Abstract
Background
Psoriasis is associated with risk of cardiovascular (CV) disease (CVD) and major adverse CV events (MACE). Whether psoriasis duration affects risk of vascular inflammation and MACE has not been well characterized.
Objectives
We utilized two resources to understand the effect of psoriasis duration on vascular disease and CV event: 1) a human imaging study and; 2) a population-based study of CVD events.
Methods
1) Patients with psoriasis (n=190) underwent 18F-fluorodeoxyglucose positron emission tomography (18-FDG PET) computed tomography (CT) (duration effect reported as a β-coefficient). 2) MACE risk was examined using nationwide registries (adjusted hazard ratios [HRs]) in patients with psoriasis (n=87,161) versus the general population (n=4,234,793).
Results
In the human imaging study, patients were young, of low CV risk by traditional risk scores and had a high prevalence of cardiometabolic diseases. Vascular inflammation by 18-FDG PET/CT was significantly associated with disease duration (β=0.171, p=0.002). In the population-based study, psoriasis duration had strong relationship with MACE (1.0% per additional year of psoriasis duration (HR 1.010; 95% confidence interval 1.007–1.013).
Limitations
These studies utilized observational data.
Conclusion
We found detrimental effects of psoriasis duration on vascular inflammation and MACE, suggesting that cumulative duration of exposure to low-grade chronic inflammation may accelerate vascular disease development and MACE. Providers should consider inquiring about duration of disease to counsel for heightened CVD risk in psoriasis.
Keywords: Psoriasis, Inflammation, Cardiovascular disease, 18-FDG PET/CT
Introduction
Patients with psoriasis have an increased incidence and prevalence of cardiovascular (CV) risk factors1 and CV disease (CVD).2–5 Vascular inflammation by 18F-fluorodeoxyglucose positron emission tomography computed tomography (18-FDG PET/CT), which is prognostic for future CV events, is increased in patients with psoriasis.6–8 Cumulative exposure to inflammation is linked to increased atherosclerosis and CV events, suggesting that longer disease duration might increase the risk for CVD and CV events. Thus, disease duration may represent a potentially easily obtainable measure of risk for CVD in inflammatory-based diseases.
We investigated whether longer duration of psoriasis would increase vascular inflammation and major adverse cardiovascular event (MACE) due to the longer exposure to chronic, low-grade systemic inflammation observed in psoriasis.
Materials
Detailed descriptions of study approvals, data sources, and methods are available online.9
Data sources
Data for the cohort study at the National Heart, Lung, and Blood Institute were obtained under the protocol titled “Psoriasis, Atherosclerosis and Cardiometabolic Disease Initiative (PACI)” (NCT01778569). Complete and accurate data (including medical conditions, pharmacotherapy, treatment interventions and surgical procedures, income, and vital statistics) on the Danish population are available from nationwide registers, which can be linked at individual-level.
Study populations
National Institute of Health (NIH) cohort
The longitudinal cohort study group (“NIH cohort”) comprised 190 consecutive patients with psoriasis, recruited from January 2013 through June 2016. A dermatologist or a certified physician confirmed the onset and duration of psoriasis, and assessed psoriasis severity using the PASI and body surface area (BSA) scores. Clinical parameters including blood pressure, height, weight, waist and hip circumferences were measured. Laboratory parameters including fasting blood glucose, fasting lipid panel, white blood count with differential, and systemic inflammatory markers including Hs-CRP and erythrocyte sedimentation rate (ESR) were evaluated in a clinical laboratory. All patients underwent 18-FDG PET/CT scans for quantification of vascular inflammation6–8.
Total Danish population cohort
The total Danish population cohort (henceforth “population cohort”) comprised all Danish citizens ≥18 years, alive and resident in Denmark on 1 January 2008 (study start).
Outcomes
NIH cohort
The primary outcome in the NIH cohort was TBR assessed by 18-FDG PET/CT (Siemens Biograph mCT PET/CT 64-slice scanner). 18-FDG uptake within the aorta was measured by utilizing dedicated analysis software for PET/CT (Extended Brilliance™ Workspace, Phillips Healthcare, Andover, MA, USA). One value of TBR was used as the primary outcome for each patient. The association between vascular inflammation and MACE was not examined. Detailed description of TBR calculation is provided in our previous work10.
Population cohort
The primary outcome in the population cohort was the first occurrence of a MACE, i.e. a composite of MI, ischemic stroke, and CV death, respectively. Secondary outcomes included specific occurrences of MI, ischemic stroke, and CV death.
Covariates
NIH Cohort
Baseline treatment for NIH cohort was defined for any of the following therapies: systemic non-biologic agents (henceforth “systemic”) or biologic therapy (systemic steroids, methotrexate, adalimumab, etanercept, and ustekinumab), statins, psoralen plus ultraviolet A (PUVA) or ultraviolet B (UVB), and topical treatments. Patients completed survey-based questionnaires regarding smoking, previous CVD, family history of CVD, and previous established diagnoses of hypertension and diabetes. Patient responses were then ascertained by interview with the physician. All parameters were assessed up to 12 months prior to study inclusion.
Population cohort
Baseline treatment up to 6 months before study inclusion was defined for the following therapies: biologics (adalimumab, efalizumab, etanercept, infliximab, and ustekinumab), cholesterol-lowering drugs, cyclosporine, methotrexate, PUVA/UVB, retinoids (acitretin/etretinate), and topical vitamin D analogues. Baseline comorbidity was assessed up to 5 years prior to study inclusion for the following conditions: alcohol abuse, smoking, CVD, diabetes, hypertension, and psoriatic arthritis. We calculated age-standardized socioeconomic status based on the average gross annual income during a 5-year period before study inclusion.
Statistical analysis
Summary statistics were presented as means with SD for normally distributed variables, medians and IQR for non-normally distributed continuous variables and frequencies for categorical variables. Normality was assessed by skewness and kurtosis. Parametric variables were compared between groups using Student’s t-test while Mann-Whitney U test was performed for non-parametric variables. Dichotomous variable comparisons were done using Pearson’s chi-square test.
NIH Cohort
Unadjusted regression analyses were performed to evaluate for potential relationships between cardiometabolic variables and psoriasis duration. We conducted multivariable linear regression analyses to evaluate the associations of vascular inflammation (TBR) with psoriasis duration. These analyses were adjusted for age at onset of psoriasis, sex, and traditional CV risk assessed by Framingham Risk Score, history of CVD, diabetes, hypertension, treatment with statins, smoking, and alcohol abuse. We assessed the ‘F’ statistics along with p-values as well as the root mean squared error values to assess for the goodness of fit for every model.
Population cohort
Incidence rates were summarized per 1,000 person-years, and Cox regression was performed to estimate hazard ratios (HRs). Multivariable models was adjusted for age, sex, socioeconomic status, previous CVD, smoking, alcohol abuse, diabetes, hypertension, and use of statins. We performed separate sensitivity analyses, where patients with psoriatic arthritis, and those with previous CVD, respectively, were excluded. We performed additional analyses where psoriasis was defined only using hospital diagnoses. Moreover, we did analyses where the population cohort was divided into older (born prior to 1960) and younger (born in or after 1960) individuals, respectively. Lastly, we repeated our analyses with adjustment for age at onset of psoriasis.
Results were presented with 95% confidence intervals (CIs) where applicable, and p-values less than 0.05 were considered statistically significant. Statistical analyses were performed with the STATA versions 12.0 and 13.0 (StataCorp, College Station, TX, USA) and SAS version 9.4 (SAS Institute Inc. Cary, NC, USA).
Results
National Institute of Health (NIH) cohort
The NIH cohort comprised 190 patients with mild-to-moderate psoriasis (Supplementary Table 1), primarily middle aged men (57% men), overweight, with mild insulin resistance (median insulin resistance of 2.77) but at low CV risk by Framingham risk score. Vascular inflammation assessed by target-to-background ratio (TBR) (mean ± standard deviation [SD] 1.70±0.26) demonstrated increased vascular inflammation.11 Strong associations with vascular inflammation were seen for male sex, smoking, Framingham 10-year risk, and BMI. The per-year incremental effect of psoriasis duration on vascular inflammation (assessed by TBR) showed that duration of psoriasis was associated with increased vascular inflammation (β=0.086, p=0.040), a relationship that persisted beyond adjustment for traditional cardiovascular risk factors (β=0.171, p=0.002) (Figure 1 and Supplementary Table 2).
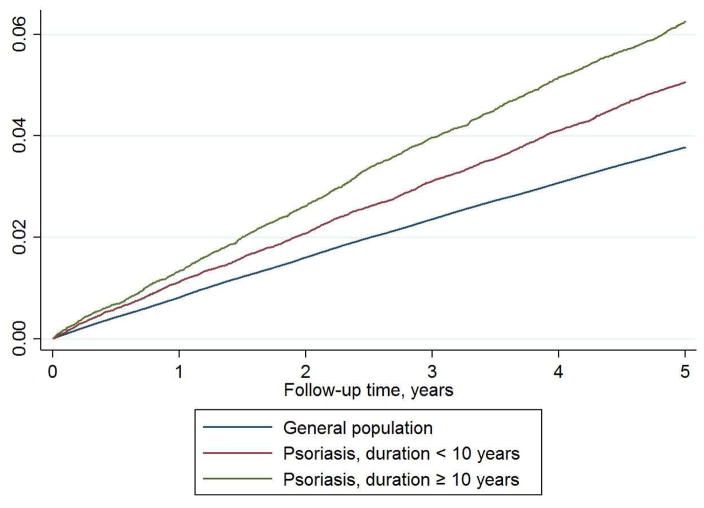
Figure 1. Nelson-Aalen cumulative hazards graph of the population cohort.
MACE risk in patients with psoriasis stratified based on disease duration less (n=57,941) or more (n=29,220) than 10 years, compared with the general population (n=4,234,793)
Population cohort
The population cohort comprised a total of 4,321,954 individuals, including 87,161 patients with psoriasis (maximum disease duration was 31.1 years) (Table 1). During a mean (SD) follow-up of 4.7 (0.9) years, a total of 152,122 and 4,472 patients experienced a MACE, in the general population (incidence rate/1,000 person-years: 7.56; 95% CI 7.52–7.60), and in patients with psoriasis (incidence rate/1,000 person-years: 10.94; 95% CI 10.62–11.26), respectively.
Table 1.
Baseline characteristics of the total population cohort
| Reference population (n=4,234,793) | Psoriasis (n=87,161) | |
|---|---|---|
|
| ||
| Age, mean (SD) | 48.6 (18.0) | 53.8 (16.3) |
| Sex, n (%) | ||
| Women | 2,148,653 (50.7) | 45,809 (52.6) |
| Men | 2,086,140 (49.3) | 41,352 (47.4) |
| Duration of psoriasis | ||
| Mean (SD) | NA | 7.8 (5.2) |
| Median (IQR) | NA | 7.7 (3.9–11.2) |
| Age at onset of psoriasis, mean (SD) | NA | 46.0 (16.6) |
| Socioeconomic status, mean (SD) | 2.0 (1.3) | 2.2 (1.3) |
| Hospitalized (inpatient) for psoriasis, n (%) | NA | 4,975 (5.7) |
| Smoking, n (%) | 337,551 (8.0) | 10,459 (12.0) |
| Comorbidity, n (%) | ||
| Alcohol abuse | 58,671 (1.4) | 1,672 (1.9) |
| Cardiovascular disease | 364,023 (8.6) | 11,737 (13.5) |
| Diabetes | 151,043 (3.6) | 5,376 (6.2) |
| Hypertension | 506,836 (12.0) | 15,908 (18.3) |
| Psoriatic arthritis | 2,515 (0.1) | 5,742 (6.6) |
| Treatment*, n (%) | ||
| Biologics | 2,760 (0.1) | 663 (0.8) |
| Cholesterol lowering drugs | 384,425 (9.1) | 12,436 (14.3) |
| Cyclosporine | 985 (0.0) | 193 (0.2) |
| Methotrexate | 13,971 (0.3) | 3,189 (3.7) |
| PUVA/UVB phototherapy | 346 (0.0) | 264 (0.3) |
| Retinoids (acitretin/etretinate) | 395 (0.0) | 553 (0.6) |
| Topical vitamin D analogues | NA | 12,621 (14.5) |
IQR, interquartile range; NA, not applicable; PUVA, psoralen plus ultraviolet A; SD, standard deviation; UVB, ultraviolet B
Within the last 6 months
In unadjusted analyses, psoriasis was associated with 3.9% increased risk of MACE per additional year of disease duration, and in multivariable analysis, the risk of MACE increased with 1.0% per additional year of psoriasis duration (HR 1.010; 95% CI 1.007–1.013) (Table 2 and Figure 2). For secondary endpoints, the multivariable HRs associated with duration of psoriasis were 1.006 (95% CI 1.001–1.012) for myocardial infarction (MI), 1.011 (95% CI 1.007–1.016) for ischemic stroke, and 1.011 (95% CI 1.007–1.015) for CV death, respectively.
Table 2.
Univariable and multivariable hazard ratios of MACE in patients with psoriasis compared with the general population
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value | |
| Duration of psoriasis | 1.039 | 1.036–1.042 | <0.0001 | 1.010 | 1.007–1.013 | <0.0001 |
| Age | 1.087 | 1.087–1.087 | <0.0001 | 1.075 | 1.075–1.076 | <0.0001 |
| Male sex | 1.230 | 1.218–1.242 | <0.0001 | 1.516 | 1.501–1.532 | <0.0001 |
| Socio-economic status | ||||||
| 0 (lowest) | 1.722 | 1.695–1.748 | <0.0001 | 1.118 | 1.101–1.136 | <0.0001 |
| 1 (below average) | 2.355 | 2.321–2.389 | <0.0001 | 1.113 | 1.096–1.130 | <0.0001 |
| 2 (average) | (reference) | (reference) | ||||
| 3 (above average) | 0.536 | 0.526–0.547 | <0.0001 | 0.855 | 0.838–0.873 | <0.0001 |
| 4 (highest) | 0.478 | 0.468–0.488 | <0.0001 | 0.744 | 0.729–0.760 | <0.0001 |
| Previous CVD | 8.667 | 8.581–8.755 | <0.0001 | 2.278 | 2.252–2.305 | <0.0001 |
| Statin use | 3.594 | 3.556–3.631 | <0.0001 | 0.908 | 0.897–0.919 | <0.0001 |
| Diabetes | 3.743 | 3.692–3.795 | <0.0001 | 1.420 | 1.399–1.441 | <0.0001 |
| Hypertension | 5.721 | 5.665–5.778 | <0.0001 | 1.394 | 1.379–1.411 | <0.0001 |
| Alcohol abuse | 1.612 | 1.565–1.659 | <0.0001 | 1.905 | 1.850–1.961 | <0.0001 |
| Smoking | 2.654 | 2.620–2.689 | <0.0001 | 1.697 | 1.676–1.720 | <0.0001 |
CVD, cardiovascular disease; CI, confidence interval
Figure 2. Association between vascular inflammation and psoriasis disease duration in the NIH cohort.
Unadjusted and fully adjusted regression plots demonstrate a direct association between psoriasis disease duration and vascular inflammation in the NIH cohort beyond traditional cardiovascular risk factors.
Discussion
We utilized a human imaging study and a population-based study to investigate whether the duration of psoriasis increases the risk of CVD and MACE, and present novel and convincing evidence to suggest a detrimental effect of psoriasis duration on CVD beyond traditional CV risk factors, even in patients with low CV risk scores. Notably, every 1 SD increase in duration of psoriasis increases the TBR by 2.5%, which roughly translates into an absolute increase of 10% in future adverse events based on a recent large population study.12
Similar to rheumatoid arthritis,13 most but not all studies have associated psoriasis with an increased risk of CVD,2–5, 14–16 yet data suggest that classical CV risk factors alone do not fully capture this heightened risk, and systemic inflammation has been put forward as a potential explanation for the link between psoriasis and CVD. Along this line, inflammation is associated with impaired insulin sensitivity, carotid intima-media thickening, endothelial dysfunction, aortic stiffness, vascular inflammation, and coronary plaque burden and such CVD markers occur more frequently in patients with psoriasis.17–19 These insults could occur due to a consistent vascular damage consequent to immune activation in those with longer duration of disease.
We opted for a translational epidemiological approach, utilizing a human imaging study combined with observational registry data to emphasize the putative clinical relevance of increased vascular inflammation observed in our study. Specifically, we found a 1% increase in future CV event risk per additional year of disease duration, an effect size similar to smoking. Each of components of the composite outcome of MACE were also increased with longer duration of disease suggesting that duration of inflammation increases all vascular disease related events. Nevertheless, we emphasize that the independent association between psoriasis and CVD remains a topic of ongoing debate,20 as does the effect of systemic therapy on the vascular inflammation and CV events.21
A limitation in the population cohort was a lack of information for body mass index and levels of physical exercise. However, such data were available in the NIH cohort, and adjustment for body mass index did not significantly change the relationship between duration of disease and vascular inflammation. Furthermore, previous CVD and diabetes, two major predictors of MACE in the population cohort did not relate to vascular inflammation in the NIH cohort. This is likely due to the low number of patients with these characteristics in the NIH cohort. Important strengths include the sheer number of participants and the high accuracy of the nationwide registries, which minimized bias due to sex, age, concurrent medication, and socioeconomic status. Observational data alone from a single study is not sufficient to establish causality. However, comparable findings across different populations, using our approach of both observational and vascular imaging data add strength and credibility to our findings and the results are consistent with prior work demonstrating that duration of psoriasis is an independent risk factor for MI and coronary artery disease measured by angiography.22, 23
In conclusion, we have provided strong novel evidence that duration of psoriasis increases vascular inflammation and the risk of MACE. Providers should consider inquiring about duration of psoriasis when assessing CV risk stratification.
Supplementary Material
Acknowledgments
Funding
The work at the NIH cohort was supported by the National Heart, Lung and Blood Institute Intramural Research Program (HL006193-02). Dr. Gelfand’s role in this study is supported by NIAMS grant K24-AR064310
| Funding/Sponsor was involved? | ||
|---|---|---|
| Design and conduct of the study | Yes__ | No _X_ |
| Collection, management, analysis and interpretation of data | Yes__ | No _X_ |
| Preparation, review, or approval of the manuscript | Yes__ | No _X_ |
| Decision to submit the manuscript for publication | Yes__ | No _X_ |
Abbreviations
- 18-FDG PET
18F-fluorodeoxyglucose positron emission tomography
- CCTA
Coronary computed tomography angiography
- CT
Computed tomography
- CV
Cardiovascular
- CVD
Cardiovascular disease
- CI
Confidence interval
- HR
Hazard ratio
- IQR
Interquartile range
- MACE
Major adverse cardiovascular events
- MI
Myocardial infarction
- NIH
National Institute of Health
- PUVA
Psoralen plus ultraviolet A
- SD
Standard deviation
- TBR
Target-to-background ratio
- UVB
Ultraviolet B
Footnotes
Study/ethical approval
Study approval was obtained from by the Danish Data Protection Agency (ref. 2007-58-0015, int. ref. GEH-2014-018, I-Suite 02736). Review of an ethics committee is not required for register studies in Denmark. Data for the cohort study at the National Heart, Lung, and Blood Institute were obtained under the protocol titled “Psoriasis, Atherosclerosis and Cardiometabolic Disease Initiative (PACI)” (NCT01778569).
Author Contributions:
Dr. Egeberg (Danish cohort) and Dr. Mehta (NIH cohort) had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Egeberg, Skov, Mallbris, Gislason, Gelfand, and Mehta. Acquisition, analysis, and interpretation of data: (NIH cohort): Joshi, Ahlman, Rodante, Lerman, Gelfand, and Mehta (Danish cohort): Egeberg and Gislason. Drafting of the manuscript: Egeberg and Mehta. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Egeberg, Gislason and Joshi. Obtained funding: Mehta. Administrative, technical, or material support: Egeberg, Skov, Gislason and Mehta. Study supervision: Egeberg and Mehta.
Disclosures/Conflicts of interest
Dr. Egeberg has received research funding from Pfizer and Eli Lilly, and honoraria as consultant and/or speaker from Pfizer, Eli Lilly, Novartis, Galderma, and Janssen Pharmaceuticals. Dr. Skov has been a paid speaker for Pfizer, AbbVie, Eli Lilly, Novartis and LEO Pharma, and has been a consultant or served on Advisory Boards with Pfizer, AbbVie, Janssen Cilag, Novartis, Eli Lilly, LEO Pharma and Sanofi. She has served as an investigator for Pfizer, AbbVie, Eli Lilly, Novartis, Amgen, Regeneron and LEO Pharma and received research and educational grants from Pfizer, AbbVie, Novartis, Sanofi, Janssen Cilag and Leo Pharma. Dr. Mallbris is currently employed by Eli Lilly. Dr. Gislason is supported by an unrestricted research scholarship from the Novo Nordisk Foundation and reports research grants from Pfizer, Bristol-Myers Squibb, AstraZeneca, Bayer and Boehringer Ingelheim. Dr. Wu received research funding from AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Coherus Biosciences, Dermira, Eli Lilly, Janssen, Merck, Novartis, Pfizer, Regeneron, Sandoz, and Sun Pharmaceutical Industries; he is a consultant for AbbVie, Amgen, Celgene, Dermira, Eli Lilly, Pfizer, Regeneron, and Sun Pharmaceutical Industries. In the previous 12 months Dr. Gelfand served as a consultant for Coherus (DSMB), Dermira, Janssen Biologics, Merck (DSMB), Novartis Corp, Regeneron, Sanofi and Pfizer Inc., receiving honoraria; and receives research grants (to the Trustees of the University of Pennsylvania) from Abbvie, Janssen, Novartis Corp, Regeneron, Sanofi, Celgene, and Pfizer Inc.; and received payment for continuing medical education work related to psoriasis that was supported indirectly by Lilly and Abbvie. Dr. Gelfand is a co-patent holder of resiquimod for treatment of cutaneous T cell lymphoma. Dr. Mehta is a full-time United States government employee.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference list
- 1.Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. Journal of the American Academy of Dermatology. 2006;55:829–35. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 2.Gelfand JM, Azfar RS, Mehta NN. Psoriasis and cardiovascular risk: strength in numbers. The Journal of investigative dermatology. 2010;130:919–22. doi: 10.1038/jid.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gelfand JM, Mehta NN, Langan SM. Psoriasis and cardiovascular risk: strength in numbers, part II. The Journal of investigative dermatology. 2011;131:1007–10. doi: 10.1038/jid.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. Jama. 2006;296:1735–41. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 5.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. European heart journal. 2010;31:1000–6. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta NN, Yu Y, Saboury B, Foroughi N, Krishnamoorthy P, Raper A, et al. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Archives of dermatology. 2011;147:1031–9. doi: 10.1001/archdermatol.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta NN, Torigian DA, Gelfand JM, Saboury B, Alavi A. Quantification of atherosclerotic plaque activity and vascular inflammation using [18-F] fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) J Vis Exp. 2012:e3777. doi: 10.3791/3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naik HB, Natarajan B, Stansky E, Ahlman MA, Teague H, Salahuddin T, et al. Severity of Psoriasis Associates With Aortic Vascular Inflammation Detected by FDG PET/CT and Neutrophil Activation in a Prospective Observational Study. Arteriosclerosis, thrombosis, and vascular biology. 2015 doi: 10.1161/ATVBAHA.115.306460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egeberg Alexander. JAAD-D-17-00307 Supplementary Materials. Mendeley Data, v1. 2017 http://dx.doi.org/10.17632/t7syfrg4yr.1. [Google Scholar]
- 10.Naik HB, Natarajan B, Stansky E, Ahlman MA, Teague H, Salahuddin T, et al. Severity of Psoriasis Associates With Aortic Vascular Inflammation Detected by FDG PET/CT and Neutrophil Activation in a Prospective Observational Study. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:2667–76. doi: 10.1161/ATVBAHA.115.306460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011;378:1547–59. doi: 10.1016/S0140-6736(11)61383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging. 2013;6:1250–9. doi: 10.1016/j.jcmg.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Annals of the rheumatic diseases. 2012;71:1524–9. doi: 10.1136/annrheumdis-2011-200726. [DOI] [PubMed] [Google Scholar]
- 14.Egeberg A, Thyssen JP, Jensen P, Gislason GH, Skov L. Risk of Myocardial Infarction in Patients with Psoriasis and Psoriatic Arthritis: A Nationwide Cohort Study. Acta dermato-venereologica. 2017 doi: 10.2340/00015555-2657. [DOI] [PubMed] [Google Scholar]
- 15.Parisi R, Rutter MK, Lunt M, Young HS, Symmons DP, Griffiths CE, et al. Psoriasis and the Risk of Major Cardiovascular Events: Cohort Study Using the Clinical Practice Research Datalink. The Journal of investigative dermatology. 2015 doi: 10.1038/jid.2015.87. [DOI] [PubMed] [Google Scholar]
- 16.Dowlatshahi EA, Kavousi M, Nijsten T, Ikram MA, Hofman A, Franco OH, et al. Psoriasis is not associated with atherosclerosis and incident cardiovascular events: the Rotterdam Study. The Journal of investigative dermatology. 2013;133:2347–54. doi: 10.1038/jid.2013.131. [DOI] [PubMed] [Google Scholar]
- 17.Boehncke WH, Boehncke S, Tobin AM, Kirby B. The ‘psoriatic march’: a concept of how severe psoriasis may drive cardiovascular comorbidity. Experimental dermatology. 2011;20:303–7. doi: 10.1111/j.1600-0625.2011.01261.x. [DOI] [PubMed] [Google Scholar]
- 18.Balci DD, Balci A, Karazincir S, Ucar E, Iyigun U, Yalcin F, et al. Increased carotid artery intima-media thickness and impaired endothelial function in psoriasis. J Eur Acad Dermatol Venereol. 2009;23:1–6. doi: 10.1111/j.1468-3083.2008.02936.x. [DOI] [PubMed] [Google Scholar]
- 19.Gyldenlove M, Storgaard H, Holst JJ, Vilsboll T, Knop FK, Skov L. Patients with psoriasis are insulin resistant. Journal of the American Academy of Dermatology. 2015;72:599–605. doi: 10.1016/j.jaad.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Ogdie A, Troxel AB, Mehta NN, Gelfand JM. Psoriasis and Cardiovascular Risk: Strength in Numbers Part 3. The Journal of investigative dermatology. 2015;135:2148–50. doi: 10.1038/jid.2015.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bissonnette R, Harel F, Krueger JG, Guertin MC, Chabot-Blanchet M, Gonzalez J, et al. TNF-alpha antagonist and vascular inflammation in patients with psoriasis vulgaris: a randomized placebo-controlled study. The Journal of investigative dermatology. 2017 doi: 10.1016/j.jid.2017.02.977. [DOI] [PubMed] [Google Scholar]
- 22.Li WQ, Han JL, Manson JE, Rimm EB, Rexrode KM, Curhan GC, et al. Psoriasis and risk of nonfatal cardiovascular disease in U.S. women: a cohort study. Br J Dermatol. 2012;166:811–8. doi: 10.1111/j.1365-2133.2011.10774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong AW, Harskamp CT, Ledo L, Rogers JH, Armstrong EJ. Coronary artery disease in patients with psoriasis referred for coronary angiography. The American journal of cardiology. 2012;109:976–80. doi: 10.1016/j.amjcard.2011.11.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.