Abstract
Clinical and laboratory studies performed over the past few decades have discovered that dry eye is a chronic inflammatory disease that can be initiated by numerous extrinsic or intrinsic factors that promote an unstable and hyperosmolar tear film. These changes in tear composition, in some cases combined with systemic factors, lead to an inflammatory cycle that causes ocular surface epithelial disease and neural stimulation. Acute desiccation activates stress signaling pathways in the ocular surface epithelium and resident immune cells. This triggers production of innate inflammatory mediators that stimulate the production of matrix metalloprotease, inflammatory cell recruitment, and dendritic cell maturation. These mediators combined with exposure of autoantigens can lead to an adaptive T-cell mediated response. Cornea barrier disruption develops by protease-mediated lysis of epithelial tight junctions leading to accelerated cell death, desquamation, an irregular poorly lubricated cornea surface and exposure and sensitization of epithelial nociceptors. Conjunctival goblet cell dysfunction and death are promoted by the T helper 1 cytokine interferon gamma (IFN-γ). These epithelial changes further destabilize the tear film, amplify inflammation and create a vicious cycle. Cyclosporine and lifitegrast, the two FDA-approved therapies inhibit T cell activation and cytokine production. While these therapies represent a major advance in dry eye therapy, they are not effective in improving discomfort and corneal epithelial disease in all patients. Preclinical studies have identified other potential therapeutic targets, biomarkers and strategies to bolster endogenous immunoregulatory pathways. These discoveries will hopefully lead to further advances in diagnostic classification and treatment.
Dry Eye – A multifactorial and self-perpetuating inflammatory disease
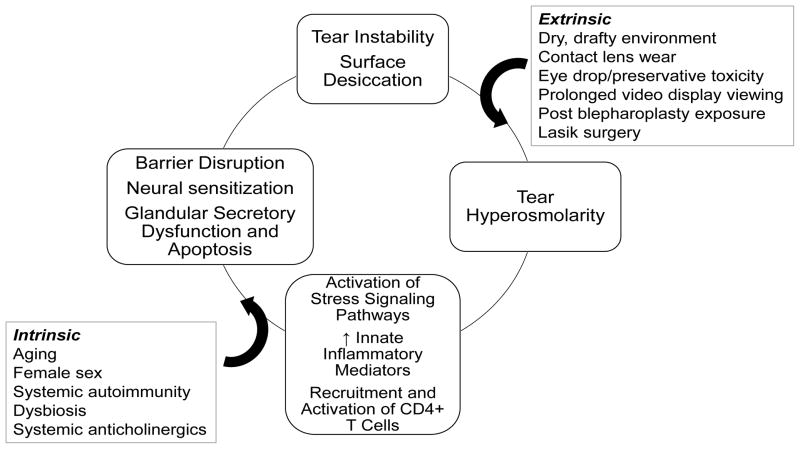
Knowledge regarding the pathophysiology of dry eye has advanced tremendously over the past two decades and continues to evolve. While tear disorders were traditionally classified by deficient component (e.g. aqueous or lipid), or as aqueous deficient or evaporative, the reality is most patients experiencing symptoms or signs of tear dysfunction have multiple risk factors and disease or dysfunction of more than one tear producing cells/glands that result in an unstable tear film.1 Tear instability is accompanied by increased tear osmolarity (either in area of tear break-up or diffusely) which activates stress signaling pathways in the ocular surface epithelium and resident immune cells and triggers production of innate inflammatory molecules that initiate a vicious self-perpetuating cycle (Figure 1) that may lead to further decline in tear function and worse symptoms.2, 3 The numerous extrinsic (e.g. desiccating environment, exposure) and intrinsic (e.g. aging, autoimmunity, drying medications) factors that can contribute to this inflammatory cycle demonstrate why it is often difficult to ascribe a single cause for most cases of dry eye disease and the importance of addressing all modifiable risk factors.
Figure 1.
Dry eye inflammatory cycle that can be initiated or amplified by extrinsic and intrinsic factors that cause tear instability and tear composition changes including hyperosmolarity that activate stress signaling pathways in the ocular surface cells which triggers production of innate inflammatory mediators which can lead to recruitment and activation of CD4+ T cells which produce cytokines that cause corneal, conjunctival and lacrimal gland epithelial disease.
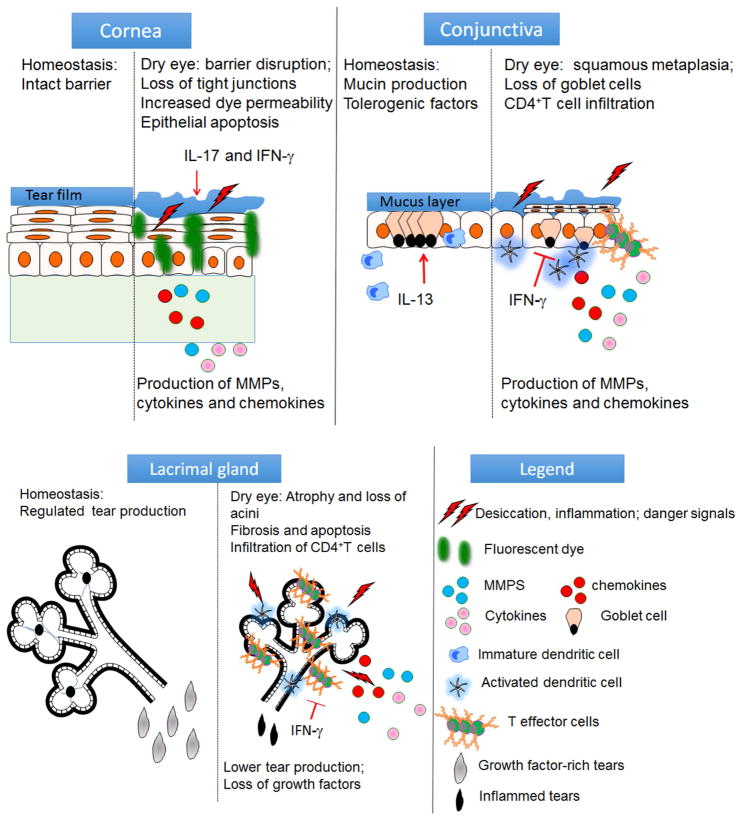
The ocular surface is a very unique exposed mucosa. It is covered with a specialized stratified epithelium that serves as a barrier to environmental, microbial and inflammatory insults. Next to the intestine, the conjunctival epithelium has the second highest density of mucus-producing goblet cells. It also harbors a variety of resident immune cells, such as natural killer, dendritic cells, macrophages, γδ and CD4 and CD8+ T cells that function primarily in antimicrobial defense but may participate in the dry eye pathogenesis.4–6 The cornea epithelium must withstand daily environmental challenges while maintaining clarity and comfort. The lacrimal glands and ocular surface epithelia produce an array of antimicrobial factors including, α and β defensins, IgA, lactoferrin, and lysozyme that are present in the tear film and function to maintain a paucibacterial microenvironment.7–20 Many of the mechanisms to maintain ocular surface and glandular homeostasis are disrupted in dry eye (Figure 2). Studies performed in animal models and dry eye patients have found that desiccation is a potent stress (in the same magnitude to microbial products) to the ocular surface that initiates a secondary immune response that can lead to a vicious cycle (Figure 1).21–27 Hyperosmolar stress has a direct pro-inflammatory effect on the ocular surface epithelium. It has been shown to activate mitogen-activated protein kinases (MAPKs), stimulate secretion of pro-inflammatory cytokines (e.g. IL-1β, TNF-α, and IL-6), chemokines and matrix metalloproteinases such as MMP-3 and MMP-9 and induce apoptosis.22, 23, 26, 28–38 The interaction of these inflammatory mediators is complex and they have been shown to upregulate each other; thus amplifying the inflammatory cascade. For example, IL-1β stimulates the production of TNF-α and MMP-3, among other factors. 31, 32, 39, 40 In turn, TNF-α stimulates MMP-9 and MMP-3 which is a physiological activator of MMP-9.41, 42 MMP-9 contributes to corneal barrier disruption by lysing tight junctions in the superficial epithelium.23, 26, 43 MMP-9 knockout mice are resistant to corneal barrier disruption when exposed to desiccating stress, and MMP inhibitors, such as corticosteroids and doxycycline have shown potential in preventing desiccation induced corneal epithelial barrier disruption in animal models.26, 43–45 A point-of-care MMP-9 detection system (InflammaDry®, RPS, Sarasota, FL) is approved for detecting elevated levels of MMP-9 in tears of dry eye patients.46–50 Increased tear MMP-9 has also been detected in other ocular surface diseases, such as atopic and vernal keratoconjunctivitis, corneal ulceration, recurrent corneal erosions and ocular burns that also have corneal barrier disruption.51–63 Detection of elevated tear MMP-9 provides a rationale for use of anti-inflammatory/protease therapies in these conditions.
Figure 2.
Function of the cornea, conjunctival and lacrimal gland in maintaining ocular surface homeostasis (left side of each tissue) and disease relevant mediators and pathological changes in each tissue (right side of each tissue). IL-17 = interleukin 17, IL-13 = interleukin 13, IFN-γ = interferon-gamma
Ocular surface epithelial cells also secrete chemokines that attract inflammatory cells. Increased levels of chemokines CCL20 (MIP3α), CXCL9 (MIG), CXCL10 (IP-10) and CXCL11 (I-TAC) and their receptors was noted in ocular surface cells and/or tears of dry eye patients and mice with the experimentally induced dry eye.64–68 Genetic deletion or pharmacological blockage of certain chemokines or chemokine receptors (CCL20, CCR6 or CXCR3) prevented the development of desiccation-induced ocular surface disease and decreased pathogenicity of autoreactive T cells in mouse models of dry eye.69, 70
Another effect of desiccation is upregulation of innate inflammatory pathways in the epithelium, including the nucleotide-binding domain, leucine-rich–containing family, pyrin domain–containing-3 (NLRP3), toll-like receptor and oxidative stress pathways.29, 30, 71–80 Antioxidants have shown therapeutic potential for treating dry eye in preclinical culture or mouse studies and in a pilot clinical trial. 30, 34, 81–85
Metaplasia and goblet cell loss in the conjunctival epithelium is a well-recognized feature of aqueous tear deficiency.86–92 The most severe ocular surface diseases, such as Stevens-Johnson syndrome, mucous membrane pemphigoid (MMP), graft vs. host disease and severe alkali burns involving the conjunctiva often have complete loss of conjunctival goblet cells.93–96 T helper cytokines have been found to modulate conjunctival goblet cell differentiation. The Th2 cytokine IL-13 stimulates proliferation and mucus production, while the Th1 cytokine IFN-γ induced goblet cell entrapment, expression of cornified envelope precursors, decreased mucus production, unresponsiveness to cholinergic stimulation, ER stress and unfolded protein response and apoptosis.27, 97–104 In addition to producing tear-stabilizing mucins, goblet cells also produce 105, 106 immunoregulatory factors, such as TGF-β and retinoic acid.104, 107, 108 Crosstalk between goblet cells and dendritic cells is critical to maintaining immune tolerance in mucosal tissues.109 Goblet cell associated-passages that deliver surface antigens to the underlying dendritic cells and promote tolerance have been identified in both intestine and conjunctiva.109, 110 Mice with deletion of the SAM pointed domain containing ETS transcription factor gene (Spdef knockout) are devoid of goblet cells, develop conjunctival inflammation and lose 111 immune tolerance to topically applied antigens, as has been found in other mouse dry eye models that are accompanied by goblet cell loss 109, 112–114 These studies indicate a critical role of goblet cell products in conditioning tolerogenic properties in conjunctival dendritic cells and maintaining ocular surface immune tolerance. 107, 108
Evidence indicates that the initial innate immune response to dryness is followed by an adaptive CD4+ T cell autoimmune response in mice exposed to desiccating stress and patients with Sjögren syndrome (SS) and non-SS associated aqueous tear deficiency.115–117 While the target autoantigen(s) in this autoimmune reaction have not been identified, members of the kallikrein family have been implicated as putative antigens in some studies.118, 119 Disrupted immune tolerance in dry eye112–114 elicits leads to dendritic cell maturation120 and generation of autoreactive T effector cells70, 101, 121–124 in mouse dry eye models. Human dry eye patients have an increased number of conjunctival dendritic cells125, 126 and a higher percentage of cells expressing the dendritic cell maturation marker HLA-DR.127–130 Depletion of dendritic cells prevented the development of dry eye disease in mice subjected to desiccating stress.120 Mature dendritic cells prime antigen-specific Th1 and Th17 effector T cells in the conjunctival draining lymph nodes. Several laboratories have identified interferon gamma (IFN-γ) and IL-17, produced by Th1 and Th-17 cells respectively, as critical effector cytokines in dry eye.27, 66, 69, 70, 99, 101, 121, 123, 124, 131–136 IFN-γ promotes conjunctival goblet cell loss and lacrimal gland acinar loss, while IL-17 cause corneal barrier disruption and lymphangiogenesis in mouse dry eye models. The disruption of immune tolerance and generation of effector T cells suggests inadequate suppression by regulatory T cells (Tregs). Indeed, dysfunctional Tregs that cannot suppress T effector activity, but produce IFN-γ and IL-17 have been observed in mouse models of dry eye induced by desiccating stress or associated with aging.123, 137 Furthermore, adoptive transfer of either T effectors or Tregs from aged mice into naïve immunodeficient recipient mice caused goblet cell loss and lacrimal gland infiltration, while the adoptive transfer T effectors or Tregs from young mice did not, suggesting that age-related Treg dysfunction may contribute to induction of dry eye disease.137
Lacrimal gland (LG) inflammation and dysfunction develop with age and in the autoimmune disease Sjögren syndrome (SS).138 The hallmarks of SS are lymphocytic infiltration of the lacrimal and salivary glands, serum autoantibodies, keratoconjunctivitis sicca and dry mouth.139 Mouse models of SS and aging have identified a pathogenic role for CD4+T 98, 137, 140–142 and B cells.143–146 Mouse SS models that develop dacryoadenitis tend to be Th1 skewed147–151, whereas those that develop sialadenitis are Th17 skewed.152–155 IFN-γ produced by the infiltrating cells increases caspase expression and causes acinar apoptosis.150, 151, 156–158 Altered nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) signaling has been implicated in SS 159–161 and increased NFκB signaling in epithelial cells was found to promote lacrimal gland acinar apoptosis that preceded lymphocytic infiltration in a mouse SS model.161 Infiltration with autoreactive T cells and oxidative stress have also been observed in the aged lacrimal gland, indicating that aging is associated with inflammation and is not simply a degenerative process. 98, 137, 162–166 These studies suggest that similar to the ocular surface, a vicious cycle of inflammation and apoptosis involving infiltrating cells and glandular acinar cells perpetuates LG inflammation leading to glandular dysfunction in SS and age-related dry eye.
There is an increased body of evidence demonstrating that the microbiome, the microbial community that inhabits the human body, has immunoregulatory functions. The presence of an ocular microbiome has long been suspected; however, traditional swab cultures of the conjunctiva are often negative.17, 167 This is in sharp contrast to cultures of the lid margin and periocular skin which often grow bacteria.168, 169 Studies using 16S genomic sequencing have demonstrated an ocular surface microbiome that may have the lowest biomass of any tissue in the body16, 170, 171 No difference in the quantity and diversity of the ocular microbiome was noted between SS and control subjects;16 however, significant alterations of the intestinal microbiome were noted in the same cohort with a significant decrease in commensal genera and an increase in pathogenic genera, such as Escherichia/Shigella and Proteobacteria. Mice that had an antibiotic-induced depletion of the microbiome with a cocktail of five oral antibiotics prior to experimental desiccating stress developed significantly worse dry eye than control mice that did not receive antibiotics, suggesting that the intestinal microbiome can modulate ocular surface inflammation and severity of dry eye disease.16
Future Directions for Research
The two approved therapies for dry eye, cyclosporine and lifitegrast, target T cells which are key contributors to the pathophysiology of chronic dry eye. Cyclosporine bound to cyclophilin inhibits the activity of the serine/threonine phosphatase calcineurin which normally dephosphorylates nuclear factor of activated T cells (NFAT) after antigen binding to the T cell receptor.172 Dephosphorylated NFAT is transported to the nucleus where it initiates transcription of T cell cytokines, notably IL-2 and IFN-γ.172 Lifitegrast is a small molecule that inhibits binding of leukocyte-associated antigen 1 (LFA-1) on T cells to its ligand intercellular adhesion molecule 1 (ICAM1) on antigen presenting, epithelial and vascular endothelial cells and prevents the formation of the immunological synapse that is required for full T cell activation.125 These molecules have improved dry eye signs and symptoms in clinical trials, but they are not effective in all dry eye patients and don’t address acute effects of desiccation on the ocular surface, including the increased production of innate mediators and activation of the MAPK stress signaling pathways.172–174 Therapies targeting the acute effects of desiccation would likely provide more rapid relief of eye irritation and prevent the effects of a dry, drafty environment such as an airplane cabin. Corticosteroids have shown efficacy in treating chronic dry eye and preventing irritation and cornea barrier disruption in response to a desiccating environmental challenge175–178; however, long-term use of corticosteroids carries risks of cataract formation and glaucoma, and therapies with steroid-like inhibitory effects on innate inflammatory pathways would represent a major advance.
Conjunctival goblet cells produce soluble mucins that stabilize the pre-corneal tear layer. They also produce factors that maintain homeostasis and immune tolerance on the ocular surface.109, 179 The worst cornea disease develops in dry eye conditions with loss of goblet cells, such as Sjögren syndrome, Stevens-Johnson syndrome and graft-vs.-host disease.134, 180, 181 The Th1 cytokine IFN-γ inhibits goblet cell secretion and promotes apoptosis of these cells.133, 182 Both cyclosporine A and serum drops have been reported to increase conjunctival goblet cell density.183, 184 Research is needed to identify therapies to maintain goblet cell number and function with aging and in dry eye conditions, particularly those associated with the most severe goblet cell loss.
Therapies to bolster endogenous natural anti-inflammatory and immunomodulatory mechanisms also appear to have promise. The Western diet is often deficient in anti-inflammatory polyunsaturated fatty acids (PUFAs).185, 186 Oral supplementation with gamma-linolenic acid (GLA, n-6) and omega-3 (n-3) PUFAs has been found to improve ocular irritation symptoms and tear stability, inhibit conjunctival dendritic cell maturation and decreased inflammatory mediators in patients with dry eye.187–189 Other nutritional supplements such as curcumin have potent anti-inflammatory effects and have been found to suppress IL-1β production by osmotically stressed cornea epithelial cells and dendritic cell maturation.190, 191 Intestinal dysbiosis has been found as a risk factor for SS dry eye and mice with antibiotic-induced depletion of their microbiome developed significantly worse ocular surface disease in response to desiccating stress.16 Supplementation with commensal microbiota have shown anti-inflammatory effects in autoimmune conditions such as inflammatory bowel disease and diabetes mellitus.192–196 It is possible that probiotics or metabolites of commensal bacteria could have future therapeutic potential for dry eye disease.
Highlights.
Multiple factors can promote tear instability and hyperosmolarity that trigger ocular surface and glandular inflammation. Cornea barrier disruption, conjunctival goblet cell loss, and glandular dysfunction are consequences of the dry eye inflammatory cascade.
Acknowledgments
This work was supported by NIH Grant EY11915 (SCP), NIH Core Grants-EY002520 & EY020799, NIH funding to Cytometry and Cell Sorting Core at Baylor College of Medicine (NIAID P30AI036211, NCI P30CA125123, and NCRR S10RR024574), Biology of Inflammation Center Baylor College of Medicine, an unrestricted grant from Research to Prevent Blindness, New York, NY (SCP), the Oshman Foundation, Houston, TX (SCP), the William Stamps Farish Fund, Houston, TX (SCP), Hamill Foundation, Houston, TX (SCP), Sid W. Richardson Foundation, Ft Worth, TX (SCP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. listed na. [DOI] [PubMed] [Google Scholar]
- 2.Pflugfelder SC, de Paiva CS, Villarreal AL, Stern ME. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-beta2 production. Cornea. 2008;27(1):64–9. doi: 10.1097/ICO.0b013e318158f6dc. [DOI] [PubMed] [Google Scholar]
- 3.Pflugfelder SC, Stern ME. Mucosal environmental sensors in the pathogenesis of dry eye. Expert Rev Clin Immunol. 2014;10(9):1137–40. doi: 10.1586/1744666X.2014.944163. [DOI] [PubMed] [Google Scholar]
- 4.Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjogren’s and non-Sjogren’s patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43(8):2609–14. [PubMed] [Google Scholar]
- 5.Hingorani M, Metz D, Lightman SL. Characterisation of the normal conjunctival leukocyte population. Exp Eye Res. 1997;64(6):905–12. doi: 10.1006/exer.1996.0280. [DOI] [PubMed] [Google Scholar]
- 6.Allansmith MR, Greiner JV, Baird RS. Number of inflammatory cells in the normal conjunctiva. Am J Ophthalmol. 1978;86(2):250–9. doi: 10.1016/s0002-9394(14)76821-7. [DOI] [PubMed] [Google Scholar]
- 7.Zavaro A, Samra Z, Baryishak R, Sompolinsky D. Proteins in tears from healthy and diseased eyes. Doc Ophthalmol. 1980;50(1):185–99. doi: 10.1007/BF00161161. [DOI] [PubMed] [Google Scholar]
- 8.Kijstra A, Jeurissen SHM, Konig KM. Lactoferrin levels in normal human tears. Br J Ophthalmol. 1983;67:1999–2005. doi: 10.1136/bjo.67.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen OL, Gluud BS, Birgens HS. The concentration of lactoferrin in tears of normals and of diabetics. Acta Ophthalmol (Copenh) 1986;64(1):83–7. doi: 10.1111/j.1755-3768.1986.tb06877.x. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz BL, Christensen GR, Ritzmann SR. Diurnal profiles of tear lysozyme and gamma A globulin. Ann Ophthalmol. 1978:75–80. [PubMed] [Google Scholar]
- 11.Vinding T, Eriksen JS, Nielsen NV. The concentration of lysozyme and secretory IgA in tears from healthy persons with and without contact lens use. Acta Ophthalmol (Copenh) 1987;65(1):23–6. doi: 10.1111/j.1755-3768.1987.tb08485.x. [DOI] [PubMed] [Google Scholar]
- 12.Kuizenga A, van Hoeringen NJ, Kijstra A. Identification of lectin binding proteins in human tears. Invest Ophthalmol Vis Sci. 1991;32:3277–84. [PubMed] [Google Scholar]
- 13.Haynes RJ, Tighe PJ, Dua HS. Antimicrobial defensin peptides of the human ocular surface. Br J Ophthalmol. 1999;83(6):737–41. doi: 10.1136/bjo.83.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonawane S, Khanolkar V, Namavari A, et al. Ocular surface extracellular DNA and nuclease activity imbalance: a new paradigm for inflammation in dry eye disease. Invest Ophthalmol Vis Sci. 2012;53(13):8253–63. doi: 10.1167/iovs.12-10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDermott AM. Antimicrobial compounds in tears. Exp Eye Res. 2013;117:53–61. doi: 10.1016/j.exer.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Paiva CS, Jones DB, Stern ME, et al. Altered Mucosal Microbiome Diversity and Disease Severity in Sjogren Syndrome. Sci Rep. 2016;6:23561–71. doi: 10.1038/srep23561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schabereiter-Gurtner C, Maca S, Rolleke S, et al. 16S rDNA-based identification of bacteria from conjunctival swabs by PCR and DGGE fingerprinting. Invest Ophthalmol Vis Sci. 2001;42(6):1164–71. [PubMed] [Google Scholar]
- 18.Moeller CT, Branco BC, Yu MC, et al. Evaluation of normal ocular bacterial flora with two different culture media. Can J Ophthalmol. 2005;40(4):448–53. doi: 10.1139/i05-014. [DOI] [PubMed] [Google Scholar]
- 19.Graham JE, Moore JE, Jiru X, et al. Ocular pathogen or commensal: a PCR-based study of surface bacterial flora in normal and dry eyes. Invest Ophthalmol Vis Sci. 2007;48(12):5616–23. doi: 10.1167/iovs.07-0588. [DOI] [PubMed] [Google Scholar]
- 20.Linden SK, Sutton P, Karlsson NG, et al. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008;1(3):183–97. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dursun D, Wang M, Monroy D, et al. A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2002;43(3):632–8. [PubMed] [Google Scholar]
- 22.Luo L, Li DQ, Corrales RM, Pflugfelder SC. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye & Contact Lens. 2005;31(5):186–93. doi: 10.1097/01.icl.0000162759.79740.46. [DOI] [PubMed] [Google Scholar]
- 23.Luo L, Li DQ, Doshi A, et al. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45(12):4293–301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 24.Corrales RM, Stern ME, de Paiva CS, et al. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Invest Ophthalmol Vis Sci. 2006;47(8):3293–302. doi: 10.1167/iovs.05-1382. [DOI] [PubMed] [Google Scholar]
- 25.de Paiva CS, Corrales RM, Villarreal AL, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis Sci. 2006;47(7):2847–56. doi: 10.1167/iovs.05-1281. [DOI] [PubMed] [Google Scholar]
- 26.de Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83(3):526–35. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 27.de Paiva CS, Villarreal AL, Corrales RM, et al. Dry Eye-Induced Conjunctival Epithelial Squamous Metaplasia Is Modulated by Interferon-{gamma} Invest Ophthalmol Vis Sci. 2007;48(6):2553–60. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z, Tong L, Li Z, et al. Hyperosmolarity-induced cornification of human corneal epithelial cells is regulated by JNK MAPK. Invest Ophthalmol Vis Sci. 2008;49(2):539–49. doi: 10.1167/iovs.07-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chi W, Hua X, Chen X, et al. Mitochondrial DNA oxidation induces imbalanced activity of NLRP3/NLRP6 inflammasomes by activation of caspase-8 and BRCC36 in dry eye. J Autoimmun. 2017 doi: 10.1016/j.jaut.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng R, Hua X, Li J, et al. Oxidative stress markers induced by hyperosmolarity in primary human corneal epithelial cells. PLoS One. 2015;10(5):e0126561. doi: 10.1371/journal.pone.0126561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li DQ, Chen Z, Song XJ, et al. Hyperosmolarity Stimulates Production of MMP-9, IL-1á and TNF- by Human Corneal Epithelial Cells Via a c-Jun NH 2- terminal kinase pathway. Invest Ophthalmol Vis Sci. 2002;2002:43. EAbstract 1981. [Google Scholar]
- 32.Li DQ, Chen Z, Song XJ, et al. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45(12):4302–11. doi: 10.1167/iovs.04-0299. [DOI] [PubMed] [Google Scholar]
- 33.Li DQ, Luo L, Chen Z, et al. JNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res. 2006;82(4):588–96. doi: 10.1016/j.exer.2005.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Ruzhi D, Hua X, et al. Blueberry Component Pterostilbene Protects Corneal Epithelial Cells from Inflammation via Anti-oxidative Pathway. Sci Rep. 2016;6:19408. doi: 10.1038/srep19408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Versura P, Profazio V, Schiavi C, Campos EC. Hyperosmolar stress upregulates HLA-DR expression in human conjunctival epithelium in dry eye patients and in vitro models. Invest Ophthalmol Vis Sci. 2011;52(8):5488–96. doi: 10.1167/iovs.11-7215. [DOI] [PubMed] [Google Scholar]
- 36.de Paiva CS, Pangelinan SB, Chang E, et al. Essential role for c-Jun N-terminal kinase 2 in corneal epithelial response to desiccating stress. Arch Ophthalmol. 2009;127(12):1625–31. doi: 10.1001/archophthalmol.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelegrino FS, Pflugfelder SC, de Paiva CS. Low humidity environmental challenge causes barrier disruption and cornification of the mouse corneal epithelium via a c-jun N-terminal kinase 2 (JNK2) pathway. Exp Eye Res. 2012;94(1):150–6. doi: 10.1016/j.exer.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solomon A, Dursun D, Liu Z, et al. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42(10):2283–92. [PubMed] [Google Scholar]
- 39.Li DQ, Lokeshwar BL, Solomon A, et al. Regulation of MMP-9 production by human corneal epithelial cells. Exp Eye Res. 2001;73(4):449–59. doi: 10.1006/exer.2001.1054. [DOI] [PubMed] [Google Scholar]
- 40.Li DQ, Shang TY, Kim HS, et al. Regulated expression of collagenases MMP-1, -8, and -13 and stromelysins MMP-3, -10, and -11 by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44(7):2928–36. doi: 10.1167/iovs.02-0874. [DOI] [PubMed] [Google Scholar]
- 41.Ogata Y, Enghild JJ, Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992;267(6):3581–4. [PubMed] [Google Scholar]
- 42.So T, Ito A, Sato T, et al. Tumor necrosis factor-alpha stimulates the biosynthesis of matrix metalloproteinases and plasminogen activator in cultured human chorionic cells. Biol Reprod. 1992;46(5):772–8. doi: 10.1095/biolreprod46.5.772. [DOI] [PubMed] [Google Scholar]
- 43.Pflugfelder SC, Farley W, Luo L, et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol. 2005;166(1):61–71. doi: 10.1016/S0002-9440(10)62232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bian F, Shin CS, Wang C, et al. Dexamethasone Drug Eluting Nanowafers Control Inflammation in Alkali-Burned Corneas Associated With Dry Eye. Invest Ophthalmol Vis Sci. 2016;57(7):3222–30. doi: 10.1167/iovs.16-19074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim HS, Luo L, Pflugfelder SC, Li DQ. Doxycycline Inhibits TGF-{beta}1-Induced MMP-9 via Smad and MAPK Pathways in Human Corneal Epithelial Cells. Invest Ophthalmol Vis Sci. 2005;46(3):840–8. doi: 10.1167/iovs.04-0929. [DOI] [PubMed] [Google Scholar]
- 46.Chan TC, Ye C, Chan KP, et al. Evaluation of point-of-care test for elevated tear matrix metalloproteinase 9 in post-LASIK dry eyes. Br J Ophthalmol. 2016;100(9):1188–91. doi: 10.1136/bjophthalmol-2015-307607. [DOI] [PubMed] [Google Scholar]
- 47.Kaufman HE. The practical detection of mmp-9 diagnoses ocular surface disease and may help prevent its complications. Cornea. 2013;32(2):211–6. doi: 10.1097/ICO.0b013e3182541e9a. [DOI] [PubMed] [Google Scholar]
- 48.Lanza NL, McClellan AL, Batawi H, et al. Dry Eye Profiles in Patients with a Positive Elevated Surface Matrix Metalloproteinase 9 Point-of-Care Test Versus Negative Patients. Ocul Surf. 2016;14(2):216–23. doi: 10.1016/j.jtos.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sambursky R. Presence or absence of ocular surface inflammation directs clinical and therapeutic management of dry eye. Clin Ophthalmol. 2016;10:2337–43. doi: 10.2147/OPTH.S121256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sambursky R, Davitt WF, 3rd, Friedberg M, Tauber S. Prospective, multicenter, clinical evaluation of point-of-care matrix metalloproteinase-9 test for confirming dry eye disease. Cornea. 2014;33(8):812–8. doi: 10.1097/ICO.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 51.St Pierre Y, Van Themsche C, Esteve PO. Emerging features in the regulation of MMP-9 gene expression for the development of novel molecular targets and therapeutic strategies. Curr Drug Targets Inflamm Allergy. 2003;2(3):206–15. doi: 10.2174/1568010033484133. [DOI] [PubMed] [Google Scholar]
- 52.Brejchova K, Liskova P, Hrdlickova E, et al. Matrix metalloproteinases in recurrent corneal melting associated with primary Sjorgen’s syndrome. Mol Vis. 2009;15:2364–72. [PMC free article] [PubMed] [Google Scholar]
- 53.Collier SA. Is the corneal degradation in keratoconus caused by matrix-metalloproteinases? Clin Experiment Ophthalmol. 2001;29(6):340–4. doi: 10.1046/j.1442-9071.2001.d01-17.x. [DOI] [PubMed] [Google Scholar]
- 54.Di Girolamo N, Verma MJ, McCluskey PJ, et al. Increased matrix metalloproteinases in the aqueous humor of patients and experimental animals with uveitis. Curr Eye Res. 1996;15(10):1060–8. doi: 10.3109/02713689609017656. [DOI] [PubMed] [Google Scholar]
- 55.Dushku N, John MK, Schultz GS, Reid TW. Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol. 2001;119(5):695–706. doi: 10.1001/archopht.119.5.695. [DOI] [PubMed] [Google Scholar]
- 56.Garrana RM, Zieske JD, Assouline M, Gipson IK. Matrix metalloproteinases in epithelia from human recurrent corneal erosion. Invest Ophthalmol Vis Sci. 1999;40(6):1266–70. [PubMed] [Google Scholar]
- 57.Leonardi A, Brun P, Abatangelo G, et al. Tear levels and activity of matrix metalloproteinase (MMP)-1 and MMP-9 in vernal keratoconjunctivitis. Invest Ophthalmol Vis Sci. 2003;44(7):3052–8. doi: 10.1167/iovs.02-0766. [DOI] [PubMed] [Google Scholar]
- 58.Markoulli M, Papas E, Cole N, Holden BA. The diurnal variation of matrix metalloproteinase-9 and its associated factors in human tears. Invest Ophthalmol Vis Sci. 2012;53(3):1479–84. doi: 10.1167/iovs.11-8365. [DOI] [PubMed] [Google Scholar]
- 59.Sakimoto T, Shoji J, Sawa M. Active form of gelatinases in tear fluidin patients with corneal ulcer or ocular burn. Jpn J Ophthalmol. 2003;47(5):423–6. doi: 10.1016/s0021-5155(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 60.Smith VA, Rishmawi H, Hussein H, Easty DL. Tear film MMP accumulation and corneal disease. Br J Ophthalmol. 2001;85(2):147–53. doi: 10.1136/bjo.85.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sobrin L, Liu Z, Monroy DC, et al. Regulation of MMP-9 activity in human tear fluid and corneal epithelial culture supernatant. Invest Ophthalmol Vis Sci. 2000;41(7):1703–9. [PubMed] [Google Scholar]
- 62.Fini ME, Cui TY, Mouldovan A, et al. An inhibitor of the matrix metalloproteinase synthesized by rabbit corneal epithelium. Invest Ophthalmol Vis Sci. 1991;32(11):2997–3001. [PubMed] [Google Scholar]
- 63.Sosne G, Christopherson PL, Barrett RP, Fridman R. Thymosin-beta4 modulates corneal matrix metalloproteinase levels and polymorphonuclear cell infiltration after alkali injury. Invest Ophthalmol Vis Sci. 2005;46(7):2388–95. doi: 10.1167/iovs.04-1368. [DOI] [PubMed] [Google Scholar]
- 64.Yoon KC, Park CS, You IC, et al. Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Invest Ophthalmol Vis Sci. 2010;51(2):643–50. doi: 10.1167/iovs.09-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoon KC, de Paiva CS, Qi H, et al. Expression of th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol Vis Sci. 2007;48(6):2561–9. doi: 10.1167/iovs.07-0002. [DOI] [PubMed] [Google Scholar]
- 66.de Paiva CS, Chotikavanich S, Pangelinan SB, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2(3):243–53. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Enriquez-de-Salamanca A, Castellanos E, Stern ME, et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010;16:862–73. [PMC free article] [PubMed] [Google Scholar]
- 68.Choi W, Li Z, Oh HJ, et al. Expression of CCR5 and its ligands CCL3, -4, and -5 in the tear film and ocular surface of patients with dry eye disease. Curr Eye Res. 2012;37(1):12–7. doi: 10.3109/02713683.2011.622852. [DOI] [PubMed] [Google Scholar]
- 69.Dohlman TH, Chauhan SK, Kodati S, et al. The CCR6/CCL20 Axis Mediates Th17 Cell Migration to the Ocular Surface in Dry Eye Disease. Invest Ophthalmol Vis Sci. 2013;54(6):4081–91. doi: 10.1167/iovs.12-11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coursey TG, Bohat R, Barbosa FL, et al. Desiccating stress-induced chemokine expression in the epithelium is dependent on upregulation of NKG2D/RAE-1 and release of IFN-gamma in experimental dry eye. J Immunol. 2014;193(10):5264–72. doi: 10.4049/jimmunol.1400016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng Q, Ren Y, Reinach PS, et al. Reactive oxygen species activated NLRP3 inflammasomes initiate inflammation in hyperosmolarity stressed human corneal epithelial cells and environment-induced dry eye patients. Exp Eye Res. 2015;134:133–40. doi: 10.1016/j.exer.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 72.Augustin AJ, Spitznas M, Kaviani N, et al. Oxidative reactions in the tear fluid of patients suffering from dry eyes. Graefe’s Arch Clin Exp Ophthalmol. 1995;233:694–8. doi: 10.1007/BF00164671. [DOI] [PubMed] [Google Scholar]
- 73.Benlloch-Navarro S, Franco I, Sanchez-Vallejo V, et al. Lipid peroxidation is increased in tears from the elderly. Exp Eye Res. 2013;115:199–205. doi: 10.1016/j.exer.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 74.Cejkova J, Ardan T, Simonova Z, et al. Decreased expression of antioxidant enzymes in the conjunctival epithelium of dry eye (Sjogren’s syndrome) and its possible contribution to the development of ocular surface oxidative injuries. Histol Histopathol. 2008;23(12):1477–83. doi: 10.14670/HH-23.1477. [DOI] [PubMed] [Google Scholar]
- 75.Choi W, Lian C, Ying L, et al. Expression of Lipid Peroxidation Markers in the Tear Film and Ocular Surface of Patients with Non-Sjogren Syndrome: Potential Biomarkers for Dry Eye Disease. Curr Eye Res. 2016;41(9):1143–9. doi: 10.3109/02713683.2015.1098707. [DOI] [PubMed] [Google Scholar]
- 76.Macri A, Scanarotti C, Bassi AM, et al. Evaluation of oxidative stress levels in the conjunctival epithelium of patients with or without dry eye, and dry eye patients treated with preservative-free hyaluronic acid 0. 15 % and vitamin B12 eye drops. Graefes Arch Clin Exp Ophthalmol. 2015;253(3):425–30. doi: 10.1007/s00417-014-2853-6. [DOI] [PubMed] [Google Scholar]
- 77.Wakamatsu TH, Dogru M, Matsumoto Y, et al. Evaluation of lipid oxidative stress status in Sjogren syndrome patients. Invest Ophthalmol Vis Sci. 2013;54(1):201–10. doi: 10.1167/iovs.12-10325. [DOI] [PubMed] [Google Scholar]
- 78.Zheng Q, Ren Y, Reinach PS, et al. Reactive oxygen species activated NLRP3 inflammasomes prime environment-induced murine dry eye. Exp Eye Res. 2014;125:1–8. doi: 10.1016/j.exer.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 79.Redfern RL, Patel N, Hanlon S, et al. Toll-like receptor expression and activation in mice with experimental dry eye. Invest Ophthalmol Vis Sci. 2013;54(2):1554–63. doi: 10.1167/iovs.12-10739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simmons KT, Xiao Y, Pflugfelder SC, de Paiva CS. Inflammatory Response to Lipopolysaccharide on the Ocular Surface in a Murine Dry Eye Model. Invest Ophthalmol Vis Sci. 2016;57(6):2443–51. doi: 10.1167/iovs.15-18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cejkova J, Trosan P, Cejka C, et al. Suppression of alkali-induced oxidative injury in the cornea by mesenchymal stem cells growing on nanofiber scaffolds and transferred onto the damaged corneal surface. Exp Eye Res. 2013;116:312–23. doi: 10.1016/j.exer.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 82.Choi W, Lee JB, Cui L, et al. Therapeutic Efficacy of Topically Applied Antioxidant Medicinal Plant Extracts in a Mouse Model of Experimental Dry Eye. Oxid Med Cell Longev. 2016;2016:4727415. doi: 10.1155/2016/4727415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hitoe S, Tanaka J, Shimoda H. MaquiBright standardized maqui berry extract significantly increases tear fluid production and ameliorates dry eye-related symptoms in a clinical pilot trial. Panminerva Med. 2014;56(3 Suppl 1):1–6. [PubMed] [Google Scholar]
- 84.Hua X, Deng R, Li J, et al. Protective Effects of L-Carnitine Against Oxidative Injury by Hyperosmolarity in Human Corneal Epithelial Cells. Invest Ophthalmol Vis Sci. 2015;56(9):5503–11. doi: 10.1167/iovs.14-16247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martins EAL, Chubatsu LS, Meneghini R. Role of antioxidants in protecting cellular DNA from damage by oxidative stress. Mutation Res. 1991;250:95–101. doi: 10.1016/0027-5107(91)90166-l. [DOI] [PubMed] [Google Scholar]
- 86.Tseng SCG, Hirst LW, Maumenee AE, et al. Possible mechanisms for the loss of goblet cells in mucin deficient disorders. Ophthalmology. 1984;91:545–52. doi: 10.1016/s0161-6420(84)34251-8. [DOI] [PubMed] [Google Scholar]
- 87.Huang AJ, Tseng SC, Kenyon KR. Morphogenesis of rat conjunctival goblet cells. Invest Ophthalmol Vis Sci. 1988;29(6):969–75. [PubMed] [Google Scholar]
- 88.Roat MI, Ohji M, Hunt LE, Thoft RA. Conjunctival epithelial cell hypermitosis and goblet cell hyperplasia in atopic keratoconjunctivitis. Am J Ophthalmol. 1993;116(4):456–63. doi: 10.1016/s0002-9394(14)71404-7. [DOI] [PubMed] [Google Scholar]
- 89.Tseng SCG. Staging of conjunctival squamous metaplasia by impression cytology. Ophthalmology. 1985;92:728–33. doi: 10.1016/s0161-6420(85)33967-2. [DOI] [PubMed] [Google Scholar]
- 90.Wittpenn JR, Tseng SCG, Sommer A. Detection of early xerophthalmia by impression cytology. Arch Ophthalmol. 1986;104:237–9. doi: 10.1001/archopht.1986.01050140091027. [DOI] [PubMed] [Google Scholar]
- 91.Lemp MA, Dohlman CH, Kuwabara T, et al. Dry eye secondary mucus deficiency. Trans Am Ophthalmol Soc. 1971;75:1223–7. [PubMed] [Google Scholar]
- 92.Ralph RA. Conjunctival goblet cell density in normal subjects and in dry eye syndromes. Invest Ophthalmol. 1975;14(4):299–302. [PubMed] [Google Scholar]
- 93.Nelson JD, Wright JC. Conjunctival goblet cell densities in ocular surface disorders. Arch Ophthalmol. 1984;102:1049–51. doi: 10.1001/archopht.1984.01040030851031. [DOI] [PubMed] [Google Scholar]
- 94.Pflugfelder SC, Huang AJW, Schuchovski PT, et al. Conjunctival cytological features of primary Sjogren syndrome. Ophthalmology. 1990;97:985–91. doi: 10.1016/s0161-6420(90)32478-8. [DOI] [PubMed] [Google Scholar]
- 95.Pflugfelder SC, Tseng SCG, Yoshino K, et al. Correlation of goblet cell density and mucosal epithelial membrane mucin expression with rose bengal staining in patients with ocular irritation. Ophthalmology. 1997;104:223–35. doi: 10.1016/s0161-6420(97)30330-3. [DOI] [PubMed] [Google Scholar]
- 96.Murube J, Rivas L. Impression cytology on conjunctiva and cornea in dry eye patients establishes a correlation between squamous metaplasia and dry eye clinical severity. Eur J Ophthalmol. 2003;13(2):115–27. doi: 10.1177/112067210301300201. [DOI] [PubMed] [Google Scholar]
- 97.de Paiva CS, Raince JK, McClellan AJ, et al. Homeostatic control of conjunctival mucosal goblet cells by NKT-derived IL-13. Mucosal Immunol. 2011;4(4):397–408. doi: 10.1038/mi.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McClellan AJ, Volpe EA, Zhang X, et al. Ocular Surface Disease and Dacryoadenitis in Aging C57BL/6 Mice. Am J Pathol. 2014;184(3):631–43. doi: 10.1016/j.ajpath.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang X, Chen W, de Paiva CS, et al. Interferon-gamma exacerbates dry eye-induced apoptosis in conjunctiva through dual apoptotic pathways. Invest Ophthalmol Vis Sci. 2011;52(9):6279–85. doi: 10.1167/iovs.10-7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang X, Chen W, de Paiva CS, et al. Desiccating Stress Induces CD4(+) T-Cell-Mediated Sjogren’s Syndrome-Like Corneal Epithelial Apoptosis via Activation of the Extrinsic Apoptotic Pathway by Interferon-gamma. Am J Pathol. 2011;179(4):1807–14. doi: 10.1016/j.ajpath.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang X, de Paiva CS, Su Z, et al. Topical interferon-gamma neutralization prevents conjunctival goblet cell loss in experimental murine dry eye. Exp Eye Res. 2014;118:117–24. doi: 10.1016/j.exer.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Corrales RM, de Paiva CS, Li DQ, et al. Entrapment of conjunctival goblet cells by desiccation-induced cornification. Invest Ophthalmol Vis Sci. 2011;52(6):3492–9. doi: 10.1167/iovs.10-5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tukler Henriksson J, Coursey TG, Corry DB, et al. IL-13 Stimulates Proliferation and Expression of Mucin and Immunomodulatory Genes in Cultured Conjunctival Goblet Cells. Invest Ophthalmol Vis Sci. 2015;56(8):4186–97. doi: 10.1167/iovs.14-15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Contreras-Ruiz L, Ghosh-Mitra A, Shatos MA, et al. Modulation of conjunctival goblet cell function by inflammatory cytokines. Mediators Inflamm. 2013;2013:636812. doi: 10.1155/2013/636812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carlstedt I, Sheehan JK, Corfield AP, Gallagher JT. Mucous glycoproteins: a gel of a problem. Essays in Biochem. 1985;20:40–76. [PubMed] [Google Scholar]
- 106.Hovenberg HW, Davies JR, Carlstedt I. Different mucins are produced by the surface epithelium and the submucosa in human trachea: identification of MUC5AC as a major mucin from the goblet cells. BiochemJ. 1996;318:319–24. doi: 10.1042/bj3180319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xiao Y, Coursey TG, Li DQ, et al. Conjunctival Goblet Cells modulate dendritic cell maturation and retinoic acid producing capacity. Invest Ophthalmol Vis Sci. 2016;57(426) [Google Scholar]
- 108.Contreras-Ruiz L, Masli S. Immunomodulatory cross-talk between conjunctival goblet cells and dendritic cells. PLoS One. 2015;10(3):e0120284. doi: 10.1371/journal.pone.0120284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barbosa FL, Xiao Y, Bian F, et al. Goblet Cells Contribute to Ocular Surface Immune Tolerance-Implications for Dry Eye Disease. Int J Mol Sci. 2017;18(5):1–13. doi: 10.3390/ijms18050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McDole JR, Wheeler LW, McDonald KG, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483(7389):345–9. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marko CK, Menon BB, Chen G, et al. Spdef null mice lack conjunctival goblet cells and provide a model of dry eye. Am J Pathol. 2013;183(1):35–48. doi: 10.1016/j.ajpath.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guzman M, Keitelman I, Sabbione F, et al. Mucosal tolerance disruption favors disease progression in an extraorbital lacrimal gland excision model of murine dry eye. Exp Eye Res. 2016;151:19–22. doi: 10.1016/j.exer.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 113.Guzman M, Keitelman I, Sabbione F, et al. Desiccating stress-induced disruption of ocular surface immune tolerance drives dry eye disease. Clin Exp Immunol. 2016;184(2):248–56. doi: 10.1111/cei.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Galletti JG, Gabelloni ML, Morande PE, et al. Benzalkonium chloride breaks down conjunctival immunological tolerance in a murine model. Mucosal Immunol. 2013;6(1):24–34. doi: 10.1038/mi.2012.44. [DOI] [PubMed] [Google Scholar]
- 115.De Paiva CS, Villarreal AL, Corrales RM, et al. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest Ophthalmol Vis Sci. 2007;48(6):2553–60. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- 116.Kunert KS, Tisdale AS, Stern ME, et al. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch Ophthalmol. 2000;118(11):1489–96. doi: 10.1001/archopht.118.11.1489. [DOI] [PubMed] [Google Scholar]
- 117.Pflugfelder SC, De Paiva CS, Moore QL, et al. Aqueous Tear Deficiency Increases Conjunctival Interferon-gamma (IFN-gamma) Expression and Goblet Cell Loss. Invest Ophthalmol Vis Sci. 2015;56(12):7545–50. doi: 10.1167/iovs.15-17627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stern ME, Schaumburg CS, Siemasko KF, et al. Autoantibodies contribute to the immunopathogenesis of experimental dry eye disease. Invest Ophthalmol Vis Sci. 2012;53(4):2062–75. doi: 10.1167/iovs.11-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jiang G, Ke Y, Sun D, et al. A new model of experimental autoimmune keratoconjunctivitis sicca (KCS) induced in Lewis rat by the autoantigen Klk1b22. Invest Ophthalmol Vis Sci. 2009;50(5):2245–54. doi: 10.1167/iovs.08-1949. [DOI] [PubMed] [Google Scholar]
- 120.Schaumburg CS, Siemasko KF, de Paiva CS, et al. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J Immunol. 2011;187(7):3653–62. doi: 10.4049/jimmunol.1101442. [DOI] [PubMed] [Google Scholar]
- 121.Coursey TG, Gandhi NB, Volpe EA, et al. Chemokine receptors CCR6 and CXCR3 are necessary for CD4(+) T cell mediated ocular surface disease in experimental dry eye disease. PLoS One. 2013;8(11):e78508. doi: 10.1371/journal.pone.0078508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.De Paiva CS, Chotikavanich S, Pangelinan SB, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2(3):243–53. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chauhan SK, El AJ, Ecoiffier T, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182(3):1247–52. doi: 10.4049/jimmunol.182.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang X, Schaumburg CS, Coursey TG, et al. CD8(+) cells regulate the T helper-17 response in an experimental murine model of Sjogren syndrome. Mucosal Immunol. 2014;7(2):417–27. doi: 10.1038/mi.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pflugfelder S, Bian F, Farley W, et al. Conjunctival dendritic cell maturation and goblet cell density in aqueous tear deficiency. ARVO Abstracts. 2017;2017:3754. [Google Scholar]
- 126.Sheppard JD, Jr, Singh R, McClellan AJ, et al. Long-term Supplementation With n-6 and n-3 PUFAs Improves Moderate-to-Severe Keratoconjunctivitis Sicca: A Randomized Double-Blind Clinical Trial. Cornea. 2013;32(10):1297–304. doi: 10.1097/ICO.0b013e318299549c. [DOI] [PubMed] [Google Scholar]
- 127.Pisella PJ, Brignole F, Debbasch C, et al. Flow cytometric analysis of conjunctival epithelium in ocular rosacea and keratoconjunctivitis sicca. Ophthalmology. 2000;107(10):1841–9. doi: 10.1016/s0161-6420(00)00347-x. [DOI] [PubMed] [Google Scholar]
- 128.Brignole F, Pisella PJ, Goldschild M, et al. Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Invest Ophthalmol Vis Sci. 2000;41(6):1356–63. [PubMed] [Google Scholar]
- 129.Brignole F, Pisella PJ, De Saint JM, et al. Flow cytometric analysis of inflammatory markers in KCS: 6-month treatment with topical cyclosporin A. Invest Ophthalmol Vis Sci. 2001;42(1):90–5. [PubMed] [Google Scholar]
- 130.Epstein SP, Gadaria-Rathod N, Wei Y, et al. HLA-DR expression as a biomarker of inflammation for multicenter clinical trials of ocular surface disease. Exp Eye Res. 2013;111:95–104. doi: 10.1016/j.exer.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen Y, Chauhan SK, Saban DR, et al. Interferon-{gamma}-secreting NK cells promote induction of dry eye disease. J Leukoc Biol. 2011;89(6):965–72. doi: 10.1189/jlb.1110611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen Y, Chauhan SK, Tan X, Dana R. Interleukin-7 and -15 maintain pathogenic memory Th17 cells in autoimmunity. J Autoimmun. 2017;77:96–103. doi: 10.1016/j.jaut.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Coursey TG, Henriksson JT, Barbosa FL, et al. Interferon-gamma-Induced Unfolded Protein Response in Conjunctival Goblet Cells as a Cause of Mucin Deficiency in Sjogren Syndrome. Am J Pathol. 2016;186(16):1547–58. doi: 10.1016/j.ajpath.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pflugfelder SC, De Paiva CS, Moore QL, et al. Aqueous Tear Deficiency Increases Conjunctival Interferon-γ (IFN-γ) Expression and Goblet Cell Loss. Invest Ophthalmol Vis Sci. 2015;56(12):7545–50. doi: 10.1167/iovs.15-17627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang X, Volpe EA, Gandhi NB, et al. NK cells promote Th-17 mediated corneal barrier disruption in dry eye. PLoS One. 2012;7(5):e36822. doi: 10.1371/journal.pone.0036822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zheng X, Bian F, Ma P, et al. Induction of Th17 differentiation by corneal epithelial-derived cytokines. J Cell Physiol. 2009 Sep 10;222(1):95–102. doi: 10.1002/jcp.21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Coursey TG, Bian F, Zaheer M, et al. Age-related spontaneous lacrimal keratoconjunctivitis is accompanied by dysfunctional T regulatory cells. Mucosal Immunol. 2016 doi: 10.1038/mi.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Whitcher JP, Shiboski CH, Shiboski SC, et al. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjogren’s Syndrome International Registry. Am J Ophthalmol. 2010;149(3):405–15. doi: 10.1016/j.ajo.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shiboski SC, Shiboski CH, Criswell L, et al. American College of Rheumatology classification criteria for Sjogren’s syndrome: a data-driven, expert consensus approach in the Sjogren’s International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken) 2012;64(4):475–87. doi: 10.1002/acr.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lieberman SM, Kreiger PA, Koretzky GA. Reversible lacrimal gland-protective regulatory T cell dysfunction underlies male-specific autoimmune dacryoadenitis in the nonobese diabetic mouse model of Sjogren syndrome. Immunology. 2015 doi: 10.1111/imm.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lin X, Rui K, Deng J, et al. Th17 cells play a critical role in the development of experimental Sjogren’s syndrome. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204584. [DOI] [PubMed] [Google Scholar]
- 142.Jin JO, Kawai T, Cha S, Yu Q. Interleukin-7 enhances the Th1 response to promote the development of Sjogren’s syndrome-like autoimmune exocrinopathy in mice. Arthritis Rheum. 2013;65(8):2132–42. doi: 10.1002/art.38007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Groom J, Kalled SL, Cutler AH, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren’s syndrome. J Clin Invest. 2002;109(1):59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Konno A, Takada K, Saegusa J, Takiguchi M. Presence of B7-2+ dendritic cells and expression of Th1 cytokines in the early development of sialodacryoadenitis in the IqI/Jic mouse model of primary Sjorgren’s syndrome. Autoimmunity. 2003;36(4):247–54. doi: 10.1080/0891693031000141077. [DOI] [PubMed] [Google Scholar]
- 145.Meng W, Li Y, Xue E, et al. B-cell tolerance defects in the B6. Aec1/2 mouse model of Sjogren’s syndrome. J Clin Immunol. 2012;32(3):551–64. doi: 10.1007/s10875-012-9663-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nakamura H, Kawakami A, Eguchi K. Mechanisms of autoantibody production and the relationship between autoantibodies and the clinical manifestations in Sjogren’s syndrome. Transl Res. 2006;148(6):281–8. doi: 10.1016/j.trsl.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 147.de Paiva CS, Hwang CS, Pitcher JD, III, et al. Age-related T-cell cytokine profile parallels corneal disease severity in Sjogren’s syndrome-like keratoconjunctivitis sicca in CD25KO mice. Rheumatology. 2010;49(2):246–58. doi: 10.1093/rheumatology/kep357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pelegrino FS, Volpe EA, Gandhi NB, et al. Deletion of interferon-gamma delays onset and severity of dacryoadenitis in CD25KO mice. Arthritis Res Ther. 2012;14(6):R234. doi: 10.1186/ar4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rahimy E, Pitcher JD, III, Pangelinan SB, et al. Spontaneous autoimmune dacryoadenitis in aged CD25KO mice. Am J Pathol. 2010;177(2):744–53. doi: 10.2353/ajpath.2010.091116. Epub 2010 Jun 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tsubota K, Fukagawa K, Fujihara T, et al. Regulation of human leukocyte antigen expression in human conjunctival epithelium. Invest Ophthalmol Vis Sci. 1999;40(1):28–34. [PubMed] [Google Scholar]
- 151.Saito I, Terauchi K, Shimuta M, et al. Expression of cell adhesion molecules in the salivary and lacrimal glands of Sjogren’s syndrome. J Clin Lab Anal. 1993;7(3):180–7. doi: 10.1002/jcla.1860070309. [DOI] [PubMed] [Google Scholar]
- 152.Gao J, Killedar S, Cornelius JG, et al. Sjogren’s syndrome in the NOD mouse model is an interleukin-4 time-dependent, antibody isotype-specific autoimmune disease. J Autoimmun. 2006;26(2):90–103. doi: 10.1016/j.jaut.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 153.Lee BH, Carcamo WC, Chiorini JA, et al. Gene therapy using IL-27 ameliorates Sjogren’s syndrome-like autoimmune exocrinopathy. Arthritis Res Ther. 2012;14(4):R172. doi: 10.1186/ar3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nguyen CQ, Hu MH, Li Y, et al. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjogren’s syndrome: findings in humans and mice. Arthritis Rheum. 2008;58(3):734–43. doi: 10.1002/art.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nguyen CQ, Yin H, Lee BH, et al. Pathogenic effect of interleukin-17A in induction of Sjogren’s syndrome-like disease using adenovirus-mediated gene transfer. Arthritis Res Ther. 2010;12(6):R220. doi: 10.1186/ar3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bian F, Barbosa FL, Corrales RM, et al. Altered balance of interleukin-13/interferon-gamma contributes to lacrimal gland destruction and secretory dysfunction in CD25 knockout model of Sjogren’s syndrome. Arthritis Res Ther. 2015;17(1):53. doi: 10.1186/s13075-015-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cha S, Nagashima H, Brown VB, et al. Two NOD Idd-associated intervals contribute synergistically to the development of autoimmune exocrinopathy (Sjogren’s syndrome) on a healthy murine background. Arthritis Rheum. 2002;46(5):1390–8. doi: 10.1002/art.10258. [DOI] [PubMed] [Google Scholar]
- 158.Doyle ME, Boggs L, Attia R, et al. Autoimmune dacryoadenitis of NOD/LtJ mice and its subsequent effects on tear protein composition. Am J Pathol. 2007;171(4):1224–36. doi: 10.2353/ajpath.2007.070388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.de Valle E, Grigoriadis G, O’Reilly LA, et al. NFkappaB1 is essential to prevent the development of multiorgan autoimmunity by limiting IL-6 production in follicular B cells. J Exp Med. 2016;213(4):621–41. doi: 10.1084/jem.20151182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Peng B, Ling J, Lee AJ, et al. Defective feedback regulation of NF-kappaB underlies Sjogren’s syndrome in mice with mutated kappaB enhancers of the IkappaBalpha promoter. Proc Natl Acad Sci U S A. 2010;107(34):15193–8. doi: 10.1073/pnas.1005533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Okuma A, Hoshino K, Ohba T, et al. Enhanced apoptosis by disruption of the STAT3-IkappaB-zeta signaling pathway in epithelial cells induces Sjogren’s syndrome-like autoimmune disease. Immunity. 2013;38(3):450–60. doi: 10.1016/j.immuni.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 162.Uchino Y, Kawakita T, Miyazawa M, et al. Oxidative stress induced inflammation initiates functional decline of tear production. PLoS One. 2012;7(10):e45805. doi: 10.1371/journal.pone.0045805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kojima T, Wakamatsu TH, Dogru M, et al. Age-related dysfunction of the lacrimal gland and oxidative stress: evidence from the Cu, Zn-superoxide dismutase-1 (Sod1) knockout mice. Am J Pathol. 2012;180(5):1879–96. doi: 10.1016/j.ajpath.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 164.Kojima T, Dogru M, Ibrahim OM, et al. Effects of Oxidative Stress on the Conjunctiva in Cu, Zn-Superoxide Dismutase-1 (Sod1)-Knockout Mice. Invest Ophthalmol Vis Sci. 2015;56(13):8382–91. doi: 10.1167/iovs.15-18295. [DOI] [PubMed] [Google Scholar]
- 165.Kawashima M, Kawakita T, Okada N, et al. Calorie restriction: A new therapeutic intervention for age-related dry eye disease in rats. Biochem Biophys Res Commun. 2010;397(4):724–8. doi: 10.1016/j.bbrc.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 166.Kawai M, Ogawa Y, Shimmura S, et al. Expression and localization of aging markers in lacrimal gland of chronic graft-versus-host disease. Sci Rep. 2013;3:2455. doi: 10.1038/srep02455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nolan J. Evaluation of conjunctival and nasal bacterial cultures before intra-ocular operations. Br J Ophthalmol. 1967;51(7):483–5. doi: 10.1136/bjo.51.7.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Willcox MD. Characterization of the normal microbiota of the ocular surface. Exp Eye Res. 2013;117:99–105. doi: 10.1016/j.exer.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 169.Dong QBJ, Iovieno A, Bates B, Garoutte A, Miller D, Revanna KV, Gao X, Antonopoulos DA, Slepak VZ, Shestopalov VI. Diversity of bacteria at healthy human conjunctiva. Invest Ophthalmol Vis Sci. 2011;52(8):5. doi: 10.1167/iovs.10-6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Huang Y, Yang B, Li W. Defining the normal “core microbiome” of conjunctival microbial communities. Clin Microbiol Infect. 2016 doi: 10.1016/j.cmi.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 171.Ozkan J, Diez-Vives C, Shaun Nielsen S, et al. The Temporal Stability of the Ocular Surface Microbiome ARVO Abstracts. 2017;2017:5615. doi: 10.1038/s41598-017-10494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Donnenfeld E, Pflugfelder SC. Topical ophthalmic cyclosporine: pharmacology and clinical uses. Surv Ophthalmol. 2009;54(3):321–38. doi: 10.1016/j.survophthal.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 173.Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107(4):631–9. doi: 10.1016/s0161-6420(99)00176-1. [DOI] [PubMed] [Google Scholar]
- 174.Perry HD, Solomon R, Donnenfeld ED, et al. Evaluation of topical cyclosporine for the treatment of dry eye disease. Arch Ophthalmol. 2008;126(8):1046–50. doi: 10.1001/archopht.126.8.1046. [DOI] [PubMed] [Google Scholar]
- 175.Marsh P, Pflugfelder SC. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjogren syndrome. Ophthalmology. 1999;106(4):811–6. doi: 10.1016/S0161-6420(99)90171-9. [DOI] [PubMed] [Google Scholar]
- 176.Pflugfelder SC. Anti-inflammatory therapy of dry eye. Am J Ophthalmol. 2004;137:337–42. doi: 10.1016/j.ajo.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 177.Moore QL, De Paiva CS, Pflugfelder SC. Effects of dry eye therapies on environmentally induced ocular surface disease. Am J Ophthalmol. 2015 doi: 10.1016/j.ajo.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pinto-Fraga J, Lopez-Miguel A, Gonzalez-Garcia MJ, et al. Topical Fluorometholone Protects the Ocular Surface of Dry Eye Patients from Desiccating Stress: A Randomized Controlled Clinical Trial. Ophthalmology. 2016;123(1):141–53. doi: 10.1016/j.ophtha.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 179.Tuckler Henricksson JC, TG, Corry DB, DePaiva CS, Pflugfelder SC. IL-13 stimulates proliferation and expression of mucins and immunomodulatory gene in cultured conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2015:56. doi: 10.1167/iovs.14-15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nelson JD, Wright JC. Conjunctival goblet cell densities in ocular surface disease. Arch Ophthalmol. 1984;102(7):1049–51. doi: 10.1001/archopht.1984.01040030851031. [DOI] [PubMed] [Google Scholar]
- 181.Pflugfelder SC, Tseng SC, Yoshino K, et al. Correlation of goblet cell density and mucosal epithelial membrane mucin expression with rose bengal staining in patients with ocular irritation. Ophthalmology. 1997;104(2):223–35. doi: 10.1016/s0161-6420(97)30330-3. [DOI] [PubMed] [Google Scholar]
- 182.Garcia-Posadas L, Hodges RR, Li D, et al. Interaction of IFN-gamma with cholinergic agonists to modulate rat and human goblet cell function. Mucosal Immunol. 2015 doi: 10.1038/mi.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pflugfelder SC, De Paiva CS, Villarreal AL, Stern ME. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-beta2 production. Cornea. 2008;27(1):64–9. doi: 10.1097/ICO.0b013e318158f6dc. [DOI] [PubMed] [Google Scholar]
- 184.Noble BA, Loh RS, MacLennan S, et al. Comparison of autologous serum eye drops with conventional therapy in a randomised controlled crossover trial for ocular surface disease. Br J Ophthalmol. 2004;88(5):647–52. doi: 10.1136/bjo.2003.026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Endres S, Ghorbani R, Kelley VE, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320(5):265–71. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 186.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71(1 Suppl):343S–8S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 187.Sheppard JD, Jr, Singh R, McClellan AJ, et al. Long-term Supplementation With n-6 and n-3 PUFAs Improves Moderate-to-Severe Keratoconjunctivitis Sicca: A Randomized Double-Blind Clinical Trial. Cornea. 2013 doi: 10.1097/ICO.0b013e318299549c. [DOI] [PubMed] [Google Scholar]
- 188.Barabino S, Rolando M, Camicione P, et al. Systemic linoleic and gamma-linolenic acid therapy in dry eye syndrome with an inflammatory component. Cornea. 2003;22(2):97–101. doi: 10.1097/00003226-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 189.Macsai MS. The role of omega-3 dietary supplementation in blepharitis and meibomian gland dysfunction (an AOS thesis) Trans Am Ophthalmol Soc. 2008;106:336–56. [PMC free article] [PubMed] [Google Scholar]
- 190.Chen M, Hu DN, Pan Z, et al. Curcumin protects against hyperosmoticity-induced IL-1beta elevation in human corneal epithelial cell via MAPK pathways. Exp Eye Res. 2010;90(3):437–43. doi: 10.1016/j.exer.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 191.Rogers NM, Kireta S, Coates PT. Curcumin induces maturation-arrested dendritic cells that expand regulatory T cells in vitro and in vivo. Clin Exp Immunol. 2010;162(3):460–73. doi: 10.1111/j.1365-2249.2010.04232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liang J, Sha SM, Wu KC. Role of the intestinal microbiota and fecal transplantation in inflammatory bowel diseases. J Dig Dis. 2014;15(12):641–6. doi: 10.1111/1751-2980.12211. [DOI] [PubMed] [Google Scholar]
- 193.Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–6. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sartor RB. The intestinal microbiota in inflammatory bowel diseases. Nestle Nutr Inst Workshop Ser. 2014;79:29–39. doi: 10.1159/000360674. [DOI] [PubMed] [Google Scholar]
- 195.Sun J, Furio L, Mecheri R, et al. Pancreatic beta-Cells Limit Autoimmune Diabetes via an Immunoregulatory Antimicrobial Peptide Expressed under the Influence of the Gut Microbiota. Immunity. 2015;43(2):304–17. doi: 10.1016/j.immuni.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 196.Razmpoosh E, Javadi M, Ejtahed HS, Mirmiran P. Probiotics as beneficial agents on the management of diabetes mellitus: a systematic review. Diabetes Metab Res Rev. 2015 doi: 10.1002/dmrr.2665. [DOI] [PubMed] [Google Scholar]