Abstract
Mate-guarding and territorial aggression (both intra- and inter-sexual) are behavioral components of social monogamy seen in male coppery titi monkeys (Callicebus cupreus) both in the field and in the laboratory. Methodology for studying these behaviors in captivity facilitates the translation of questions between field and lab. In this study, we tested whether exposure to a mirror would stimulate mate-guarding behavior in male titi monkeys, and whether this exposure was accompanied by hormonal changes. Eight males were exposed to a mirror condition (treatment) or the back of the mirror (control) for five sessions, and behavioral responses were filmed. Blood samples were taken to measure levels of cortisol, oxytocin, and vasopressin. Lipsmacks (p< 0.0001), arching (p< 0.0001), tail-lashing (p= 0.009), restraining (p= 0.015), and approaches to the female (p= 0.0002) were all higher during the mirror condition, while tail-twining tended to decline during the mirror condition (p = 0.076). Hormones did not vary by experimental treatment, but were correlated with certain behaviors during the presentation of the mirror. While social behaviors changed with mirror exposure, self-directed and mirror-guided behaviors did not, indicating a lack of self-recognition. Use of a mirror was a safe and effective means of investigating mate-guarding behavior in response to a simulated intrusion, with the added benefit of not needing another animal to serve as an intruder; and thus may be of use in providing a laboratory model for natural behavior. Especially as it eliminates the need for a stimulus animal, it would also be of possible use in investigating responses to a simulated intruder in wild populations of titis and other pithecines.
Keywords: intrasexual aggression, mate-guarding, monogamy, self-recognition
INTRODUCTION
In nature, titi monkey family groups consist of an adult male, an adult female, and 2-4 offspring, living together on small territories [Fernandez-Duque et al. , 2013; Mason, 1966]. Titi monkeys have been characterized as socially monogamous based on group composition in the wild, preference for a specific partner, distress upon separation from that partner, the ability of the partner to buffer against stress, the presence of biparental care, and mate-guarding [Anzenberger et al. , 1986; Cubicciotti and Mason, 1978; Cubiciotti and Mason, 1975; Fernandez-Duque et al. , 1997; Mason, 1975; Mason and Mendoza, 1998; Mendoza and Mason, 1986c]. In the field, ritualized encounters with neighbors may occur daily [Mason, 1966; Mason, 1968a; Robinson et al. , 1987]. Same-sex animals (typically males) engage in mutual chasing with rare but occasional grappling. Although described as aggressive, territorial encounters rarely result in injury or alteration of territorial boundaries. Males will often position themselves between their mate and potential rivals. Copulation between neighbors has been observed (though infrequently), and males will pursue and restrain mates that move away from them when a rival male is in the vicinity [Mason, 1966; Mason, 1968a]. This mate-guarding may be part of a strategy for maintenance of exclusivity of the pair.
Laboratory simulations of territorial encounters have been used frequently in the study of socially monogamous rodents (primarily prairie voles, Microtus ochrogaster) in order to examine both the neurobiological basis of mate-guarding (although often not in the presence of the mate), and to pharmacologically manipulate this behavior [Aragona et al. , 2006; Bales and Carter, 2003; Bowler et al. , 2002; Resendez et al. , 2012]. In the laboratory as in the field, titi monkeys also behave agonistically towards same-sex strangers[Fernandez-Duque et al. , 2000]. In addition to agonistic behaviors, the hypothalamo-pituitary-adrenal (HPA) axis is activated, at least measured by cortisol which is the end-product of the HPA axis. However this response varies among subjects [Mendoza and Mason, 1986b]. Eight out of ten males will display an increase in cortisol in response to exposure to a same-sex stranger, while only four out of eight females do [Mendoza and Mason, 1986b]. The agonistic reaction to same-sex strangers in male titi monkeys is enhanced when proximity of intruder to the mate decreases [Cubiciotti and Mason, 1978; Fernandez-Duque et al. , 2000]. Male titi monkeys have a greater response compared to females when their mates are exposed to strangers [Fernandez-Duque et al. , 2000]. These tests have typically been carried out with live stimulus animals, either viewed through a grate or down a long chute in a testing apparatus. Similar live intruder tests have been carried out in other New World monkey species to examine sex differences in reactions to strangers [Wolovich et al. , 2010], hormonal responses to intruders [Ross and French, 2011], and effects of group size [Schaffner and French, 1997], among other variables.
For New World monkeys, such as the titi monkey, a mirror image is likely viewed as a same sex stranger [Anderson and Gallup, 2011b]. If family members, such as the pair-mate, are also present, the stranger could represent a threat to the integrity of the established familial relationships, particularly if the mirror is positioned such that the stranger might represent a territorial intruder. Use of the mirror has the advantage of allowing for testing in the home cage, reducing the number of animals required for the experiment, sparing stimulus animals from a potential stressor, and eliminating the possibility of injury which could occur even if live animals are separated by grates. In laboratory and wild testing, it is also not always possible to have a live conspecific present, thus this represents a solution to what can be a significant logistical challenge. Mirror tests are often used in this way in other taxa such as fish [Braud and Weibel, 1969; Desjardins and Fernald, 2010]. In the present study, we proposed the use of a mirror to simulate a territorial encounter and provide a means to experimentally manipulate mate-guarding behavior in male titi monkeys; as well as to increase the knowledge base on species which do or do not respond to a mirror image.We hypothesized that arousal and mate-guarding behaviors such as tail-lashing, arching, and restraint of the pairmate, would increase upon exposure to the mirror. We chose to study males because of their higher reactivity to intruders in previous tests with live intruders [Fernandez-Duque et al. , 2000; Mendoza and Mason, 1986b].
We also measured hormonal responses to the mirror encounter to aid in further understanding of the physiology/neurobiology of territoriality. Literature on mating-induced aggression in prairie voles suggests the involvement of oxytocin (OT) and arginine vasopressin (AVP) in mating-induced territoriality [Bales and Carter, 2003; Winslow et al. , 1993]. The pituitary peptide AVP acts to augment HPA response peripherally and plays a role in augmenting social aggression centrally [Compaan et al. , 1993; DeVries et al. , 1997; DeVries and Miller, 1998; Ferris and Delville, 1994; Nelson and Chiavegatto, 2001]. Finally, OT was monitored since its involvement in facilitating affiliation and pair-bonding has been demonstrated in rodents [Cho et al. , 1999; Insel and Young, 2001; Young and Wang, 2004] and primates [Smith et al. , 2010]. It has also been implicated as a hormone that promotes in-group cohesion at the cost of out-group aggression [Anacker and Beery, 2013; De Dreu et al. , 2011;De Dreu and Kret, 2015]. Moreover, it has been suggested that OT can reduce stress responsiveness, acting as an inoculation to physiological reactions to potentially stressful social circumstances [Parker et al. , 2005]. Peripheral measurements of OT and AVP may not be reflective of central measures, but may be coordinated in response to social stimuli [Kenkel et al. , 2012; Landgraf and Neumann, 2004]. We predicted positive associations of both OT and AVP to reactions to a simulated intruder, based on their strong association with pair-bonding and social displays of aggression.
We also monitored the HPA response in order to evaluate the valence of the mirror stimulus. We hypothesized that cortisol might increase to the extent that the mirror was an aversive stimulus. However, given that cortisol increases in response to a same-sex stranger were not universally displayed even in a live intruder test [Mendoza and Mason, 1986b], to the extent that the mirror test was similar, we would expect to see variation in cortisol responses to a mirror stimulus.
Mirrors have often been used in the context of determining whether or not non-human primates and other animals can recognize themselves, i.e. demonstrate theory of mind [Anderson and Gallup, 2011b; Gallup, 1970]. Only great apes (not even lesser apes: [Suddendorf and Collier-Baker, 2009]) show undisputed self-recognition [Anderson and Gallup, 2011b], although there is some controversy regarding rhesus monkeys [Anderson and Gallup, 2011a; Rajala et al. , 2010]. Other New World monkeys tested, for example capuchins, do not show evidence of self-recognition in a mirror in several studies [Anderson and Roeder, 1989; Roma et al. , 2007], although there is some evidence for differential responses to mirrors and strangers in at least one study [de Waal et al. , 2005]. The classic method of determining self-recognition starts with spontaneous observation of mirror-guided behaviors (for instance, using a mirror to look at areas of the body that are not normally visible). Researchers have also used increased self-directed behavior (ex. increased self-grooming or touching of parts of the body) as early indicators of self-recognition. These behaviors should increase over time, as social responses to the mirror decrease. These observations are then followed by a formal mark test (marking a non-visible part of the body, such as the forehead, when the animal is unaware, and then observing mirror behavior) [Gallup, 1970]. Although testing self-recognition was not a goal of this study, we also coded the videotapes for mirror-guided and self-directed behaviors, with the prediction that titi monkeys would not show signs of self-recognition in the mirror.
METHODS
Subjects and Housing
Subjects for this study were eight paired male coppery titi monkeys (Callicebus cupreus), with a mean ± (SEM) 8.5 ± 1.3 years of age at the beginning of the project. All subjects were housed with their mates and any immature offspring (see Table I for detailed Subject information). Family groups were housed in 1.2 m × 1.2 m × 2.1 m stainless steel cages with four horizontal perches extending the width of the cage at varying heights; there was also a small U-shaped perch (15.9 cm × 15.9 cm) on the door of the cage. Animals were fed twice a day with a diet consisting of New World monkey chow, rice cereal, carrots, apples, raisins, and bananas. A more detailed account of husbandry is available in Tardif et al. [2006] and Mendoza [1986a]. Water was available ad lib. This study complied with IACUC protocols, legal requirements of the U.S., and the policies of the American Society of Primatologists on ethical treatment of animals.
TABLE I.
Subject Information including Age, Pairing Length, Number of Offspring Living with the Pair, and Oxytocin Levels in the Control Condition (Averaged from Both Samples)
| Male ID | Age (years) | Pairing Length (years) | Number of Offspring in Cage | Oxytocin (pg/ml) in Control condition |
|---|---|---|---|---|
| 29775 | 14.8 | 3.2 | 0 | 770.65 ± 21.44 |
| 31716 | 11.9 | 9.0 | 2 | 651.79 ± 51.38 |
| 32878 | 10.1 | 1.0 | 0 | 1540.71 (second sample not available) |
| 34438 | 8.5 | 3.2 | 0 | 657.89 ± 62.19 |
| 34531 | 8.2 | 3.9 | 2 | 986.07 ± 56.15 |
| 36150 | 6.4 | 0.7 | 1 | 890.21 ± 32.22 |
| 36187 | 6.1 | 1.2 | 1 | 766.08 ± 51.68 |
| 38644 | 3.2 | 0.9 | 1 | 671.43 ± 186.55 |
Mirror Exposure and Behavioral Assessment
A mirror (approximately 36 × 22 cm, on top of a movable cart 82.6 cm in height) was wheeled in front of each animal’s homecage to signal the start of the experimental session. The mirror was placed directly in front of the cage door so that an animal sitting on the small door perch would see the full image of itself sitting approximately 16.5 cm away. It is also possible that the male would see a reflection of its mate and/or offspring in the mirror as well. Each session consisted of a 5 min exposure to the cart and mirror in one of two orientations: 1. Mirror condition with the reflective surface facing towards the subject’s cage or 2. Control condition with non-reflective back of the mirror facing the subject’s cage. Each subject was tested twice per week, once in each condition with test days separated by at least one non-test day and the order of testing counter-balanced. Testing continued for 5 weeks. Subjects were filmed during the test and scored later. Blood samples were taken during both conditions on weeks 2 and 4 of the experiment. Therefore, the total experiment consisted of 10 exposures (5 Mirror, 5 Control) and 4 blood samples (2 Mirror, 2 Control). Social behaviors, as well as location in the cage, were scored using Behavior Tracker 1.5 (www.behaviortracker.com). Behavioral definitions are detailed in Table II. We also scored mirror-guided and self-directed behaviors to examine for self-recognition in the mirror. Offspring were left in the cage during testing.
TABLE II.
Stimulus Exposure Ethogram
| Behavior | Definition |
|---|---|
| a) Social Behaviors | |
| Contact | Passive physical contact between male and pair-mate that does not include tail-twining |
| Proximity | Male comes within arm’s reach of female |
| Male Groom | Male combs through fur of female |
| Female Groom | Female combs through fur of male |
| Tail-twining | Male sitting side by side with female, with tails wrapped around each other for at least one rotation |
| Male Approach | Male moves toward female and establishes contact |
| Male Leave | Male breaks contact with female |
| Lipsmack | Male repeatedly and rapidly opens and closes mouth |
| Chest Rub | Male moves chest with pressure and friction against a perch or surface; may also press downward on chest with hands or arms |
| Tail-lashing | Male repetitively swings whole tail from side to side (area greater than 40 degrees, usually sign of agitation) |
| Aggression | Male displays aggression to female |
| Mount/Sex | Male mounts female or attempts/succeeds to copulate |
| Restraining | Male forcibly restrains female by placing hands on her shoulders |
| Arching | Male arches back (as in a frightened cat), the subject may also have his arms and trunk lifted off the perch |
| b) Location | |
| Top Perch | Male located on highest perch |
| Off Camera | Male not visible in viewing area |
| Small door perch | Male located on door perch closest to mirror |
| c) Mirror-Guided Behaviors | Using the mirror to adjust movements of the body |
| Using the mirror to view body parts not normally visible | |
| Using the mirror to look at environment not normally visible | |
| Looking behind the mirror | |
| d) Self-Directed Behaviors | Self-grooming |
| Touches to the body |
Blood Collection and Processing
Four blood samples (1 ml) were collected from each subject via femoral venipuncture. Subjects were unanesthetized, and blood was collected using 1cc syringes pretreated with heparin. Subjects were captured in a transport cage which they had all been trained to voluntarily enter upon demand. Animals were then hand captured and manually restrained for sample collection. Average time of blood collection from entrance to the cage was 180.23 ± 13.16 sec, while average time from removing the monkey from the transport box until blood draw was 98.55 ± 12.68 sec. There was no difference between treatment conditions in either measure (p > 0.05). During week two of testing, blood samples were collected 20 min following first exposure to the mirror or the control. These samples were subsequently assayed for plasma cortisol concentrations. During week four, blood samples were collected 5 min after initial exposure (immediately following behavioral testing) in order to capture responses for the more rapidly reactive peptide hormones AVP and OT. Following blood collection, samples were immediately placed on ice, centrifuged at 3000 rpm at 4°C, the plasma extracted and stored at -80°C until assay.
Plasma cortisol concentrations were measured using commercial radioimmunoassay kits (Siemens Medical Solutions Diagnostics, Los Angeles, CA) previously validated for use in titi monkeys [Hoffman et al. , 1995]. Samples were diluted 1:4 in PBS-gel and all samples were run in a single assay. Intra-assay coefficient of variation was 2.45%. OT and AVP were analyzed by enzyme immunoassay (Enzo Life Sciences, Farmingdale, NY) validated for titi monkeys [Bales et al. , 2005], with samples diluted at 1:6. Intra-assay coefficients of variation were 3.72% for OT and 4.49% for AVP. Although we originally collected the 5-min samples for AVP and OT analysis and the 20-min sample for cortisol, we decided to assay AVP and OT at both time points. This allowed us to examine effects of time elapsed since the stimulus presentation on these two peptides, in order to establish a time course for future studies.
Data Analysis
Data were analyzed by general linear mixed model ANOVA [Littell et al. , 1996] in SAS 9.3 (SAS Institute, Cary, NC). Models included condition (mirror or no mirror), week, a condition by week interaction, and male identity (ID) as a random factor which accounts for the repeated measures design of the experiment. Due to very low levels of mirror-guided and self-directed behaviors, these were compared by condition but not by week. Residuals were checked for non-normality and transformed as necessary. Spearman correlations for hormone-behavior relationships were carried out on a limited number of behavioral variables (arching, tail-lashing, tail-twining, and approaches to the female, vs. OT, AVP, and cortisol).We compared only Control condition behavioral variables to Control condition hormonal variables, and Mirror condition behavior to Mirror condition hormones. We chose to use these non-parametric correlations because there were a number of zeros for certain behaviors particularly in the Control condition, causing the correlations to be more driven by a few data points. Both time points were included for correlations. Because we performed correlations on only a limited number of variables with strong a priori hypotheses regarding correlations, we did not use post-hoc corrections for multiple comparisons. All tests were two-tailed and significance was set at P < 0.05.
RESULTS
Behavioral Responses to Mirror Exposure
There was no effect of week in any of the behavioral models (P > 0.05 for all tests), and the week by condition interaction is reported below only when significant (P < 0.05). Males spent more time on the small door perch (and therefore closest to the mirror) during the Mirror condition (condition, F1,62 = 8.60, P = 0.006). During the Mirror condition, the mean duration of sitting on the door perch was 36.3 ± 0.68 times during the 5 min observation, while in the Control condition, it was 12.67 ± 0.33 times. Time spent on the highest perch (farthest from the mirror) and time off camera were not altered by experimental condition.
As predicted, agonistic displays were more frequent during the Mirror condition than the Control condition. Tail-lashing occurred at a significantly higher rate during the Mirror condition (treatment, F1,62 = 7.33, P = 0.009) (Fig. 1). Similarly, Arching occurred at a significantly higher rate during the Mirror condition (treatment, F1,62 = 19.36, P < 0.0001) (Fig. 1). Behavior toward the female pair-mate was also altered by the presence of the mirror. Lipsmacking towards the mate occurred at a significantly higher rate during the Mirror condition (treatment, F1,62 = 22.94, P < 0.0001) (Fig. 1). There was a condition by week interaction (F4,62 = 2.71, P = 0.038), with Lipsmacking increasing in the Mirror condition and decreasing in the Control condition. Males restrained their mate more often in the Mirror condition (F1,62 = 6.24, P = 0.015). (Fig. 1). Although frequency or duration of contact with the mate was not affected by condition, Tail-twining tended to be lower during the Mirror condition (treatment, F1,62 = 3.25, P = 0.076) (Fig. 1). Approaches to the female were significantly higher in the Mirror condition (F1,62 = 5.76, P = 0.019), while leaves did not differ significantly by treatment (Fig.1).
Fig. 1.
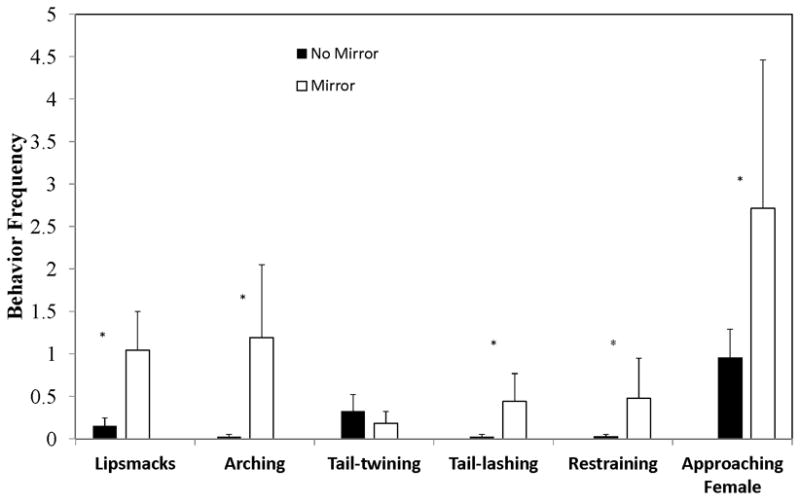
Frequency of male behaviors (mean ± standard error) for the different experimental conditions (mirror or back of mirror). Asterisks indicate p <0.05.
In all behavioral measures there was considerable variability among males (p < 0.01 for the random effect of ID). There were no differences between conditions in time spent grooming or in male chest-rubs. Aggression and mount/sex occurred too infrequently for analysis.
Endocrine Responses to Mirror Exposure
Cortisol was not significantly higher in response to the Mirror condition when compared to the Control condition (F1,13 = 0.03, P = 0.865). Cortisol levels were 35.36 ± 5.26 μg/dl (mean ± SEM) in the Control condition and 38.36 ± 5.81 μg/dl in the Mirror condition. There was no significant change in OT in response to condition (F1,25 = 0.25, P = 0.620), time to blood collection (F1,25 = 0.35, P = 0.560), or a condition by time to blood collection interaction (F1,25 = 1.72, P = 0.201). OT levels were 821.93 ± 63.46 pg/ml in the Control condition and 881.5 ± 85.65 pg/ml in the Mirror condition. There was no significant change in AVP in response to condition (F1,25 = 0.14, P = 0.710) or the condition by time interaction term (F1,25 = 0.92, P = 0.347); however, there was a significant effect of time post-mirror exposure (F1,25 = 8.06, P = 0.009), with AVP higher at 20 minutes post-exposure than at five minutes. AVP levels were 302.23 ± 23.57 pg/ml in the Control condition at 5 minutes and 507.83 pg/ml ± 88.48 in the Control condition at 20 minutes. In the Mirror condition, AVP levels were 326.45 ± 36.18 pg/ml at 5 minutes and 461.28 ± 80.95 pg/ml at 20 minutes. In all hormonal measures there was considerable variability among males (p < 0.01 for the random effect of ID).
Mirror-Guided and Self-Directed Behaviors
There were no significant effects of condition on mirror-guided or self-directed behaviors (Table 3), except for number of touches to the face (F1,71 = 4.01, P = 0.049). However, touches to the face were actually higher in the Control condition (Table 3).
Table 3.
Mirror-guided and Self-directed Behaviors (mean ± standard errors).
| Behavior | Control | Mirror |
|---|---|---|
| Look Behind the Mirror | 0.075 ± 0.042 | 0.075 ± 0.055 |
| Double-Take | 0 | 0.05 ± 0.035 |
| Examine Parts of Body Not Normally Visible | 0 | 0.075 ± 0.055 |
| Use Mirror to Adjust Movements of the Body | 0 | 0 |
| Use Mirror as a Tool to Look at Environment | 0 | 0 |
| Touch Head | 0.025 ± 0.025 | 0.075 ± 0.055 |
| Touch Face | 0.275 ± 0.101 | 0.05 ± 0.035 |
| Touch Chest | 0 | 0.075 ± 0.075 |
| Touch Arm | 0 | 0.1 ± 0.06 |
| Touch Hand | 0.025 ± 0.025 | 0.05 ± 0.025 |
| Touch Stomach | 0.025 ± 0.025 | 0.05 ± 0.05 |
| Touch Body | 0.075 ± 0.042 | 0.15 ± 0.127 |
| Touch Foot | 0.05 ± 0.035 | 0.025 ± 0.025 |
| Touch Tail | 0.025 ± 0.025 | 0 |
| Touch Back | 0.125 ± 0.082 | 0.1 ± 0.078 |
| All Touches to Body Parts | 0.625 ± 0.159 | 0.65 ± 0.296 |
| Self-Groom | 0.025 ± 0.025 | 0 |
Hormone-Behavior Relationships
In the Control condition, hormonal measures were not correlated with any behavioral variables examined (arching, tail-lashing, tail-twining, and approaches to the female). In the Mirror condition, AVP was positively correlated with tail-lashing (r14 = 0.562, P = 0.036), and there were non-significant trends for positive correlations with arching (r14 = 0.518, P = 0.057) and tail-twining (r14 = 0.530, P = 0.051) (Fig. 2). OT was positively correlated with tail-lashing (r14 = 0.665, P = 0.009)and arching (r14 = 0.626, P = 0.017), and there was a trend for a positive correlation with approaches to the female (r14 = 0.504, P = 0.066) (Fig.3).
Fig. 2.
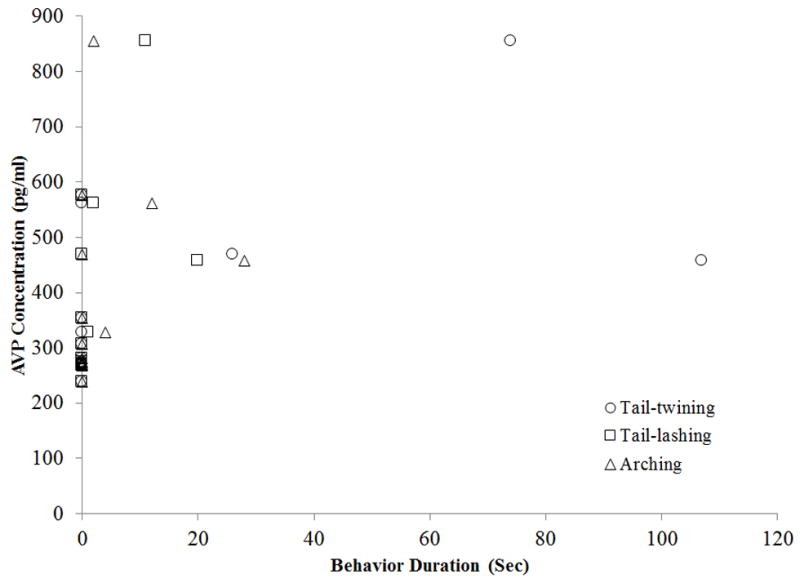
Correlation of male AVP concentration (pg/ml) to duration of tail-twining, tail-lashing, and arching in mirror condition only.
Fig. 3.
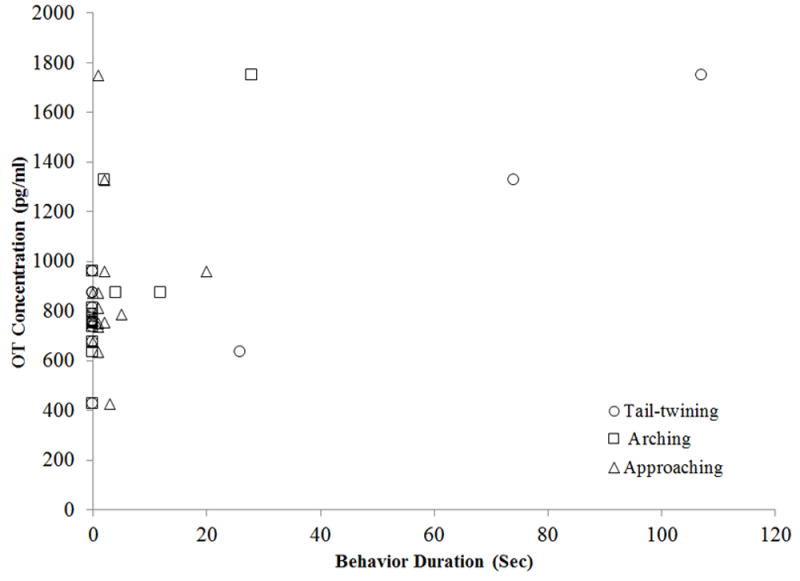
Correlations of male OT concentration (pg/ml) to durations of arching, approaching, and tail-twining in the mirror condition only.
DISCUSSION
In monogamous species, behaviors such as affiliation and mate-guarding can help maintain the pair-bond and support the reproductive success of the pair [Anzenberger, 1988; Fuentes, 1999; Kleiman, 1977; Robinson et al. , 1987]. In another socially monogamous species, owl monkeys (Aotus), a recent field study showed that replacement of pair-mates by intruders results in negative effects on the reproductive success of both partners; maintenance of the pair-bond may therefore be adaptive in many cases [Fernandez-Duque and Huck, 2013]. The present study shows that male titi monkeys will react to a mirror image as though an intruder is present. Agonistic and arousal behaviors such as arching, tail-lashing, and time spent near stimulus significantly increased during the Mirror condition, which indicates an agitated state and interest in the “other animal”. Males also increased active interactions with their pair-mates, including lip-smacking, restraint, and approaches.
In the wild, titi monkeys show a large degree of coordinated behavior [Mason, 1966], including spending large amounts of time in proximity or contact to the mate [Fernandez-Duque et al. , 2013; Mason, 1968b], grooming the mate [Fernandez-Duque et al. , 2013; Kinzey and Wright, 1982], engaging in duets [Robinson et al. , 1987], sleeping in a huddle with twined tails [Souza-Alves et al. , 2011], and performing biparental infant care [Kinzey et al. , 1977; Mason, 1966]. They also show territorial behaviors as described in the introduction, including chasing of intruders and restraint of mates [Mason, 1966; Mason, 1968b; Robinson et al. , 1987]. In our study, titi monkey males confronted by a mirror thus significantly increased the same behaviors associated with intruders in wild titi monkeys.
One limitation of this study was the lack of a live intruder control condition, which would have allowed a direct comparison between these two methods [de Waal et al. , 2005]. However, there is an extensive literature allowing us to quantitatively compare our results to those from live intruder paradigms in captive titi monkeys [Cubiciotti and Mason, 1978; Fernandez-Duque et al. , 1997; Fernandez-Duque et al. , 2000; Mendoza and Mason, 1986b; Menzel, 1986; Menzel, 1993]. When exposed to a live male stranger, male approaches to the mate increased by 2.57 times in frequency in the Mendoza and Mason paper [Mendoza and Mason, 1986b]; approaches to the mate increased by 2.87 times in this study when males were confronted with a mirror. Approaches to the male stranger (2.52 times higher than control) were also similar to approaches to a mirror, defined here as being located on the small door perch (2.03 times higher than control). With a live intruder, displays (lip-smacking and back-arching) increased by 54 times in frequency, whereas in this study back-arching increased by 47.71 times. Thus, behavior towards a simulated intruder appears quantitatively similar to that towards a live intruder.
OT did not vary consistently between the experimental conditions. However, OT was correlated with certain behaviors which promote maintenance of the pair-bond. During the mirror condition OT was positively correlated with tail-lashing, arching, and tended to correlate with approaches to female. These correlations were often driven by a few data points from males that had both high OT and high behavioral arousal. As in rodents [Carter and Keverne, 2002; Insel and Young, 2001; Young et al. , 2008; Young and Wang, 2004] these data suggest that animals that have more active OT systems also engage in more active expressions of affiliation toward the mate, and more mate-guarding in the presence of a potential challenge to the bond. In contrast to our expectations, AVP did not respond differentially to treatment conditions and did not correlate with expression of agonistic behavior toward the mirror or mate-guarding. AVP was elevated 20 min post exposure, suggesting some hormonal response to the procedure, although levels did not differ between animals exposed to the front or the back of the mirror, possibly because the pair-mate was present to buffer a stress reaction [Ragen et al. , 2013]; but this suggests that animals were mildly responsive to the novelty of the procedure as a generalized stressor. As the only previous studies on titi monkey plasma OT and AVP in the literature were focused on the effects of opioid manipulations [Ragen et al. , 2015; Ragen et al. , 2013], this paper presents the first report of OT and AVP levels in titi monkeys in the context of a behavioral manipulation. We also did not find any effect of the mirror manipulation on cortisol. However, the live intruder study [Mendoza and Mason, 1986b] also found variation between males. It is possible that variation in cortisol levels, as well as other hormonal levels, reflects differences in the strength of the pair-bond.
Were male titi monkeys responding to a simulated intruder, or to what they recognized as their own reflection? While we did not do a conclusive test of self-recognition, we examined behavior for any potential signs of self-awareness as examined in other studies of mirror behavior [Bard et al. , 2006; de Waal et al. , 2005], and did not find them. Gallup [1970] considered lowered social responses, and increased self-directed responses during mirror exposure, to be signals of self-recognition. In contrast, here we found increasing social responses to the mate across time during mirror exposure (and no change across time in other social responses), with self-directed behaviors at too low of a frequency to even analyze across time. These results are not surprising given that self-recognition has only been shown conclusively in the great apes. However, this does add another New World monkey species to the list of those that have been examined. This analysis also lends support to the assertion that titi monkey males were treating the mirror as a novel conspecific, not as their own reflection. While mirror-guided and self-directed behaviors did not differ between conditions, arousal behaviors indicative of a challenge (arching, tail-lashing), affiliative behaviors (approaches to the female, lip-smacking) and mate-guarding behaviors (restraining the female), all increased in the mirror condition. We would therefore conclude that the mirror was viewed as a territorial intruder and not as the subject himself.
While we have discussed the use of this test primarily in the context of social monogamy, it could theoretically be used to answer many questions, in different species of Pithecines, regarding social reactions to simulated same-sex conspecifics. For instance, wild male Chiropotes display a ritualized “lining-up” behavior in which they engage in affiliative contact with other in-group males [Garber and Kowalewski, 2013; Veiga and Ferrari, 2013]. Reactions to a “strange male” in the mirror may be interesting in this species, particularly depending on whether the test male is with his group-mates or not. In addition, a mirror stimulus could be relatively easily placed and used in the wild, in a manner similar to other psychological testing [Martin and Santos, 2013] or play-back experiments [Cheney and Seyfarth, 1999].
In summary, the present study confirms the hypothesis that male titi monkeys react behaviorally to a mirror image similarly to their reaction to a strange male. Our previous research has shown that male titis respond to the actual presence of a stranger with the full panoply of behavioral changes reported here [Fernandez-Duque et al. , 2000; Mendoza and Mason, 1986a]. While the simulated territorial encounter did not result in changes in cortisol, OT, or AVP, the correlation of OT and AVP to specific behaviors such as arching, lip smacking, and tail twining suggest that one avenue for future research might be in ways that individual differences in the hormones may reflect individual differences in the pair-bond. Finally, these results suggest that a simulated territorial encounter in the laboratory can be useful to address questions that are of interest to socioecologists, but difficult to answer in wild populations.
Acknowledgments
We would like to thank all of the researchers and undergraduates in the Laboratory for Comparative Neurobiology of Monogamy for help in completing this project. Funding for this study was provided by the Good Nature Institute, NIH Office of Research Infrastructure Programs grant P51OD011107 to the California National Primate Research Center, and NIH grant HD053555 to KLB and SPM. This study complied with IACUC protocols, legal requirements of the U.S., and the policies of the American Society of Primatologists on ethical treatment of animals.
References
- Anacker A, Beery A. Life in groups: the roles of oxytocin in mammalian sociality. Frontiers in Behavioral Neuroscience. 2013;7 doi: 10.3389/fnbeh.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JR, Gallup GG. Do rhesus monkeys recognize themselves in mirrors? 2011a;73:603–606. doi: 10.1002/ajp.20950. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Gallup GG. Which primates recognize themselves in mirrors? Plos Biology. 2011b;9:e1001024. doi: 10.1371/journal.pbio.1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JR, Roeder J-J. Responses of capuchin monkeys (Cebus apella) to different conditions of mirror-image stimulation. Primates. 1989;304:581–587. [Google Scholar]
- Anzenberger G. The pairbond in the titi monkey (Callicebus moloch): intrinsic versus extrinsic contributions of pairmates. Folia Primatological. 1988;50:188–203. doi: 10.1159/000156345. [DOI] [PubMed] [Google Scholar]
- Anzenberger G, Mendoza SP, Mason WA. Comparative studies of social behavior in Callicebus and Saimiri: Behavioral and physiological responses of established pairs to unfamiliar pairs. American Journal of Primatology. 1986;11:37–51. doi: 10.1002/ajp.1350110105. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang ZX. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nature Neuroscience. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Bales KL, Carter CS. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster) Hormones and Behavior. 2003;44:178–184. doi: 10.1016/s0018-506x(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Bales KL, Kramer KM, Hostetler CM, Capitanio JP, Mendoza SP. Validation of oxytocin and vasopressin blood assay for primates: what can blood tell us? American Journal of Primatology. 2005;66:73–73. [Google Scholar]
- Bard KA, Todd BK, Bernier C, Leavens DA. Self-awareness in human and chimpanzee infants: what is measured and what is meant by the mark and mirror test? Infancy. 2006;9:191–219. [Google Scholar]
- Bowler CM, Cushing BS, Carter CS. Social factors regulate female-female aggression and affiliation in prairie voles. Physiology & Behavior. 2002;76(4-5):559–566. doi: 10.1016/s0031-9384(02)00755-2. [DOI] [PubMed] [Google Scholar]
- Braud WG, Weibel JE. Acquired stimulus control of drug-induced changes in aggressive display in Betta splendens. Journal of the Experimental Analysis of Behavior. 1969;12:773–777. doi: 10.1901/jeab.1969.12-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Keverne EB. The neurobiology of social affiliation and pair bonding. In: Pfaff D, editor. Hormones, brain, and behavior. San Diego: Academic Press; 2002. pp. 299–337. [Google Scholar]
- Cheney DL, Seyfarth RM. Recognition of other individuals’ social relationships by female baboons. Animal Behaviour. 1999;58:67–75. doi: 10.1006/anbe.1999.1131. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 1999;113(5):1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Compaan JC, Buijs CW, Deruiter AJH, Koolhaas JM. Differential lateral septal vasopressin innervation in aggressive and nonaggressive male mice. Brain Research Bulletin. 1993;30:1–6. doi: 10.1016/0361-9230(93)90032-7. [DOI] [PubMed] [Google Scholar]
- Cubicciotti DDI, Mason WA. Comparative studies of social behavior in Callicebus and Saimiri: heterosexual jealousy behavior. Behavioral Ecology and Sociobiology. 1978;3:311–322. [Google Scholar]
- Cubiciotti DDI, Mason WA. Comparative studies of social behavior in Callicebus and Saimiri: male-female emotional attachments. Behavioral Biology. 1975;16:185–197. doi: 10.1016/s0091-6773(76)91296-7. [DOI] [PubMed] [Google Scholar]
- Cubiciotti DDI, Mason WA. Comparative studies of social behavior in Callicebus and Saimiri: heterosexual jealousy behavior. Behavioral Ecology and Sociobiology. 1978;3:311–322. [Google Scholar]
- De Dreu CK, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJ. Oxytocin promotes human ethnocentrism. Proceedings of the National Academy of Sciences. 2011;108:1262–1266. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu CK, Kret ME. Oxytocin conditions intergroup relations through upregulated in-group empathy, cooperation, conformity, and defense. Biological Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.03.020. [DOI] [PubMed] [Google Scholar]
- de Waal FBM, Dindo M, Freeman CA, Hall MJ. The monkey in the mirror: Hardly a stranger. Proceedings of the National Academy of Sciences. 2005;102:11140–11147. doi: 10.1073/pnas.0503935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins JK, Fernald RD. What do fish make of mirror images? Biology Letters. 2010;6:744–747. doi: 10.1098/rsbl.2010.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Young WS, Nelson RJ. Reduced aggressive behavior in mice with targeted disruption of the oxytocin gene. Journal of Neuroendocrinology. 1997;9:363–368. doi: 10.1046/j.1365-2826.1997.t01-1-00589.x. [DOI] [PubMed] [Google Scholar]
- DeVries GJ, Miller MA. Anatomy and function of the extrahypothalamic vasopressin systems in the brain. Progressive Brain Research. 1998;119:3–20. doi: 10.1016/s0079-6123(08)61558-7. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque E, Di Fiore A, De Luna AG. Pair-mate relationships and parenting in equatorial saki monkeys (Pithecia aequatorialis) and red titi monkeys (Callicebus discolor) of Ecuador. In: Veiga LM, Barnett AA, Ferrari SF, Norconk MA, editors. Evolutionary Biology and Conservation of Titis, Sakis, and Uakaris. Cambridge: Cambridge University Press; 2013. pp. 295–302. [Google Scholar]
- Fernandez-Duque E, Huck M. Till death (or an intruder) do us part: intrasexual competition in a monogamous primate. PLoS One. 2013;8:e53724. doi: 10.1371/journal.pone.0053724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Duque E, Mason WA, Mendoza SP. Effects of separation on responses to mates and strangers in the monogamous titi monkey. American Journal of Primatology. 1997;43:225–237. doi: 10.1002/(SICI)1098-2345(1997)43:3<225::AID-AJP3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque E, Valeggia CR, Mason WA. Effects of pair-bond and social context on male-female interactions in captive titi monkeys (Callicebus moloch, Primates: Cebidae) Ethology. 2000;106:1067–1082. [Google Scholar]
- Ferris CF, Delville Y. Vasopressin and serotonin interactions in the control of antagonistic behavior. Psychoneuroendorincology. 1994;19:593–601. doi: 10.1016/0306-4530(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Fuentes A. Re-evaluating primate monogamy. American Anthropologist, New Series. 1999;100(4):890–907. [Google Scholar]
- Gallup GG., Jr Chimpanzees: self recognition. Science. 1970;167:86–87. doi: 10.1126/science.167.3914.86. [DOI] [PubMed] [Google Scholar]
- Garber PA, Kowalewski MM. Male cooperation in Pitheciines: the reproductive costs and benefits to individuals of forming large multimale/multifemale groups. In: Veiga LM, Barnett AA, Ferrari SF, Norconk MA, editors. Evolutionary Biology and Conservation of Titis, Sakis, and Uakaris. Cambridge: Cambridge University Press; 2013. pp. 97–105. [Google Scholar]
- Hoffman KA, Mendoza SP, Hennessy MB, Mason WA. Responses of infant titi monkeys, Callicebus moloch, to removal of one or both parents: Evidence for paternal attachment. Developmental Psychobiology. 1995;28:399–407. doi: 10.1002/dev.420280705. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. National Review of Neuroscience. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Kenkel WM, Paredes J, Yee JR, Pournajafi-Nazarloo H, Bales KL, Carter CS. Neuroendocrine and behavioural responses to exposure to an infant in male prairie voles. Journal of Neuroendocrinology. 2012;24:874–886. doi: 10.1111/j.1365-2826.2012.02301.x. [DOI] [PubMed] [Google Scholar]
- Kinzey WG, Rosenberger AL, Heisler PS, Prowse DL, Trilling JS. A preliminary field investigation of the yellow handed titi monkey, Callicebus torquatus torquatus, in northern Peru. Primates. 1977;18:159–181. [Google Scholar]
- Kinzey WG, Wright PC. Grooming behavior in the titi monkey (Callicebus torquatus) American Journal of Primatology. 1982;3:267–275. doi: 10.1002/ajp.1350030124. [DOI] [PubMed] [Google Scholar]
- Kleiman DG. Monogamy in mammals. Quarterly Review of Biology. 1977;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in Neuroendocrinology. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Littell R, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, NC: SAS Institute Inc; 1996. [Google Scholar]
- Martin A, Santos LR. The origins of belief representation: Monkeys fail to automatically represent others’ beliefs. Cognition. 2013;130:300–308. doi: 10.1016/j.cognition.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WA. Social organization of the South American monkey, Callicebus moloch: a preliminary report. Tulane Studies in Zoology. 1966;13:23–28. [Google Scholar]
- Mason WA. Use of space by Callicebus groups. In: Jay P, editor. Primates: Studies in adaptation and variability. New York: Holt, Rinehart, and Winston; 1968a. [Google Scholar]
- Mason WA. Use of space by Callicebus groups. In: Jay PC, editor. Primates: Studies in Adaptation and Variability. New York: Holt, Rinehart, and Wilson; 1968b. pp. 200–216. [Google Scholar]
- Mason WA. Comparative studies of Callicebus and Saimiri: strength and specificity of attraction between male-female pairs. Folia Primatologica. 1975;23:11–23. doi: 10.1159/000155664. [DOI] [PubMed] [Google Scholar]
- Mason WA, Mendoza SP. Generic aspects of primate attachments: Parents, offspring and mates. Psychoneuroendocrinology. 1998;23(8):765–778. doi: 10.1016/s0306-4530(98)00054-7. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, Mason WA. Contrasting responses to intruders and to involuntary separation by monogamous and polygynous New World monkeys. Physiology and Behavior. 1986a;3:795–801. doi: 10.1016/0031-9384(86)90045-4. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, Mason WA. Contrasting responses to intruders and to involuntary separation by monogamous and polygynous New World monkeys. Physiology & Behavior. 1986b;38:795–801. doi: 10.1016/0031-9384(86)90045-4. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, Mason WA. Parental division of labour and differentiation of attachments in a monogamous primate (Callicebus cupreus) Animal Behaviour. 1986c;34:1336–1347. [Google Scholar]
- Menzel CR. An experimental study of territory maintenance in captive titi monkeys (Callicebus moloch) In: Else JG, Lee PC, editors. Primate Ecology and Conservation. New York: Cambridge University Press; 1986. pp. 133–143. [Google Scholar]
- Menzel CR. Coordination and conflict in Callicebus social groups. In: Mason WA, Mendoza SP, editors. Primate Social Conflict. Albany, N.Y.: State University of New York Press; 1993. pp. 253–290. [Google Scholar]
- Nelson RJ, Chiavegatto S. Molecular basis of agression. Trends in Neuroscience. 2001;24:713–719. doi: 10.1016/s0166-2236(00)01996-2. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology. 2005;30(9):924–929. doi: 10.1016/j.psyneuen.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Ragen BJ, Maninger N, Mendoza SP, Bales KL. The effects of morphine, naloxone, and kappa opioid manipulation on endocrine functioning and social behavior in monogamous titi monkeys (Callicebus cupreus) Neuroscience. 2015;287:32–42. doi: 10.1016/j.neuroscience.2014.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragen BJ, Maninger N, Mendoza SP, Jarcho MR, Bales KL. Presence of a pair-mate regulates the behavioral and physiological effects of opioid manipulation in the monogamous titi monkey (Callicebus cupreus) Psychoneuroendocrinology. 2013;38:2448–2461. doi: 10.1016/j.psyneuen.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala AZ, Reininger KR, Lancaster KM, Populin LC. Rhesus monkeys (Macaca mulatta) do recognize themselves in a mirror: implications for the evolution of self-recognition. PLoS One. 2010;5:e12865. doi: 10.1371/journal.pone.0012865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Kuhnmuench M, Krzywosinski T, Aragona BJ. K-opioid receptors within the nucleus accumbens shell mediate pair bond maintenance. The Journal of Neuroscience. 2012;32:6771–6784. doi: 10.1523/JNEUROSCI.5779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JG, Wright PC, Kinzey WG. Monogamous Cebids and their relatives: Intergroup calls and spacing in primate societies. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsake TT, editors. Primate Societies. Chicago: University of Chicago Press; 1987. pp. 44–53. [Google Scholar]
- Roma PG, Silberberg A, Huntsberry ME, Christensen CJ, Ruggiero AM, Suomi SJ. Mark tests for mirror self-recognition in capuchin monkeys (Cebus apella) trained to touch marks. American Journal of Primatology. 2007;69:989–1000. doi: 10.1002/ajp.20404. [DOI] [PubMed] [Google Scholar]
- Ross CN, French JA. Female marmosets’ behavioral and hormonal responses to unfamiliar intruders. American Journal of Primatology. 2011;73:1072–1081. doi: 10.1002/ajp.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner CM, French JA. Group size and aggression: ‘recruitment incentives’ in a cooperatively breeding primate. Animal Behaviour. 1997;54:171–180. doi: 10.1006/anbe.1996.0413. [DOI] [PubMed] [Google Scholar]
- Smith AS, Agmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Hormones and Behavior. 2010;57:255–262. doi: 10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Alves JP, Fontes IP, Ferrari SF. Use of sleeping sites by a titi group (Callicebus coimbrai) in the Brazilian Atlantic forest. Primates. 2011;52:155–161. doi: 10.1007/s10329-011-0235-9. [DOI] [PubMed] [Google Scholar]
- Suddendorf T, Collier-Baker E. The evolution of primate self-recognition: evidence of absence in lesser apes. Proceedings of the Royal Society of London Series B-Biological Sciences. 2009;276:1671–1677. doi: 10.1098/rspb.2008.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif S, Bales KL, Williams L, Moeller EL, Abbot D, Schultz-Darken N, Mendoza SP, Mason WA, Bourgeois S, Ruiz J. Preparing New World moneys for laboratory research. Institute for Laboratory Animal Research Journal. 2006;47:307–315. doi: 10.1093/ilar.47.4.307. [DOI] [PubMed] [Google Scholar]
- Veiga LM, Ferrari SF. Ecology and behavior of bearded sakis (genus Chiropotes) In: Veiga LM, Barnett AA, Ferrari SF, Norconk MA, editors. Evolutionary Biology and Conservation of Titis, Sakis, and Uakaris. Cambridge: Cambridge University Press; 2013. pp. 240–249. [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365(6446):545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Wolovich CK, Evans S, Green SM. Mated pairs of owl monkeys (Aotus nancymaae) exhibit sex differences in response to unfamiliar male and female conspecifics. AmJPrimatol. 2010;72(11):942–950. doi: 10.1002/ajp.20858. [DOI] [PubMed] [Google Scholar]
- Young KA, Liu Y, Wang Z. The neurobiology of social attachment: a comparative approach to behavioral, neruoanatomical, and neurochemical studies. Comparative Biochemistry and Physiology. 2008;148:401–410. doi: 10.1016/j.cbpc.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Wang ZX. The neurobiology of pair bonding. Nature Neuroscience. 2004;7(10):1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]


