Abstract
PURPOSE
To evaluate the therapeutic benefit of self-retained cryopreserved amniotic membrane (CAM) in conjunction with oral antiviral therapy in herpetic epithelial keratitis.
METHODS
Retrospective review of 4 patients with primary (1 eye) and recurrent (3 eyes) unilateral herpetic epithelial keratitis treated with CAM through the placement of PROKERA® Slim (Bio-Tissue, Miami, FL) (PKS) in conjunction with oral acyclovir. Their symptoms, conjunctival inflammation, corneal staining, and visual acuity were compared before and after treatment.
RESULTS
The herpetic epithelial keratitis presented as dendritic (3 eyes) and geographic (1 eye) epithelial lesions. Following epithelial debridement and placement of PKS for 5 ± 3.7 days, all patients reported a significant relief of symptoms, rapid corneal epithelization, and reduction of ocular surface inflammation. The visual acuity was also improved in all eyes from 0.7 ± 0.7 to 0.4 ± 0.7 logMAR (P = 0.2). They remained symptom-free during a follow-up period of 2.7 to 50.8 (20.3 ± 21.7) months.
CONCLUSIONS
PKS in conjunction with oral acyclovir facilitates the ease of early intervention to accelerate restoration of a normal corneal epithelium in herpetic epithelial keratitis.
Keywords: Amniotic membrane, Dendritic Keratitis, Geographic Keratitis, Herpetic Epithelial Keratitis, Ocular surface, Sutureless
INTRODUCTION
Herpes simplex virus (HSV) infection is an epidemiologically important cause of infectious and inflammatory disease in the world.1 Among all ocular manifestations of HSV, epithelial keratitis is the most frequent and conspicuous. Following HSV corneal infection, epithelial cells initiate immune response and inflammation.2 Activated macrophages produce cytokines such as interferon-alpha/beta (IFN-α/β) and are the main source of Interleukin-12(IL-12) and nitric oxide synthase (NOS) to produce NO.(reviewed in 3) T helper 1 (Th1) cytokines such as interferon-gamma (IFN-ɣ) and Interleukin-2 (IL-2) are elevated to enhance pro-inflammatory responses.4 Collectively, these robust immune response and toxic metabolites could cause serious damage to other corneal layers including corneal nerves.5 Therefore, it is important to formulate an effective strategy to accelerate restoration of the corneal health by promptly controlling viral infection, minimizing immune mediated damage, facilitating corneal epithelial regeneration, and preventing subsequent corneal scarring or neurotropic sequelae.
Antiviral medications are commonly used to hinder HSV replication and halt active viral infection of the cornea.6 Oral antiviral agents are as effective as topical antiviral agents in the treatment of HSV keratitis while topical antivirals may have toxic effects to the corneal epithelium.7 Additional use of topical corticosteroids or other immunosuppressive treatment in HSV epithelial keratitis is complicated by exacerbating dendritic to geographic epithelial keratitis, reactivating latent virus to trigger recurrence, and inducing side effects such as secondary bacterial infection, corneal melt, elevation of the intraocular pressure, or cataract.8 In this regard, it is innovative to consider using cryopreserved amniotic membrane (CAM) by deploying its anti-inflammatory, anti-scarring and anti-angiogenic effects known in treating many ocular surface diseases.9 This notion is further strengthened by many studies showing that CAM can effectively control inflammation in HSV stromal keratitis in a murine model of HSV1 necrotizing keratitis10–13 and in human epithelial and stromal HSV keratitis with14–16 or without17–21 an adjuvant antiviral therapy.
To facilitate early intervention by CAM, sutureless approach through the placement of PROKERA® (Bio-Tissue, Inc., Miami, FL, USA) has been developed. Within PROKERA® family, PROKERA® Slim (PKS) differs from PROKERA® Classic in the ring design where the same CAM is fastened by one polycarbonate ring and an elastic band for the former while by two polycarbonate rings for the latter. Consequently, the CAM enwraps the entire ring set in the former but partially in the latter to ensure the comfort. Herein, we present four cases of HSV epithelial keratitis to further illustrate how PKS can facilitate the ease of early intervention to accelerate restoration of the corneal health.
PATIENTS AND METHODS
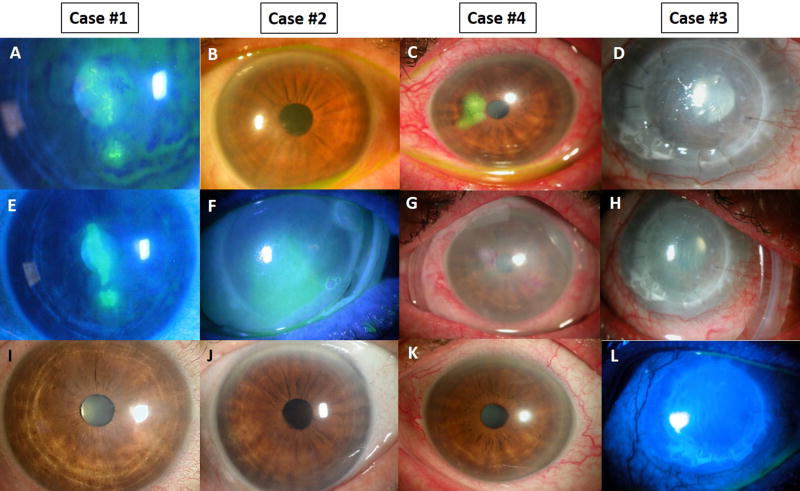
This study was approved by the ethics committee of the Ocular Surface Research and Education Foundation (Miami, FL, USA) according to the Tenets of the Declaration of Helsinki to retrospectively review four consecutive patients with HSV epithelial keratitis seen at Ocular Surface Center (Miami, FL, USA) from January 2012 to December 2016. The diagnosis of HSV infection was based primarily on the clinical findings. Three patients [Case #1 (Fig. 1A), #2 (Fig. 1B), and #4 (Fig. 1C)] had active dendritic epithelial keratitis presenting with multiple linear branches and terminal bulbs while one patient (Case #3) (Fig. 1D) developed macro-corneal ulcer of geographic epithelial keratitis. All HSV epithelial keratitis was typically unilateral without involvement of the contralateral eye. Table 1 summarizes the patients’ demographics, ocular comorbidity, number of previous episodes of epithelial keratitis and concomitant medication, and clinical manifestations including symptoms and signs at the presentation. Conjunctival inflammation was graded as none (0), mild (1), moderate (2), and severe (3). The corneal surface integrity was scored as clear (0), scattered superficial punctuate keratitis (SPK; 1+), moderate SPK (2+), and diffuse SPK or with corneal epithelial defects (3+). The debridement to remove the loosen and infected epithelium was performed in 3 patients except Case #4 with a dry Weck-Cel sponge (Beaver-Visitec International, Inc., Waltham, MA). For all four patients, self-retaining CAM via PROKERA® Slim (Bio-Tissue, Inc., Miami, FL, USA) (PKS) was placed in all 4 patients while receiving oral acyclovir 200 mg 5 times daily and topical 0.3% Ofloxacin (Allergan Irvine, CA) 3 times daily. The PKS was removed upon corneal epithelial healing and was followed by a bandage contact lens as needed. Descriptive statistics for continuous variables are reported as the mean ± SD and analyzed using SPSS software, version 20.0 (SPSS Inc., Chicago, IL). A P value less than 0.05 was considered statistically significant.
Figure 1. Clinical Outcome.
Herpetic epithelial keratitis manifested as corneal defect with multiple linear branches and terminal bulbs in Case #1, Case #2, Case #4 or geographic ulcer in Case #3 (top panel). Epithelial debridement was performed in Case #1, #2, and #3 (middle panel) in conjunction with oral acyclovir to accelerate restoration of the corneal epithelial integrity (bottom panel).
Table 1.
Summary of Relevant Clinical Data.
| Cases | Age (Years) |
Gender | Eye | Comorbidity | Prior HSV keratitis |
Number of
HSV keratitis episodes |
previous HSV
keratitis episode (months) |
prior medication |
Trigger factor(s) |
HSV Keratitis pattern |
Corneal debridement |
PKS placement duration (days) |
Symptoms | Conjunctival injection |
Visual Acuity |
Bandage contact lens replacement |
Follow up duration (months) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||
| Pre | Post | Pre | Post | Pre | Post | |||||||||||||||
|
| ||||||||||||||||||||
| 1 | 59 | F | R | − | + | 9 | 8 | Acyclovir 200 mg qd | Hordeolum | dendritic | + | 4 | pa, w, BV, irr | − | 1 | 1 | 20/50 | 20/20 | − | 2.7 |
| 2 | 83 | F | R | − | + | 5 | 5 | Acyclovir 200 mg qd | ? | dendritic | + | 5 | BV, irr | − | 1 | 0 | 20/50 | 20/25 | + | 7.0 |
| 3 | 73 | M | R | PKP | + | 1 | 53 | Acyclovir 200mg qd, Dexa bid | Dexa | geographic | + | 10 | pa, BV, irr, w | BV | 2 | 1 | HM | 20/400 | + | 20.5 |
| 4 | 70 | F | L | OCP | − | 0 | 0 | Dexa bid | Dexa | dendritic | − | 1 | pa,red, irr, BV, photo | irr | 3 | 1 | 20/30 | 20/25 | + | 50.8 |
Abbrevations: bid, twice a day; BV, blurred vision; Dexa, topical dexamethasone; F, female; HM, hand motion; HSV, herpes simplex virus; irr, irritation; L, left; M, male; mg, milligrams; OCP, ocular cicatricial phemphigoid; pa, pain; photo,; photophobia; PKP, penetrating keratoplasty; PKS, prokera slim; Post, immediately following PKS removal; Pre, before placement of PKS; qd, once every day; R, right; red, redness; VA, visual acuity; w, watery; +, present; −, absent; ?, unknown
The definition of conjuntival injection grading is detailed in the text.
RESULTS
As summarized in Table 1, these 4 patients included 3 females and one male with an average age of 71.3 ± 9.9 years. Except for Case #4, which was the first occurrence, three had a prior history of herpetic epithelial keratitis without stromal keratitis or belpharoconjunctivitis. Among these three, two patients (Case #1 and #2) experienced multiple recurrent episodes while one (Case #3) had only one previous episode that triggered corneal graft failure, thus requiring a repeated penetrating keratoplasty. For these 3 patients, herpetic epithelial keratitis recurred in an average of 6.5 ± 2.1 months while continuously receiving oral anti-viral therapy (Case #1, and #2) or 53 months after penetrating keratoplasty combined oral anti-viral treatment and topical non-preserved 0.1% dexamethasone (Ocular Surface Center, Miami, FL, USA) twice daily (Case #3).
At presentation, these patients complained of ocular pain, irritation, blurred vision, photophobia, redness, and tearing. Their vision measured by logMAR was all reduced (Table 1). Cases #1, #2, and #4 presented with a typical dendritic lesion consisting of multiple linear branches and terminal bulbs while Case #3 had a geographic ulcer (Fig. 1). Epithelial debridement followed by placement of PKS was performed uneventfully in all cases (Fig. 1). Following an average PKS placement duration of 5 ± 3.7 days (Fig. 1) in conjunction with topical Ofloxacin and oral acyclovir, all patients reported a significant relief of symptoms, rapid corneal epithelization, and reduction of ocular surface inflammation (Table 1). A single placement of PKS for 4, 5, 10 and 1 day(s) achieved complete epithelialization in 4, 5, 10, and 4 days in these four patients, respectively, based on the medical record available while all patients were not seen daily (Fig. 1). The visual acuity was also improved in all eyes from 0.7 ± 0.7 to 0.4 ± 0.7 logMAR (P = 0.2). They remained symptom-free during a follow-up period of 2.7 to 50.8 (20.3 ± 21.7) months.
DISCUSSION
Our results show that CAM through the placement of PKS in conjunction with oral acyclovir accelerates the recovery of a normal corneal epithelium in four patients with primary and recurrent HSV epithelial keratitis. Such a therapeutic benefit is consistent with what has been reported by CAM in an experimental murine model of HSV1 necrotizing keratitis.10–13 and human patients with HSV stromal keratitis,14–21 and epithelial keratitis.14,16 Our study further demonstrates that the sutureless approach via placement of PKS in the office facilitates the ease of early intervention to simplify the delivery of these therapeutic benefit of CAM. The sutureless approach of CAM is important because it can be placed in-office so as to avoid delays inherent to scheduling surgical procedures, suture-induced inflammation, and the risk of surgical complications as well as to facilitate timely intervention.
The therapeutic benefit observed herein in reducing inflammation and scarring as well as to promote epithelial wound healing is consistent with what has been summarized recently (reviewed in 22 and 23). In addition to the non-specific antiviral immunity yielded by the endogenous interferon in human placenta,24,25 recent study identified the board anti-inflammatory action in CAM applies to activated but not resting neutrophils,26,27 macrophages,27 and lymphocytes26 extending from innate to adaptive immune responses. The active matrix, termed heavy chain-hyaluronic acid/pentraxin 3 (HC-HA/PTX3), in CAM polarized macrophages toward the M2 including wound-healing (M2b) and anti-inflammatory (M2c) phenotype to significantly downregulate IL-12, TNF-α, and NOS as well as to the production of pro-inflammatory cytokines IFN-ɣ, and IL-2.28 Importantly, CAM is rich in nerve growth factor which not only reducing inflammation but also potentially facilitate the return of corneal nerves.9 Collectively, these actions explain why PKS in conjunction with oral acyclovir accelerates restoration of a normal corneal epithelium while reducing the potential adverse event from topical corticosteroids or other immunosuppressive treatment.
The average epithelialization duration of dendritic keratitis in our cases was 4.3 ± 0.6 days for dendritic keratitis and 5.75 ± 2.8 days for dendritic and geographic keratitis. The overall healing time appeared to be shorter when compared to the median time to healing with different antiviral-treated dendritic and geographic keratitis, i.e., 7 and 9 days, respectively.29 Future prospective studies to include a comparative no-CAM treatment control group will help substantiate whether the said beneficial effect of CAM can accelerate healing in herpetic epithelial keratitis.
Acknowledgments
Funding/Support: This study was supported in part by an unrestricted grant from Ocular Surface Research Education Foundation, Miami, FL. The development of PROKERA® was supported in part with grant number EY014768 from the National Institute of Health (NIH) and National Eye Institute (NEI). The content is solely the responsibility of the authors and does not necessarily represent the opinion of the NIH or the NEI.
Footnotes
Financial Disclosure: Dr. Tseng is the founder and a major shareholder of Tissue Tech Inc. that holds patents on the methods of preservation and clinical uses of amniotic membrane graft and PROKERA®.
References
- 1.Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Li H, Zhang J, Kumar A, et al. Herpes simplex virus 1 infection induces the expression of proinflammatory cytokines, interferons and TLR7 in human corneal epithelial cells. Immunology. 2006;117:167–176. doi: 10.1111/j.1365-2567.2005.02275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowe AM, St Leger AJ, Jeon S, et al. Herpes keratitis. Prog Retin Eye Res. 2013;32:88–101. doi: 10.1016/j.preteyeres.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inoue T, Inoue Y, Kosaki R, et al. Immunohistological study of infiltrated cells and cytokines in murine herpetic keratitis. Acta ophthalmologica Scandinavica. 2001;79:484–487. doi: 10.1034/j.1600-0420.2001.790511.x. [DOI] [PubMed] [Google Scholar]
- 5.Hamrah P, Sahin A, Dastjerdi MH, et al. Cellular changes of the corneal epithelium and stroma in herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2012;119:1791–1797. doi: 10.1016/j.ophtha.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones BR, Coster DJ, Fison PN, et al. Efficacy of acycloguanosine (Wellcome 248U) against herpes-simplex corneal ulcers. Lancet (London, England) 1979;1:243–244. doi: 10.1016/s0140-6736(79)90769-4. [DOI] [PubMed] [Google Scholar]
- 7.Hung SO, Patterson A, Rees PJ. Pharmacokinetics of oral acyclovir (Zovirax) in the eye. Br J Ophthalmol. 1984;68:192–195. doi: 10.1136/bjo.68.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barequet IS, Wasserzug Y. Herpes simplex keratitis after cataract surgery. Cornea. 2007;26:615–617. doi: 10.1097/ICO.0b013e318033a708. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Sheha H, Fu Y, et al. Update on amniotic membrane transplantation. Expert Rev Ophthalmol. 2010;5:645–661. doi: 10.1586/eop.10.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heiligenhaus A, Bauer D, Meller D, et al. Improvement of HSV-1 necrotizing keratitis with amniotic membrane transplantation. Invest Ophthal Vis Sci. 2001;42:1969–1974. [PubMed] [Google Scholar]
- 11.Bauer D, Wasmuth S, Hennig M, et al. Amniotic membrane transplantation induces apoptosis in T lymphocytes in murine corneas with experimental herpetic stromal keratitis. Invest Ophthal Vis Sci. 2009;50:3188–3198. doi: 10.1167/iovs.08-3041. [DOI] [PubMed] [Google Scholar]
- 12.Bauer D, Hennig M, Wasmuth S, et al. Amniotic membrane induces peroxisome proliferator-activated receptor-gamma positive alternatively activated macrophages. Invest Ophthal Vis Sci. 2012;53:799–810. doi: 10.1167/iovs.11-7617. [DOI] [PubMed] [Google Scholar]
- 13.Bauer D, Wasmuth S, Hermans P, et al. On the influence of neutrophils in corneas with necrotizing HSV-1 keratitis following amniotic membrane transplantation. Exp Eye Res. 2007;85:335–345. doi: 10.1016/j.exer.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JS, Kim JC, Hahn TW, et al. Amniotic membrane transplantation in infectious corneal ulcer. Cornea. 2001;20:720–726. doi: 10.1097/00003226-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Shi W, Chen M, Xie L. Amniotic membrane transplantation combined with antiviral and steroid therapy for herpes necrotizing stromal keratitis. Ophthalmology. 2007;114:1476–1481. doi: 10.1016/j.ophtha.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann S, Szentmary N, Seitz B. Amniotic Membrane Transplantation for the Treatment of Infectious Ulcerative Keratitis Before Elective Penetrating Keratoplasty. Cornea. 2013;32:1321–1325. doi: 10.1097/ICO.0b013e318298de10. [DOI] [PubMed] [Google Scholar]
- 17.Spira C, Szentmary N, Hasenfus A, et al. [Therapy refractory stromal Herpes keratitis under aciclovir] Ophthalmologe. 2014;111:654–659. doi: 10.1007/s00347-013-2941-8. [DOI] [PubMed] [Google Scholar]
- 18.Kruse FE, Rohrschneider K, Völcker HE. Multilayer amniotic membrane transplantation for reconstruction of deep corneal ulcers. Ophthalmology. 1999;106:1504–1511. doi: 10.1016/S0161-6420(99)90444-X. [DOI] [PubMed] [Google Scholar]
- 19.Meller D, Thomasen H, Steuhl K. [Amniotic membrane transplantation in herpetic corneal infections] Klin Monbl Augenheilkd. 2010;227:393–399. doi: 10.1055/s-0029-1245339. [DOI] [PubMed] [Google Scholar]
- 20.Heiligenhaus A, Li H, Hernandez Galindo EE, et al. Management of acute ulcerative and necrotising herpes simplex and zoster keratitis with amniotic membrane transplantation. Br J Ophthalmol. 2003;87:1215–1219. doi: 10.1136/bjo.87.10.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H-J, Pires RT, Tseng SC. Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br J Ophthalmol. 2000;84:826–833. doi: 10.1136/bjo.84.8.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tseng SC, He H, Zhang S, et al. Niche Regulation of Limbal Epithelial Stem Cells: Relationship between Inflammation and Regeneration. Ocul Surf. 2016;14:100–112. doi: 10.1016/j.jtos.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng SC. HC-HA/PTX3 Purified From Amniotic Membrane as Novel Regenerative Matrix: Insight Into Relationship Between Inflammation and Regeneration. Invest Ophthal Vis Sci. 2016;57 doi: 10.1167/iovs.15-17637. ORSFh1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franco GR, de Carvalho AF, Kroon EG, et al. Biological activities of a human amniotic membrane interferon. Placenta. 1999;20:189–196. doi: 10.1053/plac.1998.0364. [DOI] [PubMed] [Google Scholar]
- 25.Paradowska E, Blach-Olszewska Z, Sender J, et al. Antiviral nonspecific immunity of human placenta at term: possible role of endogenous tumor necrosis factors and interferons. J Interferon Cytokine Res. 1996;16:941–948. doi: 10.1089/jir.1996.16.941. [DOI] [PubMed] [Google Scholar]
- 26.He H, Li W, Tseng DY, et al. Biochemical characterization and function of complexes formed by hyaluronan and the heavy chains of inter-alpha-inhibitor (HC*HA) purified from extracts of human amniotic membrane. J Biol Chem. 2009;284:20136–20146. doi: 10.1074/jbc.M109.021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He H, Zhang S, Tighe S, et al. Immobilized Heavy Chain-Hyaluronic Acid Polarizes Lipopolysaccharide-activated Macrophages toward M2 Phenotype. J Biol Chem. 2013;288:25792–25803. doi: 10.1074/jbc.M113.479584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He H, Tan Y, Duffort S, et al. In vivo downregulation of innate and adaptive immune responses in corneal allograft rejection by HC-HA/PTX3 complex purified from amniotic membrane. Invest Ophthal Vis Sci. 2014;55:1647–1656. doi: 10.1167/iovs.13-13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilhelmus KR. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst Rev. 2015;1:Cd002898. doi: 10.1002/14651858.CD002898.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]