Abstract
The potential for radiation-induced toxicities in the brain produce significant anxiety, both among patients receiving radiation therapy and those radiation oncologists providing treatment. These concerns often play a significant role in the medical decision making process for most patients with diseases in which radiotherapy may be a treatment consideration1. While the precise mechanisms of neurotoxicity and neurodegeneration following ionizing radiation exposure continue to be poorly understood from a biological perspective, there is an increasing body of scientific and clinical literature that is producing a better understanding of how radiation causes brain injury, factors which determine whether toxicities occur, and potential preventative, treatment and mitigation strategies for patients at high risk or with symptoms of injury. This review will focus primarily on injuries and biological processes described in mature brain.
Radiobiology of therapeutic radiation on the CNS
All forms of ionizing radiation, ranging from nearly weightless photons to heavy charged particles such as protons or carbon ions, have the potential to produce toxicity in the central nervous system. Ionizing radiation particles have in common the physical ability to generate free radicals that may cause direct or indirect DNA damage, but may also provide a source of metabolic stress to which the central nervous system (CNS) is particularly susceptible as compared to other tissue types2. Although the fixation of double stranded DNA breaks leading to mitotic catastrophe is the most supported mechanism of radiation-induced cell death3, it is thought to be more relevant in cells undergoing active cell division. In normal mature CNS where mitotic potential is limited, there is growing evidence to suggest that other mechanisms of radiation-induced damage, such as oxidation of the lipid bilayer4, changes in microvascular permeability, cell-cell junctional complex rearrangements5, and mitochondrial alterations inducing additional oxidative stress6, are likely more important subcellular targets for ionizing radiation. Through the combination of DNA damage and subcellular alterations, radiation has the capacity to alter tumor microenvironment, cellular architecture, permeability of tumor vasculature and permeation of drugs within the CNS, which have the potential to simultaneously augment as well as reduce the toxicities induced by radiation treatment.
Larger fraction sizes and compressed fractionation schedules are believed to contribute disproportionately to toxicity of normal tissues in the CNS. The Radiation Therapy Oncology Group (RTOG) prospectively compared randomized whole brain radiation fractionation schedules in patients with symptomatic brain metastases to determine impact on survival7. Multiple regimens tested ranged from 10 Gray (Gy) in a single fraction up to 40 Gy delivered over 20 fractions. While there was not an appreciable difference in survival with most fractionation schedules, the delivery of 10 Gy in a single treatment to the entire brain was determined to be significantly detrimental to survival. These data suggested that above a certain threshold, large fraction sizes produce worse toxicity in the brain when treatment volumes are equivalent.
Likewise, in a retrospective evaluation of patients receiving whole brain radiotherapy for brain metastases, toxicity was observed at 20.6 months in patients who received greater than 3 Gy per fraction, many of whom also received concurrent radiosensitizing chemotherapy. More importantly, no dementia was detected in patients who received 3 Gy or less per fraction8. Subsequent evaluations suggested that reduced fraction sizes were recommended for better performing patients with a longer anticipated life expectancy when the entire brain required radiation treatment9.
As a consequence, therapeutic treatment regimens engineered to limit the amount of treated normal tissue have gained in popularity based on the hypothesis that a reduced treatment volume yields fewer and less severe toxicities. Because radiation delivery techniques such as stereotactic radiosurgery (SRS) or hypofractionated stereotactic radiotherapy (SRT) deliver ablative doses of radiation, their practical use is of benefit in limited situations to treat well-circumscribed targets that are spatially located at sufficient distance from critical structures. RTOG 90-05 defined the tolerance of SRS dosing in a volumetric fashion. As the diameter of the target lesion increased above 2 cm, the tolerated dose decreased from 24 Gy to 15 Gy when the lesion diameter was greater than 3 cm10, and was limited by severe edema and the development of radionecrosis at a median follow-up of 3 years. When SRS was combined with whole brain radiation, thereby increasing the total dose, side effects of nausea as well as central and peripheral neurologic toxicities occurred more frequently and was more severe within 90 days of radiation treatment11. Unfortunately, the use of limited radiation treatment volumes has most often been used as a tool to extend local control, not with the anticipation of the overall cure of the patient.
While the toxic effects of radiation depend on total dose, fractionation schedule, and volume treated, there is evidence to suggest that differential radiation sensitivity exists within various CNS subcompartments. Neurogenesis, a process by which new neurons are produced in the brain, persists throughout life in discrete regions of the adult brain, including the hippocampus. It has been long established that radiation exposure has a negative impact on neurogenesis12–14. It is believed that these disruptions in neurogenesis and hippocampal function are directly linked to cognitive and mood disruptions observed in patients15–16. However, what is less well-understood is to what extent spontaneous recovery of neurogenesis and hippocampal function is possible, the time course for any potential recovery, the effects of age and preexisting neurologic disease, and what therapies and interventions might benefit functional recovery.
Acute vs. Late Effects of Radiotherapy
The etiology of CNS dysfunction in patients after irradiation is multifactorial17–18, influenced by individual factors including age, medical comorbidities, psychological and genetic predispositions, characteristics of any underlying malignancy, as well as any additional injuries caused by other treatment modalities such as surgery and chemotherapy. From a radiobiological perspective, radiation-induced brain injury is described in three phases: acute (within days to weeks after irradiation), early-delayed (within 1–6 months post irradiation) and late (> 6 months post irradiation)19. From the clinical perspective, the RTOG defined acute toxicity for CNS as those symptoms attributable to radiation treatment and occurring during and within 90 days of radiation treatment, which include neurologic changes requiring corticosteroids, seizure, coma, and paralysis. Late toxicities occur after 90 days and include headache, lethargy, severe CNS dysfunction including partial loss of power and dyskinesia, and coma20. Due to the limited lifespan of many adult patients receiving radiation treatment to the CNS, it is largely unknown what the long-term consequences of most treatments would be after many years. Both conventional as well as more precise radiation treatment modes have the potential to produce side effects such as fatigue, cognitive alterations in short-term memory and concentration, pituitary dysfunction resulting in endocrinological disruptions, and in rare cases, dementia9,21–23. Thus, the goals of radiation toxicity research include improved efforts at enhancing efficacy and reducing side effects.
Radiation-induced neuropsychological function and cognition deficits evolve in a biphasic pattern with a subacute transient decline corresponding to more common symptoms, followed by a late delayed irreversible impairment several months or years later in a much smaller proportion of surviving patients24. Concerns regarding toxicity of treatment have to be balanced with data suggesting that uncontrolled tumor in the CNS has the most severe toxicities, well above those observed with radiation25. Indeed, the modality of treatment for some patients, i.e. palliative versus more aggressive treatment, may reflect an inherent bias toward an improved baseline functional well-being patients receiving more aggressive treatments as determined by FACT-Br scores26.
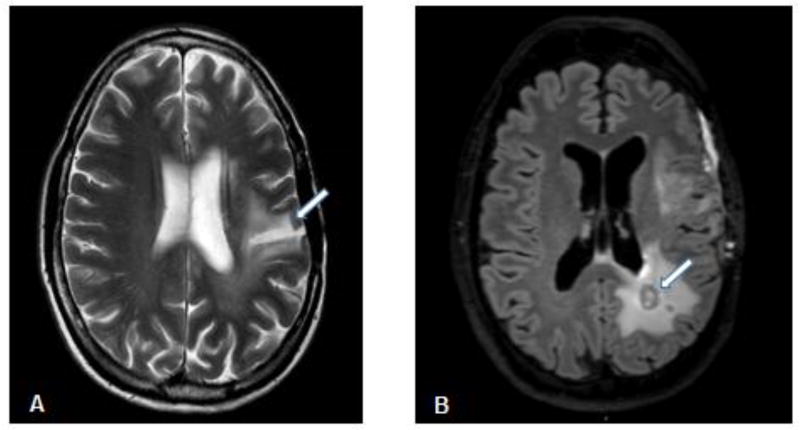
Functional toxicities are believed correlated to observed changes in the entire brain, including gray matter, white matter, ventricles, and combinations among them27–28. Hallmarks of normal tissue toxicity include vascular injury29. Radiation primarily causes coagulation necrosis of the white matter tracts and cerebral vasculature by axonal demyelination and damage to vascular endothelial cells30. Leukoencephalopathy occurs from the overproduction of myelin in oligodendrocytes and occurs as a late toxicity (Figure 1A). Demyelination can also occur in spinal cord and nerve roots. Neurodegeneration may occur directly from radiation-induced stress as well as a by-product of detrimental effects on the supporting astrocytes, and supporting astrocytes may undergo reactive gliosis. However, the most severe form of injury is radionecrosis, producing a brisk neuroinflammatory reaction (Figure 1B). Neuroinflammation is a prominent feature of many CNS diseases including stroke, Alzheimer’s disease, Parkinson’s disease, and mild cognitive impairment, and has also been hypothesized to contribute to radiation-induced cognitive losses15,31–32.
Figure 1.
(A) Axial T2 weighted axial MRI image of leukoencephalopathy (arrow) occurring in a 53 year old female patient 5 years after receiving chemoradiation (60 Gy) treatment for a high grade glioma. (B) Axial FLAIR MRI image of radionecrosis as a hypoenhancing center (arrow) surrounded by a rim of enhancement indicating active inflammation in a 48 year old male patient following chemoradiation (60 Gy) treatment for high grade glioma.
Imaging CNS radiation effects
Currently, imaging remains the technique that is most readily accessible to most clinicians to evaluate the effects of radiation. However, not all radiographic changes are directly correlated to functional neurotoxicity and neurodegeneration. Radiographic definitions are lacking as conventional magnetic resonance imaging (MRI) has a limited ability to distinguish normal tissue injury from treatment related changes, such radiation necrosis or vascular changes33. Structural changes may be present on scans in previously irradiated patients but may have no bearing on patient function, while conversely, patient function may be compromised in the absence of structural changes28.
These data demonstrate is there is a pressing need for improved understanding of the biology related to the combined effects of tumor, pharmacological therapies, and radiotherapy on short term and late changes in brain microenvironment, neuroimmunity, neural circuitry and pathways, blood-brain barrier permeability, as well as subcellular mechanisms of neurological dysfunction and neurodegeneration. Imaging-related biomarker identification for normal tissue injury and response in patients receiving radiation is one area of investigation that may show potential promise in identifying patients in whom prevention and interventional strategies are needed. MRI-based vascular hippocampal marker parameters related to blood-brain barrier permeability, K(trans), and the fraction of blood plasma volume, Vp, have recently been evaluated as markers of injury34, correlating significantly with changes in memory function at 6 and 18 months after treatment, suggesting that K(trans) could serve as one predictor of late neurocognitive dysfunction for patients.
Distinguishing recurrent CNS tumor from radiation necrosis is one of the most frustrating normal tissue considerations in imaging. A recent meta-analysis of imaging studies attempting to differentiate between the two has demonstrated that magnetic resonance spectroscopy alone has moderate diagnostic performance in differentiating tumor recurrence from radiation necrosis using metabolite ratios such as choline/creatine (Cho/Cr) and choline/N-acetylaspartate (Cho/NAA)35. However, future studies will determine how magnetic resonance spectroscopy should be combined with other advanced imaging technologies to improve diagnostic accuracy.
Imaging metabolic effects of radiation on the CNS and determining which observed changes correspond to functional toxicity are areas under active development. The use of positron emission tomography (PET)36, 13C labeled metabolites such as 13C-pyruvate37, and cation exchange saturation transfer (CEST) imaging38 may be able to allow investigators and clinicians to image altered glucose uptake, energy production, and glutamate neurotransmitter fluctuations, respectively, in acute and late toxicities.
Functional measures of toxicity
Historically, the incidence of functional toxicity from treatment to the entire brain was defined through the use of the Folstein Mini Mental Status Exam (MMSE) as a simple, rapid, and cost effective, albeit crude, measure of dementia8,9. However, more refined measures of neuropsychological testing to elucidate subtle changes in components of various aspects of CNS function such as memory, attention, executive function, processing speed, motor speed and overall sense of well-being have also been employed21. Radiation has been shown to negatively affect processing speed, attention, learning and memory, retrieval, executive function and fine motor coordination. Since white matter density is high in frontal and subcortical areas, impairment in cognition in these regions is common39.
To address the issue of standardization, a pilot study was done to evaluate the feasibility of a battery of non–physician-administered tests that measure neurocognitive function and assess activities of daily living in 30 patients with brain metastases. The selected neurocognitive function tests included the Hopkins Verbal Learning Tests (HVLT) to evaluate memory, Trailmaking (TMT) A and B to measure visual motor speed and executive function, the Grooved Pegboard for fine motor coordination, the Controlled Oral Word Association (COWA) test for verbal fluency and the Barthel Index to evaluate activities of daily living. The study demonstrated complete patient compliance, with average test completion time of 23 ± 6 minutes. It also showed that despite high functional status, most patients demonstrated baseline impairments in memory and fine motor domains, measured by the recall and delayed memory portions of the HVLT and by the pegboard test, respectively40.
Subsequently, the RTOG conducted a multi-institutional trial to test the feasibility of performing a test battery consisting of five neurocognitive measures and a quality-of-life instrument in patients with brain metastases. The test battery included the MMSE, Hopkins Verbal Learning Test, Verbal Fluency/Controlled Word Association Test, Ruff 2 and 7 test, Trailmaking Test, and Profile of Mood States-Short Form. The primary objective of the study was to establish whether patients were able to complete the test battery. The overall compliance rate for administration and completion of the five neurocognitive measures and a quality-of-life instrument before treatment, at treatment completion, and 1 month after treatment was ≥95%, ≥84%, and ≥70%. The most common causes of noncompliance were patient-related factors (e.g., performance status or inability to understand test instructions) and not institutional error41.
However, how these tests are best utilized to evaluate radiation toxicity, and which neuropsychological measures predict for radiation induced toxicities, is a matter of some debate. More typically, selected or limited neurocognitive testing at pre-selected short term time points, particularly the use of the HVLT at 4 months, already known to temporarily nadir following brain radiation at that time point, may provide only a snapshot at a given point in time and may be overgeneralized to other cognitive functions or time intervals without further data to evaluate the time course of recovery. The contributions of tumor progression to changes in these measures must also be considered.
Strategies for reducing toxicity
Prevention of radiation-induced toxicity in the CNS has focused on improvements in our technical capacity to treat patients using advanced radiation planning techniques to minimize dose to important but clinically uninvolved structures. Attempts at sparing brain subcompartments from injury are extrapolated from whole brain radiation studies. RTOG 0933 used intensity modulated radiation therapy to spare the neural stem cell population in the adult hippocampus in patients receiving whole brain radiation with brain metastases. At 4 months following treatment, a decreased mean relative decline in the HVLT-R delayed recall was observed and was significantly reduced compared to historical controls (7% vs. 30%, p<0.001), demonstrating a limited benefit in memory and quality of life in the short term42. However, it is unknown what other neurocognitive parameters might be affected by hippocampal sparing. Therefore, a phase III randomized trial evaluating the effects of hippocampal sparing is ongoing.
Reducing treatment volumes to only visible tumor through the use of stereotactic radiosurgery has also been found to have benefits in memory at short term intervals following treatment when compared to whole brain radiation. A recent multi-institutional randomized controlled trial, N0574 compared radiosurgery alone to radiosurgery combined with whole brain radiation in patients with 1–3 brain metastases43. Neurocognitive tests including HTLV-R for memory, Grooved Pegboard for fine motor control, COWA for verbal fluency, Trailmaking A and Trailmaking B for processing speed and executive function, respectively, were evaluated at 3 months and at 12 months in long-term survivors. Less cognitive deterioration was observed at 3 months with SRS alone (63.5% vs. 91.3%, p<0.001). In long-term survivors, cognitive deterioration was found to be significantly less with SRS alone at both 3 months (45.5% vs. 94.1%, p=0.007) and at 12 months (60% vs. 94.4%, p=0.04). Therefore, less normal tissue treated yields decreased functional toxicity. While increased toxicities are not acceptable for patients in whom palliation is the goal without evidence of a survival benefit, the consideration may be different if and when radiation is used with curative intent.
Although, heavy particles with high linear transfer (LET) properties, such as protons, have theoretical advantages in limiting the amount of normal tissue exposed to ionizing radiation, randomized data on their use demonstrating reduced CNS toxicities is lacking. Additionally, limited treatment options exist for most patients requiring radiation treatment to the CNS, and therefore, most questions regarding patient selection, i.e. in whom do we modify or omit radiation altogether, are questions which may be considered in the near future. Likewise, effective radioprotectors, compounds which act as radiation modifiers to reduce the damage of ionizing radiation on normal tissue, have not yet been identified to reduce CNS toxicities in a majority of patients. However, radioprotector development and exploration of radioprotection mechanisms in the CNS remain a field of active exploration and tremendous potential for future development.
Treatment and mitigation
Corticosteroids have long been employed to temper local edema and inflammatory effects of tumor on the CNS before, during, and after radiation treatment. The mechanism of action for corticosteroids is likely the consequence of direct effects on lymphocyte-mediated inflammation and reduced vascular permeability. While corticosteroids have beneficial effects in the short-term on cognition, reduction of inflammation and edema, they should be considered a temporizing measure due to the fact that long-term use can lead to multiple side effects, such as opportunistic infections, endocrinological abnormalities, weight gain, mood changes, and skin thinning.
The RTOG investigated the use of corticosteroids in symptomatic patients with brain lesions, particularly in patients with minimal neurologic difficulties, not requiring nursing care or hospitalization (neurologic function 2), and in patients seriously limited in performing normal activities, requiring nursing care or hospitalization, confined to bed or wheelchair, or with significant intellectual impairment (neurologic function 3). Patients who received steroids during irradiation showed improvement more quickly than those who did not receive steroids (at 2 weeks, p = 0.003). Patients with initial neurologic function 2 showed no difference in the overall frequency of improvement, whereas for initial neurologic function 3 patients, the overall frequency of improvement was greater for those who received steroids (p = 0.05)44. Importantly, steroid use was found to have had no influence on time to progression. Thus, the worse the neurologic function at baseline, the greater the benefit the patient is likely to receive from corticosteroids, and the more likely steroids will provide a benefit in reducing acute radiation-induced CNS toxicity.
While there have been numerous Phase I studies investigating potential avenues to treat and mitigate radiation-induced toxicity in the CNS, there have been few randomized trials (Table 1). Because of memory deficits observed following radiotherapy, there has been the assumption that Alzheimer’s associated pathways are shared with radiation-induced neurotoxicity. However, the biological basis of the process is poorly understood, and indeed in animal studies, short term changes that occur in the brain proteome leading to long-term toxicities have been determined to be a much more complex process with changes identified in neurodegenerative pathways similar to not just the Alzheimer’s canonical pathways, but also Huntington’s and Parkinsonian pathways45. Consequently, neurotransmitter-based pharmacologic therapies borrowed from Alzheimer’s treatment have been investigated in the setting of radiation-induced cognitive decline. Accordingly, limited successes have been realized. However, in the past decade, with anticipated increases in expected survival times after radiotherapy, a renewed interest in CNS late effects, early intervention, and prevention have moved to the forefront.
Table 1.
Randomized trials to prevent or minimize CNS radiation toxicity.
| Trial | Intervention | Mechanism | Administration | Result |
|---|---|---|---|---|
| RTOG 910421 | Hyperfractionation | Altered fractionation | 54.4 Gy/1.6 Gy BID vs. 30 Gy/3 Gy |
|
| RTOG 093342 | Hippocampal sparing | Preservation of hippocampal neurogenesis | Intensity modulated radiation therapy (IMRT) delivered as 30 Gy in 10 fractions |
|
| N057443 | SRS vs. WBRT | Reduction of treatment volume | SRS (18–24 Gy) +/− Whole brain irradiation (30 Gy/12 fractions) |
|
| RTOG 061446 | Memantine | NMDA receptor antagonist | 20 mg/day given during radiation and for 24 weeks post-radiation |
|
| Wake Forest47 | Donepezil | Acetylcholine esterase inhibitor | 5–10 mg/day for 24 weeks beginning at least 6 months after partial or whole brain irradiation |
|
In one example of translation of therapy from the Alzheimer’s arena is the use of memantine, an NMDA receptor antagonist used in the treatment of Alzheimer’s patients and patients with obsessive-compulsive disorder. In RTOG 0614, patients receiving whole brain radiation treatment that received concurrent and adjuvant memantine had better cognitive function over time; specifically, memantine delayed time to cognitive decline and reduced the rate of decline in memory, executive function, and processing speed in patients receiving the drug. Although there was a decreased decline in the primary endpoint of delayed recall at 24 weeks, the study may be criticized for a lack of statistical significance, possibly due to significant patient loss46.
A phase III study with donepezil, an acetylcholine esterase inhibitor, was evaluated in patients who had received partial brain irradiation or whole brain irradiation ≥ 6 months prior to enrollment. With 24 weeks of therapy, donepezil did not significantly improve composite scores for neurocognitive functioning. However, significant differences favoring donepezil were observed for memory, motor speed and dexterity. Not unexpectedly, the benefit of donepezil was found to be greater in those patients who were more cognitively impaired at baseline47.
The benefit of non-pharmacological strategies, such as exercise-induced stimulation of hippocampal neurogenesis, cognitive therapies and mesenchymal stem cell replacement to prevent or delay onset of normal tissue injury in patients are currently under investigation48,49. Importantly, clinicians and investigators have come to realize that not all equivalent interventions produce the same toxicities across multiple individuals. The reasons for this are likely complex and multifactorial, but the influence of genetic polymorphisms to individual responses is an emerging area of investigation that could allow us to preemptively stratify patients among those with low, average, and high risk of radiation-induced toxicity from a given therapy50. Alternatively, the data may allow us to stratify patients who are likely to be responders versus non-responders to a given strategy, pharmaceutical or otherwise, to ameliorate radiation-induced toxicities. In this way, precision medicine may not only be useful in determining customized treatments to combat disease, but also individualized blueprints to effectively combat potential toxic side-effects of those treatments.
Future Directions
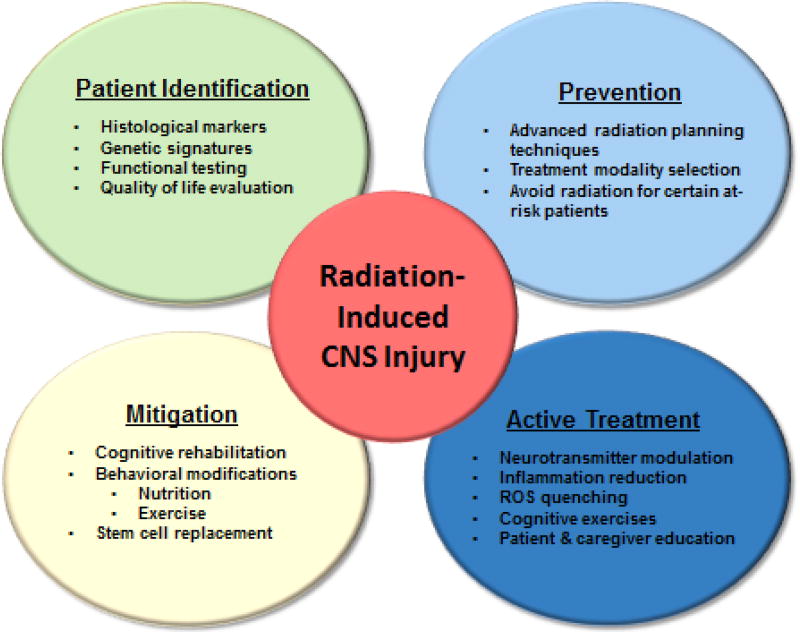
The history of investigation into radiation toxicity in the central nervous system that results from therapeutic treatment began with prioritizing toxicity over efficacy due to the limited options available to the patient at the time and the limited lifespan anticipated for most patients. Metastatic brain tumors are the most common intracranial tumor occurring in approximately 10–30% of adult cancer patients and in about 6–10% of children with cancer, with primary brain tumors occurring at increasing frequency51. While the vast majority of diseases requiring radiation treatment in the CNS suffer from poor prognosis, investigators in the field anticipate that many of these diseases will see drastic improvements in response to treatment and overall survival within the coming years. As a consequence, the development of strategies to prevent, actively treat and mitigate radiation toxicity has never been more important. Additionally, over the last four decades, our understanding of brain health and maintenance has revealed that interventions outside the CNS can have durable impacts on brain function, such as the contribution of microbial immunity to brain health and immune function, stem cell biology and the contribution of physical activity. Therefore, it is likely that many of the interventions and preventions we are able to employ to benefit the brain may be extracranial in focus and could produce benefits to brain repair via indirect effects. Identifying patients at risk, actively treating, and rehabilitating late radiation-induced CNS toxicity will require a multidisciplinary approach that will include not only the traditional approach of radiation oncologists, dosimetrists and pharmacists, but also physical therapists, nutritionists, and neuropsychologists. Improved caregiver and family support and education with treatment regimens that may be pursued as outpatients and intensive inpatient rehabilitation should be introduced when appropriate (Figure 2). It is predicted that these types of interventions to minimize radiation-induced CNS toxicity will need to begin much sooner during treatment and continue aggressively during the recovery phase after treatment. Additionally, active development of novel strategies is being pursued by investigators to reverse late effects once they have formed. As a combined program, we anticipate great improvements in patient functioning and quality of life that occur concurrently with improved disease control and increased survival.
Figure 2.
Combined strategic interventions for combating radiation-induced toxicity in the CNS.
Acknowledgments
This work was supported in part by NIH Intramural Funding Project ZIA BC 011222.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts: Nothing to disclose.
References
- 1.Cordes MC, Scherwath A, Ahmad T. Distress, anxiety and depression in patients with brain metastases before and after radiotherapy. BMC Cancer. 2014;14:731. doi: 10.1186/1471-2407-14-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tseng BP, Giedzinski E, Izadi A. Functional consequences of radiation-induced oxidative stress in cultured neural stem cells and the brain exposed to charged particle irradiation. Antioxid Redox Signal. 2014;20:1410–1422. doi: 10.1089/ars.2012.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conforth M, Bedford J. X-ray induced breakage and rejoining of human interphase chromosomes. Science. 1983;222:1141–1143. doi: 10.1126/science.6648528. [DOI] [PubMed] [Google Scholar]
- 4.Giusti A, Raimondi M, Ravagnan G. Human cell membrane oxidative damage induced by single and fractionated doses of ionizing radiation: a fluorescence spectroscopy study. Int J Radiat Biol. 1998;74:595–605. doi: 10.1080/095530098141177. [DOI] [PubMed] [Google Scholar]
- 5.Azzam E, de Toledo S, Little J. Expression of CONNEXIN43 is highly sensitive to ionizing radiation and other environmental stresses. Cancer Res. 2003;63:7128–7135. [PubMed] [Google Scholar]
- 6.Dayal D, Martin S, Owens K. Mitochondrial complex II dysfunction can contribute significantly to genomic instability after exposure to ionizing radiation. Radiat Res. 2009;172:737–745. doi: 10.1667/RR1617.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramer S, Henrickson F, Zelen M. Therapeutic trials in the management of metastatic brain tumors by different time/dose fraction schemes of radiation therapy. JNCI. 1977;46:213–221. [PubMed] [Google Scholar]
- 8.DeAngelis LM, Mandell LR, Thaler HT. The role of postoperative radiotherapy after resection of single brain metastases. Neurosurgery. 1989;24:798–805. doi: 10.1227/00006123-198906000-00002. [DOI] [PubMed] [Google Scholar]
- 9.DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789–796. doi: 10.1212/wnl.39.6.789. [DOI] [PubMed] [Google Scholar]
- 10.Shaw E, Scott C, Souhami L. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastasesfinal report of RTOG 90-05. Int J Radiat Oncol Biol Phys. 2000;47:269–271. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 11.Andrews DW, Scott CB, Sperduto PW. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: a phase III results of the RTOG 9805 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 12.Andres-Mach M, Rola R, Fike JR. Radiation effects on neural precursor cells in the dentate gyrus. Cell Tissue Res. 2008;331:251–262. doi: 10.1007/s00441-007-0480-9. [DOI] [PubMed] [Google Scholar]
- 13.Monje M. Cranial radiation therapy and damage to hippocampal neurogenesis. Dev Disabil Res Rev. 2008;14:238–242. doi: 10.1002/ddrr.26. [DOI] [PubMed] [Google Scholar]
- 14.Wojtowicz JM. Irradiation as an experimental tool in studies of adult neurogenesis. Hippocampus. 2006;16:261–266. doi: 10.1002/hipo.20158. [DOI] [PubMed] [Google Scholar]
- 15.Allen AR, Eilertson K, Sharma S. Effects of radiation combined injury on hippocampal function are modulated in mice deficient in chemokine receptor 2 (CCR2) Radiat Res. 2013;180:78–88. doi: 10.1667/RR3344.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Machida M, Lonart G, Britten RA. Low (60cGy) doses of (56)Fe HZE-particle radiation lead to a persistent reduction in the glutamatergic readily releasable pool in rat hippocampal synaptosomes. Radiat Res. 2010;174:618–623. doi: 10.1667/RR1988.1. [DOI] [PubMed] [Google Scholar]
- 17.Belka C, Budach W, Kortmann RD. Radiation induced CNS toxicity-molecular and cellular mechanisms. Br J Cancer. 2001;85:1233–1239. doi: 10.1054/bjoc.2001.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahles TA, Root JC, Ryan EL. Cancer-and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol. 2012;30:3675–3686. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res. 2000;153:357–370. doi: 10.1667/0033-7587(2000)153[0357:trotcn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 21.Regine WF, Scott C, Murray K. Neurocognitive outcome in brain metastases patients treated with accelerated-fractionation vs. accelerated-hyperfractionated radiotherapy: an analysis from Radiation Therapy Oncology Group Study 91-04. Int J Radiat Oncol Biol Phys. 2001;51:711–717. doi: 10.1016/s0360-3016(01)01676-5. [DOI] [PubMed] [Google Scholar]
- 22.Baschnagel A, Wolters PL, Camphausen K. Neuropsychological testing and biomarkers in the management of brain metastases. Radiat Oncol. 2008;3:26. doi: 10.1186/1748-717X-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appelman-Dijkstra NM, Kokshoorn NE, Dekkers OM. Pituitary dysfunction in adult patients after cranial radiotherapy: systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:2330–2340. doi: 10.1210/jc.2011-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tallet AV, Azria D, Barlesi F. Neurocognitive function impairment after whole brain radiotherapy for brain metastases: actual assessment. Radiat Oncol. 2012;7:77. doi: 10.1186/1748-717X-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aoyama H, Tago M, Kato N. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys. 2007;68:1388–1395. doi: 10.1016/j.ijrobp.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 26.Chow R, Tsao M, Pulenzas N. Do patients with brain metastases selected for whole brain radiotherapy have worse baseline quality of life as compared to those for radiosurgery or neurosurgery (with or without whole brain radiotherapy)? Ann Palliat Med. 2016;5:1–12. doi: 10.3978/j.issn.2224-5820.2015.11.01. [DOI] [PubMed] [Google Scholar]
- 27.Robbins ME, Bourland JD, Cline JM. A model for assessing cognitive impairment after fractionated whole-brain irradiation in nonhuman primates. Radiat Res. 2011;175:519–525. doi: 10.1667/RR2497.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prust MJ, Jafari-Khouzani K, Kalpathy-Cramer J. Standard chemoradiation for glioblastoma results in progressive brain volume loss. Neurology. 2015;85:683–691. doi: 10.1212/WNL.0000000000001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greene-Schloesser D, Moore E, Robbins ME. Molecular pathways: radiation-induced cognitive impairment. Clin Cancer Res. 2013;19:2294–2300. doi: 10.1158/1078-0432.CCR-11-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crossen JR, Garwood D, Glatstein E. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 31.Schnegg CI, Greene-Schloesser D, Kooshki M. The PPARdelta agonist GW0742 inhibits neuroinflammation, but does not restore neurogenesis or prevent early delayed hippocampal-dependent cognitive impairment after whole-brain irradiation. Free Radic Biol Med. 2013;61:1–9. doi: 10.1016/j.freeradbiomed.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenrow KA, Brown SL, Lapanowski K. Selective inhibition of microglia-mediated neuroinflammation mitigates radiation-induced cognitive impairment. Radiat Res. 2013;179:549–556. doi: 10.1667/RR3026.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johannesen TB, Lien HH, Hole KH. Radiological and clinical assessment of long-term brain tumour survivors after radiotherapy. Radiother Oncol. 2003;69:169–176. doi: 10.1016/s0167-8140(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 34.Farjam R, Pramanik P, Aryal MP. A radiation-induced hippocampal vascular injury surrogate marker predicts late neurocognitive dysfunction. Int J Radiat Oncol Biol Phys. 2015;93:908–915. doi: 10.1016/j.ijrobp.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Ma L, Wang Q. Role of magnetic resonance spectroscopy for the differentiation of recurrent glioma from radiation necrosis: a systematic review and meta-analysis. Eur J Radiol. 2014;83:2181–2189. doi: 10.1016/j.ejrad.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Hojjati M, Garg V, Badve CA. Differentiation of recurrent spinal ependymoma from postradiation treatment necrosis through multiparametric PET-MR and perfusion MRI. Clin Imaging. 2017;41:48–52. doi: 10.1016/j.clinimag.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Saito K, Matsumoto S, Takakusagi Y. 13C-MR spectroscopic imaging with hyperpolarized [1-13C]pyruvate detects early response to radiotherapy in SCC tumors and HT-29 tumors. Clin Cancer Res. 2015;21:5073–5081. doi: 10.1158/1078-0432.CCR-14-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kogan F, Singh A, Debrosse C. Imaging of glutamate in the spinal cord using GluCEST. Neuroimage. 2013;77:262–267. doi: 10.1016/j.neuroimage.2013.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wefel JS, Kayl AE, Meyers CA. Neuropsychological dysfunction associated with cancer and cancer therapies: a conceptual review of an emerging target. Br J Cancer. 2004;90:1691–1696. doi: 10.1038/sj.bjc.6601772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herman MA, Tremont-Lukats I, Meyers CA. Neurocognitive and functional assessment of patients with brain metastases: a pilot study. Am J Clin Oncol. 2003;26:273–279. doi: 10.1097/01.COC.0000020585.85901.7C. [DOI] [PubMed] [Google Scholar]
- 41.Regine WF, Schmitt FA, Scott CB. Feasibility of neurocognitive outcome evaluations in patients with brain metastases in a multi-institutional cooperative group setting: results of Radiation Therapy Oncology Group trial BR-0018. Int J Radiat Oncol Biol Phys. 2004;58:1346–1352. doi: 10.1016/j.ijrobp.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 42.Gondi V, Pugh SL, Tome WA. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32:3810–3816. doi: 10.1200/JCO.2014.57.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown PD, Jaeckle K, Ballman KV. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borgelt B, Gelber R, Kramer S. The palliation of brain metastases: final results of the first two studies by the Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 1980;6:1–9. doi: 10.1016/0360-3016(80)90195-9. [DOI] [PubMed] [Google Scholar]
- 45.Shukla S, Shankavaram UT, Nguyen P. Radiation-induced alteration of the brain proteome: understanding the role of the sirtuin 2 deacetylase in a murine model. J Proteome Res. 2015;14:4104–4117. doi: 10.1021/acs.jproteome.5b00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown PD, Pugh S, Laack NN. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15:1429–1437. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rapp SR, Case D, Peiffer A. Donepezil for irradiated brain tumor survivors a phase III randomized placebo controlled clinical trial. J Clin Oncol. 2015;33:1653–1659. doi: 10.1200/JCO.2014.58.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biedermann SV, Fuss J, Steinle J. The hippocampus and exercise: histological correlates of MR-detected volume changes. Brain Struct Funct. 2014;221:1353–1363. doi: 10.1007/s00429-014-0976-5. [DOI] [PubMed] [Google Scholar]
- 49.Donega V, Nijboer CH, van Tilborg G. Intranasally administered mesenchymal stem cells promote a regenerative niche for repair of neonatal ischemic brain injury. Exp Neurol. 2014;261:53–64. doi: 10.1016/j.expneurol.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 50.Liu YR, Zhou EP, Sulman ME. Genetic modulation of neurocognitive function in glioma patients. Clin Cancer Res. 2015;21:3340–3346. doi: 10.1158/1078-0432.CCR-15-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson JD, Young B. Demographics of brain metastasis. Neurosurg Clin N Am. 1996;7:337–344. [PubMed] [Google Scholar]