Summary
Background
Detention of people who use drugs into compulsory drug detention centres (CDDCs) is common throughout East and Southeast Asia. Evidence-based pharmacological therapies for treating substance use disorders, such as opioid agonist treatments with methadone, are generally unavailable in these settings. We used a unique opportunity where CDDCs coexisted with voluntary drug treatment centres (VTCs) providing methadone in Malaysia to compare the timing and occurrence of opioid relapse (measured using urine drug testing) in individuals transitioning from CDDCs versus methadone maintenance in VTCs.
Methods
We did a parallel, two-arm, prospective observational study of opioid-dependent individuals aged 18 years and older who were treated in Malaysia in the Klang Valley in two settings: CDDCs and VTCs. We used sequential sampling to recruit individuals. Assessed individuals in CDDCs were required to participate in services such as counselling sessions and manual labour. Assessed individuals in VTCs could voluntarily access many of the components available in CDDCs, in addition to methadone therapy. We undertook urinary drug tests and behavioural interviews to assess individuals at baseline and at 1, 3, 6, 9, and 12 months post-release. The primary outcome was time to opioid relapse post-release in the community confirmed by urinary drug testing in individuals who had undergone baseline interviewing and at least one urine drug test (our analytic sample). Relapse rates between the groups were compared using time-to-event methods. This study is registered at ClinicalTrials.gov (NCT02698098).
Findings
Between July 17, 2012, and August 21, 2014, we screened 168 CDDC attendees and 113 VTC inpatients; of these, 89 from CDDCs and 95 from VTCs were included in our analytic sample. The baseline characteristics of the two groups were similar. In unadjusted analyses, CDDC participants had significantly more rapid relapse to opioid use post-release compared with VTC participants (median time to relapse 31 days [IQR 26–32] vs 352 days [256–unestimable], log rank test, p<0·0001). VTC participants had an 84% (95% CI 75–90) decreased risk of opioid relapse after adjustment for control variables and inverse propensity of treatment weights. Time-varying effect modelling revealed the largest hazard ratio reduction, at 91% (95% CI 83–96), occurs during the first 50 days in the community.
Interpretation
Opioid-dependent individuals in CDDCs are significantly more likely to relapse to opioid use after release, and sooner, than those treated with evidence-based treatments such as methadone, suggesting that CDDCs have no role in the treatment of opioid-use disorders.
Funding
The World Bank Group, Doris Duke Charitable Foundation, National Institute on Drug Abuse, Australian National Health & Medical Research Council, National Institute of Mental Health, and the University of Malaya-Malaysian Ministry of Higher Education High Impact Research Grant.
Introduction
Criminalisation of drug possession and use is common worldwide, with many Asian countries confining people who use drugs, or those suspected of using them, in specialized facilities called compulsory drug detention centres (CDDCs).1 In Malaysia, CDDCs were first introduced in 1978 in response to a growing heroin epidemic and have been operated by the Malaysian National Anti-Drug Agency (NADA). As of 2010, NADA was operating 28 of these detention facilities housing 7000 individuals. For those placed in CDDCs, national drug control laws mandate 2 years of detention, followed by community supervision for another 18 months after release.2
Although Malaysia introduced opioid-agonist therapies and needle and syringe programmes in 2005 when it failed to meet its political goal of reducing HIV infections,2 CDDCs remain central to drug control efforts.3 By 2010, Malaysia’s Ministry of Health had expanded opioid agonist therapies in communities and prisons. The perceived effectiveness of community-based opioid agonist therapies in contrast to the perceived high failure rates of CDDCs resulted in NADA partially shifting its policy toward treating addiction from compulsory, institutional interventions to voluntary, evidence-based treatment in line with that provided by the Ministry of Health.4 Several CDDCs were subsequently transitioned to VTCs, called Cure and Care centres, which provided inpatient and outpatient methadone maintenance with a menu of voluntary psychosocial interventions, recreational programming, and vocational training.5,6 By contrast with CDDCs, patients could voluntarily present themselves for treatment at VTCs. After a thorough medical assessment (which was not available in the CDDCs) patients at VTCs could receive 1 to 3 months of inpatient methadone treatment, followed by continued outpatient methadone maintenance upon release.
Up to now, no findings from studies have supported any sustained rehabilitation benefits from CDDCs;7 instead, they are associated with negative health consequences, increased HIV risk-taking, compounded stigma and discrimination, human rights violations, and absence of evidence-based practices in treating drug dependence.3,8,9 Despite many international agencies calling for all countries to close CDDCs over concerns of their ineffectiveness and human-rights abuses,10 CDDCs continue to operate, and in some settings proliferate, across east and southeast Asia. Approximately 600 000 people are mandatorily detained in more than 1000 facilities annually.11–14
The pathways by which individuals enter CDDCs, the duration of detention, and the services available in these centres all vary substantially. In most countries, detention in CDDCs is predicated by a complex interplay of individual, social, and political factors.15 The main reasons for entering CDDCs include a positive urine drug test, suspicion of illicit drug use by police, or insistence by family members.7,16 Proponents argue that these centres are central policy components of a comprehensive response to opioid use, and serve to balance individuals’ needs for rehabilitation with the right to safety for families and communities.17 Individuals are held in these centres, however, which often do not have trained healthcare personnel or evidence-based drug treatments, without due process protections or judicial oversight of detention. Opioid agonist therapies such as methadone and buprenorphine, which are included in the model list of essential medicines by WHO for opioid dependence treatment, are unavailable;18 and instead, educational and vocational training programmes, and hard labour are often mandated.7
Despite more than 30 years of experience, concerns over CDDCs’ ineffectiveness and continued expansion, few studies have empirically examined how CDDCs affect drug use outcomes. A systematic review19 of compulsory inpatient and outpatient treatment strategies showed little evidence that compulsory drug treatment is effective in promoting abstention from drug use or in reducing criminal recidivism. This review did not, however, compare the effectiveness of CDDCs relative to evidence-based treatment, such as voluntary medical treatment with opioid agonist therapies.
For our study, we took advantage of a unique opportunity where CDDCs coexisted with voluntary drug treatment centres (VTCs) providing methadone in Malaysia. This transition allowed contemporaneous comparison of two divergent policies towards addressing problematic drug use in Malaysia with objective drug treatment outcomes in opioid-dependent individuals. In our analysis, we compared the timing and occurrence of relapse with opioids and other illicit drugs confirmed by urine drug testing between the two groups. Given the evidence of methadone’s effectiveness in reducing opioid use relative to treatment without opioid agonist therapies, we hypothesised that individuals transitioning to the community after release from VTCs would have fewer relapses and longer times to relapse than those released from CDDCs.
Methods
Study design
We did a parallel, two-arm, prospective study of opioid-dependent individuals treated in two settings: Malaysian CDDCs and VTCs in the Klang Valley. We did not select a randomised design because the Malaysian judicial system determined who entered CDDCs. The study was approved by institutional review boards at the University of Malaya and Yale University, and by NADA, and the protocol, questionnaires, anonymised data, and analytic code are deposited publically.
Participants and setting
Eligibility criteria included being aged 18 years or older, the ability to provide informed consent, meeting criteria for opioid dependence,20 and intending to live in the Klang Valley. Assessed individuals in CDDCs were required to participate in non-evidence-based services, including individual, group and family counselling sessions, spiritual programmes, physical exercise, manual labour, and vocational training (eg, farming or electronics). In addition to methadone therapy, individuals enrolled in the VTC arm could access many of the components of the CDDC programme, but did so voluntarily.5,6,21 Recruitment in CDDCs occurred within 90 days before expected release. Common reasons for non-participation included not returning to Klang Valley, confidentiality concerns, and concerns over potential harassment by law enforcement because of study participation.
We used sequential sampling for participant recruitment. During the recruitment periods, all facility attendees meeting eligibility criteria at three VTCs providing methadone maintenance therapy in Greater Kuala Lumpur and at six CDDCs were offered study participation. Recruitment was halted early in September, 2014, because of reversion of some VTCs to CDDCs; and because interim analyses revealed large differences between study arms in the primary outcome.
After group informational sessions, interested clients met privately with trained researchers to complete informed consent procedures. Everyone screened received referral information for healthcare and drug treatment. Consented participants were reimbursed RM50 (approximately US$15) for each visit and provided mobile phones with phone credit. Additional RM50 bonuses were provided for completing all of the first six and 12 follow-up interviews (one per month).
Procedures
Results from urine drug tests were obtained at baseline and at 1, 3, 6, 9, and 12 months post-release; baseline and monthly behavioral surveys were also obtained through 12 months post-release. We interviewed participants about their demographic and social characteristics, incarceration or detention history, lifetime and recent drug use, addiction severity,22 opioid cravings using an 11-point Likert scale, motivation for drug treatment using SOCRATES,23 HIV testing and treatment history, social support,24 and drug-related and sex-related HIV-risk behaviours. The survey was translated and back-translated to Bahasa Malaysia to ensure the accuracy of intended meaning. Researchers undertook and recorded urine drug tests for five metabolites: opioids, methamphetamines, benzodiazepines, methadone, and buprenorphine using a custom RapiDip InstaTest (Cortez Diagnostics, CA, USA). The tests have good diagnostic accuracy, interoperator reliability, and performance on interference testing with high specificity when tested with other common metabolites. We also did HIV testing, but this is the subject of a companion analysis. Urine drug test assessments occurred on the day of release for CDDC participants and baseline surveys occurred within 90 days before, or 7 days after, release from CDDC or inpatient treatment at VTCs. Follow-up visits, especially those where urine drug testing assessments were made, were scheduled (within a 2-week target window) in person in a private setting. All interviews were conducted in Bahasa Malaysia.
Outcomes
The primary outcome, specified a priori, was occurrence and timing of urine drug test-confirmed opioid use in the community, because all participants met criteria for opioid dependence, the most frequently used illicit drug in Malaysia. Timing of relapse was based on free choice in the community and not within a controlled setting. A secondary outcome was urine drug test-confirmed use of any of three illicit drug types: opioids, amphetamine-type substances, or benzodiazepines.
In our adjusted, weighted analyses, we included variables which could explain potential differences in treatment allocation or baseline risk of opioid relapse between the arms: receptiveness, ambivalence and taking steps towards change in drug use; daily heroin use before detention or inpatient VTC entry; age of first drug use and years of heroin use; addiction severity; drug injection; previous drug treatment; lifetime use of alcohol, stimulants, benzodiazepines and non-heroin opioids; age; ethnicity; marital, housing, and education status; social support; and number of times imprisoned, jailed, and previously detained in a CDDC (appendix).
Statistical analysis
We used five number summaries and Mann–Whitney U test statistics (continuous variables) or proportions and χ2 test statistics (categorical variables) to compare group characteristics. For the main analyses, we employed time-to-event approaches, for which the time origin was the first day not being in a controlled environment (release date from CDDCs or inpatient VTC units). The target event was the first positive urine drug test, assuming that any missing intervening follow-up measurements were negative (non-events). With this definition, we censored observations only at the latest non-missing negative urine drug test, given that the event had not yet occurred.
For each group, we estimated Kaplan-Meier curves for time-to-relapse, cumulative relapse-free proportions at selected intervals, and median relapse times, applying the log-rank test of equality. In our prespecified primary analysis, we employed Cox-regression with Efron’s method for ties handling. To account for potential selection effects, a logistic regression model of the propensity of seeking care at a VTC was developed using control variables measuring characteristics of participants before treatment allocation.25 Common support and balance diagnostics suggested good model performance (appendix).25 Inverse propensity of treatment scores were then incorporated as stabilised weights in the final Cox regression with the remaining control variables (ie, opioid cravings and the SOCRATES subscales) included as explanatory variables with study arm as the main variable of interest (see appendix for further description of this approach and related robustness checks).26
Detecting the presence of a time-varying effect of study arm,27 we used Akaike Information Criteria to select a piece-wise model which included a time-varying specification (appendix). We provided hazard ratio estimates for both the time-invariant and time-varying specifications, with the former interpretable as the averaged effect over the follow-up interval. We also plotted adjusted survival curves. These were implemented after a two-stage imputation approach to address partial missing information on length of inpatient stay for the VTC arm, timing of the first urine drug test measurement for the CDDC arm, and baseline control variables (appendix contains description of missingness, imputation details, and related sensitivity analyses).
Because of attrition and missing follow-up measurements, we did several additional robustness checks (sensitivity analyses) by redefining our missing follow-up measurement assumptions in several ways, such that all missing follow-up urine drug tests were equivalent to a non-event; all missing follow-up urine drug tests were equivalent to an event; and missing follow-up urine drug tests were 25% likely to be events for CDDC participants and 75% likely to be events for VTC participants (ie, a 50% absolute difference). Analyses were done in SAS version 9.4 (SAS Institute Inc., Cary, NC). This study is registered at ClinicalTrials. gov (NCT02698098).
Role of the funding source
One of the funders of the study (the World Bank) had a role in study design and review of the manuscript but had no role in data collection, data analysis, data interpretation, or decision to publish the findings. All other funders had no role in these stages. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between July 17, 2012, and August 21, 2014, we screened 281 opioid-dependent individuals in Malaysia; 168 in CDDCs and 113 in inpatient units of VTCs. 98 in both groups completed baseline interviews and 89 (CDDC) and 95 (VTC) of these individuals had at least one subsequent urine drug test, representing our analytic sample (figure 1). Loss between recruitment and baseline measurement was due to inability to locate participants (including early release) and absence of communication with the study team. Delaying the origin of time from day of entry at the VTC to day of inpatient release reduced the VTC arm sample by between 13 and 34 participants depending on the imputation model used, representing attrition before the discharge date (appendix). The number of completed outcome measurements for each group were similar, with 50% completed at month 3 and a quarter to a third completed at month 12 (appendix).
Figure 1. Participant flow chart.
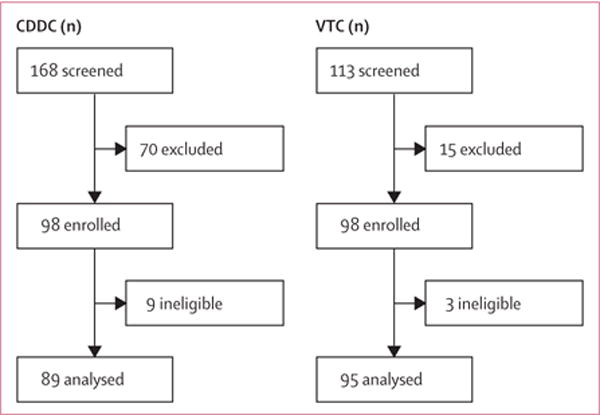
Reasons for screening failure were not systematically recorded, but common reasons included not returning to Klang Valley, and concerns regarding confidentiality and potential harassment by law enforcement due to study participation. CDDC=compulsory drug detention centre. VTC=voluntary drug treatment centre.
Participants were similarly matched for most baseline characteristics (table 1) except that CDDC participants were older, had higher education levels, were incarcerated more frequently, were less likely to have injected opioids, and were less likely to be taking steps towards changing their drug use.
Table 1.
Baseline characteristics of the study population
| Compulsory drug detention centres (n=89) |
Voluntary treatment centres (n=95) |
p value | |
|---|---|---|---|
| Age (years) | 39 (34–46); 25–56 | 37 (30–41); 21–70 | 0.0119* |
|
| |||
| Ethnic origin | 0.9232† | ||
| Malay | 65 (73%) | 67 (71%) | |
| Indian | 15 (17%) | 17 (18%) | |
| Chinese and other | 9 (10%) | 11 (12%) | |
|
| |||
| Completed secondary school | 0.0220† | ||
| No | 58 (65%) | 46 (48%) | |
| Yes | 31 (35%) | 49 (52%) | |
|
| |||
| Married | 0.1822† | ||
| No | 68 (76%) | 80 (84%) | |
| Yes | 21 (24%) | 15 (16%) | |
|
| |||
| Previous housing type | 0.3841† | ||
| Missing data | 2 (2%) | 0 | |
| Permanent | 28 (32%) | 25 (26%) | |
| Temporary | 59 (68%) | 70 (74%) | |
|
| |||
| Number of times imprisoned | 3 (2–5); 0–16 | 3 (1–4); 0–10 | 0.2480* |
|
| |||
| Number of times in lockup or jail | 7 (3–10); 0–49 | 5 (3–10); 0–60 | 0.5085* |
|
| |||
| Number of times detained in compulsory drug detention centres‡ | 1 (0–2); 0–8 | 1 (0–2); 0–10 | 0.7774* |
|
| |||
| Age at first drug use (years) | 18 (15–21); 9–40 | 18 (16–20); 12–48 | 0.5866* |
|
| |||
| Drug of choice | 0.1109† | ||
| Missing data | 2 (2%) | 0 | |
| Heroin | 82 (94.3%) | 83 (87%) | |
| Other | 5 (6%) | 12 (13%) | |
|
| |||
| Duration of heroin use (years) | 16 (10–21); 1–40 | 13 (8–20); 3–41 | 0.1430* |
|
| |||
| Daily use of heroin before entering facility | 0.4774† | ||
| Missing data | 3 (3%) | 7 (7%) | |
| No | 14 (16%) | 11 (13%) | |
| Yes | 72 (84%) | 77 (88%) | |
|
| |||
| Drug use severity | 0.5167† | ||
| Missing data | 0 | 2 (2%) | |
| Low or moderate | 19 (21%) | 14 (15%) | |
| Substantial | 59 (66%) | 65 (70%) | |
| Severe | 11 (12%) | 14 (15%) | |
|
| |||
| Opioid cravings (0–10) | 3 (1–7); 0–10 | 3 (0–7); 0–10 | 0.4550* |
|
| |||
| Ever injected drugs | 0.0944† | ||
| Missing data | 1 (1%) | 4 (4%) | |
| No | 60 (68%) | 51 (56%) | |
| Yes | 28 (32%) | 40 (44%) | |
|
| |||
| Alcohol use (lifetime) | 0.2439† | ||
| No | 17 (19%) | 25 (26%) | |
| Yes | 72 (81%) | 70 (74%) | |
|
| |||
| Non-heroin opioid use (lifetime) | 0.7269† | ||
| No | 73 (82%) | 76 (80%) | |
| Yes | 16 (18%) | 19 (20%) | |
|
| |||
| Benzodiazepine use (lifetime) | 0.99832 | ||
| No | 74 (83%) | 79 (83%) | |
| Yes | 15 (17%) | 16 (17%) | |
|
| |||
| Stimulant use (lifetime) | 0.6526† | ||
| No | 28 (32%) | 27 (28%) | |
| Yes | 61 (69%) | 68 (72%) | |
|
| |||
| Use of more than one drug at the same time (lifetime) | 01390† | ||
| Missing data | 0 | 2 (2%) | |
| No | 40 (45%) | 52 (56%) | |
| Yes | 49 (55%) | 41 (44%) | |
|
| |||
| Ever received buprenorphine treatments‡ | 0.8846† | ||
| Missing data | 0 | 11 (12%) | |
| No | 78 (88%) | 73 (87%) | |
| Yes | 11 (12%) | 11 (13%) | |
|
| |||
| Recent buprenorphine treatments‡ | 0.1670† | ||
| Missing data | 0 | 11 (12%) | |
| No | 87 (98%) | 84 (100%) | |
| Yes | 2 (2%) | 0 | |
|
| |||
| Readiness for change | |||
| Recognition | 40 (20–60); 10–70 | 50 (30–70); 10–70 | 0.1335* |
| Ambivalence | 60 (40–70); 10–90 | 60 (40–70); 10–90 | 0.6663* |
| Taking steps | 70 (50–90); 10–90 | 90 (70–90); 40–90 | 0.0001* |
|
| |||
| Recent emergent or urgent care | 0.8569† | ||
| Missing data | 1 (1%) | 0 | |
| No | 83 (94%) | 89 (94%) | |
| Yes | 5 (6%) | 6 (6%) | |
|
| |||
| Ever tested for HIV | 0.0508† | ||
| Missing data | 1 (1%) | 5 (5%) | |
| No | 7 (8%) | 16 (18%) | |
| Yes | 81 (92%) | 74 (82%) | |
|
| |||
| HIV-test result | 0.0055† | ||
| Missing data | 3 (3%) | 6 (6%) | |
| HIV-negative | 72 (84%) | 61 (69%) | |
| HIV-positive | 5 (6%) | 2 (2%) | |
| Unknown | 9 (11%) | 26 (29%) | |
|
| |||
| Social support | |||
| Significant partner | 16 (12–20); 4–24 | 16 (12–22); 4–24 | 0.7011* |
| Family | 22 (20–24); 11–24 | 23 (20–24); 10–24 | 0.2397* |
| Friends | 16 (12–20); 4–24 | 19 (12–21); 8–24 | 0.3566* |
|
| |||
| Time as inpatient (days) | ·· | 80 (58–93); 15–100 | ·· |
Data are median (IQR); range, or n (%). Denominators are different for the missing percentage calculations and the cariable percentage calculations ··=not applicable.
Kruskal-Wallis.
χ2.
12% non-response in voluntary treatment arm.
In unadjusted analyses, CDDC participants had significantly more rapid relapse to opioid use post-release compared with VTC participants (median time to relapse 31 days [95% CI 26–32] vs 352 days [256 to unestimable], log rank test, p<0·0001; table 2, vs figure 2, appendix); additional analyses with relapse to any drug use were similar (30 days [95% CI 24–32] 317 days [177 to unestimable]; table 2, appendix), favouring more rapid median time to relapse for CDDC participants (30 vs 317 days, log rank test, p<0·0001). Cox-regression modelling, including inverse propensity score weighting and adjustment for post-treatment-assignment variables revealed consistent results (adjusted curves in figure 3; unadjusted, adjusted, and adjusted with time-varying group effect hazard ratios in table 3).
Table 2.
Unadjusted probability of no drug relapse using urine drug testing
| Days from release | No opioid use
|
No illicit drug use
|
|||
|---|---|---|---|---|---|
| Voluntary treatment centres | Compulsory drug detention centres | Voluntary treatment centres | Compulsory drug detention centres | ||
| Month | |||||
| 1 | 30 | 0.90 (0.81–0.95) |
0.51 (0.39–0.61) |
0.89 (0.79–0.94) |
0.46 (0.35–0.57) |
| 3 | 90 | 0.80 (0.68–0.88) |
0.23 (0.14–0.33) |
0.78 (0.66–0.87) |
0.19 (0·11–0.28) |
| 6 | 180 | 0.69 (0·55–0.79) |
0.19 (0.11–0.28) |
0.62 (0.48–0.73) |
0.12 (0.06-0.20) |
| 9 | 270 | 0.62 (0.48–0.73) |
0.12 (0.05–0.21) |
0.53 (0.39–0.66) |
0.07 (0.02–0.16) |
| 12 | 365 | 0.50 (0.34–0.64) |
0.10 (0.04–0.19) |
0.42 (0.27–0.56) |
0.05 (0.01–0.13) |
Unadjusted Kaplan Meier estimates (95% CIs) reflecting the cumulative probability of no drug relapse for selected follow-up times.
Figure 2. Unadjusted probability of no opioid use.
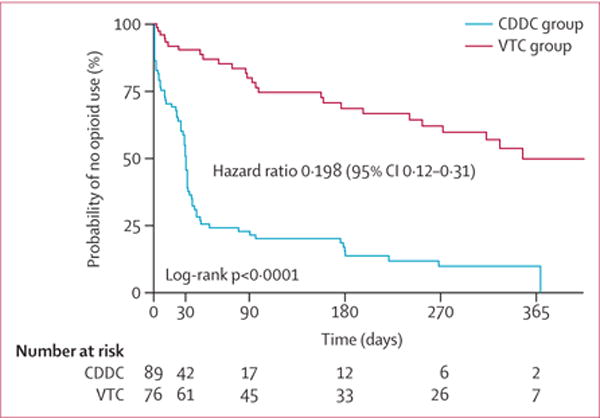
CDDC=compulsory drug detention centre. VTC=voluntary drug treatment centre.
Figure 3. Adjusted probability of no opioid use.
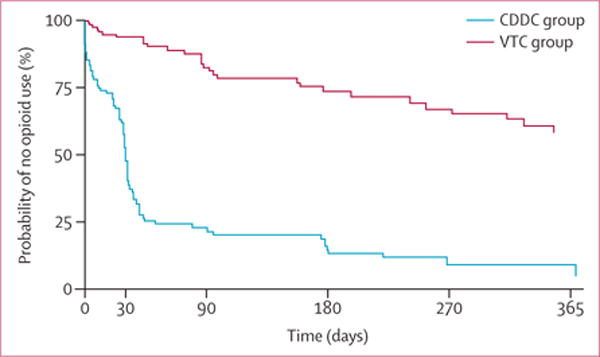
CDDC=compulsory drug detention centre. VTC=voluntary drug treatment centre.
Table 3.
Cox regression hazard ratios for opioid relapse after release
| Unadjusted
|
Adjusted
|
Adjusted, time-varying group effect
|
||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | Hazard ratio | 95% CI | Hazard ratio | 95% CI | |
| VTC exposure (CDDC exposure as reference) | 0.198 | (0.12–0.31) | 0.155 | (0.10–0.25) | ·· | ·· |
|
| ||||||
| Time-varying effects of VTC exposure (CDDC exposure as reference) | ||||||
| >0–<50 days | ·· | ·· | ·· | ·· | 0.087 | (0.04–0.17) |
| 90 days | ·· | ·· | ·· | ·· | 0.270 | (0.14–0.51) |
| 180 days | ·· | ·· | ·· | ·· | 0.388 | (0.17–0.88) |
| 270 days | ·· | ·· | ·· | ·· | 0.456 | (0.18–1.13) |
| 365 days | ·· | ·· | ·· | ·· | 0.509 | (0.19–1.35) |
|
| ||||||
| Opioid cravings | ·· | ·· | 0.903 | (0.85–0.96) | 0.904 | (0.85–0.96) |
|
| ||||||
| Recognition of change | ·· | ·· | 1.015 | (0.88–1.17) | 1.015 | (0.89–1.16) |
|
| ||||||
| Ambivalence towards change | ·· | ·· | 0.977 | (0.86–1.12) | 0.967 | (0.85–1.11) |
|
| ||||||
| Taking steps towards change | ·· | ·· | 0.991 | (0.86–1.14) | 0.993 | (0.87–1.14) |
Data compare the hazard of the specified post-release or discharge opioid relapse in the voluntary treatment centres group versus the compulsory drug detention centres group. The unadjusted hazard ratios were estimated using Cox regression models for which group was the only covariate and no additional weighting was used. The adjusted Cox models additionally adjusted for measurements of opioid cravings and ambivalence, recognition, and taking steps towards change and applied inverse propensity of treatment weights. The adjusted, time varying group effects modified the adjusted model by incorporating a time-varying (non-proportional) hazard for the group, implemented as an interaction between group and the logarithm of time beyond 50 days post-release. Stochastic regression for missing discharge and inpatient times was used; missing outcome measurements were ignored.
VTC participants had an 80% (95% CI 69–88) lower risk of opioid relapse—an effect that was accentuated to 84% (75–90) after adjustment using control variables and inverse propensity of treatment weights. Time-varying effect modelling revealed the largest hazard ratio reduction, of 91% (83–96), occurred during the first 50 days of observation. This hazard ratio reduction diminished over the post-release period to 61% (12–83) at 180 days and by 270 days a large difference remained in the arms. Moreover, increased craving for opioids at baseline corresponded to a reduction in hazard of relapse; hazard ratios for recognition of, ambivalence for, and taking steps towards changing drug use were not significantly different between the groups. Similar adjusted hazard ratio estimates were computed for any-illicit-drug use, including amphetamine-type substances (appendix). Additionally, sensitivity analyses affecting the imputation of missing dates or alternate event coding did not substantively change the results for opioid or any-illicit-drug use (appendix).
Discussion
Our study showed opioid-dependent participants treated with methadone in VTCs experienced a seven-fold decreased risk of relapse to opioids and any-illicit-drug after release, compared to similarly matched individuals released from CDDCs in Malaysia. Not only did we find that relapse was markedly faster for those released from CDDCs compared to those treated in VTCs, but considered on its own, relapse to opioid use was rapid after CDDC release, suggesting CDDCs have no role in treating opioid use disorders. This is one of the first peer-reviewed study comparing objective drug use outcomes contemporaneously for opioid-dependent persons released from CDDCs with similar participants receiving evidence-based methadone maintenance in community-based VTCs. It contributes to a growing body of evidence of how drug policies negatively impact individual and public health.28
The findings here strongly support international calls for all countries that support CDDCs to cease operations in light of the ineffectiveness of these centres in treating drug dependence. Simultaneously, these countries should scale-up evidence-based opioid agonist therapies such as methadone or buprenorphine maintenance in communities, which should be encouraged and voluntary. Promisingly, policy modifications are underway in southeast Asia where some CDDCs are transitioning to VTCs where opioid agonist therapies are available.14 Yet, these findings are also urgently needed to counter developments in Vietnam and Malaysia where VTCs are being suspended or reverted to CDDCs, in the absence of clear evidence that they reduce drug use.29
CDDCs share similarities with many prisons globally, where people who use drugs are concentrated, and transitions to the community are marked by similar high rates of drug relapse and disruptions in social networks. Findings from several countries empirically support provision of opioid agonist therapies within prisons for reducing within-prison transmission of blood-borne viruses.12 In the post-release period, opioid agonist therapies substantially reduce drug use and HIV transmission risk30 and increases retention in care,31,32 especially if the opioid agonist therapy is optimally dosed.33 Unlike prisons, however, CDDCs do not adhere to international regulatory oversight because entry ignores judicial processes, and they do not provide an equivalence of treatment available in the community, including opioid agonist therapies and medical care.3,11,34
Despite the new findings from this study, the data should be interpreted in the context of several considerations. First, the two comparison programmes differ not just by the presence and absence of methadone maintenance therapy, but also by other optional services available and the voluntariness of the two strategies. Our study was not designed to isolate the precise component(s) responsible for the difference. However, given systematic reviews that document a substantial difference in treatment outcomes between drug-free treatment and patients prescribed opioid agonist therapies,35 methadone is likely to have played a prominent role in the observed differences. In this study, we specifically compared two policy programmes to address opioid dependence that coexist in several countries in Asia. Accordingly, we have not explored nor discussed all potential policy options, such as provision of opioid agonist therapies in compulsory settings or voluntary residential treatment without opioid agonist therapies.
Second, this study was observational in nature, such that treatment exposures were allocated non-randomly. Participants in the CDDC arm were detained by police for suspected or real drug use. By contrast, VTC participants probably sought treatment of their own volition, including through support from family and friends. This difference, however, is partly mitigated by our eligibility criteria for which only those meeting opioid dependence criteria were enrolled. We characterised latent dissimilarity between these two populations by obtaining several measures associated with drug relapse. These measures yielded only small differences, especially in addiction severity, between the two groups. We further incorporated these variables in our modelling to adjust for the different propensities of seeking treatment.
Third, there was high attrition in our study. After recruitment, 53% participants in the CDDC arm completed baseline interviews and had at least one urine drug test, compared with 84% from the VTCs. Nonetheless, we noted considerable similarity between groups retained and subsequently analysed. Thus, for problematic bias to occur, we would have to believe that the full sample of CDDC participants were substantially less likely to relapse than the participants recruited from VTCs. Additionally, among those who had a baseline interview and at least one urine drug test (our analytic sample), attrition did not differ between groups. This suggests that although the analysed sample might have been predisposed to more favourable outcomes, both groups were similarly affected. For this reason, estimates of the between-group effects are still likely to be valid. Furthermore, alternative event coding which assumed a large (50%) absolute difference in risk of event for missing values between each group produced findings that remained significant. Taken together, these limitations suggest that the findings, while of strong internal validity, are likely to be most reflective of a subset of people who use drugs in these settings, and may exaggerate the overall effects experienced had all people who use drugs in CDDCs been shifted to VTCs. Despite these limitations, this study provides clear evidence of the ineffectiveness of CDDCs in addressing opioid dependence, and shows a several-fold decrease in relapse to opioid use after release among those prescribed methadone in VTCs.
Understanding the extent to which the effectiveness of methadone provided in VTCs is reflected in other outcomes such as criminal activity, rearrest, HIV transmission, mortality, and quality-of-life, is important and requires further assessment. Findings from previous studies36,37 have shown that drug-treatment effectiveness is strongly associated with improvements in these indicators. Furthermore, rapid relapse to drug use after release from controlled settings like prisons is associated with high rates of HIV risk-behaviors,38 overdose, and death.39
These results are likely to be generalisable beyond Klang Valley to greater Malaysia, and more broadly, to other regions of east and southeast Asia. This conclusion is supported by evidence suggesting that relapse is common among released detainees who are not provided opioid agonist therapies in both Malaysian CDDCs3,40 and those elsewhere in Asia.7,41 This finding would also probably hold for settings where amphetamine-type substances are prevalent. For example, use of amphetamine-type substances was common in our sample of individuals with opioid dependence (around 70% with lifetime use). Although there are currently no evidence-based pharmacological treatments for people with amphetamine use disorders, the first quartile median time to relapse to use of amphetamine-type substances in our sample was 33 versus 355 days for CDDC and VTC participants, respectively. Even though it was beyond the scope of this study to examine outcomes for those using amphetamine-type substances but without opioid dependence, findings here provide evidence that CDDCs are ineffective in preventing relapse to use of amphetamine-type substances, and should be closed even in regions where amphetamine use disorders are common.
Although the individual and societal costs of maintaining CDDCs are high and despite incontrovertible evidence that Malaysia’s harm reduction programmes are cost-effective,42 government and public resistance to closure of CDDCs or even conversion to VTCs remains high.14 Key factors sustaining CDDCs in Malaysia include the country’s anachronistic culture of zero tolerance towards people who use drugs and abstinence-based treatment. In addition, NADA’s performance metrics are focused on maintaining or increasing arrests and detentions, rather than the societal goals of rehabilitation and public health that focus on reducing drug use, crime or recidivism, and HIV transmission.14,43
Since 2010, several international agencies have provided many regional consultations on CDDCs in Asia, from which an expert working group has been established to formulate evidence-based recommendations to support the transition to a comprehensive system of voluntary, community-based treatment, harm reduction and social support services. Along with these recommendations, this group proposes a three-step strategy for transition that includes establishing a national, multisectoral decision-making mechanism with responsibility for the transition; implementing reforms to develop and strengthen the various mechanisms responsible for addressing operations and treatment of substance use disorders across different sectors; and examining related drug policies, including laws, regulations, strategies and practices, and shifting away from criminalisation and punishment, to health-based and rights-based drug policy measures.44
Despite regional consultations and a 2012 joint statement by 12 UN agencies calling for immediate closure of CDDCs and for implementation of voluntary, evidence-informed and rights-based health and social services in the community, CDDCs continue to operate in east and southeast Asia. Our study provides the first prospective, comparative evidence that CDDCs are ineffective in preventing drug relapse, especially when compared with voluntary evidence-based treatments like methadone. In light of this, and numerous studies documenting the cost-effectiveness of opioid agonist therapies in treating opioid dependence, a renewed effort by governments to transition to and expand a comprehensive system of voluntary, community-based treatment is urgently needed, especially in Asia. Ultimately, this effort should be situated within a more comprehensive review of national and regional drug policies that continue to criminalise drug use and limit people who use drugs from accessing evidence-based treatment, care, and support.
Supplementary Material
Research in context.
Evidence before this study
We sought to compare the rates of relapse after mandatory confinement in compulsory drug detention centres (CDDCs), where methadone was not available, with voluntary treatment programs providing methadone, for persons with opioid dependence. We reviewed the scientific literature by searching PubMed, EMBase, the Cochrane Database of Systematic Reviews, and Google Scholar for any original articles published through December, 2015 with no language restrictions, with the search terms “methadone”, “opioid”, opiate”, “addiction”, “opioid substitution therapy”, opioid agonist therapy”, “methadone”, ‘‘substance abuse’’, “substance use”, “dependence”, ”detention”, “forced treatment”, “compulsory treatment”, “mandated treatment”, “mandatory treatment”, “addiction”, “addiction treatment”, “involuntary treatment”, “involuntary addiction treatment”, “detained”, “compulsory”, “prison”, “jail”, “correction”, “incarc”, “effective”, “relapse”, and “urine drug testing”.
From our search we concluded that peer-reviewed research comparing the effects of voluntary opioid agonist therapies programmes with CDDCs on post-release opioid use outcomes is non-existent. The predominance of information on CDDC effectiveness is at a high risk of bias, and has equivocal findings, as documented in a systematic review. By contrast, findings from clinical trials and systematic reviews of community-based methadone maintenance therapy are available in Asia and other regions, and confirm the effectiveness of methadone maintenance therapy for treating opioid dependence in reducing illicit opioid use compared with no pharmacological therapy. Similarly, findings from several trials have shown the value of methadone provided in confined settings, with increased post-release treatment retention and decreased likelihood of relapse.
Although WHO recommends providing maintenance with opioid-agonist treatments such as methadone or buprenorphine as best practice for treating opioid dependence in prisoners with opioid dependence, CDDCs are not subjected to the same oversight, and such evidence-based pharmacological treatments for treating substance use disorders are not provided in these settings. Globally, only 40 countries provide treatment with methadone or buprenorphine in prison, albeit with low coverage rates. Many high-income countries such as Australia, Canada, and most of the European Union have made methadone maintenance therapy available in criminal justice settings. In Asia, only six countries provide methadone maintenance therapy in prisons, including Indonesia and Malaysia. In Asia where CDDCs exist, none provide methadone maintenance therapy, and because of this absence of drug treatments and the evidence of human rights abuses, many international agencies have called for their systematic closure.
Added value of this study
This is the first prospectively assessed study that directly compared post-release drug use outcomes for people who completed so-called drug rehabilitation at CDDCs with those for participants of voluntary drug treatment centres (VTCs) in Malaysia. By designing a study that simultaneously assessed two different, but coexisting, drug treatment programmes, we provided robust, previously unavailable information about the effectiveness of CDDCs and their role in relapse reduction compared with voluntary treatment with methadone. These striking findings are also urgently needed to counter the continued expansion of CDDCs across the country and recent developments in the region where VTCs are being suspended or reverted to CDDCs, in the absence of documented evidence of their benefit.
Implications of all the available evidence
The findings from our study showed that relapse to opioid use is more likely and faster after release from CDDCs compared with VTCs suggesting that CDDCs have no role in the treatment of opioid use disorders. The sum of evidence strongly supports international calls for all countries in Asia that support CDDCs to cease such human rights violations and scale-up evidence-based treatments such as opioid agonist therapies that can be accessed voluntarily and made potentially available to individuals as part of an alternative to incarceration strategy.
Acknowledgments
Funding for this research was provided by the World Bank, Malaysian Ministry of Education High Impact Research Grant (HIRGA E000001-20001), National Institute of Mental Health for career development (F30MH105153), National Institute on Drug Abuse for research (R01 DA025943 and R01 DA041271) and career development (K24 DA017072), and Doris Duke Charitable Foundation through a grant supporting the Doris Duke International Clinical Research Fellows Program at Yale University School of Medicine. We thank the participants in this study; the staff at Malaysian PUSPEN and Cure & Care facilities; our research team Muhammad Azri, Kamal Sapilo, and Nuruljannah; Jeannia Fu for her contributions to the design and initiation of the study and comments on the manuscript; the Malaysian National Anti-Drug Agency and its former Director-General Puan Seri Dato Zuraida; and support staff at the Centre of Excellence on Research In AIDS. The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Footnotes
For the protocol see https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/RO34OK
See Online for appendix
Contributors
MPW, FLA, VR, and AK did the literature search. FLA, SK, VR, SO, DW, DPW, and AK contributed to the study design and conceptualisation. VR obtained the data. MPW did the data analysis and produced the figures. MPW, FLA, DPW, and AK interpreted the data. MPW, FLA, SK, VR, SO, DW, DPW, and AK drafted and revised the report.
Declaration of interests
We declare no competing interests.
References
- 1.UN Office on Drugs and Crime, Economic and Social Commission for Asia and the Pacific, Joint United Nations Programme on HIV/AIDS. Report of the second regional consultation on compulsory centres for drug users in Asia and the Pacific. Kuala Lumpur: UNODC; 2012. [Google Scholar]
- 2.Kamarulzaman A. Impact of HIV prevention programs on drug users in Malaysia. J Acquir Immune Defic Syndr. 2009;52(suppl 1):S17–19. doi: 10.1097/QAI.0b013e3181bbc9af. [DOI] [PubMed] [Google Scholar]
- 3.Fu JJ, Bazazi AR, Altice FL, Mohamed MN, Kamarulzaman A. Absence of antiretroviral therapy and other risk factors for morbidity and mortality in Malaysian compulsory drug detention and rehabilitation centers. PLoS One. 2012;7:e44249. doi: 10.1371/journal.pone.0044249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Western Pacific Regional Office, WHO Office of the Representative for Brunei, Darussalam, Malaysia, and Singapore. Good practices in Asia: effective paradigm shifts towards an improved national response to drugs and HIV/AIDS: scale-up of harm reduction in Malaysia. Geneva: World Health Organization; 2011. [Google Scholar]
- 5.Ghani MA, Brown S-E, Khan F, et al. An exploratory qualitative assessment of self-reported treatment outcomes and satisfaction among patients accessing an innovative voluntary drug treatment centre in Malaysia. Int J Drug Policy. 2015;26:175–82. doi: 10.1016/j.drugpo.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan F, Krishnan A, Ghani M, et al. Assessment of an innovative voluntary substance abuse treatment program designed to transition from compulsory drug detention centers in Malaysia. Subst Use Misuse. 2016 doi: 10.1080/10826084.2016.1267217. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Assessment of compulsory treatment of people who use drugs in Cambodia, China, Malaysia and Vietnam: an application of selected human rights principles. Geneva: WHO; 2009. [Google Scholar]
- 8.Amon JJ, Pearshouse R, Cohen JE, Schleifer R. Compulsory drug detention in east and southeast Asia: evolving government, UN and donor responses. Int J Drug Policy. 2014;25:13–20. doi: 10.1016/j.drugpo.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Amon J, Pearshouse R, Cohen J, Schleifer R. Compulsory drug detention centers in China, Cambodia, Vietnam, and Laos: health and human rights abuses. Health Hum Rights. 2013;15:124–37. [PubMed] [Google Scholar]
- 10.UN entities. Joint statement: compulsory drug detention and rehabilition centres. 2012 https://www.unodc.org/documents/southeastasiaandpacific/2012/03/drug-detention-centre/JC2310_Joint_Statement6March12FINAL_En.pdf (accessed Sept 25, 2016).
- 11.Dolan K, Wirtz AL, Moazen B, et al. Global burden of HIV, viral hepatitis, and tuberculosis in prisoners and detainees. Lancet. 2016;388:1089–102. doi: 10.1016/S0140-6736(16)30466-4. [DOI] [PubMed] [Google Scholar]
- 12.Kamarulzaman A, Reid SE, Schwitters A, et al. Prevention of transmission of HIV, hepatitis B virus, hepatitis C virus, and tuberculosis in prisoners. Lancet. 2016;388:1115–26. doi: 10.1016/S0140-6736(16)30769-3. [DOI] [PubMed] [Google Scholar]
- 13.Mathers BM, Degenhardt L, Ali H, et al. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet. 2010;375:1014–28. doi: 10.1016/S0140-6736(10)60232-2. [DOI] [PubMed] [Google Scholar]
- 14.Tanguay P, Kamarulzaman A, Aramrattana A, et al. Facilitating a transition from compulsory detention of people who use drugs towards voluntary community-based drug dependence treatment and support services in Asia. Harm Reduct J. 2015;12:31. doi: 10.1186/s12954-015-0071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Human Rights Watch. Skin on the cable: the illegal arrest, arbitrary detention and torture of people who use drugs in Cambodia. 2010 https://www.hrw.org/report/2010/01/25/skin-cable/illegal-arrest-arbitrary-detention-and-torture-people-who-use-drugs https://www.hrw.org/sites/default/files/reports/cambodia0110webwcover.pdf (accessed Sept 25, 2016).
- 16.Human Rights Watch. Torture in the name of treatment: human rights abuses in Vietnam, China, Cambodia, and Lao PDR. 2012 https://www.hrw.org/report/2012/07/24/torture-name-treatment/human-rights-abuses-vietnam-china-cambodia-and-lao-pdr (accessed Sept 25, 2016).
- 17.Wu Z. Arguments in favour of compulsory treatment of opioid dependence. Bull World Health Organ. 2013;91:142–45. doi: 10.2471/BLT.12.108860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. 19th WHO model List of essential medicines. Geneva: World Health Organization; 2015. p. 40. [Google Scholar]
- 19.Werb D, Kamarulzaman A, Meacham MC, et al. The effectiveness of compulsory drug treatment: a systematic review. Int J Drug Policy. 2015;28:1–9. doi: 10.1016/j.drugpo.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wickersham JA, Azar MM, Cannon CM, Altice FL, Springer SA. Validation of a brief measure of opioid dependence: the rapid opioid dependence screen (RODS) J Correct Health Care. 2015;21:12–26. doi: 10.1177/1078345814557513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnan A, Brown S-E, Ghani M, Khan F, Kamarulzaman A, Altice FL. Pre-treatment drug use characteristics and experiences among patients in a voluntary substance abuse treatment center in Malaysia: a mixed methods approach. Subst Abus. 2016;37:542–49. doi: 10.1080/08897077.2016.1146648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363–71. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- 23.Miller WR, Tonigan JS. Assessing drinkers’ motivation for change: the stages of change readiness and treatment eagerness scale (SOCRATES) Psychol Addict Behav. 1996;10:81. [Google Scholar]
- 24.Zimet GD, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric characteristics of the multidimensional scale of perceived social support. J Pers Assess. 1990;55:610–17. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 25.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaughan AS, Kelley CF, Luisi N, del Rio C, Sullivan PS, Rosenberg ES. An application of propensity score weighting to quantify the causal effect of rectal sexually transmitted infections on incident HIV among men who have sex with men. BMC Med Res Methodol. 2015;15:25. doi: 10.1186/s12874-015-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleinbaum DG, Klein M. Survival analysis: a self-learning text. New York: Springer; 2012. [Google Scholar]
- 28.Csete J, Kamarulzaman A, Kazatchkine M, et al. Public health and international drug policy. The Lancet. 2016;387:1427–80. doi: 10.1016/S0140-6736(16)00619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuoi Tre News. Voluntary drug detoxification a flop in Vietnam. 2014 http://tuoitrenews.vn/features/23374/voluntary-drug-detoxification-a-flop-in-vietnam (accessed Jan 25, 2016).
- 30.Altice FL, Azbel L, Stone J, et al. The perfect storm: incarceration and the high-risk environment perpetuating transmission of HIV, hepatitis C virus, and tuberculosis in Eastern Europe and Central Asia. Lancet. 2016;388:1228–48. doi: 10.1016/S0140-6736(16)30856-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinlock TW, Gordon MS, Schwartz RP, Fitzgerald TT, O’Grady KE. A randomized clinical trial of methadone maintenance for prisoners: results at 12 months postrelease. J Subst Abuse Treat. 2009;37:277–85. doi: 10.1016/j.jsat.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rich JD, McKenzie M, Larney S, et al. Methadone continuation versus forced withdrawal on incarceration in a combined US prison and jail: a randomised, open-label trial. Lancet. 2016;386:350–59. doi: 10.1016/S0140-6736(14)62338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wickersham JA, Zahari MM, Azar MM, Kamarulzaman A, Altice FL. Methadone dose at the time of release from prison significantly influences retention in treatment: Implications from a pilot study of HIV-infected prisoners transitioning to the community in Malaysia. Drug Alcohol Depend. 2013;132:378–82. doi: 10.1016/j.drugalcdep.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO, UNODC UNAIDS. Technical guide for countries to set targets for universal access to HIV prevention, treatment and care for injecting drug Users—2012 revision. Geneva: World Health Organization; 2012. [Google Scholar]
- 35.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2:CD002207. doi: 10.1002/14651858.CD002207.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dennis BB, Naji L, Bawor M, et al. The effectiveness of opioid substitution treatments for patients with opioid dependence: a systematic review and multiple treatment comparison protocol. Syst Rev. 2014;3:105. doi: 10.1186/2046-4053-3-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ. 2012;345:e5945. doi: 10.1136/bmj.e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams LM, Kendall S, Smith A, Quigley E, Stuewig JB, Tangney JP. HIV risk behaviors of male and female jail inmates prior to incarceration and one year post-release. AIDS Behav. 2013;17:2685–94. doi: 10.1007/s10461-011-9990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merrall ELC, Kariminia A, Binswanger IA, et al. Meta-analysis of drug-related deaths soon after release from prison. Addiction. 2010;105:1545–54. doi: 10.1111/j.1360-0443.2010.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reid G, Kamarulzaman A, Sran SK. Malaysia and harm reduction: the challenges and responses. Int J Drug Policy. 2007;18:136–40. doi: 10.1016/j.drugpo.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 41.Thomson N. Detention of methamphetamine users in Cambodia, Laos, and Thailand. Melbourne: Open Society Institute; 2010. Detention as treatment. [Google Scholar]
- 42.Osornprasop S, Dahlui M, Kamarulzaman A, et al. Return on investment and cost-effectiveness of harm reduction program in Malaysia Directions in development; human development. Washington, DC: World Bank Group; 2014. http://documents.worldbank.org/curated/en/4/07/23006930/return-investment-cost-effectiveness-harm-reduction-program-malaysia (accessed Dec 12, 2015). [Google Scholar]
- 43.UN Office on Drugs and Crime Regional Centre for East Asia and the Pacific. Drug-Free ASEAN 2015 status and recommendations. 2015 http://www.unodc.org/documents/southeastasiaandpacific/Publications/ASEAN_2015.pdf (accessed Sept 25, 2016).
- 44.UNODC. Transition from compulsory centers for drug users to voluntary community-based treatment and services: discussion paper. 2015 https://www.unodc.org/documents/southeastasiaandpacific/Publications/2015/hiv/Discussion_Paper_on_Transition_from_CCDUs_Edited_Final4_04Sept15.pdf (accessed Sept 25, 2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


