Abstract
The management of choroid plexus carcinoma is challenging and multifaceted. Here, we discuss a three-year-old girl with choroid plexus carcinoma and Li-Fraumeni syndrome who achieved full remission after surgery and chemotherapy, with radiation therapy spared. At recurrence, we used a novel, standard-dose cytotoxic chemotherapy regimen, focal proton radiation therapy, and targeted agents based on morphoproteomic analysis to achieve long-term survival. We highlight the rationale for our therapy at recurrence, as well as the risk-benefit analyses necessary in decision making for these patients. Our strategy may be effective in managing other patients with recurrent choroid plexus carcinoma and Li-Fraumeni syndrome.
Keywords: choroid plexus carcinoma, Li-Fraumeni syndrome, proton radiation, morphoproteomics, long-term survival
Introduction
Choroid plexus carcinoma (CPC, WHO Grade III) is a rare, aggressive brain tumor arising from the neuro-epithelial tissue that lines the ventricles and produces cerebrospinal fluid [1,2]. CPC often shows increased vascularity and invasion into brain parenchyma, hindering the likelihood of gross total resection (GTR), which has the largest impact on prognosis [1,2]. In addition to surgery, treatment usually involves chemotherapy, as well as radiation for children over three years old [3,4]. Approximately 40 percent of CPCs occur in patients with Li-Fraumeni syndrome (LFS), a cancer predisposition syndrome defined by heterozygous germline TP53 mutation, which can influence management and confers a poorer prognosis [5,6]. Here we discuss a case encapsulating several of these factors and describe a novel treatment strategy for recurrent LFS-related CPC.
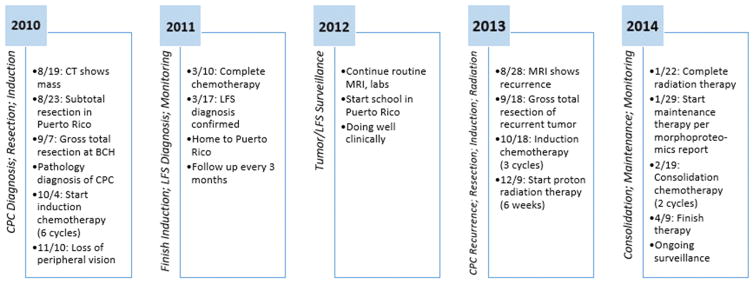
Case (Figure 1)
Figure 1.
Timeline describing the clinical course of our patient. Abbreviations: BCH - Boston Children’s Hospital; CPC - Choroid Plexus Carcinoma; LFS - Li-Fraumeni Syndrome
Our patient is a three-year-old previously healthy girl from Puerto Rico who initially presented to her local ED after falling from a high chair and suffering a closed head injury. On review of systems, her parents reported 1–2 headaches per week for three months and difficulty seeing the television. She had normal development, no prior hospitalizations, and no family history of cancer. Head CT showed a right parietal mass. Her initial surgery, performed in Puerto Rico, was complicated by severe intraoperative hemorrhage, resulting in partial resection.
Postoperatively, the patient was transferred to Boston Children’s Hospital for further management. Physical exam there was notable for normal vital signs, growth, and mental status. MRI showed a large heterogeneous mass filling the right lateral ventricle (Figure 2A). Two weeks after her initial surgery, GTR was achieved via second surgery. Pathology showed a CPC, while staging workup revealed no disseminated disease. Tumor immunohistochemical testing was positive for TP53, raising concern for LFS.
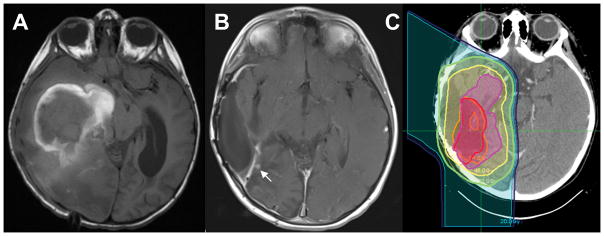
Figure 2.
T2-FLAIR MRI images at (A) initial diagnosis, and (B) recurrence, with the area of recurrent disease indicated by a white arrow. (C) The proton radiation therapy planning map, with the red volume indicating the recurrence tumor bed to receive 54 Gy RBE, and the pink volume indicating the original tumor bed to receive 45 Gy RBE, with other colors showing isodose lines indicating decreasing radiation doses with greater distance from the tumor bed, from 45 Gy RBE to 10 Gy RBE
We treated our patient as per Arm A of the International Consortium Choroid Plexus Tumor Protocol (CPT-SIOP-2009, NCT01014767), the standard therapy established by the preceding CPT-SIOP-2000 study. Treatment included six q4 week cycles of etoposide (100 mg/m2 days 1–5) and vincristine (1.5 mg/m2 day 5), with cyclophosphamide (1 g/m2 days 2–3) and carboplatin (350 mg/m2 days 2–3) alternating between cycles. Due to concern for neurodevelopmental toxicity, and increased radiation-induced second malignancy risk from potential LFS, her treatment was modified to exclude radiation therapy (RT). She instead received daily intraventricular etoposide (1 mg days 1–5) via Ommaya catheter concurrent with IV chemotherapy, the protocol-prescribed alternative for patients under 3.
During treatment, family and patient germline TP53 testing demonstrated a de novo G245S mutation in the patient consistent with LFS [7]. She completed therapy and began surveillance for recurrence, as well as for new primary malignancy per the Toronto childhood LFS protocol [8].
Thirty months off therapy, surveillance MRI showed a 4 mm enhancing nodule at the original tumor site (Figure 2B). Although there was no evidence of metastases, we presumed there to be further microscopic disease elsewhere around the original tumor bed. Our patient underwent complete surgical resection of the nodule, and pathology confirmed recurrent CPC. She began induction chemotherapy via an individualized treatment protocol based on Arm C of CPT-SIOP-2009, consisting of three q2 week cycles of high-dose methotrexate (5 g/m2 with leucovorin rescue at 36 hrs, 75 mg/m2 IV x1, then 15 mg/m2 q6 hrs until methotrexate level <0.1 μM) and weekly vincristine (1.5 mg/m2), with twice weekly intraventricular cytarabine (40 mg) and hydrocortisone (15 mg) via Ommaya. She had mild arachnoiditis from the cytarabine, successfully treated with dexamethasone. This was followed by 54 Gy RBE in 30 fractions of localized proton RT (Figure 2C), with concurrent weekly vincristine.
She then underwent consolidation chemotherapy with two q3 week cycles of doxorubicin (25 mg/m2 days 1–3), dactinomycin (45 ug/kg day 1), cisplatin (70 mg/m2 day 4), and vincristine (1.5 mg/m2 weekly), based on Arm B of CPT-SIOP-2009. A portion of her original tumor obtained before any chemotherapy during her resection at Boston Children’s Hospital was sent to the Morphoproteomics Laboratory at UTHealth, Houston for tumorigenic pathway analysis to identify additional treatment options. Morphoproteomics relies on bright field microscopy and incorporates the following with respect to the protein analytes in lesional and companionate cells: their immunohistochemical detection with the DAB chromogenic (brown signal); quantification of their signal intensity on a scale of 0 to 3+ and/or estimation of the percentage of immunopositive tumoral nuclei in the case of cell cycle-related protein analytes; their micro-anatomical regions within the specimen and subcellular compartmentalization; and an assessment of their state of activation to include phosphorylation, compartmental translocation, and functional grouping. In this patient’s specimen, 17 immunohistochemical probes were applied. Given the morphoproteomic findings showing expression of multiple upstream transducers (EGFR, IGF-1R) and downstream effectors (p-ERK, p-MTOR, p-AKT) in the context of TP53 germline mutation, and a pro-inflammatory tumor microenvironment with evidence of hypoxia (COX-2, HIF-1α), she began metformin, simvastatin, and melatonin concurrent with consolidation [9–11]. All analytes tested by morphoproteomics in this patient’s tumor, with supporting references from a National Library of Medicine’s MEDLINE Database literature review, proved to be therapeutic targets of metformin, melatonin, and/or a statin in the context of the patient’s TP53 germline mutation. She has remained on these three medications for maintenance since then.
Her only significant and unexpected toxicity overall was bilateral peripheral vision loss; this started during her initial treatment and progressed during treatment for recurrence. In the absence of other causes, we felt it was most likely due to late ongoing optic tract damage from increased intracranial pressure when she originally presented. Ongoing surveillance has shown no evidence of disease, now 83 months from initial diagnosis, 47 months from recurrence, and 40 months from the end of consolidation.
Discussion
Recent studies have improved our understanding of treatment and prognostic factors in CPC. Achievement of GTR remains the most important prognostic factor [1,2]. A widely-used chemotherapy regimen in the disease was established by the CPT-SIOP-2000 study. RT has been shown to improve overall survival (OS) but remains controversial due to concern for harmful effects on neurodevelopment, growth, and endocrine function [3,4]. LFS patients carry an increased risk of secondary malignancies from RT and alkylating agents, emphasizing the need for careful risk-benefit analyses in these cases [12].
With our patient’s CPC recurrence, therefore, we selected chemotherapy agents novel to her but also minimizing the secondary malignancy risk. We used regimens based on two of the experimental arms from CPT-SIOP-2009. For induction, we used high-dose methotrexate, given evidence for improved outcomes in children with high-risk brain tumors [13]. Because intraventricular chemotherapy is effective in CPC given its location, we added intraventricular cytarabine for its separate mechanism of action from methotrexate. Instead of using high-dose chemotherapy with autologous stem cell rescue (HDC/ASCR) – a potentially successful strategy in recurrent CPC but one that would necessitate a high dose alkylator – we chose consolidation with doxorubicin, cisplatin, and dactinomycin, based on their promising preliminary results [14].
Our initial decision to omit RT was consistent with the approach of other centers treating young children with CPC [5]. Upon relapse, though, we felt the current tumor outweighed the risk of future secondary tumors. We had extensive discussions with the family at all stages of the treatment planning process and completed written informed consents at each stage, given the long-term risks involved with her LFS, including secondary tumors. To mitigate these risks, we pursued localized proton RT in order to minimize dose distribution to non-target brain tissue [15]. Small studies have suggested that proton RT may carry reduced risk of radiation-induced secondary malignancies in children with brain tumors, although more robust studies and long-term follow-up are needed [15,16]. Furthermore, our patient’s age of six years at the time of recurrence meant a reduced risk of neurodevelopmental toxicity from radiation.
Based on morphoproteomic analysis findings, our patient started on three targeted medications that constitute her ongoing maintenance therapy. Although major outcome studies are lacking, morphoproteomics holds promise for personalized medicine [17], and we felt the potential benefit of these agents outweighed their limited risks. A previous report on relapsed brain tumors found that such personalized novel therapies could improve event-free survival [18].
With or without LFS, multiple studies have shown that CPC with somatic TP53 mutation has a significantly worse OS than wild type [6,19]. Our patient’s LFS further complicated her treatment. Reports of treatment for recurrent LFS-related CPC in the literature are very limited. One report details a patient with LFS and recurrent CPC who received HDC/ASCR without radiation and has survived five years [20]. Other studies in the literature include cases of recurrent CPC but make no mention of LFS status. Most notable among these is a case series of seven children with recurrent CPC after treatment on Head Start regimens. Four of the seven received radiation at recurrence; treatment for the others is not discussed. One of the four is alive 59 months from progression; the others died of disease [21]. Our approach is notable for its inclusion of intraventricular and non-alkylator systemic chemotherapy, proton RT, and targeted maintenance therapy, without HDC/ASCR. It is difficult to determine from this single case which components of our patient’s treatment regimen were effective or necessary. We believe, however, that this overall treatment strategy and risk-benefit analysis may be generalizable to other patients in this situation.
Footnotes
Author Disclosures: The authors have no conflicts of interest or funding to disclose.
References
- 1.Wolff JE, Sajedi M, Brant R, et al. Choroid plexus tumours. British Journal of Cancer. 2002;87(10):1086–1091. doi: 10.1038/sj.bjc.6600609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun MZ, Ivan ME, Clark AJ, et al. Gross total resection improves overall survival in children with choroid plexus carcinoma. Journal of Neuro-Oncology. 2014;116(1):179–185. doi: 10.1007/s11060-013-1281-5. [DOI] [PubMed] [Google Scholar]
- 3.Wolff JE, Sajedi M, Coppes MJ, et al. Radiation therapy and survival in choroid plexus carcinoma. Lancet. 1999;353(9170):2126. doi: 10.1016/s0140-6736(99)01744-4. [DOI] [PubMed] [Google Scholar]
- 4.Sun MZ, Ivan ME, Oh MC, et al. Effects of adjuvant chemotherapy and radiation on overall survival in children with choroid plexus carcinoma. Journal of Neuro-Oncology. 2014;120(2):353–360. doi: 10.1007/s11060-014-1559-2. [DOI] [PubMed] [Google Scholar]
- 5.Gozali AE, Britt B, Shane L, et al. Choroid plexus tumors; management, outcome, and association with the Li-Fraumeni syndrome: the Children’s Hospital Los Angeles (CHLA) experience, 1991–2010. Pediatric Blood & Cancer. 2012;58(6):905–909. doi: 10.1002/pbc.23349. [DOI] [PubMed] [Google Scholar]
- 6.Tabori U, Shlien A, Baskin B, et al. TP53 alterations determine clinical subgroups and survival of patients with choroid plexus tumors. Journal of Clinical Oncology. 2010;28(12):1995–2001. doi: 10.1200/JCO.2009.26.8169. [DOI] [PubMed] [Google Scholar]
- 7.Xu J, Qian J, Hu Y, et al. Heterogeneity of Li-Fraumeni syndrome links to unequal gain-of-function effects of p53 mutations. Sci Rep. 2014;4:4223. doi: 10.1038/srep04223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villani A, Tabori U, Schiffman J, et al. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: a prospective observational study. The Lancet Oncology. 2011;12(6):559–567. doi: 10.1016/S1470-2045(11)70119-X. [DOI] [PubMed] [Google Scholar]
- 9.Choi YK, Park KG. Metabolic roles of AMPK and metformin in cancer cells. Mol Cells. 2013;36(4):279–287. doi: 10.1007/s10059-013-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang YM, Jin BZ, Ai F, et al. The efficacy and safety of melatonin in concurrent chemotherapy or radiotherapy for solid tumors: a meta-analysis of randomized controlled trials. Cancer Chemotherapy and Pharmacology. 2012;69(5):1213–1220. doi: 10.1007/s00280-012-1828-8. [DOI] [PubMed] [Google Scholar]
- 11.Thurnher M, Nussbaumer O, Gruenbacher G. Novel aspects of mevalonate pathway inhibitors as antitumor agents. Clinical Cancer Research. 2012;18(13):3524–3531. doi: 10.1158/1078-0432.CCR-12-0489. [DOI] [PubMed] [Google Scholar]
- 12.Talwalkar SS, Yin CC, Naeem RC, et al. Myelodysplastic syndromes arising in patients with germline TP53 mutation and Li-Fraumeni syndrome. Arch Pathol Lab Med. 2010;134(7):1010–1015. doi: 10.5858/2009-0015-OA.1. [DOI] [PubMed] [Google Scholar]
- 13.Gardner SL, Asgharzadeh S, Green A, et al. Intensive induction chemotherapy followed by high dose chemotherapy with autologous hematopoietic progenitor cell rescue in young children newly diagnosed with central nervous system atypical teratoid rhabdoid tumors. Pediatric Blood & Cancer. 2008;51(2):235–240. doi: 10.1002/pbc.21578. [DOI] [PubMed] [Google Scholar]
- 14.Peters OBH, Marienhagen J, Friedrich M, et al. German Pediatric Atypical Teratoid/RHABDOID tumors: Interim analysis of the ATRT-CNS Pilot study and retrospective analysis. International Symposium on Pediatric Neuro-Oncology; Chicago. 2008. [Google Scholar]
- 15.Newhauser WD, Durante M. Assessing the risk of second malignancies after modern radiotherapy. Nature Reviews Cancer. 2011;11(6):438–448. doi: 10.1038/nrc3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stokkevag CH, Engeseth GM, Ytre-Hauge KS, et al. Estimated risk of radiation-induced cancer following paediatric cranio-spinal irradiation with electron, photon and proton therapy. Acta Oncologica. 2014;53(8):1048–1057. doi: 10.3109/0284186X.2014.928420. [DOI] [PubMed] [Google Scholar]
- 17.Brown RE. Morphoproteomics: exposing protein circuitries in tumors to identify potential therapeutic targets in cancer patients. Expert Rev Proteomics. 2005;2(3):337–348. doi: 10.1586/14789450.2.3.337. [DOI] [PubMed] [Google Scholar]
- 18.Wolff JE, Brown RE, Buryanek J, et al. Preliminary experience with personalized and targeted therapy for pediatric brain tumors. Pediatric Blood & Cancer. 2012;59(1):27–33. doi: 10.1002/pbc.23402. [DOI] [PubMed] [Google Scholar]
- 19.Merino DM, Shlien A, Villani A, et al. Molecular characterization of choroid plexus tumors reveals novel clinically relevant subgroups. Clinical Cancer Research. 2015;21(1):184–192. doi: 10.1158/1078-0432.CCR-14-1324. [DOI] [PubMed] [Google Scholar]
- 20.Mosleh O, Tabori U, Bartels U, et al. Successful treatment of a recurrent choroid plexus carcinoma with surgery followed by high-dose chemotherapy and stem cell rescue. Pediatric Hematology and Oncology. 2013;30(5):386–391. doi: 10.3109/08880018.2012.756089. [DOI] [PubMed] [Google Scholar]
- 21.Zaky W, Dhall G, Khatua S, et al. Choroid plexus carcinoma in children: the Head Start experience. Pediatric Blood & Cancer. 2015;62(5):784–789. doi: 10.1002/pbc.25436. [DOI] [PubMed] [Google Scholar]