Summary
Prior research using functional magnetic resonance imaging (fMRI) [1–4] and behavioral studies of patients with acquired or congenital amusia [5–8] suggest that the right posterior superior temporal gyrus (STG) in the human brain is specialized for aspects of music processing (for review see 9–12). Intracranial electrical brain stimulation in awake neurosurgery patients is a powerful means to determine the computations supported by specific brain regions and networks [13–21], because it provides reversible causal evidence with high spatial resolution (for review, see [22, 23]). Prior intracranial stimulation or cortical cooling studies have investigated musical abilities related to reading music scores [13, 14] and singing familiar songs [24, 25]. However, individuals with amusia (congenitally, or from a brain injury) have difficulty humming melodies but can be spared for singing familiar songs with familiar lyrics [26]. Here we report a detailed study of a musician with a low-grade tumor in the right temporal lobe. Functional MRI was used pre-operatively to localize music processing to the right STG, and the patient subsequently underwent awake intraoperative mapping using direct electrical stimulation during a melody repetition task. Stimulation of the right STG induced ‘music arrest’ and errors in pitch, but did not affect language processing. These findings provide causal evidence for the functional segregation of music and language processing in the human brain, and confirm a specific role of the right STG in melody processing.
Graphical abstract
Patient AE is a 26 year-old male saxophonist and wind instrument teacher who presented in 2015 with a brain tumor medial to the right posterior middle temporal gyrus and undercutting the superior temporal gyrus (Figure 1A, tumor in yellow fill). Over a period of six months the patient underwent extensive pre-operative fMRI and behavioral testing to localize music, language, and high-level visual processing, and to ascertain his levels of performance with judgments of musical pitch, rhythm, and contour. Patient AE had no discernible cognitive or sensorimotor impairments and was in the normal range across all pre-operative neuropsychological tests assessing language, semantic memory, visual and auditory processing, and praxis knowledge (Table S1). He exhibited typical neural organization of language, high-level visual processing, and praxis knowledge (see Figure S1A for fMRI contrast maps and Methods for all details). In this report, we focus on the relation between pre-operative fMRI studies of music processing and the behavioral effects of direct electrical stimulation to the right temporal lobe during the awake portion of his surgery.
Figure 1. Music processing in the right superior temporal gyrus.
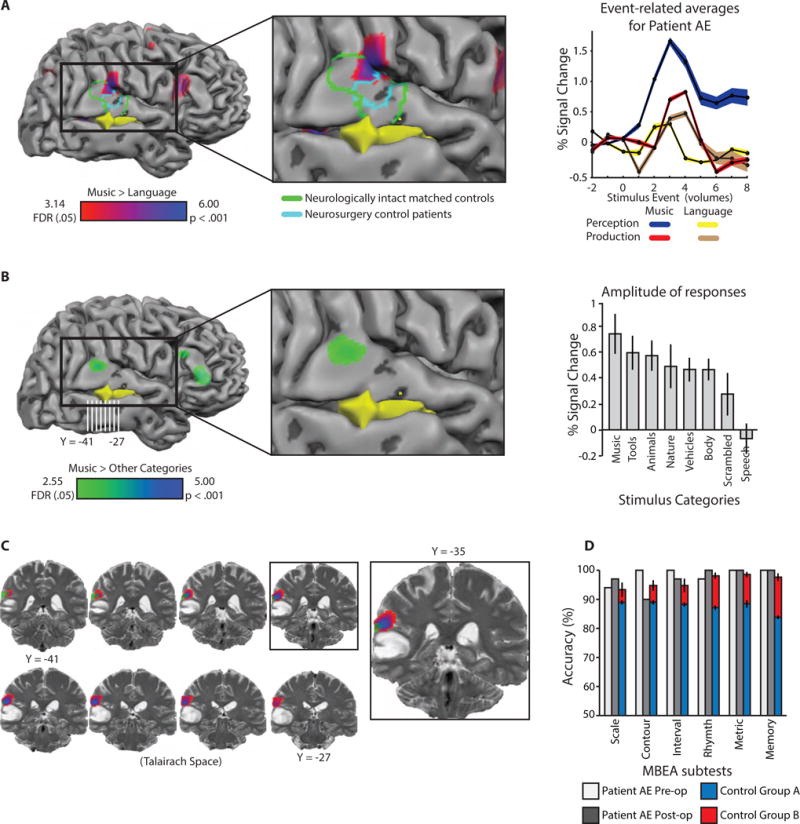
A. Voxels exhibiting increased BOLD contrast for melody compared to sentence repetition in the vicinity of the right superior temporal gyrus (red-blue color scale; t ≥ 3.14, whole-brain FDR q < .05; peak coordinate [57 -34 22]). The tumor is represented in yellow. A group of four neurologically intact age and music-education matched control participants took part in the same experiment (data are plotted in green outline on Patient AE’s brain to facilitate comparison, t ≥ 2.51, FDR q < .05). A separate group of ten neurosurgery patients also completed the same experiment pre-operatively (data plotted in cyan, t ≥ 2.26, p < .05, uncorrected). Event-related time series indicate Patient AE’s neural responses in the right superior temporal gyrus were maximal for music perception. B. There was increased BOLD contrast for music stimuli (e.g., guitar strumming, piano playing) compared to other categories of sounds (e.g., vehicle sounds, human bodily sounds, animal sounds) in the right superior temporal gyrus (green-blue color scale; t ≥ 2.55, FDR q < .05; peak coordinate [57 -37 19]). An ROI analysis (Figure 1B) demonstrated increased BOLD signal for music stimuli compared to tool, animal, nature and vehicle sounds, human bodily noises, a scrambled baseline condition, and human speech. C. Coronal images of music preferences overlaid on a pre-operative T2 anatomical image. D. Patient AE performed within control range on the Montreal Battery of Evaluation of Amusia (MBEA) pre- and post-operatively. The small decrement in AE’s performance for contour discrimination post-operatively was not significantly different from controls (t < 1). All error bars represent the standard error of the mean, across participants. See also Figure S1.
Pre-Operative functional MRI and behavioral testing
In a first fMRI experiment designed to map music processing, the patient listened to a brief (3 second) piano melody [1] or spoken sentence on each trial [27], internally ‘rehearsed’ the stimulus, and then overtly produced the stimulus (humming in the case of melodies, speaking in the case of language; task modeled directly after Hickok and colleagues [1]). Replicating prior studies using this paradigm [1, 2], there was increased blood oxygen level dependent (BOLD) contrast for melody processing compared to sentence processing in the right superior temporal gyrus (Figure 1A). A closer look at the gyral anatomy in coronal images (Figure S2) indicated that the ‘peak’ of this activity was at the posterior aspect of the Sylvian fissure, likely in the superior temporal gyrus, and potentially involving the parietal operculum (see also surface rendering in Figure 1A). Event-related responses in that region were differentially driven by perception and rehearsal as opposed to production of the melodies (Figure 1A). Two controls groups were assessed with the same fMRI experiment: One group was comprised of age- and education-matched musicians (n = 4); a second control group consisted of neurosurgery patients scanned pre-operatively (n = 10), whose lesions were in either the left or right hemisphere, but not in the right posterior superior temporal gyrus. The neurologically-intact matched controls and neurosurgery controls exhibited similar foci of increased BOLD contrast for music compared to language in the right superior temporal gyrus (green and cyan outlines, respectively, Figure 1A; see Figure S1C and Figure S1D for whole-brain contrast maps). An analysis that quantitatively assessed the similarity of Patient AE to matched healthy control participants found that he was within the range of age- and education-matched controls in terms of the location of the peak voxel in the vicinity of the right posterior superior temporal gyrus expressing ‘music preferences’ (Figure S2).
In a second fMRI experiment, the patient passively listened to melodies and other natural and environmental sounds (e.g., animal noises, human speech, tool noises; stimuli from Norman-Haignere et al., [3]). This paradigm again identified a focus within the right superior temporal gyrus that exhibited increased BOLD contrast for music stimuli compared to the other sound categories (Figure 1B). A region-of-interest (ROI) analysis demonstrated that responses to music stimuli were greater than responses to other types of sounds (Figure 1B; see Methods for details). The peak ‘music preferring’ voxel in this experiment was shifted to the lateral surface of the superior temporal gyrus compared to the peak in the posterior Sylvian fissure observed in the first experiment. A framework within which to understand that shift may be provided by Hickok and colleagues [1], who found that rehearsal of melodies, compared to general auditory processing of melodies, led to increased BOLD contrast in the deep portion of the posterior Sylvian fissure (area Spt). Nonetheless, it is important to emphasize that the peaks for music preferences in Patient AE in the two fMRI experiments were separated by less than 5mm. The close proximity indicates good agreement across two independent approaches to localizing music preferring cortex in the posterior superior temporal gyrus in Patient AE. In summary, Patient AE exhibited typical neural organization for music processing that was localized to (among other regions) the right posterior superior temporal gyrus, directly adjacent to the tumor (Figure 1C).
We also assessed the patient’s musical ability using the Montreal Battery of Evaluation of Amusia (MBEA), developed by Peretz and colleagues [5]. AE was correct on 177 out of 180 trials, which places him in the 89th percentile (normalized values from [5], see Table S1). The patient’s performance across each subtest of the MBEA was also within the range of a group of music-education matched control participants (Figure 1D). Note as well that a subset of those same controls completed the fMRI protocol to map melody processing (Figure 1A, green outline; Figure S1C).
In preparation for the awake mapping procedure, Patient AE practiced a modified version of the melody and sentence repetition task that had been used in fMRI, and also practiced playing a piece of music on his saxophone that was modified to reduce the number and duration of long notes that would be played intraoperatively (see Methods).
Intraoperative Electrical Stimulation Mapping
The awake mapping session was organized into three phases, in the following order: Picture naming, intermixed sentence and melody repetition, and melody repetition. The patient did not make any errors on any trials from the language tasks (picture naming and sentence repetition), regardless of where electrical stimulation was delivered (see below). During the melody repetition trials, AE listened to and immediately repeated 74 melodies; 36 of those trials were performed in conjunction with direct electrical stimulation to the right middle or superior temporal gyri, or inferior parietal cortex. Trials were separated, offline, into correct (completely acceptable responses), minor errors (acceptable performance but minor errors in pitch, rhythm, and/or contour), and major errors (major errors in pitch, rhythm, and contour). The category of ‘major error’ included what we refer to as ‘music arrest’— a transient inability to hum a melody (see Movie S1 for examples of errors and correct trials during intraoperative music mapping).
Patient AE made a total of 8 major errors, all of which occurred after direct electrical stimulation of the right superior temporal gyrus (see Figure 2A). On 4 additional stimulation trials AE made minor errors in pitch, rhythm, and/or contour (see Figure 2A, cyan stimulation points; see Methods for detailed discussion of error types and Movie S1 for examples). AE never made errors in rhythm or tempo in isolation; all responses marked by errors in rhythm and/or tempo also contained errors in pitch. While the exigencies of the mapping session prevented stimulation of a broad expanse of cortex, it was the case that stimulation delivered to structures other than the superior temporal gyrus, in particular the middle temporal gyrus, did not result in major errors (Figure 2C).
Figure 2. The relation between intraoperative music performance and pre-operative fMRI.
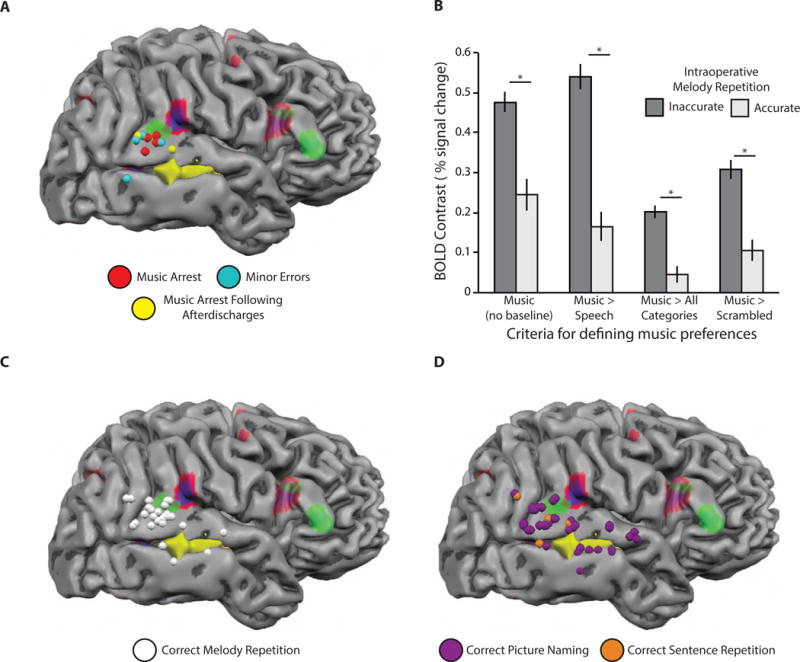
All intraoperative stimulation sites are represented as spheres on the cortical surface. A. Patient AE made 8 major and four minor errors in the melody repetition task after stimulation; 4 of the major errors were caused by electrical stimulation on the corresponding trial (red and blue spheres); the remaining 4 major errors were caused by afterdischarges propagating from stimulation events on prior trials (yellow spheres)—two spheres are shown for such trials, as one stimulation event was associated with major errors on 3 subsequent trials, and one stimulation event was associated with major errors on 1 subsequent trial; 4 minor errors were associated with stimulation sites in cyan. See Movies S1 and S2 for examples of inaccurate and accurate intraoperative trials. B. Intraoperative melody repetition during stimulation is related to pre-operative fMRI defined music activity. Functional MRI voxels corresponding to stimulation sites associated with errors exhibited stronger pre-operative BOLD contrast for musical stimuli compared to functional voxels corresponding to stimulation sites that never resulted in an error; this effect was present for a range of contrasts used to define music preferences (* p < .001, unpaired samples t-test). C. Stimulation sites associated with correct melody repetition are plotted in white. Stimulation of the right posterior superior temporal gyrus did not always elicit errors in melody repetition, but major errors were caused by stimulation of that region (panel A). D. Stimulation sites associated with correct picture naming and sentence repetition trials. There was no effect of stimulation to the right posterior superior temporal gyrus on picture naming or sentence repetition trials (see also Figure S1 and Movie S2).
It is noteworthy that the patient at times spontaneously reported when his reproduction of a melody was incorrect, and was generally aware that he was making errors. For instance, he noted, after stimulation events of the superior temporal gyrus, that his humming response ‘did not feel right,’ or that his experience on that trial ‘was weird’ (e.g., see Movie S1). However, the patient was unaware on which trials his brain was being stimulated, when stimulation was being delivered on a given trial, or where in his brain the stimulation was delivered.
An important question that can be addressed with this dataset is the degree to which pre-operative fMRI relates to behavioral accuracy after direct electrical stimulation. Coordinates in MRI-space were acquired in the operating room for each instance of brain stimulation during the mapping session (see Methods). This permitted an analysis in which we computed the fMRI-based music-related activity for stimulation sites (i.e., voxels) associated with errors and for sites that were never associated with errors (see Methods for details). We found that music-related fMRI activity was significantly stronger in voxels associated with stimulation-induced errors compared to voxels that were not associated with errors in melody repetition (Figure 2B). This finding was not dependent upon the specific contrast used to define musical preferences in the pre-operative fMRI datasets (see Figure 2B).
As shown in Figures 3 and 4, the spatial distribution of errors indicates that neither stimulation strength (Figure 3) nor the duration of electrical stimulation (Figure 4) was related to the incidence or type of errors. Specifically, major and minor errors in melody repetition were observed for the full ranges of current amplitudes (Figure 3A and 3B) and stimulation durations (Figure 4A and 4B) used throughout the mapping session. Importantly, the same values of stimulation amplitude and duration that affected melody repetition had no effect on sentence repetition or picture naming (see Figures 3D and 4D). Finally, correct melody repetition trials were associated with the full ranges of current amplitude (Figure 3C) and stimulation duration (Figure 4C) as well. These findings reinforce the conclusion that major errors in melody repetition were due to stimulation of the right superior temporal gyrus, as opposed to other parameters of the electrical stimulation.
Figure 3. Modulation of Patient AE’s intraoperative behavioral performance as a function of direct electrical stimulation strength.
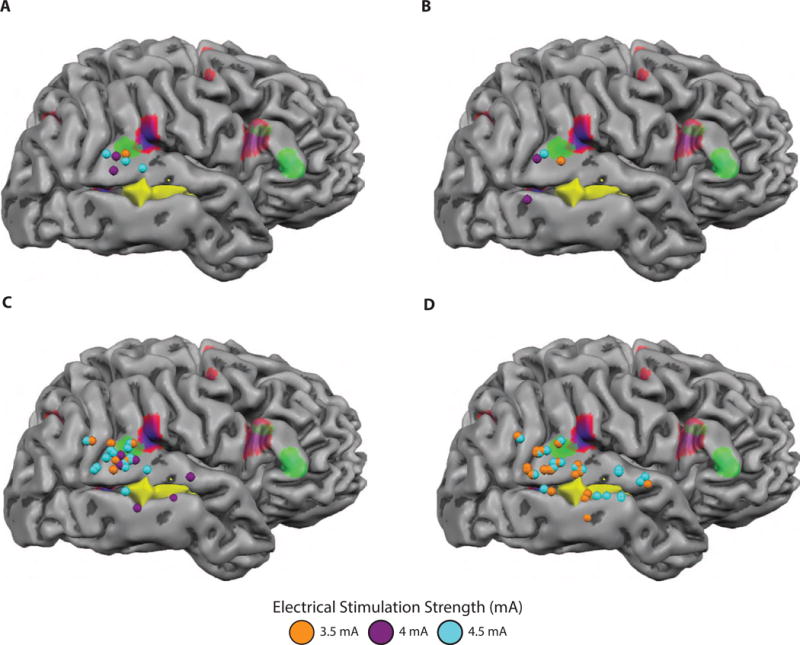
To evaluate whether the likelihood of an error was related to stimulation strength, we sought to better characterize the electrical stimulation parameters that generated major and minor errors. This analysis is possible because the surgeons vary the current amplitude throughout the mapping session to ensure that stimulation is being delivered at just below the afterdischarge threshold. Stimulation sites are recast as spheres and color-coded with respect to stimulation strength (mA). A. Stimulation sites associated with major errors in melody repetition were associated with electrical stimulation that ranged from 3.5 to 4.5 mA, indicating that Patient AE’s major errors do not derive solely from stimulations with the strongest current that was delivered. B. Stimulation sites associated with minor errors in melody repetition were present for the full range stimulation strength that was used. C. Stimulation sites associated with correct melody repetition were present for the full range stimulation strength that was used. D. Sentence repetition and picture naming performance contained both 3.5 and 4.5 mA stimulation events.
Figure 4. Modulation of Patient AE’s intraoperative behavioral performance as a function of direct electrical stimulation duration.
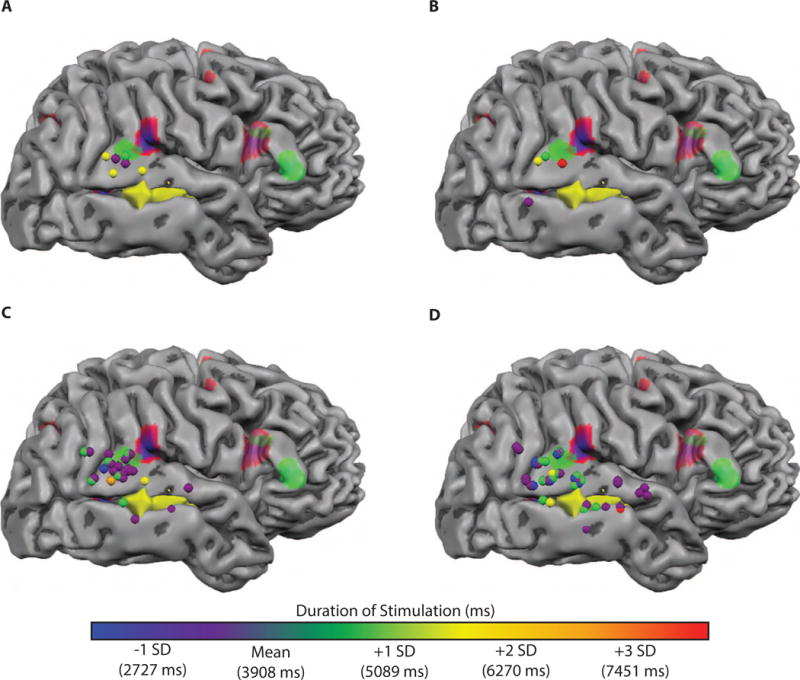
To evaluate whether error likelihood or type was related to stimulation duration, we recast stimulation sites in terms of stimulation duration. This analysis is possible because the duration of each stimulation is determined by how long the surgeon keeps the bipolar stimulator in contact with the brain. Typically, stimulation duration is 4 seconds, unless an error is elicited, in which case stimulation is discontinued. Stimulation events lasted between 2235 and 7540 milliseconds in duration (mean = 3908; SD = 1109 ms). A. Stimulation sites associated with major errors in melody repetition were associated with electrical stimulation that ranged between 3336 and 5872 milliseconds in duration (mean = 4613 ms), indicating that Patient AE’s major errors do not derive from stimulations with the longest possible duration. B. Stimulation sites associated with minor errors in melody repetition were associated with stimulation duration that ranged from 2869 to 7540 milliseconds in duration (mean = 5130 ms). C. Stimulation during Patient AE’s correct melody repetition contained stimulations from the full range of durations. D. Sentence repetition and picture naming also contained stimulations from the full range of durations.
In contrast, stimulation of the right superior temporal gyrus in AE never affected language performance (see Figure 2D for stimulation sites associated with accurate language performance). There were a total of 43 instances of direct brain stimulation during language tasks, across a combined 109 trials of picture naming and sentence repetition. Patient AE never made errors in the sentence repetition task, even after stimulation of the same region of the right posterior superior temporal gyrus that elicited errors in the similarly structured melody task (see Figure 2D; see Movie S2). A concern that may be raised is whether these intraoperative language paradigms have sensitivity to elicit language errors when critical language sites are stimulated. As a positive control, Movie S3 shows the types of language errors (phonological, speech arrest) made by Patient AG, an individual undergoing language mapping of the left temporal lobe prior to a left anterior temporal lobe resection.
Discussion
Much of the evidence that has elucidated the neural mechanisms of music processing comes from studies of individuals with impaired music ability (e.g., see [5–12]). MRI studies of the amusic brain have demonstrated structural abnormalities of the right inferior frontal gyrus (e.g., see [28, 29]) as well as reduced volume of the right arcuate fasciculus, a white matter tract that connects the right inferior frontal gyrus with the posterior superior temporal and inferior parietal areas [30]. Those studies suggest that amusia derives, at least in part, from abnormal connectivity between the right inferior frontal gyrus and right superior temporal gyrus [9]. While we were unable to record from the right inferior frontal gyrus in this study, it remains a possibility that electrical stimulation of the right posterior superior temporal gyrus resulted in current spread to the right inferior frontal gyrus via the right arcuate fasciculus ([23]). Nevertheless, the specificity of where direct brain stimulation resulted in impaired melody repetition (but not impaired language ability) provides causal evidence about a specific role for the right posterior superior temporal gyrus, perhaps together with anatomically connected structures, in melody processing.
The findings from Patient AE provide causal evidence for a key component of a neurocognitive model of music processing in the brain recently advanced by Peretz [9] in which processes local to the right superior temporal gyrus are hypothesized to support pitch processing. An important goal for future research will be to understand the real-time dynamics of functional interactions between the right inferior frontal gyrus and right superior temporal gyrus when patients are repeating melodies and sentences. Future work with electrocorticography could study the dynamics that mediate interactions between the right superior temporal gyrus and right inferior frontal gyrus during melody and language processing (e.g., for evidence in the domain of language see [31–33]; for review, see [34–35]).
Taking a step back, there is a long history of deriving inferences about the functional organization of cognitive processes from causal data provided by detailed case studies and case-series (e.g., [36 – 40], for theoretical discussion, see [41, 42]). The current report is not different in that regard, as the core causal inference extracted from the results of intraoperative stimulation is that melody processing is functionally dissociable from language processing in the right superior temporal gyrus. However, issues of cortical localization of function cannot be determined on the basis of individual cases, given the known heterogeneity of local functional organization across individuals, and other factors such as mass effects of a tumor. In this regard, our report is strengthened by the fact that Patient AE exhibited a pattern of fMRI activity that is similar to 10 other neurosurgery patients, as well as neurologically-intact age- and music-education matched controls. Furthermore, the cortical localization of music processing to the right superior temporal gyrus that we have reported is in excellent agreement with prior studies that have identified that same brain region as being differentially engaged during music processing (e.g., [1, 2, 4]).
The clinical goal of awake mapping is to facilitate a gross-total resection of the tumor while sparing eloquent areas from damage. Evidence that this was accomplished in Patient AE is provided in two forms. First, after the tumor resection was completed, but before closing of the dura, AE flawlessly played a piece of music on his saxophone (see Movie S4). Second, after his surgery (4 weeks), AE performed at a comparable level as he had pre-operatively on the MBEA (175/180; 85th percentile; Figure 1D; see Table S1); there was a slight drop in performance on the contour subtest of the MBEA, but that was within 1 standard deviation of control performance.
Our findings are a proof-of-principle that pre-operative fMRI and intraoperative mapping using a melody repetition task can be used to guide a tailored resection that preserves broader music ability in surgical interventions adjacent to cortical regions supporting music. We suggest that the melody repetition task we employed (see [1]) meets several joint constraints. First, it is a task that involves an overt and objectively quantifiable response on the part of patient, which is always preferred in an operative environment. Second, melody repetition is a task that succinctly indexes a core aspect of broader musical ability. Peretz and colleagues (e.g., see [5–12, 43) have shown that patients with amusia are better at recognizing melodies when lyrics are present [44], and that despite poor pitch discrimination, some amusic patients can sing at levels that are comparable to control participants [26]. Those findings suggest that prior intracranial stimulation and cooling studies ([24, 25]) that employed a paradigm in which patients sing familiar songs may not index the core process that is disrupted in amusia. Future work that builds on the techniques we developed could evaluate whether focusing intracranial mapping on melody processing proves critical for preserving broader music function and avoiding a postoperative amusia.
Supplementary Material
Acknowledgments
We are grateful to all individuals who participated in these studies, in particular Patients AE and AG, Gregory Hickok for sharing music stimuli [1], Sam Norman-Haignere, Nancy Kanwisher, and Josh McDermott for sharing their sound stimuli [3], Isabelle Peretz for making the testing materials from the MBEA available [5], and Duje Tadin for sharing the stimuli for the first order motion fMRI experiment. We are also grateful to Keith Parkins and the URMC NeuroIT team for technical assistance in the operating room. This research was supported by NIH grant R01NSO89069 and NSF grant BCS-1349042 to BZM, a core grant to the Center for Visual Science (P30 EY001319), and support to the Department of Neurosurgery at the University of Rochester by Norman and Arlene Leenhouts. Preparation of this ms was supported by a University of Rochester Center for Visual Science pre-doctoral training fellowship (5T32EY007125-24) to FEG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: F.E.G., B.L.C., B.D., W.L., S.L.T., M.H.S., J.L., E.M., and B.Z.M. designed the study and wrote the manuscript; F.E.G., B.L.C., B.D., W.L., S.L.T., A.T., R.B., and B.Z.M analyzed the data; S.B.G, S.E., S.O.S, J.S., L.L, T.F., B.Z.M. and W.H.P. carried out the intraoperative mapping procedure.
The authors declare no competing financial interests.
References
- 1.Hickok G, Buchsbaum B, Humphries C, Muftuler T. Auditory-motor interaction revealed by fMRI: speech, music, and working memory in area Spt. J Cogn Neurosci. 2003;15:673–82. doi: 10.1162/089892903322307393. [DOI] [PubMed] [Google Scholar]
- 2.Rogalsky C, Rong F, Saberi K, Hickok G. Functional anatomy of language and music perception: temporal and structural factors investigated using functional magnetic resonance imaging. J Neurosci. 2011;31:3843–52. doi: 10.1523/JNEUROSCI.4515-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norman-Haignere S, Kanwisher NG, McDermott JH. Distinct Cortical Pathways for Music and Speech Revealed by Hypothesis-Free Voxel Decomposition. Neuron. 2015;88:1281–96. doi: 10.1016/j.neuron.2015.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fedorenko E, McDermott JH, Norman-Haignere S, Kanwisher N. Sensitivity to musical structure in the human brain. J Neurophysiol. 2012;108:3289–3300. doi: 10.1152/jn.00209.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peretz I, Champod AS, Hyde K. Varieties of musical disorders. Ann New York Aca Sci. 2003;999:58–75. doi: 10.1196/annals.1284.006. [DOI] [PubMed] [Google Scholar]
- 6.Peretz I, Kolinsky R, Tramo M, Labrecque R, Hublet R, Demeurisse G, Belleville S. Functional dissociations following bilateral lesions of auditory cortex. Brain. 1994;117:1283–1301. doi: 10.1093/brain/117.6.1283. [DOI] [PubMed] [Google Scholar]
- 7.Peretz I, Brattico E, Jarvenpaa M, Tervaniemi M. The amusic brain: in tune, out of key, and unaware. Brain. 2009;132:1277–86. doi: 10.1093/brain/awp055. [DOI] [PubMed] [Google Scholar]
- 8.Vuvan DT, Nunes-Silva M, Peretz I. Meta-analytic evidence for the non-modularity of pitch processing in congenital amusia. Cortex. 2015;69:186–200. doi: 10.1016/j.cortex.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Peretz I. Neurobiology of Congenital Amusia. Trends Cogn Sci. 2016;20:857–867. doi: 10.1016/j.tics.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Zatorre RJ, Belin P, Penhune VB. Structure and function of auditory cortex: music and speech. Trends Cogn Sci. 2002;6:37–46. doi: 10.1016/s1364-6613(00)01816-7. [DOI] [PubMed] [Google Scholar]
- 11.Peretz I, Vuvan D, Lagrois ME, Armory JL. Neural overlap in processing music and speech. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140090. doi: 10.1098/rstb.2014.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peretz I, Zatorre RJ. Brain organization for music processing. Annu Rev Psychol. 2005;56:89–114. doi: 10.1146/annurev.psych.56.091103.070225. [DOI] [PubMed] [Google Scholar]
- 13.Roux FE, Lubrano V, Lotterie JA, Giussani C, Pierroux C, Démonet JF. When “abegg” is read and (“A, B, E, G, G”) is not: A cortical stimulation study of musical score reading. Journal of neurosurgery. 2007;106:1017–1027. doi: 10.3171/jns.2007.106.6.1017. [DOI] [PubMed] [Google Scholar]
- 14.Roux FE, Borsa S, Démonet JF. “The Mute Who Can Sing”: a cortical stimulation study on singing: Clinical article. J Neurosurg. 2009;110:282–288. doi: 10.3171/2007.9.17565. [DOI] [PubMed] [Google Scholar]
- 15.Sanai N, Mirzadeh Z, Berger MS. Functional Outcome after Language Mapping for Glioma Resection. New England Journal of Medicine. 2008;358:18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- 16.Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio-Mazoyer N, Capelle L. New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain. 2005;128:797–810. doi: 10.1093/brain/awh423. [DOI] [PubMed] [Google Scholar]
- 17.Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. J Neurosurg. 1989;71:316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- 18.Roux FE, Boulanouar K, Lotterie JA, Majdoubi M, LeSage JP, Berry I. Language Functional Magnetic Resonance Imaging in Preoperative Assessment of Language Areas: Correlation with Direct Cortical Stimulation. Neurosurgery. 2003;52:1335–1347. doi: 10.1227/01.neu.0000064803.05077.40. [DOI] [PubMed] [Google Scholar]
- 19.Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- 20.Fried I, Katz A, McCarthy G, Sass KJ, Williamson P, Spencer SS, Spencer DD. Functional organization of human supplemental motor cortex studed by electrical stimulation. J Neurosci. 1991;11:3656–3666. doi: 10.1523/JNEUROSCI.11-11-03656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desmurget M, Reilly KT, Richard N, Szathmari A, Mottolese C, Sirigu A. Movement intention after parietal cortex stimulation in humans. Science. 2009;324:811–3. doi: 10.1126/science.1169896. [DOI] [PubMed] [Google Scholar]
- 22.Giussani C, Roux FE, Ojemann J, Sganzerla EP, Pirillo D, Papagno C. Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic resonance imaging and direct cortical stimulation correlation studies. Neurosurgery. 2010;66:113–120. doi: 10.1227/01.NEU.0000360392.15450.C9. [DOI] [PubMed] [Google Scholar]
- 23.Borchers S, Himmelbach M, Logothetis N, Karnath HO. Direct electrical stimulation of human cortex - the gold standard for mapping brain functions? Nat Rev Neurosci. 2012;13:63–70. doi: 10.1038/nrn3140. [DOI] [PubMed] [Google Scholar]
- 24.Suarez RO, Golby A, Whalen S, Sato S, Theodore WH, Kufta CV, Devinsky O, Balish M, Bromfield EB. Contributions to singing ability by theh posterior portion of the superior tmeporal gyrus of the non-language dominant hemisphere: First evidence from subdural cortical stimulation, Wada testing, and fMRI. Cortex. 2010;46:343–353. doi: 10.1016/j.cortex.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katlowitz KA, Oya H, Howard MA, III, Greenlee JDW, Long MA. Paradoxical vocal changes in a trained singer by focally cooling the right superior temporal gyrus. Cortex. 2017;89:111–119. doi: 10.1016/j.cortex.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalla Bella S, Giguere JF, Peretz I. Singing in congenital amusia. J Acoust Soc Am. 2009;126:414–24. doi: 10.1121/1.3132504. [DOI] [PubMed] [Google Scholar]
- 27.Kay J, Lesser R, Coltheart M. PALPA: Psycholinguistic assessments of language processing in aphasia. Psychology Press; 1992. [Google Scholar]
- 28.Hyde KL, Zatorre RJ, Griffiths TD, Lerch JP, Peretz I. Morphometry of the amusic brain: a two-site study. Brain. 2006;129:2562–70. doi: 10.1093/brain/awl204. [DOI] [PubMed] [Google Scholar]
- 29.Hyde KL, Lerch JP, Zatorre RJ, Griffiths TD, Evans AC, Peretz I. Cortical thickness in congenital amusia: when less is better than more. J Neurosci. 2007;27:13028–32. doi: 10.1523/JNEUROSCI.3039-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loui P, Alsop D, Schlaug G. Tone deafness: a new disconnection syndrome? J Neurosci. 2009;29:10215–20. doi: 10.1523/JNEUROSCI.1701-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leonard MK, Cai R, Babiak MC, Ren A, Chang EF. The peri-Sylvian cortical network underlying single word repetition revealed by electro cortical stimulation and direct neural recordings. Brain and Language. doi: 10.1016/j.bandl.2016.06.001. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buchsbaum BR, Hickok G, Humphries C. Role of left posterior superior temporal gyrus in phonological processing for speech perception and production. Cognitive Science. 2001;25(5):663–678. [Google Scholar]
- 33.Chang EF, Niziolek CA, Knight RT, Nagarajan SS, Houde JF. Human cortical sensorimotor network underlying feedback control of vocal pitch. Proceedings of the National Academy of Sciences. 2013;110(7):2653–2658. doi: 10.1073/pnas.1216827110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchsbaum BR, Baldo J, Okada K, Berman KF, Dronkers N, D’Esposito M, Hickok G. Conduction aphasia, sensory-motor integration, and phonological short-term memory–an aggregate analysis of lesion and fMRI data. Brain and language. 2011;119(3):119–128. doi: 10.1016/j.bandl.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- 36.Caramazza A, Hillis AE. Lexical organization of nouns and verbs in the brain. Nature. 1991;349:788–790. doi: 10.1038/349788a0. [DOI] [PubMed] [Google Scholar]
- 37.Goodale MA, Milner AD, Jakobsen LS, Carey DP. A neurological dissociation between perceiving objects and grasping them. Nature. 1991;349:154–156. doi: 10.1038/349154a0. [DOI] [PubMed] [Google Scholar]
- 38.Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science. 2006;313:1402. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- 39.de Gelder B, Tamietto M, Van Boxtel G, Goebel R, Sahraie A, Van den Stock J, Pegna A. Intact navigation skills after bilateral loss of striate cortex. Curr Biol. 2008;18:R1128–R1129. doi: 10.1016/j.cub.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Finke C, Esfahani NE, Ploner CJ. Preservation of musical memory in an amnesic professional cellist. Curr Biol. 2012;22:R591–2. doi: 10.1016/j.cub.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 41.Caramazza A. The logic of neuropsychological research and the problem of patient classification in aphasia. Brain Lang. 1984;21:9–20. doi: 10.1016/0093-934x(84)90032-4. [DOI] [PubMed] [Google Scholar]
- 42.Shallice T. From Neuropsychology to Mental Structure. 1988 [Google Scholar]
- 43.Peretz I, Hyde KL. What is specific to music processing? Insights from congenital amusia. Trends Cogn Sci. 2003;7:362–367. doi: 10.1016/s1364-6613(03)00150-5. [DOI] [PubMed] [Google Scholar]
- 44.Ayotte J, Peretz I, Hyde K. Congenital amusia: A group study of adults afflicted with a music-specific disorder. Brain. 2002;125(2):238–251. doi: 10.1093/brain/awf028. [DOI] [PubMed] [Google Scholar]
- 45.Schwarzbach J. A simple framework (ASF) for behavioral and neuroimaging experiments based on the psychophysics toolbox for MATLAB. Behavior Research Methods. 2011;43(4):1194–1201. doi: 10.3758/s13428-011-0106-8. [DOI] [PubMed] [Google Scholar]
- 46.Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:377–401. [PubMed] [Google Scholar]
- 47.Chen Q, Garcea FE, Mahon BZ. The representation of object-directed action and function knowledge in the human brain. Cerebral Cortex. 2016:bhu328. doi: 10.1093/cercor/bhu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erdogan G, Chen Q, Garcea FE, Mahon BZ, Jacobs RA. Multisensory part-based representations of objects in human lateral occipital cortex. Journal of cognitive neuroscience. 2016 doi: 10.1162/jocn_a_00937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcea FE, Mahon BZ. Parcellation of left parietal tool representations by functional connectivity. Neuropsychologia. 2014;60:131–143. doi: 10.1016/j.neuropsychologia.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garcea FE, Kristensen S, Almeida J, Mahon BZ. Resilience to the contralateral visual field bias as a window into object representations. Cortex. 2016;81:14–23. doi: 10.1016/j.cortex.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Q, Garcea FE, Jacobs R, Mahon BZ. Connectivity-based constraints on category-specificity in the ventral object processing pathway. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2016.11.014. (in press—A) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Q, Garcea FE, Almeida J, Mahon BZ. Abstract representations of object-directed action in the left inferior parietal lobule. Cerebral Cortex. doi: 10.1093/cercor/bhx120. (in press—B) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- 54.Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nature neuroscience. 2009;12(5):535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith SM. Fast robust automated brain extraction. Human brain mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graham MS, Drobnjak I, Zhang H. Realistic simulation of artefacts in diffusion MRI for validating post-processing correction techniques. NeuroImage. 2016;125:1079–1094. doi: 10.1016/j.neuroimage.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical image analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 58.Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Smith SM. Characterization and propagation of uncertainty in diffusion- weighted MR imaging. Magnetic resonance in medicine. 2003;50(5):1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 59.Behrens TE, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garcea FE, Dombovy M, Mahon BZ. Preserved tool knowledge in the context of impaired action knowledge: Implications for models of semantic memory. Frontiers in Human Neuroscience. 2013;7:1–18. doi: 10.3389/fnhum.2013.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stasenko A, Garcea FE, Dombovy M, Mahon BZ. When concepts lose their color: A case of object-color knowledge impairment. cortex. 2014;58:217–238. doi: 10.1016/j.cortex.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forster KI, Forster JC. DMDX: A Windows display program with millisecond accuracy. Behavior research methods, instruments, & computers. 2003;35(1):116–124. doi: 10.3758/bf03195503. [DOI] [PubMed] [Google Scholar]
- 63.Barbarotto R, Laiacona M, Macchi V, Capitani E. Picture reality decision, semantic categories and gender: A new set of pictures, with norms and an experimental study. Neuropsychologia. 2002;40(10):1637–1653. doi: 10.1016/s0028-3932(02)00029-5. [DOI] [PubMed] [Google Scholar]
- 64.Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. Journal of experimental psychology: Human learning and memory. 1980;6(2):174. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- 65.Garcea FE, Mahon BZ. What is in a tool concept? Dissociating manipulation knowledge from function knowledge. Memory & cognition. 2012;40(8):1303–1313. doi: 10.3758/s13421-012-0236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buxbaum LJ, Veramonti T, Schwartz MF. Function and manipulation tool knowledge in apraxia: Knowing “what for” but not “how”. Neurocase. 2000;6:83–97. [Google Scholar]
- 67.Duchaine B, Nakayama K. The Cambridge Face Memory Test: Results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia. 2006;44(4):576–585. doi: 10.1016/j.neuropsychologia.2005.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


