Abstract
Amygdala and prefrontal cortex (PFC) function subserving emotional behavior has largely been examined from the perspective of their adult roles, with a tremendous focus on the regulatory influence of the PFC over amygdala activity. Here we consider the circuit’s function in its developmental context, when maximal learning about emotion and incentives from the environment is necessary. We argue that during development the amygdala exhibits an overwhelming influence over the developmental destiny of circuitry function, and the amygdala’s learning and experiential history are conveyed to the cortex to modulate subsequent PFC development. We present recent findings on the different developmental trajectories of the amygdala and PFC, their functional connectivity, and the timing of environmental influences as evidence supporting our position.
Introduction
Approximate 200 years ago, William Wordsworth noted that the “child is the father of the man.” In neuroscience, this quote reminds us that the temporal history of developmental events is utterly informative when interrogating the functioning of the mature brain. Knowledge of which regions exhibited behaviorally-relevant function earlier versus later and how their communication evolved over ontogenetic time provides a basis for understanding the construction of the mature circuit [1**]. Emotional behaviors in adulthood, including arousal, regulation, and decision making, rely heavily on the bidirectional connections between the amygdala and the prefrontal cortex (PFC)[2, 3]. The empirical research of this relationship has overwhelmingly focused on the PFC regulation or “control” over the amygdala. While this top-down relationship is clearly critical for understanding modulation of amygdala function [4–7], it is a perspective that depends on the adult vantage point of mature cortex. Moreover, this unilateral focus does not provide a complete account of amygdala-PFC connectivity since the “bottom-up” amygdala to PFC structural connections are quite robust in adulthood, even compared with top-down connections [8]. However, when the amygdala-PFC circuit is considered in its developmental context, it is clear that amygdala function is very much “in control” of the circuit’s functioning (Fig 1) and may serve as a biological “tutor” to the later developing cortex. In this paper, we argue that the amygdala’s early development, together with its high sensitivity to environmental exposures, and its massive interconnectivity with the whole cortex [9] position it for learning about the emotional world and conveying that information along with ontogenetic influences to the later developing prefrontal cortex.
Figure 1.
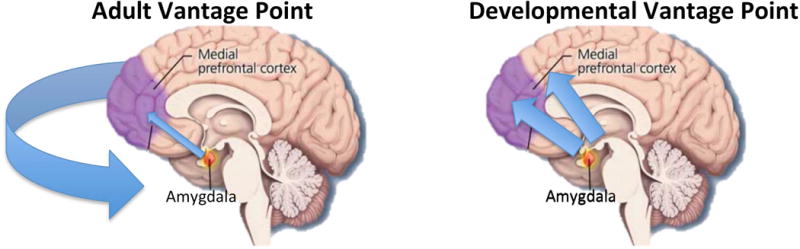
The relationship between amygdala and prefrontal cortex from different developmental vantage points.
The amygdala’s function across the lifespan is to identify and affectively learn about important events in the environment [10] that are emotionally important or motivationally relevant [11]. Both affective learning and associated amygdala activity increase under conditions of ambiguity or uncertainty [12–14]. This uncertainty is elicited, for example, by presentations of fearful [15] or surprised faces [16], which contain more ambiguous information than angry faces [17]. The amygdala is also a central mediator of threat conditioning paradigms [18–21]. Importantly, the amygdala is most reactive during the uncertain phases of affective learning, including the conditioning paradigm phases of initial acquisition or extinction [22, 23]. As predicted by traditional models of learning (e.g., Resorla-Wagner[24]), high uncertainty and arousal facilitates the learning rate. Given the importance of uncertainty in amygdala-based learning even in maturity, we can imagine the affective learning context of the young child. Never is the world as uncertain and never is there more to learn than in early life, when we are new to this planet. Compared to adulthood, childhood may be likened to the early trials of a lifelong learning paradigm – emotional stimuli should be more salient, novel, and surprising, and learning should occur at a very high rate for children, who do indeed exhibit very rapid learning, including aversive conditioning, under novel conditions [25**;26]. Here, we consider the developmental trajectories of the amygdala-PFC circuitry to illustrate how this early learning manifests in the brain. In this opinions paper, we focus on affective learning, given its significant associations with functioning of amygdala-PFC circuitry.
Amygdala shows early functionality
Structurally, the amygdala develops rapidly during postnatal life [27, 28], providing a plausible neurobiological mechanism to support this massive learning early in life. Functional magnetic resonance imaging has also revealed that the amygdala exhibits early functionality. Specifically, studies have revealed a robust functional responsiveness to emotional stimuli by early childhood, which is larger in magnitude than the responses of older individuals [29–33]. Consistent with these findings, a rich animal and human literature examining the effect of both lesions and stress on the amygdala across the lifespan suggest that this region’s role in shaping emotion and social behavior is especially important during early postnatal development, with consequences for affective behavior lasting throughout life [34–38]. These findings suggest a sensitive period for human amygdala development in the late infancy to childhood period (see Fig 2, top and middle) when the amygdala is particularly receptive to environmental stimulation.
Figure 2.
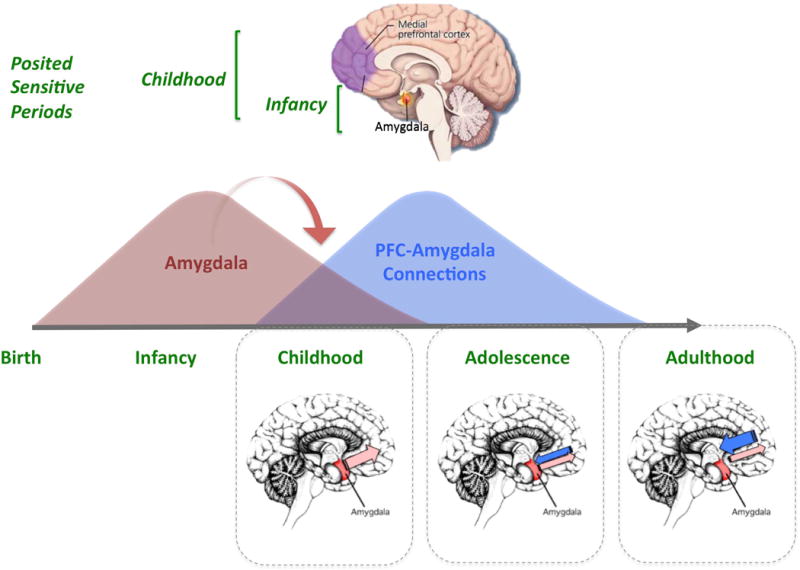
(top) Posited sensitive periods for development, with amygdala. (middle) Hierarchical organization of amygdala and amygdala-PFC development, with an influence (arrow) of amygdala development on the nature of PFC-amygdala development. (bottom) Model for the nature of amygdala-PFC connectivity across development.
The amygdala as a student
Taken together, the developmental literature has been pointing to late infancy and childhood as sensitive period when amygdala circuitry function can be phasically modulated by stimuli to elicit childhood-specific connectivity phenotypes [39–41]. So what are these important experiences that guide amygdala (and downstream cortical) development? In the case of altricial species (i.e., animals born in an underdeveloped state that require parental care for survival), such as the human, there is likely no other stimulus as powerful as the parent. The parent is the conduit of emotional learning during the sensitive period in amygdala development [42], and is itself a powerful motivating reinforcer [43]. The parent can both attenuate (e.g., parental buffering [44*]) and amplify [45*] affective behavior as well as amygdala engagement by the environment. Prior work has shown that during childhood, parental presence increases children’s likelihood of entering a novel [46] or threatening situation (e.g., new school), and decreases nighttime fears [47].
Findings from the rodent literature show that the presence/absence of the parent (always the mother for rats) has dramatic effects on the resting activity of the pup’s amygdala [48] and stimulus-elicited function. When a rat pup without its mother is presented with an odor + shock pairing, it will quickly learn an aversion to the odor. However, in the physical presence of the mother, aversive learning is effectively blocked [49], and the rat pup even shows a paradoxical preference for the odor. This point is important because it shows that the mother actively guides aversive learning, not merely distracting the pup. Blocking of aversive learning by the regulated parent (in this case, the parent is anesthetized) occurs because the parent is a powerful stimulus that prevents activation of threat-related catecholaminergic systems and the hypothalamic pituitary adrenal axis, thereby preventing amygdala engagement during learning [49]. This buffering is important because it protects the infant from potentially deleterious amygdala over-excitation during its sensitive period and from ensuing affective deregulation. On the other hand, a dysregulated parent (exhibiting fear) is highly effective in increasing amygdala engagement and aversive learning about environmental stimuli [45, 50]. Evidence from humans is strikingly consistent with the rodent work. As in the developing rodent, it has been shown that parental presence buffers against children’s elevations in stress hormones [51], parental stimuli attenuate amygdala activity in children [52*], and fear expressed by the parent directly translates into fear related behaviors in the child [50]. Thus the parent is a highly effective regulator during development, with the ability to potentiate or attenuate amygdala function.
PFC shows relatively late functionality
This strong amygdala response in childhood occurs prior to the development of regulatory connections with the prefrontal cortex (PFC)[29]. In adulthood, the medial PFC sends projections to inhibitory cells within the amygdala that reduce amygdala reactivity and are thus fundamental to mature affect regulation [53, 54]. Unlike in the adult, these regulatory influences from the medial PFC are not available to the young child. Several studies have now shown that functional connections between the amygdala and the medial PFC are immature in childhood[30, 32, 55]and switch to the adult-like state in adolescence (Fig 2, bottom)[29, 56–59]. That is, during childhood the amygdala is less likely to be regulated by the mPFC than after childhood. Instead, during infancy and childhood, external agents (e.g., caregivers) can serve as social regulators of affect and amygdala activity during this time.
The Amygdala as a teacher
Given the hierarchical nature of amygdala-PFC circuitry development and the amygdala’s learning function, we posit that affective development is highly dependent on a cascade of events that includes the early environment influencing amygdala development, and the amygdala subsequently influencing the development of the cortex [60]. This position is supported by anatomical tracings in rodent studies, which have shown that amygdala projections to the PFC emerge earlier than the reciprocal PFC projections to amygdala [61, 62]. Moreover, novel, in-vivo observation of neuroplasticity in the rodent has shown surging levels of malleable connectivity between the mPFC and amygdala during the late juvenile period, consistent with a juvenile sensitive period for the functional circuitry [63*, 64*]. Developmental lesion studies in primates also support this position. For example, amygdala lesions during the neonatal period in rhesus monkeys produce aberrant neural development in the PFC in adult monkeys, whereas analogous amygdala lesions in adulthood did not produce these same PFC alterations [65]. In humans, childhood-timed amygdala lesions resulting from the congenital disease Urbach-Wiethe are associated with impairments in explicit facial affect processing, while lesions incurred in adulthood do not result in these same impairments [66]. Similarly, childhood-timed lesions leave intact PFC-dependent somatic responses for memories of events prior to amygdala damage, but not for events that occurred later [67], consistent with the idea that the amygdala’s information transfers to the cortex across development.
While this hypothesis requires significant continued testing, patterns of amygdala-cortical development observed with functional neuroimaging suggest that early amygdala function might predict later patterns of PFC connections. First, the temporal patterning in typical development is suggestive of the amygdala’s cascading influence on later cortical development. During the transition between childhood and adolescence, adult-like connections between the PFC and amygdala begin to emerge. However, this transition is preceded developmentally by a very large amygdala response to environmental stimuli during childhood, which attenuates thereafter. This pattern could reflect the amygdala’s influence on PFC connectivity development, but it could also reflect PFC connections developing independently of amygdala input. However, work with samples of children who exhibit altered timing of amygdala development suggest the former. Under conditions of atypically high amygdala reactivity, for example following exposures to early life stress [56, 68–71], adult-like connections between the PFC and amygdala seem to appear at a developmentally earlier point [56, 72]. This pattern of accelerated PFC-amygdala connectivity following heightened amygdala reactivity is consistent with the hypothesis that amygdala function has hierarchical influences on the development of connections with the PFC.
Recently, it has been demonstrated through sequential, longitudinal tracking across a 2-year period that the functional architecture of resting-state PFC-amygdala connectivity is prospectively predicted by the nature of initial stimulus-elicited PFC-amygdala connectivity, particularly during childhood [73**]. This finding suggests that the nature of resting-state functional architecture arises from phasic patterns of functional connectivity elicited by environmental stimuli over the course of development on the order of years. Taken together, the human and the animal developmental literatures provide increasing support for the hypothesis that initial amygdala functioning “instructs” the course of PFC development.
Feldman Barrett and colleagues [74**] have proposed an Embodied Predictive Interoception Coding theory, which posits that in adulthood, limbic signals make predictions regarding the external world based on experiential histories, rather than simply reacting to environmental stimuli, and these predictions or “anticipations” are then transmitted to high-level cortical regions. The framework of this theory can be very helpful when considering the developmental model presented in this current opinions article. During development, the amygdala may be particularly active in learning about one’s unique environment. The structural and functional consequences of these early experiences on the amygdala endure and may serve as a foundation for the nature of affective predictions that transmit to cortical regions throughout maturity. That is, the early experiences can inform the development of the brain’s internal model of the world and of the internal milieu [75] that guides future behaviors in maturity. These predictions can surely be updated by subsequently occurring experiences later in life, but the impact of the early environment on subsequent amygdala development is massive.
Concluding Remarks
We have previously provided arguments for the importance of early experiences on amygdala development [41, 76] and suggested late infancy and childhood may comprise sensitive periods for development. The evidence is clear that the amygdala develops earlier than the PFC, which places it in a powerful position for helping to entrain the PFC and its connections with the amygdala (either directly through bottom-up connections or indirectly by modulating attentional mechanisms). Studies need to be performed that identify the mechanisms of this influence in humans. Here, we extend these ideas by considering the amygdala-PFC circuit and suggesting that early experiences are important not only for amygdala development, but also for their amygdala-mediated downstream effects on subsequent PFC development.
Sensitive periods posited for amygdala-prefrontal cortex circuit construction.
Early amygdala function contributes to future amygdala-prefrontal cortex function.
Heavy influences of early caregiving experiences are considered.
Acknowledgments
The project described was supported by grant number R01MH091864 (NT) from the National Institute of Mental Health, the Dana Foundation (NT), an NSF Conference Grant conference grant (BCS-1439258, co-I NT), and the Graduate Research Fellowship (LJGD) from the National Science Foundation. The content is solely the responsibility of the authors and does not necessarily represent the views of funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest to be reported for the authors, Nim Tottenham & Laurel Gabard-Durnam, of the article “The developing amygdala: a student of the world and a teacher of the cortex”/
References
- 1**.Casey BJ. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu Rev Psychol. 2015;66:295–319. doi: 10.1146/annurev-psych-010814-015156. This review presents several models depicting developmental change in subcortical and cortical regions across childhood, adolescence, and adulthood. [DOI] [PubMed] [Google Scholar]
- 2.Buhle JT, et al. Cognitive reappraisal of emotion: a meta-analysis of human neuroimagingstudies. Cereb Cortex. 2014;24(11):2981–90. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith DV, et al. Toward a cumulative science of functional integration: A meta-analysis of psychophysiological interactions. Hum Brain Mapp. 2016;37(8):2904–17. doi: 10.1002/hbm.23216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banks SJ, et al. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2(4):303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim MJ, et al. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. 2011;223(2):403–10. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motzkin JC, et al. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry. 2015;77(3):276–84. doi: 10.1016/j.biopsych.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34(3):905–23. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggleton JP, et al. Complementary Patterns of Direct Amygdala and Hippocampal Projections to the Macaque Prefrontal Cortex. Cereb Cortex. 2015;25(11):4351–73. doi: 10.1093/cercor/bhv019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hariri AR, Whalen PJ. The amygdala: inside and out. F1000 Biol Rep. 2011;3:2. doi: 10.3410/B3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herry C, et al. Processing of temporal unpredictability in human and animal amygdala. J Neurosci. 2007;27(22):5958–66. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whalen PJ. Fear, vigilance, and ambiguity: Initial neuroimaing studies of the human amygdala. Current Directions in Psychological Science. 1998;7(6):177–188. [Google Scholar]
- 13.Madarasz TJ, et al. Evaluation of ambiguous associations in the amygdala by learning the structure of the environment. Nat Neurosci. 2016;19(7):965–72. doi: 10.1038/nn.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci. 2013;14(7):488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whalen PJ, et al. Neuroscience and Facial Expressions of Emotion: The Role of Amygdala-Prefrontal Interactions. Emotion Review. 2013;5(1):78–83. [Google Scholar]
- 16.Kim H, et al. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14(18):2317–22. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- 17.Whalen PJ, et al. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1(1):70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- 18.Pine DS, et al. Methods for developmental studies of fear conditioning circuitry. Biol Psychiatry. 2001;50(3):225–8. doi: 10.1016/s0006-3223(01)01159-3. [DOI] [PubMed] [Google Scholar]
- 19.Hermans EJ, et al. Persistence of Amygdala-Hippocampal Connectivity and Multi-Voxel Correlation Structures During Awake Rest After Fear Learning Predicts Long-Term Expression of Fear. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson DC, Casey BJ. Easy to remember, difficult to forget: the development of fear regulation. Dev Cogn Neurosci. 2015;11:42–55. doi: 10.1016/j.dcn.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silvers JA, et al. Previous Institutionalization Is Followed by Broader Amygdala-Hippocampal-PFC Network Connectivity during Aversive Learning in Human Development. J Neurosci. 2016;36(24):6420–30. doi: 10.1523/JNEUROSCI.0038-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng DT, Richards J, Helmstetter FJ. Activity in the human amygdala corresponds to early, rather than late period autonomic responses to a signal for shock. Learn Mem. 2007;14(7):485–90. doi: 10.1101/lm.632007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phelps EA, et al. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 24.Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black A, Prokasy W, editors. Classical Conditioning II. Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- 25**.Gopnik A, Bonawitz E. Bayesian models of child development. Wiley Interdiscip Rev Cogn Sci. 2015;6(2):75–86. doi: 10.1002/wcs.1330. This theoretical paper uses principles from Bayesian learning to describe mechanisms of learning in young children. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, et al. The development of skin conductance fear conditioning in children from ages 3 to 8 years. Dev Sci. 2010;13(1):201–12. doi: 10.1111/j.1467-7687.2009.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilmore JH, et al. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cereb Cortex. 2012;22(11):2478–85. doi: 10.1093/cercor/bhr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Payne C, et al. Maturation of the hippocampal formation and amygdala in Macaca mulatta: A volumetric magnetic resonance imaging study. Hippocampus. 2009 doi: 10.1002/hipo.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gee DG, et al. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci. 2013;33(10):4584–93. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swartz JR, et al. Age-related changes in the structure and function of prefrontal cortex-amygdala circuitry in children and adolescents: a multi-modal imaging approach. Neuroimage. 2014;86:212–20. doi: 10.1016/j.neuroimage.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silvers JA, et al. The transition from childhood to adolescence is marked by a general decrease in amygdala reactivity and an affect-specific ventral-to-dorsal shift in medial prefrontal recruitment. Dev Cogn Neurosci. 2016 doi: 10.1016/j.dcn.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Decety J, Michalska KJ, Kinzler KD. The contribution of emotion and cognition to moral sensitivity: a neurodevelopmental study. Cereb Cortex. 2012;22(1):209–20. doi: 10.1093/cercor/bhr111. [DOI] [PubMed] [Google Scholar]
- 33.Vink M, et al. Functional differences in emotion processing during adolescence and early adulthood. Neuroimage. 2014;91:70–6. doi: 10.1016/j.neuroimage.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 34.Hanson JL, et al. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol Psychiatry. 2015;77(4):314–23. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. Journal of cognitive neuroscience. 2002;14:1264–74. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- 36.Raper J, et al. Sex-dependent role of the amygdala in the development of emotional and neuroendocrine reactivity to threatening stimuli in infant and juvenile rhesus monkeys. Horm Behav. 2013;63(4):646–58. doi: 10.1016/j.yhbeh.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Telzer EH, et al. Early experience shapes amygdala sensitivity to race: an international adoption design. J Neurosci. 2013;33(33):13484–8. doi: 10.1523/JNEUROSCI.1272-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noble KG, et al. Neural correlates of socioeconomic status in the developing human brain. Dev Sci. 2012;15(4):516–27. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyons-Ruth K, et al. Disorganized attachment in infancy predicts greater amygdala volume in adulthood. Behav Brain Res. 2016;308:83–93. doi: 10.1016/j.bbr.2016.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Callaghan BL, Tottenham N. The Neuro-Environmental Loop of Plasticity: A Cross-Species Analysis of Parental Effects on Emotion Circuitry Development Following Typical and Adverse Caregiving. Neuropsychopharmacology. 2016;41(1):163–76. doi: 10.1038/npp.2015.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tottenham N. The importance of early experiences for neuro-affective development. Curr Top Behav Neurosci. 2014;16:109–29. doi: 10.1007/7854_2013_254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Upton KJ, Sullivan RM. Defining age limits of the sensitive period for attachment learning in rat pups. Dev Psychobiol. 2010;52(5):453–64. doi: 10.1002/dev.20448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lebowitz ER, et al. Cross-generational influences on childhood anxiety disorders: pathways and mechanisms. J Neural Transm (Vienna) 2016;123(9):1053–67. doi: 10.1007/s00702-016-1565-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44*.Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol Bull. 2014;140(1):256–82. doi: 10.1037/a0032671. This paper presents a working conceptual model focused on the role of early parental buffering experiences as critical in shaping emotional development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Debiec J, Sullivan RM. Intergenerational transmission of emotional trauma through amygdala-dependent mother-to-infant transfer of specific fear. Proc Natl Acad Sci U S A. 2014;111(33):12222–7. doi: 10.1073/pnas.1316740111. With a threat conditioning paradigm, these authors show how maternal expression of emotion can be transmitted to her offspring. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorce JF, Emde RN. Mothers Presence Is Not Enough – Effect of Emotional Availability on Infant Exploration. Developmental Psychology. 1981;17(6):737–745. [Google Scholar]
- 47.Simard V, et al. Longitudinal study of preschool sleep disturbance: the predictive role of maladaptive parental behaviors, early sleep problems, and child/mother psychological factors. Arch Pediatr Adolesc Med. 2008;162(4):360–7. doi: 10.1001/archpedi.162.4.360. [DOI] [PubMed] [Google Scholar]
- 48.Sarro EC, Wilson DA, Sullivan RM. Maternal regulation of infant brain state. Curr Biol. 2014;24(14):1664–9. doi: 10.1016/j.cub.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sullivan RM, Perry RE. Mechanisms and functional implications of social buffering in infants: Lessons from animal models. Soc Neurosci. 2015;10(5):500–11. doi: 10.1080/17470919.2015.1087425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerull FC, Rapee RM. Mother knows best: effects of maternal modelling on the acquisition of fear and avoidance behaviour in toddlers. Behavior Research Therpy. 2002;40:279–287. doi: 10.1016/s0005-7967(01)00013-4. [DOI] [PubMed] [Google Scholar]
- 51.Kertes DA, et al. Inhibited temperament and parent emotional availability differentially predict young children’s cortisol responses to novel social and nonsocial events. Dev Psychobiol. 2009;51(7):521–32. doi: 10.1002/dev.20390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Gee DG, et al. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol Sci. 2014;25(11):2067–78. doi: 10.1177/0956797614550878. Using an fMRI paradigm, these authors show that amygdala response is highly modulated by parental cues in childhood, but not adolescence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delgado MR, et al. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59(5):829–38. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Motzkin JC, et al. Ventromedial prefrontal cortex damage alters resting blood flow to the bed nucleus of stria terminalis. Cortex. 2015;64:281–8. doi: 10.1016/j.cortex.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perlman SB, Pelphrey KA. Developing connections for affective regulation: age-related changes in emotional brain connectivity. J Exp Child Psychol. 2011;108(3):607–20. doi: 10.1016/j.jecp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gee DG, et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A. 2013;110(39):15638–43. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gabard-Durnam LJ, et al. The development of human amygdala functional connectivity at rest from 4 to 23years: A cross-sectional study. Neuroimage. 2014;95C:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qin S, et al. Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biol Psychiatry. 2014;75(11):892–900. doi: 10.1016/j.biopsych.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu M, et al. Age-related changes in amygdala-frontal connectivity during emotional face processing from childhood into young adulthood. Hum Brain Mapp. 2016;37(5):1684–95. doi: 10.1002/hbm.23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tottenham N. Social scaffolding of human amygdala-mPFCcircuit development. Soc Neurosci. 2015;10(5):489–99. doi: 10.1080/17470919.2015.1087424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bouwmeester H, Smits K, Van Ree JM. Neonatal development of projections to the basolateral amygdala from prefrontal and thalamic structures in rat. J Comp Neurol. 2002;450(3):241–55. doi: 10.1002/cne.10321. [DOI] [PubMed] [Google Scholar]
- 62.Bouwmeester H, Wolterink G, van Ree JM. Neonatal development of projections from the basolateral amygdala to prefrontal, striatal, and thalamic structures in the rat. J Comp Neurol. 2002;442(3):239–49. doi: 10.1002/cne.10084. [DOI] [PubMed] [Google Scholar]
- 63*.Pattwell SS, et al. Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nat Commun. 2016;7:11475. doi: 10.1038/ncomms11475. Using in vivo microprisms, these authors identify a sensitive period for fear neurobiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64*.Johnson CM, et al. Long-range orbitofrontal and amygdala axons show divergent patterns of maturation in the frontal cortex across adolescence. Dev Cogn Neurosci. 2016;18:113–20. doi: 10.1016/j.dcn.2016.01.005. Using two-photon imaging to make time-lapse observations, these authors track region specific development of afferents between amygdala and PFC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bertolino A, et al. Altered development of prefrontal neurons in rhesus monkeys with neonatal mesial temporo-limbic lesions: a proton magnetic resonance spectroscopic imaging study. Cereb Cortex. 1997;7(8):740–8. doi: 10.1093/cercor/7.8.740. [DOI] [PubMed] [Google Scholar]
- 66.Hamann SB, Adolphs R. Normal recognition of emotional similarity between facial expressions following bilateral amygdala damage. Neuropsychologia. 1999;37(10):1135–41. doi: 10.1016/s0028-3932(99)00027-5. [DOI] [PubMed] [Google Scholar]
- 67.Bechara A, Damasio H, Damasio AR. Role of the amygdala in decision-making. Ann N Y Acad Sci. 2003;985:356–69. doi: 10.1111/j.1749-6632.2003.tb07094.x. [DOI] [PubMed] [Google Scholar]
- 68.Tottenham N, et al. Elevated Amygdala Response to Faces Following Early Deprivation. Developmental Science. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCrory EJ, et al. Amygdala activation in maltreated children during pre-attentive emotional processing. Br J Psychiatry. 2013;202(4):269–76. doi: 10.1192/bjp.bp.112.116624. [DOI] [PubMed] [Google Scholar]
- 70.Maheu FS, et al. A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cogn Affect Behav Neurosci. 2010;10(1):34–49. doi: 10.3758/CABN.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Bellis MD, Hooper SR. Neural substrates for processing task-irrelevant emotional distracters in maltreated adolescents with depressive disorders: a pilot study. J Trauma Stress. 2012;25(2):198–202. doi: 10.1002/jts.21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolf RC, Herringa RJ. Prefrontal-Amygdala Dysregulation to Threat in Pediatric Posttraumatic Stress Disorder. Neuropsychopharmacology. 2016;41(3):822–31. doi: 10.1038/npp.2015.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73**.Gabard-Durnam LJ, et al. Stimulus-Elicited Connectivity Influences Resting-State Connectivity Years Later in Human Development: A Prospective Study. J Neurosci. 2016;36(17):4771–84. doi: 10.1523/JNEUROSCI.0598-16.2016. Using a longitudinal, mulitmodal imaging design, these authors show that stimulus-elicited amygdala-PFC connectivity in childhood predicts resting-state connectivity 2 years later. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74**.Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat Rev Neurosci. 2015;16(7):419–29. doi: 10.1038/nrn3950. This theoretical paper presents an argument that subcortical structures make predictions about the environment, which are sent to the cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tottenham N. Human amygdala development in the absence of species-expected caregiving. Dev Psychobiol. 2012;54(6):598–611. doi: 10.1002/dev.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barrett LF. The theory of constructed emotion: an active inference account of interoception and categorization. Soc Cog Affect Neuroscience. doi: 10.1093/scan/nsx060. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


