Abstract
Introduction
Elimination of microbial flora in cases of immature permanent teeth with necrotic pulp is both key and a challenging goal for the long-term success of regenerative therapy. Recent research has focused on the development of cell-friendly intracanal drug delivery systems. This in vitro study aimed to investigate the antimicrobial action of three-dimensional (3D) tubular-shaped triple antibiotic-eluting nanofibrous constructs against a multispecies biofilm on human dentin.
Methods
Polydioxanone (PDS) polymer solutions, antibiotic-free or incorporated with metronidazole, ciprofloxacin, and minocycline were electrospun into 3D tubular-shaped constructs. A multispecies biofilm consisting of Actinomyces naeslundii, Streptococcus sanguinis, and Enterococcus faecalis, was forced inside the dentinal tubules via centrifugation in a dentin slice in vitro model. The infected specimens were exposed to two experimental groups; i.e., 3D tubular-shaped triple antibiotic-eluting constructs and TAP, and two control groups (7-day biofilm untreated and antibiotic-free 3D tubular-shaped constructs). Biofilm elimination was quantitatively analyzed by Confocal Laser Scanning Microscopy (CLSM).
Results
CLSM analysis showed a dense population of viable (green) bacteria adhered to dentin and penetrated into the dentinal tubules. Upon 3D tubular-shaped triple antibiotic-eluting nanofibrous construct exposure, nearly complete elimination of viable bacteria on the dentin surface and inside the dentinal tubules was shown by the CLSM images, which was similar (p<0.05) to the bacterial death promoted by the TAP group but significantly greater when compared to both the antibiotic-free 3D tubular-shaped constructs and the control (saline).
Conclusion
The proposed 3D tubular-shaped antibiotic-eluting construct showed pronounced antimicrobial effects against the multispecies biofilm tested and therefore holds significant clinical potential as a disinfection strategy prior to regenerative endodontics.
Keywords: Electrospinning, nanofibers, scaffold, disinfection, regeneration, root canal, antibiotic, bacteria, stem cells, pulp
In general, endodontic infection consists of between 1 and 12 bacterial species living in symbiosis in the root canal system (1–3). This colonization encompasses mostly facultative and strict anaerobic bacteria with the ability to proliferate via interactions among bacterial cell proteins that establish a complex spatial structure known as biofilm (1–3). Several bacterial species have been identified as settling into the root canal of fully-developed (mature) permanent teeth, including E. faecalis and S. sanguinis (4, 5). Meanwhile, a recent study found A. naeslundii to be most prevalent in traumatized immature permanent teeth with necrotic pulps (6).
Over the years, numerous clinical strategies have been suggested for root canal disinfection. Mechanical and chemical approaches, including but not limited to endodontic files, irrigant solutions, and intracanal medicaments associated or not with emerging technologies (e.g., passive ultrasonic irrigation and photoactivated-induced passive irrigation) (7–12), have been used in both mature and immature permanent teeth. More specifically, among the presently available options, triple antibiotic paste (TAP, an equal parts mixture [1 g/mL] involving metronidazole [MET], ciprofloxacin [CIP], and minocycline [MINO]), has been recommended prior to evoke bleeding in regenerative-based procedures (13–16). Of note, although the intracanal application of TAP may offer some advantages, i.e., effective disinfection and a decrease in conceivable systemic complications compared with antibiotic administration (e.g., antibiotic-resistant strains, cytotoxicity, allergic reactions), TAP has been shown to promote notable tooth discoloration (13, 14, 16, 17) and significant dental stem cells’ (pulp and apical papilla) death (18, 19), when employed at considerably high (≥ 1 mg/mL) concentrations.
Due to the aforementioned concerns, recent research has focused on the development of cell-friendly intracanal drug delivery systems (20–31). Electrospinning is known to be a straightforward nanotechnology-based technique capable of fabricating not only tissue scaffolds for regenerative medicine, but also antibiotic-eluting polymer nanofibers for use in drug delivery (10, 21–25, 27–31). An accumulating body of evidence suggests that antibiotic nanofibers (single, dual, or triple antibiotics) may provide clinical benefit, as substantial antimicrobial properties have been consistently seen when evaluated against dentin biofilm models (21–23, 31). Indeed, the unique release mechanism of antibiotics from polydioxanone (PDS) nanofibers, i.e., initial burst release and sustained action for up to 14 days (22, 25, 29), has shown the ability to promote bacterial cell death inside the dentinal tubules (22, 31).
In order to mimic clinical use, a recent study (31) investigated the ability of dental pulp stem cells (DPSCs) to attach and proliferate on dentin surfaces that were previously exposed to the triple antibiotic-eluting nanofibers. No deleterious effects on stem cell attachment and viability were seen upon dentin exposure to the antibiotic-eluting nanofibers (31). By focusing on the clinical impact and translation of electrospun antibiotic-eluting polymer nanofibers for root canal disinfection prior to regenerative endodontics, this in vitro study aimed to investigate the antimicrobial action of three-dimensional (3D) tubular-shaped triple antibiotic-eluting nanofibrous constructs against a multispecies biofilm (A. naeslundii, S. sanguinis, and E. faecalis) on human dentin.
Materials and Methods
Fabrication of Triple Antibiotic-Eluting Polymer Nanofibers
Polydioxanone (PDS, Ethicon, Somerville, NJ, USA) monofilament absorbable suture processed from the polyester poly(p-dioxanone) was dissolved in hexafluoro-2-propanol (HFP, Sigma-Aldrich, St. Louis, MO, USA) at a 10 wt.% concentration (21–31). Next, MET, CIP, and MINO (Sigma-Aldrich) were added to the polymer solution at 35 wt.% relative to the total PDS weight (210 mg of each antibiotic) (22, 31). Antibiotic-free (control) PDS solution was also prepared. After overnight stirring, each solution was loaded into 5-mL plastic syringes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) fitted with a metallic 27-gauge blunt tip needle. An electrospinning system consisting of a high-voltage source (ES50P-10W/DAM, Gamma High-Voltage Research Inc., FL, USA), a syringe pump (Legato 200, KD Scientific Inc., Holliston, MA, USA), and a Teflon-coated collecting steel mandrel (1.5 ± 0.02 mm) connected to a high-speed mechanical stirrer (BDC6015, Caframo, Wiarton, ON, Canada) was used to spin, under optimized parameters, 3D tubular-shaped constructs (26, 30). All constructs were dried under vacuum for 48 h to entirely remove any remaining solvent (21–31).
Multispecies Biofilm Model and CLSM Evaluation of Bacterial Viability
This study was approved (IRB #1407656657) by the local Institutional Review Board protocol (Indiana University). Sixteen caries-free human canines were collected from de-identified patients and used to prepare the dentin slices. In brief, after thorough cleaning and crown sectioning using a low-speed water-cooled wafering diamond blade (Isomet, Buehler, Lake Bluff, IL, USA), the roots were horizontally sectioned at 3-mm apical to the cement-enamel junction to obtain 1.5 ± 0.1-mm thick dentin slices. Subsequently, the dentin slices were wet-finished with SiC papers (up to 1200-grit) to obtain a 1-mm uniform thickness. The root canals were enlarged using a round bur (2.5 mm in diameter) at low speed (300 rpm) under water-cooling. In order to remove the smear layer, all the dentin slices were incubated in an ultrasonic bath containing 2.5% sodium hypochlorite (NaOCl), followed by 17% ethylenediaminetetraacetic acid (EDTA; Inter-Med, Inc., Racine, WI, USA) solutions for 3 min each. All the dentin slices were rinsed in saline solution for 10 min and autoclaved at 121°C (22, 31). The sterile slices were then randomly placed inside microcentrifuge tubes containing 300 µL suspension (ca. 106 bacteria) of each of the following bacteria: A. naeslundii (ATCC 43146), S. sanguinis (ATCC 10556), and E. faecalis (ATCC 29212), totaling 900 µL bacteria suspension. Following a previously established protocol, an optimized sequence of centrifugal cycles (2× each) at 1400 g, 2000 g, 3600 g, and 5600 g for 5 min was performed to allow for bacterial penetration (32). The bacterial suspension was renewed between every centrifugal cycle. The infected slices were then allocated to 24-well plates containing 1 mL of fresh and sterile BHI + 1% sucrose (BHIS). The plates were incubated under aerobic conditions (37°C and 5% CO2) for 7 days for biofilm formation. The broth was replaced every other day to ensure bacterial viability. Scanning electron microscopy (SEM, JSM-5310LV, JEOL, Tokyo, Japan) was performed following a routine sample preparation protocol to qualitatively evaluate biofilm formation and overall morphology over the dentin substrate (22, 23). After 7 days, the dentin slices were gently rinsed with sterile phosphate-buffered saline (PBS, Sigma-Aldrich) to remove loosely bound bacteria.
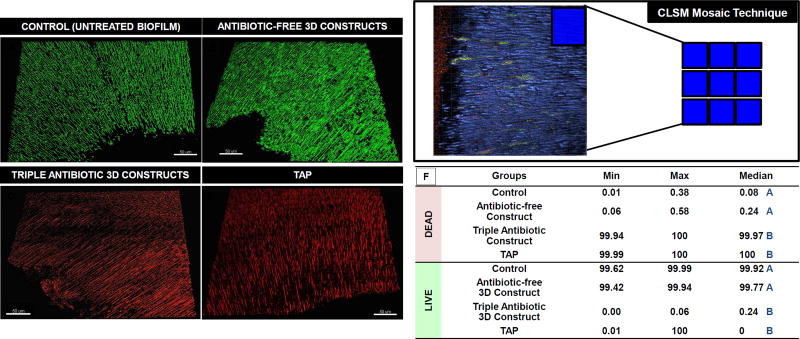
The infected dentin slices (n=4/group) were randomly allocated into 2 experimental groups: 3D tubular-shaped triple antibiotic-eluting nanofibrous constructs and TAP, and two control groups (7-day biofilm untreated and antibiotic-free 3D tubular-shaped antibiotic-free PDS nanofibrous constructs). Tubular-shaped constructs (1±0.1 mm in height and 2.5 mm in diameter, average weight 2.5 mg, i.e., ~ 900 µg of antibiotics in total) were UV-sterilized (30 min/side) and fitted inside the infected root canal space. TAP was spatulated into a creamy consistency by mixing 50 mg each of MET, CIP, and MINO with 1 mL of sterile distilled water and applied into the root canal space of the infected dentin slices. The medicaments remained for 7 days. To maintain a humid environment and prevent both the 3D constructs and TAP from drying out, a damp cotton ball saturated with 50 µL of distilled water was placed on top of each specimen. To assess the antimicrobial activity, all samples of each group (n=4/group) were prepared for confocal laser scanning microscopy (CLSM) analysis. In brief, the dentin slices were stained with the fluorescent LIVE/DEAD BacLight viability kit L-7012 (Molecular Probes Inc., Eugene, OR, USA) containing SYTO 9 and propidium iodide (PI) (32–34). Two random areas in each dentin slice were analyzed using a mosaic technique of 3D reconstruction, wherein 9 subareas of 300 × 300 µm were merged, totaling 18 areas per sample. The areas were selected starting from the root canal space toward the cementum side for imaging on CLSM (Leica SP2 CL5Mt, Leica Microsystems Inc., Heidelberg, Germany) using a 40× lens. The sequence of segments through the depth of tissue (Z-stacks) was collected by using optimal step size settings (0.35 µm); the images were composed of 512 × 512 pixels. They were evaluated and quantified using dedicated software (Imaris 7.2 software, Bitplane Inc., St. Paul, MN, USA). The excitation emission maxima for the dyes were approximately 480/500 nm for SYTO 9 and 490/635 nm for PI, respectively.
Statistical Analysis
The percentages of live/dead bacteria were compared for differences of dead cells using a mixed-model ANOVA, with a fixed effect for group and a random effect for sample, to account for measurements at multiple areas on each specimen. All tests were performed at the 5% level.
Results
Association of the 3 bacterial species (E. faecalis, A. naeslundii, and S. sanguinis) demonstrated successful multispecies biofilm formation (Figure 1). CLSM analysis showed a dense population of viable (green) bacteria adhered to dentin and penetrated into the dentinal tubules (Figure 2A). Upon contact with the 3D tubular-shaped triple antibiotic-eluting nanofibrous construct, nearly complete elimination of viable bacteria (Figure 2F) on the dentin surface and inside the dentinal tubules (Figure 2C) was shown by the CLSM images, which was similar (p<0.05) to the bacterial death promoted by the TAP group (Figure 2D) but significantly greater when compared to both the antibiotic-free 3D tubular-shaped constructs (Figure 2B) and the control/saline (Figure 2A).
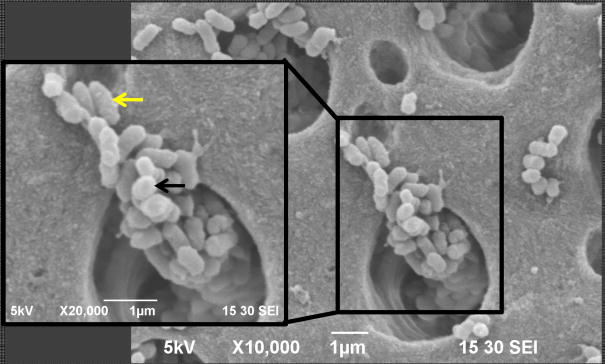
Figure 1.
Representative SEM micrograph suggesting the presence of the 3 distinct bacterial morphologies corresponding to E. faecalis (white arrow), S. sanguinis (black arrow), and A. naeslundii (yellow arrow), all of which make up the multispecies biofilm.
Figure 2.
(A) CLSM macrophotographs of 7-day multispecies biofilm (positive control) growth inside dentinal tubules. (B) Infected dentin treated with 3D tubular-shaped antibiotic-free nanofibrous constructs. Infected dentin treated for 7 days (C) 3D tubular-shaped triple antibiotic-eluting nanofibrous constructs (D) TAP. (E) CLSM images collected from inner root canal walls with a mosaic technique, allowing for deeper analysis in sequential illumination mode. (F) Table illustrating the median percentage of DEAD and LIVE bacterial cells for each group, demonstrating that 3D tubular-shaped triple antibiotic-eluting nanofibrous constructs eliminated almost all live cells, not differing from TAP paste (p>0.05). Median values, followed by distinct uppercase letters (comparing bacterial viability within each group), denote statistical difference.
Discussion
Bacterial flora throughout the root canal system is wide-ranging and its interactions might improve or disturb biofilm development. The most common bacteria found in root canals are either facultative or strict anaerobes (35). Facultative bacteria (mainly Streptococci spp.) are known for their ability to adhere to surfaces, including human tissue components, epithelial cells, and other bacterial cells (36). Their adhesion properties largely facilitate the linkage of Streptococci spp. to the first layer of biofilm formation on tooth surfaces, commonly known as the salivary glycoprotein pellicle, and to other bacterial species, including Actinomyces ssp. (36). The association between bacteria-bacteria and bacteria-substrate in the biofilm is highly dependent on the extracellular polymeric substances (EPS) matrix, which may form firm or loose connections with the cell surface (37). Besides adherence, aggregation of the different bacterial species has shown to play an important role in bacteria survival (38). Streptococcus spp. and Actinomyces spp. both hold an aggregation capacity that may be responsible for intercellular communication inside biofilm and the pathogenesis of dentinal colonization (38). In this way, the clinical relevance of these species on biofilm development has led the present investigation to select 3 bacterial species (E. faecalis, A. naeslundii, and S. sanguinis), all which are known to easily colonize human dentin and dentinal tubules (39, 40), and induce a multispecies biofilm.
Numerous studies have reported on the development of single-species (mainly E. faecalis) biofilm on dentin both on its external surface and inside dentinal tubules (23, 32, 41). However, very few studies have focused on the development of multispecies biofilm on human dentin (42–44). The multispecies model proposed herein has allowed for successful growth of the 3 bacterial species into biofilm communities inside dentinal tubules (Figure 2), exhibiting different morphologies for E. faecalis (cocci in clusters, short chains, diplococcic, and single cocci), S. sanguinis (cocci-shaped in chains), and A. naeslundii (rod-shaped) species. These species were previously responsible for causing primary and resistant intrarradicular infections (6, 45–48).
Antibiotics associated with TAP formulation have been widely used due to their ability to act against strict anaerobic bacteria (MET); Gram-positive and negative species (MINO); and specifically, Gram-negative bacteria (CIP). In recent years, a novel root canal disinfection strategy (i.e., antibiotic-eluting polymer nanofibers) has emerged in an attempt to overcome some drawbacks associated with TAP (e.g., high antibiotic concentration, damage to stem cells, and tooth discoloration) (20–31). The aforementioned drug delivery system has been exhaustively tested against E. faecalis, A. naeslundii and P. gingivalis biofilms, thus demonstrating significant antimicrobial action (21–23, 31), when compared to the standard, TAP.
In the present study, 3D tubular-shaped triple antibiotic-eluting nanofibrous constructs containing approximately 900 µg of the three antibiotics and TAP (50 mg of each antibiotic per mL of saline solution) demonstrated similar antimicrobial properties against a multispecies biofilm. Worth stressing, the dramatically low concentration of MET, CIP, and MINO within the processed nanofibers (~ 1 mg/construct), when compared to the TAP formulation used in this work, confirms the suitability of our recently developed 3D tubular-shaped drug delivery constructs as an antimicrobially effective intracanal drug delivery system. Notably, it has been previously highlighted that to achieve a paste-like consistency, which allows proper intracanal placement, antibiotics need to be manipulated at a highly cytotoxic concentration (1 g/mL) (18). In this way, the incorporation of antibiotics into electrospun nanofibers allows for the fabrication of a more cell-friendly, localized intracanal medication (10, 25). Several studies from our group have recently reported on the kinetics of antibiotics’ release. Overall, our antibiotic-containing polydioxanone (PDS) nanofibers have invariably shown an initial burst release, followed by a sustained maintenance of the antimicrobial properties for up to 14 days (22, 25, 28), which can provide long-term effects against bacteria that might have survived the initial burst of antibiotics’ release. Lastly, another key aspect of the proposed triple antibiotic-eluting nanofibers over TAP relates to its predictable degradability, which, in turn, would pose a minimal risk of dentinal tubules’ blockage and, consequently, allow for the release of dentin growth factors upon EDTA irrigation (28, 31). From a clinical standpoint, the 3D construct was able to closely adapt to the root canal walls of the dentin slices. Despite the innovation behind the proposed biofilm model, one may point out the limitations related to the absence of strict anaerobic bacteria, which is well known to be a component of intracanal microbial flora and plays an important role in the colonization and clinical signs and symptoms of periapical disease (49, 50). However, its reproducibility in vitro in association with other bacteria was not possible until recently. Collectively, these results are consistent with our previous findings, showing unequivocally that antibiotic-eluting nanofibers are able to eradicate single- (21–23), dual- (31), or multispecies-infected dentin biofilm as demonstrated herein. The present investigation further emphasizes the clinical promise of 3D tubular-shaped antibiotic-eluting nanofibers for root canal disinfection of immature teeth with pulpal necrosis and opens new treatment modalities (Figure 3) as an innovative antimicrobial and biocompatible intracanal drug delivery strategy. Furthermore, the ability to process tubular structures of various diameters allows for an intimal adaptation to the root dentin walls. Taken together, its potential is wide, not only when associated with the clinically available evoked bleeding method, but also in combination with injectable scaffolds loaded or not with stem cells and/or growth factors to encourage predictable pulp-dentin complex regeneration (Figure 3). In vivo studies are therefore warranted initially in pre-clinical animal models to select the best strategy for future human application.
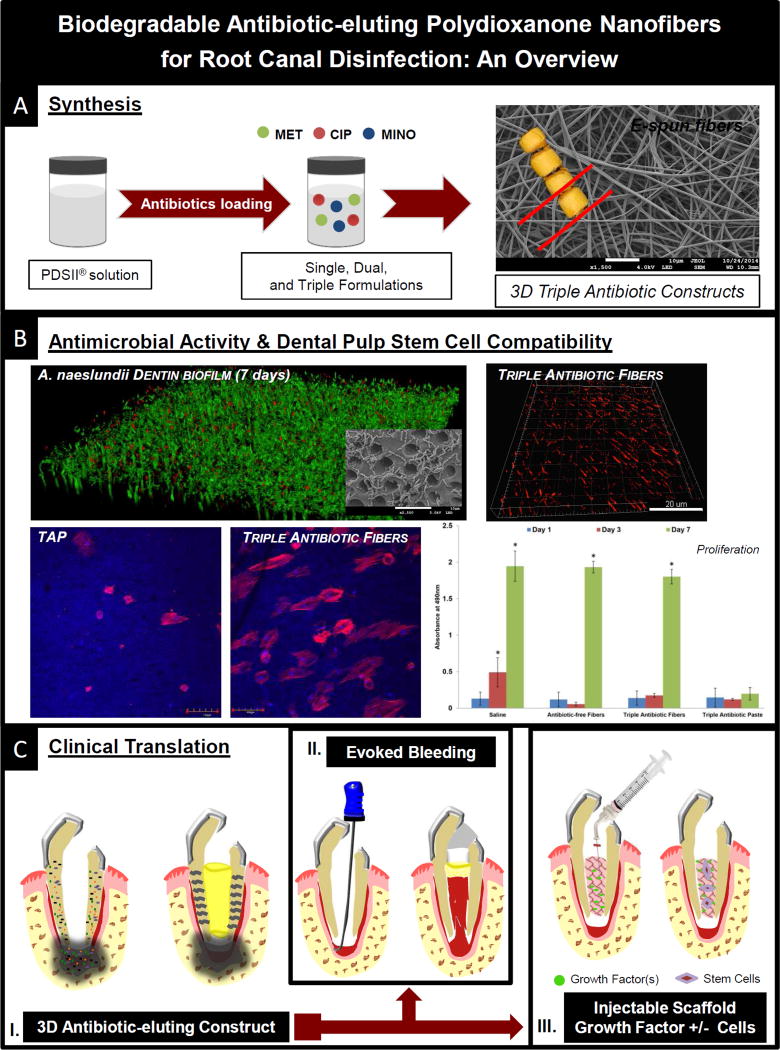
Figure 3.
Summary of future perspectives for the clinical use of the proposed 3D tubular-shaped triple antibiotic-eluting nanofibrous constructs in regenerative endodontics. (A) Synthesis of 3D tubular-shaped constructs incorporated with metronidazole (MET), ciprofloxacin (CIP), and minocycline (MINO); (B) Triple antibiotic-eluting nanofibers’ antimicrobial activity against bacterial biofilms and associated dental pulp stem cells’ (DPSCs) compatibility (31); (C) Schematic representation of an immature tooth associated with periapical lesion and placement of the proposed 3D tubular-shaped triple antibiotic-eluting construct to act as a localized drug delivery system (I); Evoked Bleeding — Blood clot evoked from the periapical tissue to the root canal space using a pre-curved K file (II); and Injectable Scaffold Strategy — with growth factors and/or stem cells (III).
Highlights.
Antibiotic nanofibers led to significant biofilm death
Antibiotic nanofibers are a more cell-friendly disinfection strategy than TAP
DPSC proliferation was higher (Day 7) on fibers-treated dentin compared to TAP.
Acknowledgments
M.C.B. acknowledges start-up funds from Indiana University School of Dentistry, the IUPUI Office of Research (RSFG grant), and the National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research (NIDCR) (Grant #DE023552). We acknowledge the expert assistance of Dr. Maria Malgorzata Kamocka with the confocal/2-photon imaging (Indiana Center for Biological Microscopy, IU School of Medicine, Indianapolis, IN, USA) and Dr. Richard L. Gregory (IU School of Dentistry, Indianapolis, IN, USA) for access to his microbiology facilities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors deny any conflicts of interest related to this study.
References
- 1.Sundqvist G, Figdor D. Life as an endodontic pathogen: Ecological differences between the untreated and root-filled root canals. Endod Topics. 2003;6:3–28. [Google Scholar]
- 2.Rickard AH, Gilbert P, High NJ, Kolenbrander PE, Handley PS. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 2003;11:94–100. doi: 10.1016/s0966-842x(02)00034-3. [DOI] [PubMed] [Google Scholar]
- 3.Mohammadi Z, Palazzi F, Giardino L, Shalavi S. Microbial biofilms in endodontic infections: an update review. Biomed J. 2013;36:59–70. doi: 10.4103/2319-4170.110400. [DOI] [PubMed] [Google Scholar]
- 4.Jacinto RC, Gomes BP, Desai M, Rajendram D, Shah HN. Bacterial examination of endodontic infections by clonal analysis in concert with denaturing high-performance liquid chromatography. Oral Microbiol Immunol. 2007;22:403–10. doi: 10.1111/j.1399-302X.2007.00378.x. [DOI] [PubMed] [Google Scholar]
- 5.Murad CF, Sassone LM, Faveri M, Hirata R, Jr, Figueiredo L, Feres M. Microbial diversity in persistent root canal infections investigated by checkerboard DNA-DNA hybridization. J Endod. 2014;40:899–906. doi: 10.1016/j.joen.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Nagata JY, Soares AJ, Souza-Filho FJ, Zaia AA, Ferraz CC, Almeida JF, Gomes BP. Microbial evaluation of traumatized teeth treated with triple antibiotic paste or calcium hydroxide with 2% chlorhexidine gel in pulp revascularization. J Endod. 2014;40:778–83. doi: 10.1016/j.joen.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 7.Soares JA, Roque de Carvalho MA, Cunha Santos SM, Mendonça RM, Ribeiro-Sobrinho AP, Brito-Júnior M, Magalhães PP, Santos MH, de Macêdo Farias L. J Endod. 2010;36:894–8. doi: 10.1016/j.joen.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Louwakul P, Saelo A, Khemaleelakul S. Efficacy of calcium oxide and calcium hydroxide nanoparticles on the elimination of Enterococcus faecalis in human root dentin. Clin Oral Investig. 2016 doi: 10.1007/s00784-016-1836-x. [in press] [DOI] [PubMed] [Google Scholar]
- 9.Shrestha A, Kishen A. Antibacterial Nanoparticles in Endodontics: A Review. J Endod. 2016;42:1417–26. doi: 10.1016/j.joen.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Albuquerque MT, Nagata JY, Diogenes AR, Azabi AA, Gregory RL, Bottino MC. Clinical perspective of electrospun nanofibers as a drug delivery strategy for regenerative endodontics. Curr Oral Health Rep. 2016;3:209–220. [Google Scholar]
- 11.Alves FR, Andrade-Junior CV, Marceliano-Alves MF, et al. Adjunctive steps for disinfection of the mandibular molar root canal system: A correlative bacteriologic, micro-computed tomography, and cryopulverization approach. J Endod. 2016 Sep 15; doi: 10.1016/j.joen.2016.08.003. pii: S0099-2399(16)30487-3. [DOI] [PubMed] [Google Scholar]
- 12.Topçuoğlu HS, Aktı A, Topçuoğlu G, Düzgün S, Ulusan Ö, Akpek F. Effectiveness of conventional syringe irrigation, vibringe, and passive ultrasonic irrigation performed with different irrigation regimes in removing triple antibiotic paste from simulated root canal irregularities. J Conserv Dent. 2016;19:323–7. doi: 10.4103/0972-0707.186452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bose R, Nummikoski P, Hargreaves K. A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod. 2009;35:1343–9. doi: 10.1016/j.joen.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Kim Y, Shin SJ, Park JW, Jung IY. Tooth discoloration of immature permanent incisor associated with triple antibiotic therapy: a case report. J Endod. 2010;36:1086–1091. doi: 10.1016/j.joen.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 15.Hargreaves KM, Diogenes A, Teixeira FB. Treatment Options: Biological Basis of Regenerative Endodontic Procedures. J Endod. 2013;39(3, Supplement):S30–S43. doi: 10.1016/j.joen.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diogenes AR, Ruparel NB, Shiloah Y, Hargreaves KM. Regenerative endodontics: A way forward. J Am Dent Assoc. 2016;147:372–80. doi: 10.1016/j.adaj.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds K, Johnson JD, Cohenca N. Pulp revascularization of necrotic bilateral bicuspids using a modified novel technique to eliminate potential coronal discolouration: a case report. Int Endod J. 2009;42:84–92. doi: 10.1111/j.1365-2591.2008.01467.x. [DOI] [PubMed] [Google Scholar]
- 18.Ruparel NB, Teixeira FB, Ferraz CC, Diogenes A. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J Endod. 2012;38:1372–1375. doi: 10.1016/j.joen.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Chuensombat S, Khemaleelakul S, Chattipakorn S, Srisuwan T. Cytotoxic effects and antibacterial efficacy of a 3-antibiotic combination: an in vitro study. J Endod. 2013;39:813– 819. doi: 10.1016/j.joen.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 20.Albuquerque MT, Valera MC, Nakashima M, Nor JE, Bottino MC. Tissue-engineering-based strategies for regenerative endodontics. J Dent Res. 2014;93:1222–1231. doi: 10.1177/0022034514549809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albuquerque MT, Evans JD, Gregory RL, Valera MC, Bottino MC. Antibacterial TAP-mimic electrospun polymer scaffold: effects on P. gingivalis-infected dentin biofilm. Clin Oral Investig. 2015 doi: 10.1007/s00784-015-1577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albuquerque MT, Ryan SJ, Munchow EA, et al. Antimicrobial Effects of Novel Triple Antibiotic Paste-Mimic Scaffolds on Actinomyces naeslundii Biofilm. J Endod. 2015;41:1337–1343. doi: 10.1016/j.joen.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albuquerque MT, Valera MC, Moreira CS, Bresciani E, de Melo RM, Bottino MC. Effects of ciprofloxacin-containing scaffolds on enterococcus faecalis biofilms. J Endod. 2015;41:710–714. doi: 10.1016/j.joen.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 24.Bottino MC, Arthur RA, Waeiss RA, Kamocki K, Gregson KS, Gregory RL. Biodegradable nanofibrous drug delivery systems: effects of metronidazole and ciprofloxacin on periodontopathogens and commensal oral bacteria. Clin Oral Investig. 2014;18:2151–2158. doi: 10.1007/s00784-014-1201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bottino MC, Kamocki K, Yassen GH, et al. Bioactive nanofibrous scaffolds for regenerative endodontics. J Dent Res. 2013;92:963–969. doi: 10.1177/0022034513505770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bottino MC, Yassen GH, Platt JA, et al. A novel three-dimensional scaffold for regenerative endodontics: materials and biological characterizations. J Tissue Eng Regen Med. 2015;9:E116–123. doi: 10.1002/term.1712. [DOI] [PubMed] [Google Scholar]
- 27.Kamocki K, Nor JE, Bottino MC. Effects of ciprofloxacin-containing antimicrobial scaffolds on dental pulp stem cell viability-In vitro studies. Arch Oral Biol. 2015;60:1131–1137. doi: 10.1016/j.archoralbio.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamocki K, Nor JE, Bottino MC. Dental pulp stem cell responses to novel antibiotic-containing scaffolds for regenerative endodontics. Int Endod J. 2015;48:1147–1156. doi: 10.1111/iej.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palasuk J, Kamocki K, Hippenmeyer L, et al. Bimix antimicrobial scaffolds for regenerative endodontics. J Endod. 2014;40:1879–1884. doi: 10.1016/j.joen.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porter ML, Munchow EA, Albuquerque MT, Spolnik KJ, Hara AT, Bottino MC. Effects of novel 3-dimensional antibiotic-containing electrospun scaffolds on dentin discoloration. J Endod. 2016;42:106–112. doi: 10.1016/j.joen.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pankajakshan D, Albuquerque MT, Evans JD, Kamocka MM, Gregory RL, Bottino MC. Triple Antibiotic Polymer Nanofibers for Intracanal Drug Delivery: Effects on Dual Species Biofilm and Cell Function. J Endod. 2016;42:1490–5. doi: 10.1016/j.joen.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma J, Wang Z, Shen Y, Haapasalo M. A new noninvasive model to study the effectiveness of dentin disinfection by using confocal laser scanning microscopy. J Endod. 2011;37:1380–5. doi: 10.1016/j.joen.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 33.Du T, Wang Z, Shen Y, Ma J, Cao Y, Haapasalo M. Effect of long-term exposure to endodontic disinfecting solutions on young and old Enterococcus faecalis biofilms in dentin canals. J Endod. 2014;40:509–14. doi: 10.1016/j.joen.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 34.Wu D, Fan W, Kishen A, Gutmann JL, Fan B. Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. J Endod. 2014;40:285–90. doi: 10.1016/j.joen.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 35.Gomes BP, Pinheiro ET, Gadê-Neto CR, Sousa EL, Ferraz CC, Zaia AA, Teixeira FB, Souza-Filho FJ. Microbiological examination of infected dental root canals. Oral Microbiol Immunol. 2004;19:71–6. doi: 10.1046/j.0902-0055.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- 36.Jenkinson HF. Adherence and accumulation of oral streptococci. Trends Microbiol. 1994:209–12. doi: 10.1016/0966-842x(94)90114-k. [DOI] [PubMed] [Google Scholar]
- 37.Khemaleelakul S, Baumgartner JC, Pruksakom S. Autoaggregation and coaggregation of bacteria associated with acute endodontic infections. J Endod. 2006;32:312–8. doi: 10.1016/j.joen.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Whitfield C, Keenleyside WJ. Regulation of expression of group IA capsular polysaccharides in Escherichia coli and related extracellular polysaccharides in other bacteria. J Ind Microbiol. 1995;15:361–71. doi: 10.1007/BF01569992. [DOI] [PubMed] [Google Scholar]
- 39.Peters LB, Wesselink PR, Buijs JF, van Winkelhoff AJ. Viable bacteria in root dentinal tubules of teeth with apical periodontitis. J Endod. 2001;27:76–81. doi: 10.1097/00004770-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Matsuo T, Shirakami T, Ozaki K, Nakanishi T, Yumoto H, Ebisu S. An immunohistological study of the localization of bacteria invading root pulpal walls of teeth with periapical lesions. J Endod. 2003;29:194–200. doi: 10.1097/00004770-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Meire MA, Coenye T, Nelis HJ, De Moor RJ. Evaluation of Nd:YAG and Er:YAG irradiation, antibacterial photodynamic therapy and sodium hypochlorite treatment on Enterococcus faecalis biofilms. Int Endod J. 2012;45:482–91. doi: 10.1111/j.1365-2591.2011.02000.x. [DOI] [PubMed] [Google Scholar]
- 42.Xie Q, Johnson BR, Wenckus CS, Fayad MI, Wu CD. Efficacy of berberine, an antimicrobial plant alkaloid, as an endodontic irrigant against a mixed-culture biofilm in an in vitro tooth model. J Endod. 2012;38:1114–7. doi: 10.1016/j.joen.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 43.Niazi SA, Clark D, Do T, Gilbert SC, Foschi F, Mannocci F, Beighton D. The effectiveness of enzymic irrigation in removing a nutrient-stressed endodontic multispecies biofilm. Int Endod J. 2014;47:756–68. doi: 10.1111/iej.12214. [DOI] [PubMed] [Google Scholar]
- 44.Mistry KS, Sanghvi Z, Parmar G, Shah S, Pushpalatha K. Antibacterial efficacy of Azadirachta indica, Mimusops elengi and 2% CHX on multispecies dentinal biofilm. J Conserv Dent. 2015;18:461–6. doi: 10.4103/0972-0707.168810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang G, Samaranayake LP, Yip HK. Molecular evaluation of residual endodontic microorganisms after instrumentation, irrigation and medication with either calcium hydroxide or Septomixine. Oral Dis. 2004;10:389–97. doi: 10.1111/j.1601-0825.2004.01015.x. [DOI] [PubMed] [Google Scholar]
- 46.Li X, Zhu XF, Zhang CF, Cathro P, Seneviratne CJ, Shen S. Endodontic bacteria from primary and persistent endodontic lesions in Chinese patients as identified by cloning and 16S ribosomal DNA gene sequencing. Chin Med J (Engl) 2013;126:634–9. [PubMed] [Google Scholar]
- 47.Pinheiro ET, Candeiro GT, Teixeira SR, Shin RC, Prado LC, Gavini G, Mayer MP. RNA-based assay demonstrated Enterococcus faecalis metabolic activity after chemomechanical procedures. J Endod. 2015;41:1441–4. doi: 10.1016/j.joen.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 48.Lew HP, Quah SY, Lui JN, Bergenholtz G, Hoon Yu VS, Tan KS. Isolation of alkaline-tolerant bacteria from primary infected root canals. J Endod. 2015;41:451–6. doi: 10.1016/j.joen.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Hashioka K, Suzuki K, Yoshida T, Nakane A, Horiba N, Nakamura H. Relationship between clinical symptoms and enzyme-producing bacteria isolated from infected root canals. J Endod. 1994;20:75–7. doi: 10.1016/S0099-2399(06)81185-4. [DOI] [PubMed] [Google Scholar]
- 50.Tennert C, Fuhrmann M, Wittmer A, Karygianni L, Altenburger MJ, Pelz K, Hellwig E, Al-Ahmad A. New bacterial composition in primary and persistent/secondary endodontic infections with respect to clinical and radiographic findings. J Endod. 2014;40:670–7. doi: 10.1016/j.joen.2013.10.005. [DOI] [PubMed] [Google Scholar]