Abstract
Purpose
The influence of childhood health on later life health outcomes is increasingly hypothesized but rarely tested. We examined the relationship between cardiometabolic indicators in childhood and risk of pregnancy induced hypertension, preeclampsia, and gestational diabetes.
Methods
Childhood measurements from 755 women in the Bogalusa Heart Study included body mass index, systolic and diastolic blood pressure (SBP and DBP), low- and high-density lipoprotein cholesterol, total cholesterol, triglycerides, insulin, and glucose. Average childhood values were estimated by area under the curve computed from longitudinal quadratic random-effects growth models to account for the unequally-spaced repeated measures. Women reported pregnancy complications, and medical records were linked to interview data where possible. Log-Poisson models predicted adjusted risk associated with an interquartile range increase in cardiometabolic indicators.
Results
Elevated childhood insulin was associated with 10–15% increased risk across the three outcomes. Elevated childhood SBP was associated with preeclampsia (SBP RR=1.50, 95% CI:1.13, 2.01) and SBP, DBP, and body mass index predicted pregnancy induced hypertension (SBP RR=2.15, 95 % CI:1.65, 2.82; DBP RR=1.83, 95% CI:1.38, 2.43; BMI RR=1.67, 95% CI:1.41, 1.98). Blood pressure mediated the association between childhood body mass index and pregnancy induced hypertension.
Conclusions
Results suggest the potential long-term impact of early-life cardiometabolic profiles on complications of pregnancy.
Keywords: pregnancy induced hypertension, gestational diabetes, preeclampsia
Introduction
All women experience physiologic changes during pregnancy including higher cardiac output, hypercoagulability, increased inflammatory activity, and insulin resistance (1). In women with an underlying predisposition to vascular endothelial dysfunction, the consequences of these changes may include development of complications during pregnancy – including pregnancy induced hypertension (PIH) and preeclampsia – or gestational diabetes mellitus (GDM). These conditions place both the woman and her fetus at risk for other serious short- and long-term morbidities and mortality (2, 3). Moreover, women who experience cardiometabolic complications during pregnancy are often at increased risk of cardiovascular disease later in life (4, 5).
Evidence in support of life-course epidemiology theory – which posits that health is shaped by exposures and experiences occurring across an individual’s life (6) – has dramatically increased in recent years. Applying this theory to women’s pregnancy health, researchers have documented the impact of risks that occur even before conception (7). Exposures from both the physical and social environment, air pollution (8, 9), neighborhood deprivation (9), and racial discrimination (10), for example, which occur prior to conception can alter the likelihood of a complication arising during pregnancy. Women entering pregnancy with a history of chronic disease (11, 12), stress (13), depression/anxiety (14), dyslipidemia (15), or high body mass index (16, 17) are more likely to experience adverse pregnancy health including development of GDM, PIH, and preeclampsia.
Preconception health studies underscore the importance of promoting women’s health during reproductive years to minimize both the woman’s own risk and that of any future children. However, while the life-course perspective of women’s risk for adverse pregnancy events extends from her infancy – possibly before, given the importance of in utero exposures – there is less empirical evidence examining relationships between these early life exposures and a woman’s health later in life during pregnancy. Maternal recall of childhood experiences– in particular adverse experiences such as abuse and neglect – have been associated with adverse birth outcomes (18, 19), excessive gestational weight gain (20), and development of GDM (21), but biological measurements from that time are rare, due in part to the difficulty and infeasibility of decades-long follow-up studies. Widespread implementation of electronic medical records and improvements in quality of electronic vital records allow for data linkage and the creation of analytic cohorts (22) for use in evaluation of longitudinal risk factors in association with adverse reproductive health (23, 24).
The purpose of this analysis was to examine how cardiometabolic profiles in childhood predict later-life health – namely adverse pregnancy complications – among women. We utilize some of the only U.S. data available with prospective measurements of childhood cardiometabolic indicators and subsequent reproductive history in a biracial cohort of women. We hypothesize that women with adverse cardiometabolic risk indicators in childhood continue on a trajectory of higher risk which is manifest in hypertensive and metabolic disorders arising during pregnancy. We additionally explore the degree to which elevated childhood body mass index (BMI) acts through cardiometabolic mediators to increase pregnancy complication risk.
Materials and Methods
Study population and data sources
The Bogalusa Heart Study is a longitudinal study of cardiovascular risk founded by Dr. Gerald Berenson that began enrolling school children in the semi-rural, biracial community of Bogalusa, Louisiana in 1973 (25). Approximately every 2 years thereafter, researchers repeated exams through 1994, enrolling new children each time and following-up those previously enrolled. Exams included interview questionnaires, venipuncture after a 12-hour fast, right-arm blood pressure measures in triplicate, and replicate anthropometric measures. Plasma glucose level was obtained as part of a multiple chemistry profile (SMA20) with the multichannel Olympus Au-5000analyzer (Olympus, Lake Success, NY). A radioimmunoassay kit was used to measure plasma insulin (Phadebas insulin kit, Pharmacia Diagnostics, Piscataway, NJ). Serum cholesterol and triglycerides levels were assayed enzymatically on the Hitachi 902 Automatic Analyzer (Roche Diagnostics, Indianapolis, IN). Further detail on Bogalusa Heart Study examinations is available elsewhere (25).
Beginning in 2013, a follow-up study sought to re-contact and enroll approximately half of the 6,000 women who have ever participated in and completed a Bogalusa Heart Study examination. Potential participants were contacted via phone call, mailing or in person at the Bogalusa Heart Study field clinic. After consenting, either verbally by phone or in writing in the clinic, each subject was interviewed for 15–30 minutes regarding her reproductive history and her family reproductive history. Participants were asked for permission to access medical records, and those consenting signed release forms for prenatal, labor and delivery records for each pregnancy. Research staff obtained and abstracted medical record data into the study database along with the self-reported interview data.
For the purposes of this analysis, we limited the dataset to women who completed an interview in adulthood and had been seen at least two Bogalusa Heart Study exams during their childhood (≤ age 16). The dataset was further restricted to the first birth among women whose first birth was a singleton and occurred after age 16 to preserve temporality. The final sample included 755 women (Figure 1).
Figure 1.
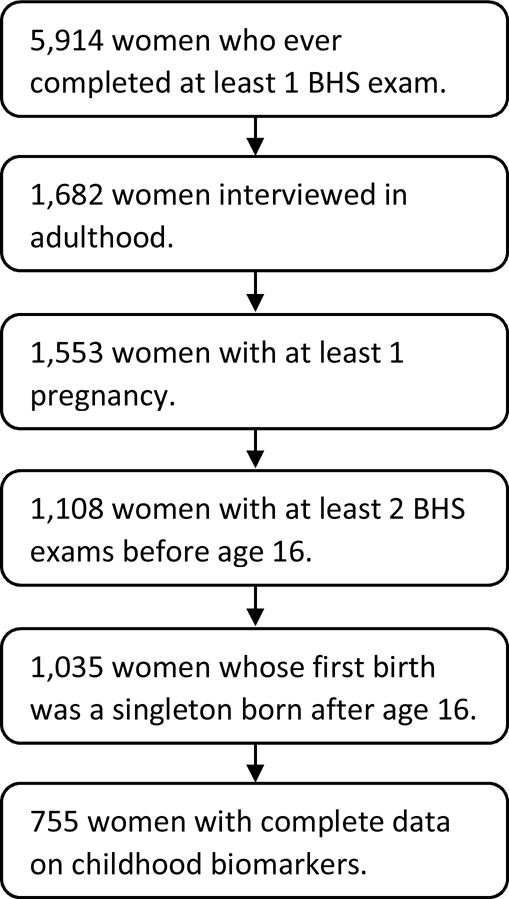
Flowchart for identification of the analytic study population.
Almost all women included in this analysis consented to release of prenatal and delivery medical records (n=729, 96.6%) associated with each pregnancy. At least partial records were obtained for 10% of the sample. Because approximately two thirds of the births included occurred during or before 1993, prior to widespread implementation of electronic medical records in Louisiana, many records may have been destroyed as typical hospital policy is to retain records for 10 years but not longer. Where available, data on participant’s reproductive history as reported during the interview was compared with obstetric medical records in order to correct for misclassification of self-reports.
Outcome
Outcomes assessed included preeclampsia, PIH, and GDM. Although maternal recall has been shown to be highly specific (>90%) for hypertensive disorders (26) and valid for reports of GDM (27, 28), we compared values to those reported in the medical record, where available, in order to ensure the most accurate case ascertainment. Following a hierarchy of validity, discrepant outcomes were resolved by classifying births based on non-missing data first from medical records, followed by maternal self-report where medical records were missing. Normotensive women (those without either PIH or preeclampsia) were the reference group for analyses of PIH and preeclampsia.
Exposure
Exposures of interest included biological markers of cardiometabolic functioning in childhood (before age 16): systolic and diastolic blood pressure (SBP and DBP, both mmHg), glucose (mg/dL), insulin (uU/mL), triglycerides, (mg/dL), total cholesterol (TC, mg/dL), high-density lipoprotein cholesterol (HDL-C, mg/dL), and low-density lipoprotein cholesterol (LDL-C, mg/dL), and body mass index (BMI, kg/m2).
In order to improve the predictive ability of these measures, we computed the area under the curve (AUC) from longitudinal growth curve models applying the methodology described in detail by Cook et al., in previous analysis of Bogalusa Heart Study data (29). Developed as a methodology to reduce measurement error in combining multiple measurements over time, the AUC values represent standardized summary measures of childhood biomarkers estimated from repeated measures on the same individuals where measures did not take place at equally-spaced intervals (29). We fit quadratic random-effects growth curves using PROC MIXED with a parameter for age centered around the approximate midpoint (age 10), as well as a quadratic age term to improve fit. The AUC was defined as the integral of the predicted biomarker curve from ages 4–16 divided by 10. AUC values for all 9 cardiometabolic indicators were computed for each individual based on a minimum of 2 measurements that occurred between age 4 and 16.
Covariates
Potentially confounding covariates known to be associated with both cardiometabolic health and pregnancy complications included maternal race (Black/White), age at delivery (continuous), educational attainment (less than high school, high school graduate, college graduate), marital status (married/not married) and smoking during pregnancy (yes/no).
Statistical analysis
We used the AUC for each biologic indicator to examine the association between childhood mean cardiometabolic profiles and complications of the women’s first pregnancy. Chi-square and t-tests compared crude differences in women with a pregnancy complication and those without across categorical and continuous covariates, respectively. For both PIH and preeclampsia, women without complications were those with neither PIH nor preeclampsia. Separate adjusted log-Poisson models estimated the relative risk associated with each cardiometabolic indicator controlling for maternal age at delivery, race, education, marital status, and smoking during pregnancy. All indicators were scaled to estimate relative risks associated with an interquartile range (IQR) increase in units.
For outcomes significantly associated with childhood BMI, we explored a potential indirect pathway through the cardiometabolic indicators. For instances where cardiometabolic indicators were associated with both childhood BMI and the outcome, we applied a counterfactual approach to estimate the natural direct effect and natural indirect effect of childhood BMI on pregnancy complications mediated through cardiovascular biomarkers.(30) Utilizing the SAS MEDIATE macro,(30) we estimated the natural indirect effect relative risk (RRNIE) and 95% confidence interval for an IQR increase in childhood BMI with potential mediators held at the 25th percentile.
Results
The 755 women included in this analysis completed an average of 3 Bogalusa Heart Study exams during childhood (before age 16) and some were seen as many as 7 times (mean=3.2, SD=1.1, min=2, max=7) at a mean age of 11. Across both sources of data, we identified a total of 70 (10.0%) cases of preeclampsia, 79 (11.1%) cases of PIH, and 35 (4.7%) cases of GDM. For all outcomes, data came primarily from maternal self-report. Among the 56 women (7.4%) with medical record data available, we identified 6 GDM cases and 3 PIH cases that had not been self-reported and re-classified 4 self-reported GDM cases and 7 PIH cases as non-cases.
Table 1 compares the average childhood cardiometabolic indicators based on the AUC as well as the simple average of measures taken at observed ages over the same age range. The simple averages are universally higher than the AUC reflecting a higher average observed age (11) than the midpoint of the age range (10). The AUC overcomes biases in correlations between measures taken at unequally spaced ages by using the population curve to extrapolate over the entire age range, independent of ages at measurement (29).
Table 1.
Simple Mean and Standard Deviation (SD) of Childhood Cardiovascular Indicators and Mean, SD, and Interquartile Range (IQR) Estimated by the Area Under the Growth Curve (AUC) for Each Individual (n=755).
| Simple Mean (SD) |
AUC Mean (SD) |
AUC IQR | |
|---|---|---|---|
| Body mass index, kg/m2 | 18.7 (3.4) | 21.7 (3.5) | 3.8 |
| Systolic blood pressure, mmHg | 101.8 (8.1) | 119.8 (6.6) | 8.4 |
| Diastolic blood pressure, mmHg | 63.0 (6.7) | 73.7 (4.0) | 5.2 |
| Low - density lipoprotein, mg/dL | 94.7 (22.9) | 113.4 (22.9) | 28.4 |
| High - density lipoprotein, mg/dL | 60.1 (14.8) | 73.3 (11.8) | 14.8 |
| Total Cholesterol, mg/dL | 164.0 (25.6) | 197.1 (25.6) | 33.3 |
| Triglycerides, mg/dL | 69.9 (26.8) | 81.7 (18.5) | 18.3 |
| Insulin, uU/mL | 11.9 (6.6) | 13.7 (1.5) | 0.7 |
| Glucose, mg/dL | 84.0 (7.7) | 98.8 (3.6) | 3.8 |
Approximately two-thirds of the women included in this analysis were White, and one-third were Black (Table 2). Two-thirds of the women were married at the time of their first child’s birth, and more than half (68.3%) had greater than a high school education. The average age of first birth among women (in this sample that included only women whose first birth was after age 16) was 23 years. There was no difference in crude outcome rates between women by race, marital status, smoking status, or educational level.
Table 2.
Maternal Characteristics of Bogalusa Babies Participants Overall and by Outcome.a
| Preeclampsia | Pregnancy Induced Hypertension | Gestational Diabetes | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Overall (N=755) | Yes (n=70, 10.0%) | Nob (n=628, 90.0%) | Yes (n=79, 11.1%) | Noc (n=635, 88.9%) | Yes (n=35, 4.7%) | No (n=716, 95.3) | ||
|
| ||||||||
| n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | ||
| Race | ||||||||
| Black | 277 (36.7) | 25 (9.9) | 228 (90.1) | 32 (12.2) | 230 (87.8) | 26 (5.5) | 449 (94.5) | |
| White | 478 (63.3) | 45 (10.1) | 400 (89.9) | 47 (10.4) | 405 (89.6) | 9 (3.3) | 267 (96.7) | |
| Marital status | ||||||||
| Married | 459 (60.9) | 46 (10.7) | 385 (89.3) | 42 (9.7) | 390 (90.3) | 25 (5.5) | 431 (94.5) | |
| Not married | 295 (39.1) | 24 (9.0) | 242 (91.0) | 37 (13.2) | 244 (86.8) | 10 (3.4) | 284 (96.6) | |
| Smoking during pregnancy | ||||||||
| Yes | 122 (16.2) | 12 (10.8) | 99 (89.2) | 15 (13.2) | 99 (86.8) | 4 (3.3) | 117 (96.7) | |
| No | 58 (9.9) | 527 (90.1) | 63 (10.6) | 534 (89.5) | 31 (4.9 | 596 (95.1) | ||
| Education | ||||||||
| Less than high school | 40 (5.3) | 2 (5.9) | 32 (94.1) | 7 (17.5) | 33 (82.5) | 3 (7.5) | 37 (92.5) | |
| High school graduate | 199 (26.4) | 17 (9.7) | 159 (90.3) | 26 (13.8) | 162 (86.2) | 11 (5.6) | 186 (94.4) | |
| Associate’s degree or some college | 267 (35.5) | 26 (10.2) | 230 (89.8) | 23 (9.0) | 232 (91.0) | 16 (6.0) | 250 (94.0) | |
| College graduate | 247 (32.8) | 25 (10.9) | 205 (89.1) | 23 (10.0) | 206 (90.0) | 5 (2.0) | 241 (98.0) | |
|
|
||||||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
|
|
||||||||
| Maternal age | 23.2 (5.1) | 24.3 (5.5) | 23.0 (5.0) | 24.3 (5.9) | 23.0 (5.0) | 24.8 (5.6) | 23.1 (5.0) | |
| Pre-pregnancy BMId | 23.6 (5.0) | 26.4 (6.0) | 23.2 (4.8) | 28.1 (4.6) | 23.0 (4.6) | 25.5 (5.3) | 23.4 (5.0) | |
| Mean childhood indicatore | ||||||||
| Body mass index, kg/m2 | 21.7 (3.5) | 21.9 (2.8) | 21.4 (3.2) | 24.3 (4.9) | 21.4 (3.2) | 22.8 (3.5) | 21.7 (3.5) | |
| Systolic blood pressure, mmHg | 119.8 (6.6) | 121.7 (6.5) | 119.3 (6.5) | 124.1 (6.5) | 119.3 (6.4) | 121.3 (6.9) | 119.8 (6.5) | |
| Diastolic blood pressure, mmHg | 73.7 (4.0) | 74.1 (4.0) | 73.5 (3.9) | 75.5 (4.0) | 73.5 (3.9) | 74.5 (4.4) | 73.7 (3.9) | |
| Low-density lipoprotein, mg/dL | 113.4 (22.9) | 118.0 (29.3) | 112.7 (22.2) | 117.2 (27.3) | 112.9 (22.2) | 118.7 (30.5) | 113.1 (22.5) | |
| High-density lipoprotein, mg/dL | 73.3 (11.8) | 73.1 (10.1) | 73.5 (11.8) | 71.4 (12.8) | 71.4 (12.8) | 70.0 (10.6) | 73.5 (11.8) | |
| Total Cholesterol, mg/dL | 197.1 (25.6) | 201.4 (31.0) | 196.7 (25.0) | 198.9 (29.8) | 196.8 (25.0) | 199.6 (30.9) | 196.9 (25.3) | |
| Triglycerides, mg/dL | 81.7 (18.5) | 80.5 (14.6) | 81.7 (18.6) | 83.7 (20.8) | 81.7 (18.6) | 89.9 (27.4) | 81.3 (18.0) | |
| Insulin, uU/mL | 13.7 (1.5) | 14.3 (2.5) | 13.6 (1.3) | 14.2 (2.5) | 13.6 (1.3) | 14.8 (3.2) | 13.6 (1.3) | |
| Glucose, mg/dL | 98.8 (3.6) | 99.1 (2.9) | 98.8 (3.7) | 98.7 (3.1) | 98.8 (3.7) | 99.7 (3.3) | 98.8 (3.6) | |
Boldface indicates groups are statistically different (P < 0.05).
Excludes women with gestational hypertension.
Excludes women with preeclampsia.
Only among women with a pre-pregnancy exam after age 16 (n=715).
Estimated by area under the growth curve for each individual based on participants with at least two visits prior to age 16.
Mean childhood SBP as estimated by AUC was elevated among women who developed preeclampsia and those with PIH during pregnancy relative to women without preeclampsia or PIH (Table 2). Childhood AUC for mean insulin was also higher among women who developed preeclampsia, PIH, and GDM compared to women without these complications, and mean childhood DBP and BMI were also higher among women with PIH.
In models adjusted for maternal age, race, educational attainment, marital status, and smoking during pregnancy, an IQR increase in mean childhood SBP was associated with a 50% increase in risk of preeclampsia (RR=1.50, 95% CI: 1.13, 2.01) and risk for PIH was increased more than two-fold (RR=2.15, 95 % CI: 1.65, 2.82; Table 3). Childhood DBP was also associated with an increased risk for PIH after adjustments (RR=1.83, 95% CI: 1.38, 2.43). An IQR increase in insulin levels across childhood was associated with significantly elevated risk for PIH (RR=1.10, 95% CI: 1.03, 1.17), preeclampsia (RR=1.14, 95% CI: 1.07, 1.23), and GDM (RR=1.15, 95 % CI: 1.06, 1.25).
Table 3.
Adjusted Relative Risks and 95% Confidence Intervals (CI) for Mean Childhood Cardiometabolic Indicators and Preeclampsia, Pregnancy Induced Hypertension, and Gestational Diabetes.a
| Preeclampsia | Pregnancy Induced Hypertension | Gestational Diabetes | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean childhood cardiometabolic indicatorc | RR | 95% CI | RR | 95% CI | RR | 95% CI |
| Body mass index | 1.15 | 0.89, 1.47 | 1.67 | 1.41, 1.98 | 1.35 | 0.99, 1.85 |
| Systolic blood pressure | 1.50 | 1.13, 2.01 | 2.15 | 1.65, 2.82 | 1.28 | 0.85, 1.93 |
| Diastolic blood pressure | 1.16 | 0.85, 1.58 | 1.83 | 1.38, 2.43 | 1.28 | 0.83, 1.98 |
| Low-density lipoprotein | 1.26 | 0.97, 1.63 | 1.21 | 0.94, 1.55 | 1.18 | 0.83, 1.69 |
| High-density lipoprotein | 0.99 | 0.72, 1.36 | 0.81 | 0.60, 1.10 | 0.80 | 0.52, 1.22 |
| Total Cholesterol | 1.25 | 0.94, 1.65 | 1.11 | 0.84, 1.46 | 1.09 | 0.74, 1.62 |
| Triglycerides | 0.93 | 0.71, 1.19 | 1.15 | 0.93, 1.42 | 1.28 | 0.97, 1.67 |
| Insulin | 1.14 | 1.07, 1.23 | 1.10 | 1.03, 1.17 | 1.15 | 1.06, 1.25 |
| Glucose | 1.06 | 0.82, 1.37 | 0.96 | 0.77, 1.20 | 1.28 | 0.85, 1.94 |
Each model is adjusted for maternal age, race, education, marital status, and smoking during pregnancy.
All RR estimates are per IQR increase in the cardiometabolic indicator.
Mean childhood BMI alone was associated with increasing risk of PIH after adjustment for maternal age, race, educational attainment, marital status, and smoking (RR =1.67, 95 % CI: 1.41, 1.98), but was not associated with preeclampsia or GDM in these data. Given significant associations between SBP, DBP, and insulin with PIH, we examined these indicators as potential mediators in the pathway between childhood BMI and PIH. SBP was the most important mediator of the childhood BMI – PIH association with an RRNIE of 1.27 (95% CI: 1.11, 1.46) followed by DBP (RRNIE= 1.12, 95% CI: 1.01, 1.24). The indirect pathway linking childhood BMI to PIH risk through childhood insulin was not significant (RRNIE= 1.06 95% CI: 0.99, 1.15).
Discussion
The results of this analysis suggest that women with elevated blood pressure prior to conception, even as early as childhood – are at greater risk for development of hypertensive disorders of pregnancy – underscoring the importance of a life course epidemiology approach to understanding the pathogenesis of these conditions. Moreover, we find that elevated BMI in childhood may be linked to increased risk for PIH by elevating blood pressure. This finding corresponds to and extends previous investigations of adulthood blood pressure as a significant mediator in the causal pathway between body mass index and cardiovascular diseases.(31, 32)
A study on preconception (adulthood) cardiovascular risk factors in Norway reported a strong relationship between blood pressure and preeclampsia (33), a finding confirmed in an more recent report based on Norwegian women from 1994–2012 (34). Egeland and colleagues (34) examined preconception (adulthood) cardiovascular risk factors for PIH and preeclampsia and found a more than two-fold increase in risk for either outcome with elevated (SBP 130–139 mmHg or DBP 85–89 mmHg) or hypertensive (SBP≥140 mmHg or DBP≥90 mmHg) blood pressure status (34). They also found triglycerides prior to pregnancy predicted preeclampsia, confirming a previous finding from the Cardiovascular Risk in Young Finns Study (35). To our knowledge this is the first study to examine childhood cardiometabolic profiles and later pregnancy complications, and we did not find an association between mean childhood triglycerides and either hypertensive disorder.
Our results included strong associations for elevated mean childhood insulin and all three pregnancy complications (36). Insulin resistance during pregnancy leading to hyperlipidemia has been associated with incident preeclampsia (36), PIH and GDM (37). Assessments of insulin and glucose levels during early pregnancy are useful predictors of preeclampsia, even among normal weight, normotensive women (38). Preconception studies of cardiometabolic risk factors have not reported an association between insulin and pregnancy complications (35), although one study reported associations with family history of diabetes mellitus and pregravid diabetes mellitus as significant risk factors for hypertensive disorders of pregnancy (34). In these data, women with elevated insulin across childhood – possible early indications for an underlying predisposition to diabetes – had a higher risk for all three outcomes.
This study utilized prospectively measured childhood cardiometabolic profiles and long-term follow up of women into their reproductive years and beyond, a rare and unique strength of these data. However, the self-reported nature of the outcomes represents a significant limitation and results should be interpreted with caution. The prevalence of preeclampsia among these women was higher than current nationally-reported rates (10% vs. 3–6%) (39), but among on the small number of women with medical records available, all self-reported cases were confirmed. Women in this cohort are predominantly overweight or obese,(40) and preeclampsia risk has been shown to progressively increase with increasing BMI, up to 2- to 3-fold higher among obese vs. normal weight women.(41) Moreover, we report lifetime prevalence of complications occurring at any pregnancy across a woman’s reproductive history which is likely to be higher than prevalence at a single pregnancy. We were not able to exclude or control for women with pregravid diabetes or chronic hypertension, and these women may have had both a worse cardiometabolic profile in childhood and been at increased risk for superimposed pregnancy hypertensive disorders. We sought to explore the influence of a range of cardiometabolic indicators on some of the most common pregnancy complications, but given the number of comparisons included in this analysis (9 exposures and 3 outcomes) some statistically significant findings may be due to chance. Finally, we have no data to characterize the pregnancy and birth experiences of women who completed a Bogalusa Heart Study exam in childhood but were not able to be contacted for interview in adulthood in order to determine how they may differ from women included in this analysis. We do know that women who were re-contacted had completed more study visits and were older at their first visit and last visit than women who had a Bogalusa Heart Study visit but were lost to follow-up in adulthood (p<0.01).
Conclusion
This study adds to the growing body of literature on the importance of childhood and early preconception health to ensure a healthy reproductive experience for girls who may later become pregnant women. Unhealthy cardiovascular and metabolic profiles established in childhood may lay the foundation for vulnerabilities to serious pregnancy morbidities that occur as a result of physiologic stresses and that jeopardize women’s health during pregnancy and in her later life.
Acknowledgments
Funding: This work was supported by the National Institutes of Health (Grants HD069587, AG041200, and AG016592).
List of abbreviations
- AUC
Area under the curve
- BMI
Body mass index
- CI
Confidence interval
- DBP
diastolic blood pressure
- GDM
Gestational diabetes
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- PIH
Pregnancy induced hypertension
- RR
Relative risk
- RRNIE
Natural indirect relative risk
- SBP
Systolic blood pressure
- SD
Standard deviation
- TC
total cholesterol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors have no conflicts of interest to report.
References
- 1.Williams D. Pregnancy: a stress test for life. Curr Opin Obstet Gynecol. 2003;15(6):465–471. doi: 10.1097/00001703-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Shih T, Peneva D, Xu X, et al. The Rising Burden of Preeclampsia in the United States Impacts Both Maternal and Child Health. Am J Perinatol. 2016;33(4):329–338. doi: 10.1055/s-0035-1564881. [DOI] [PubMed] [Google Scholar]
- 3.Kaaja R, Ronnemaa T. Gestational diabetes: pathogenesis and consequences to mother and offspring. Rev Diabet Stud. 2008;5(4):194–202. doi: 10.1900/RDS.2008.5.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet. 2001;357(9273):2002–2006. doi: 10.1016/S0140-6736(00)05112-6. [DOI] [PubMed] [Google Scholar]
- 5.Wilson BJ, Watson MS, Prescott GJ, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326(7394):845. doi: 10.1136/bmj.326.7394.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, Power C. Life course epidemiology. J Epidemiol Community Health. 2003;57(10):778–783. doi: 10.1136/jech.57.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care–United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep. 2006;55(RR-6):1–23. [PubMed] [Google Scholar]
- 8.Robledo CA, Mendola P, Yeung E, et al. Preconception and early pregnancy air pollution exposures and risk of gestational diabetes mellitus. Environ Res. 2015;137:316–322. doi: 10.1016/j.envres.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinikoor-Imler LC, Gray SC, Edwards SE, Miranda ML. The effects of exposure to particulate matter and neighbourhood deprivation on gestational hypertension. Paediatr Perinat Epidemiol. 2012;26(2):91–100. doi: 10.1111/j.1365-3016.2011.01245.x. [DOI] [PubMed] [Google Scholar]
- 10.Giurgescu C, McFarlin BL, Lomax J, Craddock C, Albrecht A. Racial discrimination and the black-white gap in adverse birth outcomes: a review. J Midwifery Womens Health. 2011;56(4):362–370. doi: 10.1111/j.1542-2011.2011.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leon MG, Moussa HN, Longo M, et al. Rate of Gestational Diabetes Mellitus and Pregnancy Outcomes in Patients with Chronic Hypertension. Am J Perinatol. 2016 doi: 10.1055/s-0036-1571318. [DOI] [PubMed] [Google Scholar]
- 12.Chuang CH, Velott DL, Weisman CS. Exploring knowledge and attitudes related to pregnancy and preconception health in women with chronic medical conditions. Matern Child Health J. 2010;14(5):713–719. doi: 10.1007/s10995-009-0518-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Ding Z, Liu H, et al. Association between mental stress and gestational hypertension/preeclampsia: a meta-analysis. Obstet Gynecol Surv. 2013;68(12):825–834. doi: 10.1097/OGX.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 14.Thombre MK, Talge NM, Holzman C. Association between pre-pregnancy depression/anxiety symptoms and hypertensive disorders of pregnancy. J Womens Health (Larchmt) 2015;24(3):228–236. doi: 10.1089/jwh.2014.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumfeld Y, Novack L, Wiznitzer A, et al. Pre-Conception Dyslipidemia Is Associated with Development of Preeclampsia and Gestational Diabetes Mellitus. PLoS One. 2015;10(10):e0139164. doi: 10.1371/journal.pone.0139164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schummers L, Hutcheon JA, Bodnar LM, Lieberman E, Himes KP. Risk of adverse pregnancy outcomes by prepregnancy body mass index: a population-based study to inform prepregnancy weight loss counseling. Obstet Gynecol. 2015;125(1):133–143. doi: 10.1097/AOG.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metsala J, Stach-Lempinen B, Gissler M, Eriksson JG, Koivusalo S. Risk of Pregnancy Complications in Relation to Maternal Prepregnancy Body Mass Index: Population-Based Study from Finland 2006–10. Paediatr Perinat Epidemiol. 2016;30(1):28–37. doi: 10.1111/ppe.12248. [DOI] [PubMed] [Google Scholar]
- 18.Christiaens I, Hegadoren K, Olson DM. Adverse childhood experiences are associated with spontaneous preterm birth: a case-control study. BMC Med. 2015;13:124. doi: 10.1186/s12916-015-0353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wosu AC, Gelaye B, Williams MA. Maternal history of childhood sexual abuse and preterm birth: an epidemiologic review. BMC Pregnancy Childbirth. 2015;15:174. doi: 10.1186/s12884-015-0606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranchod YK, Headen IE, Petito LC, Deardorff JK, Rehkopf DH, Abrams BF. Maternal Childhood Adversity, Prepregnancy Obesity, and Gestational Weight Gain. Am J Prev Med. 2016;50(4):463–469. doi: 10.1016/j.amepre.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason SM, Tobias DK, Clark CJ, Zhang C, Hu FB, Rich-Edwards JW. Abuse in Childhood or Adolescence and Gestational Diabetes: A Retrospective Cohort Study. Am J Prev Med. 2016;50(4):436–444. doi: 10.1016/j.amepre.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jutte DP, Roos LL, Brownell MD. Administrative record linkage as a tool for public health research. Annu Rev Public Health. 2011;32:91–108. doi: 10.1146/annurev-publhealth-031210-100700. [DOI] [PubMed] [Google Scholar]
- 23.Hertzman C. The role of administrative record linkage in creating trajectories of early human development. Healthc Policy. 2011;6(Spec Issue):55–62. [PMC free article] [PubMed] [Google Scholar]
- 24.Kramer MR, Dunlop AL, Hogue CJ. Measuring women’s cumulative neighborhood deprivation exposure using longitudinally linked vital records: a method for life course MCH research. Matern Child Health J. 2014;18(2):478–487. doi: 10.1007/s10995-013-1244-7. [DOI] [PubMed] [Google Scholar]
- 25.Berenson GSM,CA, Voors AW, Webber LS, Shrinivasan SR, Frank GC, Foster TA, Blonde CV. Cardiovascular Risk Factors in Children: the Early Natural History of Atherosclerosis and Essential Hypertension. New York, NY: Oxford University Press; 1980. [Google Scholar]
- 26.Stuart JJ, Bairey Merz CN, Berga SL, et al. Maternal recall of hypertensive disorders in pregnancy: a systematic review. J Womens Health (Larchmt) 2013;22(1):37–47. doi: 10.1089/jwh.2012.3740. [DOI] [PubMed] [Google Scholar]
- 27.Carter EB, Stuart JJ, Farland LV, et al. Pregnancy Complications as Markers for Subsequent Maternal Cardiovascular Disease: Validation of a Maternal Recall Questionnaire. J Womens Health (Larchmt) 2015;24(9):702–712. doi: 10.1089/jwh.2014.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunderson EP, Lewis CE, Tsai AL, et al. A 20-year prospective study of childbearing and incidence of diabetes in young women, controlling for glycemia before conception: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes. 2007 Dec;56(12):2990–6. doi: 10.2337/db07-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cook NR, Rosner BA, Chen W, Srinivasan SR, Berenson GS. Using the area under the curve to reduce measurement error in predicting young adult blood pressure from childhood measures. Stat Med. 2004;23(22):3421–3435. doi: 10.1002/sim.1921. [DOI] [PubMed] [Google Scholar]
- 30.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Y, Hajifathalian K, Rimm EB, Ezzati M, Danaei G. Mediators of the effect of body mass index on coronary heart disease: decomposing direct and indirect effects. Epidemiology. 2015;26(2):153–162. doi: 10.1097/EDE.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 32.Global Burden of Metabolic Risk Factors for Chronic Diseases C. Lu Y, Hajifathalian K, et al. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383(9921):970–983. doi: 10.1016/S0140-6736(13)61836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magnussen EB, Vatten LJ, Lund-Nilsen TI, Salvesen KA, Davey Smith G, Romundstad PR. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study. BMJ. 2007;335(7627):978. doi: 10.1136/bmj.39366.416817.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egeland GM, Klungsoyr K, Oyen N, Tell GS, Naess O, Skjaerven R. Preconception Cardiovascular Risk Factor Differences Between Gestational Hypertension and Preeclampsia: Cohort Norway Study. Hypertension. 2016 doi: 10.1161/HYPERTENSIONAHA.116.07099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harville EW, Viikari JS, Raitakari OT. Preconception cardiovascular risk factors and pregnancy outcome. Epidemiology. 2011;22(5):724–730. doi: 10.1097/EDE.0b013e318225c960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hauth JC, Clifton RG, Roberts JM, et al. Maternal insulin resistance and preeclampsia. Am J Obstet Gynecol. 2011;204(4):327, e321–326. doi: 10.1016/j.ajog.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mastrogiannis DS, Spiliopoulos M, Mulla W, Homko CJ. Insulin resistance: the possible link between gestational diabetes mellitus and hypertensive disorders of pregnancy. Curr Diab Rep. 2009;9(4):296–302. doi: 10.1007/s11892-009-0046-1. [DOI] [PubMed] [Google Scholar]
- 38.Parretti E, Lapolla A, Dalfra M, et al. Preeclampsia in lean normotensive normotolerant pregnant women can be predicted by simple insulin sensitivity indexes. Hypertension. 2006;47(3):449–453. doi: 10.1161/01.HYP.0000205122.47333.7f. [DOI] [PubMed] [Google Scholar]
- 39.Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ. 2013;347:f6564. doi: 10.1136/bmj.f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Broyles S, Katzmarzyk PT, Srinivasan SR, et al. The pediatric obesity epidemic continues unabated in Bogalusa, Louisiana. Pediatrics. 2010 May;125(5):900–5. doi: 10.1542/peds.2009-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeyabalan A. Epidemiology of preeclampsia: impact of obesity. Nutrition Rev. 2013 Oct;71(Suppl 1):S18–25. doi: 10.1111/nure.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]


