Abstract
Adverse birth outcomes such as preterm birth, low birth weight and infant mortality continue to disproportionately affect black and poor infants in the United States. Improvements in healthcare quality and access have not eliminated these disparities. The objective of this review was to consider societal factors, including suboptimal education, income inequality, and residential segregation, that together lead to toxic environmental exposures and psychosocial stress. Many toxic chemicals, as well as psychosocial stress, contribute to the risk of adverse birth outcomes and black women often are more highly exposed than white women. The extent to which environmental exposures combine with stress and culminate in racial disparities in birth outcomes has not been quantified but is likely substantial. Primary prevention of adverse birth outcomes and elimination of disparities will require a societal approach to improve education quality, income equity, and neighborhoods.
Introduction
Twenty years ago, David and Collins published groundbreaking findings demonstrating that black women in Illinois who were born in the United States gave birth to substantially smaller infants than black women who immigrated from Africa to the United States.1 Immigrant black women’s birth outcomes were much more similar to white mothers than they were to their African American counterparts. This key observation largely debunked the hypothesis that racial disparities in birth outcomes result from genetic differences between races and ethnic groups.2 There is something about being black in America over time, perhaps generations, that leads to worse birth outcomes for black infants. Over the following two decades, the search for modifiable factors that result in these disparities has been relentless, but disparities persist (Table 1). Black infants have a 50% higher risk of being born preterm (before 37 weeks of gestation), are almost twice as likely to be born low birth weight (LBW, less than 2,500 grams), and are more than twice as likely to die in the first year of life.3–5 Fetal deaths are also more than twice as common among black women compared with white women.6 Despite all of the effort and resources that have been expended to discover the reasons for birth outcome disparities, the mechanisms remain poorly understood.
Table 1.
| Black | White | Black/White disparity | |
|---|---|---|---|
| Outcome | % | % | RR |
| Preterm birth (< 37 weeks) | 13.0 | 8.9 | 1.46 |
| Very preterm (< 34 weeks) | 4.6 | 2.4 | 1.92 |
| Low birth weight (< 2,500 grams) | 12.8 | 7.0 | 1.83 |
| Very low birth weight (< 1,500 grams) | 2.8 | 1.1 | 2.55 |
| n/1,000 | n/1,000 | RR | |
| Infant mortality (< 1 year) | 11.05 | 4.93 | 2.24 |
| Neonatal mortality (< 28 days) | 7.32 | 3.37 | 2.17 |
| Fetal death (> 20 weeks) | 10.3 | 4.88 | 2.11 |
While improvements in healthcare and healthcare access can improve health overall, tackling disparities has proven difficult. Massachusetts instituted universal health insurance in 2006, but disparities persist. In 2005, black infants were 40% more likely than white infants to be born preterm (12.4% versus 8.8%) and 64% more likely to be LBW (12.0% versus 7.3%).7 In 2014, eight years after healthcare reform, black infants were still 18% more likely to be born preterm (10.0% versus 8.5%) and 48% more likely to be LBW (10.2% versus 6.9%).8 The prevention of preterm birth relies on identifying high-risk pregnancies and targeting therapies such as progesterone or cerclage placement.9 However, screening for women at high risk for preterm birth is not always universal, even when women receive prenatal care.10 Further, even if optimal screening and use of medical interventions to prevent preterm birth were uniformly distributed, only a fraction of preterm deliveries would be averted. Data demonstrate that progesterone can reduce the risk of preterm birth in high-risk women, as defined by a prior preterm delivery or short cervical length, by 43%.11 Assuming 20% of pregnancies were high risk, the overall reduction in preterm birth for the population would be just under 10%. A more comprehensive societal approach that extends beyond healthcare will be required to improve birth outcomes and eliminate their disparities.
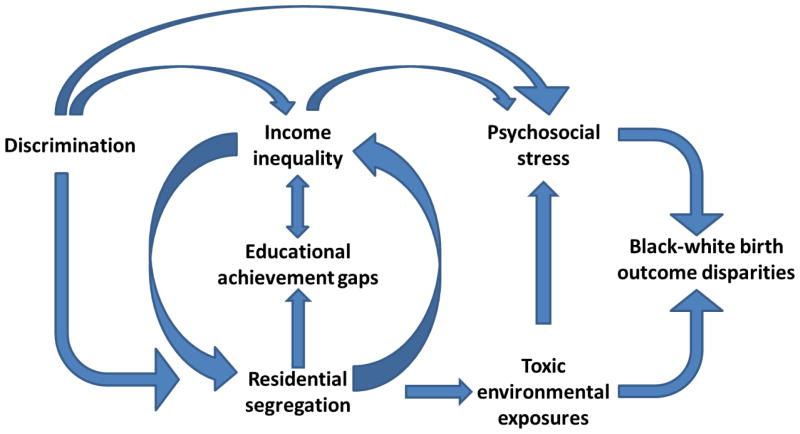
In this review, we focus on social factors such as suboptimal education and poverty, in addition to environmental exposures to air pollution, metals such as lead, and recently identified toxins such as phthalates, that differ by race and ethnicity in the United States. Lastly, we discuss the impact of psychosocial stressors, including discrimination on birth outcomes. Each of these factors may increase the risk of adverse health outcomes for all adults and children in the United States, but also likely combine to lead to unequal beginnings for the youngest members of our society (Figure 1).
Figure 1.
Conceptual model of societal factors in the United States that lead to psychosocial stress, toxic environmental exposures, and ultimately contribute to racial disparities in birth outcomes.
Education
In almost all epidemiologic studies, investigators adjust for socioeconomic position, so as to isolate the potentially causal relationship between other factors, such as smoking or diabetes, and the risk of an outcome, such a preterm birth. However, socioeconomic position itself is reproducibly associated with an increased risk of adverse birth outcomes. One of the primary contributors to socioeconomic position is education. The United States has a long history of racial segregation and inequality in schools that did not end with the 1954 Brown v. Board of Education of Topeka Supreme Court ruling outlawing segregation. In 2015, the National Assessment of Educational Progress produced a report to describe school racial composition in the United States and its association with academic achievement gaps.12 The report stated that segregation persists: white students attend schools that average 9% black students, while black students most often attend schools that are 48% black. Both black and white students underperformed at schools with a higher density of black students compared to students at schools with a lower density of black students, but the achievement gap between black and white students was similar between schools. This finding suggests that where students attend school matters for achievement overall, but that, regardless of racial composition, schools are equally poor at eliminating achievement gaps. Further, four-year high school graduation rates are substantially lower for black students (73%) than white students (87%).13 Fewer years of education are associated with increased risks of preterm birth, suboptimal fetal growth, still birth and infant mortality.14 How lower levels of education may cause poor birth outcomes is not completely understood. Associations may be partially attributable to personal habits such as smoking, which is more common among women with lower education levels, but models including both smoking and education reveal independent associations of each of these factors with birth outcomes such as birth weight.15 This suggests that low educational attainment may work through other pathways to lead to adverse birth outcomes. Additionally, equity in education is unlikely to eliminate disparities in birth outcomes. Schoendorf and colleagues compared mortality rates among infants of college educated black (n=42,230) and white (n=865,128) parents and found that even in this educated group, the mortality rate of black infants (10.2 per 100 live births) was twice that of white infants (5.4 per 100 live births).16 They concluded that this disparity was attributable to differences in LBW (7% versus 3%) because mortality did not differ by race among infants who were not LBW. These findings suggest that addressing the many sources of inequality that lead to a higher risk of LBW (a composite outcome of preterm birth and growth restriction) will be required to eliminate racial disparities in birth outcomes.
Income
Another key driver of socioeconomic position is income, which is often determined by educational attainment.17 Higher income is associated with improved birth outcomes. Parker and colleagues analyzed data from the 1988 National Maternal and Infant Health Survey, a nationally representative sample of over 6000 women.18 Poor women were from households with incomes less than 100% of the federal poverty line, whereas affluent women were from households with incomes greater than 200% of the poverty line. In race-stratified analyses, the investigators found that for both black and white women, birth outcomes were worse among poor women compared with more affluent women. Specifically, poor white women were more likely to deliver LBW (6.5%) or small-for-gestational age (SGA) (10.6%) infants than affluent white women (3.6% and 7.4%, respectively). Poor black women were more likely to delivery LBW (12.1%) and preterm (12.2%) infants compared to wealthier black women (8.8% and 7.4% respectively). Of note, income is not the only driver of racial disparities as highlighted in this same study where poor white women had better birth outcomes than affluent black women for all three adverse outcomes (LBW: 6.5% versus 8.8%, SGA: 10.6% versus 15.6%, and preterm: 3.5% versus 7.4%, respectively).
Income and race determine many aspects of life in the United States; one of the most important is where people live.19 This relationship is further complicated by the fact that where one lives can, in turn, determine income potential due to employment opportunity and educational quality differences. The interactions among race, education, income, and neighborhood can lead to health disparities. As Williams and Collins argue, residential segregation leads to disparities in health through “pathogenic residential conditions” that disproportionately affect black families, such as proximity to abandoned buildings and more commercial and industrial facilities.20 Housing quality itself also is more likely to be poor in highly segregated, non-white areas manifesting as excessive crowding, noise levels, and exposure to allergens such as dust mites and pollutants such as air particulates and lead. The extent to which environmental contamination of residential buildings affects disparities in birth outcomes remains incompletely understood.
Environmental exposures
In its report, Preterm birth: causes, consequences, and prevention, the Institute of Medicine summarized the data through 2006 on the contribution of environmental exposures to adverse birth outcomes.21 The report concluded that the contribution of environmental pollutants to preterm birth was understudied, except in the case of lead and environmental tobacco smoke, both of which have been shown in multiple studies to increase the risk of preterm birth.
It has long been established that lead exposure increases preterm birth risk. Specifically, a systematic review by Andrews and colleagues demonstrated that women who delivered preterm had higher mean blood lead levels.22 In other studies, placental lead was negatively associated with gestational age at birth and maternal blood lead was associated with lower birth weight.23,24 These latter findings are consistent with cohort data demonstrating a higher risk of LBW among women with occupational lead exposure in Norway.25 Our group recently found that this association was strongest for infants at the lower end of the birth weight-for-gestational-age spectrum, suggesting increased susceptibility to lead among fetuses that were already growing poorly.26
Despite the well-established associations between lead and adverse birth outcomes, only rarely do epidemiologic studies documenting disparities in preterm birth integrate environmental data. While lead levels generally have been declining throughout the United States, for each of the National Health and Nutrition Examination Surveys from 1999–2012 average levels have been consistently higher among non-Hispanic black compared with non-Hispanic white adults and children.27 Given these persistent differences in lead levels, it is plausible that differential lead levels may contribute to disparities in birth outcomes.
Lead is not the only environmental exposure that may contribute to adverse birth outcomes. Air pollution and its components have been shown to increase the risks of lower birth weight and shorter gestation. Stieb and colleagues performed a meta-analysis and systematic review of ambient air pollution exposure in pregnancy and birth outcomes.28 The most consistent findings were negative associations of carbon monoxide, nitrogen dioxide and particulate matter with birth weight. The most consistent estimates of increases in the risk of preterm birth were from third-trimester air pollution exposure. The authors concluded that the increased certainty regarding third-trimester exposures and preterm birth could be either biological or a result of less heterogeneity among studies focused on the third trimester. Ghosh and colleagues also reviewed the literature and concluded that fetal sex affected the association between air pollution and preterm birth, with exposed male fetuses at higher risk of preterm birth compared to exposed female fetuses.29 In sum, it is indisputable that air pollution is bad for human health and increases the risk of adverse birth outcomes. However, air pollution, like income and education, is not uniformly distributed across society.
Black adults and children experience higher levels of air pollution exposure than their white counterparts.30,31 Air pollution results from many sources, including traffic and industrial emissions from fossil fuel combustion, that lead to particulate and gaseous emissions that can harm human health. Predominantly black neighborhoods experience higher levels of exposure due to urban concentrations of populations with close residential proximity to traffic and industrial pollution.32 The extent to which differential exposure to air pollution contributes to disparities in birth outcomes has not been fully explored.
Other environmental contaminants can increase the risk of adverse birth outcomes. One class of chemicals that has garnered recent interest is phthalates. Phthalates are a class of ubiquitous man-made chemicals that are used to manufacture plastics, building materials such as flooring, adhesives, fast food packaging, and personal care products. 33–36 Phthalates are just one of several contaminants that can act as endocrine disruptors. In the United States, nearly all pregnant women have detectable levels of phthalate metabolites in urine samples.37,38 Although phthalate metabolites have short half-lives, humans are constantly exposed to varying levels of phthalates from personal care product use, indoor environments, and dietary patterns.39–41 Phthalate metabolite levels have been shown to be associated with preterm birth, specifically spontaneous preterm birth.42,43
As with lead and air pollution, phthalates are not uniformly distributed throughout society. In a review on the contribution of endocrine-disrupting chemicals and their contribution to racial disparities in reproductive outcomes, James-Todd and colleagues highlight differences in exposure to these chemicals by race.44 Specifically, non-Hispanic black women have higher levels of the low molecular weight phthalates that come from personal care products, adhesives and some medications than non-Hispanic white women.44–46 Personal care product use often is socially programmed, and one study found that vaginal douching differences might account for differences by race in levels of one particular phthalate metabolite (di-ethyl phthalate).47 This finding is interesting because douching may be associated with increased risks of preterm birth and low birth weight.48–51 Whether sources of phthalate exposure or different phthalate metabolite levels resulting from these sources are associated with excess preterm birth risk among black women has not been established. However, it is certainly plausible that differences in the built environment (i.e. flooring), personal care product use and fast food consumption could all lead to differences in phthalate levels by race.
Not all chemical exposures lead to worse birth outcomes and not all chemical exposures are higher in black women compared with white women. Perfluoroalkyl substances and organochlorines, which are chemicals used in the manufacturing of textiles and industrial farming, respectively, can pollute water and contaminate food supplies.52,53 While there is some evidence that these chemicals negatively affect fetal growth, not all data support this claim. In a recent study in Scandinavia, infants born to Swedish, but not Norwegian, women with higher exposure to perfluorooctanoate, polycholirnated biphenyl (PCB) 153 and hexacholorbenzene were more likely to be born SGA.54 Further, according to the Centers for Disease Control and Prevention National Health and Nutrition Examination Study biomonitoring data, black women have slightly lower levels of all three of these chemicals.27 With respect to PCBs and preterm birth, there have been multiple negative studies suggesting that PCBs do not increase preterm birth risk in the United States.21 It is notable that these environmental chemicals are not associated with an increased risk of adverse birth outcomes nor are they associated with race. Identifying which chemicals both increase the risk of adverse birth outcomes and disproportionately affect black women may allow for interventions that could reduce disparities.
Physiologic mechanisms by which environmental exposures lead to adverse birth outcomes are not completely described, but exposures such as lead, air pollution and phthalates can result in oxidative stress and/or inflammation,55–59 which are implicated in preterm birth and LBW.60–63 More recently, placental mitochondrial DNA content, which can be diminished by oxidative stress, has been shown to be lower in the settings of both higher exposure to air pollution and lower birth weights.64 While biologically plausible, definitively determining whether environmental factors cause adverse birth outcomes is difficult. Randomized trials of exposure, the gold standard to determine causal relationships, are unethical when an exposure is known to be harmful. However, randomized controlled trials to reduce indoor air pollution by replacing open fire cooking with stoves have been performed in developing countries to determine whether respiratory outcomes are improved. Thompson and colleagues performed subgroup analysis among pregnant participants of one such study in Guatemala.65 Women who were randomized to the stove with a chimney had infants who were an average of 89 (95% CI −27, 204) grams heavier than infants born to mothers who cooked using open fires. While the result did not reach statistical significance, studies such as these will help to determine causal relationships between environmental exposures and birth outcomes.
Psychosocial stressors
While not a toxic environmental exposure, per se, psychosocial stress can result from where people live. Racial disparities in birth outcomes may be due, in part, to excess exposure to stress.66,67 Exposure to certain stressors, specifically violence68–70 and discrimination71,72 in pregnancy, are associated with the risk of preterm birth. Further, responses to stressors, including depression and pregnancy-related anxiety, predict preterm birth.73,74 While stress in pregnancy does not affect only minority women, black women report more stress than white women, which can lead to adverse birth outcomes. 75,76 For example, black women are vastly more likely to experience racial discrimination that white women. Rankin and colleagues conducted a case-control study of black women in the U.S. and found that women who delivered preterm had over twice the odds of high exposure to interpersonal racial discrimination in public settings in the prior year (odds ratio: 2.5, 95% CI: 1.2–5.2).72 This is consistent with a prior finding from this group that women who delivered very low birth weight infants (<1,500 g) were more likely to report high levels of lifetime exposure to interpersonal discrimination, an association that persisted after adjustment for other sociodemographic factors.75
Not surprisingly, stress may result directly from the environment. For example, natural disasters,77 terrorism,78 noise79 and even perceptions of air pollution80 may affect psychosocial stress. Furthermore, psychosocial stress may increase physiologic susceptibility to environmental chemicals and exposures. Cory-Slechta and colleagues performed experiments in a rodent model using restraints as a stressor and maternal lead exposure.81 They analyzed corticosterone and neurotransmitter levels in dams and offspring. They found that maternal corticosterone increased in response to lead alone and stress alone. Further, they found that stress in combination with maternal lead exposure among female offspring increased dopamine concentrations in the frontal cortex, whereas neither stress alone nor lead alone did so. There also is recent evidence in humans that stress and lead interact during human pregnancy, although the direction of the interaction was not that of potentiation. Tamayo Y Ortiz and colleagues found that the negative association between maternal lead exposure and developmental scores in offspring at two years of age was more pronounced among women who reported fewer negative life events.82 While this would potentially suggest a buffering effect of stress against the negative effects of lead, the potential biologic interaction of the two exposures to affect offspring outcomes warrants further study. Additionally, there is evidence of prenatal air pollution and psychosocial stress interactions resulting in worse outcomes. For example, Cowell and colleagues demonstrated that among boys prenatal exposure to black carbon, a component of air pollution, and maternal stress were associated with lower Attention Concentration Index scores, whereas there was no main effect of either stress or black carbon alone.83
Whether psychosocial stress and environmental factors interact to affect birth outcomes is not well studied, although there are some data to suggest that lower socioeconomic position may increase susceptibility to air pollution with respect to preterm birth and LBW risk.84 For example, Ponce and colleagues found that traffic-related air pollution, as measured by distance-weighted traffic density, disproportionally increased the risk for preterm birth in neighborhoods of low socioeconomic position in the winter in Los Angeles County, California in the mid-1990s.85 However, the extent to which psychosocial stress reflects, predicts, or interacts with environmental exposures and social position to result in adverse birth outcomes and disparities remains unknown.
Conclusions
Recognizing that experiences of being black in America vary, certain truths require an examination of societal exposures that contribute to injustice. One of those truths is that black women in the United States have a much higher risk of adverse birth outcomes compared with white women. While some individual choices may mitigate or potentiate these risks, exposures that are not modifiable on an individual level persist. The cycle of residential segregation, educational disadvantage, income inequality and the resulting environmental exposure to unclean air and water must be interrupted to make significant progress in eliminating disparities in birth outcomes. Incremental improvements in healthcare and access to healthcare will not do this. Primary prevention of adverse birth outcomes and their disparities requires improving schools and neighborhoods, achieving income equality and enacting environmental reform. Until these changes occur, the births of small and preterm black infants will continue to embody the legacy of longstanding racial discrimination.
Acknowledgments
Dr. Burris’s work is funded by NIH: K23 ES022242 and the Chrissy and Jesse Brown family.
Footnotes
Conflicts of Interests, Financial Disclosures: The authors have no conflicts of interests or financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.David RJ, Collins JW., Jr Differing birth weight among infants of U.S.-born blacks, African-born blacks, and U.S.-born whites. The New England journal of medicine. 1997 Oct 23;337(17):1209–1214. doi: 10.1056/NEJM199710233371706. [DOI] [PubMed] [Google Scholar]
- 2.David R, Collins J., Jr Disparities in infant mortality: what’s genetics got to do with it? American journal of public health. 2007 Jul;97(7):1191–1197. doi: 10.2105/AJPH.2005.068387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamilton BE, Martin JA, Osterman MJ. Births: Preliminary Data for 2015. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2016 Jun;65(3):1–15. [PubMed] [Google Scholar]
- 4.Hamilton BE, Martin JA, Osterman MJ, Curtin SC, Matthews TJ. Births: Final Data for 2014. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2015 Dec;64(12):1–64. [PubMed] [Google Scholar]
- 5.Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. Deaths: Final Data for 2014. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2016 Jun;65(4):1–122. [PubMed] [Google Scholar]
- 6.MacDorman MF, Gregory EC. Fetal and Perinatal Mortality: United States, 2013. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2015 Jul 23;64(8):1–24. [PubMed] [Google Scholar]
- 7.Massachusetts Births 2005. Boston, MA: Division of Research and Epidemiology, Center for Health Information, Statistics, Research, and Evaluation, Massachusetts Department of Public Health; Jan, 2007. [Google Scholar]
- 8.Massachuesstes births 2014. Boston, MA: Office of Data Management and Outcomes Assessment, Massachusetts Department of Public Helath; Sep, 2015. [Google Scholar]
- 9.Committee on Practice Bulletins-Obstetrics, The American College of Obstetricians and Gynecologists. Practice bulletin no. 130: prediction and prevention of preterm birth. Obstetrics and gynecology. 2012 Oct;120(4):964–973. doi: 10.1097/AOG.0b013e3182723b1b. [DOI] [PubMed] [Google Scholar]
- 10.Haviland MJ, Shainker SA, Hacker MR, Burris HH. Racial and ethnic disparities in universal cervical length screening with transvaginal ultrasound. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2016 Dec;29(24):4078–4081. doi: 10.3109/14767058.2016.1157577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackenzie R, Walker M, Armson A, Hannah ME. Progesterone for the prevention of preterm birth among women at increased risk: a systematic review and meta-analysis of randomized controlled trials. American journal of obstetrics and gynecology. 2006 May;194(5):1234–1242. doi: 10.1016/j.ajog.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 12.Bohrnstedt G, Kitmitto S, Ogut B, Sherman D, Chan D. School Composition and the Black–White Achievement Gap (NCES 2015-018) U.S. Department of Education; Washington, DC: National Center for Education Statistics; 2015. [Accessed January 24, 2017]. [Google Scholar]
- 13.National Center for Educational Statistics. [Accessed January 24, 2017];Public high school graduation rates. https://nces.ed.gov/programs/coe/indicator_coi.asp.
- 14.Luo ZC, Wilkins R, Kramer MS, Fetal Infant Health Study Group of the Canadian Perinatal Surveillance S. Effect of neighbourhood income and maternal education on birth outcomes: a population-based study. CMAJ: Canadian Medical Association journal = journal de l’Association medicale canadienne. 2006 May 09;174(10):1415–1420. doi: 10.1503/cmaj.051096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erickson AC, Ostry A, Chan HM, Arbour L. Air pollution, neighbourhood and maternal-level factors modify the effect of smoking on birth weight: a multilevel analysis in British Columbia, Canada. BMC public health. 2016 Jul 16;16(1):585. doi: 10.1186/s12889-016-3273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoendorf KC, Hogue CJ, Kleinman JC, Rowley D. Mortality among infants of black as compared with white college-educated parents. The New England journal of medicine. 1992 Jun 04;326(23):1522–1526. doi: 10.1056/NEJM199206043262303. [DOI] [PubMed] [Google Scholar]
- 17.Earnings and unemployment rates by educational attainment. Bureau of Labor Statistics; [Accessed Januray 24, 2017]. https://www.bls.gov/emp/ep_table_001.htm. [Google Scholar]
- 18.Parker JD, Schoendorf KC, Kiely JL. Associations between measures of socioeconomic status and low birth weight, small for gestational age, and premature delivery in the United States. Annals of epidemiology. 1994 Jul;4(4):271–278. doi: 10.1016/1047-2797(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 19.Logan JR. The Persistence of Segregation in the 21st Century Metropolis. City & community. 2013 Jun 01;12(2) doi: 10.1111/cico.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public health reports. 2001 Sep-Oct;116(5):404–416. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Behrman RE, Butler AS Institute of Medicine (U.S.). Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm birth: causes, consequences, and prevention. Washington, D.C: National Academies Press; 2007. [PubMed] [Google Scholar]
- 22.Andrews KW, Savitz DA, Hertz-Picciotto I. Prenatal lead exposure in relation to gestational age and birth weight: a review of epidemiologic studies. American journal of industrial medicine. 1994 Jul;26(1):13–32. doi: 10.1002/ajim.4700260103. [DOI] [PubMed] [Google Scholar]
- 23.Falcon M, Vinas P, Luna A. Placental lead and outcome of pregnancy. Toxicology. 2003 Mar 14;185(1–2):59–66. doi: 10.1016/s0300-483x(02)00589-9. [DOI] [PubMed] [Google Scholar]
- 24.Taylor CM, Golding J, Emond AM. Adverse effects of maternal lead levels on birth outcomes in the ALSPAC study: a prospective birth cohort study. BJOG: an international journal of obstetrics and gynaecology. 2015 Feb;122(3):322–328. doi: 10.1111/1471-0528.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irgens A, Kruger K, Skorve AH, Irgens LM. Reproductive outcome in offspring of parents occupationally exposed to lead in Norway. American journal of industrial medicine. 1998 Nov;34(5):431–437. doi: 10.1002/(sici)1097-0274(199811)34:5<431::aid-ajim3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 26.Rodosthenous RS, Burris HH, Svensson K, et al. Prenatal lead exposure and fetal growth: Smaller infants have heightened susceptibility. Environment international. 2017 Feb;99:228–233. doi: 10.1016/j.envint.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Department of Health and Human Services, Centers for Disease Control and Prevention. [Accessed 02/27/2017, 2017];Fourth National Exposure Report, Updated Tables. 2014 Aug; https://www.cdc.gov/exposurereport/pdf/fourthreport_updatedtables_aug2014.pdf.
- 28.Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environmental research. 2012 Aug;117:100–111. doi: 10.1016/j.envres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh JK, Wilhelm MH, Dunkel-Schetter C, Lombardi CA, Ritz BR. Paternal support and preterm birth, and the moderation of effects of chronic stress: a study in Los Angeles county mothers. Archives of women’s mental health. 2010 Aug;13(4):327–338. doi: 10.1007/s00737-009-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pratt GC, Vadali ML, Kvale DL, Ellickson KM. Traffic, air pollution, minority and socio-economic status: addressing inequities in exposure and risk. International journal of environmental research and public health. 2015 May 19;12(5):5355–5372. doi: 10.3390/ijerph120505355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohai P, Lantz PM, Morenoff J, House JS, Mero RP. Racial and socioeconomic disparities in residential proximity to polluting industrial facilities: evidence from the Americans’ Changing Lives Study. American journal of public health. 2009 Nov;99(Suppl 3):S649–656. doi: 10.2105/AJPH.2007.131383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nachman KE, Parker JD. Exposures to fine particulate air pollution and respiratory outcomes in adults using two national datasets: a cross-sectional study. Environmental health: a global access science source. 2012 Apr 10;11:25. doi: 10.1186/1476-069X-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meeker JD, Sathyanarayana S, Swan SH. Phthalates and other additives in plastics: human exposure and associated health outcomes. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2009 Jul 27;364(1526):2097–2113. doi: 10.1098/rstb.2008.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Just AC, Adibi JJ, Rundle AG, et al. Urinary and air phthalate concentrations and self-reported use of personal care products among minority pregnant women in New York city. Journal of exposure science & environmental epidemiology. 2010 Nov;20(7):625–633. doi: 10.1038/jes.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Just AC, Miller RL, Perzanowski MS, et al. Vinyl flooring in the home is associated with children’s airborne butylbenzyl phthalate and urinary metabolite concentrations. Journal of exposure science & environmental epidemiology. 2015 Nov;25(6):574–579. doi: 10.1038/jes.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zota AR, Phillips CA, Mitro SD. Recent Fast Food Consumption and Bisphenol A and Phthalates Exposures among the U.S. Population in NHANES, 2003–2010. Environmental health perspectives. 2016 Oct;124(10):1521–1528. doi: 10.1289/ehp.1510803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environmental health perspectives. 2011 Jun;119(6):878–885. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolff MS, Engel SM, Berkowitz GS, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environmental health perspectives. 2008 Aug;116(8):1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adibi JJ, Whyatt RM, Williams PL, et al. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environmental health perspectives. 2008 Apr;116(4):467–473. doi: 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braun JM, Smith KW, Williams PL, et al. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environmental health perspectives. 2012 May;120(5):739–745. doi: 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher M, Arbuckle TE, Mallick R, et al. Bisphenol A and phthalate metabolite urinary concentrations: Daily and across pregnancy variability. Journal of exposure science & environmental epidemiology. 2015 May;25(3):231–239. doi: 10.1038/jes.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA pediatrics. 2014 Jan;168(1):61–67. doi: 10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meeker JD, Hu H, Cantonwine DE, et al. Urinary phthalate metabolites in relation to preterm birth in Mexico city. Environmental health perspectives. 2009 Oct;117(10):1587–1592. doi: 10.1289/ehp.0800522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.James-Todd TM, Chiu Y-H, Zota AR. Raical/ethnic disparities in environmental endocrine disrupting chemicals and women’s reproductive health outcomes: epidemiologic examples across the life course. Curr Epidemiol Reports. 2016;3(2):161–180. doi: 10.1007/s40471-016-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.James-Todd T, Senie R, Terry MB. Racial/ethnic differences in hormonally-active hair product use: a plausible risk factor for health disparities. Journal of immigrant and minority health. 2012 Jun;14(3):506–511. doi: 10.1007/s10903-011-9482-5. [DOI] [PubMed] [Google Scholar]
- 46.Kelley KE, Hernandez-Diaz S, Chaplin EL, Hauser R, Mitchell AA. Identification of phthalates in medications and dietary supplement formulations in the United States and Canada. Environmental health perspectives. 2012 Mar;120(3):379–384. doi: 10.1289/ehp.1103998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Branch F, Woodruff TJ, Mitro SD, Zota AR. Vaginal douching and racial/ethnic disparities in phthalates exposures among reproductive-aged women: National Health and Nutrition Examination Survey 2001–2004. Environmental health: a global access science source. 2015 Jul 15;14:57. doi: 10.1186/s12940-015-0043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Misra DP, Trabert B. Vaginal douching and risk of preterm birth among African American women. American journal of obstetrics and gynecology. 2007 Feb;196(2):140, e141–148. doi: 10.1016/j.ajog.2006.10.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiscella K, Franks P, Kendrick JS, Meldrum S, Kieke BA., Jr Risk of preterm birth that is associated with vaginal douching. American journal of obstetrics and gynecology. 2002 Jun;186(6):1345–1350. doi: 10.1067/mob.2002.122406. [DOI] [PubMed] [Google Scholar]
- 50.Bruce FC, Kendrick JS, Kieke BA, Jr, Jagielski S, Joshi R, Tolsma DD. Is vaginal douching associated with preterm delivery? Epidemiology. 2002 May;13(3):328–333. doi: 10.1097/00001648-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 51.Fiscella K, Franks P, Kendrick JS, Bruce FC. The risk of low birth weight associated with vaginal douching. Obstetrics and gynecology. 1998 Dec;92(6):913–917. doi: 10.1016/s0029-7844(98)00325-1. [DOI] [PubMed] [Google Scholar]
- 52.Clara M, Scheffknecht C, Scharf S, Weiss S, Gans O. Emissions of perfluorinated alkylated substances (PFAS) from point sources--identification of relevant branches. Water science and technology: a journal of the International Association on Water Pollution Research. 2008;58(1):59–66. doi: 10.2166/wst.2008.641. [DOI] [PubMed] [Google Scholar]
- 53.Domingo JL, Bocio A. Levels of PCDD/PCDFs and PCBs in edible marine species and human intake: a literature review. Environment international. 2007 Apr;33(3):397–405. doi: 10.1016/j.envint.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Lauritzen HB, Larose TL, Oien T, et al. Maternal serum levels of perfluoroalkyl substances and organochlorines and indices of fetal growth: a Scandinavian case-cohort study. Pediatric research. 2017 Jan;81(1–1):33–42. doi: 10.1038/pr.2016.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gurer-Orhan H, Sabir HU, Ozgunes H. Correlation between clinical indicators of lead poisoning and oxidative stress parameters in controls and lead-exposed workers. Toxicology. 2004 Feb 15;195(2–3):147–154. doi: 10.1016/j.tox.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 56.Kelly FJ. Oxidative stress: its role in air pollution and adverse health effects. Occupational and environmental medicine. 2003 Aug;60(8):612–616. doi: 10.1136/oem.60.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferguson KK, McElrath TF, Chen YH, Mukherjee B, Meeker JD. Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: a repeated measures analysis. Environmental health perspectives. 2015 Mar;123(3):210–216. doi: 10.1289/ehp.1307996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pope CA, 3rd, Hansen ML, Long RW, et al. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environmental health perspectives. 2004 Mar;112(3):339–345. doi: 10.1289/ehp.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferguson KK, Loch-Caruso R, Meeker JD. Urinary phthalate metabolites in relation to biomarkers of inflammation and oxidative stress: NHANES 1999–2006. Environmental research. 2011 Jul;111(5):718–726. doi: 10.1016/j.envres.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferguson KK, McElrath TF, Chen YH, Mukherjee B, Meeker JD. Longitudinal profiling of inflammatory cytokines and C-reactive protein during uncomplicated and preterm pregnancy. American journal of reproductive immunology. 2014 Sep;72(3):326–336. doi: 10.1111/aji.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trivedi S, Joachim M, McElrath T, et al. Fetal-placental inflammation, but not adrenal activation, is associated with extreme preterm delivery. American journal of obstetrics and gynecology. 2012 Mar;206(3):236, e231–238. doi: 10.1016/j.ajog.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lopez NJ, Smith PC, Gutierrez J. Higher risk of preterm birth and low birth weight in women with periodontal disease. Journal of dental research. 2002 Jan;81(1):58–63. doi: 10.1177/002203450208100113. [DOI] [PubMed] [Google Scholar]
- 63.Chappell LC, Seed PT, Briley AL, et al. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet. 1999 Sep 04;354(9181):810–816. doi: 10.1016/S0140-6736(99)80010-5. [DOI] [PubMed] [Google Scholar]
- 64.Clemente DB, Casas M, Vilahur N, et al. Prenatal Ambient Air Pollution, Placental Mitochondrial DNA Content, and Birth Weight in the INMA (Spain) and ENVIRONAGE (Belgium) Birth Cohorts. Environmental health perspectives. 2016 May;124(5):659–665. doi: 10.1289/ehp.1408981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thompson LM, Bruce N, Eskenazi B, Diaz A, Pope D, Smith KR. Impact of reduced maternal exposures to wood smoke from an introduced chimney stove on newborn birth weight in rural Guatemala. Environmental health perspectives. 2011 Oct;119(10):1489–1494. doi: 10.1289/ehp.1002928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roy-Matton N, Moutquin JM, Brown C, Carrier N, Bell L. The impact of perceived maternal stress and other psychosocial risk factors on pregnancy complications. Journal of obstetrics and gynaecology Canada: JOGC = Journal d’obstetrique et gynecologie du Canada: JOGC. 2011 Apr;33(4):344–352. doi: 10.1016/s1701-2163(16)34852-6. [DOI] [PubMed] [Google Scholar]
- 67.Jesse DE, Seaver W, Wallace DC. Maternal psychosocial risks predict preterm birth in a group of women from Appalachia. Midwifery. 2003 Sep;19(3):191–202. doi: 10.1016/s0266-6138(03)00031-7. [DOI] [PubMed] [Google Scholar]
- 68.Hill A, Pallitto C, McCleary-Sills J, Garcia-Moreno C. A systematic review and meta-analysis of intimate partner violence during pregnancy and selected birth outcomes. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2016 Jun;133(3):269–276. doi: 10.1016/j.ijgo.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 69.Selk SC, Rich-Edwards JW, Koenen K, Kubzansky LD. An observational study of type, timing, and severity of childhood maltreatment and preterm birth. Journal of epidemiology and community health. 2016 Jun;70(6):589–595. doi: 10.1136/jech-2015-206304. [DOI] [PubMed] [Google Scholar]
- 70.Okah FA, Oshodi A, Liu Y, Cai J. Community violence and pregnancy health behaviors and outcomes. Southern medical journal. 2014 Aug;107(8):513–517. doi: 10.14423/SMJ.0000000000000143. [DOI] [PubMed] [Google Scholar]
- 71.Mendez DD, Hogan VK, Culhane JF. Institutional racism, neighborhood factors, stress, and preterm birth. Ethnicity & health. 2014;19(5):479–499. doi: 10.1080/13557858.2013.846300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rankin KM, David RJ, Collins JW., Jr African American women’s exposure to interpersonal racial discrimination in public settings and preterm birth: the effect of coping behaviors. Ethnicity & disease. 2011 Summer;21(3):370–376. [PubMed] [Google Scholar]
- 73.Orr ST, James SA, Blackmore Prince C. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. Am J Epidemiol. 2002 Nov 1;156(9):797–802. doi: 10.1093/aje/kwf131. [DOI] [PubMed] [Google Scholar]
- 74.Dunkel Schetter C. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annu Rev Psychol. 2011;62:531–558. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
- 75.Collins JW, Jr, David RJ, Handler A, Wall S, Andes S. Very low birthweight in African American infants: the role of maternal exposure to interpersonal racial discrimination. American journal of public health. 2004 Dec;94(12):2132–2138. doi: 10.2105/ajph.94.12.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grobman WA, Parker C, Wadhwa PD, et al. Racial/Ethnic Disparities in Measures of Self-reported Psychosocial States and Traits during Pregnancy. American journal of perinatology. 2016 Dec;33(14):1426–1432. doi: 10.1055/s-0036-1586510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mills MA, Edmondson D, Park CL. Trauma and stress response among Hurricane Katrina evacuees. American journal of public health. 2007 Apr;97(Suppl 1):S116–123. doi: 10.2105/AJPH.2006.086678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schuster MA, Stein BD, Jaycox L, et al. A national survey of stress reactions after the September 11, 2001, terrorist attacks. The New England journal of medicine. 2001 Nov 15;345(20):1507–1512. doi: 10.1056/NEJM200111153452024. [DOI] [PubMed] [Google Scholar]
- 79.Gidlof-Gunnarsson A, Ohrstrom E. Noise and well-being in urban residential environments: the potential role of perceived availability to nearby green areas. Landscape and Urban Planning. 2007;83(2–3):115–126. [Google Scholar]
- 80.Zeidner M, Schechter M. Psychological responses to air pollution: Some personality and demographic correlates. J of Environ Psychology. 1988;8(3):191–208. [Google Scholar]
- 81.Cory-Slechta DA, Virgolini MB, Thiruchelvam M, Weston DD, Bauter MR. Maternal stress modulates the effects of developmental lead exposure. Environmental health perspectives. 2004 May;112(6):717–730. doi: 10.1289/ehp.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tamayo YOM, Tellez-Rojo MM, Trejo-Valdivia B, et al. Maternal stress modifies the effect of exposure to lead during pregnancy and 24-month old children’s neurodevelopment. Environment international. 2017 Jan;98:191–197. doi: 10.1016/j.envint.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cowell WJ, Bellinger DC, Coull BA, Gennings C, Wright RO, Wright RJ. Associations between Prenatal Exposure to Black Carbon and Memory Domains in Urban Children: Modification by Sex and Prenatal Stress. PloS one. 2015;10(11):e0142492. doi: 10.1371/journal.pone.0142492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shmool JL, Bobb JF, Ito K, et al. Area-level socioeconomic deprivation, nitrogen dioxide exposure, and term birth weight in New York City. Environmental research. 2015 Oct;142:624–632. doi: 10.1016/j.envres.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ponce NA, Hoggatt KJ, Wilhelm M, Ritz B. Preterm birth: the interaction of traffic-related air pollution with economic hardship in Los Angeles neighborhoods. American journal of epidemiology. 2005 Jul 15;162(2):140–148. doi: 10.1093/aje/kwi173. [DOI] [PMC free article] [PubMed] [Google Scholar]