Familial Hypercholesterolemia (FH) is an autosomal dominant disorder characterized by severe elevations in LDL-C levels and a 20-fold increased risk of premature cardiovascular disease. Although multiple causative genes have been identified, most FH cases are due to mutations in the LDLR, PCSK9 or APOB genes 1. Recent studies suggest that many FH patients fail to achieve optimal LDL-C reduction despite high intensity statin use and the recent addition of PCSK9 inhibitors 1. AAV-based gene therapy is currently approved or in clinical trials for over 80 human diseases and to address the substantial gap in treatment for patients with severe FH, hepatic AAV-LDLR based gene therapy is currently in phase II clinical trials.
Long noncoding RNA (lnc)RNAs form the vast majority of transcriptionally active regions and are arbitrary defined as transcripts greater than 200 bps that biochemically resemble mRNA, yet do not template protein2. Although multiple lines of evidence implicate lncRNAs in a range of developmental processes and diseases, few lncRNA knockout studies have yielded robust phenotypes or demonstrated that proposed mechanisms are operational in vivo. Furthermore, lncRNA regulatory circuits have not been well explored for their potential therapeutic utility.
We recently showed that the lncRNA LeXis (Liver-expressed LXR-induced sequence) orchestrates crosstalk between Liver X Receptor and SREBP transcription factors to maintain hepatic sterol content and serum cholesterol levels 3. LeXis is robustly induced by Western diet or pharmacologic LXR activation and gates the promoter binding of RALY, a transcriptional coactivator for cholesterol biosynthetic genes in mouse liver (Figure 1A). In this work we utilize a gene therapy approach to investigate the long-term effects of LeXis expression in a mouse model of Familial Hypercholesterolemia (Figure 1B). We used an AAV8 vector expressing LeXis under the control of the human liver specific thyroxine-binding globulin (TBG) promoter. A similar vector encoding LDLR is currently under clinical investigation as a therapeutic tool for patients with homozygous FH (RGX-501, ClinicalTrials.gov NCT02651675). Treatment with AAV8.hTBG.LeXis led to sustained expression of LeXis and a significant reduction of Srebp2 and its target genes involved in cholesterol biosynthesis including Hmgcr in mouse liver (Figure 1C-D). Consistent with the gene expression changes we observed a striking difference in gross appearance of livers between mice receiving AAV8.hTBG.LeXis and AAV8.hTBG.GFP (Figure 1E). Livers from AAV8.hTBG.LeXis animals showed changes consistent with reduced lipid accumulation, which was corroborated with histologic analysis of liver sections (Figure 1E). Examination of serum lipids at the end of the study showed a significant decrease in total cholesterol and triglyceride levels in LeXis treated mice (Figure 1F-G). Finally, to determine if LeXis gene therapy had an effect on atherosclerosis we performed en face lesion analysis. Ldlr-/- animals treated with AAV8.hTBG.LeXis showed significantly reduced atherosclerotic burden compared with control-treated mice (Figure 1H-I). These results demonstrate the feasibility of a lncRNA mimetic therapeutic strategy for intervention in a chronic metabolic disease such as atherosclerosis.
Figure 1.
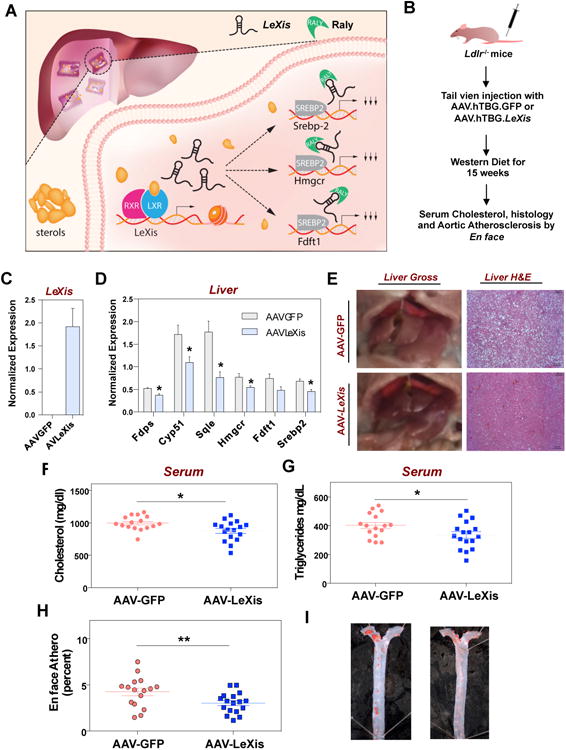
(A) Mechanism of action of LeXis in reducing cholesterol biosynthetic gene expression. (B) Schematic of AAV8 gene therapy vector. The LeXis sequence plus SV40 minimal polyadenylation signal was inserted into a pAAV8.hTBG vector and produced by the Penn Vector Core (C-D) Gene expression analysis using TRIzol (Invitrogen) isolated RNA from liver after injection with 1×1012 GC/mouse of AAV8.hTBG.GFP or AAV8.hTBG.LeXis. (E) Representative gross and histologic (H &E) appearance of livers after injection with 1×1012 GC/mouse of AAV8.hTBG.GFP or AAV8.hTBG.LeXis in Ldlr−/− mice on a Western diet. (F-G). Total serum cholesterol and triglycerides levels after 15 weeks in Western diet-fed in Ldlr−/− mice injected with AAV8.hTBG.GFP or AAV8.hTBG.LeXis (n=16-17/group). (H) Percentage of aorta surface area with atherosclerotic plaque in Ldlr−/− mice on Western diet after injection with 1×1012 GC/mouse of AAV8.hTBG.GFP or AAV8.hTBG.LeXis (n = 16-17/group). (I) Representative photographs from en face analysis of aortas Ldlr−/− mice on Western diet after injection with 1×1012 GC/mouse of AAV8.hTBG.GFP or AAV8.hTBG.LeXis. Paraffin-embedded aortic sections (en face) were quantified by computer-assisted image using Zeiss Stemi 508 microscope. Unpaired Student’s t test was used to determine statistical significance (*P < 0.05 and **P<0.01).
Although patients with FH are at considerably increased risk of cardiovascular events, the majority of these patients do not achieve guideline recommended LDL-C goals or >50% reduction in LDL-C levels. Adjunct lipid lowering therapies to established treatments are currently being evaluated in clinical trials, including the approach of LDLR cDNA to the liver. Some new agents for treatment of homozygous familial hyperlipidemia have shown substantial efficacy in reducing LDL-C but have raised concerns about long-term fatty liver development and hepatotoxicity 4. Although the magnitude of reduction in atherosclerosis was moderate in our study we did not observe evidence of hepatotoxicity. Furthermore, the efficacy was similar to miroRNA-based therapeutics in atherosclerosis5.
Our previous work has shown that a potential orthologue of LeXis exists in humans. Transcript TCONS1645 shows location and promoter conservation with LeXis, does not appear to code for any protein and is annotated as a noncoding transcript. Intriguingly TCONS1645 is induced with the activation of LXR in human hepatocyte cell lines 3. Future studies will focus on the functional conservation of LeXis and TCONS1645 transcript.
Elegant models have highlighted the contributions of lncRNAs as modulators of transcriptional control mechanisms, however their physiologic roles are less well defined owing to a lack of substantial phenotypes in vivo. The present study shows that gene therapy-facilitated expression of a lncRNA is feasible and that chronic LeXis production in the liver reduces serum cholesterol and atherosclerosis in a mouse model of familial hypercholesterolemia. The gene therapy approach we describe here may have clinical implications. Another striking finding from our study is protection against hepatic sterol overload in LeXis-treated mice. It is tempting to speculate that the same gene therapy strategy could be useful to mitigate fatty liver disease. Future studies will address that question in the relevant disease models as well as the ability of LeXis to modify cardiometabolic phenotypes in large animal models.
Acknowledgments
Funding Sources: This work was supported by grants HL066088 and HL128822 from the National Heart, Lung, and Blood Institute. Additional support provided by National Institute of Health grant 5U54GM114833, American College of Cardiology Presidential Career Development Award and the University of California, Los Angeles Cardiovascular Discovery Fund.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.deGoma EM, Ahmad ZS, O'Brien EC, Kindt I, Shrader P, Newman CB, Pokharel Y, Baum SJ, Hemphill LC, Hudgins LC, Ahmed CD, Gidding SS, Duffy D, Neal W, Wilemon K, Roe MT, Rader DJ, Ballantyne CM, Linton MF, Duell PB, Shapiro MD, Moriarty PM, Knowles JW. Treatment gaps in adults with heterozygous familial hypercholesterolemia in the united states: Data from the cascade-fh registry. Circ Cardiovasc Genet. 2016;9:240–249. doi: 10.1161/CIRCGENETICS.116.001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinn JJ, Chang HY. Unique features of long non-coding rna biogenesis and function. Nat Rev Genet. 2016;17:47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 3.Sallam T, Jones MC, Gilliland T, Zhang L, Wu X, Eskin A, Sandhu J, Casero D, Vallim TQ, Hong C, Katz M, Lee R, Whitelegge J, Tontonoz P. Feedback modulation of cholesterol metabolism by the lipid-responsive non-coding rna lexis. Nature. 2016;534:124–128. doi: 10.1038/nature17674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuchel M, Rader DJ. Lipid-lowering treatment for homozygous familial hypercholesterolaemia--authors' reply. Lancet. 2013;381:1183. doi: 10.1016/S0140-6736(13)60798-9. [DOI] [PubMed] [Google Scholar]
- 5.Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y, Fernandez-Hernando C, Fisher EA, Moore KJ. Antagonism of mir-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121:2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]


