Abstract
Objective
To study the relationship between semen quality and pregnancy loss in a cohort of couples attempting to conceive.
Design
Observational prospective cohort
Setting
Sixteen Michigan/Texas counties
Patients
Three hundred forty-four couples with a singleton pregnancy followed daily through 7 post-conception weeks of gestation.
Interventions
None.
Main Outcome Measure
Association between semen quality and pregnancy loss.
Results
Ninety-eight (28%) of the couples experience a pregnancy loss after singleton pregnancy. No differences were observed in semen volume, sperm concentration, total sperm count, sperm viability, or sperm morphology (WHO and strict) by couple’s pregnancy loss status irrespective of whether they were analyzed continuously or as dichotomous variables per the WHO 5th edition semen criteria. A dichotomous DNA fragmentation measure of ≥30% was significantly associated with pregnancy loss. No association was identified with other sperm morphometric or movement measures. Of the 70 couples who re-enrolled after a pregnancy loss, 14 experienced a second loss. Similar findings were identified when examining semen quality from couples with recurrent pregnancy loss.
Conclusions
While a few trends were identified (e.g. DNA fragmentation), general semen parameters seemed to have little relation with risk of pregnancy loss or recurrent pregnancy loss at the population level. However, given that 30% of pregnancies end in miscarriage and half of the fetal genome is paternal in origin, the findings await corroboration.
Keywords: spontaneous abortion, male infertility, fertility, DNA Fragmentation, semen analysis
Introduction
Pregnancy loss affects 30% of pregnancies.(1–3) Multifactorial in nature, most identified etiologies of loss center around the woman. However, as a man contributes 50% of the genome to an embryo, it is reasonable to assume that male factors may also contribute to pregnancy loss. Indeed, up to 50% of all cases of infertility are due to a male factor.(4, 5) To date, there is relatively limited data on male factors contributing to pregnancy loss, especially research that captures loss during the peak weeks in early gestation However, a recent study by our group using the same cohort for the present analysis did identify an association between paternal lifestyle factors (i.e., caffeine consumption) and pregnancy loss in a prospective cohort study.(6)
Recurrent pregnancy loss (RPL) is defined as two or more consecutive losses for a couple, which affects 1–5% of women.(1, 3) As with pregnancy loss, the evaluation for recurrent pregnancy loss centers around the woman. Yet even after uterine, oocyte, and chromosomal factors are excluded, an idiopathic etiology is left approximately 50% of the time.(3) Investigators have also attempted to determine male factors associated with RPL. Zidi-Draj et al reported higher levels of sperm immotility, abnormal morphology, and elevated sperm DNA fragmentation in men with RPL.(7) Elevated levels of sperm aneuploidy have also been reported among men from couples with RPL.(8) However, as these studies rely on case control design with fertile couples serving as controls, prospective studies are required to confirm the reported associations.
Surprisingly, few studies have attempted to assess semen quality and risk of incident pregnancy loss. This may reflect very few couple based preconception cohort studies ever conducted worldwide with even fewer collecting semen samples.(9) Preconception cohort studies are needed for this question given the marked concentration of losses early in pregnancy or before seeking prenatal care. Using data from the LIFE (Longitudinal Investigation of Fertility and the Environment) Study, we examined the association between semen quality and pregnancy loss in a prospective study. Given that RPL represents a unique group, we also performed a subanalysis on couples with 2 or more losses.
Methods
Study Design and Population
The study cohort comprised 347 couples (69%) whose female partners had an observed pregnancy (denoted by a positive urine pregnancy test) while participating in the LIFE Study, which was designed to examine the association between environmental and lifestyle factors and fecundity endpoints, including pregnancy loss. Three couples with twin pregnancies were excluded resulting in a cohort comprising 344 couples with singleton pregnancies. The LIFE Study utilized population-based sampling frameworks to recruit couples discontinuing contraception for purposes of becoming pregnant from 16 counties in Michigan and Texas. By design, eligibility criteria were minimal and included: 1) in a committed relationship; 2) ability to communicate in English or Spanish; 3) females’ aged 18–40 and males’ aged ≥18 years; 4) females’ menstrual cycles between 21–42 days as required by the fertility monitors; 5) no history of injectable hormonal contraception in past year; 6) no clinically diagnosed infertility in either partner; and 7) off contraception <2 months. Prior to enrollment, female partners’ urines were tested to ensure they were not already pregnant. Human subjects approval was obtained from participating institutions, and all men and women gave written informed consent before data collection. Complete details about the study design of LIFE have been previously published.(10)
Data Collection and Follow-up
Couples were interviewed individually upon enrollment to ascertain sociodemographic, lifestyle, and medical history information, followed by the measurement of height and weight to calculate body mass index (BMI). The couple was then instructed in the completion of daily journals to record their lifestyle in a manner consistent with how people think about such exposures (e.g., number of cigarettes smoked per day, number of alcoholic and caffeinated beverages consumed per day, taking daily multivitamins). Pregnant women continued journals daily through 7 post-conception weeks gestation then monthly journals until a loss or delivery. Couples experiencing a loss had the option of re-entering the study.
Biospecimen collection and analysis
Semen samples were collected via masturbation without the use of any lubricant following two days of abstinence using home collection kits which included an insulated shipping container (Hamilton Research, Beverly, MA) for maintaining sperm integrity at the time of enrollment. Other investigators have utilized such approaches.(11, 12) All semen samples were received at the study’s andrology laboratory. The complete methodology has been previously reported.(10) Briefly, an aliquot of semen was placed in a 20μm deep chamber slide (Leja, Luzemestraat, Netherlands), and sperm motility was assessed using the HTM-IVOS (Hamilton Thorne, Beverly, MA) computer assisted semen analysis system (CASA). Sperm concentration was also measured using the IVOS system and the IDENT™ stain. Microscope slides were prepared for sperm morphometry and morphology assessment. An aliquot of the whole semen was diluted in TNE buffer with glycerol and frozen for the sperm chromatin stability assay (SCSAR) analysis.(13) Sperm viability was determined by hypo-osmotic swelling (HOS assay).
To insure integrity of the 24-hour analysis, steps were taken to ensure the quality of the semen parameters. A thermometer was attached to all collection jars to ensure the temperature of the sample was within acceptable limits (all were). Upon receipt, the andrology lab assessed the integrity of the samples and all were found to be acceptable.
Home Fertility and Pregnancy Testing
Women were trained in the use of the ClearBlueR digital Fertility Monitor, which has been demonstrated to be accurate in detecting ovulation relative to the gold-standard, ultrasound visualization.(14) The monitor records the ratios of estrone-3-glucuronide (E3G) and luteinizing hormone (LH) and stores data for up to 6 months. Study personnel downloaded the data every 45 days. Day of ovulation in the study was approximated by the day of peak LH as indicated by the fertility monitor.
Women were also trained in the use of the ClearblueR digital pregnancy test (with read outs of “pregnant” or “not pregnant”), which has demonstrated sensitivity and reliability for detecting 25 mIU/mL of hCG, and demonstrated accuracy by women (16). Women tested their urine for pregnancy on the day they expected menstruation consistent with manufacturer’s guidance.
Pregnancy Loss Ascertainment
Pregnancy loss was defined as conversion to a negative pregnancy test as subsequently recorded in their journals, clinical confirmation of loss recorded on a separate pregnancy loss card, or onset of menstruation recorded in the journal, depending upon gestational age at loss.(15) Gestational age at loss was measured in days postconception, which was approximated by the day of ovulation (LH peak) as recorded by the fertility monitor.
Statistical Analysis
We summarized the distributions as mean and standard deviation (SD) or median and interquartile range (IQR) for continuous variables, and frequency and percentage for categorical variables. Table 1 included age (years, continuous), body mass index (BMI; kg/m2), race (white, non-white), education level (High school or below, Some college or above), prior paternity (Yes/No), self-reported smoking (Yes/No), and self-reported alcohol consumption (Yes/No). We dichotomized semen quality parameters (semen volume, sperm concentration, total sperm count, sperm viability, or sperm morphology (WHO and strict) using clinical cut points of male participants in the LIFE Study, based on World Health Organization standards.(16) The DNA fragmentation index (DFI) was also dichotomized (DFI ≥ 30) based on a previously reported cutpoint.(17–19)
Table 1.
Baseline characteristics of men who achieved a pregnancy, LIFE Study (n=344).
| Male (N=344) | ||
|---|---|---|
| Demographics | Age (Mean, SD) | 31.6(4.59) |
| BMI (Mean, SD) | 29.39(4.98) | |
| White, % | 285(83.33) | |
| College educated, % | 323(94.72) | |
| Prior Paternity, % | 207(60.17) | |
| Smoker, % | 37(10.76) | |
| Alcohol, % | 298(86.63) | |
| Semen Parameters | ||
| Volume (mL) | <1.5, n (%) | 32(9.64) |
| Median (IQR) | 3.4(2.3,4.4) | |
| Concentration (million/mL) | <15, n (%) | 19(5.72) |
| Median (IQR) | 66.7(37.65,97.05) | |
| Total sperm count (million) | <39, n (%) | 21(6.33) |
| Median (IQR) | 202.9(107.3,338.1) | |
| Morphology (% WHO normal) | <30, n (%) | 139(43.85) |
| Median (IQR) | 31.5(23,40) | |
| Morphology (% strict criteria) | <4, n (%) | 9(2.84) |
| Median (IQR) | 21(14,27.5) | |
| DNA (% fragmentation index) | ≥30 | 20(6.13) |
| Median (IQR) | 11.89(8.4,18.04) |
We assessed differences in semen parameters by couple’s pregnancy loss status using the nonparametric (Kruskal-Wallis) test for continuous variables and Chi-Square test for categorical variables, and reported the findings in Table 2.
Table 2.
Semen characteristics of men from couples with no pregnancy loss, one pregnancy loss, or two pregnancy losses.
| No Pregnancy Loss n=246 | Pregnancy Loss n=98 | Recurrent Pregnancy Loss n=14 | p value** | |
|---|---|---|---|---|
|
|
||||
| General semen characteristics, mean (SD) | ||||
|
|
||||
| Volume (mL) | 3.58 (1.75) | 3.28 (1.51) | 2.71 (0.78) | 0.19 |
| Sperm concentration (×106/mL) | 79.00 (59.18) | 77.18 (57.38) | 63.22 (48.10) | 0.61 |
| Total sperm count (×106/ejaculate) | 253.88 (189.83) | 242.15 (213.57) | 168.54 (135.88) | 0.54 |
| Hypo-osmotic swelling (%)a | 68.24 (8.72) | 68.18 (10.65) | 66.69 (12.35) | 0.83 |
| Straw (mm distance sperm traveled) | 10.64 (6.45) | 10.91 (6.62) | 9.75 (3.93) | 0.70 |
|
|
||||
| WHO semen parameters, n (%) | ||||
|
|
||||
| Volume <1.5 mL | 21 (8.90) | 11 (11.46) | 0.00 (0.00) | 0.79 |
| Concentration <15M/mL | 12 (5.08) | 7 (7.29) | 2 (14.29) | 0.27 |
| Total Count <39M | 13 (5.51) | 8 (8.33) | 3 (21.43)* | 0.51 |
| WHO Morphology <30% | 103 (45.98) | 36 (38.71) | 6 (42.86) | 0.19 |
| Strict Morphology <4% | 8 (3.57) | 1 (1.08) | 0 (0.00) | 0.30 |
| DFI (≥30%) | 10 (4.29) | 10 (10.75)* | 1 (7.14) | 0.04 |
|
|
||||
| Sperm motility, mean (SD) | ||||
|
|
||||
| Average path velocity (μm/sec) | 37.04 (12.54) | 36.58 (13.54) | 32.69 (19.46) | 0.70 |
| Straight line velocity (μm/sec) | 27.62 (10.26) | 27.54 (11.30) | 24.34 (15.63) | 0.89 |
| Culvilinear velocity (μm/sec) | 63.95 (21.59) | 61.94 (22.52) | 56.21 (32.12) | 0.39 |
| Amplitude head displacement (μm) | 3.21 (1.37) | 3.14 (1.38) | 2.65 (1.56) | 0.63 |
| Beat cross frequency (Hz) | 20.23 (7.23) | 19.52 (7.85) | 17.66 (10.37) | 0.38 |
| Straightness (%) | 68.70 (19.40) | 67.90 (21.56) | 56.86 (31.42) | 0.67 |
| Linearity (%) | 41.40 (13.05) | 41.27 (14.27) | 34.00 (19.52) | 0.88 |
| Percent motility (%) | 12.97 (12.87) | 14.08 (12.61) | 16.57 (16.02) | 0.58 |
|
|
||||
| Sperm head measurement, mean (SD) | ||||
|
|
||||
| Lengh (μm) | 4.88 (0.26) | 4.88 (0.32) | 4.98 (0.38) | 0.98 |
| Area (μm) | 12.25 (0.84) | 12.22 (1.04) | 12.45 (0.91) | 0.95 |
| Width (μm) | 3.18 (0.18) | 3.18 (0.18) | 3.19 (0.11) | 0.97 |
| Perimeter (μm) | 13.25 (0.48) | 13.25 (0.62) | 13.45 (0.63) | 0.89 |
| Elongation factor (%) | 66.14 (5.18) | 65.95 (5.20) | 64.90 (5.07) | 0.99 |
| Acrosome area of head (%) | 25.78 (5.06) | 26.67 (5.30) | 27.31 (3.47) | 0.26 |
|
|
||||
| Morphology, mean (SD) | ||||
|
|
||||
| Strict criteria (%) | 20.95 (9.59) | 22.01 (10.56) | 21.18 (9.45) | 0.35 |
| WHO normal (%) | 31.24 (11.99) | 33.38 (12.40) | 33.39 (12.18) | 0.12 |
| Amorphous (%) | 29.62 (10.80) | 28.60 (10.09) | 25.79 (8.59) | 0.49 |
| Round (%) | 1.14 (1.67) | 0.90 (1.09) | 0.79 (0.93) | 0.29 |
| Pyriform (%) | 6.04 (5.46) | 6.49 (7.22) | 8.50 (6.57) | 0.68 |
| Bicephalic (%) | 1.12 (1.70) | 1.04 (1.48) | 1.04 (1.43) | 0.69 |
| Tapered (%) | 2.80 (2.76) | 2.54 (2.30) | 2.39 (1.69) | 0.27 |
| Megalo head (%) | 2.32 (1.72) | 2.47 (2.22) | 3.25 (1.77)* | 0.48 |
| Micro head (%) | 1.44 (1.32) | 1.49 (1.00) | 1.75 (1.40) | 0.58 |
| Neck and midpiece abnormalities (%) | 26.02 (9.80) | 26.41 (8.42) | 26.79 (9.58) | 0.68 |
| Coiled tail (%) | 23.90 (10.70) | 21.68 (9.38) | 21.57 (9.67) | 0.07 |
| Other tail abnormalities (%) | 5.17 (4.80) | 5.17 (3.29) | 5.43 (2.20) | 0.64 |
| Cytoplasmic droplet (%) | 10.15 (5.31) | 9.69 (4.64) | 10.04 (4.31) | 0.47 |
| Immature germ cell count (%) | 4.81 (5.58) | 5.51 (10.25) | 5.93 (5.28) | 0.13 |
|
|
||||
| SCSA, mean (SD) | ||||
|
|
||||
| DNA fragmentation index (%) | 14.33 (9.72) | 15.84 (11.29) | 15.83 (17.20) | 0.10 |
| High DNA stainability (%) | 7.21 (5.15) | 6.77 (5.10) | 6.99 (5.36) | 0.69 |
p<0.05
Multivariable models adjusted for smoking and alcohol use comparing men from couples with pregnancy loss vs no pregnancy loss.
We used discrete time survival models to assess how semen quality parameters are related to pregnancy loss, with a time-to-loss as an outcome and semen quality parameters as separate independent variables. These models were run unadjusted as well as with adjustment for covariates, including self-reported smoking (Yes/No) and self-reported alcohol consumption (Yes/No). We performed several sensitivity analyses for the recurrent pregnancy loss group by varying the comparison groups but the conclusions remained unchanged. Significance was set at p-value < 0.05 without adjusting for multiple comparisons. All statistical analyses were performed by the SAS Version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
In all, ninety-eight (28%) couples who became pregnant during the study experienced an incident pregnancy loss. Demographic characteristics of the entire cohort are presented in Table 1.
No differences were observed in semen volume, sperm concentration, total sperm count, sperm viability, or sperm morphology (WHO and strict, Table 2) by couple’s pregnancy loss status irrespective of whether they were analyzed continuously or as dichotomous outcomes per the WHO 5th edition criteria.(16) Sperm motility endpoints as measured by average path velocity, straight line velocity, curvilinear velocity, amplitude head displacement, beat cross frequency, percentage with a straight trajectory, and percentage with a linear trajectory were also similar between groups. Several measures of sperm head characteristics including head length, width, area, perimeter, percentage of elongation, and percentage occupation by the acrosome were also similar relative to pregnancy loss status. Other measures of morphology such as percentage of amorphous, round, pyriform, bicephalic, tapered, megalo headed, micro headed were also similar between male partners of couples with and without a pregnancy loss. While the percent distributions of DNA fragmentation index (DFI) and high DNA stainability were similar irrespective of pregnancy loss status, dichotomizing DFI (i.e. DFI ≥ 30) was positively associated with pregnancy loss (11% and 4% of men with and without loss; p=0.03). This difference remained after adjusting for BMI, smoking, and alcohol consumption.
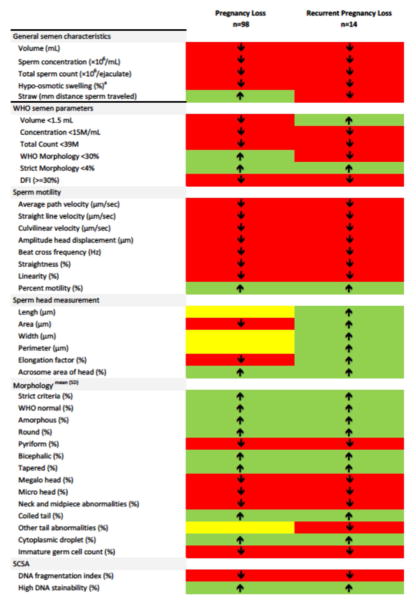
As there was little evidence for any significant differences in semen quality between men with and without a pregnancy loss, we also graphically compared semen quality patterns by couples’ pregnancy loss status. For most parameters, we found lower distributions in semen quality for couples experiencing a pregnancy loss. Figure 1 illustrates a summary of the comparison between men without a pregnancy loss, 1 pregnancy loss, and 2 or more pregnancy losses. Of the 35 semen and sperm endpoints assessed, 19 (54%) were worse among couples experiencing a pregnancy loss in comparison to couples without a loss, while 12 (34%) endpoints were better in the former group. Among the measures of motility, 7 of 9 (78%) were lower among couples with than without a loss, as were 7 (35%) morphometric endpoints.
Figure 1.
Graphical representation of semen parameters for men with pregnancy loss or recurrent pregnancy loss compared to men without pregnancy loss. Red denotes a lower semen parameter value, green denotes a higher value, and yellow denotes an equal value compared to men without pregnancy loss.
Of the 98 couples with pregnancy loss, 70 re-entered the study. Fourteen couples experienced more than one loss while being followed in the study. Similar to men in couples experiencing one loss, few associations between semen quality and recurrent loss were identified. A higher percentage of men whose female partner had recurrent losses were found to have lower total sperm counts (<39M) in comparison to male partners in couples without losses (21.4% and 5.5%, respectively; Table 2). Overall, 20 (57%) measured semen endpoints were worse in couples with than without recurrent loss (Figure 1). Eight (89%) motility parameters were worse in men with recurrent pregnancy loss, as were 6 (30%) morphologic measures.
Discussion
To our knowledge, our study is the first to evaluate a range of semen endpoints beyond traditional clinical outcomes such as count, motility and morphology in relation to risk of incident pregnancy loss. Moreover, our findings are strengthened by the preconception enrollment of couples from the general population, along with daily follow-up through 7 post-conception weeks and monthly, thereafter. We found no evidence that semen quality in men recruited from the general population was associated with incident pregnancy loss. These findings were consistent when restricting the analysis to couples with observed recurrent incident pregnancy loss. However, couples experiencing a pregnancy loss were more likely to have male partners with abnormal sperm DNA fragmentation. In addition, male partners of couples with recurrent loss were more likely to have lower total sperm counts than couples without pregnancy loss. In addition when examining all measured semen endpoints, male partners of couples experiencing pregnancy losses tended to have worse semen quality in comparison to male partners of couples without losses.
Most prior research on pregnancy loss has focused on maternal factors,(20) though there is some limited data suggesting possible male factors(6–8, 21–26) especially for recurrent pregnancy loss. Studies of recurrent loss have reported associations with DNA fragmentation, sperm aneuploidy, sperm morphology, and motility.(8, 23, 26) However, these prior studies have relied upon a case-control design. A key limitation of the case control design is that the control group is not at risk for loss, as by definition their pregnancy went to term. While these studies make important contributions to our understanding of the association between semen quality and pregnancy loss, methodologic limitations from such retrospective designs make definitive conclusions challenging. To our knowledge, our study represents the first prospective examination of semen quality and risk of pregnancy loss among couples attempting to conceive.
Similar to prior reports, we did find an association between DNA fragmentation and pregnancy loss. Importantly, the only statistically significant result was identified after dichotomizing the DNA fragmentation index based on the defined abnormal cut point for DNA fragmentation but not when examined on continuous scale. Given the variability in semen quality, it may be that defining abnormal based on strict criteria (e.g., a defined and validated cut point), may be more useful than a continuous scale, especially as our results also support a threshold effect of DFI. However given the number of parameters examined, it is also conceivable that our association was due to chance alone. Indeed for 35 tests performe, there is an 83% chance for finding at least one significant association.
When examining recurrent pregnancy loss, there was a trend identified for several semen parameters. Semen volume and total sperm count were both lower in male partners of couples with recurrent loss than those without. However, on a continuous scale, neither reached statistical significance. It is important to know that recurrent loss is a rare outcome, with less than 5% of affected pregnant couples.(1) In our cohort, only 14 (4%) couples were observed to experience RPL, which is consistent with other population estimates.(1, 27) The current report represents the first data on prospectively recruited men or men from the general population regarding semen quality and recurrent pregnancy loss.
Given the few associations identified in our primary analysis, we performed a visual analysis to examine the collective findings. Overall, we did observe that male partners of couples with (recurrent) pregnancy loss tended to have lower semen quality in comparison to unaffected couples. While many examined parameters and morphometrics are not part of routine patient care, our in depth analysis still did not identify possible signals.
Several other important limitations warrant mention. Given the close monitoring of couples, very early pregnancies and losses may be more common in this cohort than would be observed in clinical practice. Indeed, all losses occurred prior to 22 weeks gestation.(15) It is conceivable that an analysis of later pregnancies would give different results. However, given the contribution of the sperm to early embryonic development, we would expect a larger effect earlier in gestation compared to later. As genetic abnormalities are thought to contribute to early pregnancy losses, our findings of abnormal DFI in men from couples with pregnancy loss is consistent with a paternally derived genetic origin of fetal loss. While we analyzed nearly 100 pregnancy losses, it is possible that we were underpowered to identify some associations with semen quality, particularly if they are reflected in small difference that would require larger cohorts.
Nevertheless, the current report represents the first prospective examination of the association between semen quality and pregnancy loss. While a few trends were identified (e.g. DNA fragmentation), general semen parameters seemed to have little relation with risk of incident pregnancy loss at the population level. However, given that 30% of pregnancies end in miscarriage and half of the fetal genome is paternal in origin, the findings await corroboration.
Acknowledgments
Study funding/competing interest(s): Intramural research of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Contracts #N01-HD-3- 3355, N01-HD-3-3356 and N01-HD-3-3358).
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (contracts N01-HD-3-3355, N01-HD-3-3356 and N01-HD-3-3358, HHSN27500001). The authors acknowledge the Reproductive Health Assessment Team, Biomonitoring and Health Assessment Branch, National Institute of Occupational Safety and Health, for conducting the semen analyses through a Memo of Understanding with the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stirrat GM. Recurrent miscarriage. Lancet. 1990;336:673–5. doi: 10.1016/0140-6736(90)92159-f. [DOI] [PubMed] [Google Scholar]
- 2.Regan L, Rai R. Epidemiology and the medical causes of miscarriage. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:839–54. doi: 10.1053/beog.2000.0123. [DOI] [PubMed] [Google Scholar]
- 3.Practice Committee of the American Society for Reproductive M. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertility and sterility. 2012;98:1103–11. doi: 10.1016/j.fertnstert.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 4.Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, Lansac J, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989) Human reproduction. 1991;6:811–6. doi: 10.1093/oxfordjournals.humrep.a137433. [DOI] [PubMed] [Google Scholar]
- 5.Eisenberg ML, Li S, Behr B, Pera RR, Cullen MR. Relationship between semen production and medical comorbidity. Fertility and sterility. 2015;103:66–71. doi: 10.1016/j.fertnstert.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Buck Louis GM, Sapra KJ, Schisterman EF, Lynch CD, Maisog JM, Grantz KL, et al. Lifestyle and pregnancy loss in a contemporary cohort of women recruited before conception: The LIFE Study. Fertility and sterility. 2016;106:180–8. doi: 10.1016/j.fertnstert.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zidi-Jrah I, Hajlaoui A, Mougou-Zrelli S, Kammoun M, Meniaoui I, Sallem A, et al. Relationship between sperm aneuploidy, sperm DNA integrity, chromatin packaging, traditional semen parameters, and recurrent pregnancy loss. Fertility and sterility. 2015 doi: 10.1016/j.fertnstert.2015.09.041. [DOI] [PubMed] [Google Scholar]
- 8.Ramasamy R, Scovell JM, Kovac JR, Cook PJ, Lamb DJ, Lipshultz LI. Fluorescence in situ hybridization detects increased sperm aneuploidy in men with recurrent pregnancy loss. Fertility and sterility. 2015;103:906–9. e1. doi: 10.1016/j.fertnstert.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buck GM, Lynch CD, Stanford JB, Sweeney AM, Schieve LA, Rockett JC, et al. Prospective pregnancy study designs for assessing reproductive and developmental toxicants. Environmental health perspectives. 2004;112:79–86. doi: 10.1289/ehp.6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buck Louis GM, Schisterman EF, Sweeney AM, Wilcosky TC, Gore-Langton RE, Lynch CD, et al. Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction and development--the LIFE Study. Paediatr Perinat Epidemiol. 2011;25:413–24. doi: 10.1111/j.1365-3016.2011.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luben TJ, Olshan AF, Herring AH, Jeffay S, Strader L, Buus RM, et al. The healthy men study: an evaluation of exposure to disinfection by–products in tap water and sperm quality. Environmental health perspectives. 2007;115:1169–76. doi: 10.1289/ehp.10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olshan AF, Perreault SD, Bradley L, Buus RM, Strader LF, Jeffay SC, et al. The healthy men study: design and recruitment considerations for environmental epidemiologic studies in male reproductive health. Fertility and sterility. 2007;87:554–64. doi: 10.1016/j.fertnstert.2006.07.1517. [DOI] [PubMed] [Google Scholar]
- 13.Evenson DP, Larson KL, Jost LK. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl. 2002;23:25–43. doi: 10.1002/j.1939-4640.2002.tb02599.x. [DOI] [PubMed] [Google Scholar]
- 14.Behre HM, Kuhlage J, Gassner C, Sonntag B, Schem C, Schneider HP, et al. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Human reproduction (Oxford, England) 2000;15:2478–82. doi: 10.1093/humrep/15.12.2478. [DOI] [PubMed] [Google Scholar]
- 15.Sapra KJ, Buck Louis GM, Sundaram R, Joseph KS, Bates LM, Galea S, et al. Signs and symptoms associated with early pregnancy loss: findings from a population-based preconception cohort. Human reproduction (Oxford, England) 2016;31:887–96. doi: 10.1093/humrep/dew010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 17.Practice Committee of the American Society for Reproductive M. The clinical utility of sperm DNA integrity testing: a guideline. Fertility and sterility. 2013;99:673–7. doi: 10.1016/j.fertnstert.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal A, Majzoub A, Esteves SC, Ko E, Ramasamy R, Zini A. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol. 2016;5:935–50. doi: 10.21037/tau.2016.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, Purvis K, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Human reproduction (Oxford, England) 1999;14:1039–49. doi: 10.1093/humrep/14.4.1039. [DOI] [PubMed] [Google Scholar]
- 20.Brown S. Miscarriage and its associations. Semin Reprod Med. 2008;26:391–400. doi: 10.1055/s-0028-1087105. [DOI] [PubMed] [Google Scholar]
- 21.Gil-Villa AM, Cardona-Maya W, Agarwal A, Sharma R, Cadavid A. Assessment of sperm factors possibly involved in early recurrent pregnancy loss. Fertility and sterility. 2010;94:1465–72. doi: 10.1016/j.fertnstert.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 22.Bareh GM, Jacoby E, Binkley P, Chang TC, Schenken RS, Robinson RD. Sperm deoxyribonucleic acid fragmentation assessment in normozoospermic male partners of couples with unexplained recurrent pregnancy loss: a prospective study. Fertility and sterility. 2016;105:329–36. e1. doi: 10.1016/j.fertnstert.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 23.Zidi-Jrah I, Hajlaoui A, Mougou-Zerelli S, Kammoun M, Meniaoui I, Sallem A, et al. Relationship between sperm aneuploidy, sperm DNA integrity, chromatin packaging, traditional semen parameters, and recurrent pregnancy loss. Fertility and sterility. 2016;105:58–64. doi: 10.1016/j.fertnstert.2015.09.041. [DOI] [PubMed] [Google Scholar]
- 24.PK, Malini SS. Positive association of sperm dysfunction in the pathogenesis of recurrent pregnancy loss. J Clin Diagn Res. 2014;8:OC07–10. doi: 10.7860/JCDR/2014/9109.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruixue W, Hongli Z, Zhihong Z, Rulin D, Dongfeng G, Ruizhi L. The impact of semen quality, occupational exposure to environmental factors and lifestyle on recurrent pregnancy loss. J Assist Reprod Genet. 2013;30:1513–8. doi: 10.1007/s10815-013-0091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomlinson M, Lewis S, Morroll D British Fertility S. Sperm quality and its relationship to natural and assisted conception: British Fertility Society guidelines for practice. Hum Fertil (Camb) 2013;16:175–93. doi: 10.3109/14647273.2013.807522. [DOI] [PubMed] [Google Scholar]
- 27.Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–94. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]