Abstract
Introduction
Clostridium difficile is the principal infectious cause of antibiotic associated diarrhea and accounts for 12% of hospital acquired infections (HAIs). Recent literature has shown an increased risk of Clostridium difficile infection (CDI) with proton pump inhibitor (PPI) use, but a systematic assessment of the risk of hospital-acquired CDI following exposure to PPI is needed.
Methods
We searched multiple databases for studies examining the relationship between PPI and hospital-acquired CDI. Pooled odds ratios were generated and assessment for heterogeneity performed.
Results
We found 23 observational studies involving 186,033 cases that met eligibility criteria. Across studies, 10,307 cases cases of hospital-acquired CDI were reported. Significant heterogeneity was present, therefore a random effects model was used. The pooled odds ratio was 1.81 [95% CI 1.52 – 2.14], favoring higher risk of CDI with PPI use. Significant heterogeneity was present, likely due to differences in assessment of exposure and study characteristics.
Discussion
This meta-anlaysis suggests PPIs significantly increase the risk of hospital-acquired CDI. Given the significant health and economic burden of disease, optimization of PPI use should be included in a multifaceted approach to CDI prevention.
Keywords: CDI, PPI, nosocomial diarrhea, infection control, gastric acid suppression
Introduction
Clostridium difficile (C. difficile) is the principal infectious cause of antibiotic associated diarrhea and colitis1, accounting for an estimated 20–30% of cases2. The burden of disease is substantial – in a multi-state point prevalence study on healthcare-associated infections (HAIs) in 2011, C difficile diarrhea (CDI) accounted for 12% of all HAIs3. In the same year, the national burden of disease was projected at 453,000 incident infections with 83,000 recurrent cases and 29,300 deaths resulting from these recurrences4. Mortality estimates suggest attributable mortality of 6.9% and 16.7% at 30 days and one year, respectively5. This health burden also comes with a profound economic toll, estimated at greater than $1 billion per year6, further highlighting the urgency for strategies to prevent CDI.
To devise and adopt prevention strategies in inpatient settings, an understanding of the risk factors for CDI is essential. Several conventional risk factors include older age, antibiotic exposure, prolonged hospitalization, immunocompromising condition or serious underlying illness7. Recent literature has demonstrated an association between proton pump inhibitor (PPI) use and increased risk of CDI. A proposed biologic mechanism is that PPI suppresses gastric acid which is an important host defense mechanism to prevent germination of ingested C. difficile spores8. PPI use may also results in deleterious changes in the human gut microbiome, increasing the risk of CDI9,10.
Due to the observed association and plausible biologic mechanisms, the US Food and Drug Administration (FDA) released a drug safety announcement in 2012 regarding the association between C difficile and the use of PPIs and concluded that PPIs were associated with increased risk of CDI11. Despite concerns for adverse effects, PPI use remains ubiquitous12,13. Understanding the magnitude of risk for hospital-acquired CDI with PPI use would inform the potential impact of interventions to optimize PPI prescribing on hospital-acquired CDI rates. We undertook a systematic review to examine the relationship between PPI use and hospital-acquired CDI.
This systematic review evaluates the literature to answer two questions: a) are PPIs associated with an increased risk of hospital-acquired CDI? and b) if so, what is the magnitude of this association?
Methods
We conducted this analysis using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) framework14. We registered this review at the international prospective register of systematic reviews known as PROSPERO on June 21, 2015 (Registration number: CRD42015023690).
Data sources and searches
Two reviewers (V.A. and A.B.) independently searched MEDLINE (PubMed), Web of Science, EBSCO (CINAHL), Cochrane Central Register of Controlled Trials (CENTRAL), University of York Center for Reviews and Dissemination (RD), and Clinicaltrials.gov. These bibliographic databases were searched for articles between January 1, 1980 to July 30, 2015. The Web of Science search facilitated the capture of most conferences abstracts or proceedings. For completeness, we searched BIOSIS databases for conference proceedings. Details of the search strategies are available in the Supplemental Appendix A.
We also searched for ongoing systematic reviews or meta-analyses of studies with the terms “Proton Pump Inhibitor and “Clostridium difficile infection” at the Cochrane Library Online as of June 11, 2015. Two studies15,16 were identified, however, neither focused solely on hospital-acquired CDI. All medical subject headings of “proton pump inhibitors” and “Clostridium difficile” were searched in the MeSH database available from PubMed’s homepage. Twenty-five and 19 subheadings were found for the term “proton pump inhibitors” and “clostridium difficile”, respectively. Generic brand names of proton pump inhibitors such as “omeprazole”, “lansoprazole”, “dexlansoprazole”, “esomeprazole”, “pantoprazole”, “rabeprazole”, “ilaprazole” were added to the search. Studies with different type, dose, and duration of the adopted proton pump inhibitor(s) were included.
To assess articles by relevance, abstracts were screened for the following inclusion criteria: (1) observational studies or clinical trials (2) risk of hospital-acquired CDI after taking PPI was evaluated, (3) reported data was quantitative, (4) the article was published in a peer-reviewed journal, and (5) study presented data in such a way that allowed for calculation of risk or odds ratio. No language restrictions were used. Exclusion criteria consisted of: (1) studies that evaluated the risks in community-onset CDI cases, community-associated CDI cases, indeterminate onset CDI cases, and unknown outpatient cases after taking PPI, (2) reported data was qualitative, (3) the article was published as a dissertation, (4) study population had recurrent CDI defined as relapse of the original infection (i.e., endogenous persistence of the same strain) or reinfection (i.e., acquisition of a new strain from an exogenous source)17 that occurs less than or equal to 8 weeks after the onset of a previous episode18,19, and (5) pediatric, animal or lab-based studies.
Study selection
One reviewer (V.A.) merged search results using a reference management software which facilitated removal of duplicate records. Two independent reviewers (V.A. and A.B) screened all abstracts identified in the initial search.
Data extraction and quality assessment
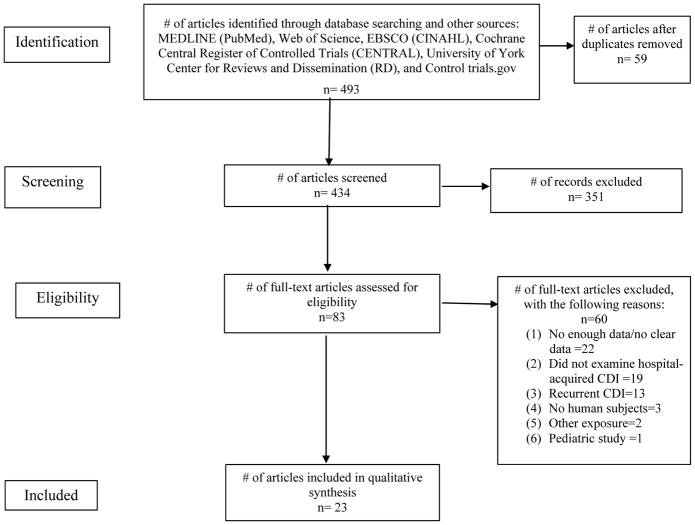
Our search, conducted on July 2, 2015, yielded 700 articles. Of these, we retrieved 493 abstracts and full-text articles that met eligibility criteria. Fifty-nine duplicate records were removed. A total of 434 articles were screened at the abstract level and 83 full-text articles were screened for eligibility (inclusion and exclusion criteria). Complete search terms, strategy, and results are described in Appendix A. Reviewers identified 23 full-text articles from which data was extracted, as shown in Figure 1. Two reviewers (V.A. and J.T.) independently extracted data from the articles. Any disagreement or discrepancy was settled in consensus with a third investigator (N.S). Reviewers extracted data using a standard electronic data sheet (Microsoft Excel). Data extracted included: study methods (study design, total study duration, methodology), participants (demographics, location, diagnostic criteria), exposure (PPI definition, regimen, dose), CDI outcome (definition, measurements), and results.
Figure 1.
PRISMA flow diagram of study selection criteria
The quality of case-control and cohort studies was assessed independently by two reviewers (V.A and A.B) using the MOOSE Guidelines for Meta-analyses and Systematic Reviews of Observational Studies20.
Outcomes
The primary outcome of interest was hospital-acquired CDI, defined in studies by positive stool toxin assay, clinical diagnosis or ICD-9 codes. For our analysis, we extracted data regarding sample size and case frequency, as well as reported odds ratios and risk ratios. Descriptive statistics were used to define the study population. Subgroup analysis was performed to determine how CDI case definition may impact risk of PPI.
Data synthesis and analysis
The relationship between PPI and CDI was examined using Review Manager Software (Rev Man, version 5.3 from Cochrane Collaboration). We calculated the Cochran Chi2 and the I2 statistic to evaluate existence and degree of heterogeneity. A p-value <0.1 for Chi2 was used as the cutoff to determine significance of heterogeneity. Significant heterogeneity would mean utilizing a random effects model, while a Chi2 that was not significant would suggest that a fixed effect model would be adequate.
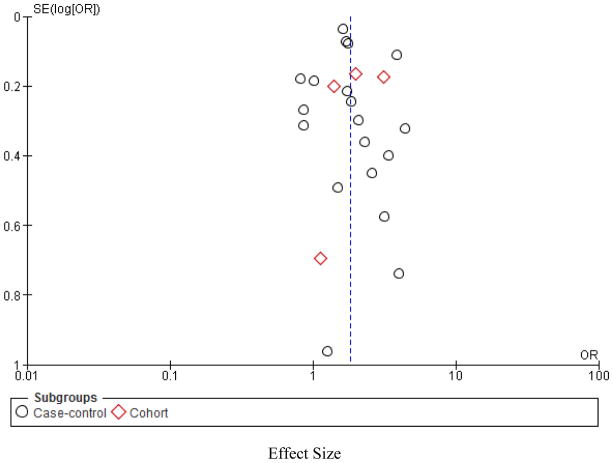
Assessment of Publication Bias
To assess for publication bias, funnel plots were generated by Rev Man. Funnel plots are used to check for asymmetry in distribution of study results, which aids in identification of studies prone to bias. If bias is present, plots of study variability or sample size against effect size are skewed and asymmetrical21. Small studies are more likely to have a poor quality and be prone to bias, thus, Duval and Tweedie’s trim and fill was to be followed to detect and correct for any publication bias present22.
Results
Study characteristics
A total of 23 studies assessing the relationship between PPI and CDI were included in this review. Table 1 shows the general characteristics of component studies in the meta-analysis. Of the 23 component studies, 19 studies were case-control studies, and four employed retrospective cohort designs. There were no RCTs that evaluated the relationship between PPI and CDI and no conference proceedings or abstracts met eligibility criteria. CDI case definitions varied, with the most common case definition being a positive stool toxin assay with associated symptoms (10 studies) or without documented symptoms (11 studies). Two studies defined cases by ICD-9 codes25,41.
Table 1.
General characteristics of included studies
| Author, year | Study location | Sample size, n | Mean age, y (SD) | Male, n (%) | Study patients | Definition of PPI Exposure | Study design |
|---|---|---|---|---|---|---|---|
| Al-Tureihi, 200523 | US | 53 | 82.3 | 13 (24.5) | LTACH patients | Duration of exposure not specified | Case-control |
| Aseeri et al, 200824 | US | 188 | NA | 82 (43.6) | Hospitalized inpatients | ≥3 days use before symptom onset | Case-control |
| Barletta et al, 201325 | US | 408 | 69 (15) | 229 (56) | ICU patients | ≥2 days use before CDI diagnosis | Case-control |
| Baxter et al, 200826 | US | 4493 | 68 | 2167 (48.2) | Hospitalized inpatients | Any exposure in 60 days preceding CDI diagnosis | Case-control |
| Beaulieu et al, 200727 | Canada | 827 | 65 | 494 (59.7) | ICU patients | Any exposure during index hospitalization | Cohort |
| Dalton et al, 200928 | Canada | 14,719 | 68.8 (17) | 7007 (47.6) | Hospitalized inpatients | Any exposure in 10 days preceding CDI diagnosis | Cohort |
| Dubberke et al, 200729 | US | 36,086 | NA | 15,159(42) | Hospitalized inpatients | Use at the time of CDI diagnosis | Case-control |
| Howell et al, 201030 | US | 101,796 | 56.6 (19.9) | 41,802 (41.1) | Hospitalized inpatients | Duration of exposure not specified | Case-control |
| Jenkins et al, 201031 | UK | 32 | 75.7 (62–85) | 14 (43.8) | Hospitalized inpatients | Duration of exposure not specified | Case-control |
| Kazakova et al, 200632 | US | 195 | NA (30–98) | 86 (44.1) | Hospitalized inpatients | Any exposure in 30 days preceding CDI diagnosis | Case-control |
| Kim et al, 201033 | South Korea | 125 | 67.6 (13.9) | 57 (45.6) | Hospitalized inpatients | ≥3 days use before CDI onset | Case-control |
| Linney et al, 201034 | Canada | 284 | 75.65 (13) | 134 (47.2) | Hospitalized inpatients | Use at the time of CDI diagnosis | Case-control |
| Loo et al, 200535 | Canada | 474 | 74.5 (11.9) | 241 (50.8) | Hospitalized inpatients | Any exposure in 6 weeks preceding CDI diagnosis | Case-control |
| Manges et al, 201036 | Canada | 75 | 69.5 (64.8–75.1) | 36 (48) | Hospitalized inpatients | Any exposure during index hospitalization | Case-control |
| McFarland, 200737 | US | 348 | NA | NA | Inpatients and outpatients | Any exposure in 3 months preceding CDI diagnosis | Case-control |
| Modena et al, 200538 | US | 250 | 59.7 (17.2) | 128 (51.2) | Hospitalized inpatients | Any exposure during index hospitalization | Case-control |
| Muto el al, 200539 | US | 406 | 61.5 (16–95) | 210 (51.7) | Hospitalized inpatients | Duration of exposure not specified | Case-control |
| Novack et al, 201440 | Israel | 556 | 68.2–69.0 (16.9) | 182 (45.8) | Hospitalized inpatients | Any exposure in 3 months preceding CDI diagnosis and during hospitalization | Case-control |
| Pakyz et al, 201341 | US | 14,134 | NA | 7,437 (52.6) | Hospitalized inpatients | Duration of exposure not specified | Case-control |
| Shah et al, 200042 | UK | 252 | 81.8 (65–96) | 85 (33.7) | Hospitalized inpatients | Any exposure in 16 weeks preceding CDI diagnosis | Case-control |
| Stevens et al, 201143 | US | 10,154 | NA | NA | Hospitalized inpatients | Any exposure during index hospitalization | Cohort |
| Yip, 200144 | Canada | 54 | 73 | 26 | Hospitalized inpatients | Duration of exposure not specified | Case-control |
| Wang et al, 201445 | China | 124 | 59–69 (30–35) | 82 (66.1) | ICU patients | Duration of exposure not specified | Cohort |
Sample sizes in studies ranged from 32 to 101,796 hospitalized patients, totaling 186,033 cases. Amongst these studies, 10,307 CDI cases were reported. Studies were from centers around the world; 12 from the United States, six from Canada, two in the United Kingdom, and one each in South Korea, Israel and China. The mean age of patients amongst the 16 studies that allowed for this calculation was 69.9 years The proportion of males in included studies ranged significantly, from as few as 24.5% to 66.1%. All studies were in hospitalized patients, and three studies25,27,45 were conducted exclusively in ICU patients.
Definition of Exposure
There was no standard definition of PPI exposure. Exposure varied from use of PPI at the time of CDI diagnosis26, to exposure during index hospitalization27,36,38,43, to any exposure in the past 90 days37,40 (table 1). Only one study commented specifically upon which PPIs were used40. In this study, PPIs used were omeprazole, lansoprazole, and pantoprazole.
Relationship between PPI and CDI
Fourteen studies identified a significant association between CDI and PPI, while the association was not statistically significant in the remaining nine. Of these nine, six27,31,35,36,44,45 had a trend toward a positive association, that is, an increased risk of CDI with PPI exposure. The remaining three37,40,42 had non-significant odds ratios less than one (0.82 – 0.86).
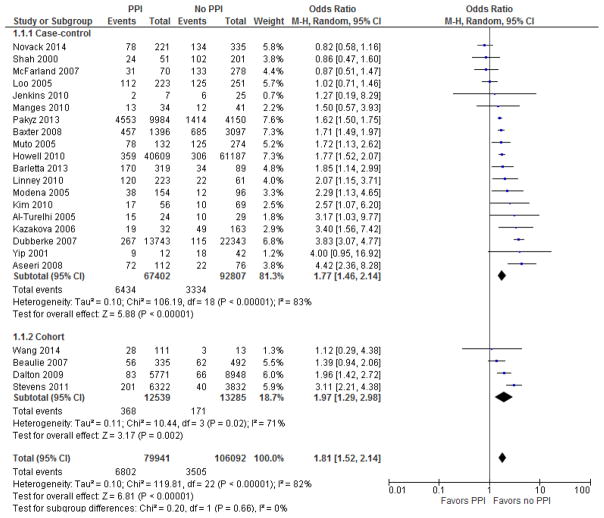
Our main analysis was performed in two subgroups – the four cohort studies and the 19 case control studies, as detailed in Figure 3. All cohort studies showed an increased risk of CDI in patients exposed to PPI, with two of four demonstrating statistical significance. All but three case control studies demonstrated a positive association between PPI and CDI, with 12 reaching statistical significance in this relationship. Pooled analysis of cohort studies demonstrated a odds ratio of 1.97 (95% CI= 1.29–2.98), which was statistically significant. Analysis of case control studies revealed an odds ratio of 1.77 (95% CI 1.46–2.14), which was also significant. There was no difference of overall effect between the subgroups (p<0.00001). Pooled odds ratio for all 23 studies was 1.81 [95% CI 1.52 – 2.14].
Figure 3.
Forest plot of the association between proton pump inhibitor and C. difficile infection. The vertical line corresponds to the no difference point between two groups. Horizontal lines represent the 95% CIs.
Subgroup analysis by definition of CDI
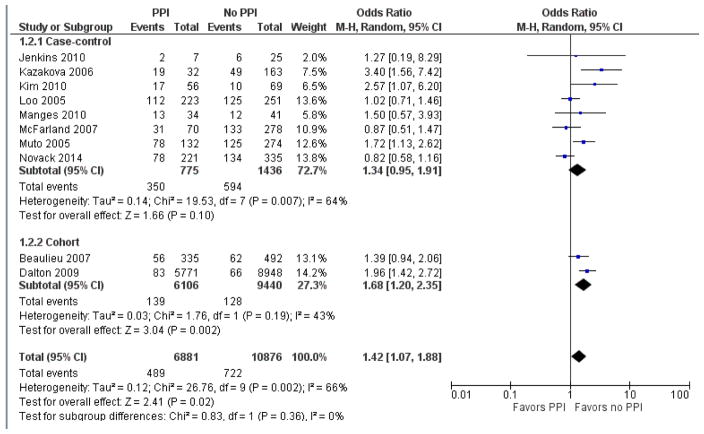
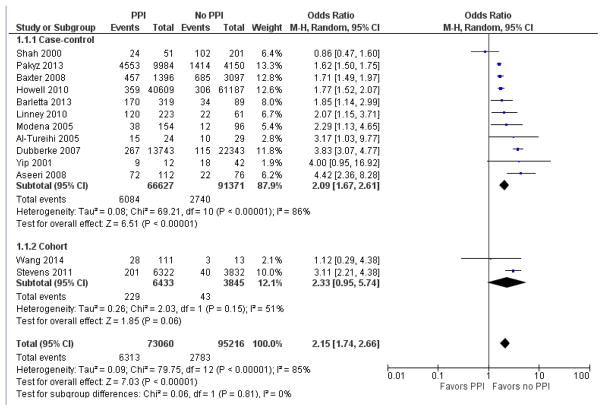
Subgroup analysis was performed to determine whether CDI case definition altered the strength of association with PPI, as detailed in Figures 4 and 5. In the 10 studies that included symptoms in the CDI case definition, pooled odds ratio was 1.42 [95% CI 1.07 – 1.88]. In the 13 studies that did not require symptoms for CDI case definition, the pooled odds ratio was 2.15 [95% CI 1.74 – 2.66].
Figure 4.
Forest plot of the association between proton pump inhibitor and C. difficile infection in those studies defining CDI cases in the presence of symptoms.
Figure 5.
Forest plot of the association between proton pump inhibitor and C. difficile infection in those studies not requiring symptoms for CDI case definition.
Effect of confounding factors on relationship between PPI and CDI
Most studies took into consideration one or more of the most common risk factors for CDI: exposure to antibiotic therapy or H2 blockers, renal failure, diabetes mellitus, immunosuppression, malignancy, and gastrointestinal disease. In addition, most studies identified sex, age, additional comorbidities such respiratory illness and length of hospitalization as potential confounding variables. Given the disparate study designs, patient populations and study locations, we did not attempt to control for the numerous confounding variables identified in component studies. Confounders identified in each of the included studies are detailed in Table 2.
Table 2.
Intra-study risk of bias, according to MOOSE Guidelines for Meta-Analyses and Systematic Reviews of Observational Studies, and confounders identified in component studies.
| Study, year | Study design | Study population clearly defined? | Clear definition of outcome and outcome assessment? | Important confounders and/prognostic factors identified? |
|---|---|---|---|---|
| Al-Tureihi, 200523 | Case-control | Yes | Yes | Age, and antibiotic treatment |
| Aseeri et al, 200824 | Case-control | Yes | Yes | Admission date, sex, age group, antibiotic use, patient location, and room type |
| Baxter et al, 200825 | Case-control | Yes | Yes | Number of days spent in the hospital, ICU days, antibiotics |
| Barletta et al, 201326 | Case-control | Yes | Yes | Prior hospital admission, intensive care unit admission, admission from a skilled nursing facility, immunosuppression, number of antibiotics received, PPI duration, and time to event |
| Beaulieu et al, 200727 | Cohort | Yes | Yes | Age, gender, length of stay, comorbidities, APACHE score, NGT feeding, tracheal tube placement, H2RA, and antibiotics |
| Dalton et al, 200928 | Cohort | Yes | Yes | Independent covariates (demographics characteristics such as age, gender, race -ethnicity), albumin and white blood cell count at the time of CDAD diagnosis, the Charlson co-morbidity score, prior admissions to Montefiore Medical Center within 180 days, and prior use of antibiotics and PPIs. ( last two were dichotomous) |
| Dubberke et al, 200729 | Case-control | Yes | Yes | Comorbid conditions that will increase the risk of CDAD (age, admissions, antibiotics, CDAD pressure, albumin level, leukemia/lymphoma, mechanical ventilations, H2RA, and anti-motility agents) |
| Howell et al, 201030 | Case-control | Yes | Yes | Age, antibiotics, and propensity score-based likelihood of receipt of acid suppression therapy |
| Jenkins et al, 201031 | Case-control | Yes | Yes | Not specified |
| Kazakova et al, 200632 | Case-control | Yes | Yes | Antibiotics, H2RA, length of stay, COPD, psychosis, and depression |
| Kim et al, 201033 | Case-control | Yes | Yes | Age, serum albumin level, and NGT feeding |
| Linney et al, 201034 | Case-control | Yes | Yes | Age, sex, discharge date and hospital unit, antibiotics, IBD, cancer, diabetes, NGT feeding, LOS, and previous residence |
| Loo et al, 200535 | Case-control | Yes | Yes | age, sex, number of days at risk for C. difficile associated diarrhea, Charlson index, and the use of chemotherapy, PPI, histamine H2 blockers and enteral feeding |
| Manges et al, 201036 | Case-control | Yes | Yes | Controlled for Bacteroidetes, and Firmicutes spp. |
| McFarland, 200737 | Case-control | Yes | Yes | Not specified |
| Modena et al, 200538 | Case-control | Yes | Yes | Antibiotic use and infections |
| Muto el al, 200539 | Case-control | Yes | Yes | Age, diabetes, organ transplantation, H2RA, and antibiotics |
| Novack et al, 201440 | Case-control | Yes | Yes | Adjusting to Charlson index |
| Pakyz et al, 201341 | Case-control | Yes | Yes | Controlling by patient level covariates NO hospital level medication covariates |
| Shah et al, 200042 | Case-control | Yes | Yes | Not specified |
| Stevens et al, 201143 | Cohort | Yes | Yes | Comorbid conditions within 48 hours following admission: diabetes, respiratory illness, kidney disease, transplant, and cancer. |
| Yip et al, 200144 | Case-control | Yes | Yes | Not specified |
| Wang et al, 201445 | Cohort | Yes | Yes | Not specified |
Assessment of heterogeneity and Publication Bias
Significant statistical heterogeneity was found (I2= 82%), as shown in Figure 2 which was not adequately explained by subgroup analyses to identify sources. Clinical heterogeneity was also present given the differing definitions across studies of exposure, and confounding variables.
Figure 2.
Funnel plot to assess the potential impact of publication bias.
Effect Size
By applying Trim and Fill, it was determined no apparent publication bias was present.
Discussion
While several reviews and studies have demonstrated an association between PPI use and CDI, and PPIs continue to be widely used among CDI susceptible populations. Our results show a significant association between PPI use and the incidence of hospital-acquired CDI, lending further evidence to PPI as a risk factor for CDI. Using the relevant available literature, we calculated a pooled odds ratio of 1.81, as shown in Figure 3.
Four previous systematic reviews of similar methodology have studied this question. Tleyhah46 and colleagues performed a meta-analysis of 51 observation studies examining both community and healthcare associated CDI, all of which demonstrated a positive association between PPI and CDI, with a pooled odds ratio of 1.65, 95% CI (1.47 – 1.85). They estimated the number needed to harm amongst patients receiving PPI concurrent with antibiotic therapy at 50, 95% CI (31, 97); this is significant given the high volume of patients exposed to both classes of medications during a hospitalization. Deshpande et al. examined the role of PPI in the development of CDI47 [Deshpande 2012], and specifically recurrent CDI48 in both the inpatient and outpatient setting. In Deshpande’s 2012 review of 30 observational studies, pooled meta-analysis demonstrated a 2.15, 95% CI (1.81, 2.55), greater odds of developing CDI amongst those on PPI. This review also performed subgroup analysis to examine the effect of concomitant antibiotic use on the relationship between PPI and CDI. They found that the higher risk of CDI among PPI users persisted across each subgroup, regardless of the frequency of antibiotic use reported on component studies. In 2015, Deshpande performed a meta-analysis examining the relationship between PPI and recurrent CDI; pooled risk ratio from eight studies was 1.58, 95% CI (1.13, 2.21). Garey et al.49 found a similar relationship when examining the association between any anti-ulcer medication (PPI and H2 blocker) and recurrent CDI, with a statistically significant pooled odds ratio from three studies 2.149, 95% CI (1.13, 4.08). Previous data have also demonstrated increased risk of severe or severe-complicated CDI in patients on PPI50.
Significant heterogeneity existed across studies which limited our ability to perform additional analysis regarding potential confounders and CDI outcomes. Despite this heterogeneity, with the exception of all but three studies demonstrated a positive association between PPI use and CDI, that is, PPI exposure appears to increase the risk of CDI significantly. Several confounders were proposed in included studies, many known to be conventional risk factors for CDI: old age, use of antibiotics, prolonged hospital course, immunosuppression and underlying chronic disease.
Inclusion of symptoms in CDI case definition appears to impact the relationship with PPI, with a less robust association when symptoms were required for CDI case identification. This may suggest colonization is an important mediator in the association between CDI and PPI. Data regarding the proportion with clinically apparent disease in the studies that did not include symptoms in the CDI case definition is not available. Without this, we cannot comment further on the frequency of colonization in these studies and the contribution to the association between PPI and CDI. The pooled odds ratio in this group remained significant, however, in line with our remaining results and previous studies demonstrating an association between CDI and PPI. Given colonization with toxigenic Clostridium difficile greatly increases the risk of clinical infection51, targeting risk for colonization are important in developing an infection prevention program.
Overuse of PPIs is widespread. In one study, 59% of general medical patients on PPI did not have a clear indication for use52. These numbers are similar amongst critically ill patients, with Farrell and colleagues citing 68.1% of patients on gastric acid suppression for stress ulcer prophylaxis did not have identifiable risk factors for stress related mucosal bleeding53. Our study highlights the importance of optimizing PPI use as an important component of a CDI reduction program. Barriers to reducing unnecessary PPI use in the inpatient setting should be studied to inform interventions to combat overuse or misuse. With the results of our meta-analysis and the results of the others on this topic, it should now be possible to predict the impact PPI optimization may have on reduction in hospital-acquired CDI rates. Intervention studies in this area are now needed.
Our study has several limitations. First, our results suffer the limitations of the component studies, such as potential selection bias when selecting controls. Secondly, studies were quite heterogeneous in their methods and outcome reporting. Given this heterogeneity, we were not able to independently adjust for potential confounders in the relationship between PPI and CDI. We attempted to control for any significant outliers by developing a priori inclusion and exclusion criteria and applying these stringently. Third, included studies used varying case definitions for CDI infection, potentially contributing to misclassification bias. We’ve addressed this by performing subgroup analysis. Finally, publication bias is always a potential concern in meta-analyses, and it is possible that studies demonstrating no association or a negative association between PPI use and CDI are less likely to be published. However, we assessed this using the Trim and Fill method for publication bias assessment, and publication bias was not identified in our review.
In conclusion, our results provide further evidence that PPIs increase the risk of CDI in hospitalized patients. Given the reported over prescription of PPIs52,54,55, focusing on optimization of PPI use in the inpatient setting should be a focus of infection prevention programs. Minimizing inappropriate use may have a significant impact on rates of hospital-acquired CDI.
Acknowledgments
From the William S. Middleton Memorial Veterans Affairs Hospital, Madison WI and the University of Wisconsin-Madison.
Financial Support. This work was supported a by the VHA National Center for Patient Safety of the United States (U.S.) Department of Veterans Affairs. The views expressed in this article are those of the author(s) and do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. NS is also supported by a VA MERIT award. AB is supported by a pre-doctoral traineeship under NIH awards UL1TR000427 and TL1TR000429.
APPENDIX A: Search strategy
The search strategy was created with the assistance of the librarians at the University of Wisconsin in Madison. EndNote software was used as reference manager.
1. PubMED
(“Proton Pump Inhibitors”[Mesh] OR Proton Pump Inhibitor* OR PPI* OR Omeprazole OR Lansoprazole OR Dexlansoprazole OR Esomeprazole OR Pantoprazole OR Rabeprazole OR Ilaprazole)) AND (Clostridium difficile OR CDI)
-
#1
[MeSH] Proton Pump Inhibitors
-
#2
Proton Pump Inhibitor*
-
#3
PPI*
-
#4
Omeprazole
-
#5
Lansoprazole
-
#6
Dexlansoprazole
-
#7
Esomeprazole
-
#8
Pantoprazole
-
#9
Rabeprazole
-
#10
Ilaprazole
-
#11
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
-
#12
[MeSH] Clostridium difficile
-
#13
CDI
-
#14
#12 OR #13
1. CINAHL
(Proton Pump Inhibitor* OR PPI* OR Omeprazole OR Lansoprazole OR Dexlansoprazole OR Esomeprazole OR Pantoprazole OR Rabeprazole OR Ilaprazole)) AND (Clostridium difficile OR CDI)
-
#1
Proton Pump Inhibitors
-
#2
Proton Pump Inhibitor*
-
#3
PPI*
-
#4
Omeprazole
-
#5
Lansoprazole
-
#6
Dexlansoprazole
-
#7
Esomeprazole
-
#8
Pantoprazole
-
#9
Rabeprazole
-
#10
Ilaprazole
-
#11
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
-
#12
Clostridium difficile
-
#13
CDI
-
#14
#12 OR #13
1. Cochrane Central Register of Controlled Trials (CENTRAL)
(Proton Pump Inhibitor OR PPI OR Omeprazole OR Lansoprazole OR Dexlansoprazole OR Esomeprazole OR Pantoprazole OR Rabeprazole OR Ilaprazole) AND (Clostridium difficile OR CDI)
-
#1
Proton Pump Inhibitors
-
#2
Proton Pump Inhibitor*
-
#3
PPI*
-
#4
Omeprazole
-
#5
Lansoprazole
-
#6
Dexlansoprazole
-
#7
Esomeprazole
-
#8
Pantoprazole
-
#9
Rabeprazole
-
#10
Ilaprazole
-
#11
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
-
#12
Clostridium difficile
-
#13
CDI
-
#14
#12 OR #13
1. Web of Science
(Proton Pump Inhibitor* OR PPI* OR Omeprazole OR Lansoprazole OR Dexlansoprazole OR Esomeprazole OR Pantoprazole OR Rabeprazole OR Ilaprazole) AND (Clostridium difficile OR CDI)
-
#1
Proton Pump Inhibitors
-
#2
Proton Pump Inhibitor*
-
#3
PPI*
-
#4
Omeprazole
-
#5
Lansoprazole
-
#6
Dexlansoprazole
-
#7
Esomeprazole
-
#8
Pantoprazole
-
#9
Rabeprazole
-
#10
Ilaprazole
-
#11
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
-
#12
Clostridium difficile
-
#13
CDI
-
#14
#12 OR #13
References
- 1.Paredes-Sabja D, Shen A, Sorg JA. Clostridium difficile spore biology: sporulation, germination and spore structural proteins. Trends in Microbiol. 2014;22(7):406–416. doi: 10.1016/j.tim.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly CP, Pothoulakis C, Lamont JT. Clostridium difficile colitis. N Eng J Med. 1994;330:257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 3.Magill SS, Edwards JR, Bamberg W, et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N Engl J Med. 2014;370:1198–208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Healthcare-associated Infections (HAIs) Tracking Clostridium difficile Infection. [Accessed March 16, 2016];Centers for Disease Control and Prevention (CDC) website. http://www.cdc.gov/hai/organisms/cdiff/tracking-Cdiff.html.
- 5.Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(suppl 2):S88–S92. doi: 10.1093/cid/cis335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott RD. The direct medical costs of healthcare-associated infections in U.S. Hospitals and the benefits of prevention. 2009. [Accessed March 16, 2016];Centers for Disease Control and Prevention website. http://www.cdc.gov/hai/pdfs/hai/scott_costpaper.pdf.
- 7.Frequently asked questions about Clostridium difficile for healthcare providers. [Accessed March 16, 2016];Centers for Disease Control and Prevention (CDC) website. http://www.cdc.gov/HAI/organisms/cdiff/Cdiff_faqs_HCP.html.
- 8.Biswal S. Proton pump inhibitors and risk for Clostridium difficile associated diarrhea. Biomed J. 2014;37(4):178–83. doi: 10.4103/2319-4170.128002. [DOI] [PubMed] [Google Scholar]
- 9.Clooney AG, Bernstein CN, Leslie WD, et al. A comparison of the gut microbiome between long-term users and non-users of proton pump inhibitors. Aliment Pharmacol Ther. 2016 doi: 10.1111/apt.13568. (e-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 10.Seto CT, Jeradlo P, Orenstein R, Chia N, DIBaise JK. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2:42. doi: 10.1186/2049-2618-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FDA Drug Safety Communication. Clostridium difficile-associated diarrhea can be associated with stomach acid drugs known as proton pump inhibitors (PPIs) [Accessed March 16, 2016];United States Food and Drug Administration website. http://www.fda.gov/Drugs/DrugSafety/ucm290510.htm.
- 12.Gawron AJ, Feinglass J, Pandolfino JE, Tan BK, Bove MJ, Shintani-Smith S. Brand name and generic proton-pump inhibitor prescriptions in the United States: Insights from the National Ambulatory Medical Care Survey (2006–2010) Gastroenterology Res Pract. 2015 doi: 10.1155/2015/689531. article ID 689531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CMS releases prescriber-level Medicare data for first time. [Accessed March 14, 2016];Centers for Medicare and Medicaid Services website. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-04-30.html.
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011–1019. doi: 10.1038/ajg.2012.108. [DOI] [PubMed] [Google Scholar]
- 16.Deshpande A, Pant C, Pasupuleti V, et al. Association between proton pump inhibitor therapy and Clostridium difficile infection in a meta-analysis. Clin Gastroenterol Hepatol. 2012;10(3):225–33. doi: 10.1016/j.cgh.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 17.Depestel DD, Aronoff DM. Epidemiology of Clostridium difficile infection. J Pharm Pract. 2013;26(5):464–75. doi: 10.1177/0897190013499521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. 2005;366(9491):1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 19.Walker AS, Eyre DW, Wyllie DH, et al. Relationship between bacterial strain type, host biomarkers, and mortality in Clostridium difficile infection. Clin Infect Dis. 2013;56(11):1589–1600. doi: 10.1093/cid/cit127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;(283):2008–10. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 21.Sterne JAC, Egger M. Funnel plots for detecting bias in meta-analysix: Guidelines on choice of axis. J Clinical Epidemiology. 2001;(54):1046–55. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 22.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 200(56):455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 23.Al-Tureihi FIJ, Hassoun A, Wolf-Klein G, Isenberg H. Albumin, length of stay, and proton pump inhibitors: Key factors in Clostridium difficile-associated disease in nursing home patients. J Am Med Dir Assoc. 2005;6:105–8. doi: 10.1016/j.jamda.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Aseeri M, Schroeder T, Kramer J, Zackula R. Gastric acid suppression by proton pump inhibitors as a risk factor for Clostridium-difficile-associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103:2308–13. doi: 10.1111/j.1572-0241.2008.01975.x. [DOI] [PubMed] [Google Scholar]
- 25.Barletta JF, Sclar DA. Proton pump inhibitors increase the risk for hospital-acquired Clostridium difficile infection in critically ill patients. Crit Care. 2014;18:714–9. doi: 10.1186/s13054-014-0714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baxter R, Ray GT, Fireman BH. Case-control study of antibiotic use and subsequent Clostridium difficile-associated diarrhea in hospitalized patients. Infect Control Hosp Epidemiol. 2008;29(1):44–50. doi: 10.1086/524320. [DOI] [PubMed] [Google Scholar]
- 27.Beaulieu M, Williamson D, Pichette G. Risk of Clostridium difficile-associated disease among patients receiving proton-pump inhibitors in a Quebec medical intensive care unit. Infect Control Hosp Epidemiol. 2007;28(11):1305–7. doi: 10.1086/521664. [DOI] [PubMed] [Google Scholar]
- 28.Dalton BR, Lye-Maccannell T, Henderson EA, Maccannell DR, Louie TJ. Proton pump inhibitors increase significantly the risk of Clostridium difficile infection in a low-endemnicity, non-outbreak hospital setting. Aliment Pharamacol Ther. 2009;29(6):626–34. doi: 10.1111/j.1365-2036.2008.03924.x. [DOI] [PubMed] [Google Scholar]
- 29.Dubberke ER, Reske KA, Yan Y, Olsen MA, McDonald LC, Fraser VJ. Clostridium difficile-associated disease in a setting of endemnicity: identification of novel risk favors. Clin Infect Dis. 2007;45(12):1543–9. doi: 10.1086/523582. [DOI] [PubMed] [Google Scholar]
- 30.Howell MD, Novack V, Grgurich P, Souilliard D, Novack L, Pencina M, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170(9):784–90. doi: 10.1001/archinternmed.2010.89. [DOI] [PubMed] [Google Scholar]
- 31.Jenkins PJ, Teoh K, Simpson PM, Dave J, Simpson AHWR, Breusch S. Clostridium difficile in patients undergoing primary hip and knee replacement. J Bone Joint Surg [Br] 2010;92-B:994–8. doi: 10.1302/0301-620X.92B7.23412. [DOI] [PubMed] [Google Scholar]
- 32.Kazakova SV, Ware K, Baughman B, Bilukha O, Paradis A, Sears S, et al. A hospital outbreak of diarrhea due to an emerging epidemic strain of Clostridium difficile. Arch Intern Med. 2006;166:2518–24. doi: 10.1001/archinte.166.22.2518. [DOI] [PubMed] [Google Scholar]
- 33.Kim JW, Lee KL, Jeong JB, Kim BG, Shin S, Kim JS, et al. Proton pump inhibitors as a risk factor for recurrence of Clostridium-difficile-associated diarrhea. World J Gastroenterol. 2010;16(28):3573–77. doi: 10.3748/wjg.v16.i28.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linney S, Fernandes T, Einarson T, Sengar A, Walker JH, Mills A. Association between use of proton pump inhibitors and a Clostridium difficile-associated disease outbreak: Case-control study. Can J Hosp Pharm. 2010;63(1):31–7. doi: 10.4212/cjhp.v63i1.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, et al. A predominantly clonal multi-institutional outbreak of Clostridium-difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442–9. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 36.Manges AR, Labbe A, Loo VG, Atherton JK, Behr M, Masson L, et al. Comparative metagenomics study of alterations to the intestinal microbiota and risk of nosocomial Clostridium difficile-associated disease. J Infect Dis. 2010;202(12):1877–84. doi: 10.1086/657319. [DOI] [PubMed] [Google Scholar]
- 37.McFarland LV, Clarridge JE, Beneda HW, Raugi GJ. Fluoroquinolone use and risk factors for Clostridium difficile-associated disease within a Veterans Administration health care system. Clin Infect Dis. 2007;45:1141–51. doi: 10.1086/522187. [DOI] [PubMed] [Google Scholar]
- 38.Modena S, Bearelly D, Swartz K, Friedenberg FK. Clostridium difficile among hospitalized patients receiving antibiotics: A case-control study. Infect Control Hosp Epidemiol. 2005;26(8):685–90. doi: 10.1086/502603. [DOI] [PubMed] [Google Scholar]
- 39.Muto CA, Pokrywka M, Shutt K, Mendelson AB, Nouri K, Posey K, et al. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005;26(3):273–80. doi: 10.1086/502539. [DOI] [PubMed] [Google Scholar]
- 40.Novack L, Kogan S, Gimpelevich L, Howell M, Borer A, Kelly CP, et al. Acid suppression therapy does not predispose to Clostridium difficile infection: The case of the potential bias. PLoS ONE. 2014;9(10):e110790. doi: 10.1371/journal.pone.0110790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pakyz AL, Jawahar R, Wang Q, Harpe SE. Medication risk factors associated with healthcare-associated Clostridium difficile infection: a multilevel model case-control study about 64 US academic medical centres. J Antimicrob Chemother. 2014;69:1127–31. doi: 10.1093/jac/dkt489. [DOI] [PubMed] [Google Scholar]
- 42.Shah W, Lewis A, Leopold D, Dunstan F, Woodhouse K. Gastric acid suppression dow not promote clostridial diarrhea in the elderly. Q J Med. 93:175–81. doi: 10.1093/qjmed/93.3.175. 200. [DOI] [PubMed] [Google Scholar]
- 43.Stevens V, Dumyati G, Brown J, Wijngaarden EV. Differential risk of Clostridium difficile infection with proton pump inhibitor use by level of antibiotic exposure. Pharmacoepidemiol Drug Saf. 2011;20:1035–42. doi: 10.1002/pds.2198. [DOI] [PubMed] [Google Scholar]
- 44.Yip C, Loeb M, Salama S, Moss L, Olde J. Quinolone use as a risk factor for nosocomial Clostridium difficile-associated diarrhea. Infect Control Hosp Epidemiol. 2001;22(9):572–5. doi: 10.1086/501954. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Cai L, Yu R, Huang W, Zong Z. ICU-onset Clostridium difficile infection in a university hospital in China: A prospective cohort study. PLoS One. 2014;9(11):e111735. doi: 10.1371/journal.pone.0111735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tleyjeh IM, Adbullhak AA, Riaz M, et al. Association between proton pump inhibitor therapy and Clostridium difficile infection: A contemporary systematic review and meta-analysis. PLoS One. 2012;7(12):e50836. doi: 10.1371/journal.pone.0050836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desphande A, Pant C, Pasupluleti V, Rolston DDK, Jain A, Desphande N, et al. Association between proton pump inhibitor therapy and Clostridium difficile infection in a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:225–33. doi: 10.1016/j.cgh.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 48.Deshpande A, Pasupuleti V, Throta P, et al. Risk factors for recurrent Clostridium difficile infection: A systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36(4):452–60. doi: 10.1017/ice.2014.88. [DOI] [PubMed] [Google Scholar]
- 49.Garey KW, Sethi S, Yadav Y, DuPont HL. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect. 2008;70:298–304. doi: 10.1016/j.jhin.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Khanna S, Aronson SL, Kammer PP, Baddour LM, Pardi DS. Gastric acid suppression and outcomes in Clostridium difficile infection: A population-based study. Mayo Clin Proc. 2012;87(7):636–42. doi: 10.1016/j.mayocp.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zacharioudaks IM, Zervou JN, Pliakos EE, Aiakas PD, Mylonakis E. Colonization with toxinogenic C difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110:381–90. doi: 10.1038/ajg.2015.22. [DOI] [PubMed] [Google Scholar]
- 52.Reid M, Keniston A, Heller C, Miller M, Medvedev S, Albert RK. Inappropriate prescribing of proton pump inhibitors in hospitalized patients. J Hosp Med. 2012;7(5):421–5. doi: 10.1002/jhm.1901. [DOI] [PubMed] [Google Scholar]
- 53.Farrell CP, Mercogliano G, Kuntz CL. Overuse of stress ulcer prophylaxis in the critical care setting and beyond. J Crit Care. 2010;25:214–20. doi: 10.1016/j.jcrc.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 54.Buckley MS, Park AS, Anderson CS, Barletta JF, et al. Impact of clinical pharmacist stress ulcer prophylaxis management program on inappropriate use in hospitalized patients. Am J Med. 2015;128(8):905–13. doi: 10.1016/j.amjmed.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 55.Tasaka CL, Burg C, VanOsdol SJ, et al. An interprofessional approach to reducing the overutilization of stress ulcer prophylaxis in adult medical and surgical intensive care units. Ann Pharmacother. 2014;48(4):462–9. doi: 10.1177/1060028013517088. [DOI] [PubMed] [Google Scholar]