Abstract
Objective
Response early in weight loss treatment predicts long-term weight change. Weight variability, independent of absolute early weight change, may also relate to long-term outcomes. This study examined whether weight variability early in treatment predicted later weight loss and maintenance.
Methods
Participants were 183 completers of a yearlong behavioral weight loss program (M age = 51, 81% female, 69% white, M body mass index = 35 kg/m2). Weight variability was calculated using weights from the first 6 and 12 weekly treatment sessions. Multiple linear regressions examined whether weight variability predicted subsequent weight change 6, 12, and 24 months later.
Results
Weight variability over 6 and 12 weeks predicted less subsequent weight loss at 12 (6-week: β = 0.18, p = 0.02; 12-week: β = 0.33, p < 0.01) and 24 (6-week: β = 0.17, p = 0.03; 12-week: β = 0.15, p = 0.05) months. Relationships held adjusting for covariates. Weight variability was more strongly associated with 6-month weight change in men than women (β = 0.27, p = 0.01).
Conclusions
Elevated weight variability early in a weight loss program predicted poor long-term outcomes, possibly reflecting inconsistent weight control behaviors. Tracking weight variability could prove useful for improving treatment outcome.
Introduction
Behavioral weight loss programs reliably produce clinically significant weight losses.1 However, weight loss outcomes are variable, and those who lose a clinically significant amount of weight (5–10%) typically regain it.2 Early identification of individuals most at risk for smaller losses and/or weight regain would allow for potential benefit from more intensive or tailored interventions. Failure to achieve meaningful weight loss early in treatment has repeatedly been associated with poor long-term outcomes.3,4 Variability in weight early in treatment, independent of total early weight loss, might provide additional predictive information about later outcome.
Higher fluctuation in weight may signal less consistency in weight control behaviors.5 However, research regarding the benefit of short-term weight consistency is mixed. On the one hand, Orsama and colleagues6 found higher weights on weekends than weekdays, a pattern that was strongest in those who lost or maintained, rather than gained, weight over time. These authors suggested that compensating for increased weekend intake contributes to successful weight control. On the other hand, successful weight loss maintainers typically report consistent daily diets, with higher consistency associated with less weight regain in this group.7 These findings are in line with the strategy taught in most behavioral weight control programs, to eat a similar amount with similar timing each day. For example, the LEARN manual, gold standard of behavioral weight loss treatment, advises meeting a daily calorie goal set 500–1000 calories below one’s energy expenditure.8 Fluctuation in body weight could be a proxy for inconsistent caloric intake, which is counter to teachings in standard behavioral programs. Additionally, Lowe and colleagues9 found that, in young women concerned with their weight, higher weight variability over 6 months predicted amount of weight gained over the following 1.5 years. Higher variability in weight may be detrimental for long-term weight control, although the association may depend on the time period over which it is measured (e.g. within-week fluctuation may be less concerning than longer-term variability).
The present study examined whether weekly weight variability early in treatment was associated with long-term weight outcomes. Based on the framework presented by Brownell8 and others,10,11 we expected that relatively consistent week-to-week weight reductions would bode well for long-term weight control. While large weekly weight losses contribute more to total weight loss and are typically viewed favorably, we hypothesized that consistency in weight changes would independently predict greater long-term weight loss. Due to the lack of consensus about the most meaningful length of time over which to measure weight variability, we chose to measure it over 6 and 12 weeks. These windows were chosen to better identify what length of time includes sufficient data to maximize the accuracy and clinical relevance of the variability measure while keeping the measurement time short enough to allow for supplementary clinical interventions as warranted. It was hypothesized that, controlling for total 6-week (or 12-week) weight loss, higher weight variability during the first 6 (or 12) weeks of treatment would predict smaller subsequent weight losses 6 months into treatment and poorer weight loss maintenance 1 and 2 years later. Gender differences in weight variability and gender as a moderator between weight variability and later weight loss were tested in an exploratory manner, as research to inform directional hypotheses is lacking. Additionally, weight variability’s relation with self-report measures of eating and appetite is largely untested, so we examined this in an exploratory manner to better understand weight variability.
Methods
Participants
Adults (N = 262) living near Philadelphia, PA were recruited to participate in a behavioral weight loss treatment study located at two local universities. Eligible participants were between age 18 and 65 with a body mass index (BMI) between 27 and 45 kg/m2. Exclusion criteria included current enrollment in another organized weight loss program; lactose intolerance (as meal replacements provided to participants include dairy); taking medications that affect appetite (unless dosage was stable for at least 6 months); history of surgical weight loss procedure; major diseases; pregnancy or pregnancy plans during the next two years; breastfeeding; and excessive alcohol consumption (unless willing and judged able by study clinicians to reduce that amount).
Procedure
Participants completed a phone screening, a group-based orientation, and an individual, in-person screening visit to confirm eligibility and provide informed consent. Enrolled participants were randomly assigned to 1 of 3 treatment conditions. Each was based on standard behavioral treatment adapted from the LEARN manual.8 Participants were given behavioral goals such as self-monitoring, calorie goals, and increasing physical activity. One condition was based strictly on standard behavioral treatment. The second built on these guidelines with instruction to use 2 meal replacements per day during the first 6 months of the study. The third focused on modifying individuals’ personal food environments by decreasing energy density of foods, increasing protein and fiber intake, and decreasing high energy density food variety (results from parent trial submitted for publication).
Treatment lasted one year, and was delivered by 2 leaders through 75-minute sessions in small groups. Treatment was administered weekly for months 1 through 6, bi-weekly for months 7 through 9, and monthly for months 10 through 12. The first 6 months focused on weight loss, with the final 6 months shifting towards weight loss maintenance. Participants attended assessment visits at 6, 12, and 24 months from study initiation. The Drexel University Institutional Review Board approved this study.
Measures
Weight was measured at every assessment and treatment session. The following self-report measures were administered at each assessment. Scores from the baseline assessment were used in the present study.
General Food Craving Questionnaire-Trait (GFCQ-T)
The GFCQ-T assessed 4 factors of food cravings: preoccupation with food; loss of control; positive outcome expectancy; and emotional craving.12 It demonstrates adequate test-retest reliability, internal consistency, and construct validity.12
Power of Food Scale (PFS)
The PFS assessed hedonic hunger, or the extent to which palatable foods in one’s environment influence their desire to consume such foods.13 It includes 3 subscales: food available, food present, and food tasted. The PFS shows adequate internal consistency, test-retest reliability, and convergent discriminant validity.13
Binge Eating Scale (BES)
The BES assessed behavioral manifestations and feelings/cognitions surrounding a binge episode.14 The BES has good test-retest reliability and is moderately associated with binge eating severity indicated from food records.15
Yale Food Addiction Scale (YFAS)
The YFAS assessed signs of addiction toward certain types of foods.16 The YFAS exhibits adequate internal reliability and good convergent and discriminant validity with other similar and dissimilar constructs, respectively.16
Weight Efficacy Lifestyle Questionnaire (WEL)
The WEL assessed self-efficacy for regulating food intake across various situations (negative emotions, availability, social pressure, physical discomfort, and positive activities).17 It shows significant validity and reliability.18
Three Factor Eating Questionnaire revised (TFEQ-18)
The TFEQ-18 assessed cognitive restraint, uncontrolled eating, and emotional eating.19 This measure has a strong factor structure and adequate reliability.19
Analysis Plan
Data were analyzed from treatment completers. Treatment dropouts were excluded to minimize variance in weight outcomes, and because they were usually missing follow-up data. Additionally, 2 participants were excluded from analyses due to missing more than 4 of the first 12 intervention sessions, limiting data available to calculate weight variability.
As neither weight variability nor early weight change differed between intervention groups, groups were combined for all analyses. All participants with available data were included in each analysis. Weight variability was calculated based on methodology from Lowe and colleagues.9 Growth curve analysis modeled weight change trajectories over the first 6 and first 12 weeks of treatment, using participants’ weekly weights. Weight variability was the root mean square error around each participant’s regression line. When data were missing, the model appropriately spaced recorded weights. An independent-samples t-test compared included and excluded participants to determine differences in demographics and early weight change/variability (for those with sufficient data for calculation).
Next the associations of the weight variability measures with potentially confounding variables (baseline BMI, 6-week (or 12-week) weight change, and number of missed sessions) were tested with Spearman’s rho correlations due to non-normal distributions. Partial correlations tested the relationship of weight variability with baseline GFCQ-T, PFS, TFEQ-18, YFAS, BES, and WEL scores, controlling for baseline BMI. A one-way ANCOVA tested for gender differences in weight variability, controlling for baseline BMI.
Bivariate linear regressions tested the independent relationship between each weight variability measure and future weight change. For 6-week weight variability, weight change was calculated from 1.5-to-6, 1.5-to-12, and 1.5-to-24 months. For 12-week weight variability, it was measured from 3-to-6, 3-to-12, and 3-to-24 months. Outcome variables started after the variability measure ended to avoid overlap in timing of the independent and dependent variables. Next, multiple linear regressions tested the relationship between each weight variability measure and the three outcome variables, controlling for covariates. In addition to weight change during the weight variability period, number of missed weights, treatment condition, and gender, all baseline measures with a significant or trending relationship to weight variability were included as covariates. Finally, the interaction between weight variability and gender was tested on the three weight change outcomes for each weight variability measure, controlling for covariates.
Results
Included (n = 183) and excluded (n = 79) participants had similar starting BMIs and gender proportions. Treatment completers were older than dropouts (t(128.41) = −2.13, p = 0.04) and groups differed in racial/ethnic breakdown, with 41.8% of black and 23.2% of white participants in the excluded group (χ2 = 14.27, p = 0.03). Included participants had a higher 6-week percent weight loss than excluded participants (t(252) = 5.35, p < 0.01), but groups did not differ on 6-week weight variability (t(243) = 0.14, p = 0.89).
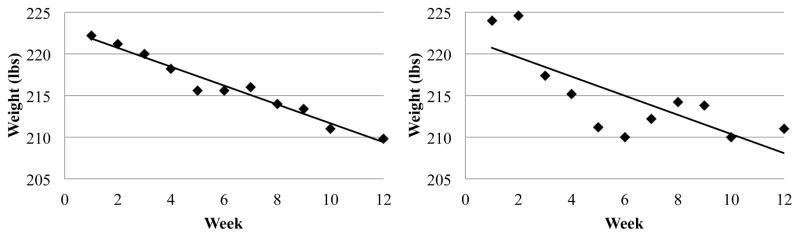
Characteristics of the included sample are shown in Table 1. Fig. 1 shows examples of an individual with low and one with high 12-week weight variability. Controlling for baseline BMI, 6- and 12-week weight variability were higher in men (6-week: M = 1.30, SD = 0.55; 12-week: M = 1.58, SD = 0.51) than women (6-week: M = 1.04, SD = 0.54; 12-week: M = 1.27, SD = 0.48; 6-week: F(1,180) = 6.14, p = 0.01; 12-week: F(1,180) = 12.11, p < 0.01). Weight variability over the first and second 6 weeks of treatment were correlated (rs = 0.23, p < 0.01). Six-week and 12-week weight variability were positively correlated with baseline BMI (rs’s = 0.19 and 0.27, respectively, p’s < 0.01). Weight variability was unrelated to number of first sessions missed and to initial percent weight change. Number of first 12 sessions missed was associated with percent weight change over the first 12 weeks (rs = 0.28, p < 0.01), such that those who missed more meetings lost less weight.
Table 1.
Descriptive information about included sample, including patterns in percent weight loss throughout the study.
| Variable | M or % | SD |
|---|---|---|
| Age | 50.89 | 10.12 |
| Baseline BMIa | 35.23 | 4.71 |
| Gender (% female) | 80.90 | |
| Race/Ethnicity (%) | ||
| White | 68.90 | |
| Black | 25.10 | |
| Asian | 1.10 | |
| Hispanic/Latino | 2.70 | |
| Other | 2.20 | |
|
| ||
| Six-week weight change (%)c | −3.62 | 2.06 |
| Six-week weight variability (lbs) | 1.09 | 0.55 |
| 1.5-to-6 month weight change (%)c | −7.25 | 4.14 |
| 1.5-to-12 month weight change (%)c | −7.69 | 6.55 |
| 1.5-to-24 month weight change (%)c | −2.76 | 7.14 |
| Number of first 6 sessions missedb | 0.22 | 0.45 |
|
| ||
| 12-week weight change (%)c | −6.85 | 3.54 |
| 12-week weight variability (lbs) | 1.33 | 0.50 |
| 3-to-6 month weight change (%)c | −4.01 | 2.73 |
| 3-to-12 month weight change (%)c | −4.48 | 5.69 |
| 3-to-24 month weight change (%)c | 0.43 | 6.66 |
| Number of first 12 sessions missedb | 0.87 | 1.01 |
BMI = body mass index (kg/m2).
Number of sessions missed refers to how many sessions the participant missed during the first 6 or first 12 weeks of treatment.
Percent weight change was calculated such that negative values indicate weight loss and positive values indicate weight gain.
Fig. 1.
Weekly weights for a participant with a low (left; root mean square error = 0.72) and high (right; root mean square error = 2.85) 12-week weight variability score. The x-axis represents weeks since the start of treatment and the y-axis represents weight in pounds. The weight variability score indicates the mean distance between each data point and the regression line for that individual, so higher weight variability score indicates a larger spread of individual data points around the best fitting line.
Both 6- and 12-week weight variability were positively associated with subsequent weight change at 12 and 24 months. Six- and 12-week weight variability were unrelated to subsequent weight change at 6 months. Percent weight change was calculated such that negative values indicate more weight loss (see Tables 2 and 3). Correlations between weight variability and baseline self-report questionnaires can be found in Table 4. Negative associations indicate that those higher in weight variability reported less of these aspects of eating behavior.
Table 2.
Results from 6-week weight variability independent and adjusted multiple regression analyses. Covariates included 6-week % weight change; number of sessions missed; treatment condition; gender; Three Factor Eating Questionnaire - Emotional Eating; General Food Craving Questionnaire-Trait - Preoccupation with food; and Power of Food Scale - Food Available and Food Present.
| Six-week weight variability | |||||
|---|---|---|---|---|---|
|
| |||||
| Independent variable | b | SE | β | p | R 2 |
| 1.5-to-6 month % weight change | |||||
|
| |||||
| Independent Model (N = 182) | 0.00 | ||||
| Weight Variability | 0.00 | 0.01 | 0.02 | 0.75 | |
| Adjusted Model (N = 174) | 0.30 | ||||
| 6-week % weight change | 1.05 | 0.14 | 0.53 | <0.01 | |
| Number of sessions missed | 0.01 | 0.01 | 0.13 | 0.05 | |
| Treatment condition | −0.01 | 0.00 | −0.10 | 0.14 | |
| Gender | 0.01 | 0.01 | 0.06 | 0.39 | |
| Emotional Eating | 0.00 | 0.00 | 0.04 | 0.58 | |
| Preoccupation with Food | 0.00 | 0.00 | 0.07 | 0.54 | |
| Hedonic Hunger – Food Available | 0.00 | 0.01 | 0.00 | 0.99 | |
| Hedonic Hunger – Food Present | 0.00 | 0.00 | −0.08 | 0.39 | |
| Weight Variability | 0.00 | 0.01 | 0.00 | 0.98 | 0.00a |
|
| |||||
| 1.5-to-12 month % weight change | |||||
|
| |||||
| Independent Model (N = 179) | 0.03 | ||||
| Weight Variability | 0.02 | 0.01 | 0.18 | 0.02 | |
| Adjusted Model (N = 171) | 0.23 | ||||
| 6-week % weight change | 1.36 | 0.24 | 0.43 | <0.01 | |
| Number of sessions missed | 0.02 | 0.01 | 0.11 | 0.11 | |
| Treatment condition | −0.01 | 0.01 | −0.11 | 0.14 | |
| Gender | 0.02 | 0.01 | 0.13 | 0.09 | |
| Emotional Eating | 0.00 | 0.01 | 0.07 | 0.38 | |
| Preoccupation with Food | 0.00 | 0.01 | 0.03 | 0.82 | |
| Hedonic Hunger – Food Available | 0.01 | 0.01 | 0.10 | 0.48 | |
| Hedonic Hunger – Food Present | −0.01 | 0.01 | −0.07 | 0.48 | |
| Weight Variability | 0.02 | 0.01 | 0.17 | 0.02 | 0.03a |
|
| |||||
| 1.5-to-24 month % weight change | |||||
|
| |||||
| Independent Model (N = 173) | 0.03 | ||||
| Weight Variability | 0.02 | 0.01 | 0.17 | 0.03 | |
| Adjusted Model (N = 165) | 0.07 | ||||
| 6-week % weight change | 0.80 | 0.29 | 0.23 | 0.01 | |
| Number of sessions missed | 0.01 | 0.01 | 0.03 | 0.69 | |
| Treatment condition | 0.00 | 0.01 | 0.02 | 0.85 | |
| Gender | 0.01 | 0.02 | 0.03 | 0.76 | |
| Emotional Eating | 0.00 | 0.00 | 0.12 | 0.22 | |
| Preoccupation with Food | 0.00 | 0.01 | −0.04 | 0.73 | |
| Hedonic Hunger – Food Available | 0.01 | 0.01 | 0.07 | 0.64 | |
| Hedonic Hunger – Food Present | 0.00 | 0.01 | −0.03 | 0.77 | |
| Weight Variability | 0.02 | 0.01 | 0.17 | 0.04 | 0.03a |
Refers to R 2 change when adding weight variability to model.
Table 3.
Results from 12-week weight variability independent and adjusted multiple regression analyses. Covariates included 12-week % weight change; number of sessions missed; treatment condition; gender; Three Factor Eating Questionnaire - Emotional Eating; General Food Craving Questionnaire-Trait Preoccupation with Food; and Weight Efficacy Lifestlye Questionnaire - Negative Emotions and Social Pressure.
| Twelve-week weight variability | |||||
|---|---|---|---|---|---|
|
| |||||
| Independent variable | b | SE | β | p | R 2 |
| 3-to-6 month % weight change | |||||
|
| |||||
| Independent Model (N = 182) | 0.02 | ||||
| Weight Variability | 0.01 | 0.00 | 0.13 | 0.08 | |
| Adjusted Model (N = 175) | 0.33 | ||||
| 12-week % weight change | 0.43 | 0.05 | 0.55 | <0.01 | |
| Number of sessions missed | 0.00 | 0.00 | 0.06 | 0.41 | |
| Treatment condition | 0.00 | 0.00 | −0.08 | 0.20 | |
| Gender | 0.01 | 0.01 | 0.13 | 0.06 | |
| Emotional Eating | 0.00 | 0.00 | 0.10 | 0.12 | |
| Preoccupation with Food | 0.00 | 0.00 | 0.08 | 0.31 | |
| Self Efficacy to Negative Emotions | 0.00 | 0.00 | −0.02 | 0.83 | |
| Self Efficacy to Social Pressure | 0.00 | 0.00 | 0.11 | 0.18 | |
| Weight Variability | 0.01 | 0.00 | 0.09 | 0.21 | 0.00a |
|
| |||||
| 3-to-12 month % weight change | |||||
|
| |||||
| Independent Model (N = 179) | 0.07 | ||||
| Weight Variability | 0.03 | 0.01 | 0.27 | <0.01 | |
| Adjusted Model (N = 172) | 0.23 | ||||
| 12-week % weight change | 0.53 | 0.12 | 0.33 | <0.01 | |
| Number of sessions missed | 0.01 | 0.00 | 0.14 | 0.05 | |
| Treatment condition | −0.01 | 0.01 | −0.08 | 0.25 | |
| Gender | 0.02 | 0.01 | 0.14 | 0.07 | |
| Emotional Eating | 0.00 | 0.00 | 0.11 | 0.12 | |
| Preoccupation with Food | 0.00 | 0.00 | 0.06 | 0.45 | |
| Self Efficacy to Negative Emotions | 0.00 | 0.00 | 0.08 | 0.47 | |
| Self Efficacy to Social Pressure | 0.00 | 0.00 | −0.03 | 0.76 | |
| Weight Variability | 0.03 | 0.01 | 0.22 | <0.01 | 0.04a |
|
| |||||
| 3-to-24 month % weight change | |||||
|
| |||||
| Independent Model (N = 173) | 0.02 | ||||
| Weight Variability | 0.02 | 0.01 | 0.15 | 0.05 | |
| Adjusted Model (N = 166) | 0.06 | ||||
| 12-week % weight change | 0.21 | 0.16 | 0.11 | 0.18 | |
| Number of sessions missed | 0.00 | 0.01 | −0.02 | 0.77 | |
| Treatment condition | 0.01 | 0.01 | 0.06 | 0.48 | |
| Gender | 0.01 | 0.02 | 0.03 | 0.75 | |
| Emotional Eating | 0.00 | 0.00 | 0.10 | 0.20 | |
| Preoccupation with Food | 0.00 | 0.01 | −0.07 | 0.47 | |
| Self Efficacy to Negative Emotions | 0.00 | 0.00 | 0.04 | 0.76 | |
| Self Efficacy to Social Pressure | 0.00 | 0.00 | −0.14 | 0.16 | |
| Weight Variability | 0.02 | 0.01 | 0.17 | 0.04 | 0.03a |
Refers to R 2 change when adding weight variability to model.
Table 4.
Partial correlations between weight variability and baseline self-report measures. All correlations are controlling for baseline BMI.
| Baseline Variable | 6-week weight variability | 12-week weight variability |
|---|---|---|
| BESa | −0.11 | −0.06 |
| GFCQ Preoccupation with Foodb | −0.21** | −0.12^ |
| GFCQ Loss of Controlb | −0.07 | −0.02 |
| GFCQ Positive Outcome Expectancyb | −0.04 | 0.03 |
| GFCQ Emotional Cravingb | −0.13^ | −0.12 |
| PFS Food Availablec | −0.17* | −0.07 |
| PFS Food Presentc | −0.15* | −0.06 |
| PFS Food Tastedc | −0.13^ | −0.03 |
| WEL Negative Emotionsd | 0.08 | 0.14^ |
| WEL Availabled | 0.07 | 0.09 |
| WEL Social Pressured | 0.11 | 0.14^ |
| WEL Physical Discomfortd | 0.09 | 0.11 |
| WEL Positive Activityd | 0.02 | 0.00 |
| YFAe | −0.03 | −0.07 |
| TFEQ Cognitive Restraintf | 0.02 | −0.02 |
| TFEQ Uncontrolled Eatingf | −0.14^ | −0.06 |
| TFEQ Emotional Eatingf | −0.23** | −0.18* |
P < 0.01;
P < 0.05;
P < 0.10.
BES = Binge Eating Scale.
GFCQ = General Food Cravings Questionnaire - Trait.
PFS = Power of Food Scale.
WEL = Weight Efficacy Lifestyle Questionnaire.
YFA = Yale Food Addiction Scale.
TFEQ = Three Factor Eating Questionnaire.
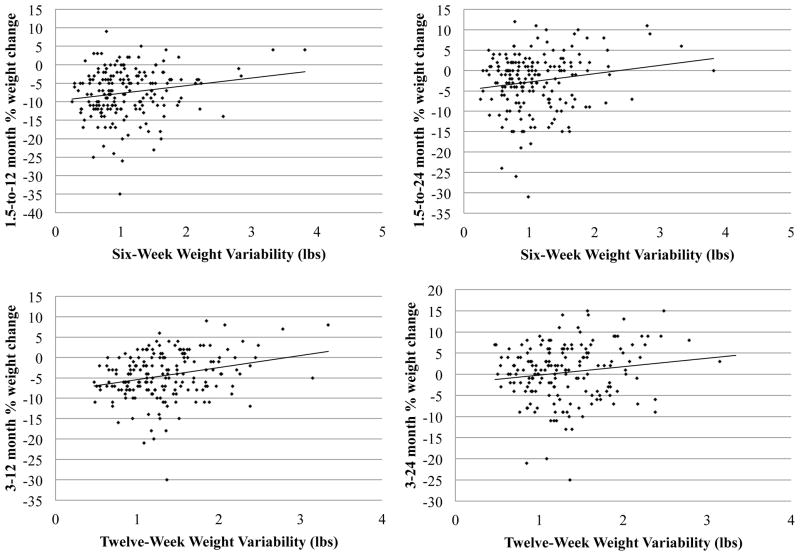
In the adjusted models, 6-week weight variability was still unrelated to 1.5-to-6 month percent weight change and continued to predict 1.5-to-12 month and 1.5-to-24 month percent weight change (see Table 2). Twelve-week weight variability still significantly predicted 3-to-12 and 3-to-24 month percent weight change and was unrelated to 3-to-6 month percent weight change (see Table 3). Fig. 2 shows scatterplots of weight variability with percent weight change at 12 and 24 months.1
Fig. 2.
Scatterplots of weight variability (x-axis) and subsequent percent weight change (y-axis). Higher weight variability was associated with less weight loss. Top: 6-week weight variability; Bottom: 12-week weight variability; Left: percent weight change at 12 months; Right: percent weight change at 24 months.
The gender-by-weight variability interaction was significant with 6-week weight variability on weight change from 1.5-to-6 months (b = 0.04, SEb = 0.01, β = 0.27, p = 0.01, R2 change = 0.03) controlling for the 6-week weight variability covariates. This interaction signifies that 6-week weight variability was more strongly associated with 6-month weight change for men than women. The interaction did not predict percent weight change over any of the time periods with 12-week weight variability, controlling for the 12-week covariates.
Discussion
This study assessed the relationship of weight variability over the initial 6 and 12 weeks of weight loss treatment with subsequent weight outcomes up to two years later. Higher weight variability measured over both the first 6 weeks and 12 weeks of treatment predicted poorer subsequent long-term weight control at 12 and 24 months. Relationships held when controlling for total weight change over the weight variability period and other covariates. Further, weight variability and percent weight change over the weight variability period were themselves unrelated. Findings suggest that the pattern of weight changes early in treatment, above and beyond amount of weight lost, is important in predicting outcomes. Those who lost similar amounts of weight each week early on were likely to have subsequently lost more weight after 12 and 24 months compared to those whose early weekly weight changes were more variable.
While weight variability did not predict weight change at the end of active treatment (6 months) controlling for covariates, a gender by 6-week weight variability interaction was significant, indicating that the relationship between 6-week weight variability and weight change at 6 months was stronger for men than for women. Further, weight variability was higher for men than women, controlling for BMI. As the sample was 81% women these findings should be interpreted with some caution; however it suggests that weekly variability in weight change may be particularly concerning for men.
The correlation between weight variability and future weight change does not necessarily indicate that weight variability causes weight gain. Research on dietary restraint supports the notion that strict dietary restriction, characterized as a “dichotomous, rule-based, all-or-nothing approach to eating,”10 which may result in large short-term weight losses, is often followed by disinhibition and regain.10,20,21 Those with higher weight variability could be using stricter, rigid dieting practices resulting in larger drops in weight. Rigid dieting could then spur periods of increased disinhibition and regain.22 Additionally, a period of regain may cause distress and a recommitment to rigid dieting. Over time, bidirectional forces between strict restraint and disinhibition could create a pattern of high weight variability. Participants following this pattern may not learn sustainable practices to maintain their weight loss over the subsequent months, as strict restraint has been associated with poorer long-term weight control.10,22 Alternatively, these results could be driven by a stronger drive to eat which produces greater energy intake and makes restraint harder to sustain. Another hypothesis is that physiological differences cause some to lose weight more consistently than others, leading to occasions where someone has followed recommendations consistently but does not immediately see the effects in their weight. Such occasions could lead one to feel disheartened, reducing motivation and causing weight regain.
Individuals reporting lower emotional eating, hedonic hunger, and preoccupation with food showed more variability in weight during the first 6 weeks of weight loss, and those with lower emotional eating also showed more weight variability in the first 12 weeks of treatment. This pattern is contrary to our expectation that individuals who reported more difficulty controlling their food intake would experience more weight variability. Lowe and colleagues similarly found that reports of disinhibition and restraint did not explain the relationship between weight variability and future weight gain.9 Thus the reason that weight fluctuation predicts future weight gain9 or poorer weight loss maintenance is enigmatic. Actual variation in body weight may provide a superior predictive ability compared to reported eating patterns that could contribute to weight patterns. However, as restraint and disinhibition ratings typically change during participation in a weight loss program,23,24 weekly measures of these and similar constructs could be more informative than baseline-only measures in testing the relationship between those dimensions and variation in weight.
A secondary aim of this study was to measure weight variability over two lengths of time in order to identify a timespan that includes sufficient data to be reliable while providing clinical information relatively early in treatment. The percent of variance in long-term outcomes explained by 6- week and 12-week weight variability were similar, and fairly small (between 2% and 7%). As weight variability over the first and second 6-week periods were correlated, and 6-week and 12-week weight variability were similarly related to long-term weight change, 6 weeks of weights are likely sufficient to identify individuals at risk for greater challenges for long-term weight loss. On the other hand, the fact that the 6-week, but not 12-week, results seemed to be influenced more by several non-statistical outliers suggests that the longer measurement of weight variability may be more stable. Using shorter periods to measure weight variability is preferable for potential clinical relevance. The sooner it is measured the earlier individuals at greater risk for weight regain can be identified and helped, should further research support the utility of weight variability as an indicator of poor outcome. More research is needed to identify the ideal balance between reliability and clinical usefulness in weight variability measurement.
While this study was not designed to examine the relationship between early weight loss and long-term outcomes, findings from this trial are consistent with others’ conclusions that weight loss early in treatment strongly predicts long-term weight change.3,4 We found that percent weight change over the first 6 weeks of treatment was significantly associated with subsequent weight loss at 6, 12, and 24 months, controlling for the covariates relevant to weight variability calculations. Those who are struggling to lose weight early on may benefit from extra intervention.
Despite the fact that early weight loss is undoubtedly easier to calculate than weight variability, and may be more strongly associated with future weight change, we see a strong rationale to study weight variability from both a theoretical, and potentially clinical, perspective. For example, even one small weight gain during treatment has been shown to predict poorer outcomes.25 Weight loss programs may be able to increase efficacy by placing a higher focus on consistency in weight losses week to week, but a better understanding of the importance of a steady weight trajectory is needed.
An important question for future research is whether losing weight in a more variable fashion itself is harmful, or whether it is a marker for something else that leads to poorer long-term weight control. The literature on intermittent fasting suggests that purposeful variety between strict and lenient restriction may produce similar outcomes to traditional behavioral weight loss prescriptions (see review).26 If reliable, this finding suggests that weight variability is not itself responsible for poorer weight outcomes, instead suggesting that another characteristic may cause both greater variability and poorer long-term weight control. For example, weight variability may indicate inconsistent adherence to dietary prescriptions. The manipulation of degree of weight variability during a weight loss program would be the best way of determining its causal status.
Strengths of this study include the relatively large sample of individuals in a behavioral weight loss program with objectively measured weights over two years. Weaknesses include the correlational nature of the data, which limits causal interpretations. Additionally, a substantial portion of those who entered the study did not contribute body weights at all assessment points. In particular, black and younger participants were more likely to drop out of treatment than white and older participants, suggesting that results may not generalize across age and race. Racial disparities in weight loss outcomes is a common pattern.27,28 Further, the fact that 6-week weight variability results weakened when excluding visual (but not statistically significant) outliers is important to acknowledge.
In conclusion, higher variability in weekly weights during the first 6 and 12 weeks of treatment predicted poorer subsequent weight loss 1 and 2 years after treatment initiation. Future research should examine replicability of these findings, and should clarify whether measuring weight variability adds to interventionists’ ability to identify individuals unlikely to achieve meaningful and sustainable weight loss. If found to be reliable and useful, weight variability early in treatment may be cause for supplemental intervention. An algorithm could be created to calculate weight variability after each week (or even each day, given the development of new technologies to monitor weight at home) of a weight loss program to be used by program leaders as a clinical indicator for risk of poor outcomes. Future research should also examine the behavioral and/or metabolic basis for weight variability, and whether increasing stability of week-to-week weight losses improves long-term outcomes. If this is the case, behavioral interventions may benefit from a stronger focus on consistent weekly weight losses.
What is already known about this subject?
Weight loss early in treatment has repeatedly predicted long-term outcomes.
Greater weight variability over 6 months has predicted weight gain two years later in college women.
What does this study add?
The present study found that weight variability in the first 6 and 12 weeks of treatment was associated with less subsequent weight loss 12 and 24 months later, above and beyond the effect of early weight loss.
Weekly variability in weight during active weight loss treatment may be a useful prognostic indicator for long-term success.
Acknowledgments
Funding: This study was supported by grant R01 DK080909 from the National Institutes of Health.
Footnotes
Scatterplots suggested several possible outliers with either high weight variability values or large amounts of weight loss. Statistical analysis of outliers suggested that these values are within a reasonable range (Cook’s D < 1 and p > 0.05 for all participants).29 However, analyses were run excluding visual outliers (defined as weight variability > 3 or % weight loss > 30). All 12-week weight variability significance levels remained the same. The relationship between 6-week weight variability and weight change weakened, with a nonsignificant relationship between weight variability and 1.5-to-12 month weight loss (p = 0.23) and a marginal independent relationship between WV and 1.5-to-24 month weight loss (p = 0.06). Both relationships were nonsignificant after controlling for covariates.
Disclosure: MRL receives compensation from funded users of the Power of Food Scale. EHF declared no conflict of interest.
References
- 1.Wadden TA, West DS, Neiberg RH, et al. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity (Silver Spring) 2009;17(4):713–722. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barte JCM, ter Bogt NCW, Bogers RP, et al. Maintenance of weight loss after lifestyle interventions for overweight and obesity, a systematic review. Obes Rev. 2010;11(12):899–906. doi: 10.1111/j.1467-789X.2010.00740.x. [DOI] [PubMed] [Google Scholar]
- 3.Unick JL, Hogan PE, Neiberg RH, et al. Evaluation of early weight loss thresholds for identifying nonresponders to an intensive lifestyle intervention. Obesity. 2014;22(7):1608–1616. doi: 10.1002/oby.20777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carels Ra, Cacciapaglia HM, Douglass OM, Rydin S, O’Brien WH. The early identification of poor treatment outcome in a women’s weight loss program. Eat Behav. 2003;4(3):265–282. doi: 10.1016/S1471-0153(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka M, Itoh K, Abe S, et al. Irregular Patterns in the Daily Weight Chart at Night Predict Body Weight Regain. Exp Biol Med. 2004;229(9):940–945. doi: 10.1177/153537020422900911. [DOI] [PubMed] [Google Scholar]
- 6.Orsama AL, Mattila E, Ermes M, Van Gils M, Wansink B, Korhonen I. Weight rhythms: Weight increases during weekends and decreases during weekdays. Obes Facts. 2014;7(1):36–47. doi: 10.1159/000356147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorin aa, Phelan S, Wing RR, Hill JO. Promoting long-term weight control: does dieting consistency matter? Int J Obes Relat Metab Disord. 2004;28(2):278–281. doi: 10.1038/sj.ijo.0802550. [DOI] [PubMed] [Google Scholar]
- 8.Brownell KD. LEARN Program for Weight Management 2000. American Health; 2000. [Google Scholar]
- 9.Lowe MR, Feig EH, Winter SR, Stice E. Short-term variability in body weight predicts long-term weight gain. Am J Clin Nutr. 2015;102(5):995–999. doi: 10.3945/ajcn.115.115402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson F, Pratt M, Wardle J. Dietary restraint and self-regulation in eating behavior. Int J Obes (Lond) 2012;36(5):665–674. doi: 10.1038/ijo.2011.156. [DOI] [PubMed] [Google Scholar]
- 11.Klein S, Sheard NF, Pi-Sunyer X, et al. Weight Management Through Lifestyle Modification for the Prevention and Management of Type 2 Diabetes: Rationale and Strategies. Diabetes Care. 2004;27(8):2067–2073. doi: 10.2337/diacare.27.8.2067. [DOI] [PubMed] [Google Scholar]
- 12.Nijs IMT, Franken IHA, Muris P. The modified Trait and State Food-Cravings Questionnaires: Development and validation of a general index of food craving. Appetite. 2007;49(1):38–46. doi: 10.1016/j.appet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Lowe MR, Butryn ML, Didie ER, et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite. 2009;53(1):114–118. doi: 10.1016/j.appet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav. 1982;7(1):47–55. doi: 10.1016/0306-4603(82)90024-7. [DOI] [PubMed] [Google Scholar]
- 15.Timmerman GM. Binge eating scale: further assessment of validity and reliability. J Appl Biobehav Res. 1999;4(1):1–12. [Google Scholar]
- 16.Gearhardt AN, Corbin WR, Brownell KD. Preliminary validation of the Yale food addiction scale. Appetite. 2009;52(2):430–436. doi: 10.1016/j.appet.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Clark MM, Abrams DB, Niaura RS, Eaton CA, Rossi JS. Self-efficacy in weight management. J Consult Clin Psychol. 1991;59(5):739–744. doi: 10.1037/0022-006X.59.5.739. [DOI] [PubMed] [Google Scholar]
- 18.Navidan A, Abedi MR, Baghban I, Fatehizade MS, Poursharifi H. Reliability and validity of the weight efficacy lifestyle questionnaire in overweight and obese individuals. Int J Behav Sci. 2009;3(3):217–222. [Google Scholar]
- 19.Karlsson J, Persson LO, Sjöström L, Sullivan M. Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. Int J Obes Relat Metab Disord. 2000;24(12):1715–1725. doi: 10.1038/sj.ijo.0801442. [DOI] [PubMed] [Google Scholar]
- 20.Herman CP, Polivy J. A boundary model for the regulation of eating. Psychiatr Ann. 1983;13(12):918–927. [PubMed] [Google Scholar]
- 21.Westenhoefer J. Dietary restraint and disinhibition: Is restraint a homogeneous construct? Appetite. 1991;16(1):45–55. doi: 10.1016/0195-6663(91)90110-E. [DOI] [PubMed] [Google Scholar]
- 22.Byrne SM, Cooper Z, Fairburn CG. Psychological predictors of weight regain in obesity. Behav Res Ther. 2004;42(11):1341–1356. doi: 10.1016/j.brat.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Foster GD, Wadden Ta, Swain RM, Stunkard aJ, Platte P, Vogt Ra. The Eating Inventory in obese women: clinical correlates and relationship to weight loss. Int J Obes Relat Metab Disord. 1998;22:778–785. doi: 10.1038/sj.ijo.0800659. [DOI] [PubMed] [Google Scholar]
- 24.Savage JS, Hoffman L, Birch LL. Dieting, restraint, and disinhibition predict women’s weight change over 6 y. Am J Clin Nutr. 2009;90(1):33–40. doi: 10.3945/ajcn.2008.26558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schumacher L, Gaspar M, Remmert J, Zhang F, Forman E, Butryn M. Small weight gains during obesity treatment: normative or cause for concern? Obes Sci Pract. 2016;2(4):366–375. doi: 10.1002/osp4.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varady KA. Intermittent versus daily calorie restriction: Which diet regimen is more effective for weight loss? Obes Rev. 2011;12(7) doi: 10.1111/j.1467-789X.2011.00873.x. [DOI] [PubMed] [Google Scholar]
- 27.Lewis KH, Edwards-Hampton SA, Ard JD. Disparities in Treatment Uptake and Outcomes of Patients with Obesity in the USA. Curr Obes Rep. 2016:1–9. doi: 10.1007/s13679-016-0211-1. [DOI] [PubMed] [Google Scholar]
- 28.Butryn M, Forman E, Lowe M, Gorin A, Zhang F, Schaumberg K. Efficacy of Environmental and Acceptance-Based Enhancements to Behavioral Weight Loss Treatment: The ENACT Trial. Obesity. 2017 doi: 10.1002/oby.21813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.RDC, Weisberg S. Residuals and influence in regression. Biometrical J. 1982 [Google Scholar]