Abstract
Objective
Few studies based in well-defined North American populations have examined the occurrence of juvenile idiopathic arthritis (JIA), and none has been based in an ethnically diverse population. We used computerized healthcare information from the Kaiser Permanente Northern California membership to validate JIA diagnoses and estimate the incidence and prevalence of the disease in this well-characterized population.
Methods
We identified children aged ≤ 15 years with ≥ 1 relevant International Classification of Diseases, 9th edition, diagnosis code of 696.0, 714, or 720 in computerized clinical encounter data during 1996–2009. In a random sample, we then reviewed the medical records to confirm the diagnosis and diagnosis date and to identify the best-performing case-finding algorithms. Finally, we used the case-finding algorithms to estimate the incidence rate and point prevalence of JIA.
Results
A diagnosis of JIA was confirmed in 69% of individuals with at least 1 relevant code. Forty-five percent were newly diagnosed during the study period. The age- and sex-standardized incidence rate of JIA per 100,000 person-years was 11.9 (95% CI 10.9–12.9). It was 16.4 (95% CI 14.6–18.1) in girls and 7.7 (95% CI 6.5–8.9) in boys. The peak incidence rate occurred in children aged 11–15 years. The prevalence of JIA per 100,000 persons was 44.7 (95% CI 39.1–50.2) on December 31, 2009.
Conclusion
The incidence rate of JIA observed in the Kaiser Permanente population, 1996–2009, was similar to that reported in Rochester, Minnesota, USA, but 2 to 3 times higher than Canadian estimates.
Key Indexing Terms: JUVENILE IDIOPATHIC ARTHRITIS, EPIDEMIOLOGY, INCIDENCE, PREVALENCE HEALTH MAINTENANCE ORGANIZATIONS, COMPUTERIZED MEDICAL INFORMATION
Juvenile idiopathic arthritis (JIA) is one of the more common chronic diseases in childhood1. It is defined by the International League of Associations for Rheumatology (ILAR) as arthritis that begins before the 16th birthday and persists for at least 6 weeks, with other known conditions excluded2. In addition to arthritis, extraarticular manifestations such as uveitis can occur3. JIA often persists into adulthood and the resulting inflammation and joint damage can lead to substantial longterm morbidity and physical disability4,5. The ILAR classification includes 7 subtypes of JIA, which are defined on the basis of symptoms and the number of involved joints. The 7 subtypes include systemic arthritis, oligoarthritis, polyarthritis [rheumatoid factor (RF)-negative and RF-positive], psoriatic arthritis (PsA), enthesitis-related arthritis, and undifferentiated arthritis2. Prior to the introduction of the ILAR classification, the most common term used in North America to describe chronic arthritis in childhood was “juvenile rheumatoid arthritis” (JRA). However, this rubric included only 4 of the subtypes defined by ILAR: systemic arthritis, oligoarthritis, and polyarthritis (RF-negative and RF-positive).
Population-based registries are important for accurately identifying cases for epidemiologic studies. These registries are used to set research priorities, investigate potentially causal associations, describe prognosis, and identify patient subgroups who share etiologic or clinical characteristics that influence treatment. Examining the frequency of JIA in diverse populations of patients is important because chronic arthritis has been shown to vary according to race and location6,7,8. In Europe, incidence rates per 100,000 range from 22.6 in Norway9 to 3.5 in the former East Berlin area of Germany10. Prevalence rates in Sweden are 86 per 100,000 as compared to 31 per 100,000 in Costa Rica and 0.83 per 100,000 in Japan7. Previous studies of JIA occurrence in the United States and Canada were conducted in somewhat homogeneous populations; their results may not be generalizable to contemporary cohorts7,11,12,13,14. The Kaiser Permanente Northern California Autoimmune Disease Registry, containing information for patients seen from 1996 through 2009, includes patients with JIA using the ILAR definition and those diagnosed using older rubrics (including JRA), as well as those with ankylosing spondylitis (AS), PsA, and inflammatory bowel disease5. In this report, we describe case-finding for JIA and provide estimates of the incidence, prevalence, and clinical characteristics of JIA in the Kaiser Permanente membership.
MATERIALS AND METHODS
We conducted our study with the approval of the Kaiser Foundation Research Institute Institutional Review Board.
Study population
Kaiser Permanente is a prepaid, comprehensive, integrated care organization that maintains computerized clinical data of all visits, procedures, pharmacy dispensings, and other medical goods and services provided to its 8.5 million members across the United States, including the 3.2 million members in Northern California. Health plan enrollees are 41.9% white, 13.5% Asian, 6.5% black, and 16.7% multiracial or other race. Hispanic ethnicity was reported by 16.1%. These databases, comprising a variety of computerized information systems as well as an electronic medical record, provide the opportunity to build disease registries for efficient study of chronic diseases that otherwise cannot be easily identified in a stable and well-characterized population. Kaiser Permanente did not have a pediatric rheumatologist on staff during much of the study period, but pediatricians and adult rheumatologists were free to refer patients to outside pediatric rheumatologists when needed. When outside referrals were made, they typically were to nearby academic centers and children’s hospitals, with claims captured by a Kaiser Permanente information system. Usually referrals were made at the time of diagnosis and during periods of disease exacerbations that were difficult for non-sub-specialists to manage.
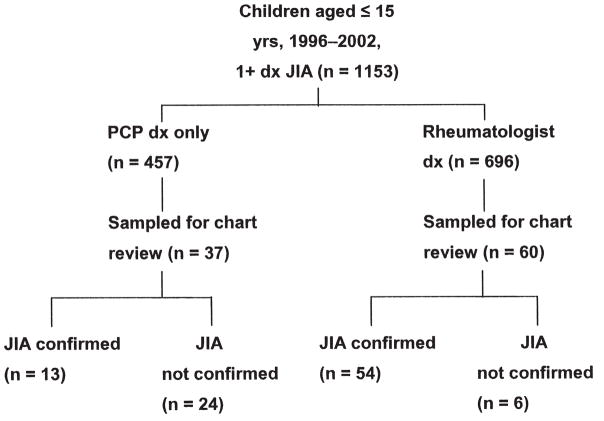
The present study included persons with > 12 months of enrollment in Kaiser Permanente Northern California between 1996 and 2009. Cases of preliminary JIA in patients age ≤ 15 years were identified using age on the date of the first diagnosis recorded during the period of observation. The upper age limit of 15 years was selected on the basis of the diagnostic criteria used by ILAR2. As indicated in Figure 1, we identified children age ≤ 15 years with at least 1 International Classification of Diseases, 9th edition (ICD-9) code of interest. Because there was no single code for JIA in the computerized outpatient or inpatient database, we used codes for chronic arthritis including 696.0 (PsA), 714 (which includes RA and JRA), and 720 (AS) to identify preliminary cases. We did not use codes for inflammatory bowel disease because few of those patients would be confirmed with JIA. A random sample of about 10% of all preliminary cases was selected, by assigning random numbers from 0 to 1.00 and selecting cases with numbers from 0 to 0.10 for validation, using detailed review of the medical record; the actual random sample was 8.4% of the total population.
Figure 1.
The process of selection of patients identified for the study. JIA: juvenile idiopathic arthritis; PCP: primary care physician.
Data collection
Data collection was accomplished during 2010. The period of observation began on the later of the patient’s first enrollment or January 1, 1996, and ended on the earlier of their disenrollment or December 31, 2009. Relevant computerized medical information was obtained for all preliminary cases and included clinical and membership data recorded during 1996–2009. These computerized data were recorded to provide clinical care and not to administer insurance claims. For care received between 2004 and 2006, electronic medical record information was available. Before the electronic medical record was established, the health plan maintained several computerized information systems that were accessed for our study. These information systems included outpatient encounters, hospital diagnoses, laboratory results, pharmacy information, and imaging examinations, among others. We also accessed outside claims that were generated when patients were referred out of the plan, for example, to children’s hospitals and academic medical centers.
Manual chart review was performed on the random sample of preliminary cases. The primary purpose of the chart review was to confirm the diagnosis recorded in the computerized data and to establish the initial diagnosis date. The initial diagnosis date was the date the patient was first diagnosed with JIA, whether or not the diagnosis was made within Kaiser Permanente or within the study period. A secondary purpose of the chart review was to obtain information on clinical characteristics of the disease. A single trained medical record abstractor reviewed each medical record after extensive training supervised by a rheumatologist. Every chart was discussed with the rheumatologist until the rheumatologist was satisfied with the quality of the medical record abstractor’s work. The abstractor accessed information from the electronic medical record, computerized information systems, and paper-based medical records. She reviewed outpatient clinic notes, hospital discharge summaries, laboratory results, radiology reports, and any other information in the medical record that was pertinent to the study. A board-certified rheumatologist was consulted when the abstractor was unsure how to interpret the medical record.
Case definition
ILAR defines JIA as being present when disease symptoms persist for ≥ 6 weeks2. Disease is classified as follows: (1) systemic onset; (2) oligoarticular (“persistent” if ≤ 4 joints over the course of disease and “extended” if > 4 joints after 6 months); (3) polyarticular (> 4 joints) and RF-negative; (4) polyarticular (> 4 joints) and RF-positive; (5) PsA; (6) enthesitis-related arthritis; and (7) undifferentiated (not meeting the criteria of any of the other subgroups, or of multiple subgroups).
For the purpose of our study, we defined a preliminary case of JIA as one for whom a diagnosis of JIA was recorded by an adult or pediatric rheumatologist. A diagnosis recorded by a primary care provider required supporting information, such as documentation of a telephone conversation between the primary care provider and a rheumatologist. Clinic notes by a primary care provider indicating joint inflammation (including synovitis or dactylitis on physical examination and lasting at least 6 weeks) in children aged ≤ 15 years were considered to be confirmed as JIA. Terms such as RA and PsA were included as cases of JIA. In addition, notes such as “history of JIA,” “JIA in remission,” or “RA diagnosed at age 15” were accepted as confirmation of JIA for all providers.
Disease manifestations
Disease manifestations ascertained from chart review included JIA subtype; joint involvement (hand/wrist, shoulder, hip, knee, feet/ankle, sacroileum, cervical spine/neck, thoracic spine, lumbar spine, spine not otherwise specified, and jaw); systemic symptoms (fever, hepatosplenomegaly, lymphadenopathy, rash, and arthritis); associated autoimmune diseases (inflammatory bowel disease, other); radiographic evidence of sacroiliitis, spondylitis, or juxtaarticular new bone formation; and presence of uveitis/iritis. We also obtained laboratory findings recorded in the computerized laboratory data.
Validity of computerized data for identifying incident and prevalent JIA
A case-finding algorithm was developed based on clinical experience and input from researchers with expertise using administrative data for this purpose. In addition, we developed a second algorithm to identify confirmed cases that were newly diagnosed, or incident. The variables examined for inclusion in the case-finding and incidence algorithms included (1) inpatient and outpatient visits with relevant codes; (2) visits specifically to a rheumatologist (adult or pediatric); (3) use of relevant drugs; (4) use of relevant radiology services; and (5) use of relevant laboratory services. We evaluated multiple possible case-finding algorithms, with “1 or more relevant codes recorded in the inpatient or outpatient setting by a primary care physician, pediatrician, or rheumatologist (adult or pediatric)” as the basis for comparison with all other algorithms.
The sensitivity and positive predictive value (PPV) were determined for each of the algorithms under consideration15. The sensitivity of the case-finding algorithms was defined as the number of confirmed cases captured by the algorithm divided by the number of confirmed cases with 1 or more relevant diagnosis codes, as described. The PPV was defined as the proportion of cases identified by the algorithm that were confirmed with JIA during the chart review. We did not compute the specificity and negative predicted value of the diagnostic codes because the focus of the study was on evaluating case-finding algorithms, and given the rarity of JIA, pursuing identification of patients without a diagnosis would have had an exceedingly low yield. The 95% CI for the rate was computed assuming a Poisson distribution16. All analyses were conducted using SAS version 9.13 (SAS Institute Inc.).
Estimation of the standardized incidence rate and point prevalence
We applied the best case-finding algorithm to all children aged ≤ 15 years in the Kaiser Permanente membership. The best algorithm was defined as the one that provided the fewest falsely classified cases, including both false-positive and false-negative cases. The false-negative cases were those not captured by the algorithm. For example, an algorithm requiring 2 or more rheumatologist diagnoses would not capture patients with only a single rheumatologist diagnosis, even if the diagnosis were correct. This would represent a false-negative case when assessing the algorithm “≥ 2 rheumatologist diagnoses.”
The incidence and point prevalence estimates were corrected by first multiplying by the PPV and then dividing by the sensitivity. For example, an uncorrected incidence rate of 13.9 per 100,000 person-years times PPV of 0.80 divided by a sensitivity of 0.95 yields a corrected incidence rate of 11.7 per 100,000 person-years.
The incidence rate of JIA among health plan members ≤ 15 years of age was computed as the number of newly diagnosed cases divided by the number of person-years of membership within Kaiser Permanente Northern California in each year. The age- and sex-specific incidence rates were calculated using as the denominator the number of boys and girls in each 5-year age group. The age- and sex-standardized incidence rates were estimated using the direct method of standardization, with the 2000 US Census population providing weights17,18. The age- and sex-standardized point prevalence rates were estimated on December 31, 2009; for this calculation, only persons who were health plan members and ≤ 15 years of age on that day were included. The 95% CI were computed assuming a Poisson distribution16.
RESULTS
Validation of the case-finding algorithm
The number of children with ≥ 1 ICD-9 diagnosis code of 696.0, 714, or 720 in the computerized data was 1153. They were enrolled in the study for an average of 8 years following their first relevant diagnosis code. Among these, 97 children were randomly sampled for chart review, of which 67 (69% with CI 59%–78%) were determined to have JIA documented in the medical record (Figure 1 and Table 1). Requiring a relevant computerized diagnosis by a rheumatologist reduced the sensitivity of case-finding from 67 true cases to 54 true cases (change in sensitivity from 100% to 81% with CI 69%–89%), but it also reduced the selection of false-positive cases from 30 to 6 (change in PPV, from 69% to 90% with CI 79%–96%). Of the 13 patients identified in the computerized data as having a primary care diagnosis, 7 had seen a pediatric rheumatologist at an academic center or children’s hospital, 4 had documentation of a diagnosis by a pediatric or adult rheumatologist that was not accurately captured in the computerized data, and 2 had longstanding disease diagnosed before their enrollment, with multiple mentions by their pediatricians and ophthalmologic evaluations for iritis. The best-performing case-finding algorithm to confirm a diagnosis of JIA required ≥ 2 diagnoses of diagnosis codes 696.0, 714, or 720 by any provider type (e.g., primary care or pediatric or adult rheumatology). Of the 1153 children, 772 had ≥ 2 diagnoses. This algorithm, which identified both incident and prevalent cases of JIA, had a sensitivity of 87% with CI 76%–93%, capturing 58 of 67 true cases (9 false-negatives); it had a PPV of 91% with CI 80%–96%, with 58 of the 64 cases in the sample captured being true cases (6 false-positive).
Table 1.
Sensitivity and PPV of various algorithms for confirming JIA and the incidence date.
| Concept | Operational Definition of Preliminary Case-finding Algorithm | No. in Population | No. in Random Sample* | Using Chart Review as the Gold Standard | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. True Positives | No. False Positives | Sensitivity (%) | 95% CI | PPV (%) | 95% CI | ||||
| Disease confirmation | ≥ 1 diagnosis code from any provider | 1153 | 97 | 67 | 30 | 100 by definition | — | 69 | 59–78 |
| ≥ 2 diagnosis codes from any provider** | 772 | 64 | 58 | 6 | 87 | 76–93 | 91 | 80–96 | |
| ≥ 1 diagnosis code from rheumatology | 696 | 60 | 54 | 6 | 81 | 69–89 | 90 | 79–96 | |
| New-onset disease | ≥ 1 diagnosis code from any provider | 1153 | 97 | 44 | 53 | 100 by definition | — | 45 | 35–56 |
| ≥ 1 diagnosis code from rheumatology | 696 | 60 | 42 | 18 | 95 | 84–99 | 70 | 57–81 | |
| ≥ 1 diagnosis code from rheumatology, ≥ 2 laboratory tests performed (ANA, RF, HLA-B27) | 581 | 52 | 39 | 13 | 93 | 79–98 | 75 | 61–86 | |
| ≥ 1 diagnosis code from rheumatology, ≥ 2 laboratory tests performed (ANA, RF, HLA-B27), ≥ 12 months of prediagnostic enrollment | 488 | 45 | 37 | 8 | 95 | 81–99 | 82 | 67–91 | |
A random sample of about 10% of all preliminary cases was selected by assigning random numbers from 0–1.00 to the 1153 cases and selecting all cases with numbers from 0–0.10 for validation using medical record review. The number in the random sample was 97 of 1153, with subsets of the 97 meeting various operational definitions tested as preliminary case-finding algorithms.
PPV: positive predictive value; JIA: juvenile idiopathic arthritis; ANA: antinuclear antibody; RF: rheumatoid factor.
Validation of incident JIA
Of the 67 children confirmed with JIA, 44 (66% with CI 53%–77%) had incident disease diagnosed while they were a Kaiser Permanente member and during the study period, while 23 (34% with CI 23%–47%) were diagnosed before the patient enrolled in Kaiser Permanente or before the study period. The best algorithm required 12 months of enrollment before the first diagnosis, a diagnosis by a rheumatologist, and 2 or more diagnostic laboratory tests. This algorithm, which identified incident JIA only, had a sensitivity of 95% with CI 81%–99%, capturing 37 of 44 true cases (9 false-negatives); it had a PPV of 82% with CI 67%–91%, with 37 of the 45 cases in the sample captured being true cases (8 false-positive).
Incidence and prevalence of JIA
We calculated the age- and sex-standardized incidence rate of JIA among children ≤ 15 years of age by applying the optimal incidence algorithm (determined through chart review of 97 children and requiring 12 months of enrollment before the first diagnosis, a diagnosis by a rheumatologist, and 2 or more diagnostic laboratory tests) to the computerized database that included 1153 persons with a relevant diagnosis code. We then corrected the number of estimated cases obtained using this algorithm by accounting for the sensitivity (95%) and PPV (82%). The corrected incidence rate per 100,000 person-years was 11.9 (CI 10.9–12.9). It was 16.4 (CI 14.6–18.1) in girls and 7.7 (CI 6.5–8.9) in boys (Table 2). Using the optimal case-finding algorithm to identify all cases (which included those with at least 1 diagnosis from any provider), the corrected prevalence of JIA among those aged ≤ 15 years among Kaiser Permanente Northern California enrollees, standardized to the 2000 U.S. Census, was 44.7 (CI 39.1–50.2) per 100,000 persons on December 31, 2009 (Table 2).
Table 2.
Standardized1 incidence (per 100,000 person-years), 1996–2009, and point prevalence (per 100,000), December 31, 2009, of juvenile idiopathic arthritis, by sex and age.
| Boys | Girls | Boys and Girls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incidence2 | N | Person- yrs | Cases per 100,000 | (95% CI) | N | Person- yrs | Cases per 100,000 | (95% CI) | N | Person- yrs | Cases per 100,000 | (95% CI) |
| Age group, yrs | ||||||||||||
| 0–5 | 51 | 781,699 | 5.6 | (1.3–9.9) | 105 | 743,786 | 12.2 | (5.9–18.6) | 156 | 1,525,484 | 8.8 | (3.5–14.2) |
| 6–10 | 51 | 522,150 | 8.5 | (3.1–13.7) | 92 | 500,196 | 15.9 | (8.6–23.1) | 143 | 1,022,346 | 12.1 | (5.8–18.4) |
| 11–15 | 58 | 543,446 | 9.2 | (3.7–14.8) | 131 | 520,994 | 21.7 | (13.2–30.2) | 189 | 1,064,441 | 15.4 | (8.2–22.4) |
| Overall | 160 | 1,847.295 | 7.7 | (6.5–8.9) | 328 | 1,764,976 | 16.4 | (14.6–18.1) | 488 | 3,612,271 | 11.9 | (10.9–12.9) |
|
| ||||||||||||
| Prevalence3 | N | Population | Cases per 100,000 | (95% CI) | N | Population | Cases per 100,000 | (95% CI) | N | Population | Cases per 100,000 | (95% CI) |
|
| ||||||||||||
| Age group, yrs | ||||||||||||
| 0–5 | 7 | 85,578 | 8.6 | (2.2–14.9) | 31 | 80,969 | 40.1 | (25.9–54.2) | 38 | 166,547 | 23.8 | (16.3–31.5) |
| 6–10 | 31 | 95,340 | 34.0 | (22.1–46.0) | 51 | 91,643 | 58.3 | (42.3–74.2) | 82 | 186,983 | 45.9 | (36.0–55.8) |
| 11–15 | 46 | 105,366 | 45.7 | (32.4–58.9) | 87 | 101,373 | 89.7 | (70.9–108.6) | 133 | 206,739 | 67.3 | (55.9–78.8) |
| Overall | 84 | 286,284 | 28.6 | (22.4–34.6) | 169 | 273,985 | 61.6 | (52.3–70.9) | 253 | 560,269 | 44.7 | (39.1–50.2) |
The age and sex distribution were standardized to that of the 2000 Census for the entire US population. The Kaiser Permanente population included 102,652 boys and 98,238 girls aged 0–5, 101,000 boys and 96,888 girls aged 6–10, and 108,747 boys and 104,399 girls aged 11–15.
The incidence rate is corrected for the sensitivity (0.95) and positive predictive value (0.82) of the validated incidence algorithm.
The point prevalence is corrected for the sensitivity (0.87) and posiive predictive value (0.91) of the validated case-finding algorithm.
Characteristics of JIA cases
Demographic characteristics of the 67 children confirmed with JIA on chart review are shown in Table 3. Forty percent were white, 26% Hispanic, 6% Asian, and 3% African American, with 25% being of other races, multiracial, or of unknown race. Forty-eight percent had oligoarticular disease, 31% had polyarticular disease, 9% systemic, 3% AS, and 8% psoriatic. The proportion receiving an antinuclear antibody test was 97%, with 70% having a positive result; for RF, 82% were tested with 9% positive; and for HLA-B27, 24% were tested with 2% positive. Among the 5 patients who were RF-positive, 4 were among the 22 patients (18%) with polyarticular disease, while 1 was among the 45 patients (0.4%) without polyarticular disease. The knee was involved in 72% of cases, the hand in 43%, and the feet in 37%. No associated autoimmune disease was recorded for 82% of children. Of note, 3% had uveitis/iritis recorded during the course of their disease.
Table 3.
Demographic and disease manifestations of confirmed cases of JIA, Kaiser Permanente, Northern California, 1996–2009.
| Characteristics | Proportion of Confirmed JIA Cases, n = 67 % |
|---|---|
| Age, yrs* | |
| 0–5 | 33 |
| 6–10 | 21 |
| 11–15 | 46 |
| Sex | |
| Female | 64 |
| Race/ethnicity | |
| White | 40 |
| African American | 3 |
| Asian | 6 |
| Hispanic | 26 |
| Other/multiracial/unknown | 25 |
| Type | |
| Oligoarticular | 48 |
| Polyarticular | 31 |
| Systemic | 9 |
| Psoriatic arthritis | 8 |
| Ankylosing spondylitis | 3 |
| Unknown | 1 |
| Laboratory findings (no. who received test) | |
| Antinuclear antibody (n = 65) | 70% positive |
| Rheumatoid factor (n = 55) | 9% positive |
| HLA-B27 (n = 16) | 2% positive |
| Joint involvement | |
| Knee | 72 |
| Hand | 43 |
| Feet | 37 |
| Hip | 9 |
| Shoulder | 7 |
| Cervical spine/neck | 3 |
| Spine | 3 |
| Other | 7 |
| Not recorded | 7 |
| Associated autoimmune disease | |
| Negtive/not mentioned | 82 |
| Other** | 13 |
| Presence during course of the disease | |
| Uveitis/iritis | 3 |
Age at the time of identification of an incident or prevalent case, thus does not correspond to the age of diagnosis for the entire population.
These conditions include type 1 diabetes, asthma, autoimmune hepatitis, and ulcerative colitis. JIA: juvenile idiopathic arthritis.
DISCUSSION
We estimated the incidence of JIA among pediatric enrollees of Kaiser Permanente Northern California during the period 1996–2009 at 11.9 cases (CI 10.9–12.9) per 100,000 person-years. This rate is consistent with population-based studies that have been conducted during the past 20 years (Table 4). Most were performed in Europe and North America, with the number of cases identified ranging from 4 to 488 (the present study).
Table 4.
Summary of articles reporting population-based JIA incidence rates*.
| Reference | Location | Period | Source | No. Cases | Annual Incidence per 100,000 (95% CI) |
|---|---|---|---|---|---|
| Kiessling, 199810 | Former East Berlin area, Germany | 1980–89 | Pediatrician reports | 78 | 3.5 (2.8–4.4) |
| Malleson, 199613 | 13 centers, Canada | 1991–1993 | Disease registry of Canadian Pediatric Rheumatology Association | 861 | 4.1 (3.6–4.6) |
| Oen, et al, 199514 | Winnipeg, Canada | 1975–92 | Disease registry of the Pediatric Rheumatology Clinic, Children’s Hospital | 261 | 5.3 (4.7–6.0) |
| Arguedas, 199824 | Costa Rica, urban area | 1993–1995 | Pediatrician reports | 48 | 6.8 (4.1–9.6) |
| Modesto, 201025 | Catalonia, Spain | 2004–2006 | Pediatrician reports | 145 | 6.9 (5.8–8.1) |
| Von Koskull, et al, 200123 | 12 southern Germany towns | 1995 | Reports from pediatricians, orthopedists, rheumatologists | 78 | 7.5 (5.8–12.6) |
| Peterson, et al, 199611 | Rochester, Minnesota, USA | 1978–93 | Rochester Epidemiology Project and previous cohort study | 65 | 11.7 (8.7–14.8) |
| Herrinton, et al (current report) | Kaiser Permanente, Northern California, USA | 1996–2009 | Computerized outpatient diagnoses (all providers) | 488 | 11.9 (10.9–12.9) |
| Hanova, 200626 | Czech Republic, 2 regions | 2002–2003 | Reports from PCP, surgeons, orthopedists, rheumatologist, and hospitals | 4 | 13 (1–20) |
| Riise, 200821 | Norway, 3 countries | 2004–2005 | Reports from pediatricians, general practitioners, orthopedists, and rheumatologist | 36 | 14 (10–19) |
| Berntson, 200319 | Iceland, Norway, Sweden, Denmark, and Finland | 1997–98 | Pediatrician reports | 315 | 15 (13–17) |
| Kaipiainen-Seppänen, 200120 | 11 of 21 hospital districts, Finland | 1995 | Pharmacy records | 114 | 19.5 (15.6–24.1) |
| Pruunsild, 200722 | 14 of 15 countries, Estonia | 1998–2000 | Pediatrician and family doctor reports | 162 | 21.7 (15.4–26.7) |
| Moe and Rygg, 19979 | Norway, 2 countries | 1985–94 | Registry | 71 | 22.6 (19.2–28.6) |
Does not include studies in which denominators are estimated from pediatric-clinic populations.
JIA: juvenile idiopathic arthritis; PCP: primary care physicians.
In Europe, studies have reported annual incidence rates per 100,000 in Scandinavia of 14 to 22.69,19,20,21, similar to the rate in Estonia (21.7 per 100,000)22, but higher than the rate in Germany (3.5 to 7.5 per 100,000)10,23. The rates in Costa Rica and Spain approached 7 per 100,000024,25.
In North America, the incidence rate reported for Canada was 4.1 to 5.3 per 100,00013,14, lower than the incidence rate estimated in our study using our case-finding algorithm, which was nearly identical to the rate of 11.7 (CI 8.7–14.8) per 100,000 person-years reported for Rochester, Minnesota, USA, 1978–199311. These rates were higher than the annual incidence rate of 4.0 per 100,000 reported by pediatric rheumatology centers in the Northeast (United States), although ascertainment at the pediatric rheumatology centers may have been incomplete12.
Differences in incidence rates reported across studies may be the result of many factors including small numbers of cases26, differences in methods of ascertainment, true population differences based on exposure to precipitating factors, or genetic predisposition. In addition, changes over time in the classification of pediatric rheumatic diseases (the adoption of the ILAR classification of JIA), greater awareness of the condition by providers in certain regions, and varying access to pediatric rheumatologists to diagnose the condition may partly explain these results.
Despite the consistency of our study with other reports, our study has limitations. Computerized information has well-known limitations relating to accuracy and completeness; it was for this reason that we validated our data and corrected our estimates for the sensitivity and PPV of case-finding27. It is possible that we underascertained mild disease, particularly among those who were enrolled in the health plan for a short time. However, the study was restricted to those Kaiser Permanente Northern California members with at least 12 months of enrollment (both to be identified as a case and to be included in the denominators of the incidence and prevalence calculations). In addition, it is possible that some patients with JIA never received a diagnosis by their providers and thus were not ascertained for this study. Because the computerized data did not contain information on symptoms recorded in a standardized manner, we cannot confirm the presence of synovitis, enthesitis, or associated autoimmune conditions.
The Kaiser Permanente Northern California population is quite representative of the general population of the state of California. The health plan’s data have been linked to the California Health Interview Survey of California residents age 20–79 years living in those postal codes served by Kaiser Permanente. When compared with persons who have medical insurance through other providers, the Kaiser Permanente membership has greater racial diversity (nonwhite, 43% vs 34%). When compared with persons who were not Kaiser Permanente members, including those who were uninsured or insured by others, Kaiser Permanente members have similar racial diversity (nonwhite, 43% vs 45%), although with fewer Latinos (16% vs 23%)28.
The point prevalence observed in the Kaiser Permanente Northern California population (44.7 per 100,000) estimates the disease burden among children ≤ 15 years of age only and does not include the burden among adults, although the disease is chronic in most. With respect to disease manifestations, we observed 48% of children with oligoarticular disease, 31% with polyarticular, and 9% systemic. The Rochester, Minnesota, cohort differed, with 72% of patients having oligoarticular, 17% polyarticular, and 11% systemic disease at onset, with progression of oligoarticular to polyarticular disease in 11% of the cases11. The prospective design they used could account for the difference, with the present study including prevalent cases, for whom disease manifestation may have been recorded following the diagnosis. Our report of uveitis/iritis in 3% of children is a bit lower than the Scandinavian study that observed uveitis/iritis in 8.6% of children, which may be due to differences in race and ethnicity19. Medical record review revealed a diagnosis of AS in only 3% of our study population. While this may reflect the true prevalence of the disease, it may also have been influenced by changes in the diagnosis and/or coding of the condition. More recently, physicians are considering spondyloarthropathies as a group rather than individual conditions and may include it as part of the JIA classification. Thus, physicians may report a diagnosis of JIA rather than AS in these patients29.
The methods used for case-finding in any particular study will depend on the nature of the research question, the research setting, and the relative costs of overascertainment and underascertainment with respect to study validity and precision. A case-finding strategy that has poor sensitivity but a high PPV, such as through recruitment of pediatric rheumatology clinics, likely will yield more severe cases. Milder cases, and those of children whose families cannot afford the time or expense to travel to specialty clinics, are more likely to be managed by adult rheumatologists or primary care providers including pediatricians and family practitioners.
The incidence rate of JIA estimated for children aged ≤ 15 years in the Kaiser Permanente population, 1996–2009, was similar to that reported in Rochester, Minnesota, but 2 to 3 times greater than Canadian estimates. Key strengths of our study include the size of the population, the diversity of the population, and the use of chart review to validate the diagnosis of JIA. Identification of an algorithm for use with computerized data enabled efficient identification of JIA cases. As a result, our study provides a foundation for further investigation including elucidation of the environmental and genetic influences on incident JIA, current treatment patterns, healthcare use, and longterm outcomes, as well as the safety of current treatments for JIA.
Acknowledgments
Supported by a grant from the National Institute of Allergy and Infectious Disease (1RC1AI086107-01). Dr. Harrold has a consulting role with the Consortium of Rheumatology Researchers of North America (CORRONA) and was supported by K23AR053856 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Herrinton has research contracts with Centocor, Genentech, and Proctor and Gamble; Dr. Asgari with Genentech; and Dr. Wu with Abbott Laboratories, Amgen, and Pfizer. Dr. Gelfand receives grants to the trustees of the University of Pennsylvania from Amgen, Abbott, and Genentech, and has consulting roles with Amgen, Abbott, Centocor, Pfizer, and Merck. Dr. Curtis receives consulting fees/honoraria and research support from Roche/Genentech, UCB/Centocor, CORRONA, Amgen, Pfizer, BMS, Crescendo, and Abbott and receives salary support from the U.S. National Institutes of Health (AR053351) and Agency for Healthcare Research and Quality (R01 R01HS018517).
References
- 1.Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767–78. doi: 10.1016/S0140-6736(07)60363-8. [DOI] [PubMed] [Google Scholar]
- 2.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–2. [PubMed] [Google Scholar]
- 3.Nordal E, Zak M, Aalto K, Berntson L, Fasth A, Herlin T, et al. Ongoing disease activity and changing categories in a long-term Nordic cohort study of juvenile idiopathic arthritis. Arthritis Rheum. 2011;63:2809–18. doi: 10.1002/art.30426. [DOI] [PubMed] [Google Scholar]
- 4.Simon D. Management of growth retardation in juvenile idiopathic arthritis. Horm Res. 2007;68(Suppl 5):122–5. doi: 10.1159/000110605. [DOI] [PubMed] [Google Scholar]
- 5.Beukelman T, Patkar NM, Saag KG, Tolleson-Rinehart S, Cron RQ, DeWitt EM, et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: Initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res. 2011;63:465–82. doi: 10.1002/acr.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gäre BA. Juvenile arthritis — Who gets it, where and when? A review of current data on incidence and prevalence. Clin Exp Rheumatol. 1999;17:367–74. [PubMed] [Google Scholar]
- 7.Oen K. Comparative epidemiology of the rheumatic diseases in children. Curr Opin Rheumatol. 2000;12:410–4. doi: 10.1097/00002281-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Manners PJ, Bower C. Worldwide prevalence of juvenile arthritis — Why does it vary so much? J Rheumatol. 2002;29:1520–30. [PubMed] [Google Scholar]
- 9.Moe N, Rygg M. Epidemiology of juvenile chronic arthritis in northern Norway: A ten-year retrospective study. Clin Exp Rheumatol. 1998;16:99–101. [PubMed] [Google Scholar]
- 10.Kiessling U, Doring E, Listing J, Meincke J, Schontube M, Strangfeld A, et al. Incidence and prevalence of juvenile chronic arthritis in East Berlin 1980–88. J Rheumatol. 1998;25:1837–43. [PubMed] [Google Scholar]
- 11.Peterson LS, Mason T, Nelson AM, O’Fallon WM, Gabriel SE. Juvenile rheumatoid arthritis in Rochester, Minnesota 1960–1993. Is the epidemiology changing? Arthritis Rheum. 1996;39:1385–90. doi: 10.1002/art.1780390817. [DOI] [PubMed] [Google Scholar]
- 12.Denardo BA, Tucker LB, Miller LC, Szer IS, Schaller JG. Demography of a regional pediatric rheumatology patient population. Affiliated Children’s Arthritis Centers of New England. J Rheumatol. 1994;21:1553–61. [PubMed] [Google Scholar]
- 13.Malleson PN, Fung MY, Rosenberg AM. The incidence of pediatric rheumatic diseases: Results from the Canadian Pediatric Rheumatology Association Disease Registry. J Rheumatol. 1996;23:1981–7. [PubMed] [Google Scholar]
- 14.Oen K, Fast M, Postl B. Epidemiology of juvenile rheumatoid arthritis in Manitoba, Canada, 1975–92: Cycles in incidence. J Rheumatol. 1995;22:745–50. [PubMed] [Google Scholar]
- 15.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3. Philadelphia: Lippincott, Williams & Wilkin; 2008. [Google Scholar]
- 16.Garwood F. Fiducial limits for the Poisson distribution. [Accessed March 6, 2013];Biometrika. 1936 28:437–42. Available from: http://www.jstor.org/discover/10.2307/2333958. [Google Scholar]
- 17.Kleinbaum D, Kupper L, Morgenstern H. Epidemiologic research: Principles and quantitative methods. New York: Van Nostrand Reinhold; 1982. [Google Scholar]
- 18.U.S. Census Bureau. [Accessed March 6, 2013];National Level Census Data for the United States: 2000. 2001 Apr 2; Available from: http://www.census.gov/population/cen2000/phc-t1/tab01.txt.
- 19.Berntson L, Andersson Gäre B, Fasth A, Herlin T, Kristinsson J, Lahdenne P, et al. Incidence of juvenile idiopathic arthritis in the Nordic countries. A population based study with special reference to the validity of the ILAR and EULAR criteria. J Rheumatol. 2003;30:2275–82. [PubMed] [Google Scholar]
- 20.Kaipiainen-Seppanen O, Savolainen A. Incidence of chronic juvenile rheumatic diseases in Finland during 1980–1990. Clin Exp Rheumatol. 1996;14:441–4. [PubMed] [Google Scholar]
- 21.Riise OR, Handeland KS, Cvancarova M, Wathne KO, Nakstad B, Abrahamsen TG, et al. Incidence and characteristics of arthritis in Norwegian children: A population-based study. Pediatrics. 2008;121:e299–306. doi: 10.1542/peds.2007-0291. [DOI] [PubMed] [Google Scholar]
- 22.Pruunsild C, Uibo K, Liivamagi H, Tarraste S, Talvik T, Pelkonen P. Incidence of juvenile idiopathic arthritis in children in Estonia: A prospective population-based study. Scand J Rheumatol. 2007;36:7–13. doi: 10.1080/03009740601089259. [DOI] [PubMed] [Google Scholar]
- 23.von Koskull S, Truckenbrodt H, Holle R, Hormann A. Incidence and prevalence of juvenile arthritis in an urban population of southern Germany: A prospective study. Ann Rheum Dis. 2001;60:940–5. doi: 10.1136/ard.60.10.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arguedas O, Fasth A, Andersson-Gare B, Porras O. Juvenile chronic arthritis in urban San Jose, Costa Rica: A 2 year prospective study. J Rheumatol. 1998;25:1844–50. [PubMed] [Google Scholar]
- 25.Modesto C, Anton J, Rodriguez B, Bou R, Arnal C, Ros J, et al. Incidence and prevalence of juvenile idiopathic arthritis in Catalonia (Spain) Scand J Rheumatol. 2010;39:472–9. doi: 10.3109/03009741003742722. [DOI] [PubMed] [Google Scholar]
- 26.Hanova P, Pavelka K, Dostal C, Holcatova I, Pikhart H. Epidemiology of rheumatoid arthritis, juvenile idiopathic arthritis and gout in two regions of the Czech Republic in a descriptive population-based survey in 2002–2003. Clin Exp Rheumatol. 2006;24:499–507. [PubMed] [Google Scholar]
- 27.Couris CM, Polazzi S, Olive F, Remontet L, Bossard N, Gomez F, et al. Breast cancer incidence using administrative data: Correction with sensitivity and specificity. J Clin Epidemiol. 2009;62:660–6. doi: 10.1016/j.jclinepi.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Gordon N. [Accessed March 6, 2013];How does the adult Kaiser Permanente membership in Northern California compare with the larger community? 2006 Jun 14; Available from: http://www.dor.kaiser.org/external/uploadedFiles/content/research/mhs/_2011_Revised_Site/Documents_Special_Reports/comparison_kaiser_vs_nonKaiser_adults_kpnc(1).pdf.
- 29.Rudwaleit M, Taylor WJ. Classification criteria for psoriatic arthritis and ankylosing spondylitis/axial spondyloarthritis. Best Pract Res Clin Rheumatol. 2010;24:589–604. doi: 10.1016/j.berh.2010.05.007. [DOI] [PubMed] [Google Scholar]