Abstract
Objectives
This study evaluated the long-term effect of carbodiimide treatments of acid-etched dentin on resin-dentin bond strength of a simplified etch-and-rinse adhesive system.
Methods
Forty-eight sound third molars were divided into three groups (n=16) according to the dentin treatment: G1: Deionized water; G2: 0.5 mol/L 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) applied for 30 seconds; and G3: 0.5 mol/L EDC applied for 60 seconds. Flat dentin surfaces were produced, etched with 37% phosphoric acid for 15 seconds and then treated with deionized water for 60s or with 0.5 mol/L EDC for 30 or 60 seconds prior to the application of Single Bond 2. Crowns were restored with resin composite and beam specimens were prepared for microtensile testing. The beams from each group were tested 24 hours or 6 or 12 months after the adhesive procedures. One slab from each tooth was prepared and analyzed for nanolaekage. Bond strength (MPa) data were submitted to analysis of variance and Tukey test (α=0.05).
Results
The treatment of dentin with 0.5 mol/L EDC for 30 seconds (24.1±6.2 MPa) and 60 seconds (25.5±5.1 MPa) did not negatively affect the immediate bond strength of Single Bond 2 when compared to the control group (24.6±7.3 MPa). Additionally, EDC prevented resin-dentin bond degradation after 12 months in artificial saliva for both periods of treatment. An increased accumulation of silver ions was seen for the control group over time, while a much lower amount of silver grains was observed for the EDC-treated groups.
Conclusions
0.5 mol/L EDC was able to prevent resin-dentin bond degradation after 12 months, especially when applied for 60 seconds.
Keywords: dentin, bond strength, collagen, adhesive, ethyldimethylaminopropyl carbodiimide
INTRODUCTION
Cross-linking agents have been reported to increase the stiffness of collagen, making it more resistant to degradation.1-3 These reagents link one peptide chain to another by covalent or ionic bonds.1,4,5 Endogenous cross-links are naturally present in collagen structure, and its mechanical properties depend on a highly regulated mechanism of intra and intermolecular cross-linking.6 Increasing the number of cross-links in dentin collagen by applying exogenous cross-linking solutions prior to adhesive bonding or incorporating these agents into adhesive systems seems to enhance dentin-resin bond durability.1,3,7,8
Degradation of resin-dentin bonds is a complex process involving the deterioration of inorganic and organic portions of the hybrid layer.9,10 Dentin matrix contains proteases, such as matrix metalloproteinases (MMPs),11-15 that are secreted as inactive proenzyme forms during dental development and are released and activated after acid etching during adhesive bonding.16 The exposed MMPs on the collagen fibrils at the base of the hybrid layer slowly destroy the collagen fibrils to which they are bound, causing the loss of the anchoring function of hybrid layers and the loss of bond strength of resin composites.17,18
1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) is a cross-linker that activates free carboxyl groups of glutamic and aspartic acids present in protein molecules19,20 to form new peptide bonds. It is able to react with collagen1 and to inactivate matrix-bound MMPs, even when applied on demineralized dentin for periods of time as short as 60 seconds.21 Therefore, EDC may provide long-lasting adhesive bonds by inactivating MMPs16,21 and by increasing collagen stiffness.1
The purpose of this study was to evaluate the long-term effect of EDC applied for short periods of time on dentin bond strength stability over a period of 12 months. The test null hypothesis was that application of 0.5 mol/L EDC has no significant effect on immediate and long-term dentin bond strength.
MATERIAL AND METHODS
Forty-eight sound human third molars were obtained under a protocol approved by the Ethics Committee of the Araraquara School of Dentistry (protocol #77/11). The occlusal enamel was completely removed from each tooth to obtain flat dentin surfaces using an ISOMET saw (Buehler Ltd, Lake Bluff, IL, USA) under water cooling. Then the smear layer thickness was standardized by wearing the dentin surface with 320-grit silicon carbide sandpaper, and the teeth were randomly divided into three groups, according to dentin treatment (n=16): G1: deionized water applied for 60 seconds (control); G2: 0.5 mol/L EDC applied for 30 seconds; and G3: 0.5 mol/L EDC applied for 60 seconds.
Bonding procedures
The dentin was etched with 35% phosphoric acid (Scotchbond etchant, 3M ESPE, St. Paul, MN, USA) for 15 seconds and then rinsed with deionized water for the same time. After blot drying, 20 μL of deionized water or 0.5 mol/L of EDC prepared in Sorensen’s buffer (pH 6) were applied on demineralized dentin for 30 or 60 seconds and then rinsed for 15 seconds. The excess of water was removed from the surface with absorbent paper prior to bonding. The adhesive system Single Bond 2 (Z350, 3M ESPE) was applied according to the manufacturer instructions, except for the dentin treatments, and photo-activated for 10 seconds (Radii Plus, SDI Limited, Bayswater, Victoria, Australia; 1000±10 mW/cm2). A 3-mm-high resin composite block (Z350, 3M ESPE) was built up incrementally, and each increment was light cured for 20 seconds using the LED curing light. The restored teeth were then stored in deionized water and kept in an incubator at 37°C for 24 hours.
Microtensile bond strength testing
After 24 hours in deionized water, dentin beams with a cross-sectional area of 0.81 mm2 were obtained in a high-precision cutting machine (Isomet 1000, Buehler, Lake Bluff, IL, USA) using a diamond saw (ISOMET, Buehler Ltd) under water cooling. One-third of the beams from each tooth were tested 24 hours after the bonding procedures. The remaining specimens were storage in 3 mL of artificial saliva at 37°C for 6 or 12 months before the microtensile test. The artificial saliva pH was monitored periodically to ensure that no significant changes occurred. The cross-sectional area of each beam was individually measured (Model 500-144B, Mytutoyo South America Ltda, SP, Brazil) and the beam fixed to a testing device with cyanoacrylate glue (Super Bond Gel, Henkel Loctile, São Paulo, SP, Brazil) and subjected to the microtensile strength test in a mechanical testing machine (DL-Digital Line, EMIC, Parana, Brazil) equipped with a 100-N load cell running at a crosshead speed of 1.0 mm/min. Immediately after testing, the debonded halves of each beam were dried and stored in closed receptacles at room temperature until analysis of the fracture pattern using a stereomiscroscope (Carl Zeiss, Oberkochen, Germany) at approximately 50x magnification. Failures were classified as cohesive in resin or dentin, adhesive or mixed.
Nanoleakage analysis
In each group, four 0.9-mm-thick slabs were randomly selected and immersed in 50 wt% ammoniacal AgNO3 solution (pH 9.5) in darkness for 24 hours according to the protocol described by Tay and others.22 After immersion in the tracer solution, followed by rinsing in deionized water for 5 minutes, the slabs were immersed in photodeveloping solution for 8 hours under a fluorescent light to reduce silver ions into metallic silver grain. The silver-stained specimens were polished with silicon carbide sand-paper with different grits (400, 600, 1200, 2400 and 4000). Then they were cleaned, mounted on aluminum stubs, and placed in a desiccator for 24 hours. Digital images were obtained using scanning electron microscopy (SEM; XL-30 FEG, Philips, Eindhoven, The Netherlands) with a back-scattered electron detector at 10 kV at 2500X magnification.
Statistical analysis
Bond strength recorded for specimens obtained from the same tooth were average in such a way that the tooth was used as the statistical unit of the study. Premature failures (failures that occurred before testing) were included in the computation of the mean as zero (0 MPa). Two-way analysis of variance (ANOVA) and Tukey tests were applied to analyze the effect of dentin treatment and storage period on microtensile bond strength (MPa). The significance level was 5% for all analyses. Failure mode and nanoleakage data were analyzed descriptively.
RESULTS
Microtensile bond strength
The ANOVA test showed a significant effect of dentin treatment (p=0.001) and interaction between dentin treatment vs storage period (p=0.041). No significant effect was detected for storage period (p=0.653). Bond strength data (MPa) are shown in Table 1 as mean and standard deviation. No statistically significant difference was observed among the immediate bond strength of Single Bond 2 after the treatment of dentin with deionized water (control) or 0.5 mol/L EDC 30 or 60 seconds. After 6 months in artificial saliva, significant bond strength decrease was observed only for the control. Bonds made to EDC-treated dentin showed bond strength values that were significantly higher than 6-month controls when EDC was applied for 60 seconds. The beams treated with deionized water (control) and stored for 12 months showed a significant reduction in bond strength when compared to that observed after 24 hours. The dentin treated with EDC for 30 or 60 seconds presented no statistically significant decrease in bond strength of Single Bond 2 after 12 months of artificial saliva storage. However, at the 12-month storage period, higher mean bond strength was seen for EDC-treated dentin at 60 seconds.
Table 1.
Bond strength (MPa) of Single Bond 2 to dentin after treatment with 0.5 mol/L EDC for 30 or 60 seconds and storage in artificial saliva for up to 12 months.A
| Dentin Treatment | Storage Period
|
||
|---|---|---|---|
| 24 hours | 6 months | 12 months | |
| Water (control) | 24.6 ± 7.3 AB | 21.1 ± 3.8 CD | 18.5 ± 6.5 D |
| EDC 30 s | 25.1 ± 6.2 AB | 22.3 ± 4.8 BC | 21.2 ± 4.7 BD |
| EDC 60 s | 26.7 ± 5.1 A | 25.9 ± 4.6 A | 27.9 ± 7.2 A |
Numbers are mean±standard-deviation, n=16. Groups identified by the same letter are statistically similar (Tukey test, p>0.05).
Failure modes and nanoleakage analysis
The distribution of failure modes is given in Table 2 as absolute values and percentage of occurrence within the group. The largest percentages of failures involved the interface, adhesive, and mixed fractures. Premature failures were observed in all groups and increased as a function of storage period. EDC seems to not influence resin-dentin bond failure mode.
Table 2.
Distribution of failure types.
| Dentin Treatment | Storage Period
|
||
|---|---|---|---|
| 24 h | 6 mo | 12 mo | |
| Water (control) | A=64 (88.9); M=1 (1.4); RC=1 (1.4); DC=1 (1.4); PF=5 (6.9) | A=47 (83.9); M=3 (5.4); PF=6 (10.7) | A=67 (82.7); M=2 (2.5); RC=1 (1.2); DC=1 (1.2); PF=10 (12.3) |
| EDC 30 s | A=78 (95.1); M=1 (1.2); DC=2 (2.4); PF=1 (1.2) | A=57 (75.0); M=15 (19.7); PF=4 (5.3) | A=76 (82.6); M=3 (3.3); RC=3 (3.3); DC=2 (2.2); PF=8 (8.7) |
| EDC 60 s | A=68 (95.8); DC=2 (2.8); PF=1 (1.4) | A=59 (83.1); M=8 (11.3); PF=4 (5.6) | A=63 (78.8); M=4 (5.0); RC=1 (1.3); DC=5 (6.3); PF=7 (8.8) |
Abbreviations: A, adhesive; M, mixed; CR, cohesive in resin; CD, cohesive in dentin; PF, premature failure. Values represent the absolute frequency (percentage of total specimens in the group).
Representative SEM photomicrographs of silver nanoleakage in adhesive bonds created by Single Bond 2 on dentin surfaces treated with water (control group) or 0.5 mol/L EDC for 30 or 60 seconds and stored for 24 hours, 6 or 12 months at 37°C are presented in Figure 1. SEM images revealed the presence of silver deposits in all groups and storage periods. The specimens analyzed 24 hours after the bonding procedures showed small silver accumulation. In the control group, a gradual increase in nanoleakage was observed over time. However, the groups treated with EDC for 30 or 60 seconds showed much lower amounts of silver nanoleakage, even after 12 months in the artificial saliva. All the SEM images exhibited the presence of polyalkenoic acid copolymer globules in the adhesive layer of Single Bond 2, indicating the occurrence of phase separation.
Figure 1.
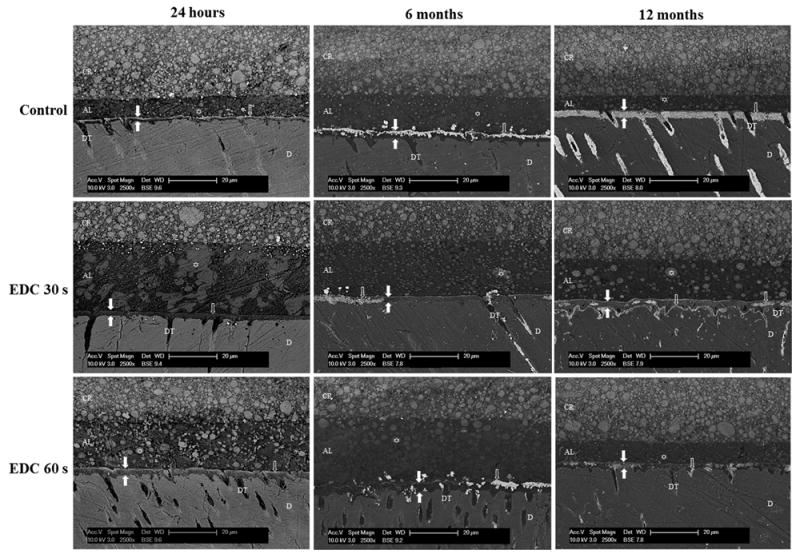
SEM photomicrographs of adhesive bonds created by Single Bond 2 on dentin surfaces pretreated with water (control group) or 0.5 mol/L EDC for 30 or 60 seconds and stored in artificial saliva for 24hours or 6 or 12months. The zone between white filled arrows represents the hybrid layer (HL). ⇧ shows silver deposits in the HL, and ✩ shows polyalkenoic acid copolymer globules. AL, adhesive layer; D, mineralized dentin; CR, composite resin; DT, dentin tubule. Magnification 2500X.
DISCUSSION
Collagen fibrils in mineralized dentin are protected from hydrolytic and enzymatic degradation by hydroxyapatite crystals. However, after acid etching, collagen fibrils become uncovered, and their bound proteases (MMPs and cathepsins) are activated23 to cleave unprotected collagen, resulting in decrease in long-term bond strength,24 as was observed in the control group of this study.
In order to reduce the activity of these proteases and preserve the integrity and durability of the adhesive interfaces, chlorhexidine (CHX) has been used as a nonspecific MMP18,24-28 and cathepsin29 inhibitor. However, chlorhexidine is highly water soluble and may be leached from the hybrid layer, compromising its antiprotease efficacy.24 The use of cross-linking agents instead of CHX has the advantage of inactivating endogenous MMPs21,30 and simultaneously increasing collagen mechanical properties1 by creating covalent cross-links that are stable over time.
The biomodification of collagen by extrinsic cross-linkers can induce the formation of additional inter and intramolecular cross-links,31,32 increasing the ultimate tensile strength and elastic modulus of demineralized dentin.33,34 Cross-linking agents such as proanthocyanidins, glutaraldehyde, and tannic acid are able to7,35 improve immediate resin-dentin bond strength after one hour of treatment, an observation that was not seen in our results. However, we used much shorter periods of treatment. The present study showed that 0.5 mol/L EDC applied on dentin for short periods of time, such as 30 and 60 seconds, was capable of preventing resin-dentin bond degradation after up to 12 months of aging in artificial saliva. These results require partial rejection of the tested null hypothesis. These findings agree with those of Mazzoni and others,36 which showed that the application of 0.3 mol/L EDC on demineralized dentin produces long-term inactivation of MMPs, contributing to bond strength preservation over time.
To degrade the organic matrix of dentin, MMPs must link their narrow binding sites37 to the substrate and unwind collagen molecules,38-40 culminating in collagen peptides cleavage. However, the treatment of demineralized dentin with cross-linking agents makes collagen more difficult to unwind, preventing the degradation by MMPs. EDC activates the free carboxylic groups of glutamic and aspartic acids present on collagen1 and MMPs structures.21 It increases collagen stiffness and inactivates MMP activation sites.21 Additionally, the cross-links created by EDC may reduce the mobility of these enzymes.
Single Bond 2 is a two-step etch-and-rinse adhesive system that contains hydrophilic resin monomers to enhance the adhesive wetting properties and avoid phase changes observed when hydrophobic monomers are added to water.41 Therefore, these adhesives have a high water affinity42,43 that favors their degradation.42 Over time, it is thought that infiltrated resins are extracted from dentin matrix44,45 and that uninfiltrated collagen fibrils are hydrolyzed and replaced by water.46 The silver nanoleakage protocol fills water spaces with silver nitrate that is later photoreduced to silver grains that can be analyzed by SEM.22 After 6 and 12 months in artificial saliva, the control showed higher silver accumulation compared to the EDC-treated groups. These results indicate that there was a greater degradation of the hybrid layer in the control group. Conversely, the cross-linking agent applied for 30 and 60 seconds on etched dentin was able to prevent the increase in nanoleakage.
Bond strength data and nanoleakage images showed that the treatment of demineralized dentin with EDC could be a simple, practical, and clinically applicable method to reduce collagen degradation in the hybrid layer, being an efficient alternative to make resin-dentin bonds more durable. How much of the increase in durability is due to cross-linking of collagen vs collagen-bound MMPs and cathepsins is unclear. Further studies are needed to better understand the effects of EDC application in vitro and to demonstrate its efficacy in vivo.
CONCLUSION
The treatment of acid-etched dentin with 0.5 mol/L EDC prior to bonding procedures was able to prevent resin-dentin bond degradation after up to 12 months especially when applied for 60 s.
Clinical significance statement.
Topical treatments of etched dentin with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) increase resin-dentin bond stability and may provide better quality and durability to adhesive restorations. EDC used in short periods of time is able to prevent the degradation of the hybrid layer over time and produce long lasting resin-dentin bonds.
Contributor Information
Débora Lopes Salles Scheffel, Department of Orthodontics and Pediatric Dentistry, Araraquara School of Dentistry, UNESP – Univ.Estadual Paulista, Araraquara, São Paulo, Brazil.
Cláudia Cristina Delgado, Department of Orthodontics and Pediatric Dentistry, Araraquara School of Dentistry, UNESP – Univ.Estadual Paulista, Araraquara, São Paulo, Brazil.
Diana Gabriela de Sousa Soares, Department of Dental Materials and Prosthodontics, Araraquara School of Dentistry, UNESP – Univ Estadual Paulista, Araraquara, São Paulo, Brazil.
Fernanda Gonçalves Basso, Department of Physiology and Patology, Araraquara School of Dentistry, UNESP – Univ Estadual Paulista, Araraquara, São Paulo, Brazil.
Carlos Alberto de Souza Costa, Department of Physiology and Patology, Araraquara School of Dentistry, UNESP – Univ Estadual Paulista, Araraquara, São Paulo, Brazil.
David H. Pashley, Department of Oral Biology, College of Dental Medicine, Georgia Regents University, Augusta, Georgia, USA
Josimeri Hebling, Department of Orthodontics and Pediatric Dentistry, Araraquara School of Dentistry, UNESP – Univ Estadual Paulista, Araraquara, São Paulo, Brazil.
References
- 1.Bedran-Russo AK, Vidal CM, Dos Santos PH, Castellan CS. Long-term effect of carbodiimide on dentin matrix and resin-dentin bonds. Journal of Biomedical Materials Research part B Applied Biomaterials. 2010;94(1):250–5. doi: 10.1002/jbm.b.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castellan CS, Pereira PN, Grande RH, Bedran-Russo AK. Mechanical characterization of proanthocyanidin-dentin matrix interaction. Dental Materials. 2010;26(10):968–73. doi: 10.1016/j.dental.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Wang Y. Proanthocyanidins’ efficacy in stabilizing dentin collagen against enzymatic degradation: MALDI-TOF and FTIR analyses. Journal of Dentistry. 2013;41(6):535–42. doi: 10.1016/j.jdent.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierpoint WS. O-Quinones formed in plant extracts: Their reactions with amino acids and peptides. Biochemical Journal. 1969;112(5):609–16. doi: 10.1042/bj1120609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loomis WD. Overcoming problems of phenolics and quinines in the isolation of plant enzymes and organelles. Methods in Enzymology. 1974;31:528–44. doi: 10.1016/0076-6879(74)31057-9. [DOI] [PubMed] [Google Scholar]
- 6.Eyre DR, Weis MA, Wu JJ. Advances in collagen cross-link analysis. Methods. 2008;45(1):65–74. doi: 10.1016/j.ymeth.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Ammar A, Drummond JL, Bedran-Russo AK. The use of collagen cross-linking agents to enhance dentin bond strength. Journal of Biomedical Materials Research part B Applied Biomaterials. 2009;91(1):419–24. doi: 10.1002/jbm.b.31417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green B, Yao X, Ganguly A, Xu C, Dusevich V, Walker MP, Wang Y. Grape seed proanthocyanidins increase collagen biodegradation resistance in the dentin/adhesive interface when included in an adhesive. Journal of Dentistry. 2010;38(11):908–15. doi: 10.1016/j.jdent.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S. Collagen degradation by host-derived enzymes during aging. Journal of Dental Research. 2004;83(3):216–21. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 10.De Munk J, Van Meerbeek B, Yoshida Y, Inoue S, Vargas M, Suzuki K, Lambrechts P, Vanherle G. Four-year water degradation of total-etch adhesives bonded to dentin. Journal of Dental Research. 2003;82(2):136–40. doi: 10.1177/154405910308200212. [DOI] [PubMed] [Google Scholar]
- 11.Martin-De Las Heras S, Valenzuela A, Overall CM. The matrix metalloproteinase gelatinase A in human dentine. Archives of Oral Biology. 2000;45(9):757–65. doi: 10.1016/s0003-9969(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 12.Mazzoni A, Mannello F, Tay FR, Tonti GA, Papa S, Mazzotti G, Di Lenarda R, Pashley DH, Breschi L. Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. Journal of Dental Research. 2007;86(8):436–40. doi: 10.1177/154405910708600509. [DOI] [PubMed] [Google Scholar]
- 13.Mazzoni A, Papa V, Nato F, Carrilho M, Tjäderhane L, Ruggeri A, Jr, Gobbi P, Mazzotti G, Tay FR, Pashley DH, Breschi L. Immunohistochemical and biochemical assay of MMP-3 in human dentine. Journal of Dentistry. 2011;39(3):231–7. doi: 10.1016/j.jdent.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sulkala M, Larmas M, Sorsa T, Salo T, Tjäderhane L. The localization of matrix metalloproteinase-20 (MMP-20, enamelysin) in mature human teeth. Journal of Dental Research. 2002;81(9):603–7. doi: 10.1177/154405910208100905. [DOI] [PubMed] [Google Scholar]
- 15.Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Archives of Oral Biology. 2007;52(2):121–7. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Tezvergil-Mutluay A, Mutluay M, Seseogullari-Dirihan R, Agee KA, Key WO, Scheffel DLS, Breschi L, Mazzoni A, Tjäderhane L, Nishitani Y, Tay FR, Pashley DH. Effect of phosphoric acid on the degradation of human dentin matrix. Journal of Dental Research. 2013;92(1):87–91. doi: 10.1177/0022034512466264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong SR, Vargas MA, Chung I, Pashley DH, Campbell JA, Laffoon JE, Qian F. Resin-dentin interfacial ultrastructure and microtensile dentin bond strength after five-year water storage. Operative Dentistry. 2004;29(6):705–12. [PubMed] [Google Scholar]
- 18.Carrilho MR, Geraldeli S, Tay F, de Goes MF, Carvalho RM, Tjäderhane L, Reis AF, Hebling J, Mazzoni A, Breschi L, Pashley D. In vivo preservation of the hybrid layer by chlorhexidine. Journal of Dental Research. 2007;86(6):529–33. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 19.Timkovich R. Detection of the stable addition of carbodiimide to proteins. Analytical Biochemistry. 1977;79(1-2):135–43. doi: 10.1016/0003-2697(77)90387-6. [DOI] [PubMed] [Google Scholar]
- 20.Zeeman R, Dijkstra PJ, van Wachem PB, van Luyn MJ, Hendriks M, Cahalan PT, Feijen J. Successive epoxy and carbodiimide cross-linking of dermal sheep collagen. Biomaterials. 1999;20(10):921–31. doi: 10.1016/s0142-9612(98)00242-7. [DOI] [PubMed] [Google Scholar]
- 21.Scheffel DLS, Hebling J, Scheffel RH, Agee KA, Turco G, de Souza Costa CA, Pashley DH. Inactivation of matrix-bound matrix metalloproteinases by cross-linking agents in acid-etched dentin. Operative Dentistry. 2013 doi: 10.2341/12-425-L. Prepublished Jun 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tay FR, Pashley DH, Yoshiyama M. Two modes of nanoleakage expression in single-step adhesives. Journal of Dental Research. 2002;81(7):472–476. doi: 10.1177/154405910208100708. [DOI] [PubMed] [Google Scholar]
- 23.Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Mannello F, Tjäderhane L, Toledano M, Pashley EL, Tay FR. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials. 2006;27(25):4470–6. doi: 10.1016/j.biomaterials.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 24.Ricci HA, Sanabe ME, de Souza Costa CA, Pashley DH, Hebling J. Chlorhexidine increases the longevity of in vivo resin-dentin bonds. European Journal of Oral Sciences. 2010;118(5):411–6. doi: 10.1111/j.1600-0722.2010.00754.x. [DOI] [PubMed] [Google Scholar]
- 25.Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine. Clinical and Diagnostic Laboratory Immunology. 1996;6(3):437–9. doi: 10.1128/cdli.6.3.437-439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. Journal of Dental Research. 2005;84(8):741–6. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 27.Brackett WW, Tay FR, Brackett MG, Dib A, Sword RJ, Pashley DH. The effect of chlorhexidine on dentin hybrid layers in vivo. Operative Dentistry. 2007;32(2):107–11. doi: 10.2341/06-55. [DOI] [PubMed] [Google Scholar]
- 28.Brackett MG, Tay FR, Brackett WW, Dip A, Dipp FA, Mai & Pashley DH. In vivo chlorhexidine stabilization of an acetone-based dentin adhesives. Operative Dentistry. 2009;34(4):381–5. doi: 10.2341/08-103. [DOI] [PubMed] [Google Scholar]
- 29.Scaffa PM, Vidal CM, Barros N, Gesteira TF, Carmona AK, Breschi L, Pashley DH, Tjäderhane L, Tersariol IL, Nascimento FD, Carrilho MR. Chlorhexidine inhibits the activity of dental cysteine cathepsins. Journal of Dental Research. 2012;91(4):420–5. doi: 10.1177/0022034511435329. [DOI] [PubMed] [Google Scholar]
- 30.Tezvergil-Mutluay A, Mutluay MM, Agee KA, Seseogullari-Dirihan R, Hoshika T, Cadenaro M, Breschi L, Vallittu P, Tay FR, Pashley DH. Carbodiimide cross-linking inactivates soluble and matrix-bound MMPs, in vitro. Journal of Dental Research. 2012;91(2):192–6. doi: 10.1177/0022034511427705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han B, Jaurequi J, Tang BW, Nimni ME. Proanthocyanidin: A natural crosslinking reagent for stabilizing collagen matrices. Journal of Biomedical Materials Research. 2003;65(1):118–24. doi: 10.1002/jbm.a.10460. [DOI] [PubMed] [Google Scholar]
- 32.Sung HS, Chang WH, Ma CY, Lee MH. Cross-linking of biological tissues using genipin and/or carbodiimide. Journal of Biomedical Materials Research. 2003;64(3):427–38. doi: 10.1002/jbm.a.10346. [DOI] [PubMed] [Google Scholar]
- 33.Bedran-Russo AK, Pereira PN, Duarte WR, Drummond JL, Yamauchi M. Application of crosslinkers to dentin collagen enhances the ultimate tensile strength. Journal of Biomedical Materials Research part B Applied Biomaterials. 2007;80(1):268–72. doi: 10.1002/jbm.b.30593. [DOI] [PubMed] [Google Scholar]
- 34.Bedran-Russo AK, Pashley DH, Agee K, Drummond JL, Miescke KJ. Changes in stiffness of demineralized dentin following application of collagen crosslinkers. Journal of Biomedical Materials Research part B Applied Biomaterials. 2008;86(2):330–4. doi: 10.1002/jbm.b.31022. [DOI] [PubMed] [Google Scholar]
- 35.Bedran-Russo AK, Yoo KJ, Ema KC, Pashley DH. Mechanical properties of tannic-acid-treated dentin matrix. Journal of Dental Research. 2009;88(9):807–11. doi: 10.1177/0022034509342556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazzoni A, Angeloni V, Apolonio FM, Scotti N, Tjäderhane L, Tezvergil-Mutluay A, Di Lenarda R, Tay FR, Pashley DH, Breschi L. Effect of carbodiimide (EDC) on the bond stability of etch-and-rinse adhesive systems. Dental Materials. 2013;29(10):1040–7. doi: 10.1016/j.dental.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, Tezvergil-Mutluay A, Carrilho MR, Carvalho RM, Tay FR, Pashley DH. Optimizing dentin bond durability: control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dental Materials. 2013;29(1):116–35. doi: 10.1016/j.dental.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung L, Dinakarpandian D, Yoshida N, Lauer-Fields JL, Fields GB, Visse R, Nagase H. Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis. The EMBO Journal. 2004;23(15):3020–30. doi: 10.1038/sj.emboj.7600318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gioia M, Monaco S, Fasciglione GF, Coletti A, Modesti A, Marini S, Coletta M. Characterization of the mechanisms by which gelatinase A, neutrophil collagenase, and membrane-type metalloproteinase MMP-14 recognize collagen I and enzymatically process the two alpha-chains. Journal of Molecular Biology. 2007;368(4):1101–13. doi: 10.1016/j.jmb.2007.02.076. [DOI] [PubMed] [Google Scholar]
- 40.Nagase H, Fushimi K. Elucidating the function of non-catalytic domains of collagenases and aggrecanase. Connective Tissue Research. 2008;49(3):169–74. doi: 10.1080/03008200802151698. [DOI] [PubMed] [Google Scholar]
- 41.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. Journal of Biomedical Materials Research. 2002;62(3):447–56. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 42.Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, Rueggeberg FA, Foulger S, Saito T, Nishitani Y, Yoshiyama M, Tay FR, Pashley DH. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials. 2005;26(33):6449–59. doi: 10.1016/j.biomaterials.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 43.Yiu CK, King NM, Carrilho MR, Sauro S, Rueggeberg FA, Prati C, Carvalho RM, Pashley DH, Tay FR. Effect of resin hydrophilicity and temperature on water sorption of dental adhesive resins. Biomaterials. 2006;27(9):1695–703. doi: 10.1016/j.biomaterials.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 44.Sano H. Microtensile testing, nanoleakage and biodegradation of resindentin bonds. Journal of Dental Research. 2006;85(1):11–14. doi: 10.1177/154405910608500102. [DOI] [PubMed] [Google Scholar]
- 45.Spencer P, Ye Q, Park J, Topp EM, Misra A, Marangos O, Wang Y, Bohaty BS, Singh V, Sene F, Eslick J, Camarda K, Katz JL. Adhesive/dentin interface: The weak link in the composite restoration. Annals of Biomedical Engineering. 2010;38(6):1989–2003. doi: 10.1007/s10439-010-9969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pashley DH, Tay FR, Breschi L, Tjäderhane L, Carvalho RM, Carrilho M, Tezvergil-Mutluay A. State of the art etch-and-rinse adhesives. Dental Materials. 2011;27(1):1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


