammatory markers and the severity of atherosclerosis (168, 169).
Bile acids represent one family of cholesterol-derived metabolites produced by the host that are found in high concentrations in the intestine, are metabolized by gut bacteria, and influence multiple inflammatory and metabolic signaling pathways. Genetic variants in one of the main bile acid-responsive transcription factor FXR (farnesoid X receptor) have been associated with the occurrence of inflammatory bowel diseases (IBD) (170), and alterations of bile acid pools have been reported in IBD patients (171). Mechanistic studies in mice demonstrated that interfering with bile acid signaling via FXR and TGR5 (also known as GPBAR1, G protein-coupled bile acid receptor 1) influence TLR-dependent pathways, chemically induced colitis, and NRLP3-dependent inflammasome activation (172–174). In vitro, bile acids regulate cytokine expression and cellular stress pathways (175–177). In contrast to these clinically or mechanistically relevant data, there is no experimental evidence for the impact of microbial conversion of bile acids on the diversity and activity of immune cell populations. In this respect, the use of minimal microbial consortia varying in their ability to produce specific secondary bile acids will be of help. Pioneering studies based on the colonization of germfree rodents with bile acid-converting strains have been reported (178, 179), but pathophysiological effects were not studied and the strains were not well characterized and are no longer available. More recent work successfully established a gnotobiotic mouse model colonized with a minimal bacterial consortium producing secondary bile acids (180, 181); however, the strains are also not readily available and the impact on immune responses was not investigated.
5 CONCLUSIONS AND OUTLOOK
Work in the 1960s first demonstrated the important influence of gut microorganisms on mouse phenotypes (182). Today there is a renewed interest in understanding the impact of changes in microbiota composition (e.g. due to animal housing) on immune responses (65, 183). It is therefore of utmost importance to dissect the effects of specific gut bacteria on the host so we can identify the microbe-host interaction networks that shape the immune system, with the ultimate goal of manipulating these interactions for positive health outcomes. The isolation and culture of microorganisms is essential to this process as it enables working with specific taxa (as single strains or simplified communities) in downstream experiments.
Catalogues of human and mouse gut bacteria have been expanded substantially thanks to recent culture-based work (123, 124, 126). Such strain collections represent an important foundation for controlled, mechanistic studies. Efforts to isolate and identify additional species should be continued or even intensified in the near future, especially with respect to efficient taxonomic description of novel taxa (184, 185) and to functional characterization of strains and their impact on immune functions (40). For the latter purpose, public accessibility of strains is paramount for performing mechanistic analyses (186). In future work, it will also be important to assess in greater detail the role of gut microorganisms other than bacteria in modulating the immune system.
Research based on the use of minimal microbiomes is now entering an accelerated phase of development. We have new opportunities to implement microbial consortia that better resemble native gut communities; such advances will also provide standardized models that are comparable between labs. Most importantly, minimal microbiomes can be used in a modular fashion—when combined with an ever-increasing availability of strains, they will facilitate determining the effects of specific taxa in the presence of endogenous microorganisms and thereby provide an important proof-of-concept in pre-clinical research. To support the modular design of minimal microbiomes, future work should implement novel bioinformatic approaches that infer the best possible mixtures of cultured strains (e.g. based on sequencing data from dysbiotic native communities). Model communities can also be used to study host specificity of colonization and immune system maturation. Without going into detail because already discussed in detail elsewhere (10, 126, 187–190), the study of underlying molecular mechanisms using strains from various host species will also be a fascinating avenue of future research.
Although microbiome and host-gut microbe interactions research has garnered much attention in the last decade, descriptive and pre-clinical studies still dominate the literature and translation into human settings has been rather slow. Clostridium difficile enteric infections represent a showcase for the efficient use of minimal microbiomes in the clinic (191); however, this advancement likely relates to exclusion of an undesirable strain by commensals via microbe-microbe interactions and does not involve modulation of host responses as is likely needed for treatment of immune-mediated diseases. Manipulation of immune functions with microbiome-based therapeutics will likely require a more tailored approach that involves targeting specific host-microbe relationships. We may also need to intervene during the host’s early life when gut communities are established and the immune system educated (18). Finally, we should also consider that targeted microbiome modulation could also improve the efficacy of conventional medications intended to augment immune responses (192). These and other microbiome-based interventions that modulate immunity represent an exciting new horizon for biomedical research.
Acknowledgments
This work was supported in part by the German Research Foundation (grant no. CL481/2-1). A.E.R.T. acknowledges funding from the National Institute of General Medical Sciences of the National Institutes of Health (P20GM104320), the Crohn’s and Colitis Foundation of America (grant # 3578), and the University of Nebraska–Lincoln.
Footnotes
CONFLICTS OF INTEREST
The authors do not have any conflicts of interest
References
- 1.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 2.Walter J, Ley R. The human gut microbiome: ecology and recent evolutionary changes. Annu Rev Microbiol. 2011;65:411–429. doi: 10.1146/annurev-micro-090110-102830. [DOI] [PubMed] [Google Scholar]
- 3.Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The intestinal microbiome in early life: health and disease. Front Immunol. 2014;5:427. doi: 10.3389/fimmu.2014.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forbes JD, Van Domselaar G, Bernstein CN. The Gut Microbiota in Immune-Mediated Inflammatory Diseases. Front Microbiol. 2016;7:1081. doi: 10.3389/fmicb.2016.01081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCoy KD, Koller Y. New developments providing mechanistic insight into the impact of the microbiota on allergic disease. Clin Immunol. 2015;159:170–176. doi: 10.1016/j.clim.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sartor RB, Mazmanian SK. Intestinal microbes in inflammatory bowel diseases. Am J Gastroenterol Suppl. 2012;1:15–21. [Google Scholar]
- 7.Bauer H, Paronetto F, Burns WA, Einheber A. The enhancing effect of the microbial flora on macrophage function and the immune response. A study in germfree mice. J Exp Med. 1966;123:1013–1024. doi: 10.1084/jem.123.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiebiger U, Bereswill S, Heimesaat MM. Dissecting the Interplay Between Intestinal Microbiota and Host Immunity in Health and Disease: Lessons Learned from Germfree and Gnotobiotic Animal Models. Eur J Microbiol Immunol (Bp) 2016;6:253–271. doi: 10.1556/1886.2016.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horowitz RE, Bauer H, Paronetto F, Abrams GD, Watkins KC, Popper H. The response of the lymphatic tissue to bacterial antigen. Studies in germfree mice. Am J Pathol. 1964;44:747–761. [PMC free article] [PubMed] [Google Scholar]
- 10.Macpherson AJ, McCoy KD. Standardised animal models of host microbial mutualism. Mucosal Immunol. 2015;8:476–486. doi: 10.1038/mi.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauer H, Horowitz RE, Levenson SM, Popper H. The response of the lymphatic tissue to the microbial flora. Studies on germfree mice. Am J Pathol. 1963;42:471–483. [PMC free article] [PubMed] [Google Scholar]
- 12.Cahenzli J, Koller Y, Wyss M, Geuking MB, McCoy KD. Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe. 2013;14:559–570. doi: 10.1016/j.chom.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Aidy S, van Baarlen P, Derrien M, et al. Temporal and spatial interplay of microbiota and intestinal mucosa drive establishment of immune homeostasis in conventionalized mice. Mucosal Immunol. 2012;5:567–579. doi: 10.1038/mi.2012.32. [DOI] [PubMed] [Google Scholar]
- 14.Hansen CH, Nielsen DS, Kverka M, et al. Patterns of early gut colonization shape future immune responses of the host. PLoS One. 2012;7:e34043. doi: 10.1371/journal.pone.0034043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olszak T, An D, Zeissig S, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto M, Yamaguchi R, Munakata K, et al. A microarray analysis of gnotobiotic mice indicating that microbial exposure during the neonatal period plays an essential role in immune system development. BMC Genomics. 2012;13:335. doi: 10.1186/1471-2164-13-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352:539–544. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torow N, Hornef MW. The Neonatal Window of Opportunity: Setting the Stage for Life-Long Host-Microbial Interaction and Immune Homeostasis. J Immunol. 2017;198:557–563. doi: 10.4049/jimmunol.1601253. [DOI] [PubMed] [Google Scholar]
- 19.Goyal MS, Venkatesh S, Milbrandt J, Gordon JI, Raichle ME. Feeding the brain and nurturing the mind: Linking nutrition and the gut microbiota to brain development. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:14105–14112. doi: 10.1073/pnas.1511465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornef M, Penders J. Does a prenatal bacterial microbiota exist? Mucosal Immunol. 2017;10:598–601. doi: 10.1038/mi.2016.141. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Munoz ME, Arrieta MC, Ramer-Tait AE, Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome. 2017;5:48. doi: 10.1186/s40168-017-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savage DC. Factors involved in colonization of the gut epithelial surface. Am J Clin Nutr. 1978;31:S131–S135. doi: 10.1093/ajcn/31.10.S131. [DOI] [PubMed] [Google Scholar]
- 23.Schaedler RW, Dubos R, Costello R. The development of the bacterial flora in the gastrointestinal tract of mice. J Exp Med. 1965;122:59–66. doi: 10.1084/jem.122.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035S-1045S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 25.Wopereis H, Oozeer R, Knipping K, Belzer C, Knol J. The first thousand days - intestinal microbiology of early life: establishing a symbiosis. Pediatr Allergy Immunol. 2014;25:428–438. doi: 10.1111/pai.12232. [DOI] [PubMed] [Google Scholar]
- 26.Hill DA, Siracusa MC, Abt MC, et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat Med. 2012;18:538–546. doi: 10.1038/nm.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reikvam DH, Erofeev A, Sandvik A, et al. Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PLoS One. 2011;6:e17996. doi: 10.1371/journal.pone.0017996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lepage P, Leclerc MC, Joossens M, et al. A metagenomic insight into our gut’s microbiome. Gut. 2013;62:146–158. doi: 10.1136/gutjnl-2011-301805. [DOI] [PubMed] [Google Scholar]
- 29.Morgan XC, Huttenhower C. Chapter 12: Human microbiome analysis. PLoS Comput Biol. 2012;8:e1002808. doi: 10.1371/journal.pcbi.1002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plovier H, Everard A, Druart C, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23:107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 31.Lagkouvardos I, Overmann J, Clavel T. Cultured microbes represent a substantial fraction of the human and mouse gut microbiota. Gut Microbes. 2017 doi: 10.1080/19490976.2017.1320468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Schillde MA, Hormannsperger G, Weiher M, et al. Lactocepin secreted by Lactobacillus exerts anti-inflammatory effects by selectively degrading proinflammatory chemokines. Cell Host Microbe. 2012;11:387–396. doi: 10.1016/j.chom.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Yan F, Liu L, Dempsey PJ, et al. A Lactobacillus rhamnosus GG-derived soluble protein, p40, stimulates ligand release from intestinal epithelial cells to transactivate epidermal growth factor receptor. J Biol Chem. 2013;288:30742–30751. doi: 10.1074/jbc.M113.492397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lakhdari O, Cultrone A, Tap J, et al. Functional metagenomics: a high throughput screening method to decipher microbiota-driven NF-kappaB modulation in the human gut. PLoS One. 2010:5. doi: 10.1371/journal.pone.0013092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yissachar N, Zhou Y, Ung L, et al. An Intestinal Organ Culture System Uncovers a Role for the Nervous System in Microbe-Immune Crosstalk. Cell. 2017;168:1135–1148 e1112. doi: 10.1016/j.cell.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ericsson AC, Franklin CL. Manipulating the Gut Microbiota: Methods and Challenges. ILAR J. 2015;56:205–217. doi: 10.1093/ilar/ilv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahern PP, Faith JJ, Gordon JI. Mining the human gut microbiota for effector strains that shape the immune system. Immunity. 2014;40:815–823. doi: 10.1016/j.immuni.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faith JJ, Ahern PP, Ridaura VK, Cheng J, Gordon JI. Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci Transl Med. 2014;6:220ra211. doi: 10.1126/scitranslmed.3008051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan TG, Sefik E, Geva-Zatorsky N, et al. Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E8141–E8150. doi: 10.1073/pnas.1617460113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geva-Zatorsky N, Sefik E, Kua L, et al. Mining the Human Gut Microbiota for Immunomodulatory Organisms. Cell. 2017;168:928–943 e911. doi: 10.1016/j.cell.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clavel T, Lagkouvardos I, Blaut M, Stecher B. The mouse gut microbiome revisited: From complex diversity to model ecosystems. International journal of medical microbiology: IJMM. 2016;306:316–327. doi: 10.1016/j.ijmm.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Schloissnig S, Arumugam M, Sunagawa S, et al. Genomic variation landscape of the human gut microbiome. Nature. 2013;493:45–50. doi: 10.1038/nature11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown Kav A, Sasson G, Jami E, Doron-Faigenboim A, Benhar I, Mizrahi I. Insights into the bovine rumen plasmidome. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5452–5457. doi: 10.1073/pnas.1116410109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins MD, Lawson PA, Willems A, et al. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol. 1994;44:812–826. doi: 10.1099/00207713-44-4-812. [DOI] [PubMed] [Google Scholar]
- 45.Hayashi A, Sato T, Kamada N, et al. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe. 2013;13:711–722. doi: 10.1016/j.chom.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 46.Kashiwagi I, Morita R, Schichita T, et al. Smad2 and Smad3 Inversely Regulate TGF-beta Autoinduction in Clostridium butyricum-Activated Dendritic Cells. Immunity. 2015;43:65–79. doi: 10.1016/j.immuni.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Lopez-Siles M, Martinez-Medina M, Abella C, et al. Mucosa-associated Faecalibacterium prausnitzii phylotype richness is reduced in patients with inflammatory bowel disease. Appl Environ Microbiol. 2015;81:7582–7592. doi: 10.1128/AEM.02006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sokol H, Seksik P, Furet JP, et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15:1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi K, Nishida A, Fujimoto T, et al. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion. 2016;93:59–65. doi: 10.1159/000441768. [DOI] [PubMed] [Google Scholar]
- 50.Rigottier-Gois L. Dysbiosis in inflammatory bowel diseases: the oxygen hypothesis. ISME J. 2013;7:1256–1261. doi: 10.1038/ismej.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rivera-Chavez F, Zhang LF, Faber F, et al. Depletion of Butyrate-Producing Clostridia from the Gut Microbiota Drives an Aerobic Luminal Expansion of Salmonella. Cell Host Microbe. 2016;19:443–454. doi: 10.1016/j.chom.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin R, Miquel S, Chain F, et al. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol. 2015;15:67. doi: 10.1186/s12866-015-0400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miquel S, Martin R, Rossi O, et al. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol. 2013;16:255–261. doi: 10.1016/j.mib.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 54.Rossi O, van Berkel LA, Chain F, et al. Faecalibacterium prausnitzii A2-165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses. Sci Rep. 2016;6:18507. doi: 10.1038/srep18507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quevrain E, Maubert MA, Michon C, et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut. 2016;65:415–425. doi: 10.1136/gutjnl-2014-307649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson CL, Vier R, Mikaelyan A, Wienemann T, Brune A. ‘Candidatus Arthromitus’ revised: segmented filamentous bacteria in arthropod guts are members of Lachnospiraceae. Environ Microbiol. 2012;14:1454–1465. doi: 10.1111/j.1462-2920.2012.02731.x. [DOI] [PubMed] [Google Scholar]
- 60.Yin Y, Wang Y, Zhu L, et al. Comparative analysis of the distribution of segmented filamentous bacteria in humans, mice and chickens. Isme j. 2013;7:615–621. doi: 10.1038/ismej.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klaasen HL, Koopman JP, Van den Brink ME, Bakker MH, Poelma FG, Beynen AC. Intestinal, segmented, filamentous bacteria in a wide range of vertebrate species. Lab Anim. 1993;27:141–150. doi: 10.1258/002367793780810441. [DOI] [PubMed] [Google Scholar]
- 62.Ericsson AC, Hagan CE, Davis DJ, Franklin CL. Segmented filamentous bacteria: commensal microbes with potential effects on research. Comp Med. 2014;64:90–98. [PMC free article] [PubMed] [Google Scholar]
- 63.Sano T, Huang W, Hall JA, et al. An IL-23R/IL-22 Circuit Regulates Epithelial Serum Amyloid A to Promote Local Effector Th17 Responses. Cell. 2015;163:381–393. doi: 10.1016/j.cell.2015.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atarashi K, Tanoue T, Ando M, et al. Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell. 2015;163:367–380. doi: 10.1016/j.cell.2015.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ivanov, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goto Y, Panea C, Nakato G, et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity. 2014;40:594–607. doi: 10.1016/j.immuni.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Panea C, Farkas AM, Goto Y, et al. Intestinal Monocyte-Derived Macrophages Control Commensal-Specific Th17 Responses. Cell Rep. 2015;12:1314–1324. doi: 10.1016/j.celrep.2015.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 69.Stepankova R, Powrie F, Kofronova O, et al. Segmented filamentous bacteria in a defined bacterial cocktail induce intestinal inflammation in SCID mice reconstituted with CD45RBhigh CD4+ T cells. Inflamm Bowel Dis. 2007;13:1202–1211. doi: 10.1002/ibd.20221. [DOI] [PubMed] [Google Scholar]
- 70.Wu HJ, Ivanov, Darce J, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 73.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 74.Shen Y, Giardino Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12:509–520. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Round JL, Lee SM, Li J, et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dasgupta S, Erturk-Hasdemir D, Ochoa-Reparaz J, Reinecker HC, Kasper DL. Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms. Cell Host Microbe. 2014;15:413–423. doi: 10.1016/j.chom.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.An D, Oh SF, Olszak T, et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell. 2014;156:123–133. doi: 10.1016/j.cell.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y, Telesford KM, Ochoa-Reparaz J, et al. An intestinal commensal symbiosis factor controls neuroinflammation via TLR2-mediated CD39 signalling. Nat Commun. 2014;5:4432. doi: 10.1038/ncomms5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ochoa-Reparaz J, Mielcarz DW, Wang Y, et al. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3:487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y, Begum-Haque S, Telesford KM, et al. A commensal bacterial product elicits and modulates migratory capacity of CD39(+) CD4 T regulatory subsets in the suppression of neuroinflammation. Gut Microbes. 2014;5:552–561. doi: 10.4161/gmic.29797. [DOI] [PubMed] [Google Scholar]
- 81.Wu S, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hecht AL, Casterline BW, Earley ZM, Goo YA, Goodlett DR, Bubeck Wardenburg J. Strain competition restricts colonization of an enteric pathogen and prevents colitis. EMBO Rep. 2016;17:1281–1291. doi: 10.15252/embr.201642282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wagner VE, Dey N, Guruge J, et al. Effects of a gut pathobiont in a gnotobiotic mouse model of childhood undernutrition. Sci Transl Med. 2016;8:366ra164. doi: 10.1126/scitranslmed.aah4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tenaillon O, Skurnik D, Picard B, Denamur E. The population genetics of commensal Escherichia coli. Nat Rev Microbiol. 2010;8:207–217. doi: 10.1038/nrmicro2298. [DOI] [PubMed] [Google Scholar]
- 85.Parry SH, Allen WD, Porter P. Intestinal immune response to E. coli antigens in the germ-free chicken. Immunology. 1977;32:731–741. [PMC free article] [PubMed] [Google Scholar]
- 86.Porter P, Kenworthy R, Noakes DE, Allen WD. Intestinal antibody secretion in the young pig in response to oral immunization with Escherichia coli. Immunology. 1974;27:841–853. [PMC free article] [PubMed] [Google Scholar]
- 87.Sprinz H, Kundel DW, Dammin GJ, Horowitz RE, Schneider H, Formal SB. The response of the germfree guinea pig to oral bacterial challenge with Escherichia coli and Shigella flexneri. Am J Pathol. 1961;39:681–695. [PMC free article] [PubMed] [Google Scholar]
- 88.Deriu E, Liu JZ, Pezeshki M, et al. Probiotic bacteria reduce salmonella typhimurium intestinal colonization by competing for iron. Cell Host Microbe. 2013;14:26–37. doi: 10.1016/j.chom.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maltby R, Leatham-Jensen MP, Gibson T, Cohen PS, Conway T. Nutritional basis for colonization resistance by human commensal Escherichia coli strains HS and Nissle 1917 against E. coli O157:H7 in the mouse intestine. PLoS One. 2013;8:e53957. doi: 10.1371/journal.pone.0053957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Darfeuille-Michaud A, Boudeau J, Bulois P, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology. 2004;127:412–421. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 91.Boudeau J, Glasser AL, Masseret E, Joly B, Darfeuille-Michaud A. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn’s disease. Infect Immun. 1999;67:4499–4509. doi: 10.1128/iai.67.9.4499-4509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Viladomiu M, Kivolowitz C, Abdulhamid A, et al. IgA-coated E. coli enriched in Crohn’s disease spondyloarthritis promote TH17-dependent inflammation. Sci Transl Med. 2017:9. doi: 10.1126/scitranslmed.aaf9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garrett WS, Gallini CA, Yatsunenko T, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garrett WS, Lord GM, Punit S, et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Seo SU, Kamada N, Munoz-Planillo R, et al. Distinct Commensals Induce Interleukin-1beta via NLRP3 Inflammasome in Inflammatory Monocytes to Promote Intestinal Inflammation in Response to Injury. Immunity. 2015;42:744–755. doi: 10.1016/j.immuni.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maggio-Price L, Bielefeldt-Ohmann H, Treuting P, et al. Dual infection with Helicobacter bilis and Helicobacter hepaticus in p-glycoprotein-deficient mdr1a−/− mice results in colitis that progresses to dysplasia. Am J Pathol. 2005;166:1793–1806. doi: 10.1016/S0002-9440(10)62489-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ward JM, Anver MR, Haines DC, et al. Inflammatory large bowel disease in immunodeficient mice naturally infected with Helicobacter hepaticus. Lab Anim Sci. 1996;46:15–20. [PubMed] [Google Scholar]
- 98.Ward JM, Fox JG, Anver MR, et al. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst. 1994;86:1222–1227. doi: 10.1093/jnci/86.16.1222. [DOI] [PubMed] [Google Scholar]
- 99.Kullberg MC, Andersen JF, Gorelick PL, et al. Induction of colitis by a CD4+ T cell clone specific for a bacterial epitope. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15830–15835. doi: 10.1073/pnas.2534546100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hue S, Ahern P, Buonocore S, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Arnold IC, Mathisen S, Schulthess J, Danne C, Hegazy AN, Powrie F. CD11c(+) monocyte/macrophages promote chronic Helicobacter hepaticus-induced intestinal inflammation through the production of IL-23. Mucosal Immunol. 2016;9:352–363. doi: 10.1038/mi.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kullberg MC, Jankovic D, Feng CG, et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buonocore S, Ahern PP, Uhlig HH, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Asquith MJ, Boulard O, Powrie F, Maloy KJ. Pathogenic and protective roles of MyD88 in leukocytes and epithelial cells in mouse models of inflammatory bowel disease. Gastroenterology. 2010;139:519–529. 529 e511–512. doi: 10.1053/j.gastro.2010.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Coccia M, Harrison OJ, Schiering C, et al. IL-1beta mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med. 2012;209:1595–1609. doi: 10.1084/jem.20111453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kullberg MC, Jankovic D, Gorelick PL, et al. Bacteria-triggered CD4(+) T regulatory cells suppress Helicobacter hepaticus-induced colitis. J Exp Med. 2002;196:505–515. doi: 10.1084/jem.20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chow J, Mazmanian SK. A pathobiont of the microbiota balances host colonization and intestinal inflammation. Cell Host Microbe. 2010;7:265–276. doi: 10.1016/j.chom.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chow J, Tang H, Mazmanian SK. Pathobionts of the gastrointestinal microbiota and inflammatory disease. Curr Opin Immunol. 2011;23:473–480. doi: 10.1016/j.coi.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Abed J, Emgard JE, Zamir G, et al. Fap2 Mediates Fusobacterium nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell Host Microbe. 2016;20:215–225. doi: 10.1016/j.chom.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gur C, Ibrahim Y, Isaacson B, et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42:344–355. doi: 10.1016/j.immuni.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pedersen HK, Gudmundsdottir V, Nielsen HB, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376–381. doi: 10.1038/nature18646. [DOI] [PubMed] [Google Scholar]
- 113.Schaubeck M, Clavel T, Calasan J, et al. Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut. 2016;65:225–237. doi: 10.1136/gutjnl-2015-309333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dewhirst FE, Chien CC, Paster BJ, et al. Phylogeny of the defined murine microbiota: altered Schaedler flora. Appl Environ Microbiol. 1999;65:3287–3292. doi: 10.1128/aem.65.8.3287-3292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wymore Brand M, Wannemuehler MJ, Phillips GJ, et al. The Altered Schaedler Flora: Continued Applications of a Defined Murine Microbial Community. ILAR J. 2015;56:169–178. doi: 10.1093/ilar/ilv012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Becker N, Kunath J, Loh G, Blaut M. Human intestinal microbiota: characterization of a simplified and stable gnotobiotic rat model. Gut Microbes. 2011;2:25–33. doi: 10.4161/gmic.2.1.14651. [DOI] [PubMed] [Google Scholar]
- 117.Norin E, Midtvedt T. Intestinal microflora functions in laboratory mice claimed to harbor a “normal” intestinal microflora. Is the SPF concept running out of date? Anaerobe. 2010;16:311–313. doi: 10.1016/j.anaerobe.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 118.Eun CS, Mishima Y, Wohlgemuth S, et al. Induction of bacterial antigen-specific colitis by a simplified human microbiota consortium in gnotobiotic interleukin-10−/− mice. Infect Immun. 2014;82:2239–2246. doi: 10.1128/IAI.01513-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ganesh BP, Klopfleisch R, Loh G, Blaut M. Commensal Akkermansia muciniphila exacerbates gut inflammation in Salmonella Typhimurium-infected gnotobiotic mice. PLoS One. 2013;8:e74963. doi: 10.1371/journal.pone.0074963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jergens AE, Wilson-Welder JH, Dorn A, et al. Helicobacter bilis triggers persistent immune reactivity to antigens derived from the commensal bacteria in gnotobiotic C3H/HeN mice. Gut. 2007;56:934–940. doi: 10.1136/gut.2006.099242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li X, Ellis ML, Dowell AE, et al. Response of germ-free mice to colonization with O. formigenes and altered Schaedler flora. Appl Environ Microbiol. 2016 doi: 10.1128/AEM.02381-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Woting A, Pfeiffer N, Loh G, Klaus S, Blaut M. Clostridium ramosum promotes high-fat diet-induced obesity in gnotobiotic mouse models. MBio. 2014;5:e01530–01514. doi: 10.1128/mBio.01530-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Browne HP, Forster SC, Anonye BO, et al. Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature. 2016;533:543–546. doi: 10.1038/nature17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lagier JC, Khelaifia S, Alou MT, et al. Culture of previously uncultured members of the human gut microbiota by culturomics. Nat Microbiol. 2016;1:16203. doi: 10.1038/nmicrobiol.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 125.Schnupf P, Gaboriau-Routhiau V, Gros M, et al. Growth and host interaction of mouse segmented filamentous bacteria in vitro. Nature. 2015;520:99–103. doi: 10.1038/nature14027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lagkouvardos I, Pukall R, Abt B, et al. The Mouse Intestinal Bacterial Collection (miBC) provides host-specific insight into cultured diversity and functional potential of the gut microbiota. Nat Microbiol. 2016;1:16131. doi: 10.1038/nmicrobiol.2016.131. [DOI] [PubMed] [Google Scholar]
- 127.Brugiroux S, Beutler M, Pfann C, et al. Genome-guided design of a defined mouse microbiota that confers colonization resistance against Salmonella enterica serovar Typhimurium. Nat Microbiol. 2016;2:16215. doi: 10.1038/nmicrobiol.2016.215. [DOI] [PubMed] [Google Scholar]
- 128.Hibberd MC, Wu M, Rodionov DA, et al. Mechanistic studies of the effects of micronutrient deficiencies on the human gut microbiota. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aal4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gonzalez LM, Moeser AJ, Blikslager AT. Porcine models of digestive disease: the future of large animal translational research. Transl Res. 2015;166:12–27. doi: 10.1016/j.trsl.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Merrifield CA, Lewis M, Claus SP, et al. A metabolic system-wide characterisation of the pig: a model for human physiology. Mol Biosyst. 2011;7:2577–2588. doi: 10.1039/c1mb05023k. [DOI] [PubMed] [Google Scholar]
- 131.Kim YB, Bradley SG, Watson DW. Ontogeny of the immune response. I. Development of immunoglobulins in germfree and conventional colostrum-deprived piglets. J Immunol. 1966;97:52–63. [PubMed] [Google Scholar]
- 132.Miniats OP, Mitchell L, Barnum DA. Response of gnotobiotic pigs to Escherichia coli. Can J Comp Med. 1970;34:269–276. [PMC free article] [PubMed] [Google Scholar]
- 133.Laycock G, Sait L, Inman C, et al. A defined intestinal colonization microbiota for gnotobiotic pigs. Vet Immunol Immunopathol. 2012;149:216–224. doi: 10.1016/j.vetimm.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 134.Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529:212–215. doi: 10.1038/nature16504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vong L, Pinnell LJ, Maattanen P, Yeung CW, Lurz E, Sherman PM. Selective enrichment of commensal gut bacteria protects against Citrobacter rodentium-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2015;309:G181–192. doi: 10.1152/ajpgi.00053.2015. [DOI] [PubMed] [Google Scholar]
- 136.Atarashi K, Tanoue T, Oshima K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 137.Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 138.Narushima S, Sugiura Y, Oshima K, et al. Characterization of the 17 strains of regulatory T cell-inducing human-derived Clostridia. Gut Microbes. 2014;5:333–339. doi: 10.4161/gmic.28572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Alvarez-Curto E, Milligan G. Metabolism meets immunity: The role of free fatty acid receptors in the immune system. Biochem Pharmacol. 2016;114:3–13. doi: 10.1016/j.bcp.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 140.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 141.Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 142.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gerard P, Beguet F, Lepercq P, et al. Gnotobiotic rats harboring human intestinal microbiota as a model for studying cholesterol-to-coprostanol conversion. FEMS Microbiol Ecol. 2004;47:337–343. doi: 10.1016/S0168-6496(03)00285-X. [DOI] [PubMed] [Google Scholar]
- 144.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 145.Thorasin T, Hoyles L, McCartney AL. Dynamics and diversity of the ‘Atopobium cluster’ in the human faecal microbiota, and phenotypic characterization of ‘Atopobium cluster’ isolates. Microbiology. 2015;161:565–579. doi: 10.1099/mic.0.000016. [DOI] [PubMed] [Google Scholar]
- 146.Donohoe DR, Garge N, Zhang X, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ji J, Shu D, Zheng M, et al. Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Scientific reports. 2016;6:24838. doi: 10.1038/srep24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Singh N, Gurav A, Sivaprakasam S, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Goverse G, Molenaar R, Macia L, et al. Diet-Derived Short Chain Fatty Acids Stimulate Intestinal Epithelial Cells To Induce Mucosal Tolerogenic Dendritic Cells. J Immunol. 2017;198:2172–2181. doi: 10.4049/jimmunol.1600165. [DOI] [PubMed] [Google Scholar]
- 150.Nastasi C, Candela M, Bonefeld CM, et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Scientific reports. 2015;5:16148. doi: 10.1038/srep16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vinolo MA, Ferguson GJ, Kulkarni S, et al. SCFAs induce mouse neutrophil chemotaxis through the GPR43 receptor. PLoS One. 2011;6:e21205. doi: 10.1371/journal.pone.0021205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vinolo MA, Rodrigues HG, Hatanaka E, Hebeda CB, Farsky SH, Curi R. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin Sci (Lond) 2009;117:331–338. doi: 10.1042/CS20080642. [DOI] [PubMed] [Google Scholar]
- 153.Correa RO, Vieira A, Sernaglia EM, et al. Bacterial short-chain fatty acid metabolites modulate the inflammatory response against infectious bacteria. Cellular microbiology. 2017 doi: 10.1111/cmi.12720. [DOI] [PubMed] [Google Scholar]
- 154.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Balmer ML, Ma EH, Bantug GR, et al. Memory CD8(+) T Cells Require Increased Concentrations of Acetate Induced by Stress for Optimal Function. Immunity. 2016;44:1312–1324. doi: 10.1016/j.immuni.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 156.Kim M, Qie Y, Park J, Kim CH. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe. 2016;20:202–214. doi: 10.1016/j.chom.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu W, Sun M, Chen F, et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2016 doi: 10.1038/mi.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Harig JM, Soergel KH, Komorowski RA, Wood CM. Treatment of diversion colitis with short-chain-fatty acid irrigation. N Engl J Med. 1989;320:23–28. doi: 10.1056/NEJM198901053200105. [DOI] [PubMed] [Google Scholar]
- 160.Yasueda A, Mizushima T, Nezu R, et al. The effect of Clostridium butyricum MIYAIRI on the prevention of pouchitis and alteration of the microbiota profile in patients with ulcerative colitis. Surg Today. 2016;46:939–949. doi: 10.1007/s00595-015-1261-9. [DOI] [PubMed] [Google Scholar]
- 161.Zhou D, Pan Q, Liu XL, et al. Clostridium butyricum B1 alleviates high-fat diet-induced steatohepatitis in mice via enterohepatic immunoregulation. J Gastroenterol Hepatol. 2017 doi: 10.1111/jgh.13742. [DOI] [PubMed] [Google Scholar]
- 162.Trompette A, Gollwitzer ES, Yadava K, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 163.Mizuno M, Noto D, Kaga N, Chiba A, Miyake S. The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS One. 2017;12:e0173032. doi: 10.1371/journal.pone.0173032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rosberg-Cody E, Stanton C, O’Mahony L, et al. Recombinant lactobacilli expressing linoleic acid isomerase can modulate the fatty acid composition of host adipose tissue in mice. Microbiology. 2011;157:609–615. doi: 10.1099/mic.0.043406-0. [DOI] [PubMed] [Google Scholar]
- 165.Chung HJ, Yu JG, Lee IA, et al. Intestinal removal of free fatty acids from hosts by Lactobacilli for the treatment of obesity. FEBS Open Bio. 2016;6:64–76. doi: 10.1002/2211-5463.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dambekodi PC, Gilliland SE. Incorporation of cholesterol into the cellular membrane of Bifidobacterium longum. J Dairy Sci. 1998;81:1818–1824. doi: 10.3168/jds.S0022-0302(98)75751-0. [DOI] [PubMed] [Google Scholar]
- 167.Lye HS, Rusul G, Liong MT. Removal of cholesterol by lactobacilli via incorporation and conversion to coprostanol. J Dairy Sci. 2010;93:1383–1392. doi: 10.3168/jds.2009-2574. [DOI] [PubMed] [Google Scholar]
- 168.Progatzky F, Sangha NJ, Yoshida N, et al. Dietary cholesterol directly induces acute inflammasome-dependent intestinal inflammation. Nat Commun. 2014;5:5864. doi: 10.1038/ncomms6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Umemoto T, Subramanian S, Ding Y, et al. Inhibition of intestinal cholesterol absorption decreases atherosclerosis but not adipose tissue inflammation. J Lipid Res. 2012;53:2380–2389. doi: 10.1194/jlr.M029264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Attinkara R, Mwinyi J, Truninger K, et al. Association of genetic variation in the NR1H4 gene, encoding the nuclear bile acid receptor FXR, with inflammatory bowel disease. BMC Res Notes. 2012;5:461. doi: 10.1186/1756-0500-5-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Duboc H, Rajca S, Rainteau D, et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013;62:531–539. doi: 10.1136/gutjnl-2012-302578. [DOI] [PubMed] [Google Scholar]
- 172.Cipriani S, Mencarelli A, Chini MG, et al. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS One. 2011;6:e25637. doi: 10.1371/journal.pone.0025637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Guo C, Xie S, Chi Z, et al. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity. 2016;45:802–816. doi: 10.1016/j.immuni.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 174.Renga B, Mencarelli A, Cipriani S, et al. The bile acid sensor FXR is required for immune-regulatory activities of TLR-9 in intestinal inflammation. PLoS One. 2013;8:e54472. doi: 10.1371/journal.pone.0054472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Berger E, Haller D. Structure-function analysis of the tertiary bile acid TUDCA for the resolution of endoplasmic reticulum stress in intestinal epithelial cells. Biochem Biophys Res Commun. 2011;409:610–615. doi: 10.1016/j.bbrc.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 176.Mobraten K, Haugbro T, Karlstrom E, Kleiveland CR, Lea T. Activation of the bile acid receptor TGR5 enhances LPS-induced inflammatory responses in a human monocytic cell line. J Recept Signal Transduct Res. 2015;35:402–409. doi: 10.3109/10799893.2014.986744. [DOI] [PubMed] [Google Scholar]
- 177.Yoneno K, Hisamatsu T, Shimamura K, et al. TGR5 signalling inhibits the production of pro-inflammatory cytokines by in vitro differentiated inflammatory and intestinal macrophages in Crohn’s disease. Immunology. 2013;139:19–29. doi: 10.1111/imm.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dickinson AB, Gustafsson BE, Norman A. Determination of bile acid conversion potencies of intestinal bacteria by screening in vitro and subsequent establishment in germfree rats. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79:691–698. doi: 10.1111/j.1699-0463.1971.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 179.Gustafsson BE, Midtvedt T, Norman A. Metabolism of cholic acid in germfree animals after the establishment in the intestinal tract of deconjugating and 7 alpha-dehydroxylating bacteria. Acta Pathol Microbiol Scand. 1968;72:433–443. doi: 10.1111/j.1699-0463.1968.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 180.Narushima S, Itoh K, Takamine F, Uchida K. Absence of cecal secondary bile acids in gnotobiotic mice associated with two human intestinal bacteria with the ability to dehydroxylate bile acids in vitro. Microbiol Immunol. 1999;43:893–897. doi: 10.1111/j.1348-0421.1999.tb01224.x. [DOI] [PubMed] [Google Scholar]
- 181.Narushima S, Itoha K, Miyamoto Y, et al. Deoxycholic acid formation in gnotobiotic mice associated with human intestinal bacteria. Lipids. 2006;41:835–843. doi: 10.1007/s11745-006-5038-1. [DOI] [PubMed] [Google Scholar]
- 182.Dubos RJ, Schaedler RW. The effect of the intestinal flora on the growth rate of mice, and on their susceptibility to experimental infections. J Exp Med. 1960;111:407–417. doi: 10.1084/jem.111.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rausch P, Basic M, Batra A, et al. Analysis of factors contributing to variation in the C57BL/6J fecal microbiota across German animal facilities. International journal of medical microbiology: IJMM. 2016;306:343–355. doi: 10.1016/j.ijmm.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 184.Abdallah RA, Beye M, Diop A, Bakour S, Raoult D, Fournier PE. The impact of culturomics on taxonomy in clinical microbiology. Antonie Van Leeuwenhoek. 2017 doi: 10.1007/s10482-017-0871-1. [DOI] [PubMed] [Google Scholar]
- 185.Oren A, Garrity GM. Then and now: a systematic review of the systematics of prokaryotes in the last 80 years. Antonie Van Leeuwenhoek. 2014;106:43–56. doi: 10.1007/s10482-013-0084-1. [DOI] [PubMed] [Google Scholar]
- 186.Overmann J. Significance and future role of microbial resource centers. Syst Appl Microbiol. 2015;38:258–265. doi: 10.1016/j.syapm.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 187.Chung H, Pamp SJ, Hill JA, et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149:1578–1593. doi: 10.1016/j.cell.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Clavel T, Lagkouvardos I, Stecher B. From complex gut communities to minimal microbiomes via cultivation. Curr Opin Microbiol. 2017 doi: 10.1016/j.mib.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 189.Frese SA, Benson AK, Tannock GW, et al. The evolution of host specialization in the vertebrate gut symbiont Lactobacillus reuteri. PLoS Genet. 2011;7:e1001314. doi: 10.1371/journal.pgen.1001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Laukens D, Brinkman BM, Raes J, De Vos M, Vandenabeele P. Heterogeneity of the gut microbiome in mice: guidelines for optimizing experimental design. FEMS Microbiol Rev. 2015;40:117–132. doi: 10.1093/femsre/fuv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Petrof EO, Gloor GB, Vanner SJ, et al. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome. 2013;1:3. doi: 10.1186/2049-2618-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Daillere R, Vetizou M, Waldschmitt N, et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity. 2016;45:931–943. doi: 10.1016/j.immuni.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 193.Derrien M, Van Baarlen P, Hooiveld G, Norin E, Muller M, de Vos WM. Modulation of Mucosal Immune Response, Tolerance, and Proliferation in Mice Colonized by the Mucin-Degrader Akkermansia muciniphila. Front Microbiol. 2011;2:166. doi: 10.3389/fmicb.2011.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hoffmann TW, Pham HP, Bridonneau C, et al. Microorganisms linked to inflammatory bowel disease-associated dysbiosis differentially impact host physiology in gnotobiotic mice. ISME J. 2016;10:460–477. doi: 10.1038/ismej.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Muller M, Fink K, Geisel J, et al. Intestinal colonization of IL-2 deficient mice with non-colitogenic B. vulgatus prevents DC maturation and T-cell polarization. PLoS One. 2008;3:e2376. doi: 10.1371/journal.pone.0002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jones-Carson J, Vazquez-Torres FA, Balish E. B cell-independent selection of memory T cells after mucosal immunization with Candida albicans. J Immunol. 1997;158:4328–4335. [PubMed] [Google Scholar]
- 197.Ivanov, de Frutos RL, Manel N, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hapfelmeier S, Lawson MA, Slack E, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Feng T, Wang L, Schoeb TR, Elson CO, Cong Y. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. J Exp Med. 2010;207:1321–1332. doi: 10.1084/jem.20092253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Geuking MB, Cahenzli J, Lawson MA, et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 201.Mosconi I, Geuking MB, Zaiss MM, et al. Intestinal bacteria induce TSLP to promote mutualistic T-cell responses. Mucosal Immunol. 2013;6:1157–1167. doi: 10.1038/mi.2013.12. [DOI] [PubMed] [Google Scholar]
- 202.Natividad JM, Hayes CL, Motta JP, et al. Differential induction of antimicrobial REGIII by the intestinal microbiota and Bifidobacterium breve NCC2950. Appl Environ Microbiol. 2013;79:7745–7754. doi: 10.1128/AEM.02470-13. [DOI] [PMC free article] [PubMed] [Google Scholar]