Abstract
Background
The venous endothelium is a key regulator of central blood volume, organ perfusion, and hemostasis in heart failure (HF). We previously reported activation of the inflammatory/oxidative program in venous endothelial cells collected from decompensated HF patients. The underlying causes are unknown. We tested the hypothesis that the pro-inflammatory state of HF and vascular strain associated with congestion can activate the endothelial inflammatory/oxidative and hemostatic programs.
Methods and Results
We studied 6 normal (NL) dogs (left ventricular ejection fraction [LVEF] >50%, central venous pressure [CVP] = 8 ± 2 mm Hg) and 6 dogs with HF (LVEF ~30%, CVP 8 ± 2 mm Hg) produced by intracoronary microembolizations. Normal dogs were studied at baseline and 1 hour after fluid load to a target CVP ≥20 mm Hg. Endothelial cells were scraped from jugular veins; mRNA expression was analyzed by reverse transcription polymerase chain reaction. The endothelial inflammatory/oxidative and hemostatic programs were significantly activated in HF dogs compared with NL. In NL dogs, fluid load significantly activated the endothelial inflammatory/oxidative and hemostatic programs, and, concurrently, caused a significant increase in plasma neurohumoral indices to levels that approached those of HF dogs.
Conclusions
The pro-inflammatory state of HF and vascular strain associated with congestion can both activate venous endothelial cells in dogs in a manner consistent with that seen in HF patients.
Keywords: Heart failure, endothelium, inflammation
Endothelial cells form the inner lining of a blood vessel with critical basal and inducible metabolic and synthetic functions that regulate vasomotor tone, hemostasis, angiogenesis, and inflammatory responses. Endothelial cells in response to biomechanical (ie, vascular strain associated with intravascular congestion) and biochemical stimuli (ie, systemic neurohormonal activation) within the circulation may switch in their synthetic profile from quiescent toward an activated state that is vasoconstricting, pro-inflammatory, and pro-thrombotic.1–3
Endothelium nitric oxide (NO)-dependent vasodilation is impaired in both arteries and veins in patients with chronic heart failure (HF),4,5 and is an independent determinant of poor prognosis.6 Endothelium-mediated control of the venous tone is critical in HF. Veins are low-pressure reservoirs that contain > 70% of the systemic blood volume.7 The marked capacity of this reservoir implies that relatively small volume changes in the peripheral veins are followed by substantial alterations in central blood volume, and, thereby, in cardiac filling pressures.7 Venous pressure is also critical for kidney perfusion and sodium excretion. An increase in renal venous pressure, while arterial pressure is unchanged, decreases sodium excretion in a canine model of isolated perfused kidney.8,9
The vascular endothelium modulates several other physiologic and pathologic processes besides NO-mediated control of the vasomotor tone. Inflammation, oxidative stress, and hemostasis are all controlled by the vascular endothelium through transitions between quiescent and activated states.10,11 The endothelial inflammatory/oxidative program (ie, inducible nitric oxide synthase [iNOS] and cyclooxygenase-2 [COX-2], and nitrotyrosine formation) is activated in patients with decompensated HF.12,13 Venous endothelial activation partially subsides with diuresis and clinical improvement during the index hospitalization. At the time of clinical decompensation, the venous endothelium is typically exposed to acute strain from intravascular congestion,14,15 in addition to chronic systemic inflammation and oxidative stress, which are typical of the HF syndrome.13,16 Whether each of these prototypic environmental (biomechanical and biochemical) “hits” are individually sufficient to cause endothelial activation in vivo is unclear. In the present study, we used a dog model of HF produced by serial coronary microembolizations17 to investigate the hypothesis that the pro-inflammatory state of HF characterized by systemic neurohormonal activation, and the vascular strain associated with congestion are both sufficient to cause endothelial activation. This animal model of HF manifests the disease syndrome in the absence of other comorbid conditions, often present in patients with HF, and is thus more amenable to mechanistic investigations.
The objectives of this study were: 1) to compare chronic levels of venous endothelial activation in normal (NL) dogs and dogs with HF; 2) to determine the acute changes in venous endothelial activation in response to intravascular volume expansion (to a target central venous pressure [CVP] ≥20 mm Hg) in NL dogs); and 3) to compare chronic levels of systemic neurohormonal activation in HF dogs with those in NL dogs before and after acute volume load. Selected markers of endothelial activation linked to the inflammation/oxidative program (ie, iNOS and COX-2, tumor necrosis factor-α [TNF-α], receptor for advanced glycation end products [RAGE], and CD40), and hemostasis (ie, tissue factor [TF] and plasminogen activator inhibitor-1 [PAI-1]) were measured. Superoxide dismutase (SOD) and glutathione peroxidase (GPX) and were also assessed to determine whether chronic HF or an acute increase in intravascular pressure may also affect compensatory expression of antioxidant enzymes. Plasma markers of systemic neurohormonal activation linked to the HF syndrome (ie, norepinephrine [NE], TNF-α, interleukin-6 (IL-6), and endothelin-1 [ET-1]) were concurrently measured.
Methods
We studied 6 normal (NL) mongrel dogs and 6 mongrel HF dogs with systolic left ventricular (LV) dysfunction produced by serial intracoronary microembolizations.
Experimental Model
Chronic LV dysfunction was produced by multiple sequential intracoronary embolizations with polystyrene latex microspheres (77 to 102 g in diameter), as previously described.17 Coronary microembolizations were performed during sequential cardiac catheterizations under general anesthesia, mechanical respiratory support, and sterile conditions. The anesthesia regimen in the present study consisted of a combination of intravenous injections of oxymorphone hydrochloride (0.22 mg/kg), diazepam (0.17 mg/kg), and sodium pentobarbital (150 to 250 mg to effect). In all dogs, coronary microembolizations were discontinued when LV ejection fraction (LVEF), determined angiographically, was ~30%. To achieve this target EF, dogs underwent an average of 5.3 microembolization procedures performed over an average period of 8.8 weeks. Microembolization procedures were performed 1 to 2 weeks apart. The first 3 embolizations consisted of 2 mL microsphere suspension injected subselectively into either the left anterior descending or left circumflex coronary artery in an alternating fashion. Subsequent embolizations consisted of 3 to 6 mL of microspheres divided equally between the left anterior descending or left circumflex coronary artery. Left ventriculograms were performed at baseline in all dogs and before each embolization in HF dogs to assess LVEF. The LVEF was calculated as the ratio of the difference of LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) to end-diastolic volume. LV volumes were measured using the area-length method as previously described.17
Hemodynamic measurements and endothelial sampling were performed 2 weeks after last embolization under general anesthesia, mechanical respiratory support with positive end-expiratory pressure, and sterile conditions (see previous section). Aortic pressure was invasively monitored throughout the procedure with a catheter-tip micromanometer (Millar Instruments, Houston, TX). Central venous pressure was measured by using a 6 Fr right Judkins catheter, introduced through the right femoral vein, with the distal edge positioned at the level of the right atrium, connected to a fluid-filled system. Heart rate was also monitored throughout the procedure. Normal dogs were studied at baseline and 1 hour after rapid fluid load (≥500 mL Dextran-40) resulting in a sustained increase in CVP to ≥20 mm Hg. The right jugular vein was excised and cut open in NL and HF dogs at baseline. The left jugular vein was excised and cut open in NL dogs 1 hour after rapid fluid load. Blood was carefully removed by washings with saline. The endothelium was collected by gentle scraping. Ten milliliters of blood were also withdrawn from the CVP catheter at the same time points.
Endothelial mRNA Expression
Total RNA was isolated from endothelial tissue using RNAqueous-4PCR Kit according to the manufacturer’s instructions (Ambion, Austin, TX). The isolated RNA in each sample was determined spectrophotometrically. RNA with an absorbance ratio (260 nm/280 nm) higher than 1.7 and that exhibited 3 major bands, namely 28S, 18S, and 5.8S on 2% agarose, with 28S being much stronger than 18S, was considered of good quality. Approximately 4 µg RNA was reverse-transcribed into cDNA in an assay volume of 80 µL. The assay contained (final concentration) 3.6 mM of each dNTP (dATP, dTTP, dGTP, and dCTP), 40 units recombinant RNasin (RNase inhibitor, Promega), 6 µM oligo (dT) primer, and 1 unit MMLV reverse transcriptase in 10 mM Tris-HCl (pH 8.3), 75 mM KCl, 10 mM DTT, and 3.0 mM MgCl2. Assay tubes were incubated at 42°C for 60 minutes and then at 96°C for 10 minute for denaturation. For each polymerase chain reaction (PCR), 2 µL first-strand cDNA was added to 18 µL of a reaction mixture containing 20 pmol of each gene-specific forward and reverse primer, 200 µM of each dNTP, 10 mM Tris-HCl (pH 8.8), 50 mM KCl, 0.1% Triton-X100, and 3.0 mM MgCl2. After heating the tube to 95°C for 5 minutes, 1-unit platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA) was added and the PCR was allowed to proceed for 20 to 40 cycles. PCR products were analyzed by subjecting 20 µL of each reaction mixture to electrophoresis on 1% to 1.5% ethidium-bromide-agarose gels. Band size of the products was compared with standard DNA size markers. The primers were based on the gene sequences reported to Gene Bank. Gene accession numbers, primer sequences, and amplicon sizes are shown in Table 1. Band intensity was quantified in arbitrary densitometric units (du) using a Bio-Rad Gel densitometer.18
Table 1.
Primer Sequences, Amplicon Sizes, and Gene Accession Numbers
| Genes | Sequences | Product Size |
Accession Number |
|---|---|---|---|
| GAPDH | F: 5′-ACCACCATGGAGAAGGCTGG-3′ R: 5′-CTCAGTGTAGCCCAGGATGC-3′ | 528 | AB038240 |
| eNOS | F: 5′-CGCTGTCTAGGCTCTCTGGT-3′ R: 5′-ATCAAACACCTGCAGCTTCC-3′ | 300 | AF077821 |
| LCA | F: 5′-GCGGTTACTCCCAGTTTTCA-3′ R: 5′-CAGAGCTACAGCAGGACACG-3′ | 301 | CD215065 |
| iNOS | F: 5′-GGCTCAAATCACAACGGAAT-3′ R: 5′-GAATAGGGAGCTGGGAGAGC-3′ | 82 | AF143503 |
| COX-2 | F: 5′-TGAAACCCACTCCAAACACA-3′’ R: 5′-AACTGATGCGTGAAGTGCTG-3′ | 385 | AY462100 |
| TNF-α | F: 5′-TGACGGGCTTTACCTCATCT-3′ R: 5′-GGCGATGATCCCAAAGTAGA-3′ | 313 | AF348421 |
| RAGE | F: 5′-GGACTCTTAGCTGGCACTGG-3′ R: 5′-CCAGTGGTCATGGGCTTACT-3′ | 382 | AY836509 |
| CD40 | F: 5′-ATATGTGCCCACCAGGAGAG-3′ R: 5′-AATGATGGGGACCACCACTA-3′ | 488 | AY333789 |
| TF | F: 5′-CCTCAGGCACTGCAGATGTA-3′ R: 5′-GGCTGTCCAAGCTTTGTCTC-3′ | 325 | AB200288 |
| PAI-1 | F: 5′-CTCTCTCTGCCCTCACCAAC-3′ R: 5′-TCCAGTGTTGTCCCAGATGA-3′ | 442 | XM_844252 |
| SOD | F: 5′-TGCAGATGACCTGTCAGAGC-3′ R: 5′-GTGCCGGTCAGAGTCCTTAG-3′ | 440 | XM_533218 |
| GPx | F: 5′-GGCATCAGGAAAACGCTAAG-3′ R: 5′-CATGGATGAAATCCCCAGAG-3′ | 430 | XM_533828 |
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; eNOS, endothelial nitric oxide synthase; iNOS, inducible nitric oxide synthase; LCA, leucocyte common antigen; COX-2, cyclooxygenase 2; TNF-α, tumor necrosis factor-alpha; RAGE, receptor for advanced glycation end products; TF, tissue factor; PAI-1, plasminogen activator inhibitor-1; SOD, superoxide dismutase; GPx, glutathione peroxidase.
Measurements of Biomarkers in Plasma
TNF-α, IL-6, and ET-1 concentration was determined in plasma on the principle of the double antibody sandwich enzyme-linked immunosorbent assay18–20 and NE based on competitive enzymelinked immunosorbent assay.20,21 All biomarkers were assayed using commercially available assay kits. Kit for NE was purchased from ALPCO Diagnostics (Salem, NH) for IL-6 and TNF-α from Assay Designs Inc (Ann Arbor, MI), and for ET-1 from R&D Systems (Minneapolis, MN). Using standard curves and software, the concentration of each biomarker was determined and expressed as pg/mL.
Data Analysis
Data are presented as mean ± SD. Measurements were compared by Student’s t-test. The study was approved by Henry Ford Health System Institutional Animal Care and Use Committee and conformed to the National Institute of Health “Guide and Care for Use of Laboratory Animals” and the “Position of the American Heart Association on Research Animal Use.”
Results
At baseline, NL dogs had higher mean arterial pressure (MAP), heart rate (HR), and LVEF, and lower LVEDV and LVESV than HF dogs with severe LV dysfunction, whereas baseline CVP was identical (Table 2).
Table 2.
Hemodynamics and Plasma Neurohormones in 6 NL Dogs before and after Acute Volume Load, and in 6 HF Dogs
| NL | NL + VO | HF | |
|---|---|---|---|
| MAP, mm Hg | 90 ± 21 | 130 ± 18* | 70 ± 10* |
| HR, beats/min | 88 ± 19 | 125 ± 13* | 64 ± 4* |
| CVP, mm Hg | 8 ± 2 | 22 ± 4* | 8 ± 2 |
| LVEDV, mL | 52 ± 1 | 67 ± 2* | |
| LVESV, mL | 23 ± 2 | 48 ± 2 | |
| LVEF, % | 56 ± 3 | 28 ± 1 | |
| NE, pg/mL | 130 ± 11 | 491 ± 128* | 825 ± 83* |
| IL-6, pg/mL | 3.3 ± 1.2 | 15.8 ± 4.3* | 27.9 ± 7.7* |
| ET-1, pg/mL | 0.2 ± 0.1 | 1.8 ± 0.2* | 2.6 ± 0.8* |
| TNF-α, pg/mL | 1.1 ± 0.7 | 2.7 ± 0.3* | 4.3 ± 0.8* |
NL, normal; VO, volume; HF, heart failure; MAP, mean arterial pressure; HR, heart rate; CVP, central venous pressure; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; NE, norepinephrine; IL-6, interleukin-6; ET-1, endothelin-1; TNF-α, tissue necrosis factor-alpha.
P < .05 vs. NL dogs.
Acute intravascular volume expansion with Dextran-40 significantly increased MAP, HR, and CVP in NL dogs (Table 2). Fluid load also caused a significant increase in plasma NE, IL-6, ET-1, and TNF-α to levels that approached those of HF dogs (Table 2).
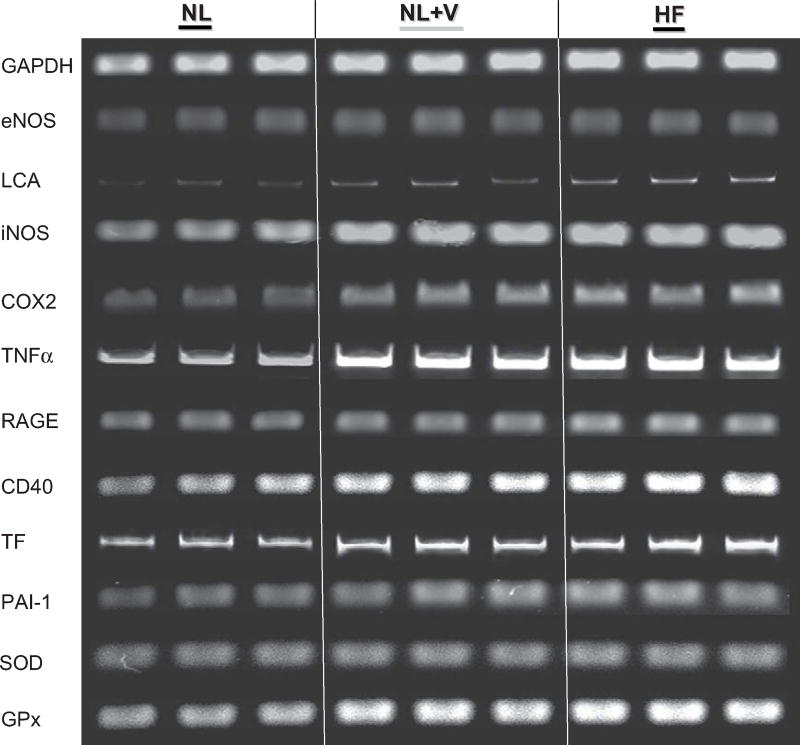
The vascular endothelium was successfully collected from internal jugular veins: eNOS (endothelial marker) was highly expressed, whereas leukocyte common antigen-1 (LCA-1) (leukocyte marker) was barely detected in all vascular samples (Fig 1).
Fig. 1.
Representative ethidium-bromide-agarose gels of reverse transcriptase-polymerase chain reaction amplification products from vascular samples in normal (NL) dogs before and after acute volume (V) load, and in heart failure (HF) dogs. Endothelial nitric oxide synthase and leukocyte common antigen expression (LCA) confirmed the endothelial nature of the sample with minimal leukocyte contamination. GADPH served as an internal control for loading conditions. Band intensity of inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX-2), tumor necrosis factor-alpha (TNF-α), receptor for advanced glycation end products (RAGE), CD40, tissue factor (TF), plasminogen activator inhibitor-1 (PAI-1), superoxide dismutase (SOD), and glutathione peroxidase (GPx) mRNA expression was quantified in arbitrary densitometric units (du) using a Bio-Rad Gel densitometer. VO.
Endothelial iNOS, COX-2 TNF-α, RAGE, CD40, TF, PAI-1, SOD, and GPx were significantly increased in HF dogs compared with NL (Fig 1, Table 3). In NL dogs, acute fluid load doubled iNOS, COX-2, TNF-α, RAGE, CD40, TF, SOD, and GPx expression to levels that approached those of HF dogs. This increase was statistically significant with the exception of TF where the P value was .07.
Table 3.
Endothelial Gene Expression in 6 NL Dogs before and after Acute Volume Load, and in 6 HF Dogs
| NL (CVP = 8 ± 2 mm Hg) |
NL+VO (CVP = 22 ± 4 mm Hg) |
HF (CVP = 8 ± 2 mm Hg) |
|
|---|---|---|---|
| iNOS (du) | 0.26 ± 0.09 | 0.48 ± 0.13* | 0.54 ± 0.18* |
| COX-2 (du) | 0.44 ± 0.14 | 0.67 ± 0.06* | 0.76 ± 0.09* |
| TNF-α (du) | 0.29 ± 0.02 | 0.47 ± 0.11* | 0.54 ± 0.09* |
| RAGE (du) | 0.35 ± 0.12 | 0.48 ± 0.07* | 0.56 ± 0.08* |
| CD40 (du) | 1.26 ± 0.30 | 1.91 ± 0.10* | 2.29 ± 0.04* |
| TF (du) | 0.57 ± 0.05 | 0.69 ± 0.12 | 0.88 ± 0.17* |
| PAI-1 (du) | 0.62 ± 0.16 | 1.05 ± 0.20* | 1.12 ± 0.25* |
| SOD (du) | 1.05 ± 0.08 | 1.23 ± 0.04* | 1.37 ± 0.11* |
| GPx (du) | 1.33 ± 0.12 | 2.10 ± 0.01* | 2.34 ± 0.12* |
NL, normal; VO, volume; HF, heart failure; CVP, central venous pressure; iNOS, inducible nitric oxide synthase; COX-2, cyclooxygenase-2; TNF-α, tumor necrosis factor-alpha; RAGE, receptor for advanced glycation end products; TF, tissue factor; PAI-1, plasminogen activator inhibitor-1; SOD, superoxide dismutase; GPX, glutathione peroxidase.
Discussion
The present data provide the first in vivo evidence that the pro-inflammatory state of HF itself, and vascular stretch from intravascular pressure overload, are both sufficient to activate venous endothelial cells in dogs in a fashion consistent with that seen in patients with HF. Molecular data in dogs expand our previous findings in humans13 by showing that 1) increased COX-2 and iNOS expression are manifestations of a coordinated and comprehensive activation of the inflammatory/oxidative and hemostatic programs with adaptive expression of antioxidant enzymes and that 2) this activation takes place in response to both biochemical (ie, systemic neurohormonal activation) and biomechanical (ie, vascular strain associated with intravascular congestion) environmental stressors, the latter being itself associated with an acute surge in plasma neurohormonal indices.
In HF patients and in experimental canine models of HF, excessive systemic inflammation and oxidative stress may result in progressive tissue injury when endogenous defenses and repair processes are overwhelmed.22–25 After completion of the embolization protocol, HF dogs continue to deteriorate as evidenced by a progressive reduction of LVEF, increase in LVEDV and filling pressures.17 As cardiac function declines, plasma neurohormonal indices increase25 in a manner consistent with that seen in patients with CHF.
In the midst of this hemodynamic and humoral turmoil, endothelial cells, can integrate and transduce biomechanical (eg, vascular strain3) and biochemical stimuli (eg, circulating cytokines,26 advanced glycation end products [AGEs],27,28 oxidized lipoproteins29) into a series of key physiologic and pathologic signaling programs that modulate not only vascular tone, but also, among the others: inflammation, oxidative stress, and hemostasis.11,30
In vitro studies have shown that cytokines,31 AGEs,27 oxidative products,32 and vascular strain3,33 may promote overlapping patterns of endothelial activation (through an increase in oxidative stress or nuclear translocation of transcription factor NF-kB)34 characterized by increased expression of iNOS,35,36 COX-2,37,38 TNF-α,35,39,40 RAGE,27,39 CD40,32,41 (inflammatory/oxidative program), TF,41,42 and PAI-143,44 (hemostatic program). In the present study, endothelial inflammatory/oxidative and hemostatic programs were activated in chronic HF dogs, and in NL dogs after acute intravascular volume expansion as compared with euvolemic NL dogs. Our experimental design specifically tested the effects of acute intravascular volume expansion on endothelial activation since venous pressures are typically increased in HF patients at the time of clinical decompensation.45 Unfortunately, because pulmonary artery catheters were not inserted, we cannot provide objective data on pulmonary congestion (ie, pulmonary artery and wedge pressures).
Inducible NOS has recently been shown to bind, nitrosylate, and activate COX-2, a key observation that links 2 major human regulatory systems, NO and prostaglandins, in their response to environmental stressors.46 iNOS may also shift from NO to superoxide production. This shift occurs when nitric oxide synthases are uncoupled because of oxidation of tetrahydrobiopterin (a critical cofactor for NO synthases)47,48 or from high levels of asymmetric dimethylarginine (ADMA),49 a competitive inhibitor that is increased in several chronic inflammatory disease states including chronic HF.50 TNF-α,51 RAGE,52 and CD4053 may also increase superoxide production by promoting NAD(P)H oxidase activity. Oxidative injury itself may cause endothelial cells to transition from a quiescent to an activated state where, in a vicious cycle, oxidative stress promotes inflammation, and inflammation, in turn, increases oxidative stress. In this context, antioxidant enzymes are the primary defense mechanism against damage, counteracting a system that has lost internal control. SOD and GPx inactivate reactive oxygen species, thereby protecting cells from the pleiotropic detrimental effects of inflammation and oxidative stress.54–56 Our observation that expression of antioxidant enzymes is increased in the HF state, and in response to increased vascular strain likely represents a compensatory attempt to counteract this bidirectional and harmful axis that links oxidative stress to inflammation.
We also observed that chronic HF dogs and NL dogs after acute intravascular volume expansion exhibit increased expression of pro-coagulant genes. Patients with HF are at an increased risk of venous thromboembolism, a risk that escalates with HF progression.57,58 Our data provide the first in vivo mechanistic evidence that both biomechemical and biomechanical stressors may independently contribute to this clinical complication of HF.
One limitation of the present study is that our experimental design included only 2 time points, baseline and 1 hour after fluid load, and, because of that, acute activation of the venous endothelium in NL dogs was not followed through its resolution. Besides, because protein and protein activity were not measured, we can only assume that protein and protein activity were also increased. On the other hand, 1-hour fluid load was chosen for mechanistic and practical purposes, rather than for clinical reasons, because fluid accumulation in decompensated patients, takes place and resolves over days (or weeks), rather than hours, in a setting of decompensation.59,60 Therefore, monitoring the resolution of endothelial activation in dogs after 1 hour of intravascular volume expansion, even if described, would not have had any direct clinical correlates.
Importantly, our study cannot identify the source of increased neurohormonal synthesis in response to intravascular volume expansion. It is possible that the endothelium itself contributes to this neurohormonal surge in response to a mechanical stressor. The strongest arguments that favor an “endothelial” contribution are: 1) ET-1 is primarily an endothelial cytokine; 2) we have documented an increase in TNF-α expression in the ECs sampled from NL dogs after volume load; and 3) previous reports indicate that ET-1,61 IL-6,62 and TNF-α63 can be secreted by the endothelium within hours in response to mechanical stress.
In conclusion, we demonstrated that chronic pro-inflammatory state of HF and acute vascular strain associated with congestion promote a common pattern of endothelial activation with coordinated expression of pro-inflammatory, pro-oxidative, and pro-coagulant genes. Compensatory upregulation of antioxidant enzymes was also observed. Future longitudinal studies may test the effect of intermittent bouts of intravascular volume expansion in experimental models of HF to determine whether they may in fact act as secondary “hits” to augment baseline levels of endothelial activation, and, eventually, promote progression of chronic HF syndrome.
Activation of the venous endothelium is a further manifestation of the systemic inflammatory/oxidative and hypercoagulable state that accompanies the syndrome of advanced HF. Chronic inflammation and acute increase in intravascular volume appear sufficient to trigger endothelial activation. Future investigations are warranted to clarify whether the venous endothelial phenotype may ultimately allow us to track in a serial manner, in the unique individual, the vascular impact of subsequent environmental (ie, biomechanical and biochemical) stressors, as well as the potential response to therapeutic interventions.
Acknowledgments
Supported by a grant from the National Heart, Lung, and Blood Institutes PO1 HL074237-05
References
- 1.Sumpio BE, Riley JT, Dardik A. Cells in focus: endothelial cell. Int J Biochem Cell Biol. 2002;34:1508–12. doi: 10.1016/s1357-2725(02)00075-4. [DOI] [PubMed] [Google Scholar]
- 2.Aird WC. Mechanisms of endothelial cell heterogeneity in health and disease. Circ Res. 2006;98:159–62. doi: 10.1161/01.RES.0000204553.32549.a7. [DOI] [PubMed] [Google Scholar]
- 3.Gimbrone MA, Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci. 2000;902:230–9. doi: 10.1111/j.1749-6632.2000.tb06318.x. discussion 239–40. [DOI] [PubMed] [Google Scholar]
- 4.Katz SD, Biasucci L, Sabba C, Strom JA, Jondeau G, Galvao M, et al. Impaired endothelium-mediated vasodilation in the peripheral vasculature of patients with congestive heart failure. J Am Coll Cardiol. 1992;19:918–25. doi: 10.1016/0735-1097(92)90271-n. [DOI] [PubMed] [Google Scholar]
- 5.Dzeka TN, Arnold JM. Prostaglandin modulation of venoconstriction to physiological stress in normals and heart failure patients. Am J Physiol Heart Circ Physiol. 2003;284:H790–7. doi: 10.1152/ajpheart.00572.2001. [DOI] [PubMed] [Google Scholar]
- 6.Katz SD, Hryniewicz K, Hriljac I, Balidemaj K, Dimayuga C, Hudaihed A, et al. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation. 2005;111:310–4. doi: 10.1161/01.CIR.0000153349.77489.CF. [DOI] [PubMed] [Google Scholar]
- 7.Pang CC. Measurement of body venous tone. J Pharmacol Toxicol Methods. 2000;44:341–60. doi: 10.1016/s1056-8719(00)00124-6. [DOI] [PubMed] [Google Scholar]
- 8.Firth JD, Raine AE, Ledingham JG. Raised venous pressure: a direct cause of renal sodium retention in oedema? Lancet. 1988;1:1033–5. doi: 10.1016/s0140-6736(88)91851-x. [DOI] [PubMed] [Google Scholar]
- 9.Blake WD, Wegria R, et al. Effect of increased renal venous pressure on renal function. Am J Physiol. 1949;157:1–13. doi: 10.1152/ajplegacy.1949.157.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 11.Esmon CT. Crosstalk between inflammation and thrombosis. Maturitas. 2004;47:305–14. doi: 10.1016/j.maturitas.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Colombo PC, Ashton AW, Celaj S, Talreja A, Banchs JE, Dubois NB, et al. Biopsy coupled to quantitative immunofluorescence: a new method to study the human vascular endothelium. J Appl Physiol. 2002;92:1331–8. doi: 10.1152/japplphysiol.00680.2001. [DOI] [PubMed] [Google Scholar]
- 13.Colombo PC, Banchs JE, Celaj S, Talreja A, Lachmann J, Malla S, et al. Endothelial cell activation in patients with decompensated heart failure. Circulation. 2005;111:58–62. doi: 10.1161/01.CIR.0000151611.89232.3B. [DOI] [PubMed] [Google Scholar]
- 14.Cotter G, Moshkovitz Y, Milovanov O, Salah A, Blatt A, Krakover R, et al. Acute heart failure: a novel approach to its pathogenesis and treatment. Eur J Heart Fail. 2002;4:227–34. doi: 10.1016/s1388-9842(02)00017-x. [DOI] [PubMed] [Google Scholar]
- 15.Gheorghiade M, De Luca L, Fonarow GC, Filippatos G, Metra M, Francis GS. Pathophysiologic targets in the early phase of acute heart failure syndromes. Am J Cardiol. 2005;96:11G–7G. doi: 10.1016/j.amjcard.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 16.White M, Ducharme A, Ibrahim R, Whittom L, Lavoie J, Guertin MC, et al. Increased systemic inflammation and oxidative stress in patients with worsening congestive heart failure: improvement after short-term inotropic support. Clin Sci (Lond) 2006;110:483–9. doi: 10.1042/CS20050317. [DOI] [PubMed] [Google Scholar]
- 17.Sabbah HN, Stein PD, Kono T, Gheorghiade M, Levine TB, Jafri S, et al. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol. 1991;260:H1379–84. doi: 10.1152/ajpheart.1991.260.4.H1379. [DOI] [PubMed] [Google Scholar]
- 18.Waage A, Brandtzaeg P, Halstensen A, Kierulf P, Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989;169:333–8. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirano T, Akira S, Taga T, Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990;11:443–9. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- 20.Bonow RO. New insights into the cardiac natriuretic peptides. Circulation. 1996;93:1946–50. doi: 10.1161/01.cir.93.11.1946. [DOI] [PubMed] [Google Scholar]
- 21.Kema IP, de Vries EG, Slooff MJ, Biesma B, Muskiet FA. Serotonin, catecholamines, histamine, and their metabolites in urine, platelets, and tumor tissue of patients with carcinoid tumors. Clin Chem. 1994;40:86–95. [PubMed] [Google Scholar]
- 22.Yndestad A, Holm AM, Muller F, Simonsen S, Froland SS, Gullestad L, et al. Enhanced expression of inflammatory cytokines and activation markers in T-cells from patients with chronic heart failure. Cardiovasc Res. 2003;60:141–6. doi: 10.1016/s0008-6363(03)00362-6. [DOI] [PubMed] [Google Scholar]
- 23.Kaneko K, Kanda T, Yamauchi Y, Hasegawa A, Iwasaki T, Arai M, et al. C-Reactive protein in dilated cardiomyopathy. Cardiology. 1999;91:215–9. doi: 10.1159/000006913. [DOI] [PubMed] [Google Scholar]
- 24.McMurray J, Chopra M, Abdullah I, Smith WE, Dargie HJ. Evidence of oxidative stress in chronic heart failure in humans. Eur Heart J. 1993;14:1493–8. doi: 10.1093/eurheartj/14.11.1493. [DOI] [PubMed] [Google Scholar]
- 25.Gupta R, Imai M, Alice J, Jiang A, Wang J, Sabbah H. Chronic therapy with selective electric vagus nerve stimulation normalizes plasma concentration of tissue necrosis factor-α, interleukin-6 and B-type natriuretic peptide in dogs with heart failure. Am Coll Cardiol. 2006 A-335172. [Google Scholar]
- 26.Pober JS. Effects of tumour necrosis factor and related cytokines on vascular endothelial cells. Ciba Found Symp. 1987;131:170–84. doi: 10.1002/9780470513521.ch12. [DOI] [PubMed] [Google Scholar]
- 27.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 28.Hartog JW, Voors AA, Schalkwijk CG, Scheijen J, Smilde TD, Damman K, et al. Clinical and prognostic value of advanced glycation end-products in chronic heart failure. Eur Heart J. 2007;28:2879–85. doi: 10.1093/eurheartj/ehm486. [DOI] [PubMed] [Google Scholar]
- 29.Jorde UP, Colombo PC, Ahuja K, Hudaihed A, Onat D, Diaz T, et al. Exercise-induced increases in oxidized low-density lipoprotein are associated with adverse outcomes in chronic heart failure. J Card Fail. 2007;13:759–64. doi: 10.1016/j.cardfail.2007.06.724. [DOI] [PubMed] [Google Scholar]
- 30.Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54:469–87. [PubMed] [Google Scholar]
- 31.Madge LA, Pober JS. TNF signaling in vascular endothelial cells. Exp Mol Pathol. 2001;70:317–25. doi: 10.1006/exmp.2001.2368. [DOI] [PubMed] [Google Scholar]
- 32.Mehta JL. The role of LOX-1, a novel lectin-like receptor for oxidized low density lipoprotein, in atherosclerosis. Can J Cardiol. 2004;20(Suppl B) 32B-6B. [PubMed] [Google Scholar]
- 33.Ungvari Z, Wolin MS, Csiszar A. Mechanosensitive production of reactive oxygen species in endothelial and smooth muscle cells: role in microvascular remodeling? Antioxid Redox Signal. 2006;8:1121–9. doi: 10.1089/ars.2006.8.1121. [DOI] [PubMed] [Google Scholar]
- 34.Canty TG, Jr, Boyle EM, Jr, Farr A, Morgan EN, Verrier ED, Pohlman TH. Oxidative stress induces NF-kappaB nuclear translocation without degradation of IkappaBalpha. Circulation. 1999;100:II361–4. doi: 10.1161/01.cir.100.suppl_2.ii-361. [DOI] [PubMed] [Google Scholar]
- 35.Ungvari Z, Csiszar A, Edwards JG, Kaminski PM, Wolin MS, Kaley G, et al. Increased superoxide production in coronary arteries in hyperhomocysteinemia: role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2003;23:418–24. doi: 10.1161/01.ATV.0000061735.85377.40. [DOI] [PubMed] [Google Scholar]
- 36.Freixes M, Rodriguez A, Dalfo E, Ferrer I. Oxidation, glycoxidation, lipoxidation, nitration, and responses to oxidative stress in the cerebral cortex in Creutzfeldt-Jakob disease. Neurobiol Aging. 2006;27:1807–15. doi: 10.1016/j.neurobiolaging.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Mark KS, Trickler WJ, Miller DW. Tumor necrosis factor-alpha induces cyclooxygenase-2 expression and prostaglandin release in brain microvessel endothelial cells. J Pharmacol Exp Ther. 2001;297:1051–8. [PubMed] [Google Scholar]
- 38.Cosentino F, Eto M, De Paolis P, van der Loo B, Bachschmid M, Ullrich V, et al. High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells: role of protein kinase C and reactive oxygen species. Circulation. 2003;107:1017–23. doi: 10.1161/01.cir.0000051367.92927.07. [DOI] [PubMed] [Google Scholar]
- 39.Huang SM, Wu CH, Yen GC. Effects of flavonoids on the expression of the pro-inflammatory response in human monocytes induced by ligation of the receptor for AGEs. Mol Nutr Food Res. 2006;50:1129–39. doi: 10.1002/mnfr.200600075. [DOI] [PubMed] [Google Scholar]
- 40.Hung TH, Charnock-Jones DS, Skepper JN, Burton GJ. Secretion of tumor necrosis factor-alpha from human placental tissues induced by hypoxia-reoxygenation causes endothelial cell activation in vitro: a potential mediator of the inflammatory response in preeclampsia. Am J Pathol. 2004;164:1049–61. doi: 10.1016/s0002-9440(10)63192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slupsky JR, Kalbas M, Willuweit A, Henn V, Kroczek RA, Muller-Berghaus G. Activated platelets induce tissue factor expression on human umbilical vein endothelial cells by ligation of CD40. Thromb Haemost. 1998;80:1008–14. [PubMed] [Google Scholar]
- 42.Wautier JL, Schmidt AM. Protein glycation: a firm link to endothelial cell dysfunction. Circ Res. 2004;95:233–8. doi: 10.1161/01.RES.0000137876.28454.64. [DOI] [PubMed] [Google Scholar]
- 43.Yamagishi S, Fujimori H, Yonekura H, Yamamoto Y, Yamamoto H. Advanced glycation endproducts inhibit prostacyclin production and induce plasminogen activator inhibitor-1 in human microvascular endothelial cells. Diabetologia. 1998;41:1435–41. doi: 10.1007/s001250051089. [DOI] [PubMed] [Google Scholar]
- 44.Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress, and inflammation. Annu Rev Nutr. 2005;25:151–74. doi: 10.1146/annurev.nutr.24.012003.132446. [DOI] [PubMed] [Google Scholar]
- 45.Gheorghiade M, Zannad F, Sopko G, Klein L, Pina IL, Konstam MA, et al. Acute heart failure syndromes: current state and framework for future research. Circulation. 2005;112:3958–68. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 46.Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 2005;310:1966–70. doi: 10.1126/science.1119407. [DOI] [PubMed] [Google Scholar]
- 47.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–9. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moens AL, Kass DA. Tetrahydrobiopterin and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2006;26:2439–44. doi: 10.1161/01.ATV.0000243924.00970.cb. [DOI] [PubMed] [Google Scholar]
- 49.Wells SM, Holian A. Asymmetric dimethylarginine induces oxidative and nitrosative stress in murine lung epithelial cells. Am J Respir Cell Mol Biol. 2007;36:520–8. doi: 10.1165/rcmb.2006-0302SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng Q, Lu X, Fortin AJ, Pettersson A, Hedner T, Kline RL, et al. Elevation of an endogenous inhibitor of nitric oxide synthesis in experimental congestive heart failure. Cardiovasc Res. 1998;37:667–75. doi: 10.1016/s0008-6363(97)00242-3. [DOI] [PubMed] [Google Scholar]
- 51.Yamagishi S, Inagaki Y, Kikuchi S. Nifedipine inhibits tumor necrosis factor-alpha-induced monocyte chemoattractant protein-1 overexpression by blocking NADPH oxidase-mediated reactive oxygen species generation. Drugs Exp Clin Res. 2003;29:147–52. [PubMed] [Google Scholar]
- 52.Basta G, Lazzerini G, Del Turco S, Ratto GM, Schmidt AM, De Caterina R. At least 2 distinct pathways generating reactive oxygen species mediate vascular cell adhesion molecule-1 induction by advanced glycation end products. Arterioscler Thromb Vasc Biol. 2005;25:1401–7. doi: 10.1161/01.ATV.0000167522.48370.5e. [DOI] [PubMed] [Google Scholar]
- 53.Sanguigni V, Ferro D, Pignatelli P, Del Ben M, Nadia T, Saliola M, et al. CD40 ligand enhances monocyte tissue factor expression and thrombin generation via oxidative stress in patients with hypercholesterolemia. J Am Coll Cardiol. 2005;45:35–42. doi: 10.1016/j.jacc.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 54.Ennezat PV, Malendowicz SL, Testa M, Colombo PC, Cohen-Solal A, Evans T, et al. Physical training in patients with chronic heart failure enhances the expression of genes encoding antioxidative enzymes. J Am Coll Cardiol. 2001;38:194–8. doi: 10.1016/s0735-1097(01)01321-3. [DOI] [PubMed] [Google Scholar]
- 55.Campese VM, Sindhu RK, Ye S, Bai Y, Vaziri ND, Jabbari B. Regional expression of NO synthase, NAD(P)H oxidase and superoxide dismutase in the rat brain. Brain Res. 2007;1134:27–32. doi: 10.1016/j.brainres.2006.11.067. [DOI] [PubMed] [Google Scholar]
- 56.Feng NH, Chu SJ, Wang D, Hsu K, Lin CH, Lin HI. Effects of various antioxidants on endotoxin-induced lung injury and gene expression: mRNA expressions of MnSOD, interleukin-1beta and iNOS. Chin J Physiol. 2004;47:111–20. [PubMed] [Google Scholar]
- 57.Chong AY, Lip GY. Viewpoint: the prothrombotic state in heart failure: a maladaptive inflammatory response? Eur J Heart Fail. 2007;9:124–8. doi: 10.1016/j.ejheart.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 58.Howell MD, Geraci JM, Knowlton AA. Congestive heart failure and outpatient risk of venous thromboembolism: a retrospective, case-control study. J Clin Epidemiol. 2001;54:810–6. doi: 10.1016/s0895-4356(00)00373-5. [DOI] [PubMed] [Google Scholar]
- 59.Yu CM, Wang L, Chau E, Chan RH, Kong SL, Tang MO, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841–8. doi: 10.1161/CIRCULATIONAHA.104.492207. [DOI] [PubMed] [Google Scholar]
- 60.Adamson PB, Magalski A, Braunschweig F, Bohm M, Reynolds D, Steinhaus D, et al. Ongoing right ventricular hemodynamics in heart failure: clinical value of measurements derived from an implantable monitoring system. J Am Coll Cardiol. 2003;41:565–71. doi: 10.1016/s0735-1097(02)02896-6. [DOI] [PubMed] [Google Scholar]
- 61.Hasdai D, Holmes DR, Jr, Garratt KN, Edwards WD, Lerman A. Mechanical pressure and stretch release endothelin-1 from human atherosclerotic coronary arteries in vivo. Circulation. 1997;95:357–62. doi: 10.1161/01.cir.95.2.357. [DOI] [PubMed] [Google Scholar]
- 62.Kawai M, Naruse K, Komatsu S, Kobayashi S, Nagino M, Nimura Y, et al. Mechanical stress-dependent secretion of interleukin 6 by endothelial cells after portal vein embolization: clinical and experimental studies. J Hepatol. 2002;37:240–6. doi: 10.1016/s0168-8278(02)00171-x. [DOI] [PubMed] [Google Scholar]
- 63.Wang BW, Chang H, Lin S, Kuan P, Shyu KG. Induction of matrix metalloproteinases-14 and-2 by cyclical mechanical stretch is mediated by tumor necrosis factor-alpha in cultured human umbilical vein endothelial cells. Cardiovasc Res. 2003;59:460–9. doi: 10.1016/s0008-6363(03)00428-0. [DOI] [PubMed] [Google Scholar]