Abstract
Parkinson´s disease (PD) is a common neurodegenerative disease characterized by a substantial decrease of dopaminergic neurons in the substantia nigra pars compacta. The neurological deterioration during PD can be, in part, attributed to elevated levels of reactive oxygen species (ROS). Radical scavengers have previously been shown to protect dopaminergic cells from toxic effects in vitro. Hence, new approaches need to be investigated to improve the administration of antioxidants in order to provide neuroprotection. Polymers exhibiting catechol structures offer one such approach due to their interesting physicochemical properties. In the present study a photocrosslinkable dopamine-containing poly(β-amino ester) (DPAE) was synthesized from poly(ethylene glycol) diacrylate (PEGDA) and dopamine hydrochloride using Michael type addition. A water-in-oil emulsion technique was used to photo-crosslink the polymer into spherical microparticles. DPAE microspheres featured excellent scavenging properties towards 1,1-Diphenyl-2-picryl-hydrazyl (DPPH) radicals in a dose dependent manner and could even reduce the dissolved oxygen content of physiological solution. Furthermore, the concentrations required for radical scavenging were shown to be non-toxic towards dopaminergic SH-SY5Y cells as well as primary astrocytes and primary embryonic rat ventral midbrain cultures.
Introduction
The death of dopaminergic neurons in patients with PD gives rise to its clinical features such as a tremor, slowness of movement and stiffness 1–2. Although genetic heritability influences the disease, its etiology is largely idiopathic with multiple pathological factors being discussed in the literature: inflammation, oxidative stress or the accumulation of toxic protein aggregates 3. Several studies associate mitochondrial mutation and dysfunction, concomitant with the unregulated production of reactive oxygen species (ROS), as factors of neurological deterioration 4. Since current PD therapies only address the symptoms and not the underlying neurodegeneration, new disease modifying strategies are being sought. Traditional molecular biology and pharmacological approaches to new therapies for neurodegenerative disorders have, more recently, been supplemented with regenerative medicine and biomaterial constructs 5–6. These include growth factor delivery systems 7–8, cell microcarriers 9, scaffolds 10, hydrogels 11 and in the interest of this work, radical scavenging materials 12–14. Neuromelanin – a by-product of dopamine oxidation, is a blackbrownish brownish pigment which is particularly concentrated in the dopaminergic neurons of the substantia nigra (SN) and in the noradrenergic neurons of locus coeruleus 15–16. Deficiencies in neuromelanin have been observed in the brain stems of patients affected by PD 16. Neuromelanin interacts with a variety of organic molecules such as lipids, pesticides and (neuro)toxic compounds like 4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), and may play a crucial role by modulating the effects and toxicity of certain compounds 17. Since natural melanin has attributed chemical complexity, it is difficult to systematically characterize, so a synthetic analog originating from dopamine (3,4- dihydroxyphenylethylamine)13 or L-DOPA (3,4-dihydroxyphenylalanine)18 has been used as a common substitute in research. Recently, Ju et al., synthesized melanin-like nanoparticles from dopamine hydrochloride which feature radical scavenging abilities 13. The catechol structure in dopamine is presumably the key to radical scavenging properties, as it is widely observed in many naturally occurring antioxidants.
The aim of this work was to produce a dopamine containing polymer that could be photo-crosslinked into biodegradable microspheres to act as a radical scavenging depot. Poly(β-amino esters) are a class of polymer that have recently received much research attention for biomedical applications 19–22. Their ease of synthesis, ability to include a wide range of monomers, biodegradability (in most cases) and cytocompatibility (of course monomer dependant), render them an attractive starting material for new therapeutics 23. Furthermore, catechol containing materials have been much studied already for biomedical applications 24–26. It was hypothesized that by combining polyethylene glycol diacrylate (PEGDA) in molar excess with dopamine hydrochloride (DOPA), a linear polymer chain containing catechol groups and free vinyl groups could be formed via Michael type addition. Furthermore it was hypothesized that crosslinked microspheres of this polymer could have ROS scavenging properties without causing cellular toxicity.
Results and Discussion
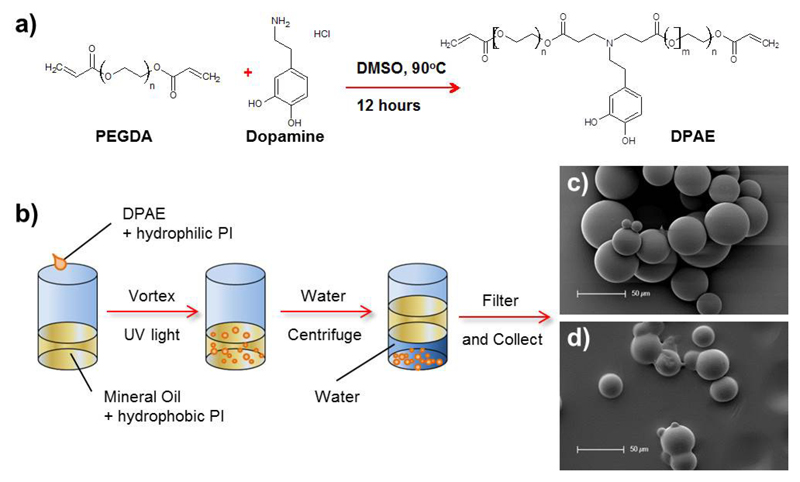
The dopamine containing poly(β-amino ester) was successfully synthesized via Michael type addition in DMSO via the reaction outlined in Figure 1a. After twelve hours of reaction time, the resulting pre-polymer from PEGDA and dopamine hydrochloride had gained viscosity and turned a pale yellow colour. After purification, a yield of 68.7 % by weight was obtained and gel permeation chromatography analysis showed the pre-polymer to have the following molecular weights: Mn = 9.6 kDa, Mw = 11.7 kDa and Đ = 1.22.
Figure 1.
Formation of the dopamine containing poly(β-amino ester)(DPAE) via Michael type addition (a). This DPAE pre-polymer could then be emulsified in oil and photocrosslinked into beads ready for filtration and collection (b). SEM images show spherical beads formed from PEG (c) or DPAE (d).
1H NMR spectroscopy confirmed that dopamine was present in the polymer structure (see Supplementary Information Figure S1 - peaks above 8 ppm). 1H NMR spectroscopy also showed the presence of free vinyl groups in the structure (peaks at 5.9 and 6.4ppm) which were used to crosslink the DPAE pre-polymer into the microsphere structures. As Anderson and co-workers have previously reported, poly(β-amino esters) make useful crosslinkable polymers since their ease of synthesis allows a combination of monomers to be used 23. Studies in our lab have previously shown that hyperbranched, dopamine containing polymers can be synthesized via Michael type addition when tri-acrylate monomers were used 21, 25. In this study PEGDA700 was used, in conjunction with the mono-amine catechol, dopamine hydrochloride to produce a linear pre-polymer with good solubility in water (required for the emulsion).
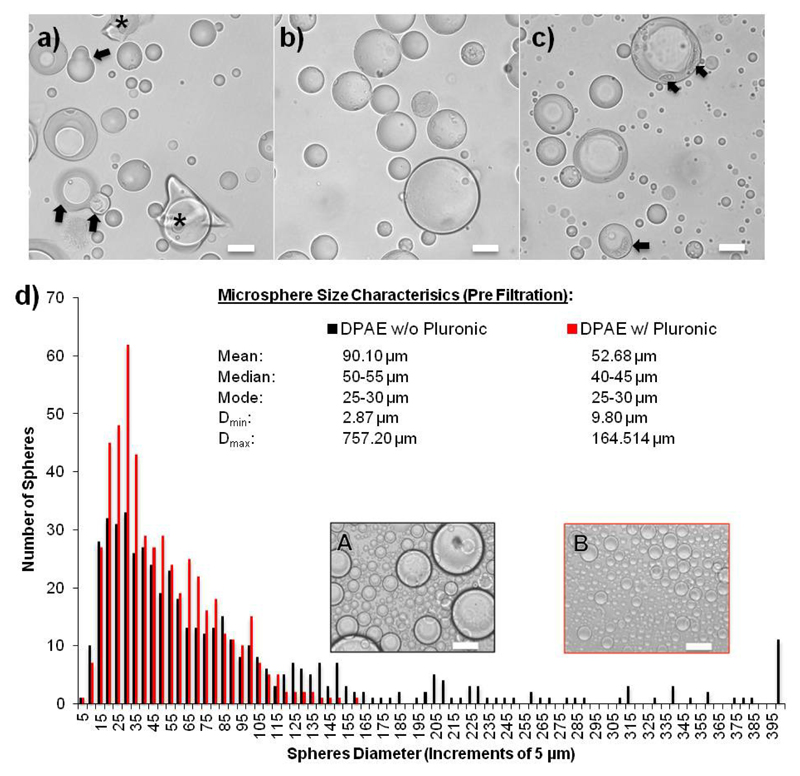
The DPAE microspheres were produced using a water-in-oil emulsion and the dual-photoinitiator technique described by Franco et al.,27 was utilized to make well-structured microspheres. Emulsion based microsphere synthesis requires efficient polymer crosslinking degrees, since phase separation and droplet coalescence collectively counteract the synthesis of single spherical polymer particles. The emulsion-based approach, when 2-hydroxy-2-methylpropio-phenone was applied as the sole photoinitiator (PI) at a concentration of 1 % (wt/wt) in the prepolymer, resulted in irregular shaped particles and debris accumulations in the samples (Fig. 2a). The addition of the hydrophobic PI irgacure I651 into the surrounding oil phase at a final concentration of 3 μg/mL supported the cross linking of the particle surface. The dual-PI system generated highly spherical particles with completely cross-linked polymer surfaces (Fig. 2b). In contrast, I651 applied solely in the oil phase in the absence of a second PI in the prepolymer phase, yielded incomplete crosslinking of the (mostly) spherical particles (Fig. 2c). The dual PI method was hence used for both the production of DPAE microspheres and the PEG control microspheres.
Figure 2.
Microsphere formation via a water-in-oil emulsion followed by UV crosslinking. Light microscopy images of microspheres formed using only the hydrophilic photoinitiator (PI) in the pre-polymer solution (a), with both the hydrophilic PI in the pre-polymer and hydrophobic PI in the oil phase (b), or only the hydrophobic PI in the oil phase (c), showing how the dual PI method results in the best micrsphere formation, without coalescence (black arrows) or debris (*). The addition of pluronic to the emulsion vastly reduced the size of the spheres formed and thus increased the usable yield.
DPAE spheres synthesized in the presence of the surfactant Pluronic® F-68 at a working concentration of 0.1 % displayed a significantly different particle size distribution (Fig. 2d). The addition of the surfactant shifts the distribution to the left resulting in a reduced mean diameter of 52.68 μm, and a smaller median value within the range of 40-45 μm. The maximum diameter was reduced to 164.51 μm allowing a far greater number of spheres after the 100 μm mesh size filtration process (see supplementary figure S2 and S3 for final size distributions of filtered DPAE and PEG spheres respectively).
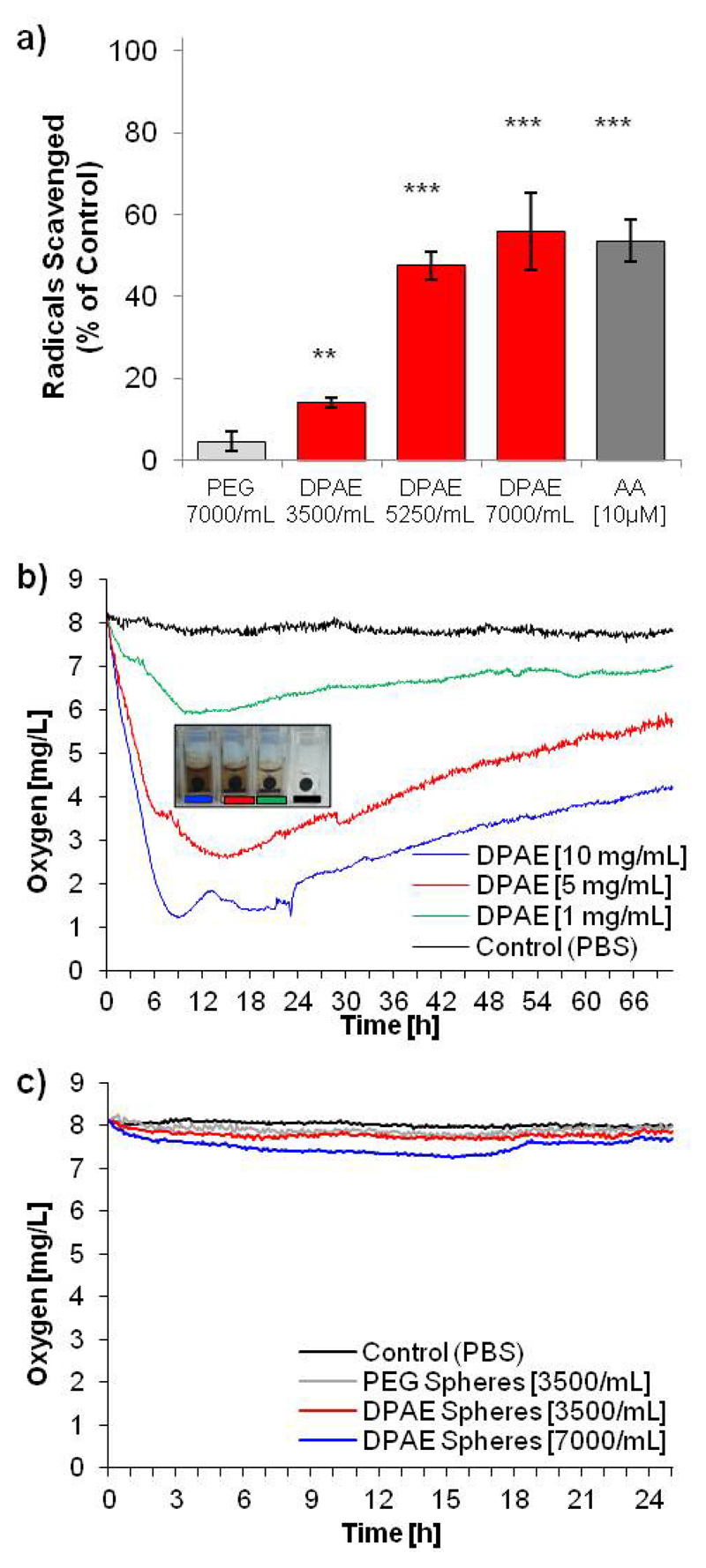
The ability of the DPAE microspheres to scavenge ROS was analysed using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay 13, 28 which gives fast detection of radical electron transfer by catechol groups 29. DPAE spheres at a concentration of [3500/mL] scavenged 14 % ±3 % of the DPPH radicals in relation to the control within 30 min of incubation (Fig. 3a).
Figure 3.
DPAE scavenges radicals and reduces dissolved oxygen content in PBS. Microspheres scavenge the DPPH stable radical (a) in a dose dependent manner up to a level scavenged by 10 μM ascorbic acid (n=4, error bars represent +/- standard deviation, two-tailed Student´s t-test marking difference to PEG spheres with p-value < 0.001 (***) and p-value < 0.01 (**)) Bulk preparations of the DPAE reduced the dissolved oxygen content of PBS (b) which results in a darkening of the DPAE due to oxidation of the catechol group after two days (images of cuvettes - insert). Microspheres of DPAE, at the same concentrations as used for the radical scavenging assay and toxicity assays, showed only a slight decrease in dissolved oxygen content (c).
At DPAE sphere concentrations of [5250/mL] and [7000/mL], 47 % ±9 % and 56 % ±5 % of the radicals were eliminated respectively. In contrast, PEG spheres added at [7000/mL] resulted in only 5 % ±1% of the radicals being. As shown in Fig. 3a, the free radical activity of DPAE microspheres increased in a dose-dependent manner up to 56 %, which was slightly higher than the activity of ascorbic acid [10 μM] – a well-known antioxidant. Bulk preparations of the crosslinked DPAE pre-polymer (i.e. not microspheres, but crosslinked macroscale droplets of 1-2μL with a pre-determined weight crosslinked on parafilm) were analysed to see if they affected the dissolved oxygen concentration of PBS. At these vastly elevated quantities, the bulk DPAE caused a decrease in the dissolved oxygen content of PBS (Fig. 3b) via the redox reaction occurring via the catechol group. The DPAE colouration (turning black) can be observed in the cuvette as shown in the insert in Fig 3b, which occurs in a dose dependent manner. However, preparations of microspheres at 3500 or 7000 spheres/mL (the same concentrations as the DPPH radical scavenging assay) caused only a slight drop in the dissolved oxygen content of PBS (Fig. 3c). Although the DPAE microspheres were originally designed for use as a biodegradable ROS scavenging depot, a degradation assay performed in PBS at 37°C for a period of three weeks showed no significant mass loss (see Supplementary Information Figure 4). Though this is not typical of poly(β-amino esters), careful analysis of the degradation profiles of the polymer library compiled by Anderson et al., showed that while most exhibited complete mass loss after 56 days, a few combinations of monomers did not23. Of particular interest for this study was that the phenol containing vinyl monomer showed a far slower degradation behaviour than most of the others tested. This lack of degradation of the DPAE microspheres means that they could also be a useful additive for in vitro cell culture for ROS scavenging without affecting intracellular processes. Conversely, the cross-linkable nature of these materials may allow smaller material formulations such as nanotubes30 or be included for the functionalization of nanomaterials31.
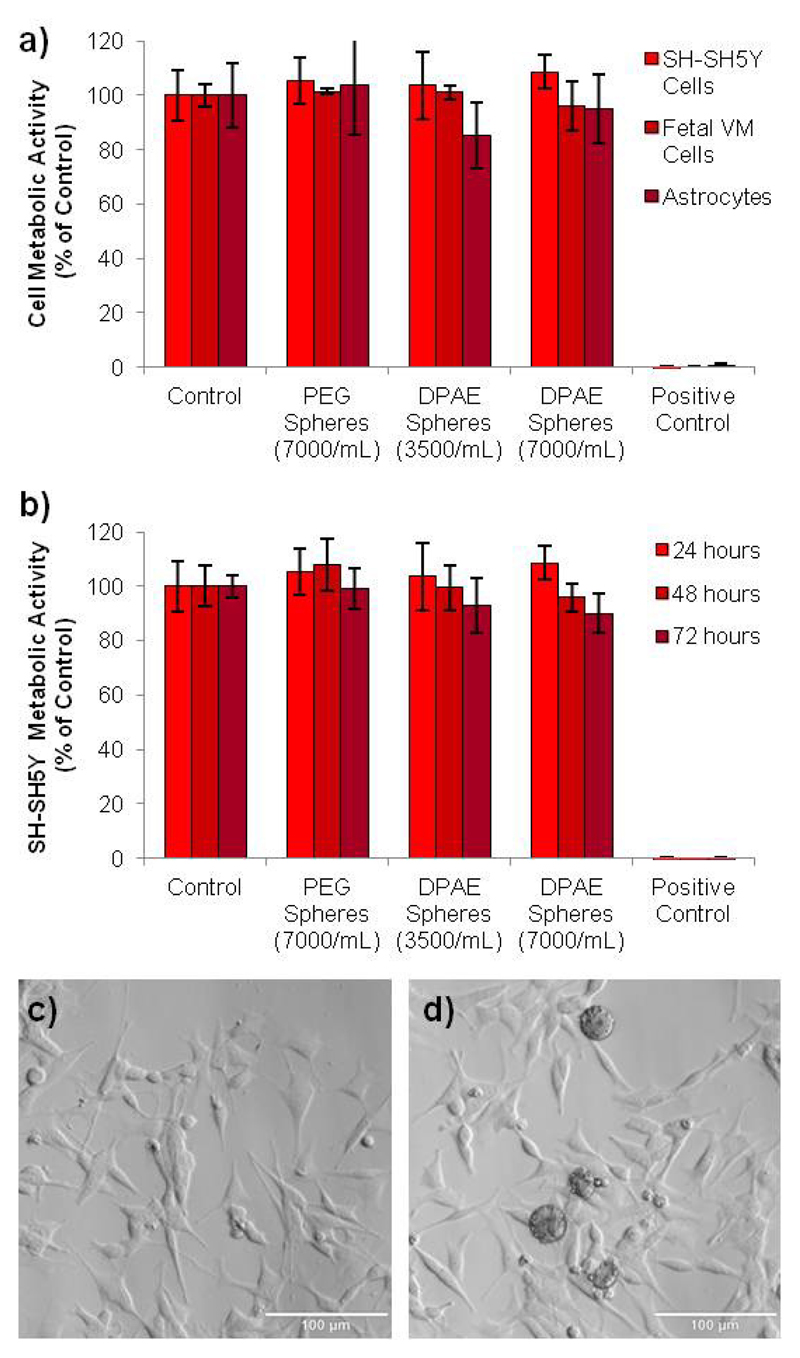
To assess the feasibility of the use of DPAE for applications in Parkinson's disease research, the cytotoxicity of the DPAE microspheres was analysed in comparison to PEG microspheres. SH-SY5Y cells were used as they are a commonly used in vitro model of dopaminergic neurons for Parkinson's disease research32. In addition, primary rat astrocytes33 and embryonic (E14) rat midbrain cultures were also used, since they are frequently transplanted into neurotoxin rodent models of Parkinson's disease 10. No reduction in cell metabolic was observed for either cell type as determined using the PrestoBlue cell viability reagent in comparison to the untreated cells (100% viable)(Fig 4a and b). No morphological differences were observed either (Figure 4c and d), and membrane integrity was not affected (see supplementary figure S5) despite a reasonably dense covering of microspheres on the well surface. The good radical scavenging capability at concentrations that do not induce toxicity, renders DPAE worthy of further investigation for neuroprotection in cell models Parkinson's disease. Biodegradability could potentially be introduced via the incorporation of other, non-catechol primary amines in the reaction procedure to act as a biodegradable “spacer” in the chemical structure.
Figure 4.
The DPAE microspheres did not affect the cell metabolic activity at the concentrations that give rise to good radical scavenging activity. Microspheres were incubated for 24 hours with SH-SY5Y cells, primary astrocytes and primary embryonic midbrain cultures (a) and viability was analysed via the PrestoBlue assay. Furthermore, the viability of SH-SY5Y cells was analysed for 72 hours, which again showed all viability to be above 80% (b). Light microscope images of the SHSY-5Y cells after 72 hours of incubation in media (control)(c) or DPAE spheres (7000/mL)(d) show the morphology of the cells appears to be unaffected. (n=4, error bars represent standard deviation, scale bars = 100 μm).
Conclusions
A dopamine containing polymer was produced via Michael type addition, which, due to free vinyl groups in the structure, could be photo-crosslinked. A dual photo-initiator, water-in-oil emulsion was used to create microspheres of the polymer, which showed excellent radical scavenging activity, the ability to reduce dissolved oxygen in PBS and showed no toxicity towards the three cell types analysed. Such materials could act as a ROS scavenging depot, and future studies will focus on degradability and ROS scavenging analysis for neuroprotection in Parkinson's disease cell models.
Supplementary Material
Electronic Supplementary Information (ESI) available: [Experimental Methods, NMR data, microsphere size distributions and degradation data]. See DOI: 10.1039/x0xx00000x
Acknowledgements
B. N. would like to thank the Wellcome Trust, Sir Henry Wellcome post-doctoral fellowship for funding this research.
References
- 1.Mori F, Nishie M, Kakita A, Yoshimoto M, Takahashi H, Wakabayashi K. Journal of Neuropathology & Experimental Neurology. 2006;65:808–815. doi: 10.1097/01.jnen.0000230520.47768.1a. [DOI] [PubMed] [Google Scholar]
- 2.Dauer W, Przedborski S. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 3.Mounsey RB, Teismann P. International Journal of Cell Biology. 2012;2012:12. doi: 10.1155/2012/983245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuo L, Motherwell MS. Gene. 2013;532:18–23. doi: 10.1016/j.gene.2013.07.085. [DOI] [PubMed] [Google Scholar]
- 5.Newland B, Newland H, Werner C, Rosser A, Wang W. Progress in Polymer Science. 2015;44:79–112. [Google Scholar]
- 6.Newland B, Dowd E, Pandit A. Biomaterials Science. 2013;1:556–576. doi: 10.1039/c3bm60030k. [DOI] [PubMed] [Google Scholar]
- 7.Garbayo E, Montero-Menei CN, Ansorena E, Lanciego JL, Aymerich MS, Blanco-Prieto MJ. J Control Release. 2009;135:119–126. doi: 10.1016/j.jconrel.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Garbayo E, Ansorena E, Lanciego JL, Blanco-Prieto MJ, Aymerich MS. Mov Disord. 2011;26:1943–1947. doi: 10.1002/mds.23793. [DOI] [PubMed] [Google Scholar]
- 9.Newland B, Welzel PB, Newland H, Renneberg C, Kolar P, Tsurkan M, Rosser A, Freudenberg U, Werner C. Small. 2015;11:5047–5053. doi: 10.1002/smll.201500898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang T-Y, Bruggeman KF, Kauhausen JA, Rodriguez AL, Nisbet DR, Parish CL. Biomaterials. 2016;74:89–98. doi: 10.1016/j.biomaterials.2015.09.039. [DOI] [PubMed] [Google Scholar]
- 11.Hoban DB, Newland B, Moloney TC, Howard L, Pandit A, Dowd E. Biomaterials. 2013;34:9420–9429. doi: 10.1016/j.biomaterials.2013.08.073. [DOI] [PubMed] [Google Scholar]
- 12.Tong J, Zimmerman MC, Li S, Yi X, Luxenhofer R, Jordan R, Kabanov AV. Biomaterials. 2011;32:3654–3665. doi: 10.1016/j.biomaterials.2011.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ju K-Y, Lee Y, Lee S, Park SB, Lee J-K. Biomacromolecules. 2011;12:625–632. doi: 10.1021/bm101281b. [DOI] [PubMed] [Google Scholar]
- 14.Karakoti A, Singh S, Dowding JM, Seal S, Self WT. Chemical Society Reviews. 2010;39:4422–4432. doi: 10.1039/b919677n. [DOI] [PubMed] [Google Scholar]
- 15.Sulzer D, Bogulavsky J, Larsen KE, Behr G, Karatekin E, Kleinman MH, Turro N, Krantz D, Edwards RH, Greene LA. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11869–11874. doi: 10.1073/pnas.97.22.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zecca L, Stroppolo A, Gatti A, Tampellini D, Toscani M, Gallorini M, Giaveri G, Arosio P, Santambrogio P, Fariello RG. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9843–9848. doi: 10.1073/pnas.0403495101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salazar M, Sokoloski T, Patil P. 1978 [PubMed] [Google Scholar]
- 18.Shanmuganathan K, Cho JH, Iyer P, Baranowitz S, Ellison CJ. Macromolecules. 2011;44:9499–9507. [Google Scholar]
- 19.Keeney M, Ong S-G, Padilla A, Yao Z, Goodman S, Wu JC, Yang F. ACS Nano. 2013;7:7241–7250. doi: 10.1021/nn402657d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson DG, Lynn DM, Langer R. Angewandte Chemie. 2003;115:3261–3266. doi: 10.1002/anie.200351244. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Bré L, Zhao T, Newland B, Da Costa M, Wang W. Journal of Materials Chemistry B. 2014;2:4067–4071. doi: 10.1039/c4tb00155a. [DOI] [PubMed] [Google Scholar]
- 22.DenizáYilmaz M, FraseráStoddart J. Nanoscale. 2015;7:7178–7183. [Google Scholar]
- 23.Anderson DG, Tweedie CA, Hossain N, Navarro SM, Brey DM, Van Vliet KJ, Langer R, Burdick JA. Advanced Materials. 2006;18:2614–2618. [Google Scholar]
- 24.Choi JS, Messersmith PB, Yoo HS. Macromolecular Bioscience. 2014;14:270–279. doi: 10.1002/mabi.201300281. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Bré LP, Zhao T, Zheng Y, Newland B, Wang W. Biomaterials. 2014;35:711–719. doi: 10.1016/j.biomaterials.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Park KM, Gerecht S. Nature Communications. 2014;5 doi: 10.1038/ncomms5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franco C, Price J, West J. Acta Biomaterialia. 2011;7:3267–3276. doi: 10.1016/j.actbio.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Brand-Williams W, Cuvelier M, Berset C. LWT-Food Science and Technology. 1995;28:25–30. [Google Scholar]
- 29.Xie J, Schaich KM. Journal of Agricultural and Food Chemistry. 2014;62:4251–4260. doi: 10.1021/jf500180u. [DOI] [PubMed] [Google Scholar]
- 30.Newland B, Leupelt D, Zheng Y, Thomas LSV, Werner C, Steinhart M, Wang W. Scientific Reports. 2015;5:17478. doi: 10.1038/srep17478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez-Yague MA, Larrañaga A, Gladkovskaya O, Stanley A, Tadayyon G, Guo Y, Sarasua J-R, Tofail SAM, Zeugolis DI, Pandit A, Biggs MJ. Bioconjugate Chemistry. 2015;26:2025–2037. doi: 10.1021/acs.bioconjchem.5b00257. [DOI] [PubMed] [Google Scholar]
- 32.Xie H-r, Hu L-S, Li G-Y. Chinese Medical Journal. 2010;123:1086–1092. [PubMed] [Google Scholar]
- 33.Newland B, Moloney TC, Fontana G, Browne S, Abu-Rub MT, Dowd E, Pandit AS. Biomaterials. 2013;34:2130–2141. doi: 10.1016/j.biomaterials.2012.11.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.