Abstract
Cystinosis is an autosomal recessive metabolic disease that belongs to the family of lysosomal storage disorders. It is caused by a defect in the lysosomal cystine transporter, cystinosin, which results in an accumulation of cystine in all organs. Despite the ubiquitous expression of cystinosin, a renal Fanconi syndrome is often the first manifestation of cystinosis, usually presenting within the first year of life and characterized by the early and severe dysfunction of proximal tubule cells, highlighting the unique vulnerability of this cell type. The current therapy for cystinosis, cysteamine, facilitates lysosomal cystine clearance and greatly delays progression to kidney failure but is unable to correct the Fanconi syndrome. This Review summarizes decades of studies that have fostered a better understanding of the pathogenesis of the renal Fanconi syndrome associated with cystinosis. These studies have unraveled some of the early molecular changes that occur before the onset of tubular atrophy and identified a role for cystinosin beyond cystine transport, in endolysosomal trafficking and proteolysis, lysosomal clearance, autophagy and the regulation of energy balance. These studies have also led to the identification of new potential therapeutic targets and here, we outline the potential role of stem cell therapy for cystinosis and provide insights into the mechanism of haematopoietic stem cell-mediated kidney protection.
Introduction
Renal Fanconi syndrome presents as a generalized dysfunction of the proximal tubule, characterized by the presence of polyuria, phosphaturia, glycosuria, proteinuria, acidosis, growth retardation and rickets1–3. The leading cause of inherited renal Fanconi syndrome in children is cystinosis, which accounts for up to 20% of cases of hereditary tubular disorders4.
Cystinosis is an autosomal recessive metabolic disease that belongs to the family of lysosomal storage disorders. It is characterized by an accumulation of cystine within all organs as a result of a deletion or mutations in CTNS, which encodes the lysosomal cystine transporter, cystinosin5. The most frequent and most severe form of cystinosis is infantile cystinosis (MIM 219800), which is the focus of this Review and is hereafter simply referred to as ‘cystinosis’. Children with this form of cystinosis appear normal at birth, but demonstrate failure to thrive by around 6–9 months of age. By 6–18 months of age, symptoms of kidney dysfunction, such as polyuria and polydipsia, develop, as well as rickets. Continuing loss of glomerular function eventually leads to renal insufficiency by ~10 years of age if untreated5 and at a median of ~20 years of age if cysteamine treatment is initiated before 5 years of age6. Deposition of cystine crystals in the cornea occurs early in the course of disease, causing photophobia and painful corneal erosions7. In their second to third decade of life, patients can develop hypothyroidism, hypogonadism, diabetes, myopathy, and deterioration of the central nervous system8–10.
Juvenile (MIM 219900)11 and ocular (MIM 219750)12 cystinosis are milder and rarer than infantile cystinosis. Juvenile cystinosis usually manifests around 12 years of age and causes photophobia and kidney dysfunction at variable ages, with a combination of glomerular and tubular alterations causing marked proteinuria, eventually leading to end-stage renal disease (ESRD). Ocular cystinosis is characterized by adult-onset mild photophobia without renal manifestations.
CTNS — which encodes the lysosomal cystine–proton co-transporter, cystinosin — was first identified as the causative gene in cystinosis in 1998 (REFS 13–15). CTNS is expressed in all tissues, and mutations in the gene therefore eventually cause multi-systemic disease. Deletion of Ctns in mice leads to the development of multiple features of cystinosis, including ocular defects with deposition of corneal cystine crystals16,17, bone demineralization and deformities18, muscle wasting19 and thyroid dysfunction20. On certain genetic backgrounds, deletion of Ctns also18 leads to the development of renal pathology, in particular a renal Fanconi syndrome between 4 and 6 months of age21. The phenotype of the Fanconi syndrome in mice is less severe than that observed in humans; nevertheless, the mice eventually develop ESRD21. This animal model has been instrumental for improving our understanding of kidney pathophysiology in cystinosis. A hallmark of renal disease in Ctns-knockout mice is the accumulation of cystine crystals within proximal tubular cells (PTCs). Interestingly, however, evidence suggests that these PTCs become dedifferentiated before crystals accumulate, losing their brush border, becoming flattened, and eventually resting on a thicker basement membrane than do healthy PTCs18,21. These tubular changes typically begin at the glomerulotubular junction and extend distally, evolving to tubular atrophy with development of characteristic swan neck deformities22. In addition, heavy inflammatory cell infiltrates can be observed in the renal interstitium21,23.
Beyond supportive therapy, the only specific therapy for cystinosis is cysteamine, which acts by depleting cystine in lysosomes. Cysteamine was first used in cystinosis patients in 1976 but wasn’t approved by the FDA until 1994 (REFS 24,25). Although early administration of cysteamine therapy delays the progression of cystinosis complications including ESRD8,10,26,27, it has no effect on established renal Fanconi syndrome. Moreover, the renal Fanconi syndrome in cystinosis is unique in that it is typically the earliest manifestation of the disease process. In addition, in cystinotic mice, the renal Fanconi syndrome manifests before structural anomalies, such as cystine crystal deposition or PTC dedifferentiation and atrophy, are detected in PTCs. These findings raise questions as to the unique features of PTCs that explain their early vulnerability in cystinosis; the molecular mechanisms that account for the development of PTC dysfunction before the development of detectable lesions; the cellular and molecular mechanisms that link cystinosin deficiency and the resulting lysosomal defects to histopathological changes and eventual tubular atrophy; and how best to translate this knowledge into better management approaches, and potentially a cure for cystinosis.
This Review summarizes decades of research into the pathogenesis of renal Fanconi syndrome in cystinosis, focusing on findings that have provided insights into the pathogenic roles of global metabolic and oxidation defects; impaired endolysosomal trafficking, proteolysis and signalling; and apical dedifferentiation in disease processes. We emphasize the importance of cystinosin in the maintenance of cellular homeostasis, beyond its function in cystine transport and discuss current treatment approaches as well as emerging therapeutic avenues that have arisen from improved understanding of the pathophysiology of cystinosis and from studies of haematopoietic stem cell transplantation in mice.
Cystinosis: an overview
Clinical course of cystinosis
Renal Fanconi syndrome is the most common initial clinical manifestation of infantile cystinosis, typically becoming evident at 6–18 months of age28,29. Although glomerular proteinuria can be detected early in the disease course (as early as 1 year of age)30,31, glomerular filtration rate begins to deteriorate from 5–6 years of age, leading to the development of ERSD. According to the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) 2010–2011 annual report, cystinosis accounts for 1.4% of children on dialysis and 2.1% of paediatric renal transplant recipients in the USA32. Cystinosis is, however, a multi-systemic disease with accumulation of cystine in lysosomes throughout the body. Deposition of cystine crystals in the cornea, which begins in infancy, gradually leads to photophobia, blepharospasm, and recurrent corneal erosions7. Retinopathy can also develop as early as 3 years of age, and can cause blindness33,34. Affected individuals often develop endocrine complications such as hypothyroidism, diabetes, pubertal delay, and male hypogonadism35. Vascular damage36,37 and hypertension38,39 have also been described in relation to hyperplasia of renin-producing cells in the juxtaglomerular apparatus39; chronic kidney disease and disruption of calcium, phosphate and parathyroid hormone homeostasis; and cystine accumulation in blood vessels. Cystinosis also causes rachitism with bone deformities and fragility40, which is attributed to massive urinary phosphate loss, defective conversion of 25-hydroxyvita-min D into active calcitriol in the kidney and deposition of cystine in bone, although the exact pathophysiology is not fully understood41. Cystinosis is also associated with the development of neuromuscular and neurocognitive dysfunction including the deterioration of fine vision, motor coordination and peripheral muscle strength, and the development of swallowing difficulties42–47. Impaired swallowing and chronic respiratory dysfunction favour the development of aspiration pneumonia, a major cause of death in affected patients48,49. Most of these complications, with the exception of established renal Fanconi syndrome, can be delayed or even prevented with cysteamine therapy8,9.
Epidemiology of cystinosis
Cystinosis is a rare, autosomal-recessive, monogenic disorder with a general incidence of 1:100,000–1:200,000 live births50. A higher local incidence has been reported in French Brittany (1:26,000 live births)51,52 and Saguenay–Québec (1:62,500 live births)53, due to distinct founder mutations in CTNS (c.898–900 + 24del27 (REFS 54,55) and p.Trp138X (REF. 56), respectively). Another founder mutation, involving a 57 kb deletion in CTNS, is the most common cause of cystinosis, affecting 65% of patients of northern European descent13,27,57 but almost never occurs in other families58–61. Cystinosis is also reported in countries with high levels of consanguinity such as some countries in the Middle East, but accurate prevalence data are lacking58,61–64. The low reported frequency of cystinosis in Russia65, India66 and China67 likely reflects underdiagnosis.
Molecular basis of cystinosis
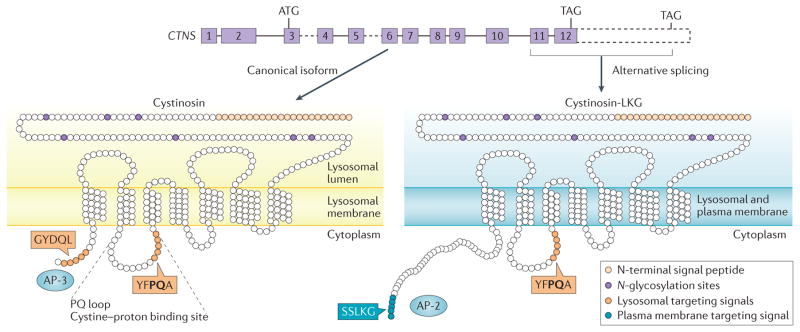
CTNS is located on the short arm of chromosome 17 (REF. 68) and comprises 12 exons, the two first of which are non-coding13. The remaining 10 exons encode a 367 amino-acid protein, called cystinosin, which is predicted to contain seven transmembrane domains, a luminal N-terminal region bearing seven N-glycosylation sites and a cystolic C-terminal GYDQL lysosomal targeting signal. A longer CTNS isoform can be generated by alternate splicing of exon 12, which removes the GYDQL motif and adds 39 amino acids to produce a 400 amino-acid protein, called cystinosin-LKG69 (FIG. 1). Like the canonical form of cystinosin, cystinosin-LKG localizes at lysosomes, but unlike canonical cystinosin, it also resides in the secretory apparatus and the plasma membrane, where it mediates proton-coupled cystine transport70. The cystinosin-LKG isoform represents 5–20% of all CTNS transcripts in most tissues (representing up to 50% of all CTNS transcripts in testes)71.
Figure 1. CTNS produces two isoforms with distinct subcellular localizations.
CTNS, which encodes the seven transmembrane lysosomal protein cystinosin, is composed of 12 exons (solid boxes) with the start codon (ATG) in exon 3 and two alternative stop codons (TAGs) in exon 12. The common, canonical form of cystinosin is shown on the left. The 3’ region of CTNS (dashed box) encodes the C-terminal extension of the less frequent isoform, cystinosin-LKG, which arises from alternative splicing in exon 12 (right). Canonical cystinosin exclusively localizes to lysosomes due to the concerted effect of two lysosome-targeting signals: a classic tyrosine-based C-terminal motif, GYDQL, and a non-classic motif, YFPQA, in the fifth transmembrane loop named the ‘PQ loop’, which also contains a cystine proton binding site. The GYDQL motif allows recognition by the adaptor protein complex 3 (AP 3), which is responsible for direct lysosomal targeting of cystinosin. The cystinosin-LKG isoform lacks the GYDQL motif but instead contains the carboxyl-terminal SSLKG sequence, which directs this isoform to the plasma membrane, with secondary endocytic trafficking to lysosomes involving AP-2 complexes.
Subcellular localization studies have confirmed the localization of the canonical cystinosin isoform to the lysosomal membrane14. The GYDQL motif interacts with the adaptor protein complex 3 (AP-3), which is responsible for direct lysosomal targeting72. An additional, non-classical lysosomal targeting motif located in the fifth cytoplasmic loop, YFPQA (FIG. 1), reinforces association with lysosomes14. Paired lysosomal sorting motives are thought to ensure robust targeting of the protein to the lysosome, and are present in other lysosomal membrane proteins, such as battenin, which is involved in the neurodegenerative disorder Batten disease73. The YFPQA sequence comprises part of a ‘PQ-loop’ motif, a defining feature of the PQ-loop family proteins, which are seven transmembrane helices with a duplicated region containing a well-conserved PQ-dipeptide motif74. The cystine and proton-binding site in cystinosin belongs to this second PQ-loop75, as seen in other PQ-loop proteins such as the SWEET sugar transporters76.
Cystinosin is a cystine–proton symporter that uses the proton gradient to transport cystine from the lysosomal lumen to the cytosol. The affinity of cystinosin for cystine at acidic pH15, is much higher than that of other lysosomal aminoacid transporters for their substrates. Indeed, the Km of cystinosin for cystine is ~0.25 mM in Cos-1 cells and down to 0.075 mM when protonated, as occurs in the acidic lysosomal lumen75, in contrast to micromolar ranges for the lysosomal amino acid transporter PQ loop repeat containing protein-2 (PQLC2; 3 mM for arginine and even higher for lysine77), and peptide/histidine transporter-2 (PHT2; 5 mM for histidine78). These values suggest that saturation of cystinosin occurs much below the level at which cystine saturation of lysosomes occurs (5 mM under acidic lysosomal conditions). Quantitative mass spectrometry studies further indicate low copy numbers of cystinosin at the lysosomal membrane (~10 transporters per lysosome)79, which is compatible with cystinosin abundance being the rate-limiting step for cystine transport, consistent with reduced transport efficiency in heterozygotes carrying one defective CNTS allele79. This rate-limiting effect of transporter number contrasts with lysosomal storage diseases caused by defective luminal hydrolases, whereby heterozygotes show no accumulation of substrate at 50% residual activity80. Moreover, although a low abundance of transporters in individual lysosomes would not be expected to cause a problem as lysosomes constantly exchange their digestive load by fission and homotypic fusion, such job sharing is compromised upon rigid content overload. In addition, genotype–phenotype correlation revealed complete disruption of cystine transport in patients with infantile cystinosis associated with point mutations in CTNS, in contrast to the partial preservation of cystine transport observed in individuals with juvenile and ocular cystinosis81. A total loss of cystine transport might not, however, be sufficient to cause nephropathic cystinosis. For example, two missense mutations in CTNS — Lys280Arg and Asn323Lys — found in patients with juvenile cystinosis, result in undetectable levels of cystine transport, yet these mutated forms of cystinosin still localize at lysosomes81. This finding indicates that the presence of cystinosin without transport capability is associated with a milder phenotype than that associated with the 57 kb deletion present in most forms of infantile cystinosis, and is compatible with an important function of cystinosin beyond cystine transport (BOX 1).
Biochemistry of cystinosis
Cystine accumulation in lysosomes
Mechanism of cystine accumulation
Impaired transporter-dependent exodus of cystine across the lysoso-mal membrane leading to intralysosomal accumulation of cystine was until a few years ago considered to be the primary cause of all cystinosis manifestations. Studies in cultured human cystinotic fibroblasts further demonstrated that intracellular cystine does not originate from oxidation of local cysteine in lysosomes82 but from lysosomal degradation of disulfide-bearing proteins82–84. Moreover, cystine loading in fibroblasts is proportional to the extracellular concentration of albumin83 and to the intrinsic abundance of disulfide bridges in various endocytic cargos82.
In particular, since albumin contains 17 disulfide bonds, each degraded albumin releases 17 cystine molecules83. Furthermore, a surprisingly low fraction of cystine released by albumin degradation (<1%) was retained, which was attributed to cystine exocytosis83. Vesicular exodus of free cystine by exocytosis should thus be regarded as a natural clearance mechanism that considerably slows the accumulation of cystine. Quantitative estimations detailed below indicate that in PTCs from Ctns-knockout mice, receptor-mediated endocytosis of ultrafiltrated plasma proteins is more than a sufficient source of cystine. Albumin, the major ultrafiltrated protein, is barely detectable in urine, indicating virtually complete reabsorption. Upon selective genetic abrogation of receptor-mediated endocytosis, mice excrete on average roughly 25 μg of albumin per day85, indicating that this amount is normally reabsorbed daily via the megalin–cubilin pathway and transferred to lysosomes, corresponding to ~12 nmol of half-cystine released from albumin alone in PTC lysosomes every day. Similar calculations can be extended to humans, on the basis of high levels of proteinuria observed in patients with homozygous deletion of the gene that encodes cubilin86. Moreover, during the first months of life, when endocytic receptors are preserved, kidneys of Ctns-knockout mice accumulate ~0.1 nmol half-cystine per mg of total protein per day (~1.5 nmol half-cystine per day)21. Thus, similarly to cystinotic cells in vitro, where only 1% of cystine released by lysosomal proteolysis is actually retained in lysosomes82, the rate of lysosomal cystine generation in PTCs from Ctns-knockout mice exceeds the rate at which it is retained by at least sevenfold. Although these calculations are rough estimates, two conclusions can be drawn: first, albumin endocytosis and lysosomal degradation in PTCs is sufficient to account for the supply of cystine precursors; and second, the amount so supplied largely exceeds the amount being stored implying that cystine is poorly retained by endolysosomes in vivo and that, in addition to cystinosin-mediated transmembrane exodus, cystine can be efficiently discharged by vesicular efflux (exocytosis). These two conclusions indicate that inhibition of megalin-dependent endocytosis and triggering vesicular exocytosis could both slow the build-up of cystine in PTCs and progression of cystinosis.
Cystine accumulates as two forms in growth-arrested cells such as PTCs and interstitial macrophages. First, as soluble cystine, which in principle cannot exceed concentrations of ~5 mM, the limit of solubility under acidic lysosomal conditions87. Soluble cystine can then organize into polyhedral crystals within the lysosomal matrix (FIG. 2). The theoretical limit of cystine solubility within the acidic lysosomal environment can be roughly converted into a maximal soluble cystine load per cell. Given that the typical protein concentration in kidney cells is 160 mg/ml, the 5 mM free cystine in acidic lysosomes, which typically occupy ~4% of cell volume, would correspond to ~0.2 μmol half-cystine per ml of tissue, or ~1 nmol half-cystine per mg of tissue protein. However, higher values of free cystine do not necessarily imply the formation of cystine crystals, since up to 20 nmol of half-cystine per mg of protein88 has been recorded in short-lived cystinotic neutrophils (which are assessed to monitor the efficiency of cysteamine therapy), without evidence of crystals. The discrepancy between the theoretical solubility threshold of cystine and the actual load might reflect enlarged lysosomal volumes, electrostatic interaction between cystine and the charged fixed residues of lysosomal proteins enabling cystine to exit the soluble free pool, or the possible contribution of variable amounts cytosolic cystine included in the tissue cystine assay. Of note, a 5 mM maximal concentration of cystine is too low to cause osmotic swelling, and therefore cannot be the primary cause of lysosomal enlargement.
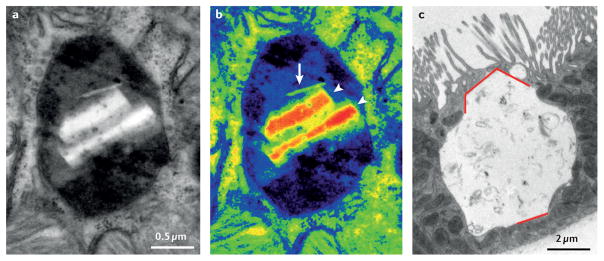
Figure 2. Cystine crystals accumulate within lysosomes of cystinotic cells.
a | Conventional electron micrograph of a proximal tubule cell from an 8-month old Ctns-knockout mouse showing a lysosome that is about twofold larger in diameter than usual. b | Pseudo-colour conversion of grey levels, to better demonstrate intertwining of the normal dense matrix (dark areas) with amorphous, less electron-dense material suggestive of undigested protein (blue areas); and the two types of crystals: a sharp, needle-shaped crystal (arrow) and two polyhedric crystals (arrow heads). Such crystals are never seen before 6 months of age and needles do not usually contact the lysosomal membrane. Note the lack of luminal membrane packing that identifies autophagic structures. c | Conventional electron micrograph of a mouse proximal tubule cell deformed by a single huge lysosome. Red lines indicate the straight borders and angles of a polyhedric crystal, close to 10 μm in height. Note the preservation of the brush border in adjacent cells. Part c reproduced with permission from the American Society of Nephrology © Gaide Chevronnay, H. P. et al. J. Am. Soc. Nephrol. 25, 1256–1269 (2014).
Crystal formation
The absence of crystals in exponentially growing cultured human and murine cystinotic fibroblasts, immortalized shed urinary cells, and short-lived cystinotic neutrophils suggests that crystallization within the heterogeneous lysosomal matrix is a rather slow process. Crystals are first detected by electron microscopy in cystinotic mouse PTCs as tiny needles that are invisible by light microscopy, which then build up as polyhedra that deform lysosomes and can reach huge dimensions (>10 μm in diameter). A build-up of crystals after the onset of renal Fanconi syndrome explains the exponential increase of cystine in kidney extracts from cystinotic mice. Large crystals have a significant impact on lysosome motility (see below) and lysosome vesicular dynamics. Enlargement and content rigidification are known to impair lysosomal fusion89,90. In addition, non-breakable micrometer-large crystals must prevent lysosomal fission. The combination of these effects should block interlysosomal exchange, and thereby arrest randomization of endocytic load between lysosomes, thus favour their conversion into residual bodies89,90. Unlike kidneys from Ctns-knockout mice, in which cystine crystals are predominantly found in PTCs21,91, crystals are absent or rarely found in PTCs from children with cystinosis92. Rather, biopsy samples from children aged 1.5–3.0 years with cystinosis show crystal deposition predominantly in interstitial macrophages39. Importantly, crystals of mouse PTCs, but not of macrophages, can be disposed into the tubular lumen, either by exocytosis (in a process known as ‘lysosomal defecation’), or by apoptotic cell fragmentation91. Of note, flattened cells in swan neck deformities in cystinotic mice are devoid of crystals91. Moreover, allografts from renal transplant recipients do not show evidence of crystals in PTCs whereas crystal formation recurs in macrophages, consistent with infiltration of the allograft by cystinosin-defective host macrophages39. Thus, cystine crystals are clearly pathognomonic of cystinosis, but should no longer be considered pathogenic in the initiation of renal Fanconi syndrome, which develops in Ctns-knockout mice before crystals appear in PTCs and is inconsistent with the absence of crystals at early stages of cystinosis in PTCs in humans. Moreover, the failure of cystine-clearing cysteamine to reverse established renal Fanconi syndrome demonstrates the involvement of additional essential mechanisms. Crystals might, however, be crucial in driving the progression of renal disease at later stages, for example, by activating the inflammasome to trigger inflammation and fibrosis93.
Energy imbalance
Loading of renal tubules with cystine dimethylester was historically used to study the effects of cystine build-up in vitro and in vivo; this approach led to a drastic decrease in cellular ATP levels in renal tubules, but this effect was later attributed to mitochondrial intoxication94–97. Low levels of ATP were, however, later found in fibroblasts from patients with cystinosis98,99, as well as upon silencing of cystinosin in rabbit PTCs with small interfering RNA100, independent of a detectable mitochondrial defect, suggesting that cystinosis is associated with altered cellular energy homeostasis. The cause of the reduced ATP levels in cystinosin-deficient cells is unknown. The presence of an energy imbalance in cystinosis is further supported by evidence that the cytosolic fuel sensor, AMP-activated protein kinase (AMPK), is activated in primary rabbit PTCs with cystinosin knockdown101. However, the interpretation of this finding must be regarded with caution since AMPK is involved in kinase-based energy sensing and adaptive crosstalk, in particular with mTORC-1, a sensor of energy supply at lysosomes (FIG. 3).
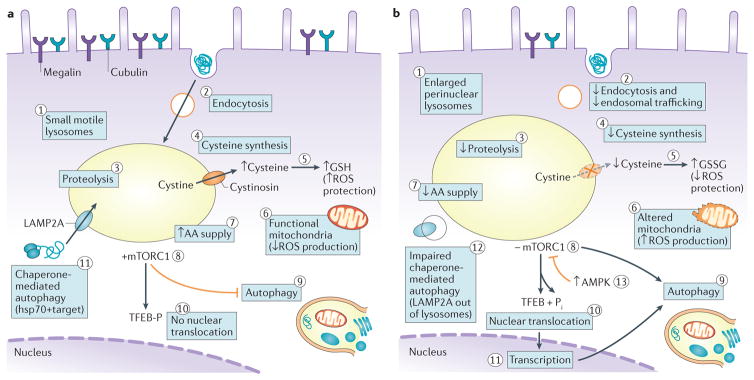
Figure 3. Cell biological alterations in cystinotic cells.
a | Lysosomes of healthy proximal tubule cells (PTCs) are small, motile and dispersed throughout the cytoplasm (1), where they can readily fuse with late endosomes. High apical expression of the tandem multiligand receptors, megalin and cubilin, and their unusually fast recycling rate result in extremely active receptor mediated endocytosis (2). Endosomal trafficking, transfer into lysosomes and proteolysis (3) of ultrafiltrated disulfide rich plasma proteins ensures an ample lysosomal supply of amino acids (AAs) together with sulfur-bearing cystine. Following cystinosin-mediated export from the lysosome, cystine becomes a source of cysteine in the cystosol (4), which favours the biosynthesis of reduced glutathione (GSH), which confers protection against reactive oxygen species (ROS; 5). Moreover, functional mitochondria do not release ROS (6). An abundance of AAs (7) keeps the mTORC1 complex at the lysosomal membrane in its active kinase form (8). Crucial physical interactions between cystinosin and mTORC1 are not indicated. Lysosomal mTORC1 inhibits macroautophagy (9) and maintains TFEB phosphorylation, thereby abolishing its nuclear translocation and transcriptional effects (10). Localization of LAMP2A at the lysosomal membrane supports the clearance of altered cytosolic proteins by chaperone-mediated autophagy (11). b | In cystinotic PTCs, lysosomes engorged by undigested proteins (evident as amorphous inclusions) become enlarged and cluster around nuclei (1); they are less motile than lysosomes of healthy PTCs and are located further from the apical membrane, rendering them less accessible to endocytic cargo and less prone to exocytic clearance. Endocytic uptake is also decreased as expression of megalin and cubilin declines (2). Impaired proteolysis (3) lowers AA supply (7). Moreover, cystine can no longer cross the lysosomal membrane due to an absence or loss-of-function of cystinosin, which presumably limits cysteine availability (4) and therefore GSH synthesis, reversing the ratio of GSH to oxidized glutathione (GSSG), leading to increased oxidative stress by ROS (5). Concomitantly, ROS production increases as mitochondria are damaged and/or are less efficiently cleared (6). The shortage of free AAs (7) inhibits mTORC1 (8), which activates autophagic programmes, including mitophagy (9). mTORC1 inactivation also leads to dephosphorylation and nuclear translocation of TFEB (10), activating transcription programmes (11) that further promote autophagy. Chaperone-mediated autophagy of altered (including oxidized) cytosolic proteins is hampered, in part because of impaired LAMP2A trafficking to lysosomes (12). Continuous autophagy eventually causes cell atrophy. In parallel, energy depletion activates AMPK, which inhibits mTORC1 thus activates autophagy (13). Enhanced apoptosis is not represented. Hsp70, heat shock protein-70.
Apoptosis
Cystinotic fibroblasts and PTCs are particularly vulnerable to apoptosis102,103. In vivo, the characteristic ‘swan neck’ deformity of the proximal tubules is associated with local apoptosis104 (FIG. 4). The propensity of PTCs to undergo apoptosis is supported by the uniform overexpression of the pro-apoptotic cysteine protease, caspase-4, in kidney biopsy samples from patients with cystinosis105. Transcripts for genes encoding caspase-1, caspase-4, and caspase-12, are significantly increased in kidneys from 15-month-old Ctns-knockout mice, suggesting global overexpression of the apoptosis cascade (S. Cherqui, unpublished work). Increased activation of the immediate apoptosis executioner, caspase-3, was also demonstrated in scattered PTCs from Ctns-knockout mice, associated with luminal shedding of apoptotic bodies and a partial compensatory response through PTC proliferation and repair91. However, apoptosis is more likely to be a common end-point of disease processes than a primary cause of proximal tubule dysfunction in cystinosis. Although iterative PTC apoptosis from the swan neck could eventually cause tubular atrophy, apoptotic luminal PTC shedding can also be viewed as an initial adaptive mechanism for the selective removal of irreversibly altered and/or crystal-overloaded cells91. Indeed, each replacement of an apoptotic PTC by a proliferating neighbour implies generation of a completely new set of lysosomes. An additional possible mechanism contributing to the swan neck deformity is sliding metaplasia, whereby the flat epithelium of the Bowman capsule would extend into the convoluted segment of the proximal tubules that has been depopulated by apoptosis. This mechanism is conceptually analogous, albeit in opposite direction, to colonization of the distal Bowman capsule by PTCs.
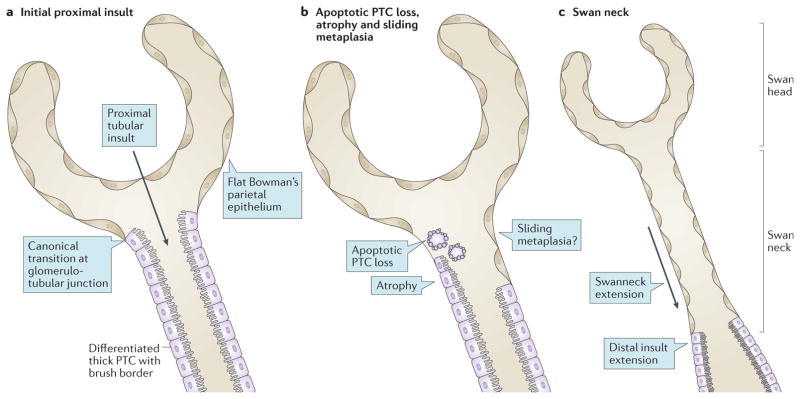
Figure 4. Origin and consequences of swan neck deformities.
a | The canonical localization at the glomerulotubular junction represents the transition between the flat parietal epithelium typical of the Bowman capsule and the thick columnar epithelium lining the proximal tubules, with a highly differentiated apical pole represented by a brush border. The transition between the two types of epithelial cells can occur within the glomerulus, with the lower third of the Bowman capsule lined by thick epithelium that is undistinguishable from differentiated proximal tubule cells (PTCs). The opposite scenario (that is, a transition from thin to thick epithelium distal to the glomerulotubular junction) is never found under normal conditions. The insult here arises from the apical pole by an ultrafiltrated and reabsorbed toxic factor. We suggest that this factor could be disulfide-bearing albumin, which is the main source of cystine, and first affects the most proximal PTCs. b | Swan neck deformities arise from the apoptotic cell death and shedding of individual PTCs; atrophy by dedifferentiation of PTCs; and possibly by replacement of dead PTCs upon expansion of the flat Bowman epithelium from the glomerular wall into the tubules (sliding metaplasia hypothesis). c | The characteristic features of a microdissected nephron exhibiting a normal-sized glomerulus (swan head) and an atrophic proximal tubule (swan neck) suggest the extension of lesions to cells at the base of the neck, as they become exposed to the ultrafiltrated and reabsorbed toxic factor, are thus governed by the same mechanisms as in part b.
Oxidative stress
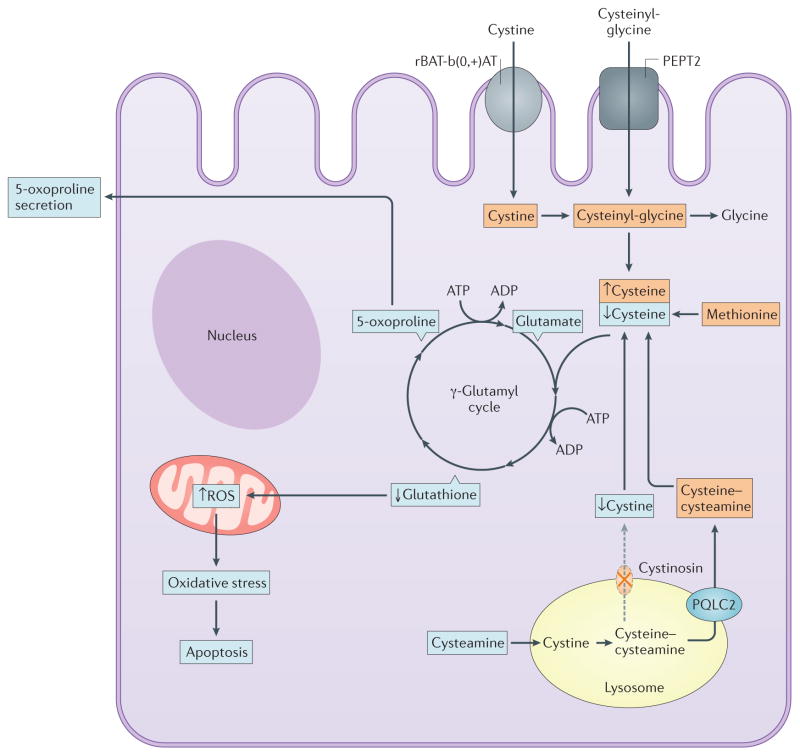
Cystine efflux from lysosomes is widely considered to serve as a source for cysteine, which itself is used in the synthesis of the tripeptide, glutathione, via the γ-glutamyl cycle. Reduced glutathione (GSH) is the major antioxidant in the cystosol, facilitating the neutralization of free radicals such as reactive oxygen species. Accordingly, altered glutathione metabolism and increased oxidative stress have been proposed to cause the Fanconi syndrome in cystinosis (FIG. 5). A significant decrease in total glutathione content and increase in superoxide dismutase activity, which enhances oxidative stress, were observed in cultured human cystinotic fibroblasts and were corrected by administration of exogenous cysteine106, raising the possibility that lowered cytosolic glutathione levels resulted from a shortage of cysteine that is normally derived from cystine (FIGS 3b,5). Further studies demonstrated altered glutathione metabolism in skin fibroblasts, polymorphonuclear cells, and cultured PTCs from patients with cystinosis107,108. Fibroblasts derived from patients were unable to increase glutathione synthesis in response to oxidative stress109. Under conditions of ATP inhibition, levels of 5-oxoproline, an intermediate of the γ-glutamyl cycle, were almost doubled in cystinotic cells compared to controls whereas that of γ-glutamyl cysteine remained unchanged, indicating that the cycle was impaired at the step of cysteine entry, further supporting the hypothesis that the supply of cysteine in cystinotic cells is limited. Of note, elevated levels of 5-oxoproline have also been identified in urine of patients with cystinosis, confirming the in vitro results110. Interestingly, one study reported that PTCs extracted from the urine of patients with cystinosis had decreased basal glutathione levels, combined with lower ATP levels and a higher apoptosis rate111, suggesting that these defects could be linked.
Figure 5. Cystinosin deficiency and oxidative stress in proximal tubular cells.
Absence of cystinosin was thought to lead to decreased cytosolic cysteine content, resulting in decreased glutathione and increased production of reactive oxygen species (ROS) and oxidative stress (blue squares). One of intermediates of the γ-glutamyl cycle is 5-oxoproline, which is increased in the urine of cystinotic patients. However, alternative sources of cysteine exist including through the reabsorption of cysteinyl-glycine by PEPT2 and cystine by the actions of the amino-acid transporter rBAT-b(0,+)AT (orange squares). Moreover, cysteamine drug, another source of cytoplasmic cysteine, does not prevent the renal Fanconi syndrome, suggesting the contribution of factors other than cysteine deficiency to this phenotype.
GSH has an important role in preventing mitochondrial oxidative damage and abnormal mitochondria have been reported in PTCs from humans and mice with cystinosis18,112,113. In Ctns-knockout mice, the increase in oxidative stress preceded the development of swan neck deformities, which could be delayed by administration of mitoquinone, an antioxidant compound that targets mitochondria104. Of interest, a pilot clinical trial in which N-acetyl-cysteine was administered for 3 months to 23 patients with nephropathic cystinosis demonstrated a significant reduction in oxidative stress and improved renal function114. However, additional studies with larger cohorts and longer follow-up are clearly needed to confirm this conclusion.
Together the above-described studies document alterations in glutathione metabolism and support the hypothesis that synthesis of cytoplasmic cysteine is limited when recycling of lysosomal cystine is abrogated. However, cells have alternative sources of cysteine. Indeed, cysteine can be synthesized from methionine, albeit to a limited extent115. More importantly, cysteine can be imported from the extracellular space116 especially in PTCs where cysteinylglycine is reabsorbed by the PEPT2 transporter117, and cystine is reabsorbed by the heterodimeric rBAT–b(0,+)AT transporter, which are active at the apical membrane118. In addition, cysteamine releases equimolar amounts of cysteine from the lysosomes into the cytosol and cysteamine is also itself a potent antioxidant, shown to increase intracellular levels of GSH119–121. However, the vexing inefficacy of cysteamine on established Fanconi syndrome in patients with cystinosis argues against a primary role for defective glutathione metabolism and increased PTC susceptibility to oxidative stress in the pathogenesis of Fanconi syndrome, suggesting the involvement of more complex cellular and molecular disease mechanisms.
Cell biological alterations
Trafficking and proteolysis defects
A host of structural and endocytic trafficking defects have been reported in cystinotic cells (FIG. 3b). First, in addition to severely decreased expression of the endocytic receptors megalin and cubilin in PTCs91,122, impaired megalin recycling has been reported in cystinotic cells123, as in two other hereditary diseases associated with renal Fanconi syndrome: Dent disease124 and Lowe oculocerebrorenal (OCRL) syndrome125. However, the finding that administration of cysteamine fails to control established Fanconi syndrome despite correction of megalin recycling in vitro123 argues against a major role for recycling defects in cystinosis.
Second, cystinotic lysosomes become enlarged and cluster to the perinuclear region of cells, reflecting impaired lysosome motility and indicating reduced accessibility to endocytic and autophagic loads122,123. Three possible explanations exist for the pericentriolar trapping of cystinotic lysosomes: first, through physical retention, since enlarged lysosomes have impaired free diffusion126 and are predicted to engage with increased numbers of microtubular contacts via their cytoskeletal motor protein, dynein, and since the microtubular network is most dense around centrioles127; second, through altered regulation of lysosomal calcium release that controls lysosomal dynamics127, although no change in the total level of lysosomal calcium store has been detected in cultured cystinotic cells128; and third, through downregulation of Rab–GTPase(s) that normally catalyse lysosomal trafficking, such as Rab27a, which is specifically expressed in PTCs and is downregulated in PTCs from mice and humans with cystinosis129.
Third, delayed transfer of endocytosed proteins into the lumen of endolysosomes that act as the degradative compartment has been documented in PTCs from Ctns-knockout mice, with evidence that cargo-loaded endosomes can dock without actually fusing with LAMP-1-labelled lysosomes91.
Fourth, proteolysis in endolysosomes is impaired, as was first suggested for cystinotic fibroblasts three decades ago based on ultrastructural evidence of ‘amorphous inclusions’ and high equilibrium density in subcellular fractionation studies as a result of protein accumulation130. Direct in situ evidence of an impaired rate of proteolysis, independent of less frequent encounters between internalized protein cargo carried by late endosomes and the set of proteolytic enzymes brought by lysosomes, has been demonstrated in primary PTC cultures from Ctns-knockout mice122, and in human cystinotic cells123. Assuming that lysosomal influx of cysteine does not increase in cystinotic cells to compensate for higher intralysosomal levels of free cystine due to impaired exodus, thus leading to luminal oxidation of lysosomal thiols, the defect in intrinsic lysosomal proteolysis could result from three possible mechanisms (FIG. 3b): first, by impairing the unfolding of disulfide-bonded substrates that are necessary to expose masked peptide bonds for endoproteolytic attack131; second, through inactivation of mature lysosomal cathepsins that belong to cysteine proteinases and critically depend on preservation of the reduced thiol in their catalytic site; and third, through impaired autoactivation and hetero-activation of pro-cathepsins by proteolytic excision of the masking pro-peptide. In turn, impaired proteolysis could affect lysosomal amino acid sensors, such as mTORC1, thereby affecting lysosomal signalling132 (FIG. 3b).
Signalling and autophagy defects
Lysosomes are no longer considered simply as bags for protein degradation, but rather, they are viewed as multipurpose, adaptive signalling organelles133 (FIG. 3a). One major function is in the control of autophagy, and increased numbers of autophagosomes, especially enclosing mitochondria, have been reported in various cystinotic cell lines as well as in liver and kidney from Ctns-knockout mice134,135. The lysosomal membrane is now recognized to be a regulated docking surface for the mTORC1 machinery, which integrates information on the nutritional status of the cell (that is, amino acid supply), to regulate autophagy and cell differentiation through various pathways including through transcription factor EB (TFEB) phosphorylation132,133. Specifically, mTOR complexes have fundamental roles in renal physiology, highlighting the importance of this pathway in kidney cell homeostasis and function136. Upon stimulation by amino acids, the small GTPases Ras-related GTP-binding proteins (Rag) and the pentameric Ragulator complex recruit cytosolic mTORC1 to the lysosomal membrane, close to its activator, Rheb GTPase137,138. Activation of mTORC1 by Rheb GTPase increases protein synthesis, while inhibiting autophagy. Conversely, starvation (amino acid deprivation) inactivates mTORC1, thereby triggering mobilization of nutrient reserves by activating autophagy139,140. The vacuolar ATPase (v-ATPase) proton pump is necessary for mTORC1 activation by amino acids, by engaging in amino acid-sensitive interactions with the Rag–Ragulator complex137,141. Co-immunoprecipitation and mass spectrometry studies have demonstrated that cystinosin interacts physically with almost all components of the v-ATPase, Ragulator complex and RagA/ RagC142; these interactions require the fifth loop in cystinosin, which contains the PQ signal. Furthermore, PTC lines derived from Ctns-knockout mice showed defective lysosomal recruitment of mTOR upon nutrient shortage (FIG. 3b). Impairment of mTOR activation was confirmed in human cystinotic cells143. Of note, as lysosomal v-ATPase–mTOR activation controls the expression of megalin and thereby regulates apical protein uptake in Drosophila epithelial cells and mouse PTCs144, these data provide a possible explanation for the decreased megalin expression in cystinotic kidney, as discussed in further detail below.
TFEB is the master transcription factor responsible for coordinating the expression of all lysosomal constituents, including cystinosin, and therefore has a key role in regulating lysosomal biogenesis and clearance, in coordination with autophagy and anabolism133 (FIG. 3b). This factor is silenced by mTOR-dependent phosphorylation, which causes it to become trapped in the cytosol, and is in turn activated by Ca2+-dependent dephosphorylation, which enables its nuclear translocation. Expression of TFEB mRNA and protein is impaired in PTCs deficient in cystinosin145. Conditionally immortalized PTCs derived from the urine of a patient with cystinosis showed persistent nuclear translocation of TFEB, indicating stimulation of lysosomal clearance. Prolonged overexpression of exogenous TFEB or its pharmacological activation by genistein lowered cystine levels and corrected cellular alterations in endocytic trafficking and rescued deficits that could not be corrected by cysteamine, demonstrating again that the absence of cystinosin affects lysosomal signalling and pointing to the potential benefits of pharmacological intervention targeting TFEB145.
Contrary to observations in many lysosomal storage disorders146 and to predictions based on evidence of defective mTOR signalling in cystinosis cells142, surprising findings from one study showed that autophagosomes in fibroblasts from Ctns-knockout mice matured normally. These autophagosomes supported fully functional macroautophagic fluxes134, but cells demonstrated impairment of chaperone-mediated autophagy (CMA)134 (FIG. 3b). CMA is a process of autophagy that involves the chaperone-dependent selection of altered cytosolic proteins and their translocation into lysosomes via lysosome-associated membrane protein type 2A (LAMP2A)147. In cells from Ctns-knockout mice, LAMP2A is mislocalized, presumably as a result of altered vesicle trafficking. In addition, CMA-dependent proteolysis by isolated lysosomes from Ctns-knockout mice was defective, whereas lysosomal proteases seemed to function normally. Importantly, CMA is activated during oxidative stress to facilitate the clearance of irreversibly modified molecules148; therefore, impaired CMA in cystinosis could increase cell sensitivity to oxidative stress. The various autophagy defects observed in cystinosis are probably interconnected, as impairment of CMA impairment might induce compensatory activation of other autophagic pathways147,149. However, the mechanisms leading to defective CMA in cystinosis are unclear and whether cystinosin stabilizes LAMP2A, a variant of LAMP2, at the lysosomal membrane is not known. Moreover, isolated autophagy deficits observed in Danon disease, a lysosomal storage disorder caused by mutations in LAMP2 (REFS 150,151), do not lead to a renal phenotype150,152, implying that this defect cannot be the primary reason for the Fanconi syndrome associated with cystinosis.
Although many questions remain unanswered with regard to the regulation and roles of autophagy in cystinosis, it is clear that cysteamine therapy does not correct many defects of cellular homeostasis, such as alterations in mTOR signalling, TFEB expression and CMA, despite improvements in cystine clearance134,142,145. These findings underscore the effects of cystinosin deletion, beyond the direct effects on cystine overload (BOX 1).
Histopathological alterations
Transporters and endocytic receptors
For all their use in investigating the biochemical and cell biological alterations in cystinosis, studies with immortalized cell lines are unable to recapitulate the exquisite apical differentiation of PTCs, nor their sequential organization as tubes with segmental specialization. As the renal Fanconi syndrome is characterized by a global defect in PTC reabsorption, a global loss of solute transporters and endocytic receptors at the apical membrane of cystinotic PTCs has been hypothesized91. Indeed, careful molecular examination of early changes in kidneys from Ctns-knockout mice revealed a loss of the major phosphate and glucose transporters (NaPi-IIa and SGLT-2, respectively) concomitant with the detection of urinary markers of Fanconi syndrome, accounting for the functional urinary loss of solutes before any detectable (ultra)structural alterations, including at the brush border. Moreover, studies revealed a parallel spatiotemporal loss of the apical multi-ligand endocytic receptors, megalin and cubilin91,122, which normally ensure near complete reuptake and lysosomal degradation of ultrafiltrated plasma proteins. This observation thus explains the characteristic tubular proteinuria of the renal Fanconi syndrome (commonly referred to as low molecular weight proteinuria). Although expression of megalin and cubilin was initially reported as being preserved in cystinosis31, subsequent reports found levels of these endocytic receptors to progressively decline91,121. Thus, the early disappearance of several apical solute transporters and endocytic receptors, in a process that could be referred to as ‘apical dedifferentiation’, might provide a common functional molecular mechanism for the renal Fanconi syndrome, well before tubule atrophy occurs.
Marked cellular heterogeneity within the same histopathological section of proximal tubules is obvious in patients39 and mice with cysinosis91. This heterogeneity distinguishes cystinosis from other causes of renal Fanconi syndrome, such as Dent disease124, and calls attention to cell-autonomous responses, such as genetic regulation, in the response of individual PTCs to cystinosin deficiency. Interestingly, studies of primary PTC cultures from Ctns-knockout mice have demonstrated loss of megalin to be associated with the nuclear translocation of ZO-1-associated nucleic acid binding protein (ZONAB)122, a transcription factor that promotes cell proliferation and downregulates megalin and cubulin expression as direct targets153. However, ZONAB is only part of a transcriptional network that integrates with mTOR-regulated pathways, including those regulated by TFEB.
Longitudinal extension and atrophy
Over time, apical dedifferentiation of PTCs extends longitudinally from the glomerulotubular junction to the more distal PTC segments91 (FIG. 4). According to the estimates provided above, endocytosis of ultrafiltrated disulfide-rich plasma proteins, in particular albumin, is the primary source of lysosomal cystine for PTCs83,84. Thus, early defective reabsorption by PTCs closest to the glomeruli followed by distal extension of the defect further suggests a causal role for defective endocytosis in disease progression. Indeed, documented transfer of endocytic load, and thus cystine insult, to more distal PTCs was predicted to cause longitudinal extension of the lesion91. Supporting this endocytosis-based model of nephropathic cystinosis, we have found that kidney- specific ablation of megalin in Ctns-knockout mice prevents accumulation of cystine only in the kidneys and protects nephrons from swan neck deformities154. On the other hand, decreased expression of endocytic receptors in proximal PTCs could be viewed as another cell-autonomous mechanism of adaptation, by lowering protein uptake to decrease the supply of cystine155. This notion is also consistent with the fact that an absence of cystinosin does not prevent primary neonatal nephron differentiation, but later induces apical dedifferentiation.
Late-stage cystinotic kidneys show extreme anatomic atrophy due to a combination of PTC atrophy with interstitial and glomerular fibrosis. Atrophied PTCs have the appearance of being replaced by flat cells resembling glomerular parietal cells, supporting the sliding metaplasia hypothesis. Interstitial fibrosis follows marked thickening of tubular basement membrane. End-stage cystinotic kidneys also show frequent glomerulotubular disconnection, due to encroachment of the junction, resulting in the development of atubular glomeruli. However, atubular glomeruli occur in a wide variety of chronic kidney diseases including glomerular, tubular, toxic obstructive and infectious syndromes156; they do not, of course, contribute to urine production and therefore cannot contribute to the phenotype of the renal Fanconi syndrome.
Treatment and therapeutic perspectives
Current treatment
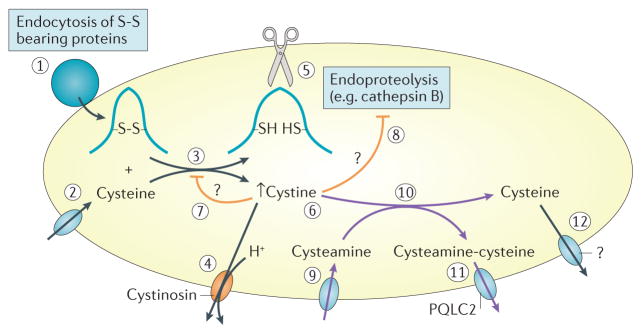
Beyond supportive therapy28, patients with cystinosis benefit from the oral drug, cysteamine (β-mercapto-ethylamine). Cysteamine depletes lysosomal cystine by cleaving cystine into free cysteine and cysteamine–cysteine mixed disulfide, which is exported from lysosomes by the cationic amino acid transporter, PQLC2, which also belongs to the PQ-loop family77 (FIG. 6). Although effective in delaying the complications of cystinosis including ESRD26, cysteamine therapy cannot correct established renal Fanconi syndrome, nor the autophagy, mTOR, TFEB and CMA defects in cystine- deficient cells134,145,157. These sobering observations highlight the fact that the pathogenic mechanisms of cystinosis involve both the lysosomal accumulation of cystine and the lack of cystinosin itself, which prevents essential, yet only partially understood, interactions with key cellular machineries. These considerations probably explain why cysteamine delays but does not prevent kidney failure even if taken compliantly from early stages of disease. Moreover, adherence to conventional immediate-release cysteamine therapy is cumbersome. Pills must be taken every 6 h, day and night, and cause body odour, halitosis and severe gastrointestinal adverse effects9,28, which hinder treatment compliance. Using enteric-release technology, a delayed and prolonged release formulation of cysteamine158–161 was approved by the FDA in 2013 (REF. 162). Although this new formulation needs to be taken only every 12 h, thus improving the quality-of-life of affected patients163,164, the impact on the disease is expected to be similar, in delaying but not arresting the complications. Topical cysteamine hydrochloride eye drops are highly effective in reducing the density of corneal crystals and alleviating the associated symptoms7,165. Treatment of the Fanconi syndrome requires careful replacement of electrolytes and vitamins. Indomethacin can reduce polyuria in patients with cystinosis, but was not adopted by consensus guidelines owing to toxicity issues9,28. Taken together, the burden of cystinosis and current approaches to its management is heavy. The combination of cysteamine therapy and multi-systemic supportive treatment requires patients to take up to 60 pills per day; young children often require a gastric tube to tolerate the medications and achieve the necessary caloric intake, and patients who develop renal failure require dialysis or transplantation. Thus any novel treatment or improvement to the current treatment regimen is eagerly awaited.
Figure 6. Lysosomal membrane transport and luminal events associated with cystinosis and cysteamine therapy.
Under normal conditions (black pathways (left)), vesicular transfer into lysosomes of disulfide-bearing proteins, such as albumin (1), which is avidly taken up by receptor-mediated endocytosis into proximal tubule cells, combined with lysosomal import of cysteine by a still unknown mechanism (2)196 leads to the reduction of disulfide bonds, resulting in protein unfolding and release of free cystine (3)197,198. Cystine is rapidly exported into the cytosol by the cystine proton symporter, cystinosin (4). Protein unfolding unmasks peptide bonds buried in globular proteins thus allows endoproteolytic attack by the concerted action of lysosomal cathepsins, such as the cysteine protease, cathepsin B (5)131. Free thiol in the catalytic site of cysteine proteinases is preserved by the reducing lysosomal environment. In the absence of cystinosin (orange pathways), cystine export across the lysosomal membrane is abolished. Cystine accumulation (6) inhibits the reduction of disulfide bonds in lysosomes, which thereby is expected to slow down further cystine generation (7) but also impairs protein unfolding and inactivates cysteine proteases (8), together leading to impairment of proteolysis (8). Cysteamine (β-mercaptoethylamine; purple pathways (right)) penetrates lysosomes by a distinct importer (9)199 and reacts with cystine to exchange its disulfide bridge into a mixed (cysteamine cysteine) disulfide (10), which exits via the cationic amino-acid exporter PQLC2 (11), and generates free cysteine, which must also exit lysosomes although this transporter is unknown (12). Question marks indicate that steps 7, 8 and 12 are still hypothetical.
New potential targets
The above-described mechanisms — involving energy depletion, oxidative stress, lysosomal enlargement, altered autophagy, lysosomal signalling and trafficking, and apoptosis — are most probably linked in the pathogenesis of the renal Fanconi syndrome in cystinosis166. Interventions that improve any step in these cascades might therefore alleviate the renal Fanconi syndrome. For example, preclinical and a limited clinical study of cysteine supplements or antioxidants indicated favourable outcomes104,114; however, larger studies with longer follow up are needed. Improved understanding of cellular dysfunctions induced by the absence of cystinosin has already identified other potential therapeutic targets. For example, small molecules that rescue CMA167 or reduce lysosomal overload by enhancing cystine excretion168 might lead to improved outcomes if they are used concomitantly with cysteamine129,134. Another approach is to enhance TFEB levels, as TFEB triggers lysosome biogenesis and promotes the clearance of accumulated protein aggregates169, in models of Huntington170 and Parkinson disease171. Induction of TFEB expression, nuclear translocation and activation of TFEB-dependent lysosome biogenesis by genistein172, has been reported to decrease lysosomal storage of glycosaminoglycans due to mucopolysaccharidosis in vitro173 and has been validated in a pilot clinical trial resulting in cognition improvement174. These findings suggest that genistein might be beneficial in other lysosomal storage diseases such as cystinosis. Indeed, activation of TFEB by genistein in cystinotic PTCs can decrease intracellular cystine levels and lysosome enlargement and rescue endocytosis defects145. Importantly, however, genistein has multiple effects, including activation of AMPK175, which as mentioned earlier is activated in primary rabbit PTCs with cystinosis knockdown101, and inhibition of mTOR signalling175, which is already reduced in cystinotic PTCs157. These varied actions call for great caution before the effects of genistein are assessed in patients with cystinosis.
PTC rescue by haematopoietic stem cells
A more radical and specific approach to those described above is the direct systemic replacement of cystinosin through administration of cystinosin-producing progenitor cells. This approach has been tested in cystinotic mice, using wild-type bone marrow-derived stem cells as a vehicle to target all tissues. Total bone marrow stem cells, or purified mesenchymal stem cells (MSCs) versus haematopoietic stem cells (HSCs), were transplanted into irradiated Ctns-knockout mice to repopulate their bone marrow with Ctns-carrying donor cells. MSCs offered only short-term benefits; however, transplantation of HSCs yielded long-term rescue of the cystinosis phenotype23,176,177. HSC transplantation led to stable engraftment of abundant bone marrow-derived cells in all organs, correlating with a dramatic reduction in cystine tissue levels (up to 94% clearance). Most importantly, this treatment led to a long-term improvement in kidney structure and function including the Fanconi syndrome, even though HSCs did not reprogramme into PTCs23. As expected, effective therapy depended on strong engraftment of Ctns-expressing HSCs (with >50% of blood cells being donor-derived). Interestingly, kidney protection was not dependent on transplantation at a young age and was still effective in 10-month old mice, suggesting the potential for established kidney dysfunction to be rescued by stem cell therapy. Whether the observed ‘rescue’ of the cystinosis phenotype actually reflects protection of previously ‘healthy tissue’ and/or reversal of organ injury is addressed in ongoing studies.
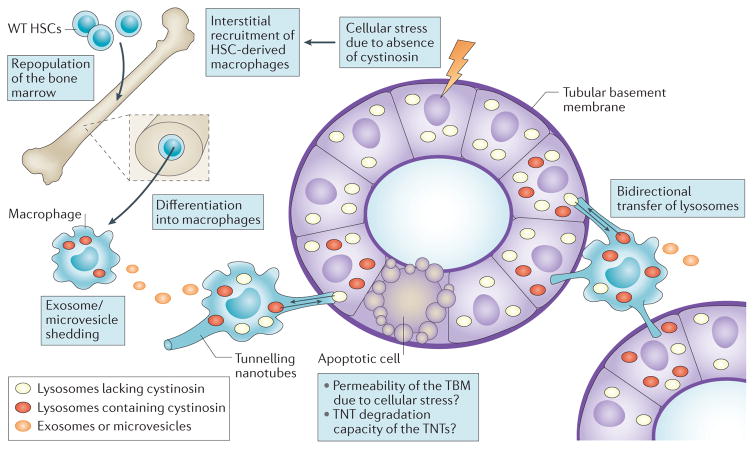
The ability of HSCs to rescue cystinosis in mice is remarkable since cystinosin is a transmembrane lysosomal protein13–15 as opposed to secreted, soluble lysosomal enzymes that can be readily recaptured by receptor-mediated endocytosis in diseased cells. Indeed, cross-correction, that is, the transfer of a functional protein from wild-type cells to deficient cells, has been well demonstrated in several lysosomal storage disorders caused by defective soluble hydrolases, after bone marrow transplantation or by recombinant enzyme infusion178. However, no cross-correction has been previously reported for lysosomal transmembrane proteins. A striking finding was the differentiation of tissue-engrafted HSCs into macrophages179. In addition to their phagocytic function, engrafted HSCs exerted paracrine effects that were responsible for effective cystine clearance179. Cystinosin-containing microvesicles and/or exosomes shed by cystinosin-expressing cells have been suggested as potential vehicles to decrease cystine in cystinotic cells79,180. However, co-culture assays of Ctns-deficient fibroblasts with wild-type MSCs or macrophages showed low correction efficiency by shed exosomes and/or microvesicles in fibroblasts (~20% clearance) contrasting with efficient cross-correction (~75% clearance) upon cell–cell contact179. Vital co-culture imaging further revealed that bone marrow-derived macrophages physically transfer cystinosin-bearing lysosomes into cystinosin-deficient cells via very long tubular extensions known as ‘tunnelling nanotubes’ (TNTs). These TNTs support long-distance intercellular communication, vesicular trafficking and pathogen spreading181–183. Amazingly, cystinosin-deficient cells exploit the same route in the opposite direction to transfer cystine-loaded lysosomes into macrophages, where they can fuse with competent lysosomes, thus providing bidirectional correction.
The mechanism of HSC-mediated renal repair in vivo has been debated184,185, in particular with regard to the route by which HSCs access PTCs across the normally continuous, thick, dense and stiff tubular basement membrane186,187. However, bone marrow-derived macrophages engrafted in cystinotic kidneys generate nanotubular extensions that readily cross the tubular basement membrane and deliver cystinosin into diseased PTCs179 (FIG. 7). This novel mechanism of genetic correction, which offers long-term kidney preservation after HSC transplantation in cystinosis, opens broad perspectives for the treatment of inherited renal disorders. Although the specific signals that trigger this effect remain to be identified, we hypothesize that inflammation is a key factor since recruitment of macrophages at the injury site is necessary for therapeutic rescue (FIG. 7). Similar protection has been demonstrated in the eyes and thyroid of Ctns-knockout mice, leading to near complete clearance of corneal cystine crystals and preservation of corneal structure188, as well as normalization of thyroid-stimulating hormone level and preservation of thyroid follicles, repectively189.
Figure 7. Proposed mechanisms by which haematopoietic stem cell (HSC) transplantation rescues cystinosis-induced Fanconi syndrome.
Injury to renal cells, including proximal tubular cells (PTCs) induced by loss of cystinosin triggers an inflammatory response, which probably aggravates the injury. Transplanted wild type (WT) cystinosin sufficient HSCs repopulate the bone marrow of Ctns knockout mice then migrate into injured tissues, where they differentiate into macrophages. Extracellular exosome or microvesicle-mediated transfer of cystinosin might correct the cystinosin deficiency of adjacent interstitial renal cells. However, rescue of PTCs, which are embedded in the tubular basement membrane, requires tunnelling nanotubes (TNTs), which extend from the macrophages across the tubular basement membrane, and are capable of bidirectional transfer of lysosomes, cystinosin-containing lysosomes (red vesicles) from wild-type HSC-derived macrophages into PTCs and cystine-loaded lysosomes (white vesicles) from Ctns deficient PTCs into macrophages. A single macrophage can generate multiple TNTs that have the ability to transfer lysosomes over long distances, which makes this route of cross-correction particularly efficient, likely accounting for the observed long-term correction of the Fanconi syndrome in Ctns-knockout mice.
Future directions
The importance of genetic context for the renal phenotype of cystinosis has long been acknowledged. In 2002, the first Ctns-knockout mouse was generated on a mixed C57BL6/129sv background18. Despite accumulation of cystine and crystal build-up in all tissues (including kidneys), no signs of renal Fanconi syndrome or renal dysfunction were observed. Subsequently generation of knockout mice on pure genetic backgrounds revealed that Ctns knockout on a C57/BL6J background led to proximal tubule dysfunction and progressive chronic renal failure, whereas Ctns knockout on a FVB background presented no renal anomaly despite some accumulation of cystine in the kidney21. These data point to the existence of modifier genes influencing the renal phenotype that could also account for the variability of disease severity in patients with identical mutations, such as patients who are homozygotes for the 57 kb deletion. Comparative genetic analyses of the two congenic Ctns-knockout mouse strains should enable the identification of such modifier genes and help unravel either the primary cause or aggravating as well as attenuating factors of the Fanconi syndrome in cystinosis. Another surprising finding was the existence of gender discrepancies in the cystine content of Ctns-knockout mice, with higher levels in females than males, specifically in the kidneys176, although no evidence exists of a gender predisposition in humans. Of note, kidney function was similar in males and female Ctns-knockout mice, which supports the current view that cystine accumulation cannot be the only factor that leads to renal defects in cystinosis. Proteins encoded by modifier genes might be involved in apical transcriptional regulation, vesicular trafficking, lysosomal signalling and autophagy, which are all impaired in cystinotic cells. Further studies of the cause of these defects might reveal the still elusive, additional cellular functions of cystinosin. In particular, the role(s) of the second PQ-loop in cystinosin, which in addition to lysosomal targeting carries a cystine–proton binding site and includes the interaction site with mTORC114,75,142, deserves further study. The challenge remains to define the mechanisms that ultimately explain the susceptibility of PTCs to cystinosin depletion and the early onset of the renal Fanconi syndrome, and to develop rational strategies for early prevention.
Rescue of PTCs by HSCs raises a new set of questions. First, whether crossing of the tubule basement membrane (TBM) by TNTs is restricted to pathologic states such as cystinosis or whether it is a so far unrecognized physiologic phenomenon that might support bidirectional exchange between PTCs and interstitial cell components during normal development or adult life. In this context, resident dendrite cells are known to closely surround tubules and to generate membrane extensions in the healthy kidney, suggesting a role as sensors of the microenvironment190. Second, whether TNTs are able to actively perforate the TBM, or simply exploit pre-existing local TBM defects due to alterations in cystinotic PTCs requires clarification. Third is whether our improved understanding of the mechanisms by which HSCs rescue cystinotic PTCs can be used to design novel treatment for other kidney disorders, in particular proximal tubulopathies.
Given the considerable risks of morbidity and mortality associated with allogeneic HSC transplantation, the current goal is to develop autologous transplantation of gene-corrected HSCs using a lentiviral vector containing CTNS for the treatment of cystinosis. Preclinical studies using such an approach have been completed in Ctns-knockout mice and showed evidence of efficacy176. Clinical trials using lentiviral vectors to transduce human HSCs have been widely undertaken in the USA and Europe, for several conditions including HIV infection, β-thalassemia, immunodeficiency diseases such as severe combined or adenosine deaminase immunodeficiency and metabolic diseases such as adrenoleukodystrophy and metachromatic leukodystrophy; no major adverse events have been reported so far, but extreme care remains necessary191–195.
Conclusions
Important new findings have improved our understanding of the renal Fanconi syndrome in cystinosis, and this entity is now understood to derive from several pathogenic mechanisms, including PTC dedifferentiation, impaired vesicular trafficking, defective mTOR signalling and autophagy. Further studies will be necessary in order to completely understand the mechanisms that lead to initiation of this syndrome. Recent insights have highlighted the fact that cystinosin is not only a lysosomal cystine transporter, but also carries other functions important for PTC homeostasis and vesicular transport. Only a combination of genomic, proteomic, and metabolomic approaches along with integrated analyses using histology, cell biology and biochemistry can help elucidate the underlying mechanisms of the renal Fanconi syndrome in cystinosis, potentially leading to new therapeutic approaches. Preclinical studies of HSC transplantation in a mouse model of cystinosis have yielded promising results, and translation of this approach into a clinical trial, which will examine to what extent this approach is also beneficial to patients, is underway.
Key points.
Cystinosis is a multi-systemic lysosomal storage disease caused by inactivating mutations in, or the absence of, the lysosomal membrane exporter for cystine, cystinosin; cystinosis is the main cause of hereditary renal Fanconi syndrome
Treatment with cysteamine efficiently depletes lysosomal cystine and delays progression to renal insufficiency; however, cysteamine does not reverse established renal Fanconi syndrome, indicating functions of cystinosin beyond cystine transport
Insights from mechanistic studies suggest that the pathological mechanisms of Fanconi syndrome in cystinosis are multifactorial, involving oxidative stress and impaired vesicular trafficking, autophagy, and mTORC1 and TFEB signalling
Haematopoietic stem cell (HSC) transplantation ameliorates renal Fanconi syndrome in cystinotic mice; HSCs differentiate into macrophages that transfer cystinosin-bearing lysosomes into proximal tubule cells via tunnelling nanotubes that cross the tubular basement membrane
Since tunnelling nanotubes contain donor-derived cytosol and carry all types of organelles, this mechanism should be generic and could be used to correct other genetic diseases that affect proximal tubule cells
Acknowledgments
S.C. and PJ.C. are funded by the Cystinosis Research Foundation, as well as NIH grants RO1-DK090058, R21-NS090066 and grant from the Sanford Stem Cell Clinical Center (to S.C.), and Belgian F.R.S./FNRS (to P.J.C.). We acknowledge Corinne Antignac, Imagine Institute, France, for her helpful comments while assembling this Review, Heloïse Gaide Chevronnay, CELL, de Duve Institute, Belgium, for her pivotal collaboration, and Patrick Van Der Smissen, CELL & PICT, de Duve Institute, Belgium, for kindly providing electron micrographs used in FIG. 2. We are also grateful to numerous colleagues who have provided so many insightful comments and contributive suggestions over the years.
Glossary
- Swan neck deformities
Tubular lesions that appear in microdissected nephrons as long atrophic tubules (the ‘swan necks’), appending below the glomeruli (the ‘swan heads’).
- Founder mutations
Mutations that appear in the DNA of one or more individuals who are founders of a distinct population.
- Km
Concentration of half-maximal rate. For a transporter, the Km value is inversely related to its affinity for its cargo.
- Half-cystine
In biochemical assays, cystine values are reported as half-molecules because cystine was originally measured in reducing conditions (as cysteine), and cysteine is half a molecule of cystine. Cystine is now directly measured as cystine using a mass spectrometer but the values are still reported as half-cystine per mg of protein.
- Lysosomal fusion
Fusion of acidified late endosomes carrying endocytic cargo with resting lysosomes bearing concentrated enzymes, or fusion between resting overloaded lysosomes (then also called interlysosomal fusion).
- Lysosomal fission
Vesicular budding from endolysosomes (to regenerate late endosomes and resting lysosomes), or division of overloaded resting lysosomes into smaller ones to randomize residual content by interlysosomal fusion.
- Residual body
A resting lysosome, filled with undigestible content, that no longer engages in vesicular trafficking.
- Lysosomal defecation
Active exocytosis of overloaded lysosomes. This lysosomal clearing activity is stimulated by transcription factor EB.
- Lysosomal amino acid sensors
Component or interacting partner of the mTOR-complex machinery that informs mTOR kinase on starvation or amino acid abundance.
- Low molecular weight proteinuria
A commonly used term to describe tubular proteinuria due to defective proximal tubular endocytosis of plasma proteins selectively ultrafiltrated according to their size and/or sieving coefficient (thus excluding IgGs and other high molecular weight (MW) proteins), as opposed to non-selective glomerular leak. This terminology is convenient, but might be misleading since pure tubular proteinuria typically includes relatively high MW proteins such as transferrin (80 kDa) and even larger globular proteins.
- Cross-correction
Transfer of functional protein from normal cells to deficient cells.
- Pathogen spreading
Transmission of bacteria, virus or parasites from one cell to another. Bacteria and virus such as HIV have been shown to colonize cells using the tunnelling nanotube route.
Footnotes
Author contributions
S.C. and P.J.C. contributed equally to researching data for the article, discussion of the content, and revising or editing the manuscript before submission.
Competing interests
The authors declare no competing interests.
DATABASES
MIM 219800: http://www.omim.org/entry/219800
MIM 219900: https://omim.org/entry/219900
MIM 219750: http://www.omim.org/entry/219750
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Fanconi G. Die nicht diabetischen glykosurien und hyperglykamiendes altem kindes. Jahrbuch Kinderheilkunde. 1931;133:257–300. [Google Scholar]
- 2.De Toni G. Remarks on the relations between renal rickets (renal dwarfism) and renal diabetes. Acta Paediatr. 1933;16:479–484. [Google Scholar]
- 3.Debre R, Marie J, Cleret J, Messimy R. Rachitisme tardif coexistant avec une nephrite chronique et une glycosurie. Arch Med Enfants. 1934;37:597–606. [Google Scholar]
- 4.Haffner D, et al. Long-term outcome of paediatric patients with hereditary tubular disorders. Nephron. 1999;83:250–260. doi: 10.1159/000045518. [DOI] [PubMed] [Google Scholar]
- 5.Gahl WA, Thoene JG, Schneider JA. Cystinosis. N Engl J Med. 2002;347:111–121. doi: 10.1056/NEJMra020552. [DOI] [PubMed] [Google Scholar]
- 6.Cohen C, et al. Excellent long-term outcome of renal transplantation in cystinosis patients. Orphanet J Rare Diseases. 2015;10:90. doi: 10.1186/s13023-015-0307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gahl WA, Kuehl EM, Iwata F, Lindblad A, Kaiser-Kupfer MI. Corneal crystals in nephropathic cystinosis: natural history and treatment with cysteamine eyedrops. Mol Genet Metab. 2000;71:100–120. doi: 10.1006/mgme.2000.3062. [DOI] [PubMed] [Google Scholar]
- 8.Brodin-Sartorius A, et al. Cysteamine therapy delays the progression of nephropathic cystinosis in late adolescents and adults. Kidney Int. 2012;81:179–189. doi: 10.1038/ki.2011.277. [DOI] [PubMed] [Google Scholar]
- 9.Langman CB, et al. Controversies and research agenda in nephropathic cystinosis: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2016;89:1192–1203. doi: 10.1016/j.kint.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 10.Nesterova G, Gahl W. Nephropathic cystinosis: late complications of a multisystemic disease. Pediatr Nephrol. 2008;23:863–878. doi: 10.1007/s00467-007-0650-8. [DOI] [PubMed] [Google Scholar]
- 11.Goldman H, Scriver CR, Aaron K, Delvin E, Canlas Z. Adolescent cystinosis: comparisons with infantile and adult forms. Pediatrics. 1971;47:979–988. [PubMed] [Google Scholar]
- 12.Cogan DG, Kuwabara T, Kinoshita J, Sheehan L, Merola L. Cystinosis in an adult. J Am Med Assoc. 1957;164:394–396. doi: 10.1001/jama.1957.02980040034009. [DOI] [PubMed] [Google Scholar]
- 13.Town M, et al. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet. 1998;18:319–324. doi: 10.1038/ng0498-319. [DOI] [PubMed] [Google Scholar]
- 14.Cherqui S, Kalatzis V, Trugnan G, Antignac C. The targeting of cystinosin to the lysosomal membrane requires a tyrosine-based signal and a novel sorting motif. J Biol Chem. 2001;276:13314–13321. doi: 10.1074/jbc.M010562200. [DOI] [PubMed] [Google Scholar]
- 15.Kalatzis V, Cherqui S, Antignac C, Gasnier B. Cystinosin, the protein defective in cystinosis, is a H(+)-driven lysosomal cystine transporter. EMBO J. 2001;20:5940–5949. doi: 10.1093/emboj/20.21.5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalatzis V, et al. The ocular anomalies in a cystinosis animal model mimic disease pathogenesis. Pediatr Res. 2007;62:156–162. doi: 10.1203/PDR.0b013e31809fda89. [DOI] [PubMed] [Google Scholar]
- 17.Simpson J, et al. Quantitative in vivo and ex vivo confocal microscopy analysis of corneal cystine crystals in the Ctns knockout mouse. Mol Vision. 2011;17:2212–2220. [PMC free article] [PubMed] [Google Scholar]
- 18.Cherqui S, et al. Intralysosomal cystine accumulation in mice lacking cystinosin, the protein defective in cystinosis. Mol Cell Biol. 2002;22:7622–7632. doi: 10.1128/MCB.22.21.7622-7632.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung WW, et al. Muscle wasting and adipose tissue browning in infantile nephropathic cystinosis. J Cachexia Sarcopenia Muscle. 2016;7:152–164. doi: 10.1002/jcsm.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaide Chevronnay HP, et al. A mouse model suggests two mechanisms for thyroid alterations in infantile cystinosis: decreased thyroglobulin synthesis due to endoplasmic reticulum stress/unfolded protein response and impaired lysosomal processing. Endocrinology. 2015;6:2349–2364. doi: 10.1210/en.2014-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nevo N, et al. Renal phenotype of the cystinosis mouse model is dependent upon genetic background. Nephrol Dial Transplant. 2010;25:1059–1066. doi: 10.1093/ndt/gfp553. [DOI] [PubMed] [Google Scholar]
- 22.Mahoney CP, Striker GE. Early development of the renal lesions in infantile cystinosis. Pediatr Nephrol. 2000;15:50–56. doi: 10.1007/pl00013448. [DOI] [PubMed] [Google Scholar]
- 23.Yeagy BA, et al. Kidney preservation by bone marrow cell transplantation in hereditary nephropathy. Kidney Int. 2011;79:1198–1206. doi: 10.1038/ki.2010.537. [DOI] [PubMed] [Google Scholar]
- 24.Schneider JA. Approval of cysteamine for patients with cystinosis. Pediatr Nephrol. 1995;9:254. doi: 10.1007/BF00860767. [DOI] [PubMed] [Google Scholar]
- 25.Thoene JG, Oshima RG, Crawhall JC, Olson DL, Schneider JA. Cystinosis. Intracellular cystine depletion by aminothiols in vitro and in vivo. J Clin Invest. 1976;58:180–189. doi: 10.1172/JCI108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cherqui S. Cysteamine therapy: a treatment for cystinosis, not a cure. Kidney Int. 2012;81:127–129. doi: 10.1038/ki.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gahl WA, Balog JZ, Kleta R. Nephropathic cystinosis in adults: natural history and effects of oral cysteamine therapy. Ann Internal Med. 2007;147:242–250. doi: 10.7326/0003-4819-147-4-200708210-00006. [DOI] [PubMed] [Google Scholar]
- 28.Emma F, et al. Nephropathic cystinosis: an international consensus document. Nephrol Dial Transplant. 2014;29(Suppl 4):87–94. doi: 10.1093/ndt/gfu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulman JD, Schneider JA. Cystinosis and the Fanconi syndrome. Pediatr Clin North Amer. 1976;23:779–793. doi: 10.1016/s0031-3955(16)33360-0. [DOI] [PubMed] [Google Scholar]
- 30.Ivanova EA, et al. Cystinosin deficiency causes podocyte damage and loss associated with increased cell motility. Kidney Int. 2016;89:1037–1048. doi: 10.1016/j.kint.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Wilmer MJ, Christensen EI, van den Heuvel LP, Monnens LA, Levtchenko EN. Urinary protein excretion pattern and renal expression of megalin and cubilin in nephropathic cystinosis. Am J Kidney Dis. 2008;51:893–903. doi: 10.1053/j.ajkd.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 32.North American Pediatric Renal Trials and Collaborative Studies. NAPRTCS Annual Reports. NAPRTCS Online [online] 2011 https://web.emmes.com/study/ped/annlrept/annlrept.html.
- 33.Dufier JL, Dhermy P, Gubler MC, Gagnadoux MF, Broyer M. Ocular changes in long-term evolution of infantile cystinosis. Ophthalm Paediatr Genet. 1987;8:131–137. doi: 10.3109/13816818709028529. [DOI] [PubMed] [Google Scholar]
- 34.Tsilou E, Zhou M, Gahl W, Sieving PC, Chan CC. Ophthalmic manifestations and histopathology of infantile nephropathic cystinosis: report of a case and review of the literature. Survey Ophthalmol. 2007;52:97–105. doi: 10.1016/j.survophthal.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gultekingil Keser A, Topaloglu R, Bilginer Y, Besbas N. Long-term endocrinologic complications of cystinosis. Minerva Pediatr. 2014;66:123–130. [PubMed] [Google Scholar]
- 36.Besouw MT, Holewijn S, Levtchenko EN, Janssen MC. Non-invasive measurements of atherosclerosis in adult cystinosis patients. J Inherited Metabol Dis. 2011;34:811–818. doi: 10.1007/s10545-011-9281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ueda M, et al. Coronary artery and other vascular calcifications in patients with cystinosis after kidney transplantation. Clin J Am Soc Nephrol. 2006;1:555–562. doi: 10.2215/CJN.01431005. [DOI] [PubMed] [Google Scholar]
- 38.Dixit MP, Greifer I. Nephropathic cystinosis associated with cardiomyopathy: a 27-year clinical follow-up. BMC Nephrol. 2002;3:8. doi: 10.1186/1471-2369-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broyer M, Guillot M, Gubler MC, Habib R. Infantile cystinosis: a reappraisal of early and late symptoms. Adv Nephrol Necker Hosp. 1981;10:137–166. [PubMed] [Google Scholar]
- 40.Klusmann M, Van’t Hoff W, Monsell F, Offiah AC. Progressive destructive bone changes in patients with cystinosis. Skeletal Radiol. doi: 10.1007/s00256-013-1735-z. http://dx.doi.org/10.1007/s00256-013-1735-z. [DOI] [PubMed]
- 41.Bacchetta J, et al. Skeletal implications and management of cystinosis: three case reports and literature review. Bonekey Rep. 2016;5:828. doi: 10.1038/bonekey.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ballantyne AO, Trauner DA. Neurobehavioral consequences of a genetic metabolic disorder: visual processing deficits in infantile nephropathic cystinosis. Cogn Behav Neurol. 2000;13:254–263. [PubMed] [Google Scholar]
- 43.Scarvie KM, Ballantyne AO, Trauner DA. Visuomotor performance in children with infantile nephropathic cystinosis. Percep Mot Skills. 1996;82:67–75. doi: 10.2466/pms.1996.82.1.67. [DOI] [PubMed] [Google Scholar]
- 44.Trauner DA, Chase C, Scheller J, Katz B, Schneider JA. Neurologic and cognitive deficits in children with cystinosis. J Pediatr. 1988;112:912–914. doi: 10.1016/s0022-3476(88)80214-2. [DOI] [PubMed] [Google Scholar]
- 45.Trauner DA, Spilkin AM, Williams J, Babchuck L. Specific cognitive deficits in young children with cystinosis: evidence for an early effect of the cystinosin gene on neural function. J Pediatr. 2007;151:192–196. doi: 10.1016/j.jpeds.2007.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trauner DA, et al. Neurological impairment in nephropathic cystinosis: motor coordination deficits. Pediatr Nephrol. 2010;25:2061–2066. doi: 10.1007/s00467-010-1589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Viltz L, Trauner DA. Effect of age at treatment on cognitive performance in patients with cystinosis. J Pediatr. 2013;163:489–492. doi: 10.1016/j.jpeds.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anikster Y, et al. Pulmonary dysfunction in adults with nephropathic cystinosis. Chest. 2001;119:394–401. doi: 10.1378/chest.119.2.394. [DOI] [PubMed] [Google Scholar]
- 49.Sonies BC, Almajid P, Kleta R, Bernardini I, Gahl WA. Swallowing dysfunction in 101 patients with nephropathic cystinosis: benefit of long-term cysteamine therapy. Medicine. 2005;84:137–146. doi: 10.1097/01.md.0000164204.00159.d4. [DOI] [PubMed] [Google Scholar]
- 50.Levy M, Feingold J. Estimating prevalence in single-gene kidney diseases progressing to renal failure. Kidney Int. 2000;58:925–943. doi: 10.1046/j.1523-1755.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- 51.Bois E, Feingold J, Frenay P, Briard ML. Infantile cystinosis in France: genetics, incidence, geographic distribution. J Med Genet. 1976;13:434–438. doi: 10.1136/jmg.13.6.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cochat P, Cordier B, Lacôte C, Saïd M-H. In: Cystinosis. Broyer M, editor. Elsevier; 1999. pp. 28–35. [Google Scholar]
- 53.DeBraekeleer M. Hereditary disorders in Saguenay-Lac-St-Jean (Quebec, Canada) Hum Hered. 1991;41:141–146. doi: 10.1159/000153992. [DOI] [PubMed] [Google Scholar]
- 54.Attard M, et al. Severity of phenotype in cystinosis varies with mutations in the CTNS gene: predicted effect on the model of cystinosin. Hum Mol Genet. 1999;8:2507–2514. doi: 10.1093/hmg/8.13.2507. [DOI] [PubMed] [Google Scholar]
- 55.Kalatzis V, et al. Characterization of a putative founder mutation that accounts for the high incidence of cystinosis in Brittany. J Am Soc Nephrol. 2001;12:2170–2174. doi: 10.1681/ASN.V12102170. [DOI] [PubMed] [Google Scholar]
- 56.McGowan-Jordan J, et al. Molecular Analysis of Cystinosis: Probable Irish Origin of the Most Common French Canadian Mutation. Eur J Hum Genet. 1999;7:671–678. doi: 10.1038/sj.ejhg.5200349. [DOI] [PubMed] [Google Scholar]
- 57.Anikster Y, et al. Identification and detection of the common 65-kb deletion breakpoint in the nephropathic cystinosis gene (CTNS) Mol Genet Metab. 1999;66:111–116. doi: 10.1006/mgme.1998.2790. [DOI] [PubMed] [Google Scholar]
- 58.Aldahmesh MA, et al. Characterization of CTNS mutations in Arab patients with cystinosis. Ophthalm Genet. 2009;30:185–189. doi: 10.3109/13816810903200953. [DOI] [PubMed] [Google Scholar]
- 59.Owen EP, et al. Common mutation causes cystinosis in the majority of black South African patients. Pediatr Nephrol. 2015;30:595–601. doi: 10.1007/s00467-014-2980-7. [DOI] [PubMed] [Google Scholar]
- 60.Shahkarami S, Galehdari H, Ahmadzadeh A, Babaahmadi M, Pedram M. The first molecular genetics analysis of individuals suffering from nephropatic cystinosis in the Southwestern Iran. Nefrologia. 2013;33:308–315. doi: 10.3265/Nefrologia.pre2012.Sep.11558. [DOI] [PubMed] [Google Scholar]
- 61.Soliman NA, et al. Mutational Spectrum of the CTNS Gene in Egyptian Patients with Nephropathic Cystinosis. JIMD reports. 2014;14:87–97. doi: 10.1007/8904_2013_288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aly R, Makar S, El Bakri A, Soliman NA. Neurocognitive functions and behavioral profiles in children with nephropathic cystinosis. Saudi J Kidney Dis Transpl. 2014;25:1224–1231. doi: 10.4103/1319-2442.144256. [DOI] [PubMed] [Google Scholar]
- 63.Elmonem MA, et al. Lysosomal Storage Disorders in Egyptian Children. Indian J Pediatr. 2016;8:805–813. doi: 10.1007/s12098-015-2014-x. [DOI] [PubMed] [Google Scholar]
- 64.Mirdehghan M, Ahmadzadeh A, Bana-Behbahani M, Motlagh I, Chomali B. Infantile cystinosis. Indian Pediatr. 2003;40:21–24. [PubMed] [Google Scholar]
- 65.Kir’ianov NA, Bazhenov EL, Stetsenko EV. Cystinosis in an adult. Arkh Patol. 1992;54:34–36. [PubMed] [Google Scholar]
- 66.Tang S, Danda S, Zoleikhaeian M, Simon M, Huang T. An Indian boy with nephropathic cystinosis: a case report and molecular analysis of CTNS mutation. Genet Test Mol Biomarkers. 2009;13:435–438. doi: 10.1089/gtmb.2008.0156. [DOI] [PubMed] [Google Scholar]
- 67.Yang YJ, et al. First report of CTNS mutations in a Chinese family with infantile cystinosis. ScientificWorldJournal. 2015;2015:309410. doi: 10.1155/2015/309410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.The Cystinosis Collaborative Research Group. Linkage of the gene for cystinosis to markers on the short arm of chromosome 17. Nat Genet. 1995;10:246–248. doi: 10.1038/ng0695-246. [DOI] [PubMed] [Google Scholar]
- 69.Taranta A, et al. Identification and subcellular localization of a new cystinosin isoform. Am J Physiol Renal Physiol. 2008;294:F1101–1108. doi: 10.1152/ajprenal.00413.2007. [DOI] [PubMed] [Google Scholar]
- 70.Bellomo F, et al. Carboxyl-Terminal SSLKG Motif of the Human Cystinosin-LKG Plays an Important Role in Plasma Membrane Sorting. PLoS ONE. 2016;11:e0154805. doi: 10.1371/journal.pone.0154805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taranta A, et al. Distribution of cystinosin-LKG in human tissues. Histochem Cell Biol. 2012;138:351–363. doi: 10.1007/s00418-012-0958-8. [DOI] [PubMed] [Google Scholar]
- 72.Andrzejewska Z, et al. Lysosomal Targeting of Cystinosin Requires AP-3. Traffic. 2015;16:712–726. doi: 10.1111/tra.12277. [DOI] [PubMed] [Google Scholar]
- 73.Kyttala A, Ihrke G, Vesa J, Schell MJ, Luzio JP. Two motifs target Batten disease protein CLN3 to lysosomes in transfected nonneuronal and neuronal cells. Mol Biol Cell. 2004;15:1313–1323. doi: 10.1091/mbc.E03-02-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saudek V. Cystinosin, MPDU1, SWEETs and KDELR belong to a well-defined protein family with putative function of cargo receptors involved in vesicle trafficking. PLoS ONE. 2012;7:e30876. doi: 10.1371/journal.pone.0030876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ruivo R, et al. Mechanism of proton/substrate coupling in the heptahelical lysosomal transporter cystinosin. Proc Natl Acad Sci USA. 2012;109:E210–217. doi: 10.1073/pnas.1115581109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee Y, Nishizawa T, Yamashita K, Ishitani R, Nureki O. Structural basis for the facilitative diffusion mechanism by SemiSWEET transporter. Nat Commun. 2015;6:6112. doi: 10.1038/ncomms7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jezegou A, et al. Heptahelical protein PQLC2 is a lysosomal cationic amino acid exporter underlying the action of cysteamine in cystinosis therapy. Proc Natl Acad Sci USA. 2012;109:E3434–3443. doi: 10.1073/pnas.1211198109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thamotharan M, Lombardo YB, Bawani SZ, Adibi SA. An active mechanism for completion of the final stage of protein degradation in the liver, lysosomal transport of dipeptides. J Biol Chem. 1997;272:11786–11790. doi: 10.1074/jbc.272.18.11786. [DOI] [PubMed] [Google Scholar]
- 79.Thoene J, et al. In vitro correction of disorders of lysosomal transport by microvesicles derived from baculovirus-infected Spodoptera cells. Mol Genet Metab. 2013;109:77–85. doi: 10.1016/j.ymgme.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 80.Leinekugel P, Michel S, Conzelmann E, Sandhoff K. Quantitative Correlation between the Residual Activity of Beta-Hexosaminidase-a and Arylsulfatase-a and the Severity of the Resulting Lysosomal Storage Disease. Hum Genet. 1992;88:513–523. doi: 10.1007/BF00219337. [DOI] [PubMed] [Google Scholar]
- 81.Kalatzis V, Nevo N, Cherqui S, Gasnier B, Antignac C. Molecular pathogenesis of cystinosis: effect of CTNS mutations on the transport activity and subcellular localization of cystinosin. Hum Mol Genet. 2004;13:1361–1371. doi: 10.1093/hmg/ddh152. [DOI] [PubMed] [Google Scholar]
- 82.Thoene JG, Lemons RM. Cystine accumulation in cystinotic fibroblasts from free and protein-linked cystine but not cysteine. Biochem J. 1982;208:823–830. doi: 10.1042/bj2080823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thoene JG, Lemons R. Modulation of the intracellular cystine content of cystinotic fibroblasts by extracellular albumin. Pediatr Res. 1980;14:785–787. doi: 10.1203/00006450-198006000-00001. [DOI] [PubMed] [Google Scholar]
- 84.Thoene JG, Oshima RG, Ritchie DG, Schneider JA. Cystinotic fibroblasts accumulate cystine from intracellular protein degradation. Proc Natl Acad Sci USA. 1977;74:4505–4507. doi: 10.1073/pnas.74.10.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amsellem S, et al. Cubilin is essential for albumin reabsorption in the renal proximal tubule. J Am Soc Nephrol. 2010;21:1859–1867. doi: 10.1681/ASN.2010050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ovunc B, et al. Exome sequencing reveals cubilin mutation as a single-gene cause of proteinuria. J Am Soc Nephrol. 2011;22:1815–1820. doi: 10.1681/ASN.2011040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oude Elferink RP, Harms E, Strijland A, Tager JM. The intralysosomal pH in cultured human skin fibroblasts in relation to cystine accumulation in patients with cystinosis. Biochem Biophys Res Commun. 1983;116:154–161. doi: 10.1016/0006-291x(83)90394-7. [DOI] [PubMed] [Google Scholar]
- 88.Elmonem MA, et al. Cystinosis: a review. Orphanet J Rare Diseases. 2016;11:47. doi: 10.1186/s13023-016-0426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Futter CE, Pearse A, Hewlett LJ, Hopkins CR. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol. 1996;132:1011–1023. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Montgomery RR, Webster P, Mellman I. Accumulation of indigestible substances reduces fusion competence of macrophage lysosomes. J Immunol. 1991;147:3087–3095. [PubMed] [Google Scholar]
- 91.Gaide Chevronnay HP, et al. Time course of pathogenic and adaptation mechanisms in cystinotic mouse kidneys. J Am Soc Nephrol. 2014;25:1256–1269. doi: 10.1681/ASN.2013060598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gubler MC, Lacoste M, Sich M, Broyer M. In: Cystinosis. Broyer M, editor. Vol. 1. Elsevier; 1999. pp. 42–48. [Google Scholar]
- 93.Prencipe G, et al. Inflammasome activation by cystine crystals: implications for the pathogenesis of cystinosis. J Am Soc Nephrol. 2014;25:1163–1169. doi: 10.1681/ASN.2013060653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baum M. The Fanconi syndrome of cystinosis: insights into the pathophysiology. Pediatr Nephrol. 1998;12:492–497. doi: 10.1007/s004670050495. [DOI] [PubMed] [Google Scholar]
- 95.Coor C, Salmon RF, Quigley R, Marver D, Baum M. Role of adenosine triphosphate (ATP) and NaK ATPase in the inhibition of proximal tubule transport with intracellular cystine loading. J Clin Invest. 1991;87:955–961. doi: 10.1172/JCI115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Foreman JW, et al. Metabolic studies of rat renal tubule cells loaded with cystine: the cystine dimethylester model of cystinosis. J Am Soc Nephrol. 1995;6:269–272. doi: 10.1681/ASN.V62269. [DOI] [PubMed] [Google Scholar]
- 97.Wilmer MJ, van den Heuvel LP, Levtchenko EN. The use of CDME in cystinosis research. Neurochem Res. 2008;33:2373–2374. doi: 10.1007/s11064-008-9709-6. [DOI] [PubMed] [Google Scholar]
- 98.Levtchenko EN, et al. Decreased intracellular ATP content and intact mitochondrial energy generating capacity in human cystinotic fibroblasts. Pediatr Res. 2006;59:287–292. doi: 10.1203/01.pdr.0000196334.46940.54. [DOI] [PubMed] [Google Scholar]
- 99.Wilmer MJ, et al. Mitochondrial complex V expression and activity in cystinotic fibroblasts. Pediatr Res. 2008;64:495–497. doi: 10.1203/PDR.0b013e318183fd67. [DOI] [PubMed] [Google Scholar]
- 100.Taub ML, Springate JE, Cutuli F. Reduced phosphate transport in the renal proximal tubule cells in cystinosis is due to decreased expression of transporters rather than an energy defect. Biochem Biophys Res Commun. 2011;407:355–359. doi: 10.1016/j.bbrc.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 101.Taub M, Cutuli F. Activation of AMP kinase plays a role in the increased apoptosis in the renal proximal tubule in cystinosis. Biochem Biophys Res Commun. 2012;426:516–521. doi: 10.1016/j.bbrc.2012.08.115. [DOI] [PubMed] [Google Scholar]
- 102.Park M, Helip-Wooley A, Thoene J. Lysosomal cystine storage augments apoptosis in cultured human fibroblasts and renal tubular epithelial cells. J Am Soc Nephrol. 2002;13:2878–2887. doi: 10.1097/01.asn.0000036867.49866.59. [DOI] [PubMed] [Google Scholar]
- 103.Park MA, Pejovic V, Kerisit KG, Junius S, Thoene JG. Increased apoptosis in cystinotic fibroblasts and renal proximal tubule epithelial cells results from cysteinylation of protein kinase Cdelta. J Am Soc Nephrol. 2006;17:3167–3175. doi: 10.1681/ASN.2006050474. [DOI] [PubMed] [Google Scholar]
- 104.Galarreta CI, et al. The swan-neck lesion: proximal tubular adaptation to oxidative stress in nephropathic cystinosis. Am J Physiol Renal Physiol. 2015;308:F1155–1166. doi: 10.1152/ajprenal.00591.2014. [DOI] [PubMed] [Google Scholar]
- 105.Sansanwal P, Kambham N, Sarwal MM. Caspase-4 may play a role in loss of proximal tubules and renal injury in nephropathic cystinosis. Pediatr Nephrol. 2010;25:105–109. doi: 10.1007/s00467-009-1289-4. [DOI] [PubMed] [Google Scholar]
- 106.Chol M, Nevo N, Cherqui S, Antignac C, Rustin P. Glutathione precursors replenish decreased glutathione pool in cystinotic cell lines. Biochem Biophys Res Commun. 2004;324:231–235. doi: 10.1016/j.bbrc.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 107.Levtchenko E, et al. Altered status of glutathione and its metabolites in cystinotic cells. Nephrol Dial Transplant. 2005;20:1828–1832. doi: 10.1093/ndt/gfh932. [DOI] [PubMed] [Google Scholar]
- 108.Wilmer MJ, et al. Elevated oxidized glutathione in cystinotic proximal tubular epithelial cells. Biochem Biophys Res Commun. 2005;337:610–614. doi: 10.1016/j.bbrc.2005.09.094. [DOI] [PubMed] [Google Scholar]
- 109.Mannucci L, et al. Impaired activity of the gamma-glutamyl cycle in nephropathic cystinosis fibroblasts. Pediatr Res. 2006;59:332–335. doi: 10.1203/01.pdr.0000196370.57200.da. [DOI] [PubMed] [Google Scholar]
- 110.Rizzo C, et al. Pyroglutamic aciduria and nephropathic cystinosis. J Inherited Metabol Dis. 1999;22:224–226. doi: 10.1023/a:1005545012776. [DOI] [PubMed] [Google Scholar]
- 111.Laube GF, et al. Glutathione depletion and increased apoptosis rate in human cystinotic proximal tubular cells. Pediatr Nephrol. 2006;21:503–509. doi: 10.1007/s00467-006-0005-x. [DOI] [PubMed] [Google Scholar]
- 112.Sakarcan A, Timmons C, Baum M. Intracellular distribution of cystine in cystine-loaded proximal tubules. Pediatr Res. 1994;35:447–450. [PubMed] [Google Scholar]
- 113.Spear GS, Slusser RJ, Tousimis AJ, Taylor CG, Schulman JD. Cystinosis. An ultrastructural and electron-probe study of the kidney with unusual findings. Arch Pathol. 1971;91:206–221. [PubMed] [Google Scholar]
- 114.Pache de Faria Guimaraes L, et al. N-Acetyl-cysteine is associated to renal function improvement in patients with nephropathic cystinosis. Pediatr Nephrol. 2014;29:1097–1102. doi: 10.1007/s00467-013-2705-3. [DOI] [PubMed] [Google Scholar]
- 115.Moldeus P, Ormstad K, Reed DJ. Turnover of cellular glutathione in isolated rat-kidney cells. Role of cystine and methionine. Eur J Biochem. 1981;116:13–16. doi: 10.1111/j.1432-1033.1981.tb05294.x. [DOI] [PubMed] [Google Scholar]
- 116.Meister A, Anderson ME, Hwang O. Intracellular cysteine and glutathione delivery systems. J Am College Nutr. 1986;5:137–151. doi: 10.1080/07315724.1986.10720121. [DOI] [PubMed] [Google Scholar]
- 117.Frey IM, et al. Profiling at mRNA, protein, and metabolite levels reveals alterations in renal amino acid handling and glutathione metabolism in kidney tissue of Pept2−/− mice. Physiol Genom. 2007;28:301–310. doi: 10.1152/physiolgenomics.00193.2006. [DOI] [PubMed] [Google Scholar]
- 118.Ahmed K, Dasgupta P, Khan MS. Cystine calculi: challenging group of stones. Postgrad Med J. 2006;82:799–801. doi: 10.1136/pgmj.2005.044156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kessler A, et al. Antioxidant effect of cysteamine in brain cortex of young rats. Neurochem Res. 2008;33:737–744. doi: 10.1007/s11064-007-9486-7. [DOI] [PubMed] [Google Scholar]
- 120.Okamura DM, et al. Cysteamine modulates oxidative stress and blocks myofibroblast activity in CKD. J Am Soc Nephrol. 2014;25:43–54. doi: 10.1681/ASN.2012090962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wilmer MJ, et al. Cysteamine restores glutathione redox status in cultured cystinotic proximal tubular epithelial cells. Biochim Biophys Acta. 2011;1812:643–651. doi: 10.1016/j.bbadis.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 122.Raggi C, et al. Dedifferentiation and aberrations of the endolysosomal compartment characterize the early stage of nephropathic cystinosis. Hum Mol Genet. 2014;23:2266–2278. doi: 10.1093/hmg/ddt617. [DOI] [PubMed] [Google Scholar]
- 123.Ivanova EA, et al. Endo-lysosomal dysfunction in human proximal tubular epithelial cells deficient for lysosomal cystine transporter cystinosin. PLoS ONE. 2015;10:e0120998. doi: 10.1371/journal.pone.0120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Christensen EI, et al. Loss of chloride channel ClC-5 impairs endocytosis by defective trafficking of megalin and cubilin in kidney proximal tubules. Proc Natl Acad Sci USA. 2003;100:8472–8477. doi: 10.1073/pnas.1432873100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vicinanza M, et al. OCRL controls trafficking through early endosomes via PtdIns4,5P(2)-dependent regulation of endosomal actin. EMBO J. 2011;30:4970–4985. doi: 10.1038/emboj.2011.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bandyopadhyay D, Cyphersmith A, Zapata JA, Kim YJ, Payne CK. Lysosome transport as a function of lysosome diameter. PLoS ONE. 2014;9:e86847. doi: 10.1371/journal.pone.0086847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li X, et al. A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat Cell Biol. 2016;18:404–417. doi: 10.1038/ncb3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ivanova EA, et al. Ca(2+) signalling in human proximal tubular epithelial cells deficient for cystinosin. Cell Calcium. 2016;60:282–287. doi: 10.1016/j.ceca.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 129.Johnson JL, et al. Upregulation of the Rab27a-dependent trafficking and secretory mechanisms improves lysosomal transport, alleviates endoplasmic reticulum stress, and reduces lysosome overload in cystinosis. Mol Cell Biol. 2013;33:2950–2962. doi: 10.1128/MCB.00417-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schulman JD, Bradley KH, Seegmiller JE. Cystine: compartmentalization within lysosomes in cystinotic leukocytes. Science. 1969;166:1152–1154. doi: 10.1126/science.166.3909.1152. [DOI] [PubMed] [Google Scholar]
- 131.Kooistra T, Millard PC, Lloyd JB. Role of thiols in degradation of proteins by cathepsins. Biochem J. 1982;204:471–477. doi: 10.1042/bj2040471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Efeyan A, Zoncu R, Sabatini DM. Amino acids and mTORC1: from lysosomes to disease. Trends Mol Med. 2012;18:524–533. doi: 10.1016/j.molmed.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Settembre C, Fraldi A, Medina DL, Ballabio A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol. 2013;14:283–296. doi: 10.1038/nrm3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Napolitano G, et al. Impairment of chaperone-mediated autophagy leads to selective lysosomal degradation defects in the lysosomal storage disease cystinosis. EMBO Mol Med. 2015;2:158–174. doi: 10.15252/emmm.201404223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sansanwal P, et al. Mitochondrial autophagy promotes cellular injury in nephropathic cystinosis. J Am Soc Nephrol. 2010;21:272–283. doi: 10.1681/ASN.2009040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fantus D, Rogers NM, Grahammer F, Huber TB, Thomson AW. Roles of mTOR complexes in the kidney: implications for renal disease and transplantation. Nat Rev Nephrol. 2016;12:587–609. doi: 10.1038/nrneph.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell. 2012;150:1196–1208. doi: 10.1016/j.cell.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sancak Y, et al. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci. 2013;126:1713–1719. doi: 10.1242/jcs.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Martina JA, Puertollano R. RRAG GTPases link nutrient availability to gene expression, autophagy and lysosomal biogenesis. Autophagy. 2013;9:928–930. doi: 10.4161/auto.24371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zoncu R, et al. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334:678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Andrzejewska Z, et al. Cystinosin is a Component of the Vacuolar H+-ATPase-Ragulator-Rag Complex Controlling Mammalian Target of Rapamycin Complex 1 Signaling. J Am Soc Nephrol. 2016;27:1678–1688. doi: 10.1681/ASN.2014090937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ivanova EA, et al. Altered mTOR signalling in nephropathic cystinosis. J Inherited Metabol Dis. 2016;39:457–464. doi: 10.1007/s10545-016-9919-z. [DOI] [PubMed] [Google Scholar]
- 144.Gleixner EM, et al. V-ATPase/mTOR signaling regulates megalin-mediated apical endocytosis. Cell Rep. 2014;8:10–19. doi: 10.1016/j.celrep.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 145.Rega LR, et al. Activation of the transcription factor EB rescues lysosomal abnormalities in cystinotic kidney cells. Kidney Int. 2016;89:862–873. doi: 10.1016/j.kint.2015.12.045. [DOI] [PubMed] [Google Scholar]
- 146.Platt FM, Boland B, van der Spoel AC. The cell biology of disease: lysosomal storage disorders: the cellular impact of lysosomal dysfunction. J Cell Biol. 2012;199:723–734. doi: 10.1083/jcb.201208152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kaushik S, Cuervo AM. Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol. 2012;22:407–417. doi: 10.1016/j.tcb.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kiffin R, Christian C, Knecht E, Cuervo AM. Activation of chaperone-mediated autophagy during oxidative stress. Mol Biol Cell. 2004;15:4829–4840. doi: 10.1091/mbc.E04-06-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Massey AC, Kaushik S, Sovak G, Kiffin R, Cuervo AM. Consequences of the selective blockage of chaperone-mediated autophagy. Proc Natl Acad Sci USA. 2006;103:5805–5810. doi: 10.1073/pnas.0507436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Balmer C, et al. Familial X-linked cardiomyopathy (Danon disease): diagnostic confirmation by mutation analysis of the LAMP2gene. Eur J Pediatr. 2005;164:509–514. doi: 10.1007/s00431-005-1678-z. [DOI] [PubMed] [Google Scholar]
- 151.Fanin M, et al. Generalized lysosome-associated membrane protein-2 defect explains multisystem clinical involvement and allows leukocyte diagnostic screening in Danon disease. Am J Pathol. 2006;168:1309–1320. doi: 10.2353/ajpath.2006.050646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Horvath J, et al. Identification of a novel LAMP2 mutation responsible for X-chromosomal dominant Danon disease. Neuropediatrics. 2003;34:270–273. doi: 10.1055/s-2003-43262. [DOI] [PubMed] [Google Scholar]
- 153.Lima WR, et al. ZONAB promotes proliferation and represses differentiation of proximal tubule epithelial cells. J Am Soc Nephrol. 2010;21:478–488. doi: 10.1681/ASN.2009070698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Janssens V, et al. Fifth International Cystinosis Research Symposium. Univ. of California; 2016. [Google Scholar]
- 155.Chevalier RL. The proximal tubule in cystinosis: fight or flight? J Am Soc Nephrol. 2014;25:1131–1132. doi: 10.1681/ASN.2014010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chevalier RL. The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am J Physiol Renal Physiol. 2016;311:F145–161. doi: 10.1152/ajprenal.00164.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Andrzejewska Z, et al. Cystinosin is a Component of the Vacuolar H+-ATPase-Ragulator-Rag Complex Controlling Mammalian Target of Rapamycin Complex 1 Signaling. J Am Soc Nephrol. 2015;6:1678–1688. doi: 10.1681/ASN.2014090937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dohil R, et al. Long-term treatment of cystinosis in children with twice-daily cysteamine. J Pediatr. 2010;156:823–827. doi: 10.1016/j.jpeds.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 159.Gangoiti JA, et al. Pharmacokinetics of enteric-coated cysteamine bitartrate in healthy adults: a pilot study. Br J Clin Pharmacol. 2010;70:376–382. doi: 10.1111/j.1365-2125.2010.03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dohil R, Cabrera BL. Treatment of cystinosis with delayed-release cysteamine: 6-year follow-up. Pediatr Nephrol. 2013;28:507–510. doi: 10.1007/s00467-012-2315-5. [DOI] [PubMed] [Google Scholar]
- 161.Dohil R, et al. Twice-daily cysteamine bitartrate therapy for children with cystinosis. J Pediatr. 2010;156:71–73. doi: 10.1016/j.jpeds.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 162.Goldenberg MM. Pharmaceutical approval update. PT. 2013;38:323–324. [PMC free article] [PubMed] [Google Scholar]
- 163.Langman CB, et al. Quality of life is improved and kidney function preserved in patients with nephropathic cystinosis treated for 2 years with delayed-release cysteamine bitartrate. J Pediatr. 2014;165:528–533. doi: 10.1016/j.jpeds.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Langman CB, et al. A randomized controlled crossover trial with delayed-release cysteamine bitartrate in nephropathic cystinosis: effectiveness on white blood cell cystine levels and comparison of safety. Clin J Am Soc Nephrol. 2012;7:1112–1120. doi: 10.2215/CJN.12321211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kaiser-Kupfer MI, et al. A randomized placebo-controlled trial of cysteamine eye drops in nephropathic cystinosis. Arch Ophthalmol. 1990;108:689–693. doi: 10.1001/archopht.1990.01070070075038. [DOI] [PubMed] [Google Scholar]
- 166.Kumar A, Bachhawat AK. A futile cycle, formed between two ATP-dependant gamma-glutamyl cycle enzymes, gamma-glutamyl cysteine synthetase and 5-oxoprolinase: the cause of cellular ATP depletion in nephrotic cystinosis? J Biosci. 2010;35:21–25. doi: 10.1007/s12038-010-0004-8. [DOI] [PubMed] [Google Scholar]
- 167.Anguiano J, et al. Chemical modulation of chaperone-mediated autophagy by retinoic acid derivatives. Nat Chem Biol. 2013;9:374–382. doi: 10.1038/nchembio.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.McNeill A, et al. Ambroxol improves lysosomal biochemistry in glucocerebrosidase mutation-linked Parkinson disease cells. Brain. 2014;137:1481–1495. doi: 10.1093/brain/awu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Medina DL, et al. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev Cell. 2011;21:421–430. doi: 10.1016/j.devcel.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sardiello M, et al. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 171.Dehay B, et al. Pathogenic lysosomal depletion in Parkinson’s disease. J Neurosci. 2010;30:12535–12544. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Moskot M, et al. The phytoestrogen genistein modulates lysosomal metabolism and transcription factor EB (TFEB) activation. J Biol Chem. 2014;289:17054–17069. doi: 10.1074/jbc.M114.555300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Piotrowska E, et al. Genistein-mediated inhibition of glycosaminoglycan synthesis as a basis for gene expression-targeted isoflavone therapy for mucopolysaccharidoses. Eur J Hum Genet. 2006;14:846–852. doi: 10.1038/sj.ejhg.5201623. [DOI] [PubMed] [Google Scholar]
- 174.Piotrowska E, et al. Genistin-rich soy isoflavone extract in substrate reduction therapy for Sanfilippo syndrome: An open-label, pilot study in 10 pediatric patients. Curr Ther Res Clin Exp. 2008;69:166–179. doi: 10.1016/j.curtheres.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lee KY, Kim JR, Choi HC. Genistein-induced LKB1-AMPK activation inhibits senescence of VSMC through autophagy induction. Vascul Pharmacol. 2016;81:75–82. doi: 10.1016/j.vph.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 176.Harrison F, et al. Hematopoietic stem cell gene therapy for the multisystemic lysosomal storage disorder cystinosis. Mol Ther. 2013;21:433–444. doi: 10.1038/mt.2012.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Syres K, et al. Successful treatment of the murine model of cystinosis using bone marrow cell transplantation. Blood. 2009;114:2542–2552. doi: 10.1182/blood-2009-03-213934. [DOI] [PubMed] [Google Scholar]
- 178.Beck M. Therapy for lysosomal storage disorders. IUBMB Life. 2010;62:33–40. doi: 10.1002/iub.284. [DOI] [PubMed] [Google Scholar]
- 179.Naphade S, et al. Brief reports: lysosomal cross-correction by hematopoietic stem cell-derived macrophages via tunneling nanotubes. Stem Cells. 2015;33:301–309. doi: 10.1002/stem.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Iglesias DM, et al. Stem cell microvesicles transfer cystinosin to human cystinotic cells and reduce cystine accumulation in vitro. PLoS ONE. 2012;7:e42840. doi: 10.1371/journal.pone.0042840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Onfelt B, et al. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol. 2006;177:8476–8483. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- 182.Marzo L, Gousset K, Zurzolo C. Multifaceted roles of tunneling nanotubes in intercellular communication. Frontiers Physiol. 2012;3:72. doi: 10.3389/fphys.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sowinski S, Alakoskela JM, Jolly C, Davis DM. Optimized methods for imaging membrane nanotubes between T cells and trafficking of HIV-1. Methods. 2011;53:27–33. doi: 10.1016/j.ymeth.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 184.Humphreys BD. Kidney injury, stem cells and regeneration. Curr Opin Nephrol Hypertens. 2014;23:25–31. doi: 10.1097/01.mnh.0000437332.31418.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yeagy BA, Cherqui S. Kidney repair and stem cells: a complex and controversial process. Pediatr Nephrol. 2011;26:1427–1434. doi: 10.1007/s00467-011-1789-x. [DOI] [PubMed] [Google Scholar]
- 186.Abrahamson DR, Leardkamolkarn V. Development of kidney tubular basement membranes. Kidney Int. 1991;39:382–393. doi: 10.1038/ki.1991.50. [DOI] [PubMed] [Google Scholar]
- 187.Halfter W, et al. Protein composition and biomechanical properties of in vivo-derived basement membranes. Cell Adhesion Migr. 2013;7:64–71. doi: 10.4161/cam.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rocca CJ, et al. Treatment of Inherited Eye Defects by Systemic Hematopoietic Stem Cell Transplantation. Investigative Ophthalmol Visual Sci. 2015;56:7214–7223. doi: 10.1167/iovs.15-17107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gaide Chevronnay HP, et al. Hematopoietic stem cell transplantation can normalize thyroid function in a cystinosis mouse model. Endocrinology. 2016;56:1363–1371. doi: 10.1210/en.2015-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Soos TJ, et al. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int. 2006;70:591–596. doi: 10.1038/sj.ki.5001567. [DOI] [PubMed] [Google Scholar]
- 191.Biffi A, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 192.Cartier N, et al. Lentiviral hematopoietic cell gene therapy for X-linked adrenoleukodystrophy. Methods Enzymol. 2012;507:187–198. doi: 10.1016/B978-0-12-386509-0.00010-7. [DOI] [PubMed] [Google Scholar]
- 193.DiGiusto DL, et al. Development of hematopoietic stem cell based gene therapy for HIV-1 infection: considerations for proof of concept studies and translation to standard medical practice. Viruses. 2013;5:2898–2919. doi: 10.3390/v5112898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Drakopoulou E, Papanikolaou E, Georgomanoli M, Anagnou NP. Towards more successful gene therapy clinical trials for beta-thalassemia. Curr Mol Med. 2013;13:1314–1330. doi: 10.2174/15665240113139990064. [DOI] [PubMed] [Google Scholar]
- 195.Zhang L, Thrasher AJ, Gaspar HB. Current progress on gene therapy for primary immunodeficiencies. Gene Ther. 2013;20:963–969. doi: 10.1038/gt.2013.21. [DOI] [PubMed] [Google Scholar]
- 196.Pisoni RL, Acker TL, Lisowski KM, Lemons RM, Thoene JG. A cysteine-specific lysosomal transport system provides a major route for the delivery of thiol to human fibroblast lysosomes: possible role in supporting lysosomal proteolysis. J Cell Biol. 1990;110:327–335. doi: 10.1083/jcb.110.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lloyd JB. Disulphide reduction in lysosomes. The role of cysteine. Biochem J. 1986;237:271–272. doi: 10.1042/bj2370271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lloyd JB. Lysosomal handling of cystine residues: stoichiometry of cysteine involvement. Biochem J. 1992;286:979–980. doi: 10.1042/bj2860979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pisoni RL, Park GY, Velilla VQ, Thoene JG. Detection and characterization of a transport system mediating cysteamine entry into human fibroblast lysosomes. Specificity for aminoethylthiol and aminoethylsulfide derivatives. J Biol Chem. 1995;270:1179–1184. doi: 10.1074/jbc.270.3.1179. [DOI] [PubMed] [Google Scholar]